SUMMARY
Lon, also known as the protease La, is a homo-oligomeric ATP-dependent protease, which is highly conserved in archaea, eubacteria and eukaryotic mitochondria and peroxisomes. Since its discovery, studies have shown that Lon activity is essential for cellular homeostasis, mediating protein quality control and metabolic regulation. This article highlights the discoveries made over the past decade demonstrating that Lon selectively degrades abnormal as well as certain regulatory proteins and thus plays significant roles in maintaining bacterial and mitochondrial function and integrity. In addition, Lon is required in certain pathogenic bacteria, for rendering pathogenicity and host infectivity. Recent research endeavors have been directed towards elucidating the reaction mechanism of the Lon protease by different biochemical and structural biological techniques. In this mini-review, the authors survey the diverse biological roles of Lon, and also place special emphasis on recent discoveries that elucidate the mechanistic aspects of the Lon reaction cycle.
Keywords: Oligomeric ATP-dependent proteases, protein, peptide, mechanism
Introduction
The ATP-dependent Lon (La) protease derives its name from the phenotype of Escherichia coli lon gene mutants that form long undivided filaments upon UV irradiation (1–3). Since its discovery, studies have shown that Lon is essential for cellular homeostasis by mediating the degradation of abnormal and damaged polypeptides, as well as short-lived regulatory proteins. Lon functions in the cytosol of prokaryotes and in mitochondria and peroxisomes of eukaryotes (2, 4–8)
Structure of Lon
Unlike other soluble ATP-dependent proteases such as the 26S proteasome, HslUV and ClpP complexes that consist of separate subunits for ATP hydrolysis and proteolysis, Lon is a homo-oligomer composed of identical subunits, which carry both ATPase and protease domains (Figure 1). Each Lon subunit has four domains - the amino-terminal (N) domain that is implicated in the binding of protein substrates, the ATPase (A) domain containing Walker A and B motifs mediating ATP-binding and hydrolysis, the substrate sensor and discriminatory domain (SSD) that discriminates the ATP versus the ADP bound enzyme form, and the carboxyl-terminal (P) domain containing the proteolytic active site (9). In Brevibacillus thermoruber Lon, the N domain may also be involved in subunit oligomerization (10). Lon belongs to the AAA+ family of proteins (ATPases Associated with a variety of cellular Activities), whose members are involved in DNA replication, transcription, membrane fusion, and proteolysis (11–15). These proteins all share a conserved ATP-binding module of ~200 amino acids that assembles into oligomeric rings. A significant degree of sequence homology is shared between the ATPase domains of Lon and the other ATP-dependent proteases, therefore it is speculated that the function of ATP binding and hydrolysis by these enzymes is mechanistically similar.
Figure 1.
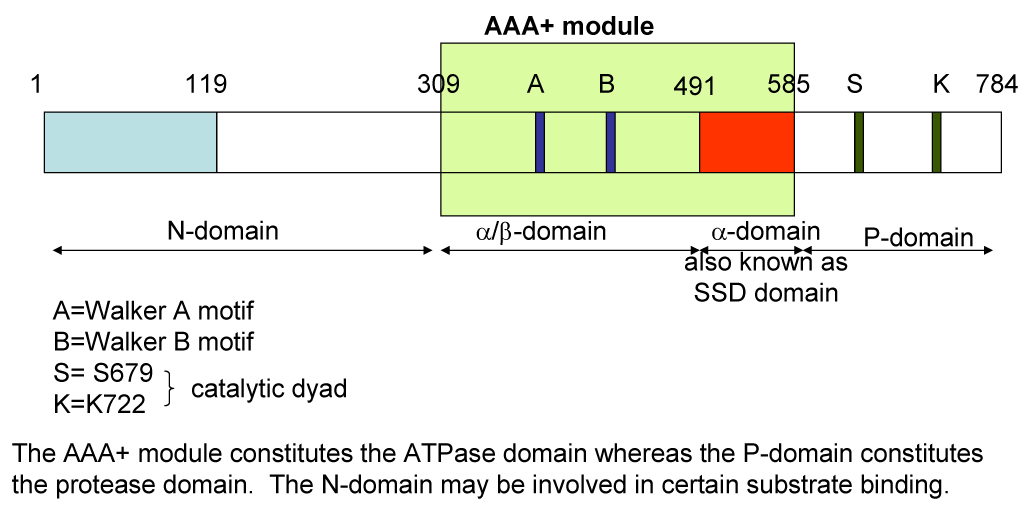
Domain organization for a subunit in Lon protease.
Structural studies demonstrate that the Lon holoenzyme is a large homo-oligomeric ring-shaped complex. Analytical ultracentrifugation analyses show that purified Lon of Mycobacterium smegmatis (MsLon) is hexameric, and that oligomerization is dependent on Mg2+, but independent of ATP, and stimulated by unfolded protein. MsLon monomers are in reversible equilibrium with oligomeric sub-complexes (e.g. dimers, trimers, tetramers) and complete hexameric complexes (16). Electron microscopy of E. coli Lon (EcLon) reveals a hexameric ring-shaped structure with a central cavity (17). Like MsLon, the formation of EcLon hexamers is Mg2+-dependent and ATP-independent (17). Cryoelectron microscopy and analytic ultracentrifugation of Lon isolated from the budding yeast Saccharomyces cerevisiae (ScLon) demonstrate that the mitochondrial protease is a heptameric ring-shaped complex with flexible subunits (18). The isolation of active heptameric ScLon complexes does not require the addition of Mg2+ or ATP. In the absence of added nucleotide, two adjacent subunits within the ScLon complex are extended resulting in asymmetric rings. Upon addition of ATP or non-hydrolyzable analogs of ATP, the percentage of symmetric ring-shaped complexes is increased, suggesting that ATP binding induces conformational changes in the ScLon holoenzyme.
Although the atomic structure of Lon has not been solved, X-ray structures of the truncated N-terminal domain, partial structure of the AAA+ domain which includes the substrate sensor and discriminatory domain (SSD), and the P-domain of several bacterial Lon homologs have been independently determined (9, 19–23) (see Figure 1 for domain organization). The truncated protease domains of various bacterial Lons show hexameric ring-shaped structures with a conserved Ser-Lys dyad at the active site. Comparisons of the various Lon P-domain crystal structures reveals that amino acids in addition to the Ser-Lys dyad may participate in catalyzing peptide bond cleavage (20, 22, 24, 25). Although these P-domains assemble to form hexameric structures, they lack proteolytic activity. Within the full-length Lon protein, mutation of either Ser or Lys to Ala in the catalytic dyad abolishes proteolytic activity, but does not affect ATPase activity (22, 26, 27). However, some mutations generated at the vicinity of the proteolytic site Ser 679 can impair the ATPase activity of EcLon (27). By contrast, mutation of the ATP-binding site abolishes both the ATPase and protease proteolytic activities of EcLon (28). These results suggest that communication between the ATPase and the proteolytic sites is crucial to Lon catalysis. In addition, the catalytic activity of Lon is also dependent on intersubunit interactions. Mixed oligomeric complexes composed of wild type EcLon and the inactive EcLon E614K mutant, results in an enzymatically inactive protein (29).
Physiological functions of Lon
In bacteria, Lon mutants exhibit a substantial decrease in the turnover of abnormal proteins, demonstrating its role as a quality control protease. In E. coli, Lon is also important for selectively degrading short-lived regulatory proteins, such as bacteriophage λ N protein, the SulA cell division regulator; the positive regulator of capsule synthesis, Rcs; and the F factor addiction system protein CcdA (for reviews see (5, 30, 31)). In the bacterium Caulobacter crescentus, Lon selectively degrades the Cell-Cycle-Regulated DNA Methyltransferase (CcrM) thereby regulating the methylation of chromosomal DNA and cellular differentiation (32). Pathogenic bacteria, such as Brucella abortus and Salmonella enterica serovar Typhimurium (S. typhimurium), Lon-mediated proteolysis of the central transcription regulatory factor HilA, controls the correct timing for the expression of virulence genes necessary for host invasion (8, 33, 34). In Pseudomonas aeruginosa, the induction of Lon has been proposed to contribute to the adaptive resistance of the organism towards antibiotic treatment (35).
The absence or down-regulation of eukaryotic Lon results in diverse phenotypes. In S. cerevisiae, the absence of the Lon ortholog, Pim1p, results in a lack of ATP-dependent proteolysis in the mitochondrial matrix, accumulation of electron dense aggregates and large mtDNA deletions (36, 37). In yeast, Pim1p is the only ATP-dependent protease within the matrix, by contrast to metazoans where ClpXP is also present. Endogenous substrates of yeast Pim1p are generally misfolded or unassembled subunits of electron transport chain complexes, ribosomal proteins and metabolic enzymes (38–41). In mammalian lung fibroblasts, Lon-depletion for 4 days using anti-sense morpholino oligonucleotides results in defects in mitochondrial membrane potential, respiration and morphology, as well as apoptotic cell death (42, 43). However, the effect of Lon-depletion may be cell type- or cell line- specific. The expression of an inducible short hairpin RNA leading to Lon depletion in a colon adenocarcinoma cell line for 14 days does not lead to cell death (7). Even after RNAi knockdown for 30 days, these cells continue to survive, although they no longer proliferate (B. Lu and C.K. Suzuki, unpublished observations). Endogenous substrates of mammalian Lon include subunits of cytochrome c oxidase (COX), which is an electron transport chain complex (44, 45); oxidized mitochondrial aconitase, which functions in the tricarboxylic acid cycle (46); and the steroidogenic acute regulatory protein StAR, which mediates cholesterol transfer from the cytosol to the mitochondrial inner membrane (47, 48).
Lon has also been shown to exhibit a chaperone-like activity that promotes the assembly of protein complexes, which is independent of its protease activity (45, 49). In mammals, a stress response network between the endoplasmic reticulum (ER), nucleus and mitochondria has been shown to up-regulate the expression of Lon, thereby providing a cytoprotective function during ER stress and hypoxia (45). ER stress and hypoxia lead to the accumulation of abnormal proteins within the ER and defects in mitochondrial function. ER stress and hypoxia stimulate the transcriptional up-regulation of ER-specific stress proteins (7). The up-regulation of Lon upon ER stress or hypoxia has been proposed to increase the assembly of subunit 2 of cytochrome c oxidase (Cox2) into functional complexes and to partially restore mitochondrial function. Studies with yeast and mammalian cells show that defects in COX assembly can be alleviated upon overexpressing wild type Lon or a protease inactive Lon mutant. Lon may thus have a chaperone-like function independent of its proteolytic activity.
In addition, recent work shows that Lon functions in the adaptive response to hypoxia by remodeling the COX holoenzyme (44). During normoxia, an isoform of the Cox4 subunit, Cox4-1, is a stable component of the fully assembled enzyme complex. However, during oxygen deprivation, the hypoxia-inducible transcription factor HIF-1α activates the expression of an alternate isoform, Cox4-2, as well as Lon. Increased levels of Lon are required for the degradation of Cox4-1, thereby permitting Cox4-2 assembly into the COX holoenzyme. It is hypothesized that subunit switching produces Cox4-1 or Cox4-2 containing complexes that are each optimized for transferring electrons under normoxic or hypoxic conditions, respectively. It is conceivable that Lon mediates not only the degradation of Cox4-1 but also the assembly of Cox4-2 into the holoenzyme complex.
Lon as a DNA binding protein
Early studies identified and purified EcLon (also referred to CapR) as a DNA binding protein (50). EcLon binds to double-stranded DNA (dsDNA) with a relative higher affinity than to single-stranded DNA (ssDNA) (51, 52). Several in vitro studies show that bacterial Lon interacts with large DNA molecules (51, 53, 54), whereas one study demonstrates sequence-specific EcLon binding to a 35 bp element referred to as pets (52). However, in a study conducted by a different group, the 35 bp pets element does not compete with the non-specific DNA binding of EcLon even at an 100-fold molar excess (54). The lack of sequence-specific DNA binding by EcLon is also suggested by the analysis of chromosomal DNA fragments co-purified with EcLon, which shows no apparent sequence similarities (54). It remains unclear whether there is a single conserved site within Lon proteins, which mediates DNA binding. The SSD sub-region of the ATPase domain of B. thermoruber Lon is sufficient for DNA binding (55), whereas the ATPase domain lacking the SSD of EcLon also binds DNA (54). Different observations have been reported for the relationship between DNA binding and the enzymatic activities of bacterial Lon (51, 53, 54, 56). One study showed that large DNA molecules such as plasmid, bacteriophage and calf thymus DNA stimulate the ATPase and protease activities of Lon (51). By contrast, another study observed that DNA stimulated ATP hydrolysis but inhibited proteolysis (53).
Nucleic acid binding is a conserved property of the Lon protease as mammalian mitochondrial Lon binds to mtDNA in vitro and in living cells (57–60) Unlike bacterial Lon, human Lon (hLon) binds to single-stranded DNA and RNA but not to double-stranded nucleic acids (57–59). In vitro studies show that hLon binds GT-rich ssDNA oligonucleotides with sequence-specificity. Protein substrate stimulates DNA binding by hLon whereas ATP-binding inhibits this interaction (58). Interaction of a protein substrate with hLon may lead to the release of bound ATP or ADP, resulting in a nucleotide -free or -depleted form of the enzyme that binds with higher affinity to DNA. ssDNA, however, has no effect on ATP hydrolysis or proteolysis. hLon exhibits not only sequence-specific binding but strand-specific binding as well, associating with guanine-rich sequences within the heavy-strand of the mitochondrial genome. Human mtDNA consists of a heavy- and light- strand; the heavy-strand is distinguished by a higher percentage of guanine and thymine residues resulting in its greater mass. The small circular human mitochondrial genome (~17 kb) encodes thirteen proteins involved in oxidative phosphorylation, two ribosomal RNAs (rRNAs) and twenty-two transfer RNAs (tRNAs). The GT-rich heavy strand encodes all of these proteins except one, as well as both rRNAs. Human mtDNA contains a single non-coding region referred to as the control region (CR) that carries the light- and heavy- strand promoters (LSP and HSP, respectively), which are required for mtDNA replication and transcription. In cultured cells, hLon is found to interact preferentially with the CR, in addition to several other regions of the mitochondrial genome (60). Analysis of the mtDNA sequences bound by Lon in living cells, reveal a G-rich consensus sequence. Biochemical and biophysical studies show that Lon specifically binds to ssDNA sequences that have a propensity for forming G-quartets (also referred to as G-quadruplexes or tetraplexes) (61). Bioinformatic analysis shows a good correlation between regions of the mitochondrial genome that are bound by Lon in cultured cells and regions that are predicted to form G-quartets (60).
Although the physiological function and importance of DNA binding by Lon remains unclear, results from bacterial and mammalian systems provide insights. In E. coli, the adaptation of amino acid starvation results in a dramatic increase in inorganic polyphosphate (polyP), which directly stimulates Lon-mediated proteolysis of free ribosomal proteins leading to the down-regulation of translation (62, 63). polyP competitively blocks the binding of DNA by EcLon in vitro and as well as in vivo, suggesting the possibility that the polyP-EcLon is released from DNA as an activated complex, which degrades free ribosomal proteins. It has been speculated that EcLon may bind to chromosomal DNA adjacent to sites bound by regulatory proteins, and thus control the turnover of such proteins. In a similar manner in mammalian cells, the preferential binding to hLon to the control region for mtDNA replication and transcription may function to recruit the protease to a site where it selectively degrades protein components of the replication and transcription machinery. hLon has been shown to interact with the mtDNA polymerase γA subunit and with the mtDNA helicase Twinkle (58), which are components of mitochondrial nucleoids that are considered to be crucial for the segregation and inheritance of mtDNA. It is conceivable that hLon mediates the proteolytic remodeling of mitochondrial nucleoids and that it also has a direct role in mtDNA metabolism and integrity. Results show that cellular levels of hLon influence the sensitivity of mtDNA to oxidative mtDNA damage (60). In control cells with normal levels of Lon, oxidative stress leads to an increased frequency of mtDNA lesions. By contrast, Lon-depleted cells show little if any mtDNA damage. These results suggest that damage is permitted when Lon is present and prevented when Lon is absent. When Lon is present and bound to mtDNA, it may facilitate oxidative mtDNA damage by stabilizing ssDNA or blocking lesion repair. Conversely, when Lon levels are reduced, single-stranded mtDNA is perhaps less vulnerable to DNA damage and more efficiently repaired. Lon-depletion may also lead to higher steady state levels of substrate proteins that function as antioxidants or repair proteins. Further experiments are required to determine the role of Lon in the maintenance of mtDNA integrity.
Modulation of Lon Protease Activity in Vivo
Lon-mediated protein turnover in vivo is likely modulated or regulated by factors that affect either the enzymatic activity of the protease and/or the conformational state of protein substrates. Unfolded protein substrates have been shown to stimulate both the peptidase and ATPase activities of Lon (2, 81). For example, unfolded casein stimulates both the ATPase and peptidase activity of Lon by interacting with an allosteric protein-binding site on Lon (82–84). In addition, the bacteriophage T4 PinA protein specifically inhibits E. coli Lon but not ClpAP or HslUV (ClpYQ) (82). PinA does not interact with the ATP-binding site, the proteolytic active site or the allosteric protein-binding site of Lon. Rather, it appears to interact at a novel regulatory or enzymatic site involved in the coupling between ATP hydrolysis and proteolysis. Results show that the binding of inorganic polyphosphate (polyP) within the ATPase domain of E. coli Lon promotes the specific association and degradation of free ribosomal proteins (54, 63, 72). Alternatively, the sensitivity of protein substrates to Lon-mediated proteolysis is influenced by their interactions with partner proteins. For example, subunits of COX or the F1Fo ATP synthase complexes, or bacterial CcdA are degraded by Lon only when they are unassembled (85).
Mechanism of Protein Degradation
The mechanism of Lon-mediated proteolysis has been studied with protein substrates that are unfolded or oxidatively modified, or with defined structural domains (46, 48, 64–71). Like other ATP-dependent proteases, Lon has been proposed to act processively in mediating ATP-dependent protein degradation (48, 67, 72). In general, enzymatic processivity refers to continued catalysis of a series of chemical transformations without enzyme dissociation from substrate, as in the case of DNA polymerase. Currently, it remains unclear the extent to which Lon-mediated degradation proceeds by processive peptide bond hydrolysis without substrate dissociation. Prokaryotic and eukaryotic Lon proteases degrade substrates by generating peptide products consisting of ~5–30 amino acids (48, 64, 70, 74). Peptide bond hydrolysis is thought to occur in a processive linear manner from the amino- to the carboxyl- termini or visa versa. It is possible that protein substrates are sequestered from the bulk solvent by the Lon complex (73) and “processively” degraded by sequential repetitive rounds of substrate binding, cleavage, release and rebinding to the proteolytic site, thereby resulting in small hydrolyzed peptide products that dissociate. Results also show that Lon does not necessarily cleave substrates at a specific peptide consensus sequence; however, it does show preference for hydrophobic residues adjacent to the scissile bond (48, 64, 66, 67, 70). In addition to rendering cleavage specificity, peptide sequences within an exposed or unstructured region of a substrate may serve to facilitate substrate recognition and interaction, thereby leading to the initiation of degradation (64, 75). The molecular details of how such interactions facilitate protein degradation are not clear and thus will require further mechanistic evaluation.
Like other ATP-dependent proteases, the general paradigm for Lon-mediated proteolysis is that ATP-dependent substrate unfolding is a pre-requisite for proteolysis. For example, Lon generally degrades folded proteins only when accompanied by ATP hydrolysis. However, if the same protein is less structured or denatured, then degradation requires only ATP-binding but not hydrolysis (64). The efficiency of unstructured protein and peptide degradation however, is maximized by the presence of ATP hydrolysis. This observation has been explained in terms of the hydrolysis of ATP by Lon is used to facilitate the translocation of unfolded polypeptide substrates, a process found in many other heterosubunit ATP-dependent proteases such as HslU/V, Clp AP or ClpXP. The heterosubunit ATP-dependent protease complexes utilize the energy from ATP hydrolysis to unfold and translocate protein and peptide substrates into the proteolytic core where they are degraded (76–78, 79). Recently a slow peptide translocation step has been detected in ClpAP, thus it is possible that a similar peptide translocation step also occurs during Lon catalysis (80). In the case of Lon, it is plausible that an unfolded protein substrate is translocated to the proteolytic active site where it is sequestered until the selective cleavage of scissile peptide bonds is complete.
Although many protein substrates of Lon are unfolded prior to degradation, recent work shows that some endogenous substrates of mitochondrial Lon are degraded when they are in the folded state (48). For example, the mitochondrial processing peptidase α subunit (MPP α) is degraded by Lon only when it is competent for assembly into active enzyme that is trypsin-resistant. By contrast, when MPPα is unfolded it is not degraded by Lon even though it is sensitive to limited trypsin digestion (48). The initial sites cleaved by Lon within folded MPPα are at hydrophobic residues that are surrounded by a highly charged environment at the surface of the protein. It is speculated that such sites may function as recognition elements or patches, which specify selective degradation by Lon. It is possible that the initial cleavage of folded MPPα by Lon destabilizes its intrinsic folded structure, which facilitates its unfolding and processive degradation. The finding that Lon degrades folded proteins suggests that the protease may have a role in regulating the activity of functional proteins, in addition to disposing of abnormal proteins.
Kinetic Coordination between the ATPase and the Proteolytic Activities in EcLon
In the presence of ATP, protein substrates are cleaved by Lon at multiple sites to yield small peptide products. During this process, Lon hydrolyzes ATP, unfold and/or translocate protein substrates and catalyze peptide bond cleavage. Therefore, when using a protein as substrate to study the kinetic mechanism of Lon protease, it is difficult to decipher the specific function for the binding and hydrolysis of ATP during each ATPase cycle. As an alternative, a systematic approach may be used. This entails first defining the kinetic mechanism for the ATP-dependent cleavage of a peptide bond and then evaluating whether the same repetitive reaction cycle to sequentially process each scissile peptide bond in an unfolded protein substrate (2). As a first step to this approach, defining how the ATPase and the protease domains of Lon are kinetically coordinated during a single peptide bond cleavage event is necessary.
To construct a minimal kinetic model for the ATP-dependent peptidase reaction mechanism, transient kinetic techniques have been utilized to detect enzyme bound reaction intermediates generated along the ATPase and peptide hydrolysis pathway. For such an analysis, relatively large proteins are not optimal substrates as they contain multiple and diverse Lon cleavage sites. Some substrates such as SulA also contain additional recognition sites within their sequences that are not cleaved by Lon. As such, protein substrates will likely interact with Lon in a complex manner (70, 75, 86), which does not allow for a clear correlation between ATP hydrolysis and a single peptide cleavage event. In the past, hydrophobic tetrapeptides have been used as model substrates to characterize the peptidase activity of Lon; however, these peptide substrates inhibit the ATPase activity of Lon and are degraded at a significantly slower rate as compared to protein substrates (2). Therefore, these model peptide substrates are also not optimal for evaluating the functional relationship between ATP hydrolysis and peptide bond cleavage.
We have developed and utilized a fluorogenic peptide substrate (FRETN 89–98, Figure 2) containing residues 89–98 of the λ N protein to determine the timing of ATP hydrolysis and peptide bond cleavage that will allow us to define a minimal kinetic mechanism of EcLon (67, 87). Like the full-length λN protein, FRETN 89–98 is efficiently degraded by Lon in the presence of ATP. ATP hydrolysis is monitored by using radiolabled α32P-ATP. Degradation of FRETN 89–98 also occurs in the presence of AMP-PNP, albeit with substantially reduced efficiency. The kcat/Km value for ATP-dependent cleavage by Lon is 3 × 104M−1s−1, which is comparable to the value measured for the degradation of λN (87). FRETN 89–98 is cleaved between Cys-Ser residues as is the full-length λN protein, indicating that this peptide contains the information needed to confer the same cleavage specificity as in λN. Although FRETN 89–98 contains only 11 residues, its ability to stimulate the ATPase activity of Lon is comparable to larger protein substrates. Collectively, these data demonstrate that FRETN 89–98 provides a unique tool for studying the kinetic mechanism associated with the activation of peptide bond cleavage by ATP binding and hydrolysis by Lon. The mechanistic characterization of FRETN 89–98 cleavage by Lon is a first step in unraveling the mechanism by which Lon catalyzes the degradation of large protein substrates containing multiple cleavage sites.
Figure 2.
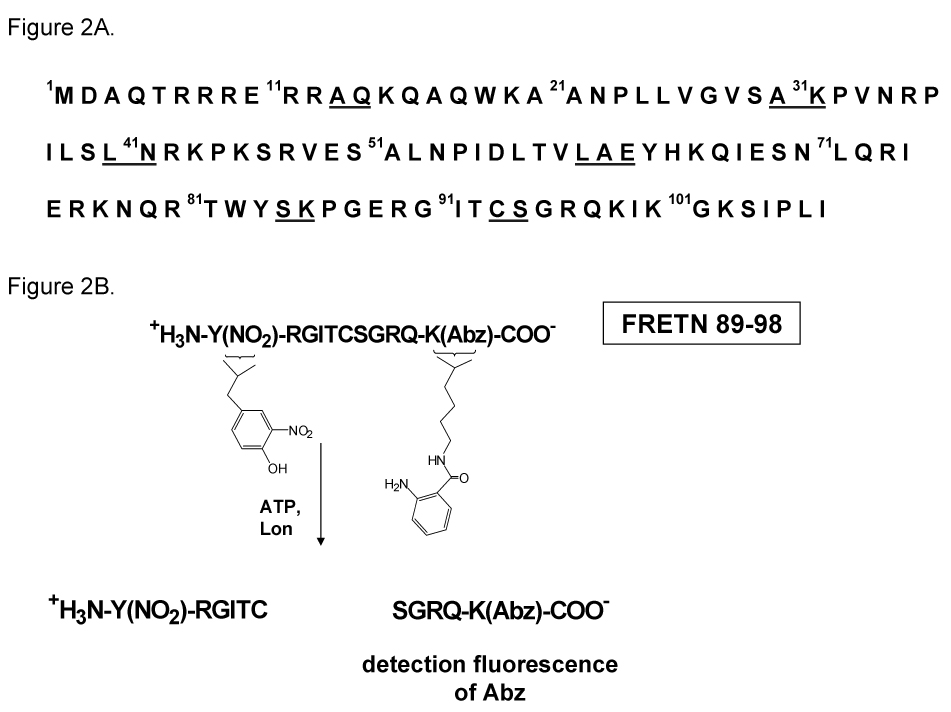
The degradation profile of λN and the sequence of the peptide substrate derived from the Lon cleavage profile of λN. Figure 2A shows the primary sequence of the λN protein, with the Lon cleavage sites underlined. Figure 2B shows the sequence of the fluorogenic peptide used for monitoring the ATP-dependent peptidase activity of Lon. This peptide contains residues 89–98 of the λN protein, the fluorophore Abz and the fluorescence quencher nitrotyrosine. An increase in the Abz fluorescence is detected upon peptide cleavage by Lon in the presence of ATP.
The first turnover of radiolabeled ATP hydrolysis and FRETN 89–98 cleavage are monitored by the rapid acid quench and the fluorescent stopped flow techniques, respectively (88). The EcLon enzyme complex exhibits two different affinities for ATP (Kd < 1 µM and 10 µM, respectively, (89, 90)). The lower affinity sites catalyze ATP hydrolysis prior to peptide bond cleavage, and hydrolyze nucleotide 1000-fold faster than the high-affinity sites. Although the high-affinity sites hydrolyze ATP at a much reduced rate than the low-affinity sites, occupancy of ATP at these sites support multiple rounds of peptide cleavage, thereby confirming that ATP and peptide hydrolysis are not stoichiometrically linked. ATP binding and hydrolysis at both the high- and low-affinity sites are necessary for optimal cleavage (90).
ADP is bound to Lon with a higher affinity than ATP; however, its rate constant of release from Lon is higher than the kcat of ATP hydrolysis in the presence of the FRETN 89–98 peptide or protein substrates (90, 91). Furthermore, ADP but not inorganic phosphate (Pi) has been shown to remain bound to Lon after sizing column chromatography (2, 89, 92). In Additional ADP is a significantly better product inhibitor of Lon as compared to Pi. The Ki of ADP is 0.3 µM against ATP, where no changes in enzyme activity are observed up to 30 mM Pi. Collectively, these results suggest that Pi release occurs prior to ADP release; however, the rate-limiting step in the ATPase turnover reaction has yet to be determined.
To determine the minimum number of steps involved in the ATP-dependent peptidase reaction as shown in Figure 3, we have used the fluorescently labeled nucleotides Mant-ATP or Mant-AMPPNP as probes, which generate changes in fluorescence intensity upon binding to Lon in the presence of peptide substrate. A dansylated peptide has also been used to monitor its binding interaction to Lon in the presence of ATP. Upon binding to Lon, the fluorescence intensity of the dansylated peptide is enhanced by the exciting the intrinsic Trp residues in the Lon:ATP complex. Using these probes a two-step mechanism for nucleotide and peptide binding have been demonstrated (93, 94). In Figure 3, free EcLon is represented by enzyme form I. For simplicity, the enzyme is shown as a dimmer with the ATPase domain in green, the SSD domain in blue and the proteolytic domain in pink. In steps 1 and 2, ATP and peptide bind to Lon independently of one another to yield the ternary complex represented by enzyme form II, which undergoes a conformational change (step 3) to yield enzyme form III. The timing of ATP and peptide hydrolysis is deduced by transient kinetic experiments. The first turnover of ATP hydrolysis displays burst kinetics with a rate constant of 10 s−1 while the hydrolysis of FRETN 89–98 under identical conditions displays lag kinetics with a rate constant of ~ 1 s−1 (88). Based on these findings, it is concluded that ATP hydrolysis by Lon precedes peptide bond cleavage (step 4, enzyme form IV). The formation of enzyme form IV is further implied by the fluorescence resonance energy transfer (FRET) signal generated from the interaction of the dansylated analog of FRETN 89–98 with the S679W, where the proteolytic site Ser679 was replaced with a Trp (93). The proteolytically inactive mutant displays wild-type ATPase activity and a first order rate constant of ~ 10 s−1 was detected for peptide interacting with S679W. This rate constant agrees with the burst rate constant detected for the first turnover of ATP hydrolysis (88). Therefore, the formation of enzyme form IV is likely facilitated by the coupled hydrolysis of ATP. Step 5, which generates enzyme form V, is detected by monitoring the FRET signal between the dansylated peptide and S679W Lon mutant (93). As the rate constant for the formation of enzyme form V approximates the pre-steady-state lag rate constant for FRETN 89–98 cleavage by wild-type Lon (88), it is proposed that step 5 contributes to the rate-limiting step in the first turnover of Lon-mediated peptide bond cleavage in the presence of ATP. Through steady-state product inhibition analyses, Lon has been shown to undergo an “isomerization step” (step 6) along its catalytic pathway to yield an enzyme form “F” (91). Hydrolysis of the FRETN 89–98 peptide yields two hydrolyzed peptide products. The hydrolyzed peptide product (PPI) that contains residues 94 to 97 of the λN protein (PPI) is a non-competitive inhibitor against the FRETN 89–98 substrate. It is proposed that PPI binds to an enzyme form in Lon (F) that does not bind the FRETN 89–98 substrate. This result could be explained by a mechanism by which Lon isomerizes following peptide hydrolysis, and the post-catalytic enzyme form does not bind FRETN 89–98. This kind of mechanism is generally known as an “iso” mechanism (95). The existence of an isomerized form of Lon (F) is further confirmed by a plateau or upturn in the double reciprocal plots of PPI inhibition versus ATP in the presence of the FRETN 89–98 substrate (91). At high nucleotide and high PPI concentrations, enzyme turnover is inhibited. This inhibition cannot be overcome by increasing the peptide concentration. Due to this observation, it is concluded that there is a post-catalytic form of Lon, designated as “F” in Figure 3, which specifically binds the hydrolyzed peptide product PPI but not the substrate; F is stabilized by the presence of ATP. Step 7 constitutes the dissociation of hydrolyzed products. At this point, it is not clear how the enzyme form “F” differs from the pre-catalytic enzyme form 1. It is conceivable that the enzyme form “F’ proposed in Figure 3 is a reflection of an enzyme form responsible for the processive protease activity of Lon. This speculation will require additional mechanistic evaluation.
Figure 3.
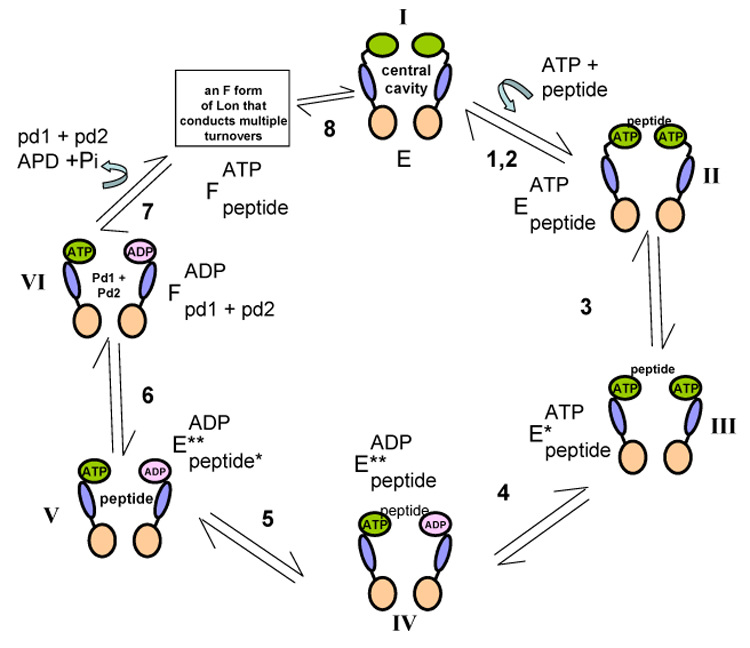
Kinetic model to account for the ATP-dependent cleavage of FRETN 89–98 by E. coli Lon.
The minimal kinetic scheme illustrated in Figure 3 can be used as a model to probe for additional steps in Lon-mediated substrate unfolding and cleavage. The detection of a slow step (step 5) prior to FRETN 89–98 cleavage is consistent with a “substrate translocation” step also observed with other ATP-dependent proteases (77–80, 96, 97). In the degradation of the full-length λN protein which contains multiple Lon cleavage sites, one wonders the same catalytic cycle depicted in Figure 3 is repeated used to catalyze the cleavage of each peptide bond in λN. That is, the hydrolysis of each peptide bond is flanked by a translocation step. Alternatively, the whole λN protein maybe translocated and sequestered within the proteolytic site before the peptide bond cleavage initiates. In the latter model, translocation occurs once whereas peptide bond cleavage events occurs multiple times via a similar mechanism proposed for the Clp proteases (80, 98).
Inhibitors of Lon Proteases
As a serine protease, Lon is inhibited non-specifically by serine protease inhibitors such as phenylmethyl sulfonyl fluoride (PMSF). Generally, millimolar concentrations are required to inhibit Lon activity by 50% (2). Recently, a collection of proteasome inhibitors has been screened as inhibitors of Lon using the ATP-dependent FRETN 89–98 peptidase assay as a detection method (99). Of all the inhibitors examined, the peptide boronate MG262 (Figure 4), exhibited the highest potency against Salmonella Lon (Ki = 6.6 nM). Derivatization of the carboxyl terminal of a hydrolyzed product of FRETN 89–98 by Lon with a boronate moiety also yields an inhibitor exhibiting a Ki of 17 nM (Dansyl 89–98 Abu boronate, Figure 4). Interestingly, the inhibition of both peptide boronates towards Lon requires the presence of ATP, and the proteolytic Ser679 is targeted by the boronate adduct (100). Presumably, the binding and hydrolysis of ATP is needed to allosterically activate the proteolytic site of Lon (enzyme form IV in Figure 3).In addition to peptide based inhibitors, a recent study using the ATP-dependent degradation of FITC-labeled casein as a screening assay reveals that certain coumarinic derivatives exhibit preferential inhibition towards human Lon compared to the 20S proteasome (101). Based on the structures of these compounds and the general mode of coumarin inhibition against serine proteases, it is proposed that the coumarin moiety reacts with the proteolytic Ser679 as well as a nearby nucleophile. Unlike the peptide boronates however, the inhibition of Lon by these compounds does not require the presence of ATP. Since these coumarin derivatives have a low molecular weight, and can be readily modified by chemical synthesis, these lead compounds should provide a starting point for structure activity relation (SAR) studies, which may lead to the development of specific cell-permeable Lon inhibitors.
Figure 4.
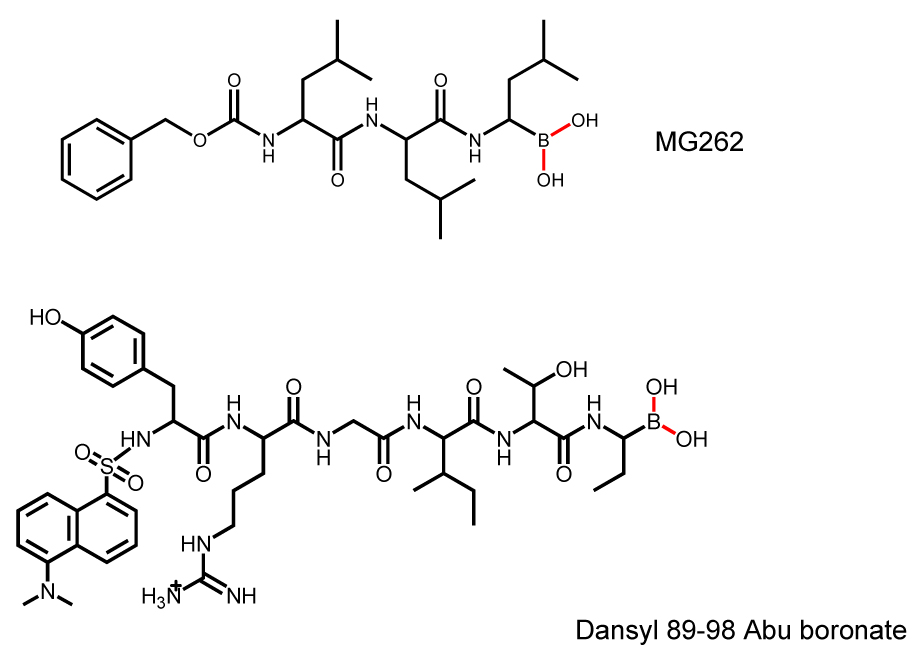
Structures of peptide boronate inhibitors against Lon proteases.
As bacterial Lon has been shown to be important for the infectivity and/or pathogenicity of certain species such as B. abortus and S. typhimurium, compounds that specifically block bacterial but not human Lon, hold promise for a new class of anti-microbial agents (8, 31, 33, 34, 99, 102). In addition, mitochondrial Lon has been implicated in the response to oxidative stress and damage to proteins and mtDNA (7, 46, 103), which has direct relevance to aging. The identification of specific inhibitors to mitochondrial Lon will provide valuable chemical genetic tools not only for cataloging the endogenous substrates of Lon, but also for defining the physiological functions of this protease in mitochondria of cells and organisms in health and disease.
Acknowledgements
The work described in the authors’ lab was supported by the National Institutes of Health (GM61095), the Basil O’Connor Scholars Award- March of Dimes and the American Heart Association to C. K. Suzuki; the National Institutes of Health (GM067172) to I. Lee
Abbreviations
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- AMPPNP
adenylyl 5-imidodiphosphate
- Abz
anthranilamide
- Bz
benzoic acid
- MANT
2’-(or 3’) O-(N-methylanthraniloyl)
- Dansyl
5-dimethylamino-1-naphthalenesulfonyl
- FITC
fluorescein isothiocyanate
- Abu
amninobutyric acid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Amerik A, Antonov VK, Gorbalenya AE, Kotova SA, Rotanova TV, Shimbarevich EV. Site-directed mutagenesis of La protease. A catalytically active serine residue. FEBS Lett. 1991;287:211–214. doi: 10.1016/0014-5793(91)80053-6. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL, Moerschell RP, Chung CH, Maurizi MR. ATP-dependent protease La (lon) from Escherichia coli. Methods Enzymol. 1994;244:350–375. doi: 10.1016/0076-6879(94)44027-1. [DOI] [PubMed] [Google Scholar]
- 3.Howard-Flanders P, Simson E, Theriot L. A Locus That Controls Filament Formation And Sensitivity To Radiation In Escherichia Coli K-12. Genetics. 1964;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman S. Proteolysis in bacterial regulatory circuits. Annu Rev Cell Dev Biol. 2003;19:565–587. doi: 10.1146/annurev.cellbio.19.110701.153228. [DOI] [PubMed] [Google Scholar]
- 5.Maurizi MR. Proteases and protein degradation in Escherichia coli. Experientia. 1992;48:178–201. doi: 10.1007/BF01923511. [DOI] [PubMed] [Google Scholar]
- 6.Maupin-Furlow JA, Gil MA, Humbard MA, Kirkland PA, Li W, Reuter CJ, Wright AJ. Archaeal proteasomes and other regulatory proteases. Curr Opin Microbiol. 2005;8:720–728. doi: 10.1016/j.mib.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Aksam EB, Koek A, Kiel JA, Jourdan S, Veenhuis M, van der Klei IJ. A peroxisomal lon protease and peroxisome degradation by autophagy play key roles in vitality of Hansenula polymorpha cells. Autophagy. 2007;3:96–105. doi: 10.4161/auto.3534. [DOI] [PubMed] [Google Scholar]
- 8.Matsui H, Suzuki M, Isshiki Y, Kodama C, Eguchi M, Kikuchi Y, Motokawa K, Takaya A, Tomoyasu T, Yamamoto T. Oral immunization with ATP-dependent protease-deficient mutants protects mice against subsequent oral challenge with virulent Salmonella enterica serovar typhimurium. Infect Immun. 2003;71:30–39. doi: 10.1128/IAI.71.1.30-39.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotanova TV, Botos I, Melnikov EE, Rasulova F, Gustchina A, Maurizi MR, Wlodawer A. Slicing a protease: structural features of the ATP-dependent Lon proteases gleaned from investigations of isolated domains. Protein Sci. 2006;15:1815–1828. doi: 10.1110/ps.052069306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AY, Hsu CH, Wu SH. Functional domains of Brevibacillus thermoruber lon protease for oligomerization and DNA binding: role of N-terminal and sensor and substrate discrimination domains. J Biol Chem. 2004;279:34903–34912. doi: 10.1074/jbc.M403562200. [DOI] [PubMed] [Google Scholar]
- 11.Heilek GM, Marusak R, Meares CF, Noller HF. Directed hydroxyl radical probing of 16S rRNA using Fe(II) tethered to ribosomal protein S4. Proc Natl Acad Sci U S A. 1995;92:1113–1116. doi: 10.1073/pnas.92.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure--diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 14.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 15.He GP, Muise A, Li AW, Ro HS. A eukaryotic transcriptional repressor with carboxypeptidase activity. Nature. 1995;378:92–96. doi: 10.1038/378092a0. [DOI] [PubMed] [Google Scholar]
- 16.Rudyak SG, Brenowitz M, Shrader TE. Mg2+-linked oligomerization modulates the catalytic activity of the Lon (La) protease from Mycobacterium smegmatis. Biochemistry. 2001;40:9317–9323. doi: 10.1021/bi0102508. [DOI] [PubMed] [Google Scholar]
- 17.Park SC, Jia B, Yang JK, Van DL, Shao YG, Han SW, Jeon YJ, Chung CH, Cheong GW. Oligomeric structure of the ATP-dependent protease La (Lon) of Escherichia coli. Mol Cells. 2006;21:129–134. [PubMed] [Google Scholar]
- 18.Stahlberg H, Kutejova E, Suda K, Wolpensinger B, Lustig A, Schatz G, Engel A, Suzuki CK. Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proc Natl Acad Sci U S A. 1999;96:6787–6790. doi: 10.1073/pnas.96.12.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botos I, Melnikov EE, Cherry S, Khalatova AG, Rasulova FS, Tropea JE, Maurizi MR, Rotanova TV, Gustchina A, Wlodawer A. Crystal structure of the AAA+ alpha domain of E. coli Lon protease at 1.9A resolution. J Struct Biol. 2004;146:113–122. doi: 10.1016/j.jsb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Botos I, Melnikov EE, Cherry S, Tropea JE, Khalatova AG, Rasulova F, Dauter Z, Maurizi MR, Rotanova TV, Wlodawer A, Gustchina A. The catalytic domain of Escherichia coli Lon protease has a unique fold and a Ser-Lys dyad in the active site. J Biol Chem. 2004;279:8140–8148. doi: 10.1074/jbc.M312243200. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Rasulova F, Melnikov EE, Rotanova TV, Gustchina A, Maurizi MR, Wlodawer A. Crystal structure of the N-terminal domain of E. coli Lon protease. Protein Sci. 2005;14:2895–2900. doi: 10.1110/ps.051736805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotanova TV, Melnikov EE, Khalatova AG, Makhovskaya OV, Botos I, Wlodawer A, Gustchina A. Classification of ATP-dependent proteases Lon and comparison of the active sites of their proteolytic domains. Eur J Biochem. 2004;271:4865–4871. doi: 10.1111/j.1432-1033.2004.04452.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith CK, Wohnert J, Sauer RT, Schwalbe H. Assignments of the 1H,13C, and 15N resonances of the substrate-binding SSD domain from Lon protease. J Biomol NMR. 2001;21:387–388. doi: 10.1023/a:1013386625751. [DOI] [PubMed] [Google Scholar]
- 24.Botos I, Melnikov EE, Cherry S, Kozlov S, Makhovskaya OV, Tropea JE, Gustchina A, Rotanova TV, Wlodawer A. Atomic-resolution crystal structure of the proteolytic domain of Archaeoglobus fulgidus lon reveals the conformational variability in the active sites of lon proteases. J Mol Biol. 2005;351:144–157. doi: 10.1016/j.jmb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Besche H, Tamura N, Tamura T, Zwickl P. Mutational analysis of conserved AAA+ residues in the archaeal Lon protease from Thermoplasma acidophilum. FEBS Lett. 2004;574:161–166. doi: 10.1016/j.febslet.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Fischer H, Glockshuber R. ATP hydrolysis is not stoichiometrically linked with proteolysis in the ATP-dependent protease La from Escherichia coli. J Biol Chem. 1993;268:22502–22507. [PubMed] [Google Scholar]
- 27.Starkova NN, Koroleva EP, Rumsh LD, Ginodman LM, Rotanova TV. Mutations in the proteolytic domain of Escherichia coli protease Lon impair the ATPase activity of the enzyme. FEBS Lett. 1998;422:218–220. doi: 10.1016/s0014-5793(98)00012-x. [DOI] [PubMed] [Google Scholar]
- 28.Fischer H, Glockshuber R. A point mutation within the ATP-binding site inactivates both catalytic functions of the ATP-dependent protease La (Lon) from Escherichia coli. FEBS Lett. 1994;356:101–103. doi: 10.1016/0014-5793(94)01244-x. [DOI] [PubMed] [Google Scholar]
- 29.Oh JY, Eun YM, Yoo SJ, Seol JH, Seong IS, Lee CS, Chung CH. LonR9 carrying a single Glu614 to Lys mutation inhibits the ATP- dependent protease La (Lon) by forming mixed oligomeric complexes. Biochem Biophys Res Commun. 1998;250:32–35. doi: 10.1006/bbrc.1998.9252. [DOI] [PubMed] [Google Scholar]
- 30.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 31.Tsilibaris V, Maenhaut-Michel G, Van Melderen L. Biological roles of the Lon ATP-dependent protease. Res Microbiol. 2006;157:701–713. doi: 10.1016/j.resmic.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Wright R, Stephens C, Zweiger G, Shapiro L, Alley MR. Caulobacter Lon protease has a critical role in cell-cycle control of DNA methylation. Genes Dev. 1996;10:1532–1542. doi: 10.1101/gad.10.12.1532. [DOI] [PubMed] [Google Scholar]
- 33.Robertson GT, Kovach ME, Allen CA, Ficht TA, Roop RM., 2nd The Brucella abortus Lon functions as a generalized stress response protease and is required for wild-type virulence in BALB/c mice. Mol Microbiol. 2000;35:577–588. doi: 10.1046/j.1365-2958.2000.01726.x. [DOI] [PubMed] [Google Scholar]
- 34.Takaya A, Tomoyasu T, Tokumitsu A, Morioka M, Yamamoto T. The ATP-dependent lon protease of Salmonella enterica serovar Typhimurium regulates invasion and expression of genes carried on Salmonella pathogenicity island 1. J Bacteriol. 2002;184:224–232. doi: 10.1128/JB.184.1.224-232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brazas MD, Breidenstein EB, Overhage J, Hancock RE. Role of Lon, an ATP-Dependent Protease Homolog, in Resistance of Pseudomonas aeruginosa to Ciprofloxacin. Antimicrob Agents Chemother. 2007;51:4276–4283. doi: 10.1128/AAC.00830-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki CK, Suda K, Wang N, Schatz G. Requirement for the yeast gene LON in intramitochondrial proteolysis and maintenance of respiration. Science. 1994;264:891. doi: 10.1126/science.8178144. [DOI] [PubMed] [Google Scholar]
- 37.Van Dyck L, Pearce DA, Sherman F. PIM1 encodes a mitochondrial ATP-dependent protease that is required for mitochondrial function in the yeast Saccharomyces cerevisiae. J Biol Chem. 1994;269:238–242. [PubMed] [Google Scholar]
- 38.Major T, von Janowsky B, Ruppert T, Mogk A, Voos W. Proteomic analysis of mitochondrial protein turnover: identification of novel substrate proteins of the matrix protease pim1. Mol Cell Biol. 2006;26:762–776. doi: 10.1128/MCB.26.3.762-776.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rep M, Grivell LA. The role of protein degradation in mitochondrial function and biogenesis. Curr. Genet. 1996;30:367–380. doi: 10.1007/s002940050145. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki CK, Rep M, van Dijl JM, Suda K, Grivell LA, Schatz G. ATP-dependent proteases that also chaperone protein biogenesis. Trends Biochem Sci. 1997;22:118–123. doi: 10.1016/s0968-0004(97)01020-7. [DOI] [PubMed] [Google Scholar]
- 41.van Dyck L, Langer T. ATP-dependent proteases controlling mitochondrial function in the yeast Saccharomyces cerevisiae. Cell Mol. Life Sci. 1999;56:825–842. doi: 10.1007/s000180050029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic Biol Med. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Ngo JK, Davies KJ. Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Ann N Y Acad Sci. 2007;1119:78–87. doi: 10.1196/annals.1404.015. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 45.Hori O, Icinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, ROn D, Tohyama M, Stern DM, Ogawa S. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J. Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 47.Granot Z, Kobiler O, Melamed-Book N, Eimerl S, Bahat A, Lu B, Braun S, Maurizi MR, Suzuki CK, Oppenheim AB, Orly J. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol Endocrinol. 2007;21:2164–2177. doi: 10.1210/me.2005-0458. [DOI] [PubMed] [Google Scholar]
- 48.Ondrovicova G, Liu T, Singh K, Tian B, Li H, Gakh O, Perecko D, Janata J, Granot Z, Orly J, Kutejova E, Suzuki CK. Cleavage site selection within a folded substrate by the ATP-dependent lon protease. J Biol Chem. 2005;280:25103–25110. doi: 10.1074/jbc.M502796200. [DOI] [PubMed] [Google Scholar]
- 49.Rep M, van Dijl JM, Suda K, Schatz G, Grivell LA, Suzuki CK. Promotion of mitochondrial membrane complex assembly by a proteolytically inactive yeast Lon. Science. 1996;274:103–106. doi: 10.1126/science.274.5284.103. [DOI] [PubMed] [Google Scholar]
- 50.Zehnbauer BA, Foley EC, Henderson GW, Markovitz A. Identification and purification of the Lon+ (capR+) gene product, a DNA-binding protein. Proc Natl Acad Sci U S A. 1981;78:2043–2047. doi: 10.1073/pnas.78.4.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung CH, Goldberg AL. DNA stimulates ATP-dependent proteolysis and protein-dependent ATPase activity of protease La from Escherichia coli. Proc Natl Acad Sci U S A. 1982;79:795–799. doi: 10.1073/pnas.79.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu GK, Smith MJ, Markovitz DM. Bacterial protease Lon is a site-specific DNA-binding protein. J Biol Chem. 1997;272:534–538. [PubMed] [Google Scholar]
- 53.Charette MF, Henderson GW, Doane LL, Markovitz A. DNA-stimulated ATPase activity on the lon (CapR) protein. J Bacteriol. 1984;158:195–201. doi: 10.1128/jb.158.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nomura K, Kato J, Takiguchi N, Ohtake H, Kuroda A. Effects of inorganic polyphosphate on the proteolytic and DNA-binding activities of Lon in Escherichia coli. J Biol Chem. 2004;279:34406–34410. doi: 10.1074/jbc.M404725200. [DOI] [PubMed] [Google Scholar]
- 55.Lee AY, Hsu CH, Wu SH. Functional domains of Brevibacillus thermoruber Lon protease for oligomerization and DNA-binding role of amino-terminal and SSD domains. J. Biol. Chem. 2004;279:34903–34912. doi: 10.1074/jbc.M403562200. [DOI] [PubMed] [Google Scholar]
- 56.Sonezaki S, Okita K, Oba T, Ishii Y, Kondo A, Kato Y. Protein substrates and heat shock reduce the DNA-binding ability of Escherichia coli Lon protease. Appl. Microbiol. Biotechnol. 1995;44:484–488. doi: 10.1007/BF00169948. [DOI] [PubMed] [Google Scholar]
- 57.Fu GK, Markovitz DM. The human LON protease binds to mitochondrial promoters in a single-stranded, site-specific, strand-specific manner. Biochemistry. 1998;37:1905–1909. doi: 10.1021/bi970928c. [DOI] [PubMed] [Google Scholar]
- 58.Liu T, Lu B, Lee I, Ondrovicova G, Kutejova E, Suzuki CK. DNA and RNA binding by the mitochondrial lon protease is regulated by nucleotide and protein substrate. J Biol Chem. 2004;279:13902–13910. doi: 10.1074/jbc.M309642200. [DOI] [PubMed] [Google Scholar]
- 59.Lu B, Liu T, Crosby JA, Thomas-Wohlever J, Lee I, Suzuki CK. The ATP-dependent Lon protease of Mus musculus is a DNA-binding protein that is functionally conserved between yeast and mammals. Gene. 2003;306:45–55. doi: 10.1016/s0378-1119(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 60.Lu B, Yadav S, Shah PG, Liu T, Tian B, Pukszta S, Villaluna N, Kutejová E, Newlon CS, Santos JH, Suzuki CK. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J. Biol. Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- 61.Han SH, Suzuki CK, Wu SH. Thermodynamic characterization of specific interactions between human Lon protease and G-quartet DNA. Nucleic Acids Res. 2007 doi: 10.1093/nar/gkm1140. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuroda A. A polyphosphate-lon protease complex in the adaptation of Escherichia coli to amino acid starvation. Biosci Biotechnol Biochem. 2006;70:325–331. doi: 10.1271/bbb.70.325. [DOI] [PubMed] [Google Scholar]
- 63.Kuroda A, Nomura K, Ohtomo R, Kato J, Ikeda T, Takiguchi N, Ohtake H, Kornberg A. Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science. 2001;293:705–708. doi: 10.1126/science.1061315. [DOI] [PubMed] [Google Scholar]
- 64.Van Melderen L, Thi MHD, Lecchi P, Gottesman S, Couturier M, Maurizi MR. ATP-dependent degradation of CcdA by Lon protease. Effects of secondary structure and heterologous subunit interactions. J Biol Chem. 1996;271:27730–27738. doi: 10.1074/jbc.271.44.27730. [DOI] [PubMed] [Google Scholar]
- 65.Laachouch JE, Desmet L, Geuskens V, Grimaud R, Toussaint A. Bacteriophage Mu repressor as a target for the Escherichia coli ATP- dependent Clp Protease. Embo J. 1996;15:437–444. [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez M, Frank EG, Levine AS, Woodgate R. Lon-mediated proteolysis of the Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maurizi MR. Degradation in vitro of bacteriophage lambda N protein by Lon protease from Escherichia coli. J Biol Chem. 1987;262:2696–2703. [PubMed] [Google Scholar]
- 68.Waxman L, Goldberg AL. Protease La from Escherichia coli hydrolyzes ATP and proteins in a linked fashion. Proc Natl Acad Sci U S A. 1982;79:4883–4887. doi: 10.1073/pnas.79.16.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 70.Nishii W, Maruyama T, Matsuoka R, Muramatsu T, Takahashi K. The unique sites in SulA protein preferentially cleaved by ATP-dependent Lon protease from Escherichia coli. Eur J Biochem. 2002;269:451–457. doi: 10.1046/j.0014-2956.2001.02668.x. [DOI] [PubMed] [Google Scholar]
- 71.Dervyn E, Canceill D, Huisman O. Saturation and specificity of the Lon protease of Escherichia coli. J Bacteriol. 1990;172:7098–7103. doi: 10.1128/jb.172.12.7098-7103.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishii W, Suzuki T, Nakada M, Kim YT, Muramatsu T, Takahashi K. Cleavage mechanism of ATP-dependent Lon protease toward ribosomal S2 protein. FEBS Lett. 2005;579:6846–6850. doi: 10.1016/j.febslet.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 73.Van Melderen L, Gottesman S. Substrate sequestration by a proteolytically inactive Lon mutant. Proc Natl Acad Sci U S A. 1999;96:6064–6071. doi: 10.1073/pnas.96.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menon AS, Goldberg AL. Protein substrates activate the ATP-dependent protease La by promoting nucleotide binding and release of bound ADP. J. Biol. Chem. 1987;262:14929–14934. [PubMed] [Google Scholar]
- 75.Ishii Y, Amano F. Regulation of SulA cleavage by Lon protease by the C-terminal amino acid of SulA, histidine. Biochem J. 2001;358:473–480. doi: 10.1042/0264-6021:3580473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 77.Reid BG, Fenton WA, Horwich AL, Weber-Ban EU. ClpA mediates directional translocation of substrate proteins into the ClpP protease. Proc Natl Acad Sci U S A. 2001;98:3768–3772. doi: 10.1073/pnas.071043698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Song JJ, Franklin MC, `, Kamtekar S, Im YJ, Rho SH, Seong IS, Lee CS, Chung CH, Eom SH. Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure. 2001;9:177–184. doi: 10.1016/s0969-2126(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 79.Ishikawa T, Beuron F, Kessel M, Wickner S, Maurizi MR, Steven AC. Translocation pathway of protein substrates in ClpAP protease. Proc Natl Acad Sci U S A. 2001;98:4328–4333. doi: 10.1073/pnas.081543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee C, Schwartz MP, Prakash S, Iwakura M, Matouschek A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol Cell. 2001;7:627–637. doi: 10.1016/s1097-2765(01)00209-x. [DOI] [PubMed] [Google Scholar]
- 81.Menon AS, Goldberg AL. Protein substrates activate the ATP-dependent protease La by promoting nucleotide binding and release of bound ADP. J Biol Chem. 1987;262:14929–14934. [PubMed] [Google Scholar]
- 82.Hilliard JJ, Maurizi MR, Simon LD. Isolation and characterization of the phage T4 PinA protein, an inhibitor of the ATP-dependent lon protease of Escherichia coli. J Biol Chem. 1998;273:518–523. doi: 10.1074/jbc.273.1.518. [DOI] [PubMed] [Google Scholar]
- 83.Rudyak SG, Shrader TE. Polypeptide stimulators of the Ms-Lon protease. Protein Sci. 2000;9:1810–1817. doi: 10.1110/ps.9.9.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Downs D, Waxman L, Goldberg AL, Roth J. Isolation and characterization of lon mutants in Salmonella typhimurium. J Bacteriol. 1986;165:193–197. doi: 10.1128/jb.165.1.193-197.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Dijl JM, Kutejova E, Suda K, Perecko D, Schatz G, Suzuki CK. The ATPase and protease domains of yeast mitochondrial Lon: roles in proteolysis and respiration-dependent growth. Proc Natl Acad Sci U S A. 1998;95:10584–10589. doi: 10.1073/pnas.95.18.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishii Y, Sonezaki S, Iwasaki Y, Miyata Y, Akita K, Kato Y, Amano F. Regulatory role of C-terminal residues of SulA in its degradation by Lon protease in Escherichia coli. J Biochem (Tokyo) 2000;127:837–844. doi: 10.1093/oxfordjournals.jbchem.a022677. [DOI] [PubMed] [Google Scholar]
- 87.Lee I, Berdis AJ. Adenosine triphosphate-dependent degradation of a fluorescent lambda N substrate mimic by Lon protease. Anal Biochem. 2001;291:74–83. doi: 10.1006/abio.2001.4988. [DOI] [PubMed] [Google Scholar]
- 88.Vineyard D, Patterson-Ward J, Berdis AJ, Lee I. Monitoring the Timing of ATP Hydrolysis with Activation of Peptide Cleavage in Escherichia coli Lon by Transient Kinetics. Biochemistry. 2005;44:1671–1682. doi: 10.1021/bi048618z. [DOI] [PubMed] [Google Scholar]
- 89.Menon AS, Goldberg AL. Binding of nucleotides to the ATP-dependent protease La from Escherichia coli. J Biol Chem. 1987;262:14921–14928. [PubMed] [Google Scholar]
- 90.Vineyard D, Patterson-Ward J, Lee I. Single-Turnover Kinetic Experiments Confirm the Existence of High- and Low-Affinity ATPase Sites in Escherichia coli Lon Protease. Biochemistry. 2006;45:4602–4610. doi: 10.1021/bi052377t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas-Wohlever J, Lee I. Kinetic characterization of the peptidase activity of Escherichia coli Lon reveals the mechanistic similarities in ATP-dependent hydrolysis of peptide and protein substrates. Biochemistry. 2002;41:9418–9425. doi: 10.1021/bi0255470. [DOI] [PubMed] [Google Scholar]
- 92.Goff SA, Goldberg AL. Production of abnormal proteins in E. coli stimulates transcription of lon and other heat shock genes. Cell. 1985;41:587–595. doi: 10.1016/s0092-8674(85)80031-3. [DOI] [PubMed] [Google Scholar]
- 93.Patterson-Ward J, Huang J, Lee I. Detection and Characterization of Two ATP-Dependent Conformational Changes in Proteolytically Inactive Escherichia coli Lon Mutants by Stopped Flow Kinetic Techniques. Biochemistry. 2007;46:13593–13605. doi: 10.1021/bi701649b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vineyard D, Zhang X, Lee I. Transient kinetic experiments demonstrate the existence of a unique catalytic enzyme form in the peptide-stimulated ATPase mechanism of Escherichia coli Lon protease. Biochemistry. 2006;45:11432–11443. doi: 10.1021/bi060809+. [DOI] [PubMed] [Google Scholar]
- 95.Rebholz KL, Northrop DB. Kinetics of iso mechanisms. Methods Enzymol. 1995;249:211–240. doi: 10.1016/0076-6879(95)49037-x. [DOI] [PubMed] [Google Scholar]
- 96.Park E, Rho YM, Koh OJ, Ahn SW, Seong IS, Song JJ, Bang O, Seol JH, Wang J, Eom SH, Chung CH. Role of the GYVG pore motif of HslU ATPase in protein unfolding and translocation for degradation by HslV peptidase. J Biol Chem. 2005;280:22892–22898. doi: 10.1074/jbc.M500035200. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Song JJ, Seong IS, Franklin MC, Kamtekar S, Eom SH, Chung CH. Nucleotide-dependent conformational changes in a protease-associated ATPase HsIU. Structure (Camb) 2001;9:1107–1116. doi: 10.1016/s0969-2126(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 98.Choi KH, Licht S. Control of peptide product sizes by the energy-dependent protease ClpAP. Biochemistry. 2005;44:13921–13931. doi: 10.1021/bi0505060. [DOI] [PubMed] [Google Scholar]
- 99.Frase H, Hudak J, Lee I. Identification of the proteasome inhibitor MG262 as a potent ATP-dependent inhibitor of the Salmonella enterica serovar Typhimurium Lon protease. Biochemistry. 2006;45:8264–8274. doi: 10.1021/bi060542e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frase H, Lee I. Peptidyl boronates inhibit Salmonella enterica serovar Typhimurium Lon protease by a competitive ATP-dependent mechanism. Biochemistry. 2007;46:6647–6657. doi: 10.1021/bi7002789. [DOI] [PubMed] [Google Scholar]
- 101.Bayot A, Basse N, Lee I, Gareil M, Pirotte B, Bulteau AL, Friguet B, Reboud-Ravaux M. Towards the control of intracellular protein turnover: Mitochondrial Lon protease inhibitors versus proteasome inhibitors. Biochimie. 2007 doi: 10.1016/j.biochi.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 102.Eguchi M, Sekiya Y, Suzuki M, Yamamoto T, Matsui H. An oral Salmonella vaccine promotes the down-regulation of cell surface Toll-like receptor 4 (TLR4) and TLR2 expression in mice. FEMS Immunol Med Microbiol. 2007;50:300–308. doi: 10.1111/j.1574-695X.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 103.Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]


