There is increasing evidence that presence and location of neovascular vasa vasorum play an important role in atherosclerotic plaque pathogenesis and stability. This paper describes a method to detect vasa vasorum with high contrast and high spatial resolution. It uses second harmonic or subharmonic intravascular ultrasound, in combination with ultrasound contrast agents. The same technology in combination with targeted contrast agents is suited for molecular imaging. The potential for vasa vasorum imaging is illustrated using an atherosclerotic animal model and the potential for molecular imaging is illustrated using phantom experiments. While at present these approaches are only available for animal experiments, they are fully compatible with implementation on commercial platforms, which will enable their widespread clinical use. It is anticipated that they will ultimately be used to gain further insight into the natural history of atherosclerosis, the guidance of novel therapeutic strategies, and possibly as a marker of plaque vulnerability.
Background
Intravascular ultrasound (IVUS) is an established clinical tool for assessing coronary artery atherosclerosis. IVUS has contributed to an improved understanding of the natural history of atherosclerosis1 and IVUS data are increasingly being used as an endpoint in therapeutic trials.2 For diagnostic purposes it is employed as an adjunct to angiography, in order to provide additional insight into the extent and severity of atherosclerosis and frequently reveals the presence of angiographically occult (i.e. nonstenotic) lesions.3 Such ‘nonculprit’ lesions are now recognised to be responsible for a high proportion of ensuing cardiac events resulting in either fatalities or requiring further interventional treatment.4 A significant issue in cardiology is, therefore, developing imaging methods to identify specific atherosclerotic lesions that are at high risk, or vulnerable, to rupture and should therefore receive therapy.5,6 Candidate markers for lesion vulnerability currently under investigation include plaque morphology, volume, mechanical integrity, and composition (e.g. fatty or calcified).7 IVUS is well suited to indicate morphology and plaque burden, which has been the primary form of image analysis.3 Mechanical properties relevant to plaque vulnerability can be derived using IVUS elastography techniques,8 and efforts are being made to perform radiofrequency (RF) IVUS signal analysis to gain insight into plaque composition.9 More recently, there is a growing recognition of the significance of two other potential markers of plaque vulnerability: molecular expression and neovascular vasa vasorum. These are areas of coronary plaque assessment that have yet to be addressed by imaging, and where IVUS in combination with microbubble contrast agents has the potential to play a significant role.
Microbubble contrast agents have been employed extensively at lower diagnostic ultrasound frequencies (∽2 to 5 MHz),10 where they have enabled microvascular flow detection in a range of applications such as myocardial perfusion imaging. While microbubbles can in some circumstances be visualised through simple echogenicity enhancement, the sensitive and specific detection of agent is invariably achieved with the use of techniques that cause the echoes from bubbles to be substantially different to those from tissue.11 These approaches are generally referred to as nonlinear imaging. One means of achieving this is through ‘harmonic’ imaging, which stimulates resonant bubble vibrations to emit energy at twice the transmitted frequency. A second technique is called ‘subharmonic’ imaging, whereby images are formed from bubble echoes at half the ultrasound transmit frequency. Commercial contrast agents are therefore designed to be comprised primarily of bubble sizes that resonate in the 2 to 5 MHz diagnostic ultrasound frequency range (∽2 to 6 microns in diameter).12 It has been widely assumed that it is not possible to conduct harmonic imaging at high ultrasound frequencies.13 However, contrast harmonic imaging of microvessels was recently demonstrated at 20 MHz14 using instrumentation and extracorporeal transducers compatible with applications in ophthalmology and small animal imaging.
We recently described a prototype nonlinear IVUS system and showed the feasibility of harmonic and subharmonic contrast IVUS imaging.15 The system was a modified version of one developed to perform tissue harmonic imaging.16 With these new approaches, two applications are possible with IVUS: coronary vasa vasorum imaging and molecular imaging.
Vasa vasorum imaging
There is increasing evidence that the vasa vasorum play an important role in atherosclerotic plaque pathogenesis and stability.17,18 It is well established that pathological neovascularisation occurs during plaque development, and that the resulting microvessels have abnormal spatial distributions and branching patterns.19,20 Neovascular vasa vasorum are correlated with the presence of inflammatory cells and infiltrate within plaques,21,22 and are implicated in a positive feedback loop of inflammation and angiogenesis.23 Plaque neovessels have been associated with intraplaque haemorrhage, and thereby with rupture.24,25 Elevated plaque microvessel counts have been found in the coronary arteries of patients with acute myocardial infarctions26,27 and in the carotid arteries of those with symptomatic carotid occlusive disease.28
There is, therefore, a rapidly growing demand for the development of sensitive, robust and quantitative techniques for in vivo and clinical imaging of the vasa vasorum. Such techniques can potentially contribute to the identification of vulnerable plaques,29 and in monitoring therapeutic response. Due to the heterogeneous nature of plaque vasa vasorum and the potential significance of localised measures of vascularisation as markers of vulnerability,30 high resolution imaging may also be required. In an ex vivo setting, microCT techniques are capable of generating high resolution images of coronary vasa vasorum in animal studies, and have provided considerable insight into their structure and function.31,32 In the carotid artery, contrast-enhanced MRI33 and transcutaneous contrast ultrasound34 have been shown to be sensitive to plaque neovascularisation, though they do not have sufficient resolution to directly image microvascular morphology. While signs of plaque ‘blush’ are sometimes evident with angiography,35 there are no established clinical techniques for examining coronary artery vasa vasorum with high resolution and sensitivity.
Due to the severe relative tissue-catheter motion and weak signal strength from the microcirculation, coronary vasa vasorum imaging is a challenging problem for imaging. As a result, IVUS imaging systems developed to examine flow within the lumen of larger arteries36 are not be capable of detecting vasa vasorum. It has recently been reported that bolus injections of contrast agents can give rise to IVUS echogenicity enhancement in the adventitia of coronary arteries, consistent with the detection of vasa vasorum.37 While these results are encouraging, they ultimately rely upon the assumption of similarity between images acquired at the same point in the cardiac cycle and as such are susceptible to issues such as noncyclical catheter-vessel motion or nonuniform rotation of the transducer element. Since these issues can be expected to reduce the sensitivity and robustness of such measures, this motivates the development of contrast IVUS detection techniques based on bubble-specific nonlinear acoustic signatures. To this end, we have conducted phantom in vivo feasibility studies for vasa vasorum imaging with contrast harmonic IVUS with a 20 MHz transmit signal.38
Animal studies were carried out to investigate the use of microbubble contrast agents in combination with prototype nonlinear IVUS systems as a means of imaging vasa vasorum.38 In these experiments, the IVUS catheter was situated in a region of interest in an atherosclerotic rabbit aorta and contrast agent (Definity™, Bristol Myers Squibb) was released proximally in the form of a bolus through a delivery catheter. Agent was first detected within the main lumen, and then (after 5-10 s delay) within the adventia surrounding the plaque (figure 1). A quantification of the enhancement was found to be statistically significant. The general spatial pattern of agent presence within the adventitia and not the plaque itself was consistent with the microvascular distribution revealed by histological sections (figure 2) taken in the vicinity of the imaging planes. These results indicate that contrast harmonic IVUS is a tool capable of imaging vasa vasorum.
Figure 1.
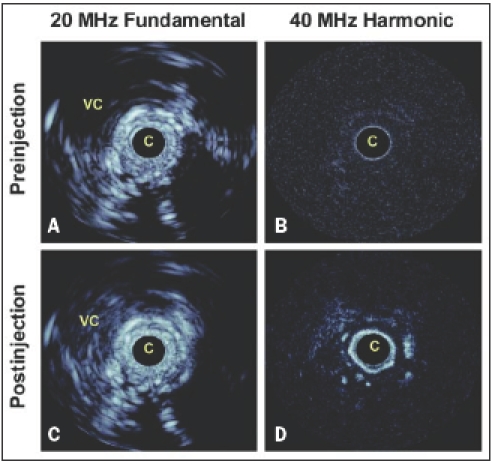
In vivo results in an atherosclerotic rabbit aorta using decanted Definity™. A. Fundamental mode prior to agent injection. B. Fundamental mode 15 seconds postinjection where changes in adventitial enhancement are not evident, except for in a region at 4 o’clock and within the vena cava. C. Harmonic mode prior to injection shows the tissue signals to be largely suppressed. D. 15 second postinjection harmonic mode shows significant adventitial enhancement, consistent with the detection of adventitial microvessels. Scale of images is 12 mm across. The dynamic range of the fundamental and harmonic images is 40 and 25 dB respectively. C=catheter, VC=vena cava.
Figure 2.
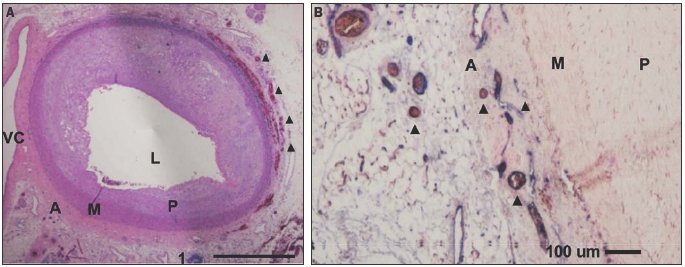
A. Overview haematoxylin/eosin stained section from the aorta examined by IVUS. A large eccentric plaque is evident, which thins towards the vena cava. B. CD31 stained section highlights endothelial cells (purple) and reveals the presence of numerous adventitial microvessels (many containing erythrocytes), some examples of which are denoted with triangles. VC=vena cava, L=aortic lumen, M=media, P=atherosclerotic plaque, A=adventitia.
Molecular imaging
The use of targeted microbubble contrast agents in conjunction with IVUS holds considerable potential for gaining insight into the molecular status of atherosclerotic plaques. A number of reports have indicated the feasibility of imaging microbubbles targeted to endothelial cell adhesion molecules39 and fibrin.40 These studies relied upon simple enhancement of echogenicity to determine the presence of microbubbles and as such require the accumulation of considerable amounts of agent at the target sites. We have investigated the feasibility of performing bubble-specific imaging of targeted agent using a prototype nonlinear IVUS system.41,42 The agent examined was an experimental biotinated, lipid encapsulated formulation comprised substantially of micron and submicron sized bubbles (BG3039; Bracco Research, Geneva) which were targeted to an avidin coated agar substrate. Example images are shown in figure 3. In fundamental (i.e. conventional ‘linear’ imaging) mode (figure 3A), the agent is difficult to distinguish from background tissue signals. However, in harmonic (not shown) and subharmonic (figure 3B) modes the agent is clearly detected, with tissue signals being suppressed to below the noise floor. These results demonstrate the feasibility of harmonic imaging as a strategy for improving the sensitivity and specificity of targeted contrast agent detection at high ultrasound frequencies.
Figure 3.
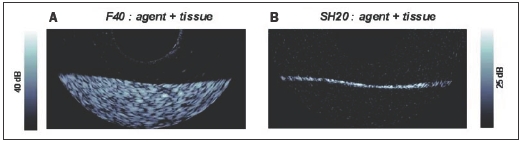
Agent targeted to tissue phantom in A. 40 MHz fundamental (F40) mode and B. in 20 MHz subharmonic (SH20) imaging modes. Image size 10 mm laterally, displaying half of rotational IVUS image.
Conclusion
The development of nonlinear contrast IVUS has the potential to provide unique information about the microvascular and molecular status of atherosclerotic coronary plaques. Technical advances continue to be made, and can be expected to further improve sensitivity. While at present these approaches are only available for animal experiments, they are fully compatible with implementation on commercial platforms which will enable their widespread clinical use.43 It is anticipated that they will ultimately be used to gain further insight into the natural history of atherosclerosis, the effects of novel therapeutic strategies, and possibly as a marker of plaque vulnerability.
Acknowledgement
This project is financially supported by ICIN 32, ICIN 49 and the 2000 NWO PIONIER Technische Wetenschappen (STW). Contrast agents were provided by BMS imaging and Bracco.
References
- 1.Schoenhagen P, Ziada KM, Vince DG, Nissen SE, Tuzcu EM. Arterial remodeling and coronary artery disease: the concept of “dilated” versus “obstructive” coronary atherosclerosis J Am Coll Cardiol 2001;38:297-306. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls SJ, Sipahi I, Schoenhagen P, Crowe T, Tuzcu EM, Nissen SE. Application of intravascular ultrasound in anti-atherosclerotic drug development. Nat Rev Drug Discov 2006;5:485-92. [DOI] [PubMed] [Google Scholar]
- 3.Nissen SE, Yock P. Novel pathophysiological insights and current clinical applications. Intravascular ultrasound. Circulation 2001;103:604-16. [DOI] [PubMed] [Google Scholar]
- 4.Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005;111:143-9 [DOI] [PubMed] [Google Scholar]
- 5.Schaar JA, Muller JE, Falk E, Virmani R, Fuster V, Serruys PW, et al. Terminology for high-risk and vulnerable coronary artery plaques – Report of a meeting on the vulnerable plaque. Santorini, Greece, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Muller JE, Tawakol A, Kathiresan S, Narula J. New opportunities for identification and reduction of coronary risk - Treatment of vulnerable patients, arteries, and plaques. J Am Coll Cardiol 2006;47:Suppl:C2-6. [DOI] [PubMed] [Google Scholar]
- 7.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47:C13-8. [DOI] [PubMed] [Google Scholar]
- 8.Schaar JA, de Korte CL, Mastik F, Strijder C, Pasterkamp G, Boersma E, et al. Characterizing vulnerable plaque features with intravascular elastography. Circulation 2003;108:2636-41. [DOI] [PubMed] [Google Scholar]
- 9.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002;106:2200-6. [DOI] [PubMed] [Google Scholar]
- 10.Ultrasound contrast agents: basic principles and clinical applications echocardiography. Goldberg BB, Raichlen JS, Forsberg F (editors). Martin Dunitz. 2001. [Google Scholar]
- 11.de Jong N, Frinking PJA, Buoakaz A, Ten Cate FJ. Detection procedures of ultrasound contrast agents. Ultrasonics 2000;38:87-92. [DOI] [PubMed] [Google Scholar]
- 12.Gorce JM, Arditi M, Schneider M. Influence of bubble size distribution on the echogenicity of ultrasound contrast agents - A study of SonoVue™. Invest Radiol 2000;35:661-71. [DOI] [PubMed] [Google Scholar]
- 13.Cachard C, Finet G, Bouakaz A, Tabib A, Francon D, Gimenez G. Ultrasound contrast agents in intravascular echography: An in vitro study. Ultrasound Med Biol 1997;23:705-17. [DOI] [PubMed] [Google Scholar]
- 14.Goertz DE, Cherin E, Needles A, Karshafian R, Duckett A, Burns PN, et al. High frequency nonlinear b-scan imaging of microbubble contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control 2005;52:65-79. [DOI] [PubMed] [Google Scholar]
- 15.Goertz DE, Frijlink ME, de Jong N, van der Steen AF. Nonlinear intravascular ultrasound contrast imaging. Ultrasound Med Biol 2006;32:491-502. [DOI] [PubMed] [Google Scholar]
- 16.Frijlink ME, Goertz DE, van Damme LC, Krams R, van der Steen AFW. Intravascular ultrasound tissue harmonic imaging in vivo. IEEE Trans Ultrason Ferroelectr Freq Control 2006;53:1844-52. [DOI] [PubMed] [Google Scholar]
- 17.Zamir M, Silver MD. Vasculature in the walls of human coronary arteries. Arch Pathol Lab Med 1985;109:659-62. [PubMed] [Google Scholar]
- 18.Barger AC, Beeuwkes R, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of coronary arteries. A possible role in the pathophyiology of atherosclerosis. N Engl J Med 1984;310:175-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol 1993;143:164-73. [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Schwartz RS, et al. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest 1998;101:1551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumamoto M, Nakashimi Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis- its origin and pathophysiological significance. Hum Pathol 1995;26:450-6. [DOI] [PubMed] [Google Scholar]
- 22.de Boer OJ, van der Wal AC, Teeling P, et al. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: a prominent role for neovascularization? Cardiovascular Res 1999;41:443-9. [DOI] [PubMed] [Google Scholar]
- 23.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Nat Acad Sciences 2003;100;4736-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary Atheroma. N Engl J Med 2002;349:2316-25. [DOI] [PubMed] [Google Scholar]
- 25.Milei J, Parodi JC, Alonso GF, et al. Carotid rupture and intraplaque hemorrhage: Immunophenotype and role of cells involved. Am Heart J 1998;136: 1096-105. [DOI] [PubMed] [Google Scholar]
- 26.Barger AC, Beeuwkes R. Rupture of vasa vasorum as trigger of acute myocardial infarction. Am J Cardiol 1990;66:G41-3. [DOI] [PubMed] [Google Scholar]
- 27.Tenaglia AN, Peters KG, Sketch MH, et al. Neovascularization in atherectomy specimens from patients with unstable angina: Implications for pathogenesis of unstable angina. Am Heart J 1998;135:10-4. [DOI] [PubMed] [Google Scholar]
- 28.Mofidi R, Crotty TB, McCarthy P, et al. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg 2001;88:945-50. [DOI] [PubMed] [Google Scholar]
- 29.Schaar JA, Muller JE, Falk E, Virmani R, Fuster V, Serruys PW, et al. Terminology for high-risk and vulnerable coronary artery plaques. Eur Heart J 2004;25:1077-82. [DOI] [PubMed] [Google Scholar]
- 30.Moreno PR, Purushothaman R, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta - Implications for plaque vulnerability. Circulation 2004;110:2032-8. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen SM, Demirkaya O, Ritman EL. Three-dimensional imaging of vasculature and parenchyma in intact rodent organs with X-ray micro-CT. Am J Physiol 1998;275:H1103-4. [DOI] [PubMed] [Google Scholar]
- 32.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Richardson DM, Ritman EL, et al. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res 2001;51:762-6. [DOI] [PubMed] [Google Scholar]
- 33.Kerwin W, Hooker A, Spilker M, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 2003;107:851-6. [DOI] [PubMed] [Google Scholar]
- 34.Feinstein SB. The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am J Physiol 2004;287:H450-7. [DOI] [PubMed] [Google Scholar]
- 35.Casscels W, Haasan G, Vasegi MF, et al. Plaque blush, branch location, and calcification are angiographic predictors of progression of mild to moderate coronary stenosis. Am Heart J 2003;145:813-20. [DOI] [PubMed] [Google Scholar]
- 36.Li W, van der Steen AFW, Lancee CT, Cespedes EI, Bom N. Blood flow imaging and volume flow quantitation with intravascular ultrasound. Ultrasound Med Biol 1998;24:203-14. [DOI] [PubMed] [Google Scholar]
- 37.Carlier SG, Kakadiaris A, Dib N, et al. Vasa vasorum imaging: a new window to the clinical detection of vulnerable atherosclerotic plaques. Curr Atherosclerosis Reports 2005;7:164-9. [DOI] [PubMed] [Google Scholar]
- 38.Goertz DE, Frijlink ME, Tempel D, van Damme LC, Krams R, Schaar JA, et al. Contrast harmonic intravascular ultrasound: a feasibility study for vasa vasorum imaging. Invest Radiol 2006;41:631-8. [DOI] [PubMed] [Google Scholar]
- 39.Villanueva FS, Jankowski RJ, Klibanov AL, Brandenburger GH, Wagner WR. Microbubble targeted to intercellular adhesion molecule-1 bind to activated coronary endothelial cells. Circulation 2004;98:1-5. [DOI] [PubMed] [Google Scholar]
- 40.Demos SM, Alkan-Onyuksel H, Kane BJ, Ramani K, Nagaraj A, Greene R, et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol 1999; 33:867-75. [DOI] [PubMed] [Google Scholar]
- 41.Goertz DE, van Wamel A, Frijlink ME, de Jong N, van der Steen AFW. Nonlinear Imaging of Targeted Microbubbles with Intravascular Ultrasound. IEEE Ultrasonics Symp., Rotterdam, 2005:2003-6. [Google Scholar]
- 42.van der Steen AFW, Baldewsing RA, Degertekin FL, Emelianov S, Frijlink ME, Furukawa Y, et al. IVUS beyond the horizon. EuroIntervention 2006;2:132-42. [PubMed] [Google Scholar]
- 43.van der Steen AFW, Goertz D. Kontiki revisited. Eur J Echocardiog. In press 2007. [DOI] [PubMed] [Google Scholar]


