Abstract
Background
U.S. epidemiologic surveys have consistently found higher lifetime prevalence of alcohol dependence among younger subjects than among older groups. Because lifetime prevalence is cumulative, such patterns are suggestive of strong secular trends; i.e., more-recently born subjects have developed more disease in a shorter period of time than their elders. However, it remains unclear whether such patterns truly reflect secular trends or are confounded by age-dependent factors such as differential recall, differential mortality, and other effects.
Methods
Using data from two large, national epidemiological surveys, a repeated cross-sectional analysis was conducted to compare lifetime prevalence of alcohol dependence across temporally adjacent birth cohorts surveyed at the same age, thus enabling estimates of cross-cohort differences while controlling for age-related factors.
Results
In contrast with results from single cross-sectional analyses, there were few significant cross-cohort differences among groups of men compared at similar ages. On the other hand, women born between 1954 and 1963 were at 1.2 fold higher odds for lifetime drinking, and those who drank were at 1.5-fold higher odds for lifetime alcohol dependence, compared with the immediately preceding birth cohort (1944–53). The 1944–53 cohort was also at elevated odds for lifetime drinking compared with their predecessors (1934–43). These results were largely due to changes among White and Hispanic women.
Conclusions
These results suggest that there have been substantial secular increases in drinking and alcohol dependence among women, but not men. Analyses of single cross-sectional studies may tend to over-estimate secular trends by failing to account for age-dependent effects. Nonetheless, secular increases in drinking and alcohol dependence among women are evident after taking age-related factors into account.
Keywords: alcohol dependence, prevalence, secular trends, race, women
Introduction
Cross-sectional epidemiologic studies of the U.S. population have consistently documented higher lifetime prevalence of alcohol dependence among younger age cohorts than among older groups. For example, in the National Longitudinal Alcohol Epidemiologic Survey (NLAES), conducted in 1991–92, the prevalence of lifetime alcohol dependence using DSM-IV criteria was 19.0% among 25–34 year olds, compared with 12.1% among 45–54 year olds. Moreover, the youngest (18–24 year) age cohort reported rates as high as the 25–34 year olds, despite the fact that they had not aged completely through the period of risk for development of alcohol dependence (Grant, 1997). Qualitatively similar trends were observed in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a survey conducted ten years later (2001–02), which utilized methods very similar to those used in the NLAES (Hasin and Grant, 2004). The National Comorbidity Survey, conducted in 1991–92, also reported higher lifetime prevalence among younger age cohorts, using DSM-III-R lifetime diagnostic criteria (SAMHDA; 2007; Kessler et al., 1994). Patterns such as these have also been noted in a study of relatives of alcohol dependent probands (Rice et al., 2003) and in an earlier study of three sites in the Epidemiologic Catchment Area survey (ECA; Robins et al., 1984). Because lifetime prevalence is cumulative; i.e., an individual is counted as having the disorder even if in remission, these patterns suggest strong secular trends because younger people report higher level of disorder than older subjects despite having had less time to develop the disease. However, results from cross-sectional surveys have generally been interpreted with caution because of the possibility that the apparent epidemic might stem from age-dependent recall effects or other measurement issues.
Further complicating the interpretation of lifetime prevalence estimates reported from various ages, a number of reports have expressed skepticism about the validity of survey prevalence estimates of alcohol dependence among younger people. After reviewing findings from two studies, Caetano and Babor pointed out that certain criteria are disproportionately endorsed by young adults and cautioned that survey instruments might not sufficiently differentiate consequences of occasional heavy drinking, such as hangovers and the development of normal tolerance, from symptoms of alcohol dependence, such as extremely high tolerance and severe withdrawal symptoms (Caetano and Babor, 2006; Harford et al., 2005; Ungemack et al., 2001). They argued that young adults who experience relatively minor consequences of heavy drinking episodes might over-report symptoms, leading to inflated prevalence estimates for younger cohorts. Furthermore, secondary analysis of data from the ECA showed that the tendency for older subjects to report fewer problems was observed across all psychiatric disorders and symptoms, including alcohol dependence, which suggested a general age-related recall or response effect, rather than actual secular changes in specific disorders (Simon and VonKorff, 1992).
One method of controlling for age-related effects is to analyze cross-sectional surveys conducted at different points in time, and to compare groups of subjects from different birth cohorts surveyed at the same age (Firebaugh, 1997; Menard, 1991). If the cross-sectional surveys utilize the same disease definition and sample the same target population, then differences between similarly aged birth cohorts are more likely to reflect true secular change in disease patterns rather than age-related reporting artifacts or other age-dependent effects. This repeated cross-sectional approach is analogous to a retrospective cohort design in which comparison groups are defined on the basis of time-of-survey, which is equivalent to the “exposure”, and compared on the basis of disease outcome, which is the dependent variable (Aschengrau and Seage, 2003). In this paper, we utilize data from the NESARC (2001–02) and the NLAES (1991–92) to accomplish such an analysis; the two cross-sectional samples were ascertained ten-years apart and both are representative of the U.S. residential population. In contrast to analyses of single cross-sectional surveys, in which age and year-of-birth are perfectly correlated, the repeated cross-sectional analysis allows comparison of cohorts born at different times while controlling for age at interview. Our goal was to determine whether cross-cohort differences in lifetime prevalence of drinking and of alcohol dependence among drinkers would be observed when comparing similarly aged, temporally adjacent birth cohorts from two surveys, conducted a decade apart.
Methods
Subjects
Survey Description
The National Longitudinal Alcohol Epidemiologic Survey (NLAES) was conducted in 1991 and 1992; the National Epidemiological Survey on Alcohol and Related Conditions (NESARC) was conducted in 2001 and 2002. There were many methodological similarities between the two surveys, including the sampling universe and instrumentation used to assess alcohol dependence and other disorders. Both surveys focused on alcohol and drug use, DSM-IV substance use disorders, associated impairment and comorbid disorders in samples representative of the adult (18 and older), non-institutionalized, civilian population of the United States. Blacks were oversampled in both surveys and Hispanics were oversampled in the NESARC. Face-to-face interviews were administered by experienced lay interviewers from the U.S. Census Bureau. Respondents were informed about measures taken to ensure the confidentiality of the information they provided and informed consent was obtained from all subjects. Ethical review and approval of all procedures was conducted by the U.S. Census Bureau and U.S. Office of Management and Budget. The final NESARC sample consisted of 43,093 persons; overall raw response rate was 81%. The final NLAES sample consisted of 42,862 persons with a response rate of 90%. Further details for both surveys, and comparative descriptions of methods are available elsewhere (Compton et al., 2004; Grant, 1997; Grant et al., 2004; Grant et al., 2003).
Analytical Design
Because of similarities in sampling universe, definitions of outcome variables, and other methodological characteristics, simultaneous analysis of the NLAES and NESARC constitutes a repeated cross-sectional analysis. When subjects from the NESARC, conducted in 2000–01, are grouped by age and compared with subjects of the same age range from the NLAES, conducted in 1990–91, the primary difference between the two is the range of birth years, which differs by ten years (See Figure 1). The present analyses focus on the subset of subjects aged 18 to 57 at the time of survey (see below for rationale in determining the upper age limit). Hence, cohorts born between 1944 and 1973 were represented in both surveys, while those born between 1974 and 1983 were represented in the NESARC only, and those born between 1934 and 1943 are represented in the NLAES only.
Figure 1.
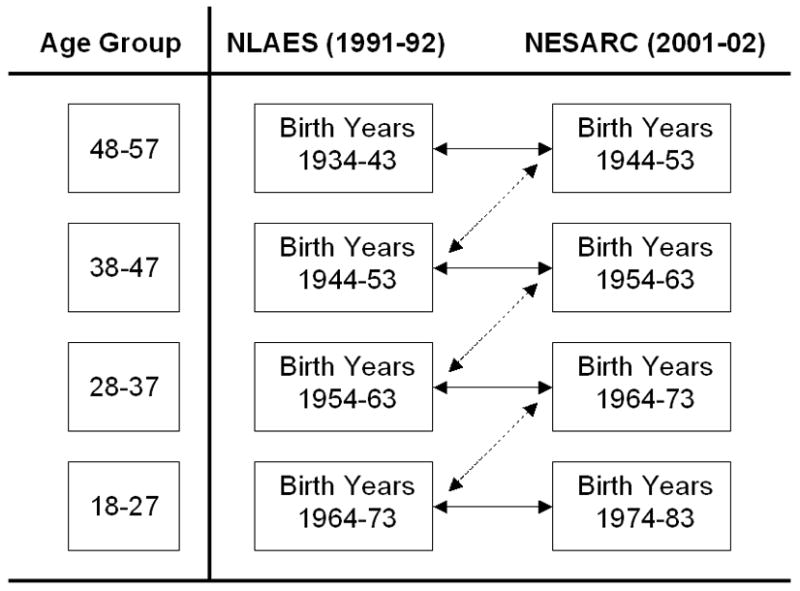
Schematic illustration of cross-survey comparisons. Dashed line corresponds to comparisons of the same birth cohort at different ages (Intra-cohort comparisons, Table 1), which assesses the magnitude of age-related effects on reported prevalence. Solid lines correspond to age-matched comparisons of temporally adjacent birth cohorts (Cross-cohort comparisons, Tables 2 and 3), which are used to provide data on differences in lifetime prevalence between birth cohorts, while controlling for the effects of age by comparing similarly aged cohorts.
Cross-survey comparisons in repeated cross-sectional analyses can be confounded by population change between surveys (Ware, 1985); sources of population change include in-migration, out-migration and differential mortality. To limit the effects of in-migration, analyses were limited to U.S. born subjects Out-migration can be considered to be statistically negligible for U.S. born adults (population loss of ~0.02% per year; Fernandez, 1995; Lauderdale, 2001).
One of the objectives of this study is to determine whether the lifetime prevalence of alcohol dependence changes with age in a given birth cohort. Change in cohort composition due to alcohol-related mortality is a potential confounder for these analyses. The decision to limit analyses to subjects aged 57 and younger at the time of the NESARC serves to mitigate the potential effects of differential mortality due to alcohol dependence on cohort composition; differential mortality is addressed more fully in the Discussion section.
Some reports suggest differences in secular trends by sex and race/ethnicity (Holdcraft and Iacono, 2002, Caetano and Clark, 1998). Hence, birth cohorts were stratified by sex and race/ethnicity to examine whether any secular changes would differ by these variables. Men and women were examined separately for all analyses, comparisons were first conducted with all racial/ethnic groups combined, and subsequently on groups stratified by race/ethnicity.
Measures
Diagnostic Measures
Outcome variables in these analyses include lifetime drinking and lifetime alcohol dependence. Subjects were considered to be lifetime drinkers if they reported ever having consumed 12 or more alcoholic drinks in a single year. Lifetime alcohol dependence in the NLAES and NESARC was assessed with the Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV version (AUDADIS-IV, Grant et al., 2001) which covers DSM-IV substance use syndromes for past 12-month and life time frames. To minimize the effect of changes to the AUDADIS-IV interview between the NLAES and NESARC, we derived a diagnostic algorithm that maximized overlap between the two administrations and eliminated items appearing in one survey questionnaire, but not the other (2 NLAES items and 1 NESARC item). Items incorporated into the algorithm assessed all seven DSM-IV diagnostic criteria and whether or not symptoms clustered together in a given year (analyses were also run using the diagnoses as coded by the survey administrators; results were not significantly changed).
Additional Variables
Race/ethnicity was assessed by self-report. Analyses stratified by race/ethnicity were limited to subjects whose racial/ethnic membership could be categorized as White, Black, or Hispanic because of low subsample sizes for other racial/ethnic groups. Subjects were categorized into ten-year age groups (18–27, 28–37, 38–47, and 48–57); this allows comparison of adjacent, non-overlapping birth cohorts across surveys, while minimizing the loss of statistical power due to stratification. The sample sizes for the present analyses were 27,485 for the NLAES and 25,384 for the NESARC. Subjects not born in the U.S. (n = 4,401/7,471) and those ages 58 and older (n = 10,976/10,238) were excluded from the analyses.
Statistical Analyses
All statistical analyses were conducted using the SUDAAN statistical software package (RTI International, 2004). Variance estimation utilized a Taylor linearization method appropriate for the complex design of each survey. Significance of between-survey differences in prevalence estimates were assessed using two-sample Z-tests. Chi-square tests were used to assess significance of within-survey age-cohort effects.
Reported P-values reflect comparison-wise error rates; i.e., no adjustment for multiple testing was introduced (Bender and Lange, 2001). This decision was made because Type-I (false positive) and Type-II (false negative) errors were of equal concern, given the study aims. Standard errors and annotations for unadjusted p-values are presented for all comparisons, so that readers may evaluate both the public health relevance and nominal statistical significance of each comparison (Feise, 2002; Perneger, 1998; Rothman, 1990).
Results
Cross-survey comparisons
A schematic illustration of the analytical approach for cross-survey comparisons is provided in Figure 1. In order to evaluate the magnitude of the influence of age on reported prevalence of alcohol dependence, equivalent birth cohorts from the two surveys were compared; these intra-cohort comparisons correspond to the dashed arrows in Figure 1. Comparison of temporally adjacent NLAES and NESARC birth cohorts at similar ages are illustrated by the solid arrows in Figure 1. These cross-cohort comparisons were conducted to evaluate changes that are primarily due to birth cohort differences, rather than confounded age-related effects.
Lifetime Prevalence of Alcohol Dependence by Age (Within Survey Comparisons)
Table 1 lists the prevalence of lifetime alcohol dependence from the NLAES and the NESARC, stratified by age group. Within-survey (column-wise) comparisons of lifetime prevalence of alcohol dependence in Table 1 confirm that, in both surveys, there was a strong association between age and lifetime prevalence, such that younger age groups report higher lifetime prevalence (Grant, 1997; Hasin and Grant, 2004).
Table 1.
Lifetime Prevalence of DSM-IV Alcohol Dependence by Birth Cohort and Survey
| NLAES (1990–91)
|
NESARC (2000–01)
|
Intra- Cohort Differencea |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Birth Years | Age in 1991 | N | % | (SE) | Age in 2001 | N | % | (SE) | |
| Birth Cohort | |||||||||
| 1974–1983 | ------ | ------ | ------ | ------ | 18–27 | 5,822 | 18.7 | (0.7) | ------ |
| 1964–1973 | 18–27 | 6,882 | 19.5 | (0.6) | 28–37 | 6,585 | 17.8 | (0.7) | −9%+ |
| 1954–1963 | 28–37 | 9,023 | 18.0 | (0.5) | 38–47 | 7,181 | 16.4 | (0.6) | −9%* |
| 1944–1953 | 38–47 | 7,017 | 13.9 | (0.5) | 48–57 | 5,796 | 12.0 | (0.6) | −14%* |
| 1934–1943 | 48–57 | 4,563 | 9.7 | (0.5) | ------ | ------ | ------ | ------ | ------ |
| Total (1943–1973) | 18–47 | 22,922 | 17.2 | (0.3) | 28–57 | 19,562 | 15.5 | (0.4) | −12%** |
| χ2b | 65*** | 45*** | |||||||
Note: Difference between 2000–01 (NESARC) and 1990–91 (NLAES) lifetime prevalence estimates for a given birth cohort.
χ2 for association between birth cohort and lifetime prevalence within each survey. +p < 0.10
p < 0.05
p < 0.01
p < 0.001
Intra-Cohort Comparisons
Comparisons of equivalent birth cohorts across the two surveys with respect to lifetime prevalence of alcohol dependence are also made in Table 1, across rows. These analyses compare the lifetime prevalence of alcohol dependence of each birth cohort in the NLAES with the same cohort in the NESARC, conducted ten years later (dashed line comparisons in Figure 1). Because lifetime alcohol dependence is a cumulative diagnosis, and we have controlled for the effects of population change, new cases of alcohol dependence that developed between the NLAES and the NESARC could only contribute to positive changes in lifetime prevalence for a birth cohort. As shown in Table 1, however, all changes in prevalence are negative. Overall, lifetime prevalence for the 1944–1973 cohorts decreased by 12.0% in the NESARC compared to the NLAES (p < 0.01). Hence, these analyses demonstrate that within a given birth cohort, the reported lifetime prevalence of alcohol dependence is reduced as the cohort ages. The primary sources of negative changes in prevalence with time would be recall bias, other measurement error, and differential mortality.
Cross-Cohort Comparisons
Birth cohorts from the NESARC were categorically matched with similarly-aged birth cohorts from the NLAES in order to determine whether cross-cohort differences in lifetime prevalence of (1.) drinking and (2.) alcohol dependence among lifetime drinkers would remain after controlling age-related factors. Same-aged subgroups from the NLAES and NESARC correspond to temporally adjacent birth cohorts whose birth year range differs by ten years.
Results of cross-cohort comparisons of male subgroups are presented in Table 2 for both outcomes (lifetime drinking and lifetime alcohol dependence among lifetime drinkers); few cross-cohort differences were observed. The exceptions were for 48–57 year old men unstratified by race/ethnicity, and 18–27 year old White men. The former group had a higher lifetime prevalence of drinking in the NESARC than the NLAES (OR = 1.3, Z = 2.29, p = 0.024), suggesting an increase in drinking for those born between 1944 and 53, relative to those in the preceding cohort (1934–1943). No significant differences were observed in lifetime prevalence of alcohol dependence among lifetime drinkers in this group. The other significant difference was that 18–27 year old White males had a lower lifetime prevalence of drinking in the NESARC than in the NLAES (OR = 0.8, Z = 2.27, p = 0.02), suggesting a slight decrease in drinking for the most recent birth cohort of White men (1974–83), compared with their 1964–73 predecessors. While these may reflect genuine cross-cohort differences, it should be noted that Table 2 comprises 24 independent comparisons; accordingly these p-values may reflect false-positive results.
Table 2.
Cross-Cohort Comparisons of Lifetime Prevalences of Drinking and DSM-IV Alcohol Dependence -- Men
| Drinking
|
Alcohol Dependence Among Drinkers
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth Years
|
N
|
NLAES
|
NESARC
|
NLAES
|
NESARC
|
|||||
| Age | NLAES | NESARC | NLAES | NESARC | % (SE) | % (SE) | OR‡ | % (SE) | % (SE) | OR‡ |
| All Men | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 3,045 | 2,539 | 76.4 (0.9) | 74.1 (1.1) | 0.9 | 32.7 (1.1) | 33.1 (1.4) | 1.0 |
| 28–37 | 1954–63 | 1964–73 | 3,709 | 2,716 | 85.8 (0.7) | 84.2 (0.9) | 0.9 | 27.6 (0.9) | 27.0 (1.2) | 1.0 |
| 38–47 | 1944–53 | 1954–63 | 3,061 | 3,274 | 86.1 (0.8) | 84.5 (0.9) | 0.9 | 24.4 (1.0) | 26.1 (1.1) | 1.1 |
| 48–57 | 1934–43 | 1944–53 | 2,010 | 2,596 | 80.3 (1.1) | 83.8 (1.1) | 1.3* | 18.0 (1.1) | 20.5 (1.1) | 1.2 |
| Whites | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 2,378 | 1,479 | 80.0 (0.9) | 76.3 (1.4) | 0.8* | 33.8 (1.3) | 35.3 (1.7) | 1.1 |
| 28–37 | 1954–63 | 1964–73 | 3,038 | 1,741 | 87.8 (0.7) | 85.8 (1.1) | 0.8 | 28.5 (1.0) | 28.1 (1.4) | 1.0 |
| 38–47 | 1944–53 | 1954–63 | 2,539 | 2,269 | 87.6 (0.8) | 85.7 (1.0) | 0.8 | 24.2 (1.1) | 26.1 (1.2) | 1.1 |
| 48–57 | 1934–43 | 1944–53 | 1,677 | 1,796 | 81.6 (1.1) | 84.3 (1.2) | 1.2 | 17.4 (1.2) | 20.0 (1.2) | 1.2 |
| Blacks | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 434 | 467 | 58.0 (2.9) | 63.1 (2.9) | 1.2 | 22.1 (3.2) | 19.9 (3.1) | 0.9 |
| 28–37 | 1954–63 | 1964–73 | 418 | 507 | 72.6 (2.7) | 76.4 (2.3) | 1.2 | 21.8 (2.8) | 18.8 (2.0) | 0.8 |
| 38–47 | 1944–53 | 1954–63 | 352 | 578 | 77.9 (2.8) | 77.6 (2.2) | 1.0 | 21.2 (2.8) | 23.3 (2.1) | 1.1 |
| 48–57 | 1934–43 | 1944–53 | 243 | 513 | 70.1 (4.0) | 77.3 (2.2) | 1.4 | 24.5 (4.0) | 19.2 (2.3) | 0.7 |
| Hispanics | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 175 | 532 | 72.6 (3.7) | 73.2 (2.7) | 1.0 | 36.5 (4.8) | 31.4 (3.4) | 0.8 |
| 28–37 | 1954–63 | 1964–73 | 174 | 429 | 83.8 (3.4) | 80.9 (2.7) | 0.8 | 25.6 (4.1) | 26.8 (3.5) | 1.1 |
| 38–47 | 1944–53 | 1954–63 | 115 | 387 | 80.8 (4.6) | 82.8 (2.5) | 1.1 | 30.3 (5.4) | 31.4 (4.3) | 1.1 |
| 48–57 | 1934–43 | 1944–53 | 60 | 250 | 88.0 (4.6) | 87.8 (2.6) | 1.0 | 21.2 (6.0) | 21.9 (4.5) | 1.0 |
Notes: Odds Ratio for NESARC relative to NLAES; corresponds to OR for birth years included in NESARC age group (third column), relative to the preceding birth cohort, which is represented in the NLAES (second column).
p < 0.05,
p < 0.01,
p < 0.001.
Cross-cohort comparisons between categorically matched subsamples of women from the NLAES and NESARC are made in Table 3. In contrast with men, a number of highly significant differences between birth cohorts were observed. In the combined race/ethnicity comparisons, women aged 48–57 (born between 1944 and 1953) in the NESARC had a higher lifetime prevalence drinking (OR = 1.3, Z = 3.29, p < 0.001) than those in the NLAES (born between 1934 and 1943). The next youngest cohort was at higher risk for both outcomes; 38–47 year old women in the NESARC (born between 1954 and 1963) had higher risk than their NLAES counterparts (born 1944–1953) for lifetime drinking (OR = 1.2, Z = 2.91, p = 0.0037), and had 1.5-fold higher odds for lifetime alcohol dependence among lifetime drinkers (Z = 4.18, p < 0.001). In summary, women born between 1944 and 1953 had higher lifetime prevalence of drinking than the preceding birth cohort (based on the 48–57 year old comparisons), and the following birth cohort, born between 1954 and 1963, had even higher lifetime rates of drinking and higher lifetime prevalence of alcohol dependence among those who reported lifetime drinking compared with the preceding birth cohort (based on the 38–47 year comparisons). No further increases were seen for more recent birth cohorts, but these groups were at equally high risk as the 1954–63 group; that is, after the elevation in risk observed for the 1954–63 group, there was no return to the baseline level of risk for later cohorts.
Table 3.
Cross-Cohort Comparisons of Lifetime Prevalences of Drinking and DSM-IV Alcohol Dependence -- Women
| Drinking
|
Alcohol Dependence Among Drinkers
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth Years
|
N
|
NLAES
|
NESARC
|
NLAES
|
NESARC
|
|||||
| Age | NLAES | NESARC | NLAES | NESARC | % (SE) | % (SE) | OR‡ | % (SE) | % (SE) | OR‡ |
| All Women | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 3,837 | 3,283 | 63.3 (0.9) | 61.0 (1.3) | 0.9 | 22.4 (1.0) | 21.3 (1.1) | 0.9 |
| 28–37 | 1954–63 | 1964–73 | 5,314 | 3,869 | 69.3 (0.8) | 70.2 (1.1) | 1.0 | 18.2 (0.7) | 18.8 (1.0) | 1.0 |
| 38–47 | 1944–53 | 1954–63 | 3,956 | 3,907 | 64.2 (1.0) | 68.5 (1.1) | 1.2** | 11.1 (0.7) | 16.0 (1.0) | 1.5*** |
| 48–57 | 1934–43 | 1944–53 | 2,553 | 3,200 | 56.7 (1.2) | 62.6 (1.1) | 1.3*** | 9.3 (0.9) | 11.2 (0.9) | 1.2 |
| Whites | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 2,695 | 1,762 | 71.1 (1.0) | 66.3 (1.5) | 0.8** | 23.4 (1.1) | 22.0 (1.4) | 0.9 |
| 28–37 | 1954–63 | 1964–73 | 3,955 | 2,213 | 74.0 (0.8) | 74.7 (1.2) | 1.0 | 18.4 (0.8) | 19.3 (1.1) | 1.1 |
| 38–47 | 1944–53 | 1954–63 | 3,146 | 2,408 | 67.4 (1.1) | 71.9 (1.3) | 1.2** | 10.9 (0.8) | 15.8 (1.1) | 1.5*** |
| 48–57 | 1934–43 | 1944–53 | 2,017 | 2,071 | 58.7 (1.4) | 64.9 (1.3) | 1.3*** | 8.9 (1.0) | 11.2 (1.0) | 1.3 |
| Blacks | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 758 | 780 | 30.7 (1.9) | 40.9 (2.0) | 1.6*** | 11.5 (2.3) | 13.8 (2.5) | 1.2 |
| 28–37 | 1954–63 | 1964–73 | 991 | 973 | 47.6 (2.0) | 53.7 (2.1) | 1.3* | 15.6 (2.1) | 12.5 (1.9) | 0.8 |
| 38–47 | 1944–53 | 1954–63 | 608 | 956 | 46.2 (2.5) | 50.7 (2.0) | 1.2 | 12.2 (2.3) | 13.4 (1.9) | 1.1 |
| 48–57 | 1934–43 | 1944–53 | 401 | 801 | 44.5 (3.3) | 50.0 (2.4) | 1.2 | 9.2 (2.4) | 10.2 (1.9) | 1.1 |
| Hispanics | ||||||||||
| 18–27 | 1964–73 | 1974–83 | 280 | 675 | 52.7 (3.4) | 55.7 (3.5) | 1.1 | 15.1 (3.3) | 24.6 (3.2) | 1.8* |
| 28–37 | 1954–63 | 1964–73 | 253 | 596 | 57.8 (3.4) | 64.7 (3.1) | 1.3 | 18.3 (3.6) | 21.7 (3.0) | 1.2 |
| 38–47 | 1944–53 | 1954–63 | 130 | 495 | 54.3 (5.3) | 64.4 (3.6) | 1.5 | 7.6 (2.8) | 21.5 (3.8) | 3.3** |
| 48–57 | 1934–43 | 1944–53 | 91 | 285 | 48.7 (6.1) | 56.7 (3.8) | 1.4 | 9.5 (4.0) | 15.7 (3.9) | 1.8 |
Notes: Odds Ratio for NESARC relative to NLAES; corresponds to OR for birth years included in NESARC age group (third column), relative to the preceding birth cohort, which is represented in the NLAES (second column).
p < 0.05,
p < 0.01,
p < 0.001.
Inspection of the results for women stratified by race/ethnicity shows that the overall differences in the 38–47 and 48–57 year old comparisons correspond largely to changes among White and Hispanic women; no significant differences were seen across surveys for Black women in these age groups. Additional significant differences for specific race/ethnicity groups were observed. The youngest group of White women in the NESARC (birth years 1974–1983) had lower lifetime rates of drinking than those in the NLAES (OR = 0.8, Z = 2.6, p = 0.009), while the youngest group of Black women (ages 18–27 in the NESARC) had higher lifetime rates of drinking than their predecessors (OR = 1.6, Z = 3.7, p < 0.001). Notably, however, young Black women still have a much lower lifetime prevalence of drinking (40.9%) than their male or non-Black counterparts. Finally, the youngest cohort of Hispanic women (1974–83) had higher lifetime rates of alcohol dependence among drinkers than their immediate predecessors in the NLAES (1964–73, OR = 1.8, Z = 2.0, p = 0.04). Though the significance is marginal due to the small sub-sample size, further research into the drinking patterns of this demographic group may be warranted.
Discussion
While epidemiologic surveys in the U.S. have consistently found higher lifetime prevalence of alcohol dependence among younger subjects than among older groups, these results have generally been interpreted cautiously because such analyses necessarily compare retrospectively reported behaviors among different birth cohorts reporting from vastly different ages. In this report, we control for the influence of age-related factors by comparing subgroups of subjects from two different surveys, conducted ten years apart, categorically matched on the basis of age and other demographic factors. This approach allowed different birth cohorts to be compared at the same age. Among women, significant increases in the lifetime prevalence of drinking were observed for the 1944–53 birth cohort, compared to their immediate predecessor (1934–43, see 48–57 year old comparisons, Table 3). There was a further increase in the lifetime prevalence of drinking and, among drinkers, there was an increase in lifetime prevalence of alcohol dependence for the following birth cohort (1954–1963; see 38–47 year old comparisons). Hence, after controlling for age-related factors, secular changes in alcohol dependence for women remain significant. Among men, however, only a few, marginally significant differences in drinking or alcohol dependence were observed when temporally adjacent birth cohorts were compared, suggesting that these outcomes have remained fairly constant across the birth cohorts of men sampled in these surveys.
The findings in women and patterns by race/ethnicity can be summarized as follows: an increase in risk for drinking and alcohol dependence among drinkers has apparently taken place beginning with the first cohorts born after World-War II, with no reduction to earlier baseline levels observed for more recent birth cohorts. The changes seem to have affected White and Hispanic women, but not Black women. We also note a marginally significant, but potentially substantial increase in alcohol dependence for Hispanic women born between 1974 and 1983, the most recent birth cohort sampled in the two surveys, compared to their predecessors. While caution is in order due to low statistical power for this comparison, further monitoring of trends in this demographic group are clearly in order. Likewise, elevated rates of drinking were seen in the youngest cohort of Black women, compared to their predecessors.
These findings extend the current literature in several ways. First, by taking an analytical approach that has not been previously applied to secular analyses of alcohol dependence, we have shown that secular increases in lifetime alcohol dependence for women remain significant after adjusting for age-related effects, but that few secular changes among men are apparent after accounting for age-related effects. These findings confirm previous reports that have suggested a narrowing of the gender gap in alcohol dependence (Greenfield et al., 2003; Holdcraft and Iacono, 2002) while showing that the increase among women is observed primarily among Whites and Hispanics. Additionally, we have shown that the secular trends suggested by analyses of single cross-sectional epidemiological surveys are likely to be confounded by age-related factors, such that the increase in risk for more recently born cohorts has been overestimated. This finding underscores the importance of controlling for such effects as has been done here.
Inverse Association Between Age and Lifetime Prevalence of Alcohol Dependence
The importance of considering age-related factors when estimating secular trends is demonstrated by the cross-survey comparisons of subjects from the same birth cohorts, who, by design, have different age ranges in the two surveys. Cohorts in the NESARC consistently reported 9–14% lower prevalence of lifetime alcohol dependence than equivalent birth cohorts in the earlier NLAES. Because lifetime alcohol dependence is a cumulative diagnosis, and we controlled for population change by limiting analyses to U.S. born subjects, the major contributors to decreased lifetime prevalence of alcohol dependence within a cohort over time are expected to be differential mortality and age-dependent measurement error. Differential mortality is likely to play a role in the within-cohort reduction in lifetime prevalence of alcohol dependence between surveys, but is unlikely to account for the majority of the change. The estimated number of U.S. deaths attributable to excessive alcohol use in 2001 was 75,000 or 0.025% of the population (Midanik et al., 2004). This corresponds to about 0.25% of the population over ten years. According to Table 1, the population reports 12% fewer cases of alcohol dependence after aging ten years; the number of cases lost corresponds to 2.1% of the total population. Hence, even under a “worst case” assumption in which alcohol-related mortality effects cases only, differential mortality can account for only a minor proportion of the total reduction in reported cases.
Although the results presented here cannot be used to determine the specific reasons that cohort prevalence declines with age, systematic measurement error, such as recall or response bias, is one likely source. Such measurement error could reflect a number of factors. Older subjects may under-report remitted symptoms due to recall problems or response bias and may, in general, have a different perspective on symptom reporting. Also, as suggested elsewhere, younger subjects may misinterpret survey questions so as to report consequences of occasional heavy drinking episodes as symptoms of alcohol dependence. Hence, over-reporting of alcohol dependence symptoms among younger subjects is also a possibility (Caetano and Babor, 2006).
Increase in Alcohol Dependence Risk for Women
Altogether, these data suggest that there has been a substantial change in the demographic distribution of drinking and alcohol dependence. The increase in prevalence among White and Hispanic women contributes to a substantial narrowing in the gap between male and female alcohol dependence. These findings have important implications for physicians because female alcoholics are under-identified in primary care settings, with under-identification in older women being especially problematic (CASA), 1998; Brienza and Stein, 2002; Green, 2006). Furthermore, women perceive more stigmatization and face greater barriers to treatment than men with alcohol dependence, and may be under-represented in specialized treatment settings (Brady and Ashley, 2005; Green, 2006.; Thom, 1987) This is particularly disturbing because women with alcohol problems face more severe health-related consequences and possibly more years of life lost than their male counterparts (Bradley et al., 1998; Jarque-Lopez et al., 2001; Smith et al., 1994). In addition to improved treatment access, primary prevention efforts directed toward young women are also needed.
Why have lifetime drinking and alcohol dependence prevalence in women become closer to those for men? It may be that through entry into the work force and other steps toward gender equity, cultural and economic conditions for women born after World War II in the U.S. more closely resemble those of men. Indeed, such factors have been invoked to explain the narrowing gender gap found in other studies (Holdcraft and Iacono, 2002). Identification of specific environmental factors responsible for these changes is of interest for the rational design of prevention strategies. Among the myriad candidates are greater economic power for women, facilitating purchasing power for alcohol, (Becker and Murphy, 1999), the correlation between drinking behavior and occupational subcultures (Ames and Rebhun, 1996), and changes in women’s attitudes toward alcohol in the post-prohibition era (Murdock, 1998). As a result of potentially numerous social and cultural changes, the stigma associated with drinking behavior among women may have been reduced in recent years. Unfortunately, a corresponding reduction in barriers to alcoholism treatment has apparently not been realized.
Limitations
Although this report extends the existing literature in several ways, there are a number of limitations to these results. One such limitation is that the two surveys analyzed used similar methods; while this is necessary for the types of analyses conducted here, it also means that any method-specific biases will be common to both surveys. Ideally, these findings should be replicated with data collected using a variety of methods. In addition, we were not able to examine minority groups other than from Blacks and Hispanics, due to low statistical power for stratified groups. Finally, the exclusion of older subjects due to mortality concerns, and the unavailability of data on younger subjects limits the window of birth years that could be analyzed here, making it difficult to interpret our findings in a broader historical context.
Conclusions
National epidemiological surveys in the U.S. have consistently shown an inverse association between age and the lifetime prevalence of alcohol dependence, but whether such trends are confounded by age-related recall or response effects has been unclear. By comparing temporally adjacent birth cohorts from two such surveys, while controlling for age and other demographic factors through category-matching, we have shown that important differences between birth cohorts remain after matching for age, but that such differences are limited to women. The largest change detected was that women born after 1953 were at higher risk for drinking, and those who drank had higher risk for alcohol dependence, compared with earlier birth cohorts. These differences were observed among White and Hispanic women, but not Black women. Analyses of single cross-sectional studies may tend to over-estimate secular trends by failing to account for such age-dependent effects.
Acknowledgments
Analysis and manuscript preparation were supported by NIH-K01DA16618 (RAG), NIH AA12640, DA14363, AA11998 (KKB); NIH-U10AA08401, K02-DA021237 (LJB). NLAES and NESARC data were obtained from CSR, Incorporated and the NIAAA, (http://niaaa.census.gov), respectively. The author’s have no financial interest in this work. We are grateful to Dr. Karen Norberg for helpful suggestions in the final revisions of this manuscript.
Support: NIH-K01DA16618 (RAG), AA12640, DA14363, AA11998 (KKB), U10AA08401 (LJB).
References
- Ames GM, Rebhun LA. Women, alcohol and work: interactions of gender, ethnicity and occupational culture. Soc Sci Med. 1996;43(11):1649–63. doi: 10.1016/s0277-9536(96)00081-0. [DOI] [PubMed] [Google Scholar]
- Aschengrau A, Seage GR. Essentials of Epidemiology for Public Health. Jones & Bartlett; Boston, MA: 2003. [Google Scholar]
- Becker GS, Murphy KM. A Theory of Rational Addiction. The economics of corruption and illegal markets Volume 3. The economics of illegal markets and organized crime. 1999:401–26. [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing--when and how? J Clin Epidemiol. 2001;54(4):343–9. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Badrinath S, Bush K, Boyd-Wickizer J, Anawalt B. Medical risks for women who drink alcohol. J Gen Intern Med. 1998;13:627–39. doi: 10.1046/j.1525-1497.1998.cr187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady TM, Ashley OS. Women in Substance Abuse Treatment: Results from the Alcohol and Drug Services Study (ADSS) Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2005. [Google Scholar]
- Brienza RS, Stein MD. Alcohol use disorders in primary care: do gender-specific differences exist? J Gen Intern Med. 2002;17:387–97. doi: 10.1046/j.1525-1497.2002.10617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano R, Clark CL. Trends in alcohol-related problems among whites, blacks, and Hispanics: 1984–1995. Alcohol Clin Exp Res. 1998;22(2):534–8. [PubMed] [Google Scholar]
- Caetano R, Babor TF. Diagnosis of alcohol dependence in epidemiological surveys: an epidemic of youthful alcohol dependence or a case of measurement error? Addiction. 2006;101(Suppl 1):111–4. doi: 10.1111/j.1360-0443.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- CASA: National Center on Addiction and Substance Abuse at Columbia University, New York (1998) Under the Rug: Substance Abuse and The Mature Woman.
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E. Estimation of the annual emigration of US born persons by using foreign censuses and selected administrative data: Circa 1980. US Bureau of the Census; Washington, DC: 1995. [Google Scholar]
- Firebaugh G. Analyzing Repeated Surveys. Sage; Thousand Oaks, CA: 1997. [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–73. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Hasin DS. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-DSM-IV Version. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2001. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004a;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Moore TC, Shepard J, Kaplan K. Source and Accuracy Statement: Wave 1 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) National Institute on Alcohol Abuse and Alcoholism; Bethesda, Md: 2003. [Google Scholar]
- Green CA. Gender and use of substance abuse treatment services. Alcohol Res Health. 2006;29:55–62. [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Manwani SG, Nargiso JE. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30:413–46. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Harford TC, Grant BF, Yi HY, Chen CM. Patterns of DSM-IV alcohol abuse and dependence criteria among adolescents and adults: results from the 2001 National Household Survey on Drug Abuse. Alcohol Clin Exp Res. 2005;29:810–28. doi: 10.1097/01.alc.0000164381.67723.76. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. The co-occurrence of DSM-IV alcohol abuse in DSM-IV alcohol dependence: results of the National Epidemiologic Survey on Alcohol and Related Conditions on heterogeneity that differ by population subgroup. Arch Gen Psychiatry. 2004;61:891–6. doi: 10.1001/archpsyc.61.9.891. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cohort effects on gender differences in alcohol dependence. Addiction. 2002;97:1025–36. doi: 10.1046/j.1360-0443.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Jarque-Lopez A, Gonzalez-Reimers E, Rodriguez-Moreno F, Santolaria-Fernandez F, Lopez-Lirola A, Ros-Vilamajo R, Espinosa-Villarreal JG, Martinez-Riera A. Prevalence and mortality of heavy drinkers in a general medical hospital unit. Alcohol Alcohol. 2001;36:335–8. doi: 10.1093/alcalc/36.4.335. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS. Education and Survival: Birth Cohort, Period, and Age Effects. Demography. 2001;38:551–561. doi: 10.1353/dem.2001.0035. [DOI] [PubMed] [Google Scholar]
- Menard S. Longitudinal Research. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Midanik L, Chaloupka F, Saitz R, Toomey T, Fellows J, Dufour M, Landen M, Brounstein P, Brewer R, Naimi T, Miller J. Alcohol-Attributable Deaths and Years of Potential Life Lost --- United States 2001. MMWR Weekly. 2004;53:866–870. [PubMed] [Google Scholar]
- Murdock CG. Domesticating Drink: Women, Men, and Alcohol in America. Johns Hopkins University Press; Baltimore, MD: 1998. [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Neuman RJ, Saccone NL, Corbett J, Rochberg N, Hesselbrock V, Bucholz KK, McGuffin P, Reich T. Age and birth cohort effects on rates of alcohol dependence. Alcohol Clin Exp Res. 2003;27:93–9. doi: 10.1097/01.ALC.0000047303.89421.AA. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD, Jr, Regier DA. Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatry. 1984;41:949–58. doi: 10.1001/archpsyc.1984.01790210031005. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- RTI International. SUDAAN Language Manual, Release 9.0. Research Triangle Institute; Research Triangle Park, NC: 2004. [Google Scholar]
- SAMHDA: Substance Abuse and Mental Health Data Archive. National Comorbidity Survey [database online]. Inter-University Consortium for Political and Social Research. 2007
- Simon GE, VonKorff M. Reevaluation of secular trends in depression rates. Am J Epidemiol. 1992;135:1411–22. doi: 10.1093/oxfordjournals.aje.a116252. [DOI] [PubMed] [Google Scholar]
- Smith EM, Lewis CE, Kercher C, Spitznagel E. Predictors of mortality in alcoholic women: a 20-year follow-up study. Alcohol Clin Exp Res. 1994;18:1177–86. doi: 10.1111/j.1530-0277.1994.tb00101.x. [DOI] [PubMed] [Google Scholar]
- Thom B. Sex differences in help-seeking for alcohol problems--2. Entry into treatment. Br J Addict. 1987;82:989–97. doi: 10.1111/j.1360-0443.1987.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Ungemack J, Babor TF, Bidiorini A. Connecticut Compendium on Substance Abuse Treatment Need. Connecticut Department of Mental Health and Addiction Services; Connecticut: 2001. [Google Scholar]
- Ware JH. Linear Models for the Analysis of Longitudinal Studies. The American Statistician. 1985;39:95–101. [Google Scholar]


