Abstract
A prolonged swimming trial is the most common approach in studying steady-state changes in oxygen uptake, cardiac output and tissue oxygen extraction as a function of swimming speed in salmonids. The data generated by these sorts of studies are used here to support the idea that a maximum oxygen uptake is reached during a critical swimming speed test. Maximum oxygen uptake has a temperature optimum. Potential explanations are advanced to explain why maximum aerobic performance falls off at high temperature. The valuable information provided by critical swimming tests can be confounded by non-steady-state swimming behaviours, which typically occur with increasing frequency as salmonids approach fatigue. Two major concerns are noted. Foremost, measurements of oxygen uptake during swimming can considerably underestimate the true cost of transport near critical swimming speed, apparently in a temperature-dependent manner. Second, based on a comparison with voluntary swimming ascents in a raceway, forced swimming trials in a swim tunnel respirometer may underestimate critical swimming speed, possibly because fish in a swim tunnel respirometer are unable to sustain a ground speed.
Keywords: critical swimming speed, oxygen consumption, cost of swimming, heart, cardiac output, temperature optimum
1. Introduction
Fish exercise has been categorized according to the duration of the swim as sustained, prolonged or burst swimming (Hoar & Randall 1978). In terms of cardiorespiratory studies during swimming, by far the majority have focused on prolonged swimming and have used salmonids as the model fish. The critical swimming speed test is commonly used to assess prolonged swimming performance (see Beamish (1978) for details). During the test, the fish are forced to swim against a water current within a swimming chamber of a fixed length while water velocity is increased incrementally until the fish fatigues. Critical swimming speed (Ucrit) is interpolated from this final level of swimming performance. Prolonged swimming speed is defined as one that can be maintained for 15–200 min (Hoar & Randall 1978) although most, but not all, Ucrit tests use increment durations of 15–60 min. The duration of the speed increment is important because a minimum time-interval of 15 min is probably needed for cardiorespiratory activity to reach a steady state. Even though heart rate can change quickly, cardiac output, blood pressure and blood gas tensions can take several minutes to reach a steady state at a new speed increment (Kiceniuk & Jones 1977; figure 1). Nevertheless, when performed correctly, Ucrit tests have afforded researchers the opportunity to derive relationships between prolonged swimming speeds and many cardiorespiratory variables, including oxygen uptake (Mo2), cardiac and respiratory frequencies, blood gas tensions and cardiac output.
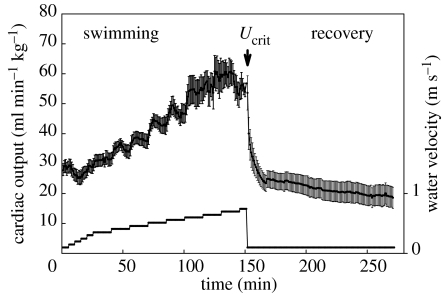
Figure 1.
An example of the changes in cardiac output in response to step increases in water velocity during a critical swimming speed test. The data (courtesy of Simonot 2006) are taken from triploid rainbow trout (Oncorhynchus mykiss; n=8; body mass=823 g; fork length, 37.4 cm; water temperature, 12–14°C). Cardiac output (mean±SEM) generally increases incrementally with swimming speed up to a maximum value and shows signs of a collapse at the critical swimming speed (Ucrit). In addition, the continuous record clearly shows that at several of the intermediate swimming speeds cardiac output can initially overshoot the steady-state cardiac output. Also note that at the start of the swim test routine cardiac output was clearly elevated, as indicated by a lower steady-state cardiac output at the end of the 100 min recovery period following fatigue at Ucrit.
The cardiorespiratory responses to prolonged swimming have been reviewed previously (Jones & Randall 1978; Farrell & Jones 1992; Farrell 1996). Likewise, the effects of temperature on cardiorespiratory performance have been considered (Farrell 1997, 2002; Taylor et al. 1997; Pörtner 2002). An important point emerging from these reviews is the idea of a thermal optimum for maximum cardiorespiratory performance in fishes, above which there is a cardiorespiratory system collapse. Therefore, an objective of this perspective is to briefly present evidence in support of the idea that the cardiorespiratory system is operating maximally at or near Ucrit, and then examine what factors might cause the cardiorespiratory collapse above the thermal optimum. These insights into the thermal limits of cardiorespiratory performance will probably find application in understanding the potential consequences of climate change, which has already resulted in fish facing higher water temperatures in both polar (A. P. Farrell 2003, personal observation, Danish Arctic Marine Station) and temperate climates (Foreman et al. 2001).
This perspective also focuses on the importance of gait transition during the critical swimming speed test. As salmonids approach Ucrit, they change swimming gait. They recruit more powerful and faster muscle types when they switch from aerobic to anaerobic swimming. However, studying cardiorespiratory responses after gait transition is difficult and data will be used to illustrate some associated limitations and potential analytical pitfalls. In addition, it will be shown that important performance differences exist between fish forced to swim in typical aquatic treadmills and fish with a free choice to decide if and when they swim against water currents. This perspective will close by concluding that while critical swimming tests continue to be extremely useful for investigating cardiorespiratory performance during prolonged swimming and can do a good job of assessing maximum aerobic and cardiovascular performance, our understanding of gait transition needs to be expanded for correct interpretation of data obtained from critical swimming tests.
2. Maximum cardiorespiratory performance, temperature effects and temperature optima
Underpinning the idea of a thermal optimum for maximum cardiorespiratory performance in fish is the need to be able to reliably measure maximum Mo2. There is good evidence to suggest that salmonids reach their maximum Mo2 near Ucrit, provided the critical swim test is properly performed. The key features that allow us to conclude that the internal oxygen convection system is operating maximally near Ucrit are: (i) arterial blood is being pumped at the maximum flow rate by the heart, (ii) arterial blood is fully saturated with oxygen at the gills, and (iii) venous blood has been maximally depleted of oxygen by the tissues. Key evidence in support of each of these three features is presented below.
Cardiac output increases with increasing swimming speed (figure 1). Fish can continue to increase their swimming speed even though cardiac output has reached a plateau before Ucrit (figure 1). Thus, the suggestion has been made that cardiac output is either at or very near its physiological maximum when Ucrit is reached (Farrell 2002). To support this suggestion are in vitro measurements of the maximum pumping capacity of the heart. There is an excellent agreement between direct measurements in vivo of the maximum cardiac output near Ucrit in rainbow trout, and the in vitro measurements of maximum cardiac pumping capacity when the heart has been maximally stimulated with adrenaline (figure 2). Moreover, the agreement between in vitro and in vivo data spans a wide temperature range. Therefore, it seems unlikely that the rainbow trout heart is able to pump blood around the circulation at a higher rate than that observed when rainbow trout are swimming near their Ucrit. An important exception is at high temperature, when this agreement does break down, a critical feature that I return to later.
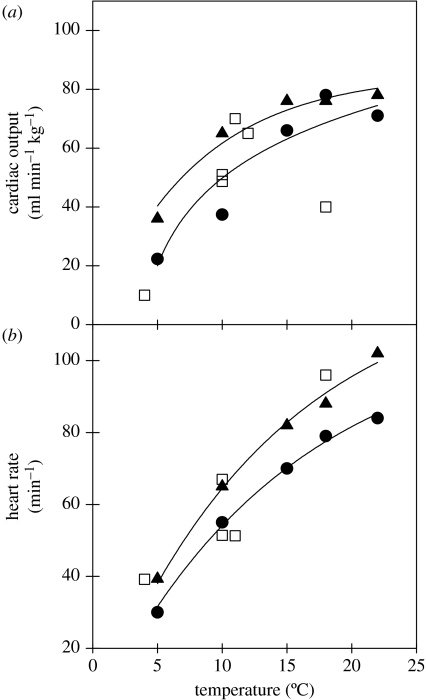
Figure 2.
A comparison of in vivo and in vitro maximum cardiac output and heart rate values for rainbow trout (Oncorhynchus mykiss). The in vivo values (open square symbols) were from critical swimming speed tests over a range of acclimation temperatures. The in vitro cardiac performance values (closed symbols) were from experiments with the ‘Farrell’ perfused heart preparation in which maximum performance was measured with either minimal (closed circles and solid line) or maximum (closed triangles and solid line) adrenergic stimulation over a range of acclimation temperatures. There are two important points to note from these comparisons. First, the in vitro data with maximum adrenergic stimulation are generally as good as or better than the in vivo data. Second maximum cardiac output in vitro clearly plateaus at warm temperatures, but collapses in vivo. Data sources were as follows. In vivo data: Kiceniuk & Jones 1977; Taylor et al. 1996; Thorarensen et al. 1996; Brodeur et al. 2001. In vitro data: Farrell et al. 1986, 1991, 1996; Graham & Farrell 1989; Milligan & Farrell 1991; Keen & Farrell 1994.
Arterial blood in resting salmonids is essentially fully saturated with oxygen, and it remains this way during, and even at, the completion of critical swimming tests with salmonids (Kiceniuk & Jones 1977; Randall & Daxboeck 1982; Thorarensen et al. 1993; Gallaugher et al. 2001). Consequently, with arterial blood fully saturated and the heart pumping maximally, arterial oxygen transport (i.e. the product of the two variables) must be maximal as salmonids approach Ucrit unless blood haemoglobin concentration changes appreciably. However, blood haemoglobin concentration also appears to be near an optimum for swimming in rainbow trout. Only minor improvements in Ucrit and maximum Mo2 occurred when rainbow trout were made polycythemic (using red blood cells from a donor fish) in an attempt to increase maximum arterial oxygen transport by increasing their arterial oxygen carrying capacity (Gallaugher et al. 1995; Gallaugher & Farrell 1998). Polycythemia, however, does induce a mild arterial hypoxemia at Ucrit (Gallaugher et al. 1995), an observation that suggests oxygen diffusion, between the water and the blood contained in the gill secondary lamellae, may be approaching some optimum when normocythemic rainbow trout are swimming near their maximum Mo2. This is not to say that haemoglobin concentration cannot be influenced by temperature acclimation and by release of red blood cells from the spleen during swimming (Gallaugher & Farrell 1998). Consequently, the increase in arterial oxygen transport associated with prolonged swimming in salmonids not only reaches a maximum near Ucrit, but it comes almost entirely from the increase in cardiac output.
In addition to supplying more oxygen to working muscles through increased cardiac output, salmonids can increase tissue oxygen extraction during prolonged swimming tests by an equivalent amount. As a result, venous oxygen content and oxygen tension decrease as swimming speed increases (Stevens & Randall. 1967; Kiceniuk & Jones 1977; Farrell & Clutterham 2003). Continuous measurements of venous oxygen content are technically difficult, and so it is not known if venous oxygen content reaches a plateau. However, venous oxygen tension (Pvo2) has been continuously measured in rainbow trout, and it seems that a minimum plateau for Pvo2 is reached before Ucrit (figure 3). While this result has been interpreted to mean that tissue oxygen extraction is either approaching or has reached a maximum near Ucrit, a caveat is the possibility that increases in CO2 and/or H+ outputs from skeletal muscle could increase tissue oxygen extraction without decreasing Pvo2 through Bohr and Root effects on haemoglobin.
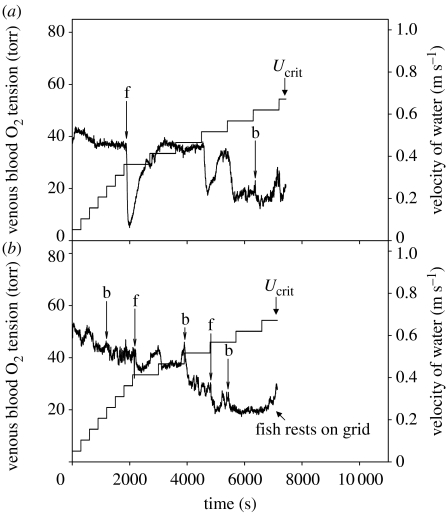
Figure 3.
Examples of continuous recording of the partial pressure of oxygen in venous blood of individual rainbow trout (Oncorhynchus mykiss) to illustrate the progressive changes that occur during a Ucrit test and the abrupt changes associated with the fish fighting (f) and burst-and-glide swimming (b). (a) Illustration of how non-steady-state swimming can profoundly alter venous oxygen tension. (b) Illustration of how venous oxygen tension can plateau before fish quit swimming at their critical swimming speed and an increase in venous oxygen tension can be resolved when the fatigued fish rests on the grid at the back of the swim chamber. Venous oxygen tension was recorded by a fibreoptic oxygen electrode implanted in the ductus Cuvier. The increments in swimming speed are indicated separately. Data were taken from Farrell & Clutterham (2003).
This minimum plateau for Pvo2 is important in another regard. The heart is a terminal organ in fish circulation in terms of oxygen supply; the myocardial oxygen supply in rainbow trout relies largely (and completely in some other species) on the oxygen contained in venous blood (Davie & Farrell 1991; Farrell 1993). It is Pvo2 that is important in terms of oxygen delivery to the heart and not venous oxygen content because, even during exercise, venous oxygen content greatly exceeds the myocardial oxygen needs (Farrell 1987). What ultimately sets the lower limit for Pvo2 returning to the heart during swimming is unclear, but there would certainly be dire consequences to the heart if venous blood was completely stripped of oxygen by somatic tissues. Thus, it has been suggested that somatic tissue oxygen extraction needs to be limited during swimming, otherwise the heart will become hypoxic and maximum cardiac performance will fall off (Farrell 1997; Farrell & Clutterham 2003).
Given that arterial oxygen transport and tissue oxygen extraction both approach maximum levels when salmonids perform critical swimming speed tests, it is not unreasonable to conclude that Mo2 is also maximal when salmonids approach Ucrit. The question now addressed is how is this maximum cardiorespiratory performance influenced by water temperature?
Maximum cardiorespiratory performance is temperature dependent (Brett 1964, 1971; Randall & Brauner 1991; Taylor et al. 1996). Cardiac output and heart rate in salmonids typically have Q10 values of 1.2–2.0 over much of their temperature range (see figure 2; Farrell 2002). In addition, maximum Mo2 has a temperature optimum in salmonids, which is close to their upper temperature limit, like many other physiological processes. Figure 4 illustrates how temperature optima for maximum Mo2 can vary among adult Pacific salmon stocks. Nonetheless, the temperature optima for these salmon stocks were reasonably broad (Lee et al. 2003a) because 90% of the maximum Mo2 could be maintained over a 4–5°C range. A broad temperature optimum for maximum Mo2 is perhaps expected for eurythermal fish such as Pacific salmon since year-to-year the population encounters a fairly wide range of temperatures (figure 4) when they migrate upstream in the Fraser River watershed (Farrell 1997). A broad temperature optimum represents a challenge, however, when calculating and comparing Q10 values, especially if just two temperature measurements lie on either side of the temperature optimum (i.e. an erroneous conclusion of thermal compensation at the higher temperature might be reached when, in fact, cardiorespiratory performance had declined from an unmeasured optimum). A proper appreciation of temperature effects near the thermal optimum will require that temperature is treated as a continuous variable, just as the original data were treated to generate the temperature curves in figure 4.
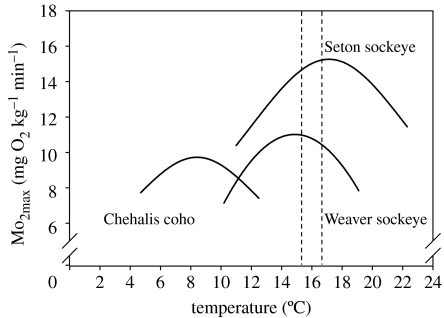
Figure 4.
Examples of the temperature optima for maximum oxygen uptake in adult (2–3 kg) sockeye (Oncorhynchus nerka) and coho (Oncorhynchus kisutch) salmon from the Fraser River watershed, BC, Canada (N=30–40 fish per stock). The higher Mo2max (and Ucrit; figure 5) for the Seton stock is presumed to be related to the fact that this stock has a longer and more arduous up-river migration. There is variability among the temperature optima for the two salmonid species, as well as the two stocks of the same species. Also, there is a reasonable match between the temperature optima of the two sockeye salmon stocks and 60 year average water temperature encountered when these stocks migrate up the main stem of the Fraser River (temperature bounded by the vertical broken lines). Data were adapted from Lee et al. (2003a) and are based on field studies.
Routine Mo2 does not plateau at the same temperature as Mo2max, but continues to increase exponentially (Lee et al. 2003a). Therefore, at high temperature, there is a theoretical intersection between the curves that describe the increasing routine Mo2 and the collapsing maximum Mo2. This theoretical intersection is the temperature at which aerobic scope is zero and above which fish should die, i.e. a critical temperature, Tcrit. Interestingly, in some years when the Fraser River temporarily reaches temperatures higher than the range indicated in figure 4, many salmon do not complete their migration.
The question of what causes maximum cardiorespiratory performance to decline above an optimum temperature has received detailed consideration elsewhere (Brett 1971; Farrell 1997, 2002; Taylor et al. 1997; Pörtner 2002). Several explanations have been put forward (gill related, cardiac related and muscle related).
In terms of the gills, Brett (1971) suggested that salmonids may be unable to sufficiently increase gill ventilation to compensate for the decrease in water oxygen content that naturally occurs as temperature increases. However, hyperoxic water does not increase Ucrit (Davis et al. 1963; Jones 1971). Therefore, water delivery to, and oxygen extraction by, the gills may not be an immediate problem.
Based on studies with perfused rainbow trout gills, it has been concluded that gas exchange is perfusion limited on the blood side of the gills under resting normoxic conditions, whereas diffusion limitations come more into play during exercise even though gill oxygen diffusing capacity increases with exercise (Daxboeck et al. 1982; Randall & Daxboeck 1982). With increasing water temperature in resting rainbow trout, arterial blood saturation decreases (Heath & Hughes 1973), a finding that could reflect a perfusion limitation (blood transit time in rainbow trout secondary lamellae is estimated to be approximately 1 s and maximum stroke volume of the heart—approx. 1 ml kg−1—does not exceed the lamellar blood volume; Randall & Daxboeck 1982). Conversely, polycythemia induces arterial hypoxemia in exercising rainbow trout (Gallaugher et al. 1995), a finding that supports the idea of a diffusion limitation at the secondary lamellae. Whether or not this diffusion limitation at the gill increases with temperature needs to be tested experimentally.
In terms of the heart, three factors may contribute to problems at high temperature. First, although mass-specific myocardial power output does increase with temperature (e.g. Overgaard et al. 2004), relative ventricular mass decreases with acclimation temperature in rainbow trout (Farrell et al. 1988). Thus, with less cardiac muscle mass available to pump blood, any accrued benefit of high temperature is diminished and, not surprisingly, maximum cardiac stroke volume decreases at high acclimation temperatures (Farrell et al. 1996). The second problem is that salmonids appear to use anaerobic swimming to a greater extent in critical swimming speed tests (see below; Brett 1964; Jain & Farrell 2003; Lee et al. 2003b). Anaerobic swimming leads to acidosis, hypoxia and hyperkalemia of the venous blood (Kiceniuk & Jones 1977; Holk & Lykkeboe 1998), conditions individually and collectively debilitating for maximum cardiac pumping because they inhibit contractility (Driedzic & Gesser 1994). These adverse conditions worsen at warm temperatures in rainbow trout (Jain & Farrell 2003). The third problem is that high temperature diminishes the effects of adrenaline on cardiac tissue. Adrenergic stimulation mobilizes the reserve capacity for cardiac pumping and protects the cardiac tissues against the negative effects of acidosis, hypoxia and hyperkalemia. Both of these beneficial cardiac effects are attenuated at high temperature (Farrell et al. 1996).
Earlier, a lack of correspondence was noted for the comparison of in vivo and in vitro measurements of maximum cardiac performance at high temperature (figure 2). This is probably a good expression of cardiac collapse in vivo because in vitro perfused heart preparations assess (even assure) maximum performance using an optimal extracellular (perfusate) environment. This clearly contrasts with the debilitating acidotic, hypoxic and hyperkalemic venous conditions noted above for the in vivo situation near Ucrit.
Taylor et al. (1997) suggested that the evidence supporting cardiac collapse does not necessarily preclude maximum Mo2 being limited by oxygen delivery to somatic tissues. Indeed, the Pvo2 plateau near Ucrit in rainbow trout (figure 3) is consistent with the possibility of limited oxygen delivery to swimming muscles. But whether this reflects a limitation of blood flow to the muscle, as noted above (i.e. the decreasing cardiac output and arterial oxygen content), or a diffusion limitation set by tissue capillary density and geometry will require experimental data beyond those that are currently available.
Future studies would also benefit from: (i) treating experimental temperature as a continuous not a discrete variable, (ii) acknowledging upper temperature tolerance is probably an individual trait, and (iii) measuring variables continuously to ensure a steady state. The importance of temperature tolerance being an individual trait has been acknowledged in two recent studies. In one study, maximum cardiac performance was found to be similar for 14°C- and 18°C-acclimated triploid brown trout (Salmo trutta; Altimiras et al. 2002). While this result is consistent with a broad temperature optimum (Mercier et al. 2002), the problem of more individuals dying due to seasonal temperature spikes in the warm temperature group leaves open the possibility that selective mortality for the less temperature-tolerant individuals resulted in performance for the 18°C group being overestimated. Similarly, as compared to the typical success rate of approximately 100% with 15°C-acclimated rainbow trout when maximum cardiac performance was assessed with the ‘Farrell’ in situ heart preparation, the success rate declined to 85% at 18°C and to 60% at 22°C (Farrell et al. 1996), again raising the possibility that maximum cardiac performance was overestimated at the higher temperatures.
Continuous measurement of gas tensions in blood vessels, tissues and respiratory media is now possible using fibre-optic oxygen probe technology, replacing the need for periodic sampling. In fact, the oscillations in Pvo2 shown in figure 3 would not be resolved using the blood sampling technique used more commonly to assess blood gas tensions. Moreover, coupling continuous monitoring of cardiorespiratory variables during swimming with online data acquisition systems is invaluable for resolving steady states, e.g. measurements for red-muscle-tissue oxygen tension during swimming suggest that red muscle remains well perfused up to Ucrit (McKenzie et al. 2004). After counting many millions of squares on chart paper as a graduate student to determine blood flow and pressures, I needed little convincing to develop my own off-line analysis using in-house algorithms programmed in machine language and only 32 K of available memory (Farrell & Bruce 1987) when computers first became readily available.
This section closes by acknowledging that the above account for prolonged swimming in salmonids is not necessarily applicable to all fish species. Foremost, fish have evolved a repertoire of swimming modes, and critical swimming speed tests are not suited to all fish species and environmental conditions. Moreover, different measures of swimming performance can respond quite differently to temperature acclimation among fish species (O'Steen & Bennett 2003). In addition, Kolok et al. (1993) discovered to their dismay that the large-scale sucker (Catostomus macrocheilus) is adept at taking advantage of the boundary layer of water in the swim chamber, with the result that steady-state swimming was rarely performed with low speeds at a low temperature.
In view of these limitations, the next section briefly considers non-steady-state swimming behaviours from three perspectives: (i) what is known about the cardiorespiratory changes during this type of swimming activity, (ii) how the swimming behaviour might alter the interpretation of results from prolonged swimming tests, and (iii) whether or not the critical swimming speed test properly evaluates swimming performance after a shift in swimming gait.
3. Cardiorespiratory consequences of gait transition during prolonged swimming tests
Ucrit and maximum Mo2 are highly repeatable among individual fish. This is true whether the test uses velocity increments of 10% or the ramp-Ucrit protocol (i.e. salmonids are accelerated to approximately 50% of their Ucrit before continuing with velocity increments of 10%; Randall et al. 1987; Farrell et al. 1998, 2003; Jain & Farrell 2003). Similarly, individual rank order of swimming performance is maintained after temperature acclimation (Kolok 1992), surgery (Kolok & Farrell 1994) and growth (Claireaux et al. 2005). This level of individual repeatability must underscore the reliability of the critical swimming speed test. However, non-steady swimming states are part and parcel of a critical swimming test and they can introduce cardiorespiratory variability. As noted already, there can be a brief cardiovascular ‘overshoot’ (e.g. the temporary elevation of cardiac output; figure 1) associated with the transition to a new velocity increment and the size of the velocity increment probably affects the extent and duration of this overshoot. In addition, unsteady swimming behaviours can appear periodically during a swim test. Both fighting and burst-and-glide swimming can decrease Pvo2 at a constant water velocity (figure 3), and burst activity can produce bradycardia followed by hypertension (Farrell 1982). Ideally, critical swimming tests should minimize these types of swimming behaviours.
What cannot be avoided, however, is the change in swimming gait that ultimately takes salmonids to fatigue in a critical swim speed test. For some fish, this gait change may be restricted to the final speed increment, making it so short that it precludes a steady state. However, other fish change their swimming gait at 80% Ucrit, as judged by the appearance of lactate in the blood (Burgetz et al. 1998). The consequence of this gait transition on cardiorespiratory performance is now the focus for the remainder of this perspective. It needs to be stressed that after gait transition, this final swimming effort is powered by white anaerobic muscle, which constitutes an approximately 20-times greater mass than the red aerobic muscle in rainbow trout. In addition, individual white fibres generate a higher force than red muscle fibres and can relax much faster, thereby permitting a higher tail-beat frequency (Syme 2006).
The first important point to consider is the oxygen cost of anaerobic swimming activity. Theoretically, the metabolic cost of swimming increases exponentially with velocity. Indeed, many of the published relationships between whole fish Mo2 and swimming velocity are exponential curves (figure 5; or linear when using the logarithm of Mo2). However, the fish's oxygen requirements should either plateau (as shown in figure 5) or decrease after gait transition because there is no question that white muscle is activated (based on electro-myography recordings) and that glycolysis is occurring (based on glycogen depletion and lactate accumulation in white muscle). Therefore, an exponential relationship between Mo2 and swimming velocity is paradoxical when white muscle is powering swimming after gait transition. Two possibilities could explain this discrepancy.
White muscle contraction has a significant aerobic component that drives up Mo2 near Ucrit. White fibres have an aerobic capacity and they are activated at speeds well below Ucrit (Johnston & Moon 1980; Jones 1982). However, their aerobic potential has not been directly measured and only indirect estimates are available. Randall & Daxboeck (1982) examined and rejected the possibility that increased blood flow to white muscle could supply an increased oxygen requirement. For their analysis, they considered a rainbow trout in which mosaic muscle constituted 66% of the body mass, of which 64.5% was white glycolytic fibres and 1.5% was red (pink), fatigue-resistant fibres. Lateral red muscle accounted for an additional 2.5% of body mass. The threefold increase in cardiac output at 80% Ucrit supported a 15-fold increase in blood flow to lateral red muscle (increasing from 9 to 42% of cardiac output) and a doubling of mosaic-muscle blood flow. However, the mosaic-muscle blood flow accounted for only 27% of cardiac output at 80% Ucrit, unlike 43% of cardiac output at rest. Therefore, they concluded that blood flow to the white muscle fibres was curtailed some 10-fold because a majority of the increased blood flow to mosaic muscle would have been used by the pink muscle fibres, assuming that lateral red and pink mosaic-muscle fibres receive similar blood flows. This analysis rejects the idea that white muscle fibre activity is predominantly aerobic at 80% Ucrit. Indeed, at these speeds lactate begins to accumulate in the blood (Burgetz et al. 1998; Jain & Farrell 2003). Furthermore, the finding that peak lactate appearance in the blood occurs approximately 1 h after exhaustive exercise (Milligan 1996) suggests that blood flow to white muscle continues to be curtailed even during recovery. Also, definitive experimental evidence is needed concerning red muscle activity during intense white muscle activity because the theoretical calculations suggest that the partial pressure of oxygen in red muscle should be around 2 kPa, a suggestion that has not been borne out by recent direct measurements of red-muscle oxygen tensions in rainbow trout (McKenzie et al. 2004).
There are artefacts associated with relationships between oxygen uptake and swimming velocity. If artefacts exist, they could occur in one of several forms. One problem could be caused by pooling fish that vary considerably in their individual Ucrit values, as this would tend to obscure any individual plateaus in Mo2. Individual variability could be retained by plotting Mo2 against a per cent of Ucrit, but not as a logarithmic plot. In addition, poor respirometry can lead to measurement artefacts, an issue dealt with elsewhere (Steffensen 1989). A third possibility is that fish may prematurely stop swimming rather than becoming metabolically fatigued at Ucrit. 31P-NMR has already been used to observe a sudden peak in inorganic phosphate (indicating a switch to glycolysis) when Atlantic cod (Gadus morhua) reached Ucrit (Pörtner et al. 2002). Thus, detailed 31P-NMR studies with salmonids during gait transition could be particularly informative since the alternative of taking muscle biopsies during the terminal stage of Ucrit swimming will stress fish. I will return to the possibility of a premature stop under volitional gait transition momentarily.
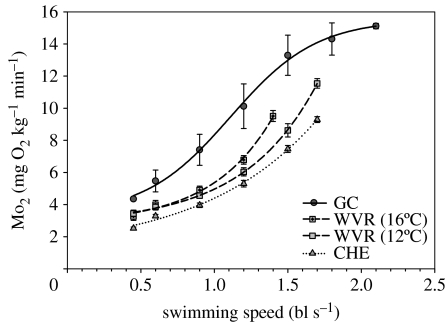
Figure 5.
Relationships between oxygen consumption (Mo2) and swimming speed (in body lengths per second) for adult (2–3 kg) salmon (N=12–20 fish per stock/temperature) during critical swimming speed tests. While three of the fish stocks show exponential increases in Mo2 with swimming speed, there is a distinct plateau in Mo2 for one of the stocks. The data from the Gates Creek (GC) sockeye salmon acclimated to 18°C and Chehalis (CHE) coho salmon acclimated to 8°C are used in subsequent figures for further analysis. WVR, Weaver Creek sockeye salmon acclimated to 12 and 16°C. Data were taken from Lee et al. (2003a).
Regardless of whether the relationship between swimming speed and Mo2 is exponential or sigmoidal (figure 5), there is absolutely no question that the relationship underestimates the true cost of transport (COT) near Ucrit. This is because such curves do not account for the anaerobic and deferred oxygen costs of swimming (e.g. depletion of normal myoglobin and haemoglobin oxygen stores). These additional costs of swimming are clearly evident when excess post-exercise oxygen consumption (EPOC) is measured (figure 6; Brett 1964; Scarabello et al. 1992; Reid et al. 1995; Lee et al. 2003b). The most recent of these EPOC studies show that Mo2 remains elevated for at least 40 min post-Ucrit. Even so, the real challenge is in deciding how EPOC should be applied to Mo2 measurements made during swimming to obtain a proper estimate of COT. Lee et al. (2003b) approached this problem by assuming that most of the EPOC was either a result of, or associated with, non-steady swimming near to Ucrit. They then modelled the aerobic portion of the Mo2 curve (i.e. less than 50% Ucrit) with an exponential equation, as predicted by the cost of moving through water, and extrapolated this curve to Ucrit (figure 6). The precise exponent of this curve was determined using mathematical iterations that forced the measured EPOC to be equal to the difference between the integrals for the modelled and measured equations for Mo2 versus time (with time being bounded by the duration of the Ucrit swim). While this approach has promise as a better estimate of COT near Ucrit (figure 7), with COT being 20–50% higher than that derived from just measuring Mo2 at Ucrit, it can probably benefit from mathematical refinement. An important point to emerge from this analysis is that, by failing to account for it, EPOC can produce a sigmoid relationship between Mo2 and swimming speed. Thus, factors that influence EPOC will also affect the relationship between Mo2 and swimming speed.
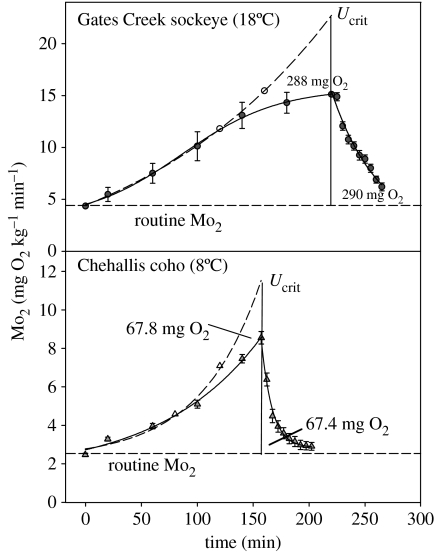
Figure 6.
An analysis of the oxygen cost of swimming to the critical swimming speed (Ucrit) by incorporating the excess post-exercise oxygen consumption (EPOC). Data were adapted from Lee et al. (2003b), where details of the methodology are presented. It is assumed during the later stages of swimming that anaerobic swimming results in the measured oxygen consumption (Mo2; solid line) being an underestimate of the true oxygen constant at these higher swimming speeds. It was further assumed that the EPOC measured during recovery after Ucrit (solid line) reflected the deferred oxygen costs of swimming and that the overall cost of locomotion through water is exponentially related to swimming speed. To allow a continuous plot of Mo2 measured during the swim test and during the recovery period (solid line), data for Gates Creek sockeye salmon and Chehallis coho salmon (taken from figure 5) were transformed by plotting Mo2 against the experimental time (this is possible because the speed increments occur at fixed time-intervals). EPOC (indicated numerically) was then derived from the integral of the Mo2 measured post-Ucrit and bounded by routine Mo2. The integrated EPOC could then be applied to the measured Mo2 during the swim test (through iterative mathematical modelling) to generate the derived oxygen cost of swimming (broken line) such that the integrated EPOC matched the numerical difference (as indicated) between the derived and measured Mo2. The net result is an estimate of the extent to which direct measurements on Mo2 during swimming by themselves underestimate the oxygen cost of swimming at Ucrit.
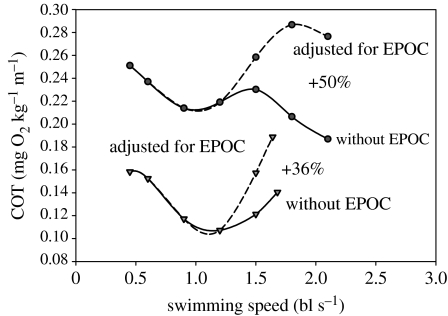
Figure 7.
An analysis of the effect of incorporation excess post-exercise oxygen consumption (EPOC) on the cost of transport (COT) in adult Pacific salmon (data derived from figures 5 and 6). The solid curves represent the COT derived from the direct measurement of Mo2 during swimming without any adjustment for EPOC (figure 6), and the broken curves represent COT with the derived Mo2 during swimming with the adjustment for EPOC as shown in figure 6. The derived value for COT at Ucrit was 36, 50% higher than the COT measured at Ucrit. Data were adapted from Lee et al. (2003b).
Temperature has a clear effect on EPOC. Brett (1964) reported that EPOC nearly doubled between 5 and 15°C for juvenile sockeye salmon (table 1). Similarly, the EPOC for adult salmon is highest in a salmon stock at the warmest temperature and which swam faster than other stocks (table 1). In addition, the presence of a plateau in the relationship between Mo2 and swimming speed is related to the extent of the anaerobic swimming effort, which is in part temperature dependent (Lee et al. 2003b).
Table 1.
A comparison of excess post-exercise oxygen consumption (EPOC) for juvenile and adult salmonids at different temperatures.
| temperature (°C) | EPOC (mg O2 kg−1) | |
|---|---|---|
| adult coho salmona | 8 | 67 |
| adult sockeye salmona | 12 | 62 |
| adult sockeye salmona | 16 | 105 |
| adult sockeye salmona | 18 | 254 |
| juvenile sockeye salmonb | 5 | 252 |
| juvenile sockeye salmonb | 15 | 504 |
| juvenile rainbow troutc | 15 | 454 |
Data taken from Lee et al. (2003b).
Data taken from Brett (1964).
Data taken from Scarabello et al. (1992).
The basis for the temperature effect on EPOC is unclear. It could simply be that glycolysis is more extensive during anaerobic swimming in Ucrit tests at warm temperature. This would require either a greater muscle glycogen store or a greater depletion of the store. Interestingly, salmonids show a faster metabolic recovery after being chased to exhaustion at higher temperatures (Kieffer & Tufts 1996; Galloway & Kieffer 2003), and this appears at odds with the EPOC results for Ucrit swimming. Furthermore, Jain & Farrell (2003) showed that, along with a higher Ucrit for warm-acclimated rainbow trout, there were greater elevations of plasma levels of lactate, K+, Na+ and cortisol at Ucrit compared with cold-acclimated fish. Thus, in agreement with the EPOC data, rainbow trout appeared to make a greater anaerobic effort at the warmer temperature, with the added consequence that they did not perform as well on a repeat Ucrit test. Consequently, salmonids appear to be more willing to exploit and draw down their muscle glycogen stores when Ucrit tests are performed at warm temperature rather than recovering faster after the Ucrit tests at warm temperature. Also, rainbow trout did not seek warmer water in a thermal gradient to accelerate the recovery process (Clutterham et al. 2004), as one might predict from the metabolic recovery data. This finding may be a simple temperature-dependent behavioural response with salmonids at warm temperature being more ‘willing’ to swim harder or longer, perhaps with the expectation that the rate of metabolic recovery for anaerobic activity is faster. The potential importance of fish behaviour in Ucrit tests and its ramifications on the interpretation of Ucrit data is considered further in the section on volitional gait transition.
Salmonids have a remarkable ability to recover from exhaustive exercise. Brett (1964) suggested a 3 h recovery period between testing a fish and introducing it to a swim tunnel. This recovery period may be excessive based on more recent estimates of EPOC (figure 6). In addition, repeated critical swimming tests are certainly possible after either 1 h of burst swimming to fatigue (Randall et al. 1987) or a 40 min recovery period between a fish fatiguing in one Ucrit test and starting a second test (Jain et al. 1997). Even lethargic salmon landed on commercial gill net vessels can be revived in recovery boxes such that they are able to perform a swim test after as little as an hour (Farrell et al. 2001a,b). To what degree recovery periods need to be extended at warm temperature to accommodate the associated increase in EPOC is unknown.
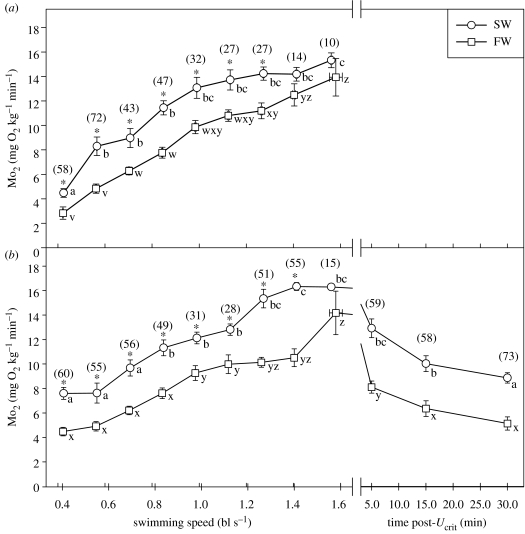
Recovery of routine Mo2, which can occur after approximately an hour post-Ucrit (Farrell et al. 1998, 2003; Lee et al. 2003b), may not be the best measure of a fish's ability to re-perform. Light swimming during the recovery, which would increase metabolic rate, actually accelerates metabolic recovery in salmonids (Milligan et al. 2000; Farrell et al. 2001a,b). Moreover, it appears that certain forms of metabolic loading do not necessarily limit maximum Mo2. As can be seen from figure 8, routine Mo2 is 40–50% higher in seawater- compared with freshwater-acclimated adult sockeye salmon. Yet, when both groups of fish swim to a similar Ucrit, the difference in maximum Mo2 is statistically insignificant and only 10%. Even so, a 50% difference in Mo2 quickly reappears during the recovery period, and the whole phenomenon is repeatable with a subsequent swim (figure 8). Clearly, certain oxygen costs can be deferred during swimming, but maybe not during EPOC. What these oxygen costs might be is unclear. Likely candidates include tissue repair (protein synthesis) and recovery (glycogen resynthesis).
Figure 8.
Examples of the increase in oxygen uptake (Mo2) of seawater-acclimated (open circles) and freshwater-acclimated (open squares) adult (2–3 kg) sockeye salmon during critical swimming speed tests (N=9 and 10 fish; 13°C). Fish were captured during their spawning migration, just prior to entry into the Fraser River and held for at least one month. (a) Results of the first critical swimming test, illustrating a higher Mo2 for seawater fish compared with freshwater fish at all swimming speeds except those very close to Ucrit (% difference between seawater and freshwater values at a common velocity is indicated numerically and the presence of a statistical difference is indicated by an asterisk). (b) Similar results of the same fish performing a second critical swimming test. Although neither group of fish had fully recovered routine Mo2 after the 45 min recovery period, they performed at similar levels for Ucrit and maximum Mo2. Data were adapted from Wagner et al. (2006).
A complete contrast is provided by post-prandial metabolic costs, which cannot be deferred in salmonids. The elevated Mo2 associated with digesting a meal is present at all swimming velocities and this type of metabolic loading ultimately limits swimming performance resulting in a lower Ucrit (Alsop & Wood 1997; Thorarensen & Farrell 2006). Thus, when fish exercise, food assimilation may be more important than tissue repair and recovery.
4. Volitional gait transition during swimming
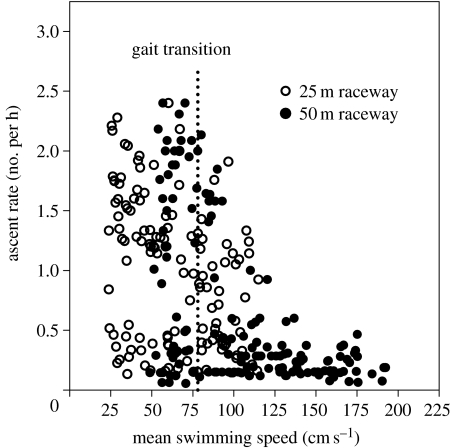
Throughout this perspective, I have alluded to the potential importance of fish behaviour. Therefore, this final section considers recent studies of volitional swimming ascents up a 25–50 m raceway by smallmouth bass (Micropterus dolomieu). This work provides evidence that the gait transition from steady aerobic swimming to unsteady burst-and-glide swimming is probably a behavioural event, triggered by a fish being required to swim faster than the red muscle can support, but one that may be triggered earlier when fish are confined in a swim tunnel rather than in a raceway. Furthermore, it seems that detection of ground speed by the fish plays an important role in gait transition, a suggestion that has important consequences when interpreting data collected during the final stages of Ucrit tests, in determining the ecological relevance of the Ucrit value, and in designing the swimming chambers of a swim tunnel respirometer.
The switch from salmonids to smallmouth bass requires a brief explanation. Studies on sprint performance exist for a wide variety of species, including salmonids (e.g. Weaver 1963, 1965; Colavecchia et al. 1998; Haro et al. 2004; Castro-Santos 2004a,b), and on the apparent efficiency benefit when switching gait to an unsteady burst-and-glide swimming gait (e.g. Videler 1981). However, most of these studies had the primary aim of providing detailed information on the duration of voluntary sprint performance against different velocities so that fishways could be optimally engineered relative to the biological capabilities of the fish that encountered them. Often the ascents were incomplete (i.e. the fish fatigued part way and fell back) and the completed ascents rarely lasted a minute. Moreover, they were not intended to carefully compare gait transition in a forced swimming behaviour (Ucrit test in a swim tunnel respirometer) with voluntary ascents in a raceway, which requires usage of the same population of fish at the same location with the same water quality characteristics. To the best of my knowledge, such a comparison is available only for the smallmouth bass making volitional swimming ascents through a water raceway built on the Winnipeg River, Manitoba, Canada.
For these experiments, smallmouth bass were placed in a tank at the base of either 25 or 50 m long raceways (cross-sectional area was approximately 0.25 m2) and exposed to different water velocities in the flume. Fish could choose to either remain in the tank or enter the raceway and ascend. If they did ascend, an array of light-gate sensors followed their progress, which allowed calculations of swimming speed, ground speed, swim duration and the number of completed ascents per hour. One result was that the number of swimming attempts per hour was independent of the water velocity up to a threshold velocity, above which successful swimming attempts fell off precipitously, as shown in figure 9. Previous raceway ascent studies with other fish species also suggest that attempt rate falls off with the encountered water velocity (Castro-Santos 2004a,b). Consequently, fish are either increasingly less willing to ascend the raceway as water speed increases, or require a longer recovery period following a failed ascent. Regardless, smallmouth bass were still capable of completing the ascent against the highest water speed in the raceway (approx. 120 cm s−1), which when combined with their ground speed results in a swimming speed in excess of 170 cm s−1. Moreover, data from both raceways (25 and 50 m long) are superimposable (figure 9), a finding that suggests swimming distance (hence swim duration) had not become a limiting factor even at these remarkably high swimming speeds. The duration of the fastest swim was 2 min, as shown in figure 10a. (Note that even longer and faster ascents may be possible because the 50 m long raceway may not have been long enough to elicit the maximum swimming performance.)
Figure 9.
Number of voluntary ascents made by smallmouth bass (Micropterus dolomieu) against different water velocities in either a 25 m (open symbols) or a 50 m (closed symbols) raceway. The willingness of fish to ascend the raceway decreased dramatically after the fish switched swimming gait (indicated by the vertical broken line). One hundred and thirty-one fish (mean fork length: 35.3 cm) were used in the 25 m raceway, while 148 individuals (mean fork length: 36.8 cm) were used in the 50 m raceway. All fish were naive to the raceway. Ascent rate was calculated by dividing the number of complete ascents by the exposure period. Gait transition speed was assessed using video recordings of fish as they swam past an observation window.
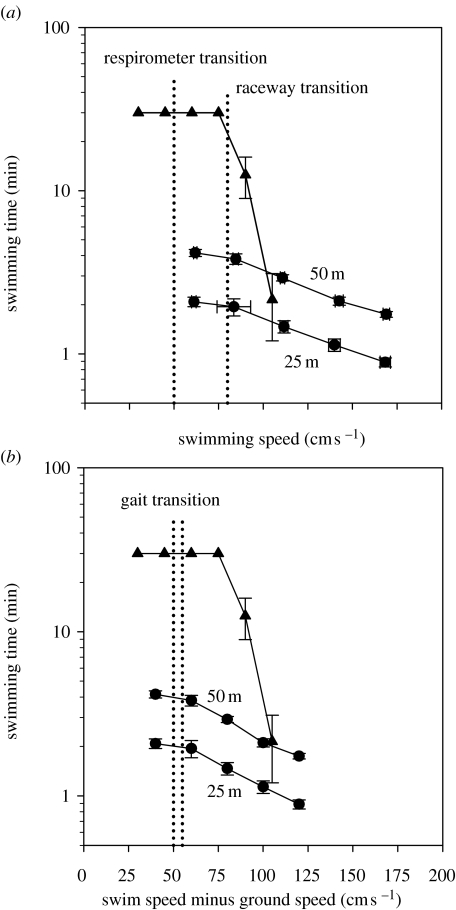
Figure 10.
(a) A comparison of voluntary and forced swimming times versus swimming speeds for smallmouth bass (M. dolomieu). Data are presented for the duration of the ascent in 25 and 50 m raceways at swimming speeds at and above the gait transition. Once the fish voluntarily switch swimming gait (broken vertical line), the swimming time decreases significantly, indicating that fish are increasing their ground speed as well as swimming speed to overcome increasing water velocity. When fish perform a critical swimming speed test in a respirometer (time-intervals and speed increments were 30 min and 15 cm s−1, respectively: N=8; mean fork length was 32.1 cm), they make a gait transition (broken vertical line) at a lower swimming speed than with voluntary ascents. Data were adapted from Peake & Farrell (2004, 2005). (b) This graph shows how removing the effect of ground speed alters the data presented in a. To account for the differences in ground speed between critical swimming speed tests in respirometers and voluntary ascents in raceways, the data are presented as swimming speed minus ground speed. Gait transition occurs at the same swimming speed minus ground speed for both types of experiment. This result is taken as evidence that fish incorporate ground speed into their decision about swimming speed and, in particular, gait transition. Data were adapted from Peake & Farrell (2004, 2005).
The decline in voluntary ascent frequency in smallmouth bass corresponded to a change in swimming gait, visible through an observation window in the raceway. The new gait was a burst-and-glide swimming (an accelerative burst followed by no swimming, and a continuous but oscillatory ground speed). This gait change also involved switching from aerobically to anaerobically powered swimming, as confirmed by post-ascent detection of lactate accumulation (Peake & Farrell 2004, 2005). It is important to note here that gait transition, while presumably involving a switch from red-muscle- to white-muscle-powered locomotion, is not entirely analogous to the burst-and-glide behaviour of salmonids in swim tunnels because the confined length of the swim chamber forces the fish to fall back at some point, otherwise it cannot burst forward again. During voluntary ascents, smallmouth bass only occasionally fell back during burst-and-glide swimming behaviours, and this typically occurred at speeds around the gait transition. If anything, smallmouth bass appeared to avoid falling back during gait transition in voluntary ascents. For adult salmon, some volitional swimming ascents in a raceway apparently involved burst swimming lasting 4–12 s (but no longer) and then holding station before either bursting again or falling back completely (Colavecchia et al. 1998). Given the above consideration of EPOC, it seems unlikely that gait transition provides an energy saving, other than the rates of contraction, which may be better matched to the optimal power bands for red and white muscle. Nevertheless, Videler (1981) has argued that burst and glide is a more efficient form of swimming, although I would contend that no one has yet attempted to include the inevitable EPOC into such calculations of swimming efficiency. Instead, it seems more probable that a gait transition simply provides a biomechanical advantage; powering locomotion with white muscle increases the power capability of fish similar to changing up a gear in an automobile. However, gait change also increases a fish's ground speed as well as swimming speed, which is an unexpected finding.
Figure 10a illustrates voluntary ascent durations versus water speed. The shorter the ascent duration, the faster ground speed. Ascent duration clearly decreased after the gait transition. In addition, smallmouth bass chose to progressively increase their ground speed with increasing water velocity during white-muscle-powered swimming (figure 10a). In contrast, ground speed was independent of water velocity when red muscle was powering locomotion below the gait transition speed; a single, constant ground speed was selected and as a result the ascent duration for the 50 m long raceway was exactly twice that of the 25 m long raceway.
With the smallmouth bass study it is possible to superimpose the swimming performance in the raceway with that in a respirometer (figure 10a). In doing so, an inescapable conclusion emerges: swimming behaviour differs for the two experimental set-ups. Three points are immediately obvious from the comparison: (i) the swimming speed for gait transition in the swim tunnel is considerably lower than that in the raceway, (ii) swimming activity close to the gait transition speed can be maintained for at least 30 min, and (iii) raceway fish swim 75% faster for similar durations. A swim duration lasting 2 min (the maximum duration observed in the raceway) can be resolved in a swim chamber and so the theoretical expectation is that Ucrit values should be of this magnitude for smallmouth bass. Clearly, this is not the case. Thus, smallmouth bass voluntarily swim considerably faster than when forced to do so in a respirometer. In reaching this conclusion, it is essential to remember that the raceway fish generate a ground speed, whereas the respirometer fish do not. Therefore, the impact of ground speed needs to be considered in the comparison.
The impact of ground speed can be considered by replotting the data in figure 10a as a function of swimming speed minus ground speed. The result is that gait transition speeds in the raceway and the respirometer are essentially indistinguishable and that raceway and respirometer data for the maximum swimming speed are comparable once ground speed has been removed from the raceway results (figure 10b). Consequently, the difference in swimming performance between forced swimming in the respirometer and volitional swimming in the raceway most probably relates to a difference in ‘swimming with no ground speed’ in the respirometer versus ‘swimming with a ground speed’ in the raceway.
This finding has a number of important implications. Smallmouth bass performing a Ucrit test clearly do not perform at their maximum swimming speed. As shown above, swimming above the gait transition speed has a consequence to EPOC. Therefore, not only does EPOC need to be incorporated into COT, but EPOC needs to be re-evaluated with volitional swimming, if at all possible. Layered onto this re-evaluation will be the effect of temperature on EPOC because, as noted earlier, salmon appear to be more willing or able to swim anaerobically at higher temperature. What will also require further investigation is how well the results for smallmouth bass apply directly to salmonids (and other fish species). One concern is that salmonids may not rely on anaerobic swimming as extensively as smallmouth bass.
Another implication is the possibility that the length of the swim chamber in a respirometer probably plays an important role in determining gait transition and will directly influence Ucrit if premature gait transition in a respirometer is related to the inability of fish to either sense or generate a ground speed. With a longer swim chamber, fish may be better able to behaviourally engage a ground speed and this may enable a gait transition at swimming speed more comparable to that seen with volitional swimming. Consequently, a respirometer with a longer swim chamber relative to fish length may be more desirable for measuring Ucrit than the one not much longer than the fish itself. This in turn may have an impact on whether or not a plateau in Mo2 is observed in Ucrit tests.
Ultimately, it is highly unlikely that a respirometer can exactly mimic the volitional swimming behaviour observed in a raceway. Both types of studies have advantages and disadvantages. In terms of the physiological data collected from fish in a respirometer, it appears that the ecological significance of Ucrit needs to be re-evaluated given that anaerobic abilities are undoubtedly underestimated based on the facts presented above (some of this work is already underway; C. Tudorache 2005, personal communication). In contrast, it is unlikely that the maximum aerobic performance measured in a swim tunnel respirometer needs to be challenged at this time. Therefore, until emerging biotelemetry tools have advanced to a stage where respiratory variables can be accurately measured in raceways, it might be best to focus on examining the effects of swim chamber length, and extending the data collected for smallmouth bass in raceways to other species, including salmonids for which the greatest physiological database exists.
Acknowledgments
The research by the author was supported by the Natural Sciences and Engineering Research Council of Canada. Dr Steve Peake & Christian Tudorache are thanked for sharing their thoughts (2006) and unpublished data (2006), which were important in shaping some of the ideas presented above.
Footnotes
One contribution of 18 to a Theme Issue ‘Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms’.
References
- Alsop D.H, Wood C.M. The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss) J. Exp. Biol. 1997;200:2337–2346. doi: 10.1242/jeb.200.17.2337. [DOI] [PubMed] [Google Scholar]
- Altimiras J, Axelsson M, Claireaux G, Lefrancois C, Mercier C, Farrell A.P. Cardiorespiratory status of triploid brown trout (Salmo trutta) during swimming at two acclimation temperatures. J. Fish Biol. 2002;60:102–116. doi:10.1111/j.1095-8649.2002.tb02390.x [Google Scholar]
- Beamish F.W.H. Swimming capacity. In: Hoar W.S, Randall D.J, editors. Fish physiology. vol. 7. Academic Press; New York, NY: 1978. pp. 101–187. [Google Scholar]
- Brett J.R. The respiratory metabolism and swimming performance of young sockeye salmon. J. Fish. Res. Board Can. 1964;21:1183–1226. [Google Scholar]
- Brett J.R. Energetic responces of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka) Am. Zool. 1971;11:99–113. [Google Scholar]
- Brodeur J.C, Dixon G.D, McKinley R.S. Assessment of cardiac output as a predictor of metabolic rate in rainbow trout. J. Fish Biol. 2001;58:439–452. doi:10.1111/j.1095-8649.2001.tb02263.x [Google Scholar]
- Burgetz I.J, Rojas-Vargas A, Hinch S.G, Randall D.J. Initial recruitment of anaerobic metabolism during sub-maximal swimming in rainbow trout (Oncorhynchus mykiss) J. Exp. Biol. 1998;201:2711–2721. doi: 10.1242/jeb.201.19.2711. [DOI] [PubMed] [Google Scholar]
- Castro-Santos T. Quantifying the combined effects of attempt rate and swimming capacity on passage through velocity barriers. Can. Fish. Aquat. Sci. 2004a;61:1601–1615. [Google Scholar]
- Castro-Santos T. Optimal swim speeds for traversing velocity barriers: an analysis of volitional high-speed swimming behaviour of migratory fishes. J. Exp. Biol. 2004b;208:421–432. doi: 10.1242/jeb.01380. doi:10.1242/jeb.01380 [DOI] [PubMed] [Google Scholar]
- Claireaux G, McKenzie D.J, Genge A.G, Chatelier A, Aubin J, Farrell A.P. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J. Exp. Biol. 2005;208:1775–1784. doi: 10.1242/jeb.01587. doi:10.1242/jeb.01587 [DOI] [PubMed] [Google Scholar]
- Clutterham S, Gamperl A.K, Wallace H.L, Crawshaw L.I, Farrell A.P. Exhaustive exercise does not affect the preferred temperature for recovery in juvenile rainbow trout (Oncorhynchus mykiss) Physiol. Biochem. Zool. 2004;77:611–618. doi: 10.1086/422053. doi:10.1086/422053 [DOI] [PubMed] [Google Scholar]
- Colavecchia M, Katopodis C, Goosney R, Scruton D.A, McKinley R.S. Measurement of burst swimming performance in wild Atlantic salmon (Salmo salar L.) using digital telemetry. Regul. Rivers Res. Manage. 1998;14:41–51. doi:10.1002/(SICI)1099-1646(199801/02)14:1<41::AID-RRR475>3.0.CO;2-8 [Google Scholar]
- Davie P.S, Farrell A.P. The coronary and luminal circulations of the myocardium of fishes. Can. J. Zool. 1991;69:1993–2001. [Google Scholar]
- Davis E.D, Foster J, Warren C.E, Doudorouff P. The influence of oxygen concentration on the swimming performance of juvenile Pacific salmon at various temperatures. Trans. Am. Fish. Soc. 1963;92:111–124. doi:10.1577/1548-8659(1963)92[111:TIOOCO]2.0.CO;2 [Google Scholar]
- Daxboeck C, Davie P.S, Perry S.F, Randall D.J. Oxygen uptake in a spontaneously ventilating, blood perfused trout preparation. J. Exp. Biol. 1982;101:25–46. [Google Scholar]
- Driedzic W.R, Gesser H. Energy metabolism and contractility in ectothermic vertebrate hearts: hypoxia, acidosis, and low temperature. Physiol. Rev. 1994;74:221–258. doi: 10.1152/physrev.1994.74.1.221. [DOI] [PubMed] [Google Scholar]
- Farrell A.P. Cardiovascular changes in the unanesthetized ling cod, Ophiodon elongatus, during short-term, progressive hypoxia and spontaneous activity. Can. J. Zool. 1982;60:933–941. [Google Scholar]
- Farrell A.P. Coronary flow in a perfused rainbow trout heart. J. Exp. Biol. 1987;129:107–123. doi: 10.1242/jeb.129.1.107. [DOI] [PubMed] [Google Scholar]
- Farrell A.P. Cardiac output: regulation and limitations. In: Bicudo E, editor. The vertebrate gas transport cascade: adaptations to environment and mode of life. CRC Press; Boca Raton, FL: 1993. pp. 208–214. [Google Scholar]
- Farrell A.P. Features heightening cardiovascular performance in fishes, with special reference to tunas. Comp. Biochem. Physiol. A. 1996;113:61–67. doi:10.1016/0300-9629(95)02058-6 [Google Scholar]
- Farrell A.P. Effects of temperature on cardiovascular performance. In: Wood C.M, McDonald D.G, editors. Global warming implications for freshwater and marine fish. Cambridge University Press; Cambridge, UK: 1997. pp. 135–158. [Google Scholar]
- Farrell A.P. Cardiorespiratory performance in salmonids during exercise at high temperature: insights into cardiovascular design limitations in fishes. Comp. Biochem. Physiol. A. 2002;132:797–810. doi: 10.1016/s1095-6433(02)00049-1. doi:10.1016/S1095-6433(02)00049-1 [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Bruce F. Data acquisition and analysis of pulsatile signals using a personal computer: an application in cardiovascular physiology. Comput. Biol. Med. 1987;17:151–156. doi: 10.1016/0010-4825(87)90039-4. doi:10.1016/0010-4825(87)90039-4 [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Clutterham S.M. On-line venous oxygen tensions in rainbow trout during graded exercise at two acclimation temperatures. J. Exp. Biol. 2003;206:487–496. doi: 10.1242/jeb.00100. doi:10.1242/jeb.00100 [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Jones D.R. The heart. In: Hoar W.S, Randall D.J, Farrell A.P, editors. Fish physiology. vol. 12a. Academic Press; San Diego, CA: 1992. pp. 1–88. [Google Scholar]
- Farrell A.P, MacLeod K.R, Chancey B. Intrinsic mechanical properties of the perfused rainbow trout heart and the effects of catecholamines and extracellular calcium under control and acidotic conditions. J. Exp. Biol. 1986;125:319–345. doi: 10.1242/jeb.125.1.319. [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Hammons A.M, Graham M.S, Tibbits G.F. Cardiac growth in rainbow trout, Salmo gairdneri. Can. J. Zool. 1988;66:2368–2373. [Google Scholar]
- Farrell A.P, Johansen A.J, Suarez R.K. Effects of exercise-training on cardiac performance and muscle enzymes in rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 1991;9:303–312. doi: 10.1007/BF02265151. doi:10.1007/BF02265151 [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Gamperl A.K, Hicks J.M.T, Shiels H.A, Jain K.E. Maximum cardiac performance of rainbow trout, Oncorhynchus mykiss, at temperatures approaching their upper lethal limit. J. Exp. Biol. 1996;199:663–672. doi: 10.1242/jeb.199.3.663. [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Gamperl A.K, Birtwell I.K. Prolonged swimming, recovery and repeat swimming performance of mature sockeye salmon Oncorhynchus nerka exposed to moderate hypoxia and pentachlorophenol. J. Exp. Biol. 1998;201:2183–2193. doi: 10.1242/jeb.201.14.2183. [DOI] [PubMed] [Google Scholar]
- Farrell A.P, Gallaugher P.E, Routledge R. Rapid recovery of exhausted adult coho salmon after commercial capture by troll fishing. Can. J. Fish. Aquat. Sci. 2001a;58:2319–2324. doi:10.1139/cjfas-58-12-2319 [Google Scholar]
- Farrell A.P, Gallaugher P.E, Fraser J, Pike D, Bowering P, Hadwin A.K.M, Parkhouse W, Routledge R. Successful recovery of the physiological status of coho salmon on-board a commercial gillnet vessel by means of a newly designed revival box. Can. J. Fish. Aquat. Sci. 2001b;58:1932–1946. doi:10.1139/cjfas-58-10-1932 [Google Scholar]
- Farrell A.P, Lee C.G, Tierney K, Hodaly A, Clutterham S, Healey M.C, Hinch S.G, Lotto A. Field-based measurements of oxygen uptake and swimming performance with adult Pacific salmon using a mobile respirometer swim tunnel. J. Fish. Biol. 2003;62:64–84. doi:10.1046/j.1095-8649.2003.00010.x [Google Scholar]
- Foreman M.G.G, Lee D.K, Morrison J, Macdonald S, Barnes D, Williams I.V. Simulations and retrospective analyses of Fraser watershed flows and temperatures. Atmos. Ocean. 2001;39:89–105. [Google Scholar]
- Gallaugher P, Farrell A.P. Hematocrit and blood oxygen-carrying capacity. In: Perry S.F, Tufts B, editors. Fish respiration. vol. 17. Academic Press; San Diego, CA: 1998. pp. 185–227. [Google Scholar]
- Gallaugher P, Thorarensen H, Farrell A.P. Hematocrit in oxygen transport and swimming in rainbow trout, Oncorhynchus mykiss. Respir. Physiol. 1995;102:279–292. doi: 10.1016/0034-5687(95)00065-8. doi:10.1016/0034-5687(95)00065-8 [DOI] [PubMed] [Google Scholar]
- Gallaugher P.E, Thorararensen H, Kiessling A, Farrell A.P. Effects of high intensity exercise training on cardiovascular function, oxygen uptake, internal oxygen transfer and osmotic balance in chinook salmon (Oncorhynchus tshawytscha) during critical speed swimming. J. Exp. Biol. 2001;204:2861–2872. doi: 10.1242/jeb.204.16.2861. [DOI] [PubMed] [Google Scholar]
- Galloway B.J, Kieffer J.D. The effects of an acute temperature change on the metabolic recovery from exhaustive exercise in juvenile Atlantic salmon (Salmo salar) Physiol. Biochem. Zool. 2003;76:652–662. doi: 10.1086/376921. doi:10.1086/376921 [DOI] [PubMed] [Google Scholar]
- Graham M.S, Farrell A.P. The effect of temperature acclimation and adrenaline on the performance of a perfused trout heart. Physiol. Zool. 1989;62:38–61. [Google Scholar]
- Haro A, Castro-Santos T, Noreika J, Odeh M. Swimming performance of upstream migrant fishes in open-channel flow: a new approach to predicting passage through velocity barriers. Can. J. Fish. Aquat. Sci. 2004;61:1590–1601. doi:10.1139/f04-093 [Google Scholar]
- Heath A.G, Hughes G.M. Cardiovascular and respiratory changes during heat stress in rainbow trout (Salmo gairdneri) J. Exp. Biol. 1973;59:323–338. doi: 10.1242/jeb.59.2.323. [DOI] [PubMed] [Google Scholar]
- Hoar W.S, Randall D.J. Terminology to describe swimming activity in fish. In: Hoar W.S, Randall D.J, editors. Fish physiology. vol. 7. Academic Press; New York, NY: 1978. pp. 13–14. [Google Scholar]
- Holk K, Lykkeboe G. The impact of endurance training on arterial plasma potassium and swimming performance in rainbow trout. J. Exp. Biol. 1998;201:1373–1380. doi: 10.1242/jeb.201.9.1373. [DOI] [PubMed] [Google Scholar]
- Jain K.E, Farrell A.P. Influence of seasonal temperature on the repeat swimming performance of rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 2003;206:3569–3579. doi: 10.1242/jeb.00588. doi:10.1242/jeb.00588 [DOI] [PubMed] [Google Scholar]
- Jain K.E, Hamilton J.C, Farrell A.P. Use of a ramp velocity test to measure critical swimming speed in rainbow trout, Onchorhynchus mykiss. Comp. Biochem. Physiol. A. 1997;117:441–444. doi:10.1016/S0300-9629(96)00234-4 [Google Scholar]
- Jones D.R. The effect of hypoxia and anaemia on the swimming performance of rainbow trout (Salmo gairdneri) J. Exp. Biol. 1971;55:541–551. doi: 10.1242/jeb.55.2.541. [DOI] [PubMed] [Google Scholar]
- Jones D.R. Anaerobic exercise in teleost fish. Can. J. Zool. 1982;60:1131–1134. [Google Scholar]
- Jones D.R, Randall D.J. The respiratory and circulatory systems during exercise. In: Hoar W.S, Randall D.J, editors. Fish physiology. vol. 7. Academic Press; New York, NY: 1978. pp. 425–501. [Google Scholar]
- Johnston I.A, Moon T.W. Exercise training in skeletal muscle of brook trout (Salvelinus fontinalis) J. Exp. Biol. 1980;87:177–194. doi: 10.1242/jeb.87.1.177. [DOI] [PubMed] [Google Scholar]
- Keen J.E, Farrell A.P. Maximum prolonged swimming speed and maximum cardiac performance of rainbow trout, Oncorhynchus mykiss, acclimated to two different water temperatures. Comp. Biochem. Physiol. A. 1994;108:287–295. doi:10.1016/0300-9629(94)90097-3 [Google Scholar]
- Kiceniuk J.W, Jones D.R. The oxygen transport system in trout (Salmo gairdneri) during sustained exercise. J. Exp. Biol. 1977;69:247–260. [Google Scholar]
- Kieffer J.D, Tufts B.L. The influence of environmental temperature on the role of the rainbow trout gill in correcting the acid–base disturbance following exhaustive exercise. Physiol. Zool. 1996;69:1301–1323. [Google Scholar]
- Kolok A.S. The swimming performances of largemouth bass are repeatable. J. Exp. Biol. 1992;170:265–270. [Google Scholar]
- Kolok A.S, Farrell A.P. Individual variation in the swimming performance and cardiac performance of northern squawfish, Ptytocheilus oregonensis. Physiol. Zool. 1994;67:706–722. [Google Scholar]
- Kolok A, Spooner M, Farrell A.P. The effect of exercise on the cardiac output and blood flow distribution of the large-scale sucker, Catostomus macrocheilus. J. Exp. Biol. 1993;183:301–321. [Google Scholar]
- Lee C.G, Farrell A.P, Lotto A, MacNutt M.J, Hinch S.G, Healey M.C. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J. Exp. Biol. 2003a;206:3239–3251. doi: 10.1242/jeb.00547. doi:10.1242/jeb.00547 [DOI] [PubMed] [Google Scholar]
- Lee C.G, Farrell A.P, Lotto A, Hinch S.G, Healey M.C. Excess post-exercise oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon following critical speed swimming. J. Exp. Biol. 2003b;206:3253–3260. doi: 10.1242/jeb.00548. doi:10.1242/jeb.00548 [DOI] [PubMed] [Google Scholar]
- McKenzie D.J, Wong S, Randall D.J, Egginton S, Taylor E.W, Farrell A.P. The effects of sustained exercise and hypoxia upon oxygen tensions in the red muscle of rainbow trout. J. Exp. Biol. 2004;207:3629–3637. doi: 10.1242/jeb.01199. doi:10.1242/jeb.01199 [DOI] [PubMed] [Google Scholar]
- Mercier C, Axelsson M, Imbert N, Claireaux G, Lefrancois C, Altimiras J, Farrell A.P. In vitro cardiac performance in triploid brown trout (Salmo trutta) at two acclimation temperatures. J. Fish Biol. 2002;60:117–133. doi:10.1111/j.1095-8649.2002.tb02391.x [Google Scholar]
- Milligan C.L. Metabolic recovery from exhaustive exercise in rainbow trout. Comp. Biochem. Physiol. A. 1996;113:51–60. doi:10.1016/0300-9629(95)02060-8 [Google Scholar]
- Milligan C.L, Farrell A.P. Lactate utilization by an in situ perfused trout heart: effects of workload and blockers of lactate transport. J. Exp. Biol. 1991;155:357–373. [Google Scholar]
- Milligan C.L, Hooke B, Johnson C. Sustained swimming at low velocity following a bout of exhaustive exercise enhances metabolic recovery in rainbow trout. J. Exp. Biol. 2000;203:921–926. doi: 10.1242/jeb.203.5.921. [DOI] [PubMed] [Google Scholar]
- O'Steen S, Bennett A.F. Thermal acclimation effects differ between voluntary, maximum, and critical swimming velocities in two cyprinid fishes. Physiol. Biochem. Biol. 2003;76:484–496. doi: 10.1086/376421. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Stecyk J.A, Gesser H, Wang T, Gamperl A.K, Farrell A.P. Preconditioning stimuli do not benefit the myocardium of hypoxia-tolerant rainbow trout (Oncorhynchus mykiss) J. Comp. Physiol. 2004;174:329–340. doi: 10.1007/s00360-004-0418-4. [DOI] [PubMed] [Google Scholar]
- Peake S.J, Farrell A.P. Post-exercise physiology and oxygen consumption in relation to swimming speed and gait transition in free-swimming smallmouth bass (Micropterus dolomieu) J. Exp. Biol. 2004;207:1563–1575. doi: 10.1242/jeb.00927. doi:10.1242/jeb.00927 [DOI] [PubMed] [Google Scholar]
- Peake S, Farrell A.P. Repeat performance capacity and locomotory behaviour reflect exercise physiology in free-swimming smallmouth bass in an experimental raceway. Physiol. Biochem. Zool. 2005;78:801–807. doi: 10.1086/432148. doi:10.1086/432148 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Climate change and temperature dependant biography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A. 2002;123:739–761. doi: 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pörtner, H. O., Webber, D. M., Bock, C. & Wittig, R.-M. 2002 In vivo31P-NMR studies of speeding fish: online monitoring of muscular energetics in Atlantic cod (Gadus morhua). Proc. Int. Soc. Magnetic Resonance Medicine, 10.
- Randall D.J, Brauner C. Effects of environmental factors on exercise in fish. J. Exp. Biol. 1991;160:113–126. [Google Scholar]
- Randall D.J, Daxboeck C. Cardiovascular changes in the rainbow trout (Salmo gairdneri) during exercise. Can. J. Zool. 1982;60:1135–1140. [Google Scholar]
- Randall D.J, Mense D, Boutilier R.G. The effects of burst swimming on aerobic swimming in chinok salmon (Oncorhyncus tshawytscha) Mar. Behav. Physiol. 1987;13:77–88. [Google Scholar]
- Reidy S.P, Nelson A.J, Tang Y, Kerr S.R. Post-exercise metabolic rate in Atlantic cod and its dependence upon the method of exhaustion. J. Fish Biol. 1995;47:377–386. doi:10.1111/j.1095-8649.1995.tb01907.x [Google Scholar]
- Scarabello M, Heigenhauser G.J.F, Wood C.M. Gas exchange, metabolite status and excess post-exercise oxygen consumption after repetitive bouts of exhaustive exercise in juvenile rainbow trout. J. Exp. Biol. 1992;167:155–169. doi: 10.1242/jeb.167.1.155. [DOI] [PubMed] [Google Scholar]
- Simonot, D. L. 2006. Cardiac remodelling in diploid and triploid rainbow trout (Oncorhynchus mykiss Walbaum), pp. 1–133. MSc thesis, University of British Columbia, Vancouver, Canada.
- Steffensen J.F. Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol. Biochem. 1989;6:49–59. doi: 10.1007/BF02995809. [DOI] [PubMed] [Google Scholar]
- Stevens E.D, Randall D.J. Changes of gas concentrations in blood and water during moderate swimming activity in rainbow trout. J. Exp. Biol. 1967;46:329–337. doi: 10.1242/jeb.46.2.329. [DOI] [PubMed] [Google Scholar]
- Syme D.A. Functional properties of skeletal muscle. In: Shadwick R.E, Lauder G.V, editors. Fish biomechanics. Fish physiology. vol. 23. Elsevier; San Diego, CA: 2006. [Google Scholar]
- Taylor S, Egginton S, Taylor E.W. Seasonal temperature acclimatisation of rainbow trout: cardiovascular and morphometric influences on maximal sustainable exercise level. J. Exp. Biol. 1996;199:835–845. doi: 10.1242/jeb.199.4.835. [DOI] [PubMed] [Google Scholar]
- Taylor E.W, Eggington S, Taylor S.E, Butler P.J. Factors which may limit swimming performance at different temperatures. In: Wood C.M, MacDonald D.G, editors. Global warming implications for freshwater and marine fish. Cambridge University Press; Cambridge, UK: 1997. p. 135. [Google Scholar]
- Thorarensen H, Farrell A.P. Postprandial intestinal blood flow, metabolic rates and exercise in chinook salmon (Oncorhynchus tshawytscha) Physiol. Biochem. Zool. 2006;79:688–694. doi: 10.1086/505512. [DOI] [PubMed] [Google Scholar]
- Thorarensen H, Gallaugher P, Kiessling A, Farrell A.P. Intestinal blood flow in swimming chinook salmon, Oncorhynchus kisutch: the effects of hematocrit on blood flow distribution. J. Exp. Biol. 1993;179:115–129. [Google Scholar]
- Thorarensen H, Gallaugher P.E, Farrell A.P. Cardiac output in swimming rainbow trout, Oncorhynchus mykiss. Physiol. Zool. 1996;69:139–153. [Google Scholar]
- Videler J.J. Swimming movements, body structure and propulsion in cod Gadus morhua. Symp. Zool. Soc. Lond. 1981;48:1–27. [Google Scholar]
- Wagner G.N, Kuchel L.J, Lotto A, Patterson D.A, Shrimpton M, Hinch S.G, Farrell A.P. Routine and active metabolic rates of migrating, adult wild sockeye salmon (Oncorhynchus nerka Walbaum) in seawater and freshwater. Physiol. Biochem. Zool. 2006;79:100–108. doi: 10.1086/498186. doi:10.1086/498186 [DOI] [PubMed] [Google Scholar]
- Weaver C.R. Influence of water velocity upon orientation and performance of adult migrating salmonids. Fish. Bull. 1963;63:97–121. [Google Scholar]
- Weaver C.R. Observations on the swimming ability of adult American shad (Alosa sapidissima) Trans. Am. Fish. Soc. 1965;94:383–385. doi:10.1577/1548-8659(1965)94[382:OOTSAO]2.0.CO;2 [Google Scholar]