Abstract
Feline and primate immunodeficiency viruses (FIVs, SIVs, and HIV) are transmitted via direct contact (e.g. fighting, sexual contact, and mother–offspring transmission). This dynamic likely poses a behavioral barrier to cross-species transmission in the wild. Recently, several host intracellular anti-viral proteins that contribute to species-specificity of primate lentiviruses have been identified revealing adaptive mechanisms that further limit spread of lentiviruses between species. Consistent with these inter-species transmission barriers, phylogenetic evidence supports the prediction that FIV transmission is an exceedingly rare event between free-ranging cat species, though it has occurred occasionally in captive settings. Recently we documented that puma and bobcats in Southern California share an FIV strain, providing an opportunity to evaluate evolution of both viral strains and host intracellular restriction proteins. These studies are facilitated by the availability of the 2× cat genome sequence annotation. In addition, concurrent viral and host genetic analyses have been used to track patterns of migration of the host species and barriers to transmission of the virus within the African lion. These studies illustrate the utility of FIV as a model to discover the variables necessary for establishment and control of lentiviral infections in new species.
Keywords: FIV, Lentiviruses, Cross-species transmission
Feline immunodeficiency virus in domestic cats (FIVfca) produces an AIDS like pathology characterized by CD4 depletion, immune suppression, and death (Bendinelli et al., 1995, Willett et al., 1997). As such, it is the only natural model for HIV/AIDS. Similar to the primate lentiviruses, closely related FIVs circulate in non-domestic cat species (Olmsted et al., 1992, Troyer et al., 2005). While antibodies that cross-react to FIV are detectable at a low percentage in most feline species and in the Hyeanidae (Troyer et al., 2005), little is known about these viruses. The current paradigm, based mainly on studies in puma and lion, is that these viruses are benign, having little or no clinical effect on the host species (Carpenter and O’Brien, 1995). However, relevant clinical data from wild populations has not been collected in most cases due to the difficulty of prolonged surveillance, sample collection, and clinical monitoring of such rare, solitary, and evasive species. Therefore, although seropositive animals (confirmed in many cases by sequencing) have been documented at low frequencies in all South American species, at high frequencies in large African carnivores, and in one Asian species: the Pallas’ cat (Troyer et al., 2005), there is a paucity of epidemiologic, genetic, viralogic, or clinical data available for most infected species.
In the absence of comprehensive datasets, what can be discerned about these non-domestic cat lentiviruses and how does this knowledge add to our overall understanding of lentiviral infection and pathology? One important source of information has been DNA sequence data from the infecting FIVs, in most cases amplified from integrated provirus and thus obtainable from blood samples. A comparison of the phylogenetic relationships of FIVs to that of their host species can often contribute to our understanding of the biology and evolution of these viruses. Free-ranging individuals of many species harbor monophyletic, species-specific strain(s) of FIV (Troyer et al., 2005). However, viruses isolated from different species seem to group more by geographic region of the host than in groupings concordant with the phylogenetic relationships of host species (Fig. 1 ). For example, puma and cheetah are closely related, belonging to the puma linage, while lions and leopards are members of the panthera linage (Johnson et al., 2006); cheetah FIV (FIVaju) is most closely related to leopard FIV (FIVppa) and falls within a branch of the FIV tree that contains primarily viruses from other African species (Fig. 1).
Fig. 1.
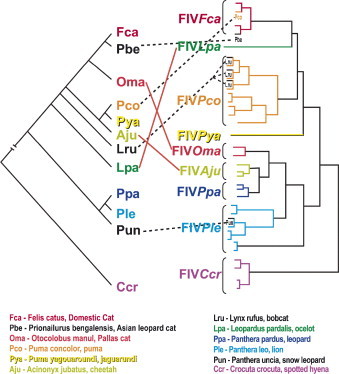
Viral–host co-evolution. Colors correspond to the host species. The tree on the left represents host species relationships (Johnson et al., 2006) and the tree on the right shows observed viral sequence relationships (Carpenter et al., 1996, Nishimura et al., 1999, Troyer et al., 2005, Franklin et al., 2007). The majority of the branches reflect within-species monophyly. However, this tree also reflects both ancient cross-species transmissions that disrupt the concordance of the host and viral trees (shown in solid red lines) as well as recent cross-species transmission events that disrupt within host monophyly of viral sequences (shown in dashed black lines).
Puma and lion FIVs have the highest divergence, and multiple highly divergent strains circulate within these species suggesting a long period of evolution within their respective hosts (Carpenter et al., 1996, Troyer et al., 2004, Troyer et al., 2005, O’Brien et al., 2006). These genetic observations correlate to ecological observations that there is no reduced lifespan, fecundity, or increased disease incidence in FIV-infected puma or lions and has led to speculation that lion and puma FIVs are relatively harmless to their hosts (Carpenter and O’Brien, 1995, Hofmann-Lehmann et al., 1996, Packer et al., 1999, Biek, 2006).
There are several caveats that should be considered when drawing conclusions from phylogenetic data. First, most samples have been collected during capture for ecological studies. Therefore, samples come from limited geographic ranges for some host species leading to potential sampling bias. Without viral sequence from samples throughout the range of a given species, as is available for lion and puma viruses, it is difficult to estimate the overall FIV diversity across a species. Second, only a small portion of the FIV pol gene has been sequenced for most non-domestic FIVs. It is probable, as with primate lentiviruses (Bailes et al., 2003, VandeWoude and Apetrei, 2006), that recombination plays a significant role in the evolution of FIV (Slattery, this issue) and that different evolutionary patterns would be seen within different viral regions. In addition, since not all species are widely sampled, there are important gaps in the understanding of the natural history of the virus and host. Finally, although there has been a presumption that long branch lengths reflecting high genetic divergence within a species is indicative of long term viral–host adaptation and gradual attenuation of viral pathogenicity, there is an increasing body of literature suggesting that at least lion FIV (FIVple) is associated with CD4 depletion, immunological decline, and some clinical health consequences (Poli et al., 1995, Bull et al., 2003, Brennan et al., 2006, Roelke et al., 2006). Therefore, it is premature to extrapolate assumptions concerning viral pathogenicity or virulence based on genetic data alone. For example, while it may be tempting to suggest that FIVs with short within-species branch lengths reflecting low genetic diversity would be more pathogenic than those with long within-species branch lengths and high diversity (for example, those infecting Pallas’ cats versus those infecting puma; Troyer et al., 2005), there is currently insufficient clinical data from FIV endemic populations to make this claim.
However, based on phylogenetic data, several conclusions can be made concerning the natural history of FIV within its hosts. First, the presence of FIV in both old and new world felids suggests that the current viruses may have descended from transmission events that occurred the last time felid species crossed the Bering Straits in the late Pleistocene (>12,000 years ago; Johnson et al., 2006), or earlier. Second, the monophyly of FIV sequences within each species suggests that, in most cases, FIV has been successfully introduced once and adapted, expanded, and evolved within the host. Third, although cross-species transmissions have been rare, they likely did occur in the past to produce a pattern of viral evolution in felids that does not completely match the evolution of the Felidae. Finally, there are now several examples of modern inter-species transmissions (Fig. 1; Carpenter et al., 1996, Nishimura et al., 1999, Troyer et al., 2005, Franklin et al., 2007).
While there is one case of a free-ranging leopard cat that acquired FIVfca from a domestic cat (Nishimura et al., 1999), most cross-species transmissions of FIV have been documented in captive settings. These include an instance of FIVfca in a captive puma in Argentina as well as lion FIV clade A sequences (FIVpleA) amplified from a snow leopard and a tiger in Asian zoos (Carpenter et al., 1996, Troyer et al., 2005). While exact transmission events cannot be verified without access to the historical housing records of captive animals, these examples support the tenant that animals in captivity may come into direct or indirect (via dental or surgical instruments) contact with multiple individuals from other species that they would never otherwise encounter, increasing the probability that exposure to non-native viruses would occur.
In natural settings there are substantial behavioral and ecological barriers to cross-species transmission of FIV, a pathogen requiring direct contact for infection to occur (Fig. 2 ). The major mode of transmission for FIV in domestic cats is believed to be biting, although vertical transmission can also occur (Elder et al., 1998, Pedersen and Barlough, 1991, Rogers and Hoover, 1998, Allison and Hoover, 2003a, Allison and Hoover, 2003b). Assuming non-domestic cat FIVs have similar modes of transmission and infection levels seen in other lentiviruses, sufficient contact between individuals of different species is probably a rare event, especially for more solitary species that generally avoid contact with each other. However, in situations where related species have frequent aggressive encounters and may even prey on each other, occasional cross-species infections might occur. We have recently discovered nearly identical FIV strains in pumas and bobcats occupying the same habitat in both Florida and California, strongly suggestive of cross-species transmission and the emergence of an FIV with expanded host tropism (Fig. 1b; Franklin et al., 2007).
Fig. 2.
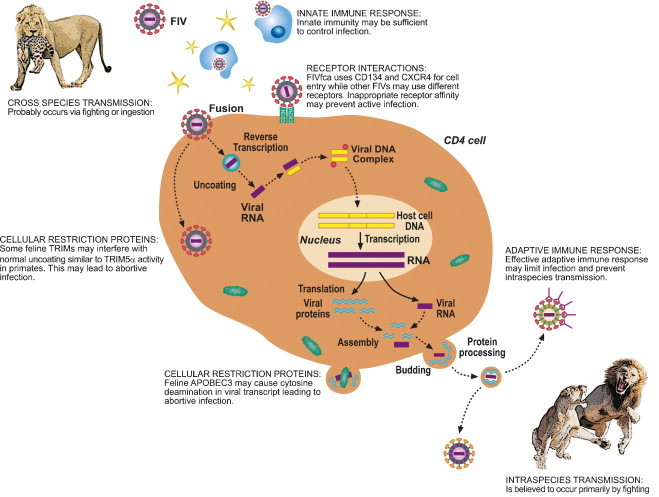
Cartoon representing possible barriers to successful cross-species transmission of lentiviruses.
When a lentivirus infects a new host species, there are several lines of defense for the newly infected species. First, the virus must evade the humoral and adaptive immune systems of the new host (for a review of this topic see: Johnson and Desrosiers, 2002, Nolan et al., 2006). In addition, the virus must find cells that express the right combination of receptors and co-receptors (Fig. 2). While CD134 and CXCR4 have been well established as essential for FIVfca receptor-mediated cell entry, the receptor interactions of puma and lion FIVs are not identified, but in some cases appear to involve other cell surface determinants (de Parseval et al., 2004, de Parseval et al., 2006, Shimojima et al., 2004, Smirnova et al., 2005, Willett et al., 2006).
Recent discoveries in primate lentiviruses have shown that in addition to conventional immune responses, there are also potent intra-cellular viral restriction factors that effectively limit non-adapted viruses (Harris et al., 2003, Lee and KewalRamani, 2004). Human APOBEC3F and G and TRIM5α are effective barriers to infection by other primate lentiviruses, but HIV has evolved viral proteins that circumvent these defenses in a highly host-specific manner (Bogerd et al., 2004, Hatziioannou et al., 2004, Lee and KewalRamani, 2004, Schrofelbauer et al., 2004). Non-native viruses are blocked shortly after cell entry (in the case of TRIM5α) or mutated during reverse transcriptase (APOBEC3; Fig. 2). An APOBEC3 homolog in domestic cats, fe3, has been shown to have anti-viral properties similar to its primate counterparts (Lochelt et al., 2005) and cytosine deaminase activity suggestive of APOBEC3 appears to be effective in limiting experimental cross-species infections of felid lentiviruses (see below and Poss et al., 2006). While felids are not known to have an active TRIM5α, 53 homologs to members of the TRIM family have been found in the 2× cat genome sequence (Table 1 ; Pontias, et al., this issue). Of these, 31 have upstream and downstream neighboring genes that are conserved with respect to the human genome organization. This orthologous synteny provides confidence that gene family members from different species are true orthologs. Thirty-nine of the cat TRIMs have open reading frames that, when translated to amino acids, produce hypothetical proteins similar to their human counterparts; 17 have a SPRY domain, the active anti-viral portion of TRIM5α (Li et al., 2006, Perron et al., 2006). Although the function of most TRIMs is unknown in any species, many of the SPRY-containing TRIM family members probably have anti-viral activity (Lee and KewalRamani, 2004, Si et al., 2006, Zhang et al., 2006).
Table 1.
TRIM gene orthogous (1–68) detected in the cat genome using GARField; a comparitive genomics approacha
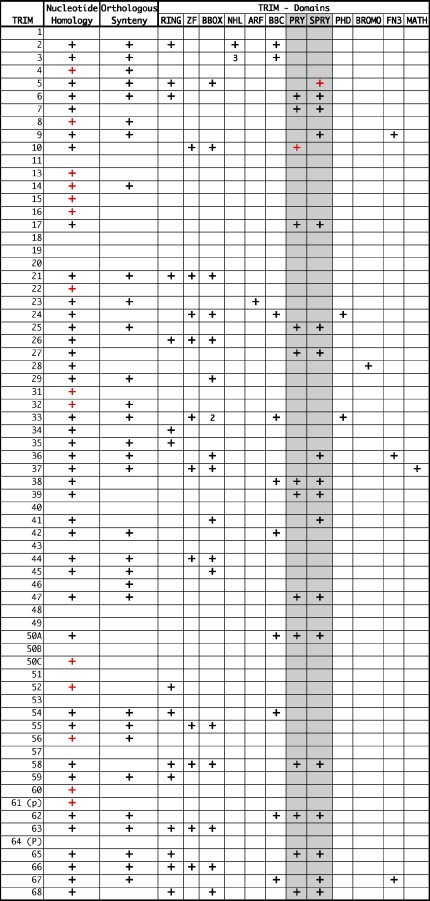
Summary of TRIM results from GARfield (Pontias, this issue). Open reading frames (ORFs) are in black, apparent pseudogenes are in red. A blank means that this sequence was not found in the 2× cat genome.
Given the multitude of defenses in the host, new infections are often abortive. This has been shown in an experimental cross-species infection where puma FIVs (FIVpco) productively but apathogenically infect domestic cats via parental and mucosal routes of infection (VandeWoude et al., 1997a, VandeWoude et al., 1997b, VandeWoude et al., 2003). Initial infection results in increased viral load and transient lymphadenopathy, but does not produce a significant drop in CD4 cells (VandeWoude et al., 1997a, Terwee et al., 2005). Preceding the development of serum neutralizing antibodies (that occur only in a subset of infections) and other indicators of effective immune response, viral loads decreased over time; in 3 out of 4 mucosal infections, loads decreased to undetectable levels in circulating lymphocytes and in most tissues (Terwee et al., 2005). An IV route of infection increased the viral load set point, however, viral loads declined significantly throughout the study, suggesting that these infections would also ultimately abort (Terwee et al., 2005). FIVpco isolates from these experimentally infected domestic cats demonstrate relatively high mutational rates in situ, notably an increase of G to A mutations, changes reminiscent of APOBEC3 activity (Poss et al., 2006).
In order to successfully emerge in a new host, lentiviruses must evolve quickly to evade these host defenses and then successfully transmit to other individuals of the same species (Fig. 2). This process may be augmented when emerging in species that display extreme genetic homogeneity, as was shown in an feline coronavirus outbreak in a colony of captive cheetahs that spread quickly through the entire population and caused 60% mortality, presumably facilitated by the genetic homogeneity of the cheetah's immune defense genes (Pearks Wilkerson et al., 2004).
Conversely, a virus may find itself in a dead-end host if the population demonstrates sufficient immunological diversity. Social animals such as humans, other primates and lions must provide many opportunities for transmission within a species and therefore should be exceptional hosts for lentiviruses. Lions present a particularly interesting model for transmission studies. Their behavior is ideally suited for FIV transmission; biting is an important social signal and occurs within prides as a means of establishing dominance, as part of the mating ritual, and incidentally around a kill, as well as during conflicts between prides (Packer et al., 1999, Packer et al., 2005, West and Packer, 2002, Kissui and Packer, 2004, West et al., 2006). In addition, lions may disperse extremely long distances (up to 350 km), engaging in conflict with other individual lions and prides along the way (Sunquist and Sunquist, 2002). Dispersed lions take up residence in a new pride, often via violent takeover, where they resume normal pride relations that are rife with aggressive interactions. When seen in this light, it is not surprising that in some dense populations close to 100% of adult lions are infected with FIVple throughout much of their range.
The benefit of studying host and viral evolutionary histories together is demonstrated in lions, where FIVple sequences cluster by geographic origin in a pattern that reflects both the current population structure and the natural history of their host species (Antunes, submitted). Genetic studies of lion population structure and natural history suggest that the current distribution of lions is a result of several population contractions, expansions, and long-range migrations relating to the historical rise and fall of water levels in Africa. As a result, most lions throughout their range have a recent genetic history. In addition, small numbers of long-range migrations continue to occur, resulting in “hybrid” individuals of mixed ancestry in all populations sampled (Antunes, submitted). Therefore, it is interesting that there are African lion populations completely devoid of FIV (Brown et al., 1994, O’Brien et al., 2006). These sero-negative populations have been found in the Kalahari, Etosha, and Namibia, suggesting that the Kalahari may present an ecological barrier to FIV transmission. One hypothesis is that there is a lion-density threshold necessary for effective transmission within a species that is not reached in these sparsely populated areas (Winterbach, personal communication).
These results demonstrate that naturally occurring FIVs are an invaluable model system to investigate questions of lentiviral transmission, natural history, evolution, and host-tropism. This system can now be investigated on the background of a well-defined host phylogeny containing multiple species with known population structure, migration patterns, and natural history. In addition, the genetic resources available from the full cat genome sequence allow for the investigation of host genes instrumental in viral control and restriction of cross-species infection. The ability to look at the evolution of host and virus simultaneously in an entire family is unprecedented and promises insight into the complex process of viral emergence, co-evolution, and adaptation.
Conflict of interest statement
None of the authors has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the paper entitled “FIV cross-species transmission: an evolutionary prospective”.
Acknowledgements
We would like to thank Melody Roelke, Christiaan and Hanlie Winterbach, Craig Packer, Kevin Crooks, Seth Riley, and Walter Boyce, for providing samples, ecological data and insights that were necessary for the discoveries discussed in this review. Samples were collected in full compliance with specific federal permits (CITES; Endangered and Threatened Species) issued to the National Cancer Institute, principle investigator S.J. O’Brien, by the U.S. Fish and Wildlife Service of the Department of the Interior. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Allison R.W., Hoover E.A. Covert vertical transmission of feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 2003;19:421–434. doi: 10.1089/088922203765551764. [DOI] [PubMed] [Google Scholar]
- Allison R.W., Hoover E.A. Feline immunodeficiency virus is concentrated in milk early in lactation. AIDS Res. Hum. Retrovir. 2003;19:245–253. doi: 10.1089/088922203763315759. [DOI] [PubMed] [Google Scholar]
- Bailes E., Gao F., Bibollet-Ruche F., Courgnaud V., Peeters M., Marx P.A., Hahn B.H., Sharp P.M. Hybrid origin of SIV in chimpanzees. Science. 2003;300:1713-. doi: 10.1126/science.1080657. [DOI] [PubMed] [Google Scholar]
- Bendinelli M., Pistello M., Lombardi S., Poli A., Garzelli C., Matteucci D., Ceccherini-Nelli L., Malvaldi G., Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek R. Examining effects of persistent retroviral infection on fitness and pathogen susceptibility in a natural feline host. Can. J. Zool. 2006;84:365–373. [Google Scholar]
- Bogerd H.P., Doehle B.P., Wiegand H.L., Cullen B.R. A single amino acid difference in the host APOBEC3G protein controls the primate species specificity of HIV type 1 virion infectivity factor. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3770–3774. doi: 10.1073/pnas.0307713101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan G., Podell M.D., Wack R., Kraft S., Troyer J.L., Bielefeldt-Ohmann H., VandeWoude S. Neurologic disease in captive lions (Panthera leo) with low-titer lion lentivirus infection. J. Clin. Microbiol. 2006;44:4345–4352. doi: 10.1128/JCM.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.W., Yuhki N., Packer C., O’Brien S.J. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J. Virol. 1994;68:5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull M.E., Kennedy-Stoskopf S., Levine J.F., Loomis M., Gebhard D.G., Tompkins W.A. Evaluation of T lymphocytes in captive African lions (Panthera leo) infected with feline immunodeficiency virus. Am. J. Vet. Res. 2003;64:1293–1300. doi: 10.2460/ajvr.2003.64.1293. [DOI] [PubMed] [Google Scholar]
- Carpenter M.A., Brown E.W., Culver M., Johnson W.E., Pecon-Slattery J., Brousset D., O’Brien S.J. Genetic and phylogenetic divergence of feline immunodeficiency virus in the puma (Puma concolor) J. Virol. 1996;70:6682–6693. doi: 10.1128/jvi.70.10.6682-6693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M.A., O’Brien S.J. Coadaptation and immunodeficiency virus: lessons from the Felidae. Curr. Opin. Genet. Dev. 1995;5:739–745. doi: 10.1016/0959-437x(95)80006-q. [DOI] [PubMed] [Google Scholar]
- de Parseval A., Chatterji U., Sun P., Elder J.H. Feline immunodeficiency virus targets activated CD4+ T cells by using CD134 as a binding receptor. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13044–13049. doi: 10.1073/pnas.0404006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A., Grant C.K., Sastry K.J., Elder J.H. Sequential CD134-CXCR4 interactions in feline immunodeficiency virus (FIV): soluble CD134 activates FIV Env for CXCR4-dependent entry and reveals a cryptic neutralization epitope. J. Virol. 2006;80:3088–3091. doi: 10.1128/JVI.80.6.3088-3091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J.H., Dean G.A., Hoover E.A., Hoxie J.A., Malim M.H., Mathes L., Neil J.C., North T.W., Sparger E., Tompkins M.B., Tompkins W.A., Yamamoto J., Yuhki N., Pedersen N.C., Miller R.H. Lessons from the cat: feline immunodeficiency virus as a tool to develop intervention strategies against human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 1998;14:797–801. doi: 10.1089/aid.1998.14.797. [DOI] [PubMed] [Google Scholar]
- Franklin S.P., Troyer J.L., Terwee J.A., Lyren L.M., Boyce W.M., Riley S.P.D., Roelke M.E., Crooks K.R., VandeWoude S. Frequent transmission of immunodeficiency viruses among bobcats and pumas. J. Virol. 2007;81:10961–10969. doi: 10.1128/JVI.00997-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.S., Bishop K.N., Sheehy A.M., Craig H.M., Petersen-Mahrt S.K., Watt I.N., Neuberger M.S., Malim M.H. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T., Perez-Caballero D., Yang A., Cowan S., Bieniasz P.D. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10774–10779. doi: 10.1073/pnas.0402361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Fehr D., Grob M., Elgizoli M., Packer C., Martenson J.S., O’Brien S.J., Lutz H. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin. Diagn. Lab. Immunol. 1996;3:554–562. doi: 10.1128/cdli.3.5.554-562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.E., Desrosiers R.C. Viral persistance: HIV's strategies of immune system evasion. Annu. Rev. Med. 2002;53:499–518. doi: 10.1146/annurev.med.53.082901.104053. [DOI] [PubMed] [Google Scholar]
- Johnson W.E., Eizirik E., Pecon-Slattery J., Murphy W.J., Antunes A., Teeling E., O’Brien S.J. The late Miocene radiation of modern Felidae: a genetic assessment. Science. 2006;311:73–77. doi: 10.1126/science.1122277. [DOI] [PubMed] [Google Scholar]
- Kissui B.M., Packer C. Top-down population regulation of a top predator: lions in the Ngorongoro Crater. Proc. R. Soc. London Ser. B: Biol. Sci. 2004;271:1867–1874. doi: 10.1098/rspb.2004.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., KewalRamani V.N. In defense of the cell: TRIM5alpha interception of mammalian retroviruses. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10496–10497. doi: 10.1073/pnas.0404066101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li Y., Stremlau M., Yuan W., Song B., Perron M., Sodroski J. Functional replacement of the RING, B-box 2, and coiled-coil domains of tripartite motif 5alpha (TRIM5alpha) by heterologous TRIM domains. J. Virol. 2006;80:6198–6206. doi: 10.1128/JVI.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochelt M., Romen F., Bastone P., Muckenfuss H., Kirchner N., Kim Y.B., Truyen U., Rosler U., Battenberg M., Saib A., Flory E., Cichutek K., Munk C. The antiretroviral activity of APOBEC3 is inhibited by the foamy virus accessory Bet protein. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7982–7987. doi: 10.1073/pnas.0501445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Goto Y., Yoneda K., Endo Y., Mizuno T., Hamachi M., Maruyama H., Kinoshita H., Koga S., Komori M., Fushuku S., Ushinohama K., Akuzawa M., Watari T., Hasegawa A., Tsujimoto H. Interspecies transmission of feline immunodeficiency virus from the domestic cat to the Tsushima cat (Felis bengalensis euptilura) in the wild. J. Virol. 1999;73:7916–7921. doi: 10.1128/jvi.73.9.7916-7921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan D., Gaudieri S., Mallal S. Host genetics and viral infections: immunology taught by viruses, virology taught by the immune system. Curr. Opin. Immunol. 2006;18:413–421. doi: 10.1016/j.coi.2006.05.015. [DOI] [PubMed] [Google Scholar]
- O’Brien S.J., Troyer J.L., Roelke M., Marker L., Pecon-Slattery J. Plagues and adaptation: lessons from the Felidae models for SARS and AIDS. Biol. Conserv. 2006;131:255–267. doi: 10.1016/j.biocon.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R.A., Langley R., Roelke M.E., Goeken R.M., Adgerjohnson D., Goff J.P., Albert J.P., Packer C., Laurenson M.K., Caro T.M., Scheepers L., Wildt D.E., Bush M., Martenson J.S., Obrien S.J. Worldwide prevalence of lentivirus infection in wild feline species—epidemiologic and phylogenetic aspects. J. Virol. 1992;66:6008–6018. doi: 10.1128/jvi.66.10.6008-6018.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer C., Altizer S., Appel M., Brown E., Martenson J., O’Brien S.J., Roelke-Parker M., Hofmann-Lehmann R., Lutz H. Viruses of the Serengeti: patterns of infection and mortality in African lions. J. Anim. Ecol. 1999;68:1161–1178. [Google Scholar]
- Packer C., Hilborn R., Mosser A., Kissui B., Borner M., Hopcraft G., Wilmshurst J., Mduma S., Sinclair A.R.E. Ecological change, group territoriality, and population dynamics in Serengeti lions. Science. 2005;307:390–393. doi: 10.1126/science.1105122. [DOI] [PubMed] [Google Scholar]
- Pearks Wilkerson A.J., Teeling E.C., Troyer J.L., Bar-Gal G.K., Roelke M., Marker L., Pecon-Slattery J., O’Brien S.J. Coronavirus outbreak in cheetahs: lessons for SARS. Curr. Biol. 2004;14:R227–R228. doi: 10.1016/j.cub.2004.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Barlough J.E. Clinical overview of feline immunodeficiency virus. J. Am. Vet. Med. Assoc. 1991;199:1298–1305. [PubMed] [Google Scholar]
- Perron M.J., Stremlau M., Sodroski J. Two surface-exposed elements of the B30.2/SPRY domain as potency determinants of N-tropic murine leukemia virus restriction by human TRIM5alpha. J. Virol. 2006;80:5631–5636. doi: 10.1128/JVI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli A., Abramo F., Cavicchio P., Bandecchi P., Ghelardi E., Pistello M. Lentivirus infection in an African lion: a clinical, pathologic and virologic study. J. Wildlife Dis. 1995;31:70–74. doi: 10.7589/0090-3558-31.1.70. [DOI] [PubMed] [Google Scholar]
- Poss M., Ross H.A., Painter S.L., Holley D.C., Terwee J.A., Vandewoude S., Rodrigo A. Feline lentivirus evolution in cross-species infection reveals extensive G-to-A mutation and selection on key residues in the viral polymerase. J. Virol. 2006;80:2728–2737. doi: 10.1128/JVI.80.6.2728-2737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelke M.E., Pecon-Slattery J., Taylor S., Citino S., Brown E., Packer C., Vandewoude S., O’Brien S.J. T-lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J. Wildlife Dis. 2006;42:234–248. doi: 10.7589/0090-3558-42.2.234. [DOI] [PubMed] [Google Scholar]
- Rogers A.B., Hoover E.A. Maternal-fetal feline immunodeficiency virus transmission: timing and tissue tropisms. J. Infect. Dis. 1998;178:960–967. doi: 10.1086/515692. [DOI] [PubMed] [Google Scholar]
- Schrofelbauer B., Chen D., Landau N.R. A single amino acid of APOBEC3G controls its species-specific interaction with virion infectivity factor (Vif) Proc. Natl. Acad. Sci. U.S.A. 2004;101:3927–3932. doi: 10.1073/pnas.0307132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojima M., Miyazawa T., Ikeda Y., McMonagle E.L., Haining H., Akashi H., Takeuchi Y., Hosie M.J., Willett B.J. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–1195. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- Si Z., Vandegraaff N., O’Huigin C., Song B., Yuan W., Xu C., Perron M., Li X., Marasco W.A., Engelman A., Dean M., Sodroski J. Evolution of a cytoplasmic tripartite motif (TRIM) protein in cows that restricts retroviral infection. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7454–7459. doi: 10.1073/pnas.0600771103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova N., Troyer J.L., Schissler J., Terwee J., Poss M., VandeWoude S. Feline lentiviruses demonstrate differences in receptor repertoire and envelope structural elements. Virology. 2005;342:60–76. doi: 10.1016/j.virol.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Sunquist M., Sunquist F. The University of Chicago Press; 2002. Wild Cats of the World. 452 pp. [Google Scholar]
- Terwee J.A., Yactor J.K., Sondgeroth K.S., Vandewoude S. Puma lentivirus is controlled in domestic cats after mucosal exposure in the absence of conventional indicators of immunity. J. Virol. 2005;79:2797–2806. doi: 10.1128/JVI.79.5.2797-2806.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer J.L., Pecon-Slattery J., Roelke M.E., Black L., Packer C., O’Brien S.J. Patterns of feline immunodeficiency virus multiple infection and genome divergence in a free-ranging population of African lions. J. Virol. 2004;78:3777–3791. doi: 10.1128/JVI.78.7.3777-3791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer J.L., Pecon-Slattery J., Roelke M.E., Johnson W., VandeWoude S., Vazquez-Salat N., Brown M., Frank L., Woodroffe R., Winterbach C., Winterbach H., Hemson G., Bush M., Alexander K.A., Revilla E., O’Brien S.J. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J. Virol. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeWoude S., Apetrei C. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 2006;19:728–762. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeWoude S., Hageman C.L., Hoover E.A. Domestic cats infected with lion or puma lentivirus develop anti-feline immunodeficiency virus immune responses. J. Acquir. Immune Defic. Syndr. 2003;34:20–31. doi: 10.1097/00126334-200309010-00003. [DOI] [PubMed] [Google Scholar]
- VandeWoude S., O’Brien S.J., Hoover E.A. Infectivity of lion and puma lentiviruses for domestic cats. J. Gen. Virol. 1997;78(Pt 4):795–800. doi: 10.1099/0022-1317-78-4-795. [DOI] [PubMed] [Google Scholar]
- VandeWoude S., O’Brien S.J., Langelier K., Hardy W.D., Slattery J.P., Zuckerman E.E., Hoover E.A. Growth of lion and puma lentiviruses in domestic cat cells and comparisons with FIV. Virology. 1997;233:185–192. doi: 10.1006/viro.1997.8587. [DOI] [PubMed] [Google Scholar]
- West P.M., Maccormick H., Hopcraft G., Whitman K., Ericson M., Hordinsky M., Packer C. Wounding, mortality and mane morphology in African lions Panthera leo. Anim. Behav. 2006;71:609–619. [Google Scholar]
- West P.M., Packer C. Sexual selection, temperature, and the lion's mane. Science. 2002;297:1339–1343. doi: 10.1126/science.1073257. [DOI] [PubMed] [Google Scholar]
- Willett B.J., Flynn J.N., Hosie M.J. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- Willett B.J., McMonagle E.L., Ridha S., Hosie M.J. Differential utilization of CD134 as a functional receptor by diverse strains of feline immunodeficiency virus. J. Virol. 2006;80:3386–3394. doi: 10.1128/JVI.80.7.3386-3394.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Hatziioannou T., Perez-Caballero D., Derse D., Bieniasz P.D. Antiretroviral potential of human tripartite motif-5 and related proteins. Virology. 2006;353:396–409. doi: 10.1016/j.virol.2006.05.035. [DOI] [PubMed] [Google Scholar]


