Abstract
The review summarizes and integrates findings from 40 years of event-related potential (ERP) studies using pictures that differ in valence (unpleasant-to-pleasant) and arousal (low-to-high) and that are used to elicit emotional processing. Affective stimulus factors primarily modulate ERP component amplitude, with little change in peak latency observed. Arousal effects are consistently obtained, and generally occur at longer latencies. Valence effects are inconsistently reported at several latency ranges, including very early components. Some affective ERP modulations vary with recording methodology, stimulus factors, as well as task-relevance and emotional state. Affective ERPs have been linked theoretically to attention orientation for unpleasant pictures at earlier components (< 300 ms). Enhanced stimulus processing has been associated with memory encoding for arousing pictures of assumed intrinsic motivational relevance, with task-induced differences contributing to emotional reactivity at later components (> 300 ms). Theoretical issues, stimulus factors, task demands, and individual differences are discussed.
Keywords: P300, affect, emotion, arousal, valence, habituation, event-related potentials, ERPs
1.0 Introduction
1.1. Present review
Affective processing in the human brain is receiving increased interest (Calder et al., 2001; LaBar and Cabeza, 2006; LeDoux, 2000). Event-related potentials (ERPs) enable the assessment of neural responses to affective events with millisecond temporal resolution. However, even though ERP affect assessment has a comparatively long history—since the late 1960's—relatively little integration of findings has been made. The present review attempts to summarize affective ERP studies using picture stimuli1. The major goals are to: (1) describe the characteristic ERP effects from affective picture stimulation; (2) evaluate their possible dependency on methodology, stimulus characteristics, and task procedures; and (3) provide a critical discussion of limitations in the empirical literature as a guide for future investigations. The review is organized by describing ERP effects in terms of primary affective stimulus dimensions (valence and arousal), specific components defined by their latency range, and scalp topography. The review begins with a sketch of the historical background, which is followed by a highlighting of contemporary findings through examples from a summary table.
1.2. Cortical processing of affect
The current enthusiasm for assessing affective processing contrasts with the history of experimental psychology in which emotion research has been largely neglected due to its traditional focus on subjective emotional states (LeDoux, 2000). In addition, it has been assumed that cognitive and emotional processes had separate neuroanatomical localizations in the neocortex and the limbic system, respectively (MacLean, 1949, 1952). However, this initial dichotomization has eroded because of accumulating evidence that several neocortical regions are crucial for intact affective functioning (Bechara et al., 2000; Bush et al., 2000; Phillips et al., 1997, 1998).
Processing of affective information can be assayed by analyzing amplitudes (size) and latencies (timing) of ERP components (Rugg and Coles, 1995). Although psychophysiological measures such as heart rate and skin conductance provide useful indices of affective reactions, a major advantage for using ERP measures is that these electrophysiological processes can be defined temporally in contrast to other neuroimaging technologies such as fMRI and PET. Rapid processing of affective stimuli is a critical aspect of emotional responsivity, with the perception of potentially dangerous events facilitated by a fast processing route involving the thalamus and amygdala (LeDoux, 2000; Morris et al., 1999). Indeed, characterizing the temporal order of affective ERPs can contribute to theoretical development through models of the affective time course (Codispoti et al., 2007; Schupp et al., 2006). Thus, scalp-recorded brain potentials offer a powerful means to characterize affective processing in the human brain (Batty and Taylor, 2003; Britton et al., 2006; Maratos and Rugg, 2001; Smith et al., 2004).
2.0 Early ERP Affect Investigations
Classic ERP studies have found that neuroelectric responses are influenced by whether the participant is required to respond to the stimuli or not, thereby emphasizing task-relevance as a major determinant of component measures (Chapman and Bragdon, 1964; Davis, 1964; Davis et al., 1964; Larsson, 1960). Assessing affective picture processing with ERPs occurred in part because of the assumed intrinsic motivational relevance of emotional stimuli. An early ERP finding suggested that unpleasant (repulsive medical images) and pleasant (erotic) pictures, compared to neutral (scenic) pictures, produced a positive-going waveform at about 350-450 ms after stimulus onset (Lifshitz, 1966). However, only qualitative assessments of waveform morphologies were provided, with little emphasis on the affect-related positivity.
Begleiter and colleagues (1967, 1969) elicited ERPs with neutral visual stimuli (line figures) that were affectively conditioned by using words of unpleasant, neutral, and pleasant valence. In three experiments, ERPs were recorded during passive figure viewing, and peak-to-peak amplitudes varied with affective category. If participants were notified of an association between words and figures just before the ERP session, amplitude was largest for stimuli associated with unpleasant words, whereas the opposite pattern (lowest peak-to-peak amplitudes for unpleasantly conditioned stimuli) was obtained from naïve subjects. No affective ERP modulation was found in participants who were fully informed of the conditioning procedure. The combined results were interpreted as highlighting the role of awareness as a modulating factor in affective conditioning.
These initial reports spurred the use of affective picture stimuli to assess emotional reactions with ERPs. Radilova and colleagues reported enhanced P300 amplitudes for strongly unpleasant (dead bodies) and pleasant (erotic) pictures, compared to neutral (natural scenes/landscapes) pictures under passive viewing conditions (Radilova, 1982; Radilova et al., 1983). The findings suggested that intensive emotional pictures elicit an increased P300 “irrespective of the pleasant or unpleasant nature of the emotion induced” (Radilova, 1989, p. 365). Since these early ERP affect studies, the use of pictures as emotional stimuli has increased in part because image presentation on computers and use of normative picture stimuli have been well developed. A major goal has been to characterize ERP component modulations related to affective valence and arousal. These findings are outlined below.
3.0 Empirical and Theoretical Background
3.1. Affective ERP studies
Most affective ERP studies have employed stimuli from the International Affective Picture System (IAPS), which was constructed to provide a standardized stimulus set for attention and emotion research (Lang et al., 1999). These images are rated with respect to their valence category (unpleasant-to-pleasant) and arousal level (low-to-high) on a nine-point scale by both female and male young adults. The availability of this organized stimulus set was a major impetus for affective ERP studies, as the images vary systematically in valence and arousal.
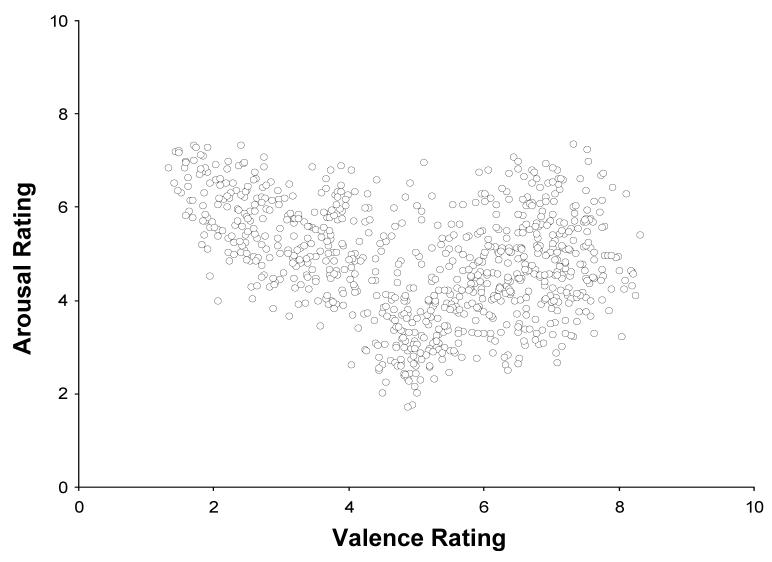
Figure 1 illustrates the distribution of mean ratings across female and male raters for each image by plotting the image valence and arousal ratings in a two-dimensional space. Note that extreme valence levels are typically associated with high arousal for both unpleasant and pleasant pictures. Arousal is somewhat stronger for unpleasant stimuli—a stimulus characteristic that may reflect a motivational function of adaptive utility (Ito et al., 1998). Valence effects are obtained by comparing ERPs elicited with IAPS images rated as unpleasant and pleasant and have comparable arousal levels.
Figure 1.
Mean of female and male ratings of the arousal (low to high) and valence (unpleasant to pleasant) values from a 1-9 scale for each stimulus image from the International Affective Picture System (IAPS).
Table 1 summarizes the major affect ERP studies in chronological order, and lists demographic information, stimulus/study-design characteristics, the primary affect results, and component topographic distribution. This listing conveys the appreciable variability for stimulus and task conditions that have been employed, even for the more recent reports. Despite the paradigm heterogeneity, some consistent valence and arousal manipulations have been demonstrated even though affective amplitude topographies are less consistent. Table 1 also suggests that systematic assessments of stimulus and task factors generally have not been performed within the study sessions, although several contemporary studies have begun to dissect how procedural factors contribute to the obtained ERP modulations. It is also noteworthy that many affective ERP studies have employed high-arousing unpleasant/pleasant stimuli that were compared to neutral pictures of low arousal value. These issues are discussed below.
Table 1.
Description and main results of ERP studies in chronological order using affective picture stimuli.
| Study (year) | Female/male (F/M) number, age (A) range or mean (yrs) |
Stimulus characteristics (C) [duration in ms] and basis for its selection (B) |
Special study features (F) and task (T) during ERP recording |
Effects of stimulus affect (A) and topography interaction (T) at latency [component] |
|---|---|---|---|---|
| Lifshitz (1966) | F/M: 0/10 A: 18-33 |
C: High-arousing neg, low-arousing neu [1000-2000] B: Not reported |
F: Emotion and P300 T: Passive viewing |
A: ∼400 ms: neg/pos > neu; no statistical analysis reported T: Not reported |
| Begleiter et al. (1967) |
F/M: 0/31 A: 19.0 |
C: Line figures conditioned by unpleasant, neutral and pleasant words [1500] B: Not reported |
F: ERPs to “unconsciously conditioned” visual stimuli T: Passive viewing |
A: ∼100-220 ms [peak-to-peak amplitudes]: neu > pos > neg T: Not reported |
| Begleiter et al. (1969, Study 1) |
F/M: 0/31 A: 19.7 |
C: Line figures conditioned by unpleasant, neutral and pleasant words [1600] B: Not reported |
F: ERPs to conditioned visual stimuli in partly informed participants T: Passive viewing |
A [when informed of a non-specific word-figure association]: ∼100-220 ms [peak-to-peak amplitudes]: neg > pos > neu T: Not reported |
| Begleiter et al. (1969, Study 2) |
F/M: 0/16 A: 19.2 |
C: Line figures conditioned by unpleasant, neutral and pleasant words [1600] B: Not reported |
F: ERPs to conditioned visual stimuli in fully informed participants T: Passive viewing |
A [when informed of the specific word-figure association]: no effects T: Not reported |
| Radilova (1982) | F/M: 1/9 A: 20-50 |
C: High-arousing neg, low-arousing neu [300] B: Not reported |
F: Emotion and P300 T: Passive viewing |
A: ∼300 ms [peak-to-peak N2/P3]: neg > neu T: Not reported |
| Radilova et al., (1983) | F/M: 0/12 A: 24.3 |
C: High-arousing pos (erotica), low- arousing neu (landscapes, etc.) [300] B: Not reported |
F: ERPs for sexually arousing stimuli T: Passive viewing |
A: ∼300 ms [P3]: erotic > neutral; no statistical analysis reported T: Not reported |
| Johnston et al. (1986) |
F/M: 10/10 A: 18-35 |
C: Pictures and CVC trigrams [100] B: Previous study |
F:ERPs to affective pictures paired with CVC trigrams T: Count stimuli |
A: 300-600 P3 and slow wave components, PCA, neg > pos T: Parietal maximum; hemispheric differences |
| Johnston & Wang (1991) | F/M: 30/0 A: 20-35 |
C: Neg (dermatological cases), neu (people), pos (babies, male models, female models) [130] B: Used in previous study |
F: Different menstrual phases T: Passive viewing |
A: 410 ms [P3]: female models > babies/male models/dermatology cases > people T: Not reported |
| Mini et al. (1996) | F/M: 8/5 A: 24 |
C: High-arousing neg/pos, low-arousing neu [2000] B: Not reported |
F/T: Memorization of stimuli |
A: 300-400 and 400-500 ms: neg/pos > neu T: No effects |
| Kayser et al. (1997) | F/M: 23/0 A: 22 |
C: High-arousing neg (dermatological cases before treatment), neu (cases after treatment) [250] B: Pilot study |
F: Laterally presented stimuli T: Passive viewing |
A: 225 ms [N225], 285 ms [P285] and 450 ms [Sw]: neg > neu; 380 ms [P380]: neu > neg T: 225 ms: largest valence effect at medial sites; 285 ms: right-parietal sites; 380 ms: fronto-central sites; 450 ms: centro-posterior sites |
| Palomba et al. (1997) | F/M: 17/3 A: 25.3 |
C: Neg (mutilations), neu (objects), pos (puppies, babies) [6000] B: IAPS normative data |
F: Emotional memory T: Memorization of stimuli |
A: 282 ms [N2], 351 ms [P3] and 600-900 ms: neg/pos > neu; 400-600 ms: pos > neu T: Not reported |
| Ito et al. (1998, Study 1) | F/M: 25 A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [1000] B: IAPS normative data validated in pilot study |
F: “Negativity bias” in emotional categorizaton T: Emotional categorization of valenced target stimuli |
A: 400-900 ms [LPP]: neg > pos T: No effects |
| Ito et al. (1998, Study 2) | F/M: 14 A: Undergraduates |
C: High-arousing neg/pos (dead animals/food), low-arousing neu [1000] B: Same as Study 1 |
F: Same as Study 1 T: Emotional categorization |
A: 400-900 ms [LPP]: neg > pos T: No effects |
| Cuthbert et al. (2000) | F/M: 14/23 A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [6000] B: IAPS normative data |
F: Autonomic arousal T: Arousal and valence rating of stimuli |
A: 200-300 ms: pos > neu; 300-400 ms: pos > neg/neu; 400- 700 ms: pos > neg > neu; 700-5000 ms: neg/pos > neu T: Not reported |
| Schupp et al. (2000) | F/M: 23 (not specified) A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [1500] B: IAPS normative data |
F: Emotional stimulus context effects T: Valence categorization of stimuli |
A: 350-750 ms: neg/pos > neu T: 350-750 ms: largest arousal effect at Cz/Pz sites |
| Carretié et al. (2001) |
F/M: 23/6 A: 22.4 |
C: High-arousing neg, low-arousing neu, high- and low-arousing pos [1750] B: Participant ratings |
F: Cues indicating stimulus category T: Matching of picture stimuli to schematic cues |
A: 176-224 ms [P200]: pos > neg/neu; 312-368 ms [P340]: neg > pos/neu T: 176-224 and 312-368 ms: largest valence effect at visual association cortex (temporal lobe; LORETA algorithm) |
| Junghöfer et al. (2001) | F/M: 17 (not specified) A: 12 students, 5 researchers |
C: High-arousing, low-arousing [200-333] B: IAPS normative data |
F: Rapid stimulus presentation (3-5 Hz) T: Passive viewing |
A: 168-232 ms [P200]: high arousal > low arousal (at 3 Hz); 232-296 ms [N260]: high arousal > low arousal (at 3 Hz) T: 168-232 and 232-296 ms: largest arousal effect at right- hemisphere (occipital and parietal) sites |
| Keil et al. (2001) | F/M: 7/3 A: 26.1 |
C: Arousal: neg > pos > neu [1000] B: IAPS normative data |
F: Laterally presented pictures T: Passive viewing |
A: 160 ms [N1]: neg/pos > neu; 400-600 ms [Sw]: neg/pos > neu T: 160 ms: no effect; 400-600 ms: largest arousal effect at parietal sites |
| Dolcos & Cabeza (2002) | F/M: 15/0 A: Students |
C: High-arousing neg/pos, low-arousing neu [2000] B: IAPS normative data |
F: Emotional memory T: Emotion rating and memorization of stimuli |
A: 500-800 ms: neg/pos > neu T: 500-800 ms: largest valence effect at right-hemisphiere sites; largest arousal effect at parietal sites |
| Keil et al. (2002) | F/M: 0/11 A: 26 |
C: High-arousing neg/pos, low-arousing neu: neg > pos > neu [6000] B: IAPS normative data |
F: Source analysis T: Passive viewing |
A: 120-150 ms [N1]: pos > neg/neu; 300-340 ms [early P3], 380-440 ms [late P3] and 550-900 ms [SW]: neg/pos > neu T: 380-900 ms: largest valence effect at centro-parietal sites, largest arousal effect at right hemisphere; 550-900 ms: largest valence effect at right-parietal sites |
| Carretié et al. (2003) | F/M: 19/6 A: 22.3 |
C: High-arousing neg/pos, low-arousing neu [200] B: Participant ratings |
F: Habituation to cued affective stimuli T: Valence discrimination |
A: 176 ms [N1]: habituation larger for pos/neu than neg T: 176 ms: habituation/affect interaction largest at frontal sites |
| Schupp et al. (2003a) | F/M: 8/7 A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [333] B: IAPS normative data |
F: Task-irrelevant affective pictures T: Detection of non-picture stimuli |
A: 160-224 ms [N1] and 232-292 ms [N2]: neg/pos > neu; erotica > other pos, mutilations/threat > other neg T: 160-224 ms: largest valence effect at occipito-temporal sites; 232-292 ms: largest valence effect at right temporo- occipital sites |
| Schupp et al. (2003b) | F/M: 12/8 A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [1500] B: IAPS normative data |
F: Multi-channel ERP T: Emotional categorization |
A: 312 ms: neg/pos > neu; 416-456 ms: neg/pos > neu T: 312 ms: neg > neu largest at temporo-occipital sites, pos > neu largest at centro-medial sites |
| Smith et al. (2003) | F/M: 16/16 A: Undergraduates |
C: Neg/pos, matched for arousal level [1000] B: IAPS normative data |
F: Affective target pictures presented on affective background pictures T: Emotional discrimination |
A: 117 ms [P1]: neg > pos T: 117 ms: largest valence effect at occipital sites |
| Amrhein et al. (2004) | F/M: 6/10 A: 29.5 |
C: High-arousing neg > pos, low-arousing neu [8000] B: Participant ratings |
F: Habituation of psychophysiological responses T: Passive viewing |
A: 200-300, 300-400 and 400-700 ms: neg/pos > neu T: No effects |
| Carretié et al. (2004) | F/M: 28/9 A: 21.5 |
C: Neg (snarling wolf), neu (wristwatch), pos (opposite-gender nude) [200] B: Participant ratings |
F: Source analysis T: Count of target stimuli (oddball paradigm) |
A: 105 ms [P1]: neg > pos/neu; 180 ms [P2]: neg/pos > neu; 240 ms [N2]: neg > pos T: 105 ms: largest valence effect in occipital cortex; 180 ms and 240 ms: anterior cingulate (LORETA algorithms) |
| Delplanque et al. (2004) | F/M: 12/0 A: 20.6 |
C: Neg/neu/pos balanced for arousal [1000] B: IAPS normative data |
F: Valence effects T: Detection of affective picture target stimuli |
A: 150-165 ms [late P1]: neg > pos; 180-213 ms [P2]: neg > pos > neu; 406-603 ms [P3b]: neg < pos T: 150-165 and 180-213 ms: largest valence effect at occipito-temporal sites; 406-603 ms: fronto-central site |
| Schupp et al. (2004) | F/M: 14/2 A: 21.2 |
C: High-arousing neg > pos, low-arousing neu [120] B: Participant categorization |
F: Short stimulus presentation T: Emotional categorization of stimuli |
A: 200-350 ms [EPN]: erotic > babies; 400-500 ms [LPP]: erotic > babies/mutilations > threat T: 200-350 ms: largest valence effect at occipital-temporal sites; 400-500 ms: centro-parietal sites |
| Delplanque et al. (2005) | F/M: 17/0 A: 21.7 |
C: High-arousing neg/pos, low-arousing neu [750] B: IAPS normative data |
F: Task-irrelevant affective picture stimuli T: Detection of non- affective target stimuli |
A: 411-599 ms [P3b]: neg/pos > neu T: 411-599 ms: largest valence effect for neg at posterior sites, largest effect for pos at anterior sites |
| Pollatos et al. (2005) | F/M: 28/16 A: 25.5 |
C: High-arousing neg/pos, low-arousing neu [6000] B: IAPS normative data |
F: Interoceptive awareness T: Rating of valence and arousal |
A: 290-500 ms [P3]: pos > neg > neu; 550-900 ms [SW]: pos/neg > neu T: 290-500 ms: largest valence effect for pos > neg/neu at frontal/centro-lateral sites and for arousal neg/pos > neu at posterior sites; 550-900 ms: global effect |
| Carretié et al. (2006) | F/M: 21/ 9 A: 21.7 |
C: High-arousing neg/pos, low-arousing neu [1500] B: IAPS normative data |
F: Source analysis T: Non-emotional categorization |
A: 160 and 680 ms: neg > neu/pos; 400 ms: pos > neg/neu T: 160 ms: largest valence effect in prefrontal cortex; 400 ms: visual association cortex; 680 ms: left precentral gyrus (LORETA algorithm) |
| Codispoti et al. (2006) | F/M: 26/24 A: 22-34 |
C: High-arousing neg/pos, low-arousing neu [6000] B: IAPS normative data |
F: Habituation of psychophysiological responses T: Passive viewing |
A: 400-800 ms [LPP]: neg/pos > neu [also effects of habituation] T: 400-800 ms: largest arousal effect at posterior sites |
| De Cesarei & Codispoti (2006) | F/M: 8/8 A: 22.7 |
C: High-arousing neg/pos, low-arousing neu [100] B: Not reported |
F: Stimulus size T: Non-emotional categorization |
A: 150-300 ms: pos > neg/neu; 400-600 [LPP]: pos/neg > neu T: 400-600: largest arousal effect at parietal sites |
| Delplanque et al. (2006) | F/M: 17/0 A: 21.5 |
C: High-arousing neg/pos, low-arousing neu [750] B: IAPS normative data |
F: Affective influences on P3a and P3b T: Emotional categorization |
A: 333-384 ms [P3a]: neg > pos/neu; 439-630 ms [P3b]: pos > neg > neu; T: 333-384 ms: largest valence effect at parietal site; 439- 630 ms: pos > neg effect largest at frontal/central sites |
| Gasbarri et al. (2006) | F/M: 24/24 A: 28 |
C: High-arousing neg, low-arousing neu [10000] B: Participant ratings |
F: Memory and gender T: Affective rating of picture sequences |
A: 250-700 ms [P3]: females > males for arousing stimuli; T: 250-700 ms: largest gender effect at left-hemisphere sites |
| Hajcak & Nieuwenhuis (2006) | F/M: 11/3 A: Students |
C: High-arousing neg/pos, low-arousing neu [1000] B: IAPS normative data |
F: Emotional reappraisal vs passive viewing T: Emotional rating |
A: 200-400, 600-1000, 1200-1800 [LPP]: reappraisal < passive viewing for arousing pictures T: largest effects of reappraisal at centro-parietal sites |
| Hajcak et al. (2006) | F/M: 10/6 A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [1000] B: IAPS normative data |
F/T: Emotional vs. non- emotional categorization of stimuli |
A: 500-650 ms [LPP]: emotional categorization task > non- emotional categorization task T: Not reported |
| Hot et al. (2006) | F/M: 16/14 A: 22.6 |
C: High-arousing neg/pos, low-arousing neu [1500] B: IAPS normative data validated in pilot study |
F: Cultural differences: French/Japanese T: Passive viewing |
A: 105-140, 176-230, 255-455 ms: pos/neg > neu; 176-230 and 255-455 ms: French > Japanese (parieto-occipital area) T: 105-140 ms: largest arousal effect at frontal-occipital sites; 176-230 ms: largest arousal effects at frontal sites; 255-455 ms: largest arousal and group effects at parieto- occipital[b]r sites |
| Moser et al. (2006) | F/M: 16/3 A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [1000] B: IAPS normative data |
F/T: Emotional enhancement vs. suppression |
A: 250-350 and 350-600 ms [LPP]: emotional enhancement/passive viewing instructions > emotional suppression instructions T: Not reported |
| Schupp et al. (2006) | F/M: 4/4 A: 24.0 |
C: High-arousing neg > pos, low-arousing neu [330] B: Not reported |
F: Habituation effects under rapid presentation T: Passive viewing |
A: 200-300 ms [EPN]: pos/neg vs neu T: 200-300 ms: largest arousal effect at occipito-temporal and fronto-central sites, and over the right hemisphere |
| Spreckelmeyer et al. (2006) | F/M: 8/6 A: 21 |
C: High-arousing neg/pos, low-arousing neu [302-515] B: IAPS normative data validated in pilot study |
F: Multi-sensory processing (pictures/voices) T: Emotional ratings of stimuli |
A: 150-250 [P2]: pos > neg/neu; 380-420 [P3]: pos/neg > neu; 500-1400 ms [LPP]: larger for congruent pairing (sad pictures/sad voices) than other combinations T: >380 ms: largest effect of pos > neu/neg at frontal sites |
| Wood & Kisley (2006) | F/M: 15/5 (young), 12/8 (old) A: 21.0 (Y), 68.5 (O) |
C: High-arousing neg/pos, low-arousing neu [1200] B: IAPS normative data, validated by participant ratings |
F: Age effects T: Emotional ratings of pictures |
A: 400-900 [LPP]: neg > pos only for young adults. Neg/pos > neu overall, but stronger effect in young adults T: 400-900: not reported |
| Bradley et al. (2007) | F/M: 31/9 A: Undergraduates |
C: High-arousing neg/pos, low-arousing neu [6000] B: IAPS normative data, picture composition, ratings of complexity |
F: Picture composition; figure-ground vs. complex scenes T: Passive viewing |
A: 150-250: figure-ground vs complex scenes; 400-700 [LPP]: arousal effect; for pos/neg, figure-ground vs complex scenes T: 150-250: largest effect of composition at fronto-occipital sites; 400-700: parietal locus of arousal effect |
| Codispoti et al. (2007) | F/M: 12/12 A: 21-28 |
C: High-arousing neg/pos, low-arousing neu [1000] B: IAPS normative data |
F: Habituation of early and late affective processes T: Passive viewing |
A: 150-300: valence/arousal effects; overall habituation within stimulus blocks, least habituation for pos; 300-600: valence/arousal effects; habituation within/between blocks T: 150-300; 300-600: valence/arousal effects and their interactions with habituation vary with scalp region |
| Cano & Polich (2007) |
F/M: 16/0 | C: Moderate identical arousal for neg, neut, pos [1000] B: IAPS normative data |
F: Normal vs. scrambled pictures; color vs. bl/w T: Detection of affective stimuli |
A: Exp. 1, 400 ms valence effects for color not bl/w; Exp 2 valence differences eliminated with scrambled images T: 400 ms valence effects strongest over frontal areas |
| Carretié et al. (2007) | F/M: 27/4 A: 23.4 |
C: High-arousing neg/pos, low-arousing neu [100] B: Participant ratings of valence/arousal |
F: Spatial frequency (fuzzy vs clear images) T: Non-emotional categorization |
A: 135: spatial frequency effect; 135, 180, 240: spatial frequency interacts with emotional content. T: 135: largest spatial frequency effect in occipito-parietal cortex [LORETA]; 180, 240: interaction effects largest at frontal sites |
| Conroy & Polich (2007) | F/M: 12/0 | C: Moderately arousing neg, neut, pos [1000] B: IAPS normative data |
F: Arousal level controlled across valence; T: Detection of affective stimuli |
A: 400 ms left frontal amplitude decreased P300 amplitude for neg relative to neut or pos stimuli T: P300 strongest valence effect over frontal areas |
| Olofsson & Polich (2007) | F/M: 18/0 A: 18-27 |
C: High-arousing neg/pos, low-arousing neu [1000] B: IAPS normative data |
F: Repetition and time-on- task T: Detection of affective picture stimuli |
A: 160-220 ms [P2]: neg > neu; 300-450 ms [P3] and 500- 850 ms [SW]: neg/pos > neu T: Largest arousal effects at posterior sites |
| Rozenkrants & Polich (2007) | F/M: 16/16 | C: Low/high arousal at neg/pos valence, scrambled control stimuli for each picture condition [1000] B: IAPS normative data |
F: Gender effects in arousal and valence parameters T: Detection of affective picture stimuli, repeated stimulus conditions |
A: 400-800 [P300, SW]: high arousal > low arousal, weak valence effects, no gender differences T: Valence effects strongest over frontal areas |
| Rozenkrants et al. (2007) | F/M: 16/16 | C: Low/high arousal at neg/pos valence, scrambled control stimuli for each picture condition [1000] B: IAPS normative data |
F: Independent manipulation of arousal and valence parameters T: Detection of affective picture stimuli, repeated stimulus conditions |
A: 400-800 [P300, SW]: high arousal > low arousal differences that were found for both normal and scrambled stimuli, no valence effects, no gender differences T: 220-300: Arousal effect largest at parietal sites, stable across repetions |
| Sabatinelli et al. (2007) | F/M: 10/8 A: 20 |
C: High-arousing neg/pos, low-arousing neu [6000] B: IAPS normative data; categories balanced on perceptual dimensions |
F: ERP-fMRI correlations T: Passive viewing |
A: 400-900 [LPP]: neg/pos > neu T: 400-900: largest effect at centro-parietal sites; correlations to hemodynamic activity in occipital, parietal and temporal lobes |
| Schupp et al. (2007) | F/M: 8/8 A: 25 |
C: High-arousing neg (mutilations)/pos (erotica), low-arousing neu (people) [333] B: IAPS normative data |
F: Attention manipulation through varying target categories T: Count target stimuli |
A: 200-350 [EPN]: neg/pos < neu; effect of attention similar across affect categories; 400-600 [LPP]; neg/pos > neu; effect of attention largest for neg/pos T: 200-350; 400-600: largest effect at posterior sites |
Neg = stimuli of unpleasant valence; neu = stimuli of neutral valence; pos = stimuli of pleasant valence. In the results column, only statistically significant contrasts are reported. In description of stimuli, x < y refers to lower affective ratings of x compared to y. In description of effects, x < y refers to smaller positive-going amplitude (or factor loading) for x compared to y. Complex interaction effects are not presented.
3.2. Theoretical perspective
The present review attempts to organize affective ERP methodological variability by using representative studies from the table to characterize the major empirical outcomes. The organizational scheme follows ERP component generation order from short (P1 and N1) to middle (N2 and P2) to long (P300 and slow wave) latencies. This approach facilitates at least a broad generalization of affective influences on the waveform, such that the affective ERP timing pattern serves as theoretical scaffolding that supports the empirical valence and arousal ERP effects. In general, ERPs from IAPS images suggest that more attention is garnered by affective content compared to neutral conditions, with amplitude modulations observed for the early and late components (Carretie et al., 2001, 2004; Conroy and Polich, 2007; Delplanque et al., 2006; Schupp et al., 1997, 2000). The temporal courses of ERP valence and arousal effects differ, however, as valence appear to influence relatively early (100-250 ms) and arousal influences relatively late (200-1000 ms) components (Codispoti et al., 2007; Olofsson and Polich, 2007). Such effects can be obtained in passive viewing as well as active response tasks (Bernat et al., 2001; Cuthbert et al., 2000; Roschmann and Wittling, 1992; Yee and Miller, 1987). Thus, affective processing can be described as an automatic feature of perception (Esteves et al., 1994; Fox, 1991; Kunst-Wilson and Zajonc, 1980; LeDoux, 1989; Öhman and Soares, 1998).
Support for this hypothesis comes from findings suggesting that valence category reflects initial selective attention capture by salient image content (appetitive, threatening). In general, unpleasant stimuli can produce stronger emotional effects than pleasant stimuli (Cacioppo et al., 1999; Crawford and Cacioppo, 2002; Öhman and Mineka, 2001), and such a “negativity-bias” may reflect rapid amygdala processing of aversive information (LeDoux, 1995; Morris et al., 1998). Arousal level is thought to determine attentional resource allocation for emotional picture processing (low, medium, high). This increase in attentional resources for arousing stimuli has been explained in terms of high intrinsic motivational properties of picture stimuli (e.g., viewer-directed threat), as these appear to facilitate encoding and memory storage of the affective event (Bradley et al., 1992; Lang et al., 1993). In sum, stimulus valence activates selective attention, whereas arousal is elicited by stimulus motivational qualities that engages the attentional resources that contribute to memory encoding (Dolcos and Cabeza, 2002; Schupp et al., 2004).
4.0 Contemporary ERP Affect Investigations
4.1. Short latency (100-200 ms)
Many affect-related ERP modulations for picture stimuli have been reported in the 100-200 ms range. The P1 and subsequent N1 components are sensitive to physical stimulus factors and index early sensory processing within the extrastriate visual cortex. When a stimulus discrimination task is employed, these potentials also respond to manipulations of selective attention (Clark and Hillyard, 1996; Hillyard et al., 1973; Luck et al., 2000; Mangun et al., 1997; Thorpe et al., 1996; Vogel and Luck, 2000). As Table 1 indicates, studies assessing these potentials have employed a wide range of valence/arousal levels and a variety of processing requirements, which have produced variable affective ERP outcomes for the P1 and N1 components.
The P1 component has been obtained in studies where affective pictures stimuli were varied in unpleasant and pleasant valence. These pictures either (1) occurred within a temporal sequence of neutral stimuli (Carretie et al., 2004; Delplanque et al., 2004), (2) were presented as single-stimuli under a non-emotional categorization task (Carretie et al., 2006), or (3) were superimposed on larger background pictures that varied in valence under an emotional discrimination task (Smith et al., 2003). In the last study, the P1 (117 ms) potential elicited by the affective target pictures was found to be larger for unpleasant than pleasant target stimuli over the occipital sites. As pleasant and unpleasant valence categories were matched on arousal level, the findings suggested that unpleasant valence pictures engage more focal attentional processing than pleasant valence pictures. A similar valence effect was reported when single targets of unpleasant (snarling wolf) and pleasant (opposite-gender nude) motifs were presented within a sequence consisting of a repeated neutral picture during a simple target counting task (Carretie et al., 2004). Although this study has limited generalizability due to the few picture stimuli used, this effect was replicated at parietal-occipital sites for a “late P1” (150-165 ms) component when using an oddball task with multiple picture targets (Delplanque et al., 2004). Another study found enlarged ERP positivity (160 ms) for unpleasant valence pictures at frontal sites when using a non-emotional discrimination task (Carretie et al., 2006). Taken together, these findings imply that unpleasant valence images can produce larger P1 amplitudes than pleasant and neutral images, which is consonant with a processing system that is sensitive to unpleasant stimuli (Cacioppo et al., 1999; Crawford and Cacioppo, 2002; LeDoux, 1995; Morris et al., 1998; Öhman and Mineka, 2001). Although most studies report occipital topography distributions for this negativity effect, discrepancies across papers likely originate from a combination of methodological, stimulus, task, and analysis differences (Carretie et al., 2006).
The 150-200 ms latency range further contains the onset of an arousal-related positivity that reaches its maximum effect at the longer latencies as described below. A later N1 (176 ms) component has been found resistant to habituation with increases of time-on-task for high-arousing unpleasant images compared to other stimulus categories when a single picture for each valence occurred multiple times (Carretie et al., 2003). However, later studies using multiple pictures within each category have not supported this effect, implying that the original findings might not be generalizable across stimuli (Codispoti et al., 2007; Olofsson and Polich, 2007). Hence, the early ERP latency findings suggest that amplitude modulations are largest for unpleasant valence pictures, which may indicate that unpleasant valence preferentially attracts attention early in the information processing stream. Habituation of affective ERP amplitudes has not been found consistently within this latency range, and after 150 ms stimulus arousal level also begins to influence the ERP waveform.
Table 1 demonstrates that the affective ERP findings evince a great deal of variability across studies in the early latency range. Different mixes of stimulus valence categories and arousal levels might induce processing differences that have not yet been investigated systematically. The varying number of stimulus repetitions could further modulate these affective ERP effects. As previous studies ranges from using only one picture per affective category to only novel picture stimulation, tonic arousal level and motivation of the participants might differ among studies in ways that could interact with affective reactivity (Polich and Kok, 1995). Furthermore, early ERPs are influenced by the perceptual characteristics of the pictures: Simple figure-ground compositions produce larger amplitudes than complex scenes (Bradley et al., 2007), color contributes to ERP affective outcomes (Cano et al., 2007), and stimulus spatial frequency affects ERP responsivity in this latency range (Carretie et al., 2007). No lateralization of early potentials is apparent, and occasional reports of timing changes for these early potentials are inconclusive (De Cesarei and Codispoti, 2006; Keil et al., 2001).
4.2. Middle latency (200-300 ms)
Processing within the 200-300 ms latency range reflects early stimulus discrimination and response selection processes (Di Russo et al., 2006). ERP modulation by affective arousal has been observed, primarily with high-density electrode arrays that employ an “average” reference derived from active-to-Cz recordings. An “early posterior negativity” (EPN) has been reported at 200-300 ms for arousing compared to neutral stimuli. The EPN consists of a negative amplitude deflection over fronto-central sites and a positive-going waveform over temporo-occipital sites (Schupp et al., 2003a, 2003b, 2004, 2006). The main theoretical interpretation of the EPN is that it indexes “natural selective attention,” such that evaluation of image features is guided by perceptual qualities that select affectively arousing stimuli for further processing (Dolcos and Cabeza, 2002; Schupp et al., 2004).
Stimulus arousal level contributes to EPN since highly arousing pictures (mutilations and erotica) elicit larger amplitude EPNs than less arousing pictures for both unpleasant and pleasant categories (Schupp et al., 2003a). Moreover, middle latency arousal-related ERP modulations have been obtained across tasks (passive viewing, target detection, neutral non-picture target-detection), picture presentation inter-stimulus intervals (0 ms to 6 s), and stimulus durations (120-1500 ms). These findings imply that the middle latency arousal modulation can occur automatically during affective picture viewing even when processing resource availability is limited by rapid presentation rate (Junghöfer et al., 2001; Schupp et al., 2003a, 2003b). This sensitivity for arousal level may reflect rapid affective amygdala processing of aversive information (LeDoux, 1995; Morris et al., 1998)
Stimulus valence also has been shown to influence the middle latency N2 component, which overlaps with the EPN. Unpleasant stimuli elicit a decreased N2 negativity compared to pleasant valence stimuli—an effect that was localized to the anterior cingulate cortex by means of source analysis algorithms (Carretie et al., 2004). This result, although not consistently found, resembles valence effects found at early latencies (100-150 ms) and the EPN component (Codispoti et al., 2007), however methodological differences among studies preclude strong inferences. Given that stimulus discrimination and response selection are thought to occur during the 200-300 ms range, it seems likely that affective visual stimuli would influence neural activation before behavioral response execution stages. N2 amplitude modulation between arousal levels sometimes (but not consistently) demonstrate larger amplitude changes over the right compared to left hemisphere (Junghöfer et al., 2001; Schupp et al., 2006). Habituation patterns that differentiate between affective picture categories within this latency range are not observed, and there is little evidence of N2 timing changes that can be attributed to affective picture category (Codispoti et al., 2007; Olofsson and Polich, 2007; Schupp et al., 2006).
Scalp electrode arrays using linked earlobe or mastoid references have demonstrated affective modulation in the 200-300 ms latency range, but this montage does not share the properties of the unpleasant-to-pleasant shift obtained with an average reference montage. Differences in direction and magnitude of ERP effects from affective pictures as a function of reference type have to date attracted little empirical interest. However, standard ERP recording methods find that the P2 component and adjacent N2 component are sensitive to the onset of pleasant-going arousal-related amplitude modulation that persists until stimulus offset (Amrhein et al., 2004; Carretie et al., 2001a, 2001b, 2004; Cuthbert et al., 2000; Olofsson and Polich, 2007).
Theoretically, the underlying factor determining middle latency amplitude modulations is selective attention to objects within the affective image that are assumed to be of intrinsic relevance (Schupp et al., 2006). This interpretation has received indirect support from studies demonstrating non-affect perceptual and category-related ERP modulations in the middle latency range that were attributed to selective attention mechanisms (Bradley et al., 2007; Codispoti et al., 2006). Additional assessment of how valence and arousal effects interact, how non-affective stimulus parameters contribute to affective outcomes, and how recording methods may produce ERP differences are needed to further clarify these issues.
4.3. Long latency (>300 ms)
The later segment of the affective ERP is dominated by the P300 component and following positive slow wave. These potentials are often elicited using some variant of the oddball paradigm in which a covert or overt response is made to a designated target stimulus presented in a series of non-target stimuli. The P300 is composed of the P3a and P3b subcomponents that are hypothesized to index attentional and initial memory storage events (Polich, 2007), whereas the subsequent slow wave activity appears related to task demands involving working memory operations (Azizian and Polich, 2007; Mecklinger and Pfeifer, 1996; Ruchkin et al., 1988, 1995). The P300/slow wave elicited with affective pictures is sometimes denoted as a “late positive potential” (LPP), with the finding that the latter portion of the ERP waveform over a broad latency interval demonstrates elevated positivity to high-arousing stimuli (Cuthbert et al., 2000). Table 1 summarizes the relevant results wherein similar affect outcomes often encompass both P300 and slow wave ERPs. A systematic account of similarities and possible differences between P300 and slow wave for affective stimulus parameters has not yet emerged.
4.3.1. P300 processes
Major determinants of P300 amplitude are task-relevance, motivational significance, arousal level and the influence of these factors on mental resource allocation (Duncan-Johnson and Donchin, 1977; Polich and Kok, 1995; Squires et al., 1977). Recent reviews have proposed P300 reflects dopaminergic modulatory effects exerted by phasic activity of the locus coeruleus-norepinephrine system as associated with decision-making processes (Nieuwenhuis et al., 2005). P300 emotional arousal effects have been obtained for passive (viewing) and active (affect discrimination) procedures as well as for images presented as distracting or target stimuli in an oddball task (Delplanque et al., 2004, 2005; Keil et al., 2002; Mini et al., 1996; Schupp et al., 2000). Affective ERP results are typically of maximum amplitude over the parietal cortex, suggesting that emotional arousal amplifies activity in cortical structures that are normally engaged for target processing (Sabatinelli et al., 2007). Valence level also can modulate P300 when arousal level is controlled (Cano and Polich, 2006; Conroy and Polich, 2007; Rozenkrants and Polich, 2007), which may be related to affective EEG findings of approach/withdrawal stimulus evaluation (Davidson, 2001).
The relationship between affective stimulus conditions to the P3a and P3b subcomponents has been addressed using temporal principal component analysis. In this context, image valence level does not alter P3a but does influence P3b amplitude, such that pleasant pictures elicit larger components than unpleasant pictures when the pictures are task-relevant (Carretie et al., 2006; Delplanque et al., 2004). However, when the affective pictures are task-irrelevant distractors, P3a amplitude becomes larger over frontal/central sites for unpleasant and pleasant stimuli relative to neutral images (Delplanque et al., 2005). A theoretical integration of affective processing influences on P3a in relation to P3b has not been developed in part because affect ERP studies using a passive viewing condition have not reported such valence-dependent effects (Amrhein et al., 2004; Codispoti et al., 2006; Cuthbert et al., 2000). Thus, only the P3b appears sensitive to both valence and arousal variation, which suggests that these factors specifically influence target processing (Conroy and Polich, 2007; Delplanque et al., 2006).
4.3.2. Slow wave processes
A long-lasting elevated ERP positivity to arousing pictures is a common finding (Amrhein et al., 2004; Cuthbert et al., 2000; Ito et al., 1998; Keil et al., 2002; Mini et al., 1996; Olofsson and Polich, 2007; Palomba et al., 1997; Schupp et al., 2000). Evidence suggests that the slow wave arousal positivity is involved in memory formation. Palomba and colleagues (1997) reported that arousing stimuli (unpleasant/pleasant) elicited more positive-going ERPs in the 300-900 ms range (including the P300) and were recalled more often than neutral and relatively low-arousing stimuli. This finding has been interpreted as indicating enhanced encoding processing for arousing stimuli since memory performance also is associated with late latency ERP amplitude (Azizian and Polich, 2007; Karis et al., 1984; Paller et al., 1988). Dolcos and Cabeza (2002) replicated the affective slow wave arousal effects during an incidental encoding phase and found that the subsequent memory ERPs—higher positivity during the encoding of items that were later recalled—over centroparietal sites was larger for arousing pictures than for neutral pictures (400-600 ms). The amplitude increases corresponded to improved recognition memory performance, and the results can be interpreted as ERP memory formation effects for affective stimuli. This finding illustrates the benefits of using paradigms that combine affective ERPs with behavioral assessment for functional interpretation of ERP effects.
Additional findings also suggest that ERP arousal modulations within the slow wave latency range (>500 ms) are susceptible to top-down processing influences. This effect is related to evaluation of the affective stimuli (Hajcak et al., 2006; Moser et al., 2006): When categorizing affective pictures along a non-affective dimension (e.g., number of people in the pictures), ERP positivity for arousing (unpleasant and pleasant) pictures was decreased during a 500-650 ms interval compared to when participants performed an affective categorization task. Furthermore, an experimentally induced suppression emotional reactions to unpleasant stimuli has been associated with diminished ERP positivity compared to other viewing conditions (Moser et al., 2006).
Carretie and colleagues (2006) presented affective picture stimuli during a non-affective discrimination task. An increase in ERP amplitude (680 ms) following stimulus onset was present for unpleasantly arousing stimuli. The effect was located in the left precentral gyrus by means of LORETA (low resolution tomography algorithm) source localization, leading the authors to speculate on a possible “motor-related bias” when responding to unpleasant stimuli. Affective stimuli may therefore elicit not only attention and perceptual processes but also motor effects. Taken together, top-down processes such as emotional evaluation or suppression appear to interact with affective stimulus activation during slow wave ERP ranges as do memory encoding processes.
5.0 Discussion
Component amplitude is influenced by affective stimulus value from around 100 ms to several seconds after stimulus onset, and peak latency is typically not changed. Affective valence and arousal can independently modulate ERP outcomes at several partially overlapping latency ranges, mainly between 200 and 400 ms. Valence exerts influence predominantly for early and middle-range components, whereas arousal induces a positive shift in the ERP waveform that is the primary affective influence of the middle-range and later ERP component amplitudes. Table 1 indicates that arousal-related ERP modulation is observed across a wide range of studies, using different picture stimuli and experimental procedures, whereas valence yields fewer consistent effects. Thus, ERP affective processing begins early, remains sustained over time, and occurs across several processing stages.
5.1. Theoretical implications
A few theoretical interpretations have been proposed to account for affective ERP modulations produced by complex picture stimuli. The “negativity-bias” framework emphasizes the intrinsic relevance for unpleasant and threatening stimuli and has been used to account for valence-related ERP modulations (Cacioppo et al., 1999; Crawford and Cacioppo, 2002; Öhman and Mineka, 2001). Such negativity bias may reflect rapid activity by amygdala processing of aversive information (LeDoux, 1995; Morris et al., 1998), so that attentional resources are engaged more readily for unpleasant relative to neutral or pleasant stimuli (Cacioppo and Berntson, 1994; Ito et al., 1998). The negativity-bias framework has been linked to attention processing through findings of picture valence effects in ERPs (Carretie et al., 2001, 2004; Wood and Kisley, 2006). According to this view, attention is automatically oriented towards events that might pose a threat to the perceiver in an evolutionarily adaptive fashion (Öhman et al., 2001). Thus, valence ERP effects may originate from a predisposition towards rapid orienting of attention to threatening stimuli in order to facilitate processing efficiency.
Another approach suggests that an early selective attention mechanism produces an enhanced EPN depending on arousal value across valence categories (Schupp et al., 2006). This perspective implies that emotional processing may reflect a default mechanism that does not require overt valence categorization (Schupp et al., 2004). This outcome has led to the theoretical view that stimuli of high motivational relevance facilitate processing independent of the motivational direction (Schupp et al., 2000). Stimulus motifs that are particularly erotic or threatening, for example, differ in their intrinsic motivational properties and may induce “motivated attention” from their physical contents. Since highly motivating stimuli also generate the strongest arousal IAPS ratings, this theoretical viewpoint provides a connection with the physiological concept of arousal. Findings of non-affective picture factors that influence neuroelectric measures ERPs in ways similar to affective parameters are likely to become important for understanding early affective ERPs (Bradley et al., 2007; Codispoti et al., 2006).
The hypothesis of an attentional bias to affective events, whether affect is varied along the valence or arousal dimension, needs to be evaluated by manipulating attention and obtaining performance measures to complement early ERP amplitude modulation. Most stimulus-driven affective ERP modulations can be generated automatically in conditions wherein the participant is viewing the images. Passive viewing paradigms, however, do not produce overt performance measures. The major advantage of obtaining behavioral measures is that they can validate the theoretical interpretation of the ERP outcomes by providing an overt index of attention. Integrating affective ERPs with behavioral paradigms should help reveal the functional significance of waveform modulations by arousal and valence. For example, although it is unclear how early affective ERP modulations will be influenced by divided attention, the EPN to arousing stimuli may be attenuated under high task-demands (Schupp et al., 2007). Mapping the correlates between behavioral performance measures and affective ERP changes will help to identify the psychological mechanisms underlying affective neuroelectric changes.
Table 1 indicates that long-latency ERPs are strongly influenced by arousal, since later ERP positivity covaries with subjective arousal levels (Cuthbert et al., 2000). Arousal has been shown to be relevant for encoding and long-term memory storage (Bradley et al., 1992; Lang et al., 1993). Successful attempts have been made to associate the arousal-related influence on long-latency ERPs with emotional memory (Dolcos and Cabeza, 2002; Maratos and Rugg, 2001) and emotion regulation (Hajcak and Nieuwenhuis, 2006).
The P300 component may be associated with phasic activity of the locus coeruleus-norepinephrine (LC-NE) neuromodulatory system (Nieuwenhuis et al., 2005; Pineda et al., 1989). This hypothesis proposes that the LC-NE system is activated by events that are relevant for goal-directed behavior. In addition, it has been speculated that the long-latency ERPs to affective pictures are secondary to LC-NE activity (Schupp et al., 2006). P300 amplitude is related to tonic arousal as well as to phasic modulations by stimulus features, and testable hypotheses are likely to be generated from the LC-NE account of P300 (Cuthbert et al., 2000; Polich and Kok, 1995). A challenge for the LC-NE theory is to account for effects on both P3a and P3b subcomponents (Polich and Criado, 2006), given the differential susceptibility of these subcomponents to affective modulation (Delplanque et al., 2004, 2005, 2006).
5.2. Empirical considerations
The listing of ERP affective picture studies in Table 1 suggests certain topics that warrant consideration. These points will be discussed in the subsections below to highlight issues related to methodological disparities among reported studies and characterize issues for further investigation. As each topic encompasses a relatively specific area, the presentation order and contents are necessarily somewhat idiosyncratic. However, the choice of topics presents a snapshot view of adjacent affective ERP issues that are emerging.
5.2.1. Affective ERP neural sources
As the above review suggests, neural origins of affective ERP effects have been broadly painted, but a variety of approaches are attempting to define the generator parts and pathways observed at the scalp. Evidence from fMRI methods suggests that the amygdala is a key structure in affective processing (Dalgleish, 2004; Vuilleumier et al., 2004). This structure receives initial sensory input directly through the thalamus and enhances cortical and subcortical processing of the visual stimulus based on its affective arousal value, which leads to improved memory for emotional stimuli (Cahill et al., 1995, 1996; Hamann et al., 1996; LeDoux, 2000; McGaugh, 2004, 2005; McGaugh et al., 2002). Such a mechanism can be conceived of an “overall heightened perceptual vigilance” for affectively arousing stimuli (Phelps et al., 2004). Several studies have attempted to localize affective ERP modulations to specific intra-cranial sources (Carretie et al., 2001, 2004, 2006). However, integration of hemodynamic neuroimaging and scalp-recorded ERPs only recently has been conducted. For example, the arousal-related slow wave ERP positivity was localized by means of fMRI-ERP correlation analyses (Sabatinelli et al., 2007). Results reveal a distributed network of brain regions in the lateral occipital, inferotemporal, and parietal visual areas that likely underlie scalp-recorded ERP modulation. In addition, contributions of intra-cranial recordings of key affective regions could facilitate development of a more comprehensive theoretical framework for the spatio-temporal dynamics of affective processing (Krolak-Salmon et al., 2004).
Traditional ERP technology using low-density electrode arrays might not provide sufficient sensitivity to detect subtle affective modulations. Findings of early arousal effects (200 ms) are obtained primarily by means of high-density electrode arrays and reference-free recordings (Codispoti et al., 2007; Junghöfer et al., 2001). Application of advanced analysis methods (ICA, PCA, LORETA) could prove important for detecting, localizing, and explaining complex ERP patterns related to affective picture responsivity (Delplanque et al., 2004; Dien et al., 2004; Makeig et al., 2004).
5.2.2. Subjective stimulus assessment
Figure 1 illustrates the IAPS ratings and demonstrates that the images reflect a wide variety of affective influence that permits experimental manipulations through the use of picture subsets. The fundamental affect dimensions of valence category and arousal level are typically determined by normative stimulus judgments. The pictures are composed of various themes within broad emotional categories, some of which are more arousing than others. For example, images of erotica (pleasant/arousing) and mutilations/threat (unpleasant/arousing) are perceived as particularly arousing and produce large ERP modulations within both middle- and long-latency intervals (Schupp et al., 2003b 2004). Affective responsivity is determined in part by the evolutionary significance of the image content, so that ERP variation to these pictures is mediated by a picture's inherent arousal level or motivational relevance (Schupp et al., 2003a).
Most ERP studies using IAPS have relied on the associated stimulus ratings by undergraduate female and male students who examined the pictures during free viewing (Lang et al., 1999). However, as Table 1 indicates, affective ERP studies differ appreciably in stimulus parameters and task requirements. For example, unpleasant and pleasant pictures used in an ERP task were equally arousing according to IAPS ratings. However, stimulus assessment of arousal during the experiment by the participants differentiated between the two categories, which suggests that procedural differences between the IAPS normative rating and ERP recording sessions (e.g., stimulus duration and size) might be of importance (Pollatos et al., 2005). Subjective ratings of valence and arousal could therefore be performed within the ERP session to assess stimulus effects (Dolcos and Cabeza, 2002; Spreckelmeyer et al., 2006).
Experimental designs that carefully manipulate and control valence/arousal parameters perceived by the participants within the laboratory setting will be useful (Conroy and Polich, 2007; Rozenkrants and Polich, 2007). Uncertainties regarding stimulus assessment complicate the interpretation of the resulting ERP modulation. Studies sometimes employ rating scales that may be conceptually different from other rating procedures, such as the pictorial Self-Assessment Manikin (Amrhein et al., 2004; Bradley and Lang, 1994). ERP effects attributed to differences in picture valence might be influenced by relatively subtle and unappreciated differences in arousal level. Careful evaluation of affective stimuli using methods derived from psychophysical scaling might further improve the disentangling of valence and arousal effects (Cuthbert et al., 2000; De Cesarei and Codispoti, 2006; Schupp et al., 2004).
5.2.3. Stimulus repetition
In a similar vein, experimental manipulations of repeating stimuli to induce valence and arousal affective ERP changes is a common procedure, but systematic assessment of repetition number, non-affect stimulus controls, or habituation is generally lacking. Studies have used reoccurring affective pictures to increase the number of stimuli, thereby effectively obtaining a high signal/noise ratio in the ERP recording (Carretie et al., 2001a, 2001b; Schupp et al., 2000). However, a limited number of picture motifs restricts the generalizability of any obtained findings. Moreover, multiple stimulus repetitions are associated with a short-term amplitude decline observed in an early (150-300 ms) and a slower decline in later (300-600ms) processing stages (Codispoti et al., 2006, 2007). In contrast, repetition of IAPS images within an oddball discrimination paradigm revealed increased positivity (200-450 ms) as a function of affective stimulus repetition, regardless of whether the stimulus was reoccurring with a short or long lag. Decreases in response time and peak P300 latency over repeated trials also have been observed (Olofsson and Polich, 2007; Rozenkrants et al., 2007). Characterization of repetition-induced affective changes may prove useful in distilling how valence/arousal relationships contributes to memory-related ERP effects (Bentin and McCarthy, 1994; Segalowitz et al., 1997).
5.2.4. Physical stimulus attributes
Affective stimulus categories are generally interpreted as originating from the semantic properties or “meaning” of the affective stimuli, with the physical aspects of the images left to vary. Affect ERP studies that have evaluated variables such as stimulus complexity, color, spatial frequency, etc. find some influences of physical variables on affective waveforms (Cano and Polich, 2006; Carretie et al., 2004; Junghöfer et al., 2001). In addition, participant ratings of familiarity and physical complexity have not supported the interpretation that such variables help determine ERP valence/arousal effects (Carretie et al., 2004). Picture composition such as the relationship between a central figure and background image relative to complex scenes composed of several items appears to influence ERPs at a very early stage (150 ms), whereas no effect of picture composition was found at long latencies. These effects are largely additive to the image affective value, implying little confound from perceptual factors (Bradley et al., 2007). Evaluation of stimulus features by removing semantic content but maintaining perceptual characteristics can be obtained by using “scrambled” images of the affective item in the same task. This procedure appears to attenuate, but entirely remove, affective outcomes and can help determine the sources of stimulus affective influences such as separation of local from global features (Maljkovic and Martini, 2005; Rozenkrants et al., 2007; Rozenkrants and Polich, 2007).
Table 1 suggests that most affective ERP studies use IAPS stimuli that are typically selected by using the ratings from bipolar scales (valence: unpleasant-to-pleasant; arousal: low-to-high). However, this bipolar structure has been criticized, because unpleasantness and pleasantness might not be reciprocal and antagonistic such that some stimuli could engage both unpleasant and pleasant motivational systems (Ito et al., 1998) . Humans are predisposed to exploration of neutral environments, described as a “positivity offset”, which is complemented by a “negativity bias” that makes unpleasant encounters induce a stronger emotional impact (Cacioppo and Berntson, 1994). As evident from Table 1 and the review above, ERP valence findings are appreciably less consistent than manipulations of the arousal dimension. Alternative or empirically validated assessment of valence and arousal dimensions might help to clarify the nature of how these primary affective dimensions influence ERP outcomes.
5.25. Behavioral assessment
Related to the identification and evaluation of stimulus characteristics are task effects. Many ERP affective studies have used passive viewing methods, others have employed detection time measurements (Carretie et al., 2001, 2003; Olofsson and Polich, 2007; Smith et al., 2003). Speeded emotional categorization tasks also have been employed (Delplanque et al., 2005; Schupp et al., 2004), but these studies display ERP findings similar to those from detection tasks and passive perception (Schupp et al., 2006). Affective ERP modulations by comparing task conditions on the same stimulus set would help identify behavioral correlates of emotional processes. For example, the EPN component is attenuated by increasing task load (Schupp et al., 2007), and long-latency affective ERPs can be altered by changing the stimulus evaluation task (Hajcak et al., 2006; Moser et al., 2006). Such studies would help to clarify the functional significance underlying affective ERP outcomes.
5.3. Individual differences and affective ERPs
Individual variability in affective reactivity is a topic that is receiving increased interest: for example, normal variation in fMRI amygdala activation by emotional face and word stimuli has been associated with genetic and personality factors, suggesting fundamental and systematic differences in affective perception (Canli et al., 2002, 2005). However, affective ERP waveform variability across individuals has received very little consideration. Although such variability is likely multifactorial in nature, finding correlations with demographic factors and psychological traits are important first steps towards a theoretical characterization of affective ERP variability. One major source of individual variation may stem from gender differences in IAPS ratings for certain pictures, most of which are of pleasant valence and containing humans (female/male nudes, babies) as content (Lang et al., 1999). fMRI findings indicate stronger activation to arousing erotic pictures in males compared to females, but an opposite pattern for mutilations (Sabatinelli et al., 2004). Larger positive potentials in the 250-700 ms range have been found in females compared to males for arousing pictures with unpleasant affect (Gasbarri et al., 2006). Not all studies find gender effects, but systematic assessment of gender could prove fruitful in characterizing stimulus- and task factors (Cahill, 2004; Rozenkrants and Polich, 2007).
Participant age is another potential factor of affective import. When young and older adult subjects were compared using startle-elicited ERPs as a measure of arousal from emotional pictures, smaller N1 and P3 amplitudes were obtained for the older adults irrespective of affective stimulation (Smith et al., 2005). IAPS images produced a decrease in LPP amplitude for arousing pictures in older adults (particularly for unpleasant pictures) but no age-related difference for earlier components (Wood and Kisley, 2006). The results imply that a “negativity bias” is present in younger adults, but decreased in elderly adults. Since corresponding differences in affective ratings were not found, and as valence effects are absent in many comparable studies, this finding should be replicated using different unique IAPS pictures before firm conclusions can be made. Given the relatively large literature on cognitive ERPs and aging for neutral stimuli (Fjell and Walhovd, 2005; Kok and Zeef, 1991; Kugler et al., 1993; Polich, 1997), age-related affect changes have not been well defined and are not yet conclusive.
A relatively new topic is cultural affective processing effects, which could provide clues to the mechanisms underlying cultural differences. For example, Hot et al. (2006) compared Japanese and French individuals on their reactivity to affective pictures. Smaller affective ERP components in the mid-range (170-450 ms) were obtained in the Japanese compared to French subjects, which may suggest that emotional expressivity differs among cultural backgrounds. In addition, individuals with high interoceptive awareness (self-perception of cardiac signals) demonstrated increased late ERP amplitudes to affective pictures (Pollatos et al., 2005).
Individual ERP variation to affective pictures remains a collection of interesting but disparate findings, and such investigations may contribute to understanding the functional significance of affective ERP responsivity. Indeed, amplitude modulations associated with instructed emotional reappraisal correspond to subjectively perceived affective stimulus change (Hajcak and Nieuwenhuis, 2006). Strategic abilities to cope with unpleasant situations could be reflected in the associated waveforms, and the affective ERP reactivity might therefore vary with general executive control capabilities. Explanations for individual differences in affective ERPs also will be helpful for characterizing clinical affective dysfunction. Future affective ERP applications could include assessment of individual differences as associated with cultural idiosyncrasies, demographic, and personality factors related to affective disposition (Davidson, 2001, 2003; Ito and Cacioppo, 2005).
6. Conclusion
The primary affective dimensions of valence and arousal influence ERP amplitudes at several processing stages that occur at separate and overlapping latencies. Valence effects, when reported, have been mainly found at short latencies (100-300 ms) and appear associated with rapid selective attention processes within the “negativity bias” framework are modulated by target detection as indexed by the P3b subcomponent. However, ERP valence effects are less consistently obtained than arousal effects, and the heterogeneity of results prevents strong conclusion about the nature of the effects of pictorial valence on ERPs. Arousal elicits a positive-going waveform from about 200 ms until stimulus offset. This effect is consistently obtained but varies with task relevance within the P300 range. Arousal-related ERP modulation has been linked with automatic attention at middle-range latencies, and intrinsically motivational stimuli facilitate processing for subsequent memory storage at longer latencies. ERP arousal effects can be inhibited by emotional reappraisal and task instructions, especially at longer latencies.
Further development of affective ERP paradigms will help to characterize brain processes produced by emotional picture stimuli. Complementary behavioral evidence is needed to clarify the functional significance of affective ERP modulations, especially for short-latency components related to attentional processing. Additional investigations of task-demands and picture composition as attention-related modulators of affective influences and emotional regulation would be informative (Bradley et al., 2007; Hajcak et al., 2006; Hajcak and Nieuwenhuis, 2006; Moser et al., 2006; Schupp et al., 2007). The susceptibility of early ERP components to non-affective perceptual features particularly highlights the need for balanced stimulus categories. Behavioral findings of affective influences on perception and visuo-spatial attention (Carrasco et al., 2004; Lundqvist and Öhman, 2005; Phelps et al., 2006; Öhman et al., 2001), as well as the evidence from non-affective ERPs that correlate with visuo-spatial attention (Hillyard and Anllo-Vento, 1998; Luck et al., 2000; Mangun and Hillyard, 1991; Mangun et al., 1993; McDonald et al., 2005), will contribute to a theoretical synthesis of ERP findings on attention, perception, and affect. Theoretical advances are likely to benefit from integration across different neurophysiological methods and stimulus types (Vuilleumier, 2005). Finally, additional topics for further investigation are the demographic, cognitive, procedural, and non-affective stimulus factors that interact with the affective ERP modulation.
Acknowledgements
The first author was supported by a fellowship from the American-Scandinavian Foundation and Thord-Gray Memorial Fund. This study was supported by RO1-DA018262 to JP and by grants from the Swedish Research Council to SN. We thank Bella Rozenkrants and Stefan Wiens for helpful comments. We thank the reviewers for their extraordinary critiques. This paper is publication number 16680 from The Scripps Research Institute.
Footnotes
The approach was focused and excluded topics such as non-visual stimuli, affective pathologies, and fear learning (Karl et al., 2006; LaBar et al., 1998). Excluded also were studies employing emotional words or pictures of facial expressions, as these stimuli involve highly specific neurophysiological processing (Britton et al., 2006; Horovitz et al., 2004; Puce et al., 1996), stimulus assessment, and specific procedures not employed in most affective ERP studies (Batty and Taylor, 2003; Campanella et al., 2002; Ekman and Friesen, 1971; Esslen et al., 2004; Smith et al., 2004).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amrhein C, Muhlberger A, Pauli P, Wiedemann G. Modulation of event-related brain potentials during affective picture processing: a complement to startle reflex and skin conductance response? International Journal of Psychophysiology. 2004;54:231–240. doi: 10.1016/j.ijpsycho.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Azizian A, Polich J. Evidence for attentional gradient in the serial position memory curve from ERPs. Journal of Cognitive Neuroscience. 2007 doi: 10.1162/jocn.2007.19.12.2071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Cognitive Brain Research. 2003;17:613–620. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bentin S, Mccarthy G. The effects of immediate stimulus repetition on reaction-time and event-related potentials in tasks of different complexity. Journal of Experimental Psychology: Learning, Memory and Cognition. 1994;20:130–149. [Google Scholar]
- Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. International Journal of Psychophysiology. 2001;42:11–34. doi: 10.1016/s0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Low A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion - the self-assessment mannequin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space - a critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: Form follows function. Journal of Personality and Social Psychology. 1999;76:839–855. [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, Mcgaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nature Reviews Neuroscience. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Campanella S, Gaspard C, Debatisse D, Bruyer R, Crommelinck M, Guerit JM. Discrimination of emotional facial expressions in a visual oddball task: an ERP study. Biological Psychology. 2002;59:171–186. doi: 10.1016/s0301-0511(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Cano ME, Polich J. Affective Visual Stimuli (IAPS) and ERPs: Valence, color, and control conditions; Paper presented at the Cognitive Neuroscience Society; San Francisco, CA. 2006. [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Albert J, Mercado F. Neural response to sustained affective visual stimulation using an indirect task. Experimental Brain Research. 2006;174:630–637. doi: 10.1007/s00221-006-0510-y. [DOI] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Lopez-Martin S, Tapia M. An electrophysiological study on the interaction between emotional content and spatial frequency of visual stimuli. Neuropsychologia. 2007;45:1187–1195. doi: 10.1016/j.neuropsychologia.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Martin-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Human Brain Mapping. 2004;22:290–299. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Mercado F. Cerebral patterns of attentional habituation to emotional visual stimuli. Psychophysiology. 2003;40:381–388. doi: 10.1111/1469-8986.00041. [DOI] [PubMed] [Google Scholar]
- Carretie L, Martin-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience. 2001a;13:1109–1128. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Carretie L, Mercado F, Hinojosa JA, Martin-Loeches M, Sotillo M. Valence-related vigilance biases in anxiety studied through event-related potentials. Journal of Affective Disorders. 2004;78:119–130. doi: 10.1016/s0165-0327(02)00242-2. [DOI] [PubMed] [Google Scholar]
- Carretie L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. International Journal of Psychophysiology. 2001b;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Chapman RM, Bragdon HR. Evoked responses to numerical and non-numerical visual stimuli while problem solving. Nature. 1964;203:1155–1157. doi: 10.1038/2031155a0. [DOI] [PubMed] [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: autonomic and cortical correlates. Brain Research. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19:577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Junghofer M, Schupp HT. The categorization of natural scenes: brain attention networks revealed by dense sensor ERPs. Neuroimage. 2006;32:583–591. doi: 10.1016/j.neuroimage.2006.04.180. [DOI] [PubMed] [Google Scholar]
- Conroy MA, Polich J. Affective valence and P300 when stimulus arousal level is controlled. Cognition & Emotion. 2007;21:891–901. [Google Scholar]
- Crawford LE, Cacioppo JT. Learning where to look for danger: Integrating affective and spatial information. Psychological Science. 2002;13:449–453. doi: 10.1111/1467-9280.00479. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. The emotional brain. Nature Reviews Neuroscience. 2004;5:582–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Toward a biology of personality and emotion. In Unity of knowledge: the convergence of natural and human science. 2001;935:191–207. doi: 10.1111/j.1749-6632.2001.tb03481.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davis H. Enhancement of evoked cortical potentials in humans related to a task requiring a decision. Science. 1964;145:182–183. doi: 10.1126/science.145.3628.182. [DOI] [PubMed] [Google Scholar]
- Davis H, Engebretson M, Lowell EL, Mast T, Satterfield J, Yoshie N. Evoked responses to clicks recorded from the human scalp. Annals of the New York Academy of Sciences. 1964;112:224–225. doi: 10.1111/j.1749-6632.1964.tb26751.x. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. When does size not matter? Effects of stimulus size on affective modulation. Psychophysiology. 2006;43:207–215. doi: 10.1111/j.1469-8986.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Lavoie ME, Hot P, Silvert L, Sequeira H. Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neuroscience Letters. 2004;356:1–4. doi: 10.1016/j.neulet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Rigoulot S, Sequeira H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. International Journal of Psychophysiology. 2006;60:315–322. doi: 10.1016/j.ijpsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Sequeira H. Event-related P3a and P3b in response to unpredictable emotional stimuli. Biological Psychology. 2005;68:107–120. doi: 10.1016/j.biopsycho.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Sequeira H. Attentional modulation of appraisal in emotion: the case of the P3b. Psychophysiology. 2006;43:S7–S7. [Google Scholar]
- Di Russo F, Taddei F, Apnile T, Spinelli D. Neural correlates of fast stimulus discrimination and response selection in top-level fencers. Neuroscience Letters. 2006;408:113–118. doi: 10.1016/j.neulet.2006.08.085. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Cabeza R. Event-related potentials of emotional memory: encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective, and Behavioral Neuroscience. 2002;2:252–263. doi: 10.3758/cabn.2.3.252. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. Quantifying surprise - variation of event-related potentials with subjective-probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Constants across cultures in the face and emotion. Journal of Personality and Social Psychology. 1971;17:124–129. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Esslen M, Pascual-Marqui RD, Hell D, Kochi K, Lehmann D. Brain areas and time course of emotional processing. Neuroimage. 2004;21:1189–1203. doi: 10.1016/j.neuroimage.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Esteves F, Parra C, Dimberg U, Öhman A. Nonconscious associative learning: Pavlovian conditioning of skin conductance responses to masked fear-relevant facial stimuli. Psychophysiology. 1994;31:375–385. doi: 10.1111/j.1469-8986.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Age-sensitivity of P3 in high-functioning adults. Neurobiology of Aging. 2005;26:1297–1299. doi: 10.1016/j.neurobiolaging.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it's not left, it's right. Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Arnone B, Pompili A, Marchetti A, Pacitti F, Calil SS, et al. Sex-related lateralized effect of emotional content on declarative memory: an event related potential study. Behavioral Brain Research. 2006;168:177–184. doi: 10.1016/j.bbr.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive Affective & Behavioral Neuroscience. 2006;6:291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Stefanacci L, Squire LR, Adolphs R, Tranel D, Damasio H, et al. Recognizing facial emotion. Nature. 1996;379:497. doi: 10.1038/379497a0. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo-Vento L. Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:781–787. doi: 10.1073/pnas.95.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Rossion B, Skudlarski P, Gore JC. Parametric design and correlational analyses help integrating fMRI and electrophysiological data during face processing. Neuroimage. 2004;22:1587–1595. doi: 10.1016/j.neuroimage.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Hot P, Saito Y, Mandai O, Kobayashi T, Sequeira H. An ERP investigation of emotional processing in European and Japanese individuals. Brain Research. 2006;1122:171–178. doi: 10.1016/j.brainres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Ito T, Cacioppo JT, Lang PJ. Eliciting affect using the international affective picture system: Trajectories through evaluative space. Personality and Social Psychology Bulletin. 1998;24:855–879. [Google Scholar]
- Ito TA, Cacioppo JT. Variations on a human universal: Individual differences in positivity offset and negativity bias. Cognition & Emotion. 2005;19:1–26. [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Johnston VS, Wang XT. The relationship between menstrual phase and the P3 component of ERPs. Psychophysiology. 1991;28:400–409. doi: 10.1111/j.1469-8986.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38:175–178. [PubMed] [Google Scholar]
- Karis D, Fabiani M, Donchin E. P300 and memory - individual differences in the von Restorff effect. Cognitive Psychology. 1984;16:177–216. [Google Scholar]
- Karl A, Malta LS, Maercker A. Meta-analytic review of event-related potential studies in post-traumatic stress disorder. Biological Psychology. 2006;71:123–147. doi: 10.1016/j.biopsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke C, Nordby H, Hammerborg D, Hugdahl K, Erdmann G. Event-related potential (ERP) asymmetries to emotional stimuli in a visual half-field paradigm. Psychophysiology. 1997;34:414–426. doi: 10.1111/j.1469-8986.1997.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clinical Neurophysiology. 2001;112:2057–2068. doi: 10.1016/s1388-2457(01)00654-x. [DOI] [PubMed] [Google Scholar]
- Kok A, Zeef EJ. Arousal and effort - a review and theoretical synthesis of studies of age-related-changes in event-related potentials. Electroencephalography and Clinical Neurophysiology. 1991:324–341. [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff MA, Vighetto A, Bertrand O, Mauguiere F. Early amygdala reaction to fear spreading in occipital, temporal, and frontal cortex: A depth electrode ERP study in human. Neuron. 2004;42:665–676. doi: 10.1016/s0896-6273(04)00264-8. [DOI] [PubMed] [Google Scholar]
- Kugler CFA, Taghavy A, Platt D. The Event-Related P300 Potential Analysis of Cognitive Human Brain Aging - a Review. Gerontology. 1993;39:280–303. doi: 10.1159/000213544. [DOI] [PubMed] [Google Scholar]
- Kunst-Wilson WR, Zajonc RB. Affective discrimination of stimuli that cannot be recognized. Science. 1980;207:557–558. doi: 10.1126/science.7352271. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert B. International Affective Picture System (IAPS): Instruction manual and affective ratings. (Technical Report No. A-4) The Center for Research in Psychophysiology, University of Florida; Gainsville, Florida: 1999. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larsson LE. Correlation between the psychological significance of stimuli and the magnitudes of the startle blink and evoked EEG potentials in man. Acta Physiologica Scandinavia. 1960;48:276–294. doi: 10.1111/j.1748-1716.1960.tb01862.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Cognitive emotional interactions in the brain. Cognition & Emotion. 1989;3:267–289. Special Issue: Development of emotion cognition relations. [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annual Review of Psychology. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lifshitz K. The averaged evoked cortical response to complex visual stimuli. Psychophysiology. 1966;3:55–68. doi: 10.1111/j.1469-8986.1966.tb02680.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Öhman A. Emotion regulates attention: the relation between facial configurations, facial emotion, and visual attention. Visual Cognition. 2005;12:51–84. [Google Scholar]
- MacLean PD. Psychosomatic disease and the “visceral brain”: recent developments bearing on the Papez theory of emotion. Psychosomatic Medicine. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Some psychiatric implications of physiological studies on fronto-temporal portion of limbic system (visceral brain) Electroencephalography and Clinical Neurophysiology. 1952;4:407–418. doi: 10.1016/0013-4694(52)90073-4. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Sciences. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Martini P. Short-term memory for scenes with affective content. Journal of Vision. 2005;5:215–229. doi: 10.1167/5.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual spatial priming. Journal of Experimental Psychology-Human Perception and Performance. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA, Luck SJ. Electrocortical Substrates of Visual Selective Attention. Attention and Performance. 1993:219–243. [Google Scholar]
- Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher EM, Heinze HJ. Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Human Brain Mapping. 1997;5:273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Rugg MD. Electrophysiological correlates of the retrieval of emotional and non-emotional context. Journal of Cognitive Neuroscience. 2001;13:877–891. doi: 10.1162/089892901753165809. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Teder-Salejarvi WA, Di Russo F, Hillyard SA. Neural basis of auditory-induced shifts in visual time-order perception. Nature Neuroscience. 2005;8:1197–1202. doi: 10.1038/nn1512. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Emotional arousal and enhanced amygdala activity: new evidence for the old perseveration-consolidation hypothesis. Learning & Memory. 2005;12:77–79. doi: 10.1101/lm.93405. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiology of Learning and Memory. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Pfeifer E. Event-related potentials reveal topographical and temporal distinct neuronal activation patterns for spatial and object working memory. Cognitive Brain Research. 1996;4:211–224. doi: 10.1016/s0926-6410(96)00034-1. [DOI] [PubMed] [Google Scholar]
- Mini A, Palomba D, Angrilli A, Bravi S. Emotional information processing and visual evoked brain potentials. Perception and Motor Skills. 1996;83:143–152. doi: 10.2466/pms.1996.83.1.143. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the p3, and the locus coeruleus-norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Soares JJ. Emotional conditioning to masked stimuli: Expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. Journal of Experimental Psychology: General. 1998;127:69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Polich J. Affective visual event-related potentials: arousal, repetition, and time-on-task. Biological Psychology. 2007;75:101–108. doi: 10.1016/j.biopsycho.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, McCarthy G, Wood CC. ERPs predictive of subsequent recall and recognition performance. Biological Psychology. 1988;26:269–276. doi: 10.1016/0301-0511(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings in the Biological Sciences. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Foote SL, Neville HJ. Effects of locus coeruleus Lesions on Auditory, Long-latency, event-related potentials in monkey. Journal of Neuroscience. 1989;9:81–93. doi: 10.1523/JNEUROSCI.09-01-00081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. EEG and ERP assessment of normal aging. Evoked Potentials: Electroencephalography and Clinical Neurophysiology. 1997;104:244–256. doi: 10.1016/s0168-5597(97)96139-6. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and Biological Determinants of P300: an Integrative Review. Biological Psychology. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Kirsch W, Schandry R. On the relationship between interoceptive awareness, emotional experience, and brain processes. Cognitive Brain Research. 2005;25:948–962. doi: 10.1016/j.cogbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radilova J. The late positive component of visual evoked response sensitive to emotional factors. Activitas Nervosa Superior Supplement. 1982;3:334–337. [PubMed] [Google Scholar]
- Radilova J. P300 and emotional states studied by psychophysiological methods. International Journal of Psychophysiology. 1989;7:364–366. [Google Scholar]
- Radilova J, Figar S, Radil T. Sexual arousal and visual perception. Activitas Nervosa Superior. 1983;25:168–170. [Google Scholar]
- Roschmann R, Wittling W. Topographic brain mapping of emotion-related hemisphere asymmetries. International Journal of Neuroscience. 1992;63:5–16. doi: 10.3109/00207459208986656. [DOI] [PubMed] [Google Scholar]
- Rozenkrants B, Olofsson JK, Polich J. Affective visual event-related potentials: arousal, valence and repetition effects for normal and distorted pictures. International Journal of Psychophysiology. 2007 doi: 10.1016/j.ijpsycho.2007.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenkrants B, Polich J. IAPS and ERPs from an oddball task: strong arousal, weak valence, and minimal gender effects. 2007 Manuscript submitted for publication. [Google Scholar]
- Ruchkin DS, Canoune HL, Johnson R, Ritter W. Working-Memory and Preparation Elicit Different Patterns of Slow-Wave Event-Related Brain Potentials. Psychophysiology. 1995;32:399–410. doi: 10.1111/j.1469-8986.1995.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R, Mahaffey D, Sutton S. Toward a functional categorization of slow waves. Psychophysiology. 1988;25:339–353. doi: 10.1111/j.1469-8986.1988.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-related Brain Potentials and Cognition. Oxford University Press; New York: 1995. [Google Scholar]
- Sabatinelli D, Flaisch T, Bradley MM, Fitzsimmons JR, Lang PJ. Affective picture perception: gender differences in visual cortex? Neuroreport. 2004;15:1109–1112. doi: 10.1097/00001756-200405190-00005. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: Correlation of functional MRI and event-related potentials. Cerebral Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Schupp H, Flaisch T, Stockburger J, Junghöfer M. Anders S, Ende M, Junghöfer M, Kissler J, Wildgruber D, editors. Emotion and attention: event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer N, Lang PJ. Probe P3 and blinks: Two measures of affective startle modulation. Psychophysiology. 1997;34:1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Hillman CH, Hamm AO, Lang PJ. Brain processes in emotional perception: Motivated attention. Cognition & Emotion. 2004;18:593–611. [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003a;14:1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Emotional facilitation of sensory processing in the visual cortex. Psychological Science. 2003b;14:7–13. doi: 10.1111/1467-9280.01411. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Bublatzky F, Junghofer M, Weike AI, Hamm AO. Explicit attention interferes with selective emotion processing in human extrastriate cortex. Bmc Neuroscience. 2007;8 doi: 10.1186/1471-2202-8-16. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghofer M, Weike AI, Hamm AO. Selective visual attention to emotion. Journal of Neuroscience. 2007;27:1082–1089. doi: 10.1523/JNEUROSCI.3223-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Stimulus novelty and emotion perception: the near absence of habituation in the visual cortex. Neuroreport. 2006;17:365–369. doi: 10.1097/01.wnr.0000203355.88061.c6. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, VanRoon P, Dywan J. The ERP late positivity: a graduated response to stimulus repetition. Neuroreport. 1997;8:757–760. doi: 10.1097/00001756-199702100-00035. [DOI] [PubMed] [Google Scholar]
- Smith AP, Dolan RJ, Rugg MD. Event-related potential correlates of the retrieval of emotional and nonemotional context. J Cogn Neurosci. 2004;16:760–775. doi: 10.1162/089892904970816. [DOI] [PubMed] [Google Scholar]
- Smith DP, Hillman CH, Duley AR. Influences of age on emotional reactivity during picture processing. Journal of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:49–56. doi: 10.1093/geronb/60.1.p49. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: Electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41:171–183. doi: 10.1016/s0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Kutas M, Urbach TP, Altenmuller E, Münte TF. Combined perception of emotion in pictures and musical sounds. Brain Research. 2006;1070:160–710. doi: 10.1016/j.brainres.2005.11.075. [DOI] [PubMed] [Google Scholar]
- Squires KC, Donchin E, Herning RI, Mccarthy G. Influence of task relevance and stimulus probability on event-related-potential components. Electroencephalography and Clinical Neurophysiology. 1977;42:1–14. doi: 10.1016/0013-4694(77)90146-8. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Wood S, Kisley MA. The negativity bias is eliminated in older adults: Age-related reduction in event-related brain potentials associated with evaluative categorization. Psychology and Aging. 2006;21:815–820. doi: 10.1037/0882-7974.21.4.815. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Yee CM, Miller GA. Affective valence and information processing. Electroencephalography and Clinical Neurophysiology Supplement. 1987;40:300–307. [PubMed] [Google Scholar]