Abstract
Background and purpose:
Drug-induced prolongation of the QT interval can lead to torsade de pointes, a life-threatening ventricular arrhythmia. Finding appropriate assays from among the plethora of options available to predict reliably this serious adverse effect in humans remains a challenging issue for the discovery and development of drugs. The purpose of the present study was to develop and verify a reliable and relatively simple approach for assessing, during preclinical development, the propensity of drugs to prolong the QT interval in humans.
Experimental approach:
Sixteen marketed drugs from various pharmacological classes with a known incidence—or lack thereof—of QT prolongation in humans were examined in hERG (human ether a-go-go-related gene) patch-clamp assay and an anaesthetized guinea-pig assay for QT prolongation using specific protocols. Drug concentrations in perfusates from hERG assays and plasma samples from guinea-pigs were determined using liquid chromatography-mass spectrometry.
Key results:
Various pharmacological agents that inhibit hERG currents prolong the QT interval in anaesthetized guinea-pigs in a manner similar to that seen in humans and at comparable drug exposures. Several compounds not associated with QT prolongation in humans failed to prolong the QT interval in this model.
Conclusions and implications:
Analysis of hERG inhibitory potency in conjunction with drug exposures and QT interval measurements in anaesthetized guinea-pigs can reliably predict, during preclinical drug development, the risk of human QT prolongation. A strategy is proposed for mitigating the risk of QT prolongation of new chemical entities during early lead optimization.
Keywords: drug development, drug-induced QT prolongation, patch clamping, surface ECG (electrocardiogram), TdP (torsade de pointes), hERG potassium channel
Introduction
Drug-induced QT prolongation leading to serious ventricular arrhythmias, such as torsade de pointes (TdP), poses a major safety consideration for the development and use of new drug candidates (Crumb and Cavero, 1999). TdP is a unique form of polymorphic ventricular tachycardia that may result in a worsening of the patient's cardiac condition and/or sudden death. The incidence of TdP with cardiac pro-arrhythmic drugs is 1–8%, but the incidence with non-cardiac drugs is considerably lower, approximately 1:10 000–1:100 000 (Darpo, 2001). TdP is typically not seen in clinical trials because of its infrequent occurrence. The mechanism of TdP remains unclear and this uncertainty makes it difficult to predict it in the early stages of drug development. However, TdP is always associated with prolongation of the QT interval of the surface ECG. Consequently, the presence of a prolonged QT interval has become a surrogate marker for the possible development of TdP. Thus, any evidence indicating a QT prolongation by a new drug is a significant concern in drug development, as it raises the risk of TdP. QT prolongation issues have significantly affected the pharmaceutical industry. The clinical development of candidate compounds has been halted as a result of QT prolongation and marketed drugs have been withdrawn due to a small number of TdP incidents in humans. Some notable examples include cisapride, grepafloxicin and terfenadine. Because of this significant safety issue, regulatory agencies including the Food and Drug Administration (FDA), the Committee for Proprietary Medicinal Products (CPMP) and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) have increasingly required pharmaceutical companies to take serious measures to minimize the risk of drug-induced TdP (Committee for Proprietary Medicinal Products, 1997; ICH S7A, B; ICH E14).
To protect patients and address the concerns of regulatory agencies, pharmaceutical companies have employed a number of preclinical models to evaluate the likelihood of a compound prolonging the QT interval. Clearly, no single assay is sufficient to predict the risk of TdP in humans. This concept is also reflected in the final preclinical guidelines (ICH S7A and S7B, http://www.ich.org), where both an in vitro IKr (rapidly activating delayed rectifier potassium current) (hERG (human ether a-go-go-related gene)) assay and an in vivo QT assay are recommended to assess a candidate's QT prolongation risk. Among the in vitro hERG assays, functional hERG testing using conventional patch-clamp electrophysiology is still the most relevant methodology and remains the gold standard. The telemetered dog and monkey in vivo models are frequently used for ECG investigations (Hammond et al., 2001). However, these assays are often delayed until the late stage of preclinical development, due to cost and resource constraints. Ideally, potential QT prolongation issues need to be identified earlier in drug development to guide structure–activity relationship to minimize the risk of QT prolongation in new chemical entities. Thus, there is a critical need for a reliable approach for accurately and expeditiously predicting the QT prolongation liability of many compounds during the early stage of drug development using a less expensive, but predictive, alternative to the dog or monkey. Among small species, the guinea-pig, unlike rat and mouse, has a cardiac action potential and ECG that are similar to humans, and thus represents a practical, small animal model for assessing QT interval prolongation early in the drug development process. This species is also listed as a suitable model for QT assessment in the ICH S7B guidelines.
Thus, to predict potential QT issues preclinically, we set out to develop a strategy of using a guinea-pig QT model in combination with a hERG functional assay, which we describe herein. This strategy stems from a systematic examination of the relationships between the potency of hERG inhibition in conjunction with drug exposures, the change of QT interval in a guinea-pig model and QT prolongation/TdP in humans for a variety of therapeutic agents. Sixteen marketed drugs were examined in the hERG electrophysiology and anaesthetized guinea-pig QT measurement assays, protocols for which were optimized to yield consistent, robust data with ease of use. Eight drugs that have been associated with QT prolongation and TdP incidents in humans, three drugs that caused QT prolongation without TdP reported in humans and five drugs that had no association with QT prolongation and TdP in humans were included.
Methods
Selection of drugs
Sixteen marketed drugs were selected to cover a wide range of cardiovascular and non-cardiovascular therapeutic classes, including antiarrythmics, a gastrointestinal prokinetic, antihistamines, an anti-oestrogen, antibiotics, antipsychotics, antithrombotics and angiotensin-converting enzyme inhibitors. As shown in Table 1, these drugs were divided into three groups based on their QT prolongation effects and the numbers of TdP incidents in humans. Group A contained eight drugs that induce QT prolongation with TdP incidents reported. The three drugs in group B cause QT prolongation but have not been reported to cause TdP. Five group C drugs do not cause QT prolongation or TdP incidents. Commercial availability was also considered for the selection of drugs. The source of each drug is listed in Supplementary Table 1.
Table 1.
Published data regarding QT prolongation (LQT) and TdP incidence, maximal plasma concentrations and protein binding in human clinical use
| Incidence | Human total plasma Cmax (μM) | Protein binding (%) | Reference | |
|---|---|---|---|---|
| Group A | ||||
| Amiodarone | LQT, TdP | 3.67 | 96 | Hardman and Limbird (2006a) |
| Cisapride | LQT, TdP | 0.13 | 97.5 | Meuldermans et al. (1988); Hardman and Limbird (2006a) |
| Droperidol | LQT, TdP | 4.098 | 90 | Fischler et al. (1986) |
| Haloperidol | LQT, TdP | 0.106 | 90 | Hardman and Limbird (2006a) |
| Quinidine | LQT, TdP | 12 | 84 | Hardman and Limbird (2006a) |
| D,L-Sotalol | LQT, TdP | 18.4 | 0 | Hardman and Limbird (2006b) |
| Sparfloxacin | LQT, TdP | 9.68 | 37 | Shimada et al. (1993); Morganroth et al. (1999) |
| Terfenadine | LQT, TdP | 0.12 | 97 | Monahan et al. (1990); González and Estes (1998) |
| Group B | ||||
| Moxifloxacin | LQT | 8.68 | 37 | Hardman and Limbird (2006a) |
| Tamoxifen | LQT | 0.493 | >98 | Hardman and Limbird (2006a) |
| Ziprasidone | LQT | 0.228 | >99.8 | Aweeka et al. (2000); Miceli et al. (2000) |
| Group C | ||||
| Amoxicillin | None | 158.7 | 18 | Hardman and Limbird (2006a) |
| Aspirin | None | 110.8 | 80 | Hardman and Limbird (2006c) |
| Captopril | None | 3.61 | 25 | Song and White (2002) |
| Propranolol | Nonea | 0.22 | 85 | Hardman and Limbird (2006a) |
| Verapamil | None | 0.55 | 88 | Hardman and Limbird (2006a) |
Abbreviation: TdP, torsade de pointes.
Case report of LQT during overdose.
Medline searches were performed to identify the QT effects and TdP incidents for the drugs used in this study. The majority of Cmax (maximum plasma concentration) values and ranges for protein binding were found in Goodman and Gilman's The Pharmacological Basis of Therapeutics, 11th Edition (Hardman and Limbird, 2006a, 2006b). Otherwise, they were found in individual publications indicated in Table 1. The highest Cmax values and the lowest values of protein binding were chosen when more than two values were available. Maximum free drug concentration (free Cmax) was calculated using the following equation:
where the total Cmax was the peak total plasma concentration and the protein-bound fraction is the proportion of compound bound to plasma proteins. These plasma levels are summarized in Table 1.
Cell preparation and voltage-clamp recordings of hERG currents
The hERG potassium channels were stably expressed in the Chinese hamster ovary (CHO)-K1 cells. The CHO-K1 cells were maintained in cell media that contained 90% Iscove's modified Dulbecco's medium, 10% foetal bovine serum, 1% HT supplement, 1% non-essential amino acid (NEAA), penicillin G sodium 100 U mL−1, streptomycin sulphate 100 μg mL−1 and geneticin 500 μg mL−1. Confluent cells in flasks were rinsed once with PBS prior to passage. The flasks were incubated with Versene (EDTA) 1:5000 for 5 min at 37 °C to detach the cells from the flasks. Cells used in electrophysiology experiments were plated on glass cover slips 24–48 h prior to use.
hERG current recordings were performed using the whole-cell patch clamp configuration with an AxonMultiClamp 700A amplifier (Axon Instruments, Union City, CA, USA). Voltage clamp protocols were controlled using pClamp9 (Axon Instruments) acquisition and analysis software as previously described (Yao et al., 2005). The currents were stable for up to 45 min, and were recorded in control condition and during the application of the drugs at different concentrations (n=4–8 cells for each concentration). Borosilicate glass patch pipettes were pulled to obtain a tip resistance of 2–4 MΩ when filled with (mM): KCl 126, MgSO4 2, CaCl2 0.5, EGTA 5, Mg-ATP 4 and HEPES 25 (pH 7.3). External bath solution consisted of (mM) NaCl 150, CaCl2 1.8, KCl 4, MgCl2 1, glucose 5 and HEPES 10 (pH 7.4). The temperature was controlled at 25±0.5 °C for all experiments using a temperature controller (SHM-6 Multi-Line Solution Heater) from Warner Instruments (Hamden, CT, USA).
Sotalol, aspirin and amoxicillin were dissolved directly in the external bath solution to the desired concentrations. All other drugs were prepared as either a 10 or 100 mM stock solution in dimethylsulphoxide depending on the solubility limitations and stored at −20 °C. On the day of experiments, the stock solution was diluted to the desired concentrations with bath solution. The final concentration of dimethylsulphoxide in bath solution was 0.1%.
The inhibition of hERG is determined by measuring the peak amplitude of the tail currents at −40 mV before and after compound application. The half-maximal inhibitory concentration (IC50) is determined from a curve fit of Hill equation to the data points:
where Y is the percent inhibition and n is a coefficient that determines the slope of the curve.
Animal use and care
All animal procedures in this study were approved by the GlaxoSmithKline Institutional Animal Care and Use Committee according to IACUC protocol 593.03. The guinea-pigs (Charles River, Raleigh, NC, USA) arrived at the animal service department at least 1 week before the study. The guinea-pigs were housed in fibreglass cages with food and water available ad libitum. They were fasted overnight before the study procedure.
Experimental procedures and recordings of ECG, blood pressure and heart rate in guinea-pigs
Male Hartley guinea-pigs weighing 450–550 g were fasted 18 h prior to experimental procedures. The guinea-pigs were anaesthetized using an intraperitoneal injection of Inactin (thiobutabarbital sodium salt) 100–120 mg kg−1 and urethane 1.2 g kg−1. A tracheotomy was performed and the guinea-pigs were mechanically ventilated (Harvard Apparatus, Holliston, MA, USA). Tidal volumes were set at 3 mL with an initial ventilation rate of 40–42 strokes per minute and supplemental oxygen was added. Final ventilation settings were determined using expired CO2 measurements obtained from a micro-capnograph (Columbus Instruments, Columbus, OH, USA). Oxygen saturation values were monitored using a pulse oximeter (Surgivet, Waukesha, WI, USA). The animal was placed on a heat pad with circulating water at a temperature of 37–39 °C. The jugular vein and the carotid artery were cannulated for drug administration and blood pressure monitoring, respectively. ECG pin electrodes were positioned for the standard limb lead and chest lead configuration. ECG signals were measured from lead II or V4 lead depending on the integrity of the signal response. The carotid arterial catheter through a pressure transducer and ECG cable signals were relayed to a Gould Physiograph with outputs to a data acquisition system (Ponemah, St Paul, MN, USA). The blood pressure and ECG were sampled at rates of 250 and 1000 Hz, respectively. The heart rate values were obtained from the ECG channel.
All animals were allowed to stabilize for a 40- to 50-min period after being instrumented prior to the start of data collection. Data were collected over a 5-min control period before drug administration. A vehicle was used to dissolve the compound initially. Normal physiological saline was then added to reach the desired concentration for the 1 mL infusion volume. Guinea-pigs received a single dose of compound or vehicle at a time. To minimize indirect haemodynamic effects, all animals within each dose group were given the same infusion volume. All compounds were infused in all animals at a rate of 1 mL over a 10-min period for each dose given. Data were recorded for 30 min post-infusion. Each drug (or an equal volume of vehicle) was administered intravenously at the doses shown in Supplementary Table 1 to five animals per dose, infused over 10 min. After infusion, the animals were monitored for 30 min to assess compound effects. Blood samples (0.4 mL) for determination of drug concentrations were collected 1 and 31 min post-infusion. For each vehicle type, five control animals were infused with equal volumes of vehicle following an identical protocol to the drug-treated groups.
The QT interval is corrected for heart rate changes (QTc) by using Bazett's formula:
where QTc represents the QT interval corrected for heart rate, QT represents the interval from the beginning of the Q wave (onset of ventricular depolarization) through the end of the T wave (completion of ventricular repolarization) and the R–R interval is the time between two consecutive heart beats and is the inverse of the heart rate.
All parameters were averaged on a beat-to-beat basis every 10 s and reported out every 30 s to a data table. The baseline values of mean arterial blood pressure, heart rate or QTc interval consisted of a 5 min average of measurements before drug administration.
For each animal, the post-infusion QTc measurements (60 measurements recorded over 30 min) are summarized in the single number ‘baseline-corrected QTc', which we defined as the percent change in mean QTc from baseline (pre-infusion) to post-infusion.
Doses and vehicles
For each dose of a given drug or vehicle, a separate group of animals (n=5) was used; thus, each animal received only a single treatment of either drug or vehicle. All doses of all compounds or vehicles were infused intravenously at a rate of 0.1 mL min−1 over a 10-min period (total volume=1 mL). Drugs, doses and vehicles are summarized in Supplementary Table 1. The drug doses were selected to achieve reported human clinical plasma levels. Our past experience with this guinea-pig model indicated that a dose of 10 mg kg−1 of a compound with molecular weight of about 400 resulted in plasma levels (Cmax) of 2–10 μM. In some instances, drug solubility in an appropriate intravenous formulation dictated the upper limit of the dose that could be administered. Physiological saline (0.9%) was used to dissolve water-soluble drugs. Three other vehicles were employed to achieve appropriate dosing for the drugs with poor solubility (see Table 4).
Table 4.
Effects of vehicles on baseline-corrected QTc in the anaesthetized guinea pig
| Vehicles | Composition | Baseline-corrected QTc (Δ%)(n) |
|---|---|---|
| 1 | 0.9% NaCl | 1.3±0.8 (5) |
| 2 | 15% HP-β-cyclodextrin in 25 mM methanesulphonic acid | 1.5±0.1 (5) |
| 3 | 5% DMSO–10% SBE–cyclodextrin in 5 mM methanesulphonic acid | 1.6±0.8 (5) |
| 4 | 10 mg mL−1 lactic acid in 5% dextrose in water | 0.4±1.2 (5) |
Abbreviation: QTc, heart rate corrected QT interval of the ECG.
Analyses of drug concentrations in hERG solutions and in plasma of guinea-pigs
Drug concentrations were determined by atmospheric pressure ionization-liquid chromatography mass spectrometry (LCMS). Three samples of 1 mL for each concentration were taken from the reservoir and perfusate during the experiments for hERG. The samples were diluted with hERG buffer and analysed, or extracted with methyl tert-butyl ether (1 mL; Sigma-Aldrich, St Louis, MO, USA) before analysis. After extraction/dilution, the sample was injected onto the analytical column of an LCMS instrument (Hewlett-Packard Series 1100 Liquid Chromatograph Mass Selective Detector). Drugs and internal standards were detected as molecular ions ([M+H]+) in the positive selected ion-monitoring mode of the instrumentation. Drug concentration (nanogram or microgram per millilitre) in the samples was calculated with a calibration curve constructed from the peak area ratios (drug peak area/internal standard peak area) using a weighted linear regression. The concentration of drug in the sample was determined by interpolation from the regression line. The average differences between measured concentrations and expected concentrations were less than 8% (data not shown).
Internal standards of various compounds from this study (20 μL) were added to plasma samples (50–100 μL), vortexed and then precipitated with acetonitrile (Sigma-Aldrich; 1:3 v/v) or extracted with methyl tert-butyl ether (1 mL), centrifuged at 7280 g for 5 min, dried down in a TurboVap under nitrogen and reconstituted in mobile phase prior to analysis.
Statistical considerations
All statistical calculations were carried out using JMP 7.0 or SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA). For each compound, we performed ANOVA to compare baseline-corrected QTc for the low, medium and high doses to vehicle (compound-matched). Because we were interested in QTc increases, we performed one-sided comparisons to vehicle. We used P=0.05 as our cutoff for determining statistically significant increases.
Results
Effects of the drugs on hERG
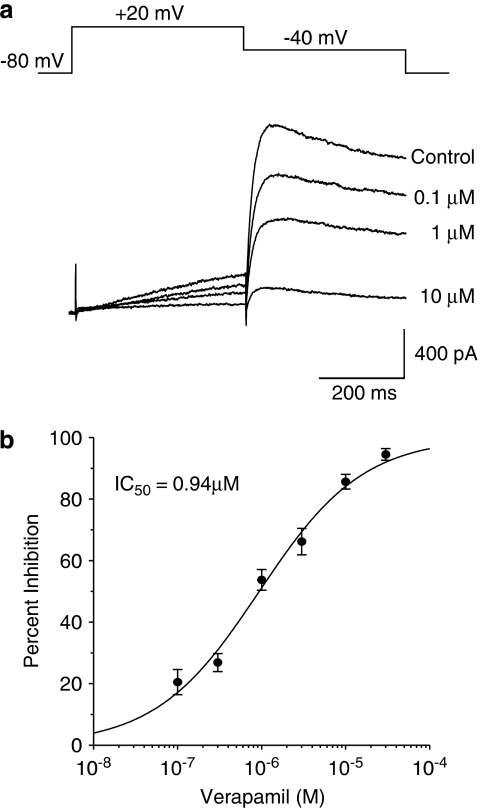
hERG currents were recorded under control conditions and during the application of the drugs at different concentrations. An example is shown in Figure 1, hERG tail currents were blocked by verapamil in a concentration-dependent manner with an IC50 value of 0.94 μM. An IC50 value was generated for each drug, except for class C drugs (amoxicillin, aspirin and captopril) where no hERG inhibition was observed. The percent inhibition of hERG current was taken for these three drugs at the highest concentration permitted by their solubility. The effects on hERG currents of the drugs evaluated in this study are summarized in Table 2. The IC50 values for the drugs in all three groups are widely scattered. All drugs, except sotalol in group A, that induce QT prolongation and TdP incidence in humans had significant inhibitory effects on hERG. The IC50 values for group A drugs ranged from 15 nM to 556 μM. Most of the drugs in group A had potent hERG inhibitory effects. The drugs in group B that induced QT prolongation without TdP incidence showed significant hERG inhibitory effects as well. In general, the drugs in group C, which do not prolong QT and have no TdP reports, demonstrated significantly higher hERG IC50 values. The hERG IC50 values were not clearly separated among these three groups although there was a trend towards a decrease in hERG potency when moving from group A to group B.
Figure 1.
Effects of verapamil on hERG (human ether a-go-go-related gene) potassium currents. (a) Stably transfected Chinese hamster ovary (CHO)-K1 cells were held at −80 mV, then stepped to +20 mV for 400 ms followed by a second pulse to −40 mV for 400 ms to elicit large, slowly deactivating hERG tail currents. This pulse protocol was repeated every 10 sec while the cells were exposed to verapamil at different concentrations. (b) The percent inhibition at each dose was calculated (n=4–8 cells for each concentration), and the concentration–response curve was then fitted to a Hill equation as described in the Methods. In this example, the IC50 for verapamil inhibition of hERG was 0.94 μM. The IC50 values for the other drugs evaluated in this study are listed in Table 2.
Table 2.
Summary of hERG IC50 values and safety margins
| hERG IC50 (μM) | Total safety margin (hERG IC50/ total Cmax) | Free safety margin (hERG IC50/ free Cmax) | |
|---|---|---|---|
| Group A | |||
| Amiodarone | 0.546 | 0.15 | 3.7 |
| Cisapride | 0.091 | 0.7 | 28 |
| Droperidol | 0.307 | 0.075 | 0.749 |
| Haloperidol | 0.015 | 0.1 | 1.4 |
| Quinidine | 1.5 | 0.125 | 0.78 |
| D,L-Sotalol | 556 | 30.2 | 30.2 |
| Sparfloxacin | 24 | 2.477 | 3.757 |
| Terfenadine | 0.061 | 0.5 | 16.9 |
| Group B | |||
| Moxifloxacin | 235 | 27 | 42 |
| Tamoxifen | 0.777 | 1.58 | 78.88 |
| Ziprasidone | 0.165 | 0.573 | 286.45 |
| Group C | |||
| Amoxicillin | >50 000 | >315 | >384 |
| Aspirin | >100 000 | >902 | >4512 |
| Captopril | >3000 | >831 | >1382 |
| Propranolol | 9.9 | 49.5 | 330 |
| Verapamil | 0.94 | 1.6 | 14.2 |
Abbreviation: hERG, human ether a-go-go-related gene.
Calculation of safety margins between hERG IC50 and plasma Cmax of drugs in clinical use in conjunction with QT prolongation and TdP incidence in humans
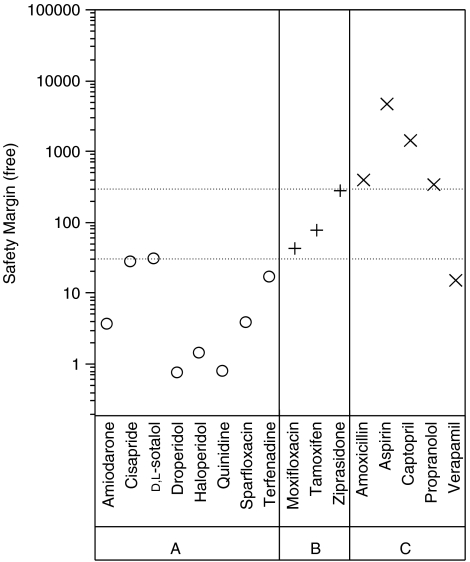
In a survey of the literature, Redfern et al. reported that the margin between the hERG IC50 value and plasma exposure of the drug might be a valuable parameter for predicting the risk of QT prolongation and TdP induced by a hERG blocker. We calculated this margin, termed safety margin (SM), as the mean of IC50 value divided by the highest quoted value of the maximal plasma concentration in clinical use. In the case of terfenadine, the plasma concentration is from a case report of a drug interaction causing inhibition of cytochrome P450 enzymes; normally, the plasma levels of terfenadine are very low due to extensive first pass metabolism through this mechanism (Monahan et al., 1990). Two SMs were calculated for each drug using the total plasma concentration and unbound or free plasma concentration, which we refer to as the total SM (SM-total) and free SM (SM-free), respectively. As shown in Table 2, the total SMs for groups A and B were similar, which ranged from 0.07 to 30.2 for group A and from 0.6 to 27 for group B. Sotalol showed a markedly larger total margin of 30.2. The free SMs for group A were generally lower than group B (Figure 2). The free SMs for group A were in the range of 0.7 and 28. All of the free SMs for group B were greater than 30 with values ranging from 42 to 286. In group C, all free SMs were greater than 300, and the total SMs were larger than 50 with the exception of verapamil, which had smaller free (1.6) and total (14) SMs. The relationships between the free SMs for groups A, B and C are shown in Figure 2. Interestingly, groups B and C had significantly higher free SMs than group A. Each group differs from its neighbour by approximately one log unit (that is, 10-fold), which provides some heuristic support for considering cutoff values of 30 and 300 to distinguish between the free SMs of the three groups. These cutoff values are indicated by dashed lines in Figure 2. In fact, the difference of free SM between groups A and B was statistically significant (P=0.0005) (data not shown).
Figure 2.
Free safety margins were calculated by dividing the hERG (human ether a-go-go-related gene) IC50 values (Table 2) by the therapeutic free Cmax plasma concentrations calculated using the total Cmax and the protein binding from Table 1. Group A (open circles, ○) contains eight drugs that induce QTc (heart rate corrected QT interval of the ECG) prolongation with TdP incidents reported. The three drugs in group B (plus symbols, +) cause QTc prolongation but have not been reported to cause TdP. Neither QTc prolongation nor TdP incidents were found for the five drugs in group C (multiplication symbols, × ). The dashed horizontal lines represent free safety margins of 30 and 300.
Effects of drugs on QTc interval in guinea-pigs
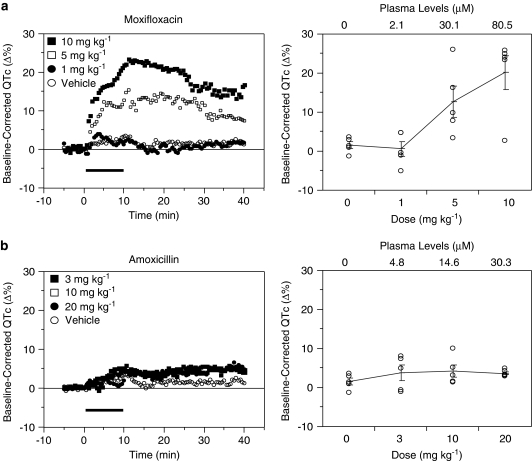
Each of the drug doses was administered on a milligram per kilogram basis by slow intravenous infusion over 10 min. For each animal, baseline-corrected QTc was calculated as the percent change in mean QTc from baseline (that is, pre-infusion) to post-infusion (that is, from 10.5 to 40 min after the start of the 10-min infusion period). Examples for a drug from groups B and C are shown in Figure 3, and the baseline-corrected QTc results are listed in Table 3.
Figure 3.
Example of a drug that prolongs the QT interval (moxifloxacin) (a) versus another that does not (amoxicillin) (b). Baseline-corrected QTc (heart rate corrected QT interval of the ECG) time courses (left panel) and averaged responses (right panel) in each case are depicted. Each of the drug doses was administered to anaesthetized guinea-pigs on a milligram per kilogram basis by slow intravenous infusion over 10 min (horizontal bars). For each animal, baseline-corrected QTc interval was calculated by comparing the mean QTc interval during baseline (that is, pre-infusion) with the mean QTc interval post-infusion (that is, from 10.5 to 30 min after the start of the 10-min infusion period). Baseline-corrected QTc is plotted as a function of the dose in the right panel graphs. The mean values are connected by lines and the error bars represent the standard error of the mean. The vehicle data correspond to the 0 mg kg−1 dose. Sixteen drugs were evaluated in this model, and the data are summarized in Figure 4.
Table 3.
Summary of baseline-corrected QTc and peak plasma levels in the anaesthetized guinea-pig
| Dose (mg kg−1) | Peak plasma level (μm)(n) | Baseline-corrected QTc (Δ%)(n) |
|---|---|---|
| Group A | ||
| Amiodarone (vehicle 1) | ||
| 5 | 6.0±1.4 (4) | 8.0±5.1 (5) |
| 10 | 58.3±9.6 (3) | 13.0±5.2 (4) |
| 20 | 240±52.7 (5) | 6.9±3.8 (5) |
| Cisapride (vehicle 2) | ||
| 0.1 | 0.037±0.023 (4) | 8.5±3.6 (5) |
| 0.3 | 0.129±0.032 (4) | 6.6±2.4 (5) |
| 1 | 0.496±0.085 (5) | 18.2±1.9 (5) |
| Droperidol (vehicle 1) | ||
| 0.03 | 0.55±0.07 (3) | 2.1±3.2 (5) |
| 0.1 | 0.62±0.05 (5) | 6.7±2.3 (5) |
| 0.3 | 1.21±0.34 (5) | 19.1±4.8 (5) |
| Haloperidol (vehicle 3) | ||
| 0.01 | 0.010±0.003 (3) | 1.5±0.8 (5) |
| 0.1 | 0.113±0.012 (4) | 4.3±1.2 (5) |
| 1 | 0.712±0.137 (5) | 15.8±3.6 (5) |
| Quinidine (vehicle 2) | ||
| 3 | 6.2±0.08 (3) | 8.5±1.5 (5) |
| 10 | 27.8±8.0 (5) | 13.7±2.9 (5) |
| 30 | Not determined | 15.6±4.9 (4) |
| Sotalol (vehicle 1) | ||
| 1 | 18.2±1.5 (5) | 18.1±5.3 (5) |
| 3 | 41.3±4.8 (4) | 10.9±1.8 (5) |
| 10 | 145.9±16.6 (5) | 18.9±6.6 (5) |
| Sparfloxacin (vehicle 4) | ||
| 3 | 23.5±4.2 (3) | 6.6±0.9 (5) |
| 10 | 80.4±9.1 (5) | 9.8±1.7 (5) |
| 30 | 159.4±22.4 (4) | 20.0±3.3 (5) |
| Terfenadine (vehicle 3) | ||
| 0.3 | 0.23±0.03 (3) | 2.8±0.8 (5) |
| 1 | 1.28±0.07 (3) | 8.0±2.0 (5) |
| 3 | 3.04±±1.07 (3) | 11.7±1.2 (5) |
| Group B | ||
| Moxifloxacin (vehicle 3) | ||
| 1 | 2.08±0.28 (4) | 0.9±1.7 (5) |
| 5 | 30.6±7.2 (4) | 11.2±3.0 (5) |
| 10 | 80.5±4.0 (5) | 18.3±4.4 (5) |
| Tamoxifen (vehicle 3) | ||
| 3 | 3.8±0.8 (4) | 2.2±1.0 (5) |
| 10 | 10.5±2.1 (5) | 10.2±3.9 (5) |
| 30 | 60.0±20.0 (5) | 8.4±1.4 (5) |
| Ziprasidone (vehicle 1) | ||
| 0.1 | 1.1±0.2 (4) | 4.9±2.0 (5) |
| 0.3 | 1.2±0.1 (2) | 7.0±1.9 (5) |
| 1 | 4.0±2.2 (2) | 9.1±1.0 (5) |
| Group C | ||
| Amoxicillin (vehicle 3) | ||
| 3 | 4.8±0.5 (5) | 4.2±2.0 (5) |
| 10 | 14.6±1.4 (4) | 4.4±1.6 (5) |
| 20 | 30.3±2.5 (5) | 3.9±0.3 (5) |
| Aspirin (vehicle 1) | ||
| 1 | 31.1±2.3 (5) | 0.5±0.8 (5) |
| 3 | 74.6±2.1 (5) | 1.3±0.6 (5) |
| 10 | 468±78 (4) | 2.5±1.8 (5) |
| Captopril (vehicle 1) | ||
| 3 | 57.8±1.6 (2) | 0.0±0.9 (5) |
| 10 | 142±15 (2) | −1.3±1.4 (5) |
| 30 | 313±112 (2) | −6.0±1.9 (4) |
| Propranolol (vehicle 1) | ||
| 0.3 | 0.50±0.03 (3) | −1.6±1.2 (5) |
| 1 | 1.84±0.41 (3) | 2.7±1.3 (5) |
| 3 | 4.65±0.40 (5) | 1.1±1.4 (5) |
| Verapamil (vehicle 1) | ||
| 0.25 | 0.48±0.04 (4) | −0.9±0.7 (5) |
| 0.5 | 0.73±0.03 (2) | −2.3±1.7 (5) |
| 1 | 1.97±0.37 (4) | −0.4±2.0 (5) |
Abbreviation: QTc, heart rate corrected QT interval of the ECG.
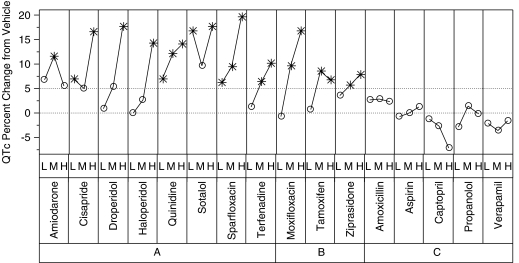
Group A drugs (agents associated with prolonged QT interval as well as TdP in humans), which included amiodorone, cisapride, droperidol, haloperidol, quinidine, sotalol, sparfloxacin and terfenadine caused significant (P⩽0.05) increases in QTc above vehicle for at least one of the doses administered (Figure 4). Additionally, clear dose–response curves were noted for five of the eight group A drugs. The QTc changes with amiodarone were highly variable, as were those observed with the low dose of cisapride; this variability may have contributed to the lack of a clear dose–response relationship. Because of the marked bradycardia and resultant hypotension, the maximum dose of terfenadine was limited to 3 mg kg−1, thus limiting the potential overall maximum changes. For the highest dose level of the eight drugs in group A, the mean baseline-corrected QTc values ranged from 6.9% (amiodarone) to 20.0% (sparfloxacin). The corresponding increases over vehicle ranged from 5.6% (amiodarone) to 19.6% (sparfloxacin) (Figure 4).
Figure 4.
Summary of QTc (heart rate corrected QT interval of the ECG) expressed as differences from vehicle. The y axis shows the mean difference from vehicle (of baseline-corrected QTc) for each drug and the low (L), medium (M) and high (H) doses (Table 3) that were tested in the anaesthetized guinea-pig model. QTc values that are significantly higher than vehicle (P<0.05) are indicated by asterisks. Every drug in groups A and B showed at least one significant increase over vehicle, but none of the drugs in group C had any significant increases over vehicle.
Group B drugs (agents associated with QTc prolongation, but not TdP in humans) included moxifloxacin, tamoxifen and ziprasidone. These agents elicited significant increases in QTc of a similar magnitude to the group A compounds. For all three group B drugs, QTc increased significantly above vehicle at both the second and third doses given. For the highest dose level of the three drugs in group B, the mean baseline-corrected QTc values ranged from 8.4% (tamoxifen) to 18.3% (moxifloxican). The corresponding increases over vehicle ranged from 6.8% (tamoxifen) to 16.8% (moxifloxican).
Group C drugs (agents not associated with QTc prolongation or TdP in clinical use) were captopril, verapamil, propranolol, amoxicillin and aspirin. For the highest dose level of these five drugs, the mean baseline-corrected QTc values ranged from −6.0% (captopril) to 3.9% (amoxicillin). The corresponding changes with vehicle ranged from −7.3% (captopril) to +2.4% (amoxicillin). No drugs in group C showed a significant increase in QTc over vehicle. In fact, QTc actually dropped below vehicle for captopril and verapamil. These decreases probably reflect significant haemodynamic changes and multiple ion channel (that is, calcium channel inhibition) effects, respectively.
In the past, when using this guinea-pig protocol with these or similar vehicles, we considered QTc increases of 5% over vehicle as pharmacologically important safety signals. Figure 4 shows baseline-corrected QTc, expressed as difference from vehicle, for every compound and dose. Doses with a significant (P⩽0.05) increase over vehicle are marked with an asterisk. Every compound in groups A and B showed a statistically significant increase over vehicle for one or more doses, but none of the compounds in group C showed a statistically significant increase over vehicle. Additionally, every compound in groups A and B but none of the group C drugs exceeded the 5% risk signal for one or more doses.
No significant heart rate or blood pressure changes occurred in any of the vehicles used in these studies. The baseline blood pressures were stable and maintained within the ranges of 33–47 mm Hg for all groups, with mean values of 39±3 mm Hg. Baselines heart rate ranges were 194–267 b.p.m. (beats per minute) with an overall group mean of 230±16 b.p.m. QTc, heart rate and mean blood pressure changes for all drugs and vehicles are provided in Supplementary Tables 2–5.
Discussion and conclusions
Numerous reviews and studies have attempted to evaluate relationships between hERG potency, QTc changes in mammals and QT prolongation/TdP incidence in humans (Carlsson, 2001; Hammond et al., 2001; Champeroux et al., 2005). However, comparisons of data from different sources and experimental protocols have been difficult. hERG IC50 values for a drug can vary by up to 20-fold (Crumb, 2000; Kirsch et al., 2004). Often, as drug concentrations in the hERG perfusate are not analysed, their contribution to discrepancies in data from different sources is impossible to ascertain. The QTc data are even harder to compare across studies due to differences in species and experimental protocols. The present study is the first effort to correlate the outcomes of both in vitro functional hERG and in vivo QTc assays under defined conditions using specific protocols within the same laboratory for a variety of therapeutic agents with human data.
All drugs in groups A and B that caused QT prolongation in humans also showed concentration-dependent and pharmacologically relevant hERG inhibition. However, the IC50 values were not clearly separated between groups A and B, suggesting that a risk of TdP cannot be predicted from hERG IC50 values alone. The hERG liability may be placed in context with TdP incidence in humans by calculating an SM based on the IC50 for inhibition of hERG divided by the maximal free plasma concentration of drug in clinical conditions (Webster et al., 2002; Redfern et al., 2003; De Bruin et al., 2005). In the present study, we demonstrated that an SM-free of 30 may differentiate between group A and B drugs (Figure 2), that is, drugs that induce TdP versus drugs that only cause QT prolongation. A 30-fold SM-free has also been derived by other groups based on literature surveys and analyses (Webster et al., 2002; Redfern et al., 2003).
Three group C drugs that do not cause QT prolongation in humans (aspirin, amoxicillin and captopril) also failed to potently inhibit hERG (IC50 values above 1000 μM) and had SM-free above 300. These findings suggest that an SM-free of 300 or more may denote a compound devoid of a potential for causing QTc prolongation. Propranolol has not been reported to cause QT prolongation in clinical use. However, a recent report indicated that an overdose of propranolol in a patient led to QT prolongation (Farhangi and Sansone, 2003). We previously demonstrated that propranolol inhibits hERG potassium channels with an IC50 of 10 μM, which is about 300- to 530-fold higher than its maximum free plasma concentration at therapeutic doses. Therefore, propranolol is unlikely to induce QTc prolongation at therapeutic doses (Yao et al., 2005). In general, the SM-free value of a drug candidate should exceed 300 to be considered safe from causing QT prolongation. In our experience, for typical drug projects in metabolic diseases, a compound that satisfies SM-free >300 usually must have a hERG IC50 greater than 10 μM. The free plasma Cmax of the drug at therapeutic exposures can be obtained from appropriate animal models of efficacy utilized during the early stages of drug development.
The guinea-pig is an established small animal model for cardiac research because its cardiac ion channel profile (with the exception of ITO) resembles that of humans (Busch et al., 1994), as does its ECG, with easily identifiable P, QRS and T waves. During the lead-optimization phase of drug discovery, drug availability is usually limited and a small, inexpensive animal model such as the guinea-pig that can provide QTc prolongation data alongside pharmacokinetic measures is highly desirable. Both conscious and anaesthetized guinea-pigs have been used to examine the effects of pro-arrhythmic drugs on the QT interval or the monophasic action potential duration in vivo (Lu et al., 1993; Sosunov et al., 1996; Minematsu et al., 1999). The draft guideline ICH S7B Step 2 Revision (2004) allows an anaesthetized model to be used for QT measurements, and the Japanese Guidelines for Nonclinical Studies of Drugs Manual (1995) advises the use of anaesthetized animals. Data from the Japan Manufacturers' Association PRODACT Initiative confirmed that the anaesthetized dog is a sensitive model for detecting drug-induced effects on QT interval (Tashibu et al., 2005). QTc prolongation has been shown in guinea-pigs for terfenadine, cisapride and sotalol, as it has in humans (Gras and Llenas, 1999; Kii et al., 2001; Hamlin et al., 2003; Fossa et al., 2004; Testai et al., 2004; Hauser et al., 2005). Isolated guinea-pig heart preparations have been used for studying pro-arrhythmic effects and TdP potential of compounds (Gerhardy et al., 1998).
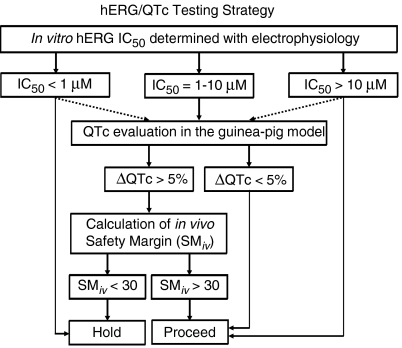
Certain elements of the specific protocols used in our hERG and guinea-pig studies are critical to the sensitivity, precision and accuracy of the readouts, which in turn, directly impact the correlation analyses. We employed a 400-ms pulse protocol in our hERG protocol because the duration is similar to the cardiac action potential. Longer duration pulse protocols are commonly used to test drug effects during supramaximal channel activation. Although our shorter pulse duration protocol could potentially underestimate the IC50 values for some compounds, the strategy that we have outlined here is based on correlation analyses using these IC50 values; one could use longer pulse protocols, provided correlation analyses are done and appropriate adjustments are made to the evaluation strategy outlined in Figure 5.
Figure 5.
A testing strategy incorporating the hERG (human ether a-go-go-related gene) electrophysiology and anaesthetized guinea-pig QTc (heart rate corrected QT interval of the ECG) assays. The solid lines represent the standard progression path, whereas the dashed lines represent exceptions on a case-by-case basis. Compounds are initially evaluated in a hERG electrophysiology assay conducted using the defined protocol described in Methods. Compounds with hERG IC50 values of >10 μM are usually progressed, whereas compounds with IC50 values of <1 μM are usually withheld. Compounds with hERG IC50 values between 1 and 10 μM are evaluated for their potential to prolong the QTc in a specific anaesthetized guinea-pig model (see Methods for protocol details). If a compound is found to prolong QTc in this model, an in vivo safety margin (SMiv) is calculated by dividing the peak drug concentration obtained from an anaesthetized guinea-pig study (where the QTc increased by more than 5%) by the peak drug concentration obtained during an efficacy study in an appropriate animal model. If the SMiv is greater than 30, the compound is further progressed; if the SMiv is less than 30, the compound is put on ‘hold', and research continues for an alternative clinical candidate.
Specific elements of the guinea-pig protocol contributing to the precision of the data include anaesthesia, ventilation and a single-dose testing regimen. Inactin provides a rapid induction, whereas urethane provides a uniform maintenance of anaesthesia with minimal inhibition of the autonomic system. Both anaesthetics lack any reported effects on QTc, unlike other anaesthetic agents, such as pentabarbitone, isoflurane and halothane. Artificial ventilation ensures that cardiovascular effects secondary to respiratory depression are minimized. We avoided multiple dosing to the same animal (Hauser et al., 2005) and instead used a single dose per animal to enable accurate assessments of plasma concentrations of drug. We also limited the volume infused (1 mL) to minimize effects on haemodynamic function and reduce the experimental duration of the preparation to minimize its deterioration over time. Despite its many advantages, one limitation of this guinea-pig model is the requirement that the test compound be soluble in a vehicle suitable for slow intravenous infusion.
The present study showed that our anaesthetized guinea-pig model mimics drug-induced QTc prolongation in humans. An important finding of our study was that a 5% increase in QTc (over vehicle) is a useful cutoff value for detecting significant QTc prolongation in this model. Group C drugs, including amoxicillin, aspirin, captopril, propranolol and verapamil, which are not associated with QT prolongation in humans, did not reach the 5% increase of QTc in the guinea-pig model, whereas every compound in groups A and B exceeded the 5% risk signal for one or more doses. These observations would indicate that the cutoff value of 5% is a reasonable threshold for detecting compounds with potential clinical QT risk. Furthermore, our guinea-pig model elicited dose-dependent QTc prolongation with moxifloxacin, a commonly utilized positive control in ‘Thorough QT Studies' for detecting QT effects of investigational drugs in clinical trials. A single 400 mg dose of moxifloxacin, which prolongs the QT interval in humans with a plasma Cmax of approximately 7 μM, was mimicked by our guinea-pig model (Figure 3a and Table 3).
A poor understanding of the mechanisms underlying TdP has always imposed limitations for predicting its risk through evaluation of QT interval. Our experience suggests that excessive QT prolongation in conjunction with drug exposure is likely to be associated with TdP. Pro-arrhythmic paradigms, such as isolated canine Purkinje fibres (Champeroux et al., 2005), and rabbit heart (Hondeghem and Hoffmann, 2003; Liu et al., 2006) or other electrocardiographic measures such as transmural dispersion have been claimed to be better surrogate markers for TdP; the latter has not been extensively validated in the clinic (Antzelevitch, 2001). Hondeghem and colleagues proposed triangulation, reverse use dependency, electrical instability of the action potential, and dispersion as a potential specific TdP predictor (Shah and Hondeghem, 2005). A major limitation of all in vitro preparations is the absence of any plasma drug exposure data for estimating in vivo safety ratios. To date, measurement of the QT interval in the clinic remains the only acceptable indicator of TdP liability. Moreover, the requirement of the Thorough QT Study in humans for many drugs is a reflection of the inefficiency of preclinical guidelines to predict QT prolongation in humans, even though studies of 19 compounds showed a satisfactory correlation between TQT and preclinical results (Cavero, 2008). Using these analyses of hERG inhibitory potency in conjunction with drug exposures and QTc interval changes in the anaesthetized guinea-pig model, we have developed a strategy, schematically depicted in Figure 5, for selecting drug candidates during early drug development with a reduced risk of human QT prolongation. In our experience, compounds with hERG IC50 values of less than 1 μM usually prolong QTc in the anaesthetized guinea-pig. Such compounds are usually not progressed unless they are potent at the primary target and efficacious in an animal model at low plasma exposures. Compounds with hERG IC50 values above 10 μM usually do not prolong QTc in anaesthetized guinea-pigs. However, certain classes of compounds, such as antibiotics, can achieve high plasma levels and prolong QTc, although they might be weak hERG inhibitors. Compounds with hERG IC50 values between 1 and 10 μM are evaluated in anaesthetized guinea-pigs. If a compound is found to prolong QTc in this model, then an in vivo SM (SMiv) is calculated for decision-making, as elaborated in Figure 5. Although hERG inhibition may be useful for predicting QTc prolongation, it cannot be considered in isolation. Verapamil is not associated with QT prolongation and TdP in humans, but inhibits hERG with an IC50 of 0.94 μM (Figure 1 and Table 2). However, as verapamil blocks cardiac calcium channels—its primary mechanism, leading to QT shortening—as well as hERG, the net effect on cardiac action potential duration is not markedly changed. In agreement with clinical observations, verapamil did not prolong the QT interval in our guinea-pig model. These findings underscore the importance of considering other cardiac ion channels for evaluation of drug safety (Fermini and Fossa, 2003; Bass et al., 2005; Salama and London, 2007). At the GlaxoSmithKline Center for Excellence in Drug Discovery (Research Triangle Park, NC, USA) we have used this strategy to successfully evaluate and select a variety of drug candidates for human clinical trials over the past 10 years. Subsequent data have repeatedly confirmed our predictions of risk for QTc prolongation of these drugs in humans.
External data objects
Acknowledgments
We thank Dr Paul Feldman for his review of the paper and helpful comments and suggestions.
Abbreviations
- hERG
human ether a-go-go-related gene
- QTc
the heart rate corrected QT interval of the ECG
- TdP
torsade de pointes
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Antzelevitch C. Tpeak−Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest. 2001;31:555–557. doi: 10.1046/j.1365-2362.2001.00849.x. [DOI] [PubMed] [Google Scholar]
- Aweeka F, Jayesekara D, Horton M, Swan S, Lambrecht L, Wilner KD, et al. The pharmacokinetics of ziprasidone in subjects with normal and impaired renal function. Br J Clin Pharmacol. 2000;49:27S–33S. doi: 10.1046/j.1365-2125.2000.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AS, Tomaselli G, Bullingham R, III, Kinter LB. Drugs effects on ventricular repolarization: a critical evaluation of the strengths and weaknesses of current methodologies and regulatory practices. J Pharmacol Toxicol Methods. 2005;52:12–21. doi: 10.1016/j.vascn.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Busch AE, Malloy K, Groh WJ, Varnum MD, Adelman JP, Maylie J. The novel class III antiarrhythmics NE-10064 and NE10133 inhibit IsK channels expressed in Xenopus oocytes and IKs in guinea pig cardiac myocytes. Biochem Biophys Res Commun. 1994;202:265–270. doi: 10.1006/bbrc.1994.1922. [DOI] [PubMed] [Google Scholar]
- Carlsson L. Drug-induced torsade de pointes: the perspectives of industry. Eur Heart J (Supplement K) 2001;3:K114–K120. [Google Scholar]
- Cavero I. Safety Pharmacology Society: 7th annual meeting 19–20 September. Expert Opin Drug Safety. 2008;7:91–100. doi: 10.1517/14740338.7.1.91. [DOI] [PubMed] [Google Scholar]
- Champeroux P, Viaud K, El Amrani AI, Fowler JS, Martel E, Le Guennec JY, et al. Prediction of the risk of torsade de pointes using the model of isolated canine Purkinje fibres. Br J Pharmacol. 2005;144:376–385. doi: 10.1038/sj.bjp.0706070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for Proprietary Medicinal Products Points to consider: the assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products 1997. CPMP document number: CPMP/986/96. European Agency for the Evaluation of Medicinal Products: London
- Crumb W, Cavero I. QT interval prologation by non-cardiovascular drugs: issues and solutions for novel drug development. Pharm Sci Technol Today. 1999;2:270–280. doi: 10.1016/s1461-5347(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Crumb WJ. Loratadine blockade of K+ channels in human heart: comparison with terfenadine under physiological conditions. J Pharmacol Exp Ther. 2000;292:261–264. [PubMed] [Google Scholar]
- Darpo B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J (Supplement K) 2001;3:K70–K80. [Google Scholar]
- De Bruin ML, Pettersson LM, Meyboom RHB, Hoes AW, Leufkens HGM. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J. 2005;26:590–597. doi: 10.1093/eurheartj/ehi092. [DOI] [PubMed] [Google Scholar]
- Editorial Supervision by Pharmaceutical and Cosmetics Division, Pharmaceutical Affairs Bureau, Japanese Ministry of Health and Welfare Japanese Guidelines for Nonclinical Studies of Drugs Manual. 1995.
- Farhangi V, Sansone RA. QTc prolongation due to propranolol overdose. Int J Psychiatry Med. 2003;33:201–202. doi: 10.2190/KLBE-UWHT-TARV-8E0M. [DOI] [PubMed] [Google Scholar]
- Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2:439–447. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- Fischler M, Bonnet F, Trang H, Jacob L, Levron JC, Flaisler B, et al. The pharmacokinetics of droperidol in anesthetized patients. Anesthesiology. 1986;64:486–489. doi: 10.1097/00000542-198604000-00012. [DOI] [PubMed] [Google Scholar]
- Fossa AA, Wisialowski T, Wolfgang E, Wang E, Avery M, Raunig DL, et al. Differential effect of HERG blocking agents on cardiac electrical alternant in the guinea pig. Eur J Pharmacol. 2004;486:209–221. doi: 10.1016/j.ejphar.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Gerhardy A, Scholtysik G, Schaad A, Haltiner R, Hess T. Generating and influencing torsades de pointes––like polymorphic ventricular tachycardia in isolated guinea pig hearts. Basic Res Cardiol. 1998;93:285–294. doi: 10.1007/s003950050097. [DOI] [PubMed] [Google Scholar]
- González MA, Estes KS. Pharmacokinetic overview of oral second-generation H1 antihistamines. Int J Clin Pharmacol Ther. 1998;36:292–300. [PubMed] [Google Scholar]
- Gras J, Llenas J. Effects of H1 antihistamines on animal models of QTc prolongation. Drug Safety. 1999;21:39–44. doi: 10.2165/00002018-199921001-00006. [DOI] [PubMed] [Google Scholar]
- Hamlin RL, Kijtawornrat A, Keene BW, Hamlin DM. QT and RR intervals in conscious and anaesthetized guinea pigs with highly varying RR intervals and given QTc-lengthening test articles. Toxicol Sci. 2003;76:437–442. doi: 10.1093/toxsci/kfg254. [DOI] [PubMed] [Google Scholar]
- Hammond TG, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Redfern WS, et al. Methods of collecting and evaluating non-clinical cardiac electrophysiology data in the pharmaceutical industry: results of an international survey. Cardiovasc Res. 2001;49:741–750. doi: 10.1016/s0008-6363(00)00310-2. [DOI] [PubMed] [Google Scholar]
- Hardman JG, Limbird LE. Goodman and Gilman's The Pharmacological Basis of Therapeutics 2006aMcGraw-Hill: New York; 1927–2020.11th edn. [Google Scholar]
- Hardman JG, Limbird LE. Goodman and Gilman's The Pharmacological Basis of Therapeutics 2006bMcGraw-Hill: New York; 95511th edn. [Google Scholar]
- Hardman JG, Limbird LE. Goodman and Gilman's The Pharmacological Basis of Therapeutics 2006cMcGraw-Hill: New York; 70011th edn. [Google Scholar]
- Hauser DS, Stade M, Schmidt A, Hanauer G. Cardiovascular parameters in anaesthetized guinea pigs: a safety pharmacology screening model. J Pharmacol Toxicol Methods. 2005;52:106–114. doi: 10.1016/j.vascn.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Hoffmann P. Blinded test in isolated female rabbit heart reliably identifies action potential duration prolongation and proarrhythmic drugs: importance of triangulation, reverse use dependency and instability. J Cardiovasc Pharmacol. 2003;41:14–24. doi: 10.1097/00005344-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Kii Y, Nakatsuji K, Nose I, Yabuuchi M, Mizuki Y, Ito T. Effects of 5-HT(4) receptor agonists, cisapride and mosapride citrate on electrocardiogram in anaesthetized rats and guinea-pigs and conscious cats. Pharmacol Toxicol. 2001;89:96–103. doi: 10.1034/j.1600-0773.2001.d01-142.x. [DOI] [PubMed] [Google Scholar]
- Kirsch GI, Trepakova ES, Brimecombe JC, Sidach SS, Erickson HD, Kochlan MC, et al. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J Pharmacol Toxicol Methods. 2004;50:93–101. doi: 10.1016/j.vascn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan GX. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm. 2006;3:948–956. doi: 10.1016/j.hrthm.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Remeysen P, De Clerck D. Inhibition of Na+/Ca2+ overload with R56865 protects against cardiac arrhythmias elicited by ouabain in vivo in guinea-pigs. Eur J Pharmacol. 1993;235:89–93. doi: 10.1016/0014-2999(93)90824-2. [DOI] [PubMed] [Google Scholar]
- Meuldermans W, Van Peer A, Hendrickx J, Lauwers W, Swysen E, Bockx M, et al. Excretion and biotransformation of cisapride in dogs and humans after oral administration. Drug Metab Dispos. 1988;16:403–409. [PubMed] [Google Scholar]
- Miceli JJ, Smith M, Robarge L, Morse T, Laurent A. The effects of ketoconazole on ziprasidone pharmacokinetics––a placebo-controlled crossover study in healthy volunteers. Br J Clin Pharmacol. 2000;49:71S–76S. doi: 10.1046/j.1365-2125.2000.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minematsu T, Ohtani H, Sato H, Iga T. Sustained QT prolongation induced by tacrolimus in guinea pigs. Life Sci. 1999;65:PL197–PL202. doi: 10.1016/s0024-3205(99)00396-3. [DOI] [PubMed] [Google Scholar]
- Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR. Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788–2790. [PubMed] [Google Scholar]
- Morganroth J, Talbot GH, Dorr MB, Johnson RD, Geary W, Magner D. Effect of single ascending, supratherapeutic doses of sparfloxacin on cardiac repolarization (QTc interval) Clin Ther. 1999;21:818–828. doi: 10.1016/s0149-2918(99)80004-6. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Salama G, London B. Mouse models of long QT syndrome. J Physiol. 2007;578:43–53. doi: 10.1113/jphysiol.2006.118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RR, Hondeghem LM. Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm. 2005;2:758–772. doi: 10.1016/j.hrthm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Shimada J, Nogita T, Ishibashi Y. Clinical pharmacokinetics of sparfloxacin. Clin Pharmacokinet. 1993;25:358–369. doi: 10.2165/00003088-199325050-00002. [DOI] [PubMed] [Google Scholar]
- Song JC, White CM. Clinical pharmacokinetics and selective pharmacodynamics of new angiotensin converting enzyme inhibitors. Clin Pharmacokinet. 2002;41:207–224. doi: 10.2165/00003088-200241030-00005. [DOI] [PubMed] [Google Scholar]
- Sosunov EA, Anyukhovsky P, Rosen MR. Chronic in vivo and in vitro effects of amiodarone on guinea pig hearts. J Pharmacol Exp Thera. 1996;278:906–912. [PubMed] [Google Scholar]
- Tashibu H, Miyazaki H, Aoki K, Akie Y, Yamamoto K. QT PRODACT: in vivo QT assay in anesthetized dog for detecting the potential for QT interval prolongation by human pharmaceuticals. J Pharm Sci. 2005;99:473–486. doi: 10.1254/jphs.qt-a3. [DOI] [PubMed] [Google Scholar]
- Testai L, Calderone V, Salvadori A, Breschi MC, Nieri P, Martinotti E. QT prolongation in anaesthetized guinea-pigs: an experimental approach for preliminary screening of torsadogenicity of drugs and drug candidates. J Appl Toxicol. 2004;24:217–222. doi: 10.1002/jat.975. [DOI] [PubMed] [Google Scholar]
- Webster R, Leishman D, Walker D. Towards a drug concentration effect relationship for QT prolongation and torsade de pointes. Curr Opin Drug Discov Dev. 2002;5:116–126. [PubMed] [Google Scholar]
- Yao X, McIntyre MS, Lang DG, Song IH, Becherer JD, Hashim MA. Propranolol inhibits the human ether-a-go-go-related gene potassium channels. Eur J Pharmacol. 2005;519:208–211. doi: 10.1016/j.ejphar.2005.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.