Abstract
A better understanding of the unique cellular and functional properties of the superficial zone of articular cartilage may aid current strategies in tissue engineering which attempts a layered design for the repair of cartilage lesions to avert or postpone the onset of osteoarthritis. However, data pertaining to the cellular organization of non-degenerated superficial zone of articular cartilage is not available for most human joints. The present study analyzed the arrangement of chondrocytes of non-degenerated human joints (shoulder, elbow, knee, and ankle) by using fluorescence microscopy of the superficial zone in a top-down view. The resulting horizontal chondrocyte arrangements were tested for randomness, homogeneity or a significant grouping via point pattern analysis and were correlated with the joint type in which they occurred. The present study demonstrated that human superficial chondrocytes occurred in four distinct patterns of strings, clusters, pairs or single chondrocytes. Those patterns represented a significant grouping (p<0.0001) with horizontal alignment. Each articular joint surface was dominated by only one of these four patterns (p<0.001). Specific patterns correlated with specific diarthrodial joint types (p<0.001). Further studies need to establish whether these organizational patterns are a consequence of their surrounding environment or whether they are linked to a functional purpose.
Keywords: Superficial zone, articular cartilage, human, chondrocyte, cluster, clustering, chondron, point pattern, knee, ankle, joint type, cell organization
Introduction
Articular cartilage is an avascular, aneural, alymphatic, and viscoelastic connective tissue that functions hydrodynamically to bear loads and provide almost friction-free movement of diarthrodial joints (Kuettner et al., 1991). Its histomorphology is dominated by an extracellular matrix (ECM) consisting mainly of proteoglycan and type II collagen. Although cells of articular cartilage are represented by relatively sparsely distributed chondrocytes (Kuettner et al., 1991; Stockwell, 1971), articular chondrocytes and their pericellular microenvironment, termed chondrons, function as the primary metabolic units of articular cartilage to maintain matrix homeostasis via a synchronized balance between anabolism and catabolism (Kuettner et al., 1991; Poole, 1997).
Four major cartilage layers exist in articular cartilage: the superficial, transitional, radial, and calcified zones. Each can be distinguished based on differences in morphology (Aydelotte and Kuettner, 1988; Jadin et al., 2005; Kuettner et al., 1991), content of glycosaminoglycans (Venn and Maroudas, 1977), collagen (Nieminen et al., 2001), water (Maroudas and Venn, 1977), and mineral (Burr, 2004). The zonal differences in organization and biochemical content are directly associated with depth-dependant changes in biomechanical and bioelectrical tissue properties (Chen et al., 2001; Klein et al., 2007; Krishnan et al., 2003; Schinagl et al., 1997). This association is relevant both scientifically and clinically because current strategies for repair of traumatic cartilage lesions attempt to utilize the depth-dependent organization of articular cartilage (Klein et al., 2007). Additionally, engineering of layered tissues raises the hope to improve the wear characteristics of surrogate tissues and avert or postpone the frequent development of secondary osteoarthritis (OA) (Davis et al., 1989).
The superficial zone of articular cartilage in particular may be important for engineering of layered tissues due to the unique role that superficial chondrocytes play in joint lubrication (Jay et al., 2001; Schumacher et al., 1994), its biomechanical function as a tension resisting diaphragm (Meachim and Stockwell, 1979) and its distinctive proliferative and biosynthetic response to mechanical stimuli (Lee et al., 1998). Studies that were undertaken to further clarify the unique role of the superficial zone thus far have concentrated on cell shape (Hunziker et al., 2002; Jadin et al., 2005), cell density (Bywaters, 1937; Hunziker et al., 2002; Jadin et al., 2005; Quinn et al., 2005; Stockwell, 1971), cytoskeletal organization (Kim and Spector, 2000), growth-related changes in the bovine chondrocyte organization (Jadin et al., 2007), single cell mechanical properties (Jurvelin et al., 1996), gene expression (Darling et al., 2004; Jay et al., 2001; Khan et al., 2001; Schumacher et al., 1994), and metabolism (Eger et al., 2002). While it is widely accepted that the organization of human chondrocytes of the radial zones occur in vertical columns and the transitional zone in small “random” groups (Aydelotte and Kuettner, 1988; Brighton et al., 1984; Kuettner et al., 1991), it remains unclear how the human chondrocytes of the superficial zone are organized.
Studies investigating the cellular organization of the superficial zone of human articular cartilage are rare. Few groups have access to non-degenerative human donor tissue in which the superficial zone is still intact. Furthermore, despite an abundant availability of surgical specimens, most of these specimens are often degenerated and therefore have partially lost their superficial zone. However, organizational information on non-degenerative human tissue may prove vital to modern cartilage repair strategies that attempt to engineer the layered structure of articular cartilage. An additional potential problem is that conventional histological analyses on tissue sections are looked at in the vertical plane. When viewed vertically, superficial chondrocytes appear as single cells (Hunziker et al., 2002). However, we recently showed that chondrocytes within the superficial zone of normal human ankle cartilage were arranged in horizontal clusters parallel to the articular surface (Schumacher et al., 2002). Although this study suggests that human superficial chondrocytes, in a top-down view, may appear in horizontally oriented groups, particular patterns in the superficial chondrocyte organization have not been investigated.
To further elucidate the spatial arrangement of superficial chondrocytes, the present study quantitatively analyzed fluorescent microscopic images of the entire intact superficial zone of four major human joints (shoulder, elbow, knee, and ankle) that were photographed from top-down views. Further vertical or horizontal tissue sectioning was avoided to prevent any potential loss of three-dimensional information. The resulting arrangements of the chondrocytes were tested for randomness, homogeneity or a significant grouping in the context of a point pattern analysis (Diggle et al., 2000). The presence of specific chondrocyte patterns were also correlated with the joint type in which they occurred.
Methods
Human Articular Cartilage
Articular cartilages from joints of the upper (elbow and shoulder joints) and lower (knee and ankle joints) extremities were obtained with institutional approval from healthy adult human tissue donors within 24 hours after death through the Gift of Hope Organ and Tissue Donor Network (Illinois, USA) and graded according to a 5-point scale by a modified Collins grading (Muehleman et al., 1997), using the following criteria: grade 0 (normal cartilage without signs of degeneration), grade 1 (minor surface roughening), grade 2 (fibrillations and fissuring), grade 3 (full defects covering less than 30% of the articular surface), and grade 4 (full defects covering more than 30% of the articular surface). Only joints with the grades 0–1 were included into this study. No other pathology other than articular degeneration was observed in any of the joints.
Tissues from eight human donors (age 65.9 ± 5.4 years, range 52–85 years) were obtained from the proximal humerus (center of the humeral head articular surface facing the glenoid, n = 6, grade 0: n = 4, grade 1: n = 2), distal humerus (capitulum center, n = 4, all grade 0), ulna (semilunar notch, convex medial portion, n = 4, all grade 0), radius (radial head, concave fossa, n = 4, all grade 0), distal femur (condyles, weight bearing region in knee joint extension; patellofemoral groove, medial part, n = 7, grade 0: n = 5, grade 1: n = 2), proximal tibia (edge of the medial plateau, that is covered by the medial meniscus body, n = 5, grade 0: n = 3, grade 1: n = 2), distal tibia (center, n = 4, all grade 0), and talus (dome center, n = 8, grade 0: n = 5, grade 1: n = 3).
Fluorescence Microscopy
In order to visualize the arrangement of the superficial chondrocytes through the depth of the superficial zone cartilage, samples of approximately 200 µm thickness and 1 cm2 area were obtained by manual horizontal dissection from the weight-bearing area of each joint surface and not further sectioned to preserve intact chondrocyte patterns. The orientation of the cartilage slices was maintained by cutting one border of the sample slightly angulated producing an arrowhead shape. The samples were directly fixed in 4% paraformaldehyde and stained as previously described (Schumacher et al., 2002). Briefly, samples were washed with 1% Triton X-100 in phosphate buffered saline (PBS) and treated with 5% aqueous CaCl2 for 30 minutes. All samples were stained for 60 minutes with propidium iodine (10 µg/ml in PBS) and washed for 20 minutes with Tris buffered saline (1 M Tris, 15 M NaCl, pH 7.5). The samples were viewed perpendicular to the articular surface, providing a top-down view. Two-dimensional images were digitally recorded for each articulating surface (Nikon Eclipse TE200, Melles-Griot 43 series ion laser) at the sample center as a single topographical location beneath the surface when cells first came into focus and at successive depths when further cells came into focus within the superficial zone.
Quantitative Image Analysis
Images of the superficial zone were imported into Adobe Photoshop (San Jose, CA). The images were digitally filtered by using the contour threshold function of Fovea Pro in Photoshop (Reindeer Graphics, Asheville, NC) to exclude cells that were out of focus and therefore in different planes. Based on proximity, the depicted nuclei were then grouped numerically into single cells or groups of 2, 3, 4, 5, 6 to 8, or 9 to 12. Based on their spatial arrangement, groups were classified as singles, pairs, clusters, and strings. Singles were defined as single cells, pairs as two neighboring chondrocytes, clusters as a circular arrangement of chondrocytes, and strings as chondrocytes that were aligned in a line.
Point Pattern Analysis
To determine if the spatial arrangement of cells was random, homogeneous or significantly grouped in the context of a point pattern analysis (Diggle et al., 2000), the nearest neighbor method was used (Skellam, 1951) for which grouped data was defined as a sub-collection of data that is proximate based on distance (Rousseeuw, 1987). Briefly, nuclear centroids (one pixel marking the geometrical nucleus center) were determined with Fovea Pro in Photoshop (Reindeer Graphics, Asheville, NC), and the mean nearest neighbor distance between the centroids of two neighboring chondrocytes was calculated. The ratio of observed values of the distance to the nearest neighbor and their expected values in an assumed homogeneous distribution was used to determine the presence or absence of grouping. To quantify the departure from random, the appropriate test of significance for this method was used (Clark and Evans, 1954).
Correlation of Chondrocyte Patterns with Diarthrodial Joint Types
To test the correlation between specific chondrocyte patterns and the particular diarthrodial joint type (Gray, 1995) in which they occurred, the articular surfaces were coded according to their joint function (1 = uni-axial hinge joint [talus, distal tibia]; 2 = bi-axial joint with a secondary rotational axis [condyles, humeral head, distal humerus capitulum]; and 3 = concave surface [patellofemoral groove, radial head]).
Cell Density and Split Lines
To separately analyze the cell densities of the superficial and deeper zones, the entire articular surface was dissected to a depth of 200 µm and defined as the superficial zone. The remaining non-calcified cartilage was dissected and defined as deeper zones. The wet weights of both the superficial and deeper zones were determined. The chondrocytes were isolated using an established method (Aydelotte and Kuettner, 1988). Briefly, the superficial and deeper zones were digested in 0.2% pronase (Calbiochem, La Jolla, CA) in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10 % fetal bovine serum (FBS; HyClone, Logan, UT) for 90 min at 37°C, followed by overnight digestion with 0.025% collagenase-P (Boehringer Mannheim, Indianapolis, IN) in DMEM containing 10 % FBS.
The cell numbers were determined for each joint surface and for the superficial and deeper zones by averaging three manual counts of the released chondrocytes. Before counting, the complete digestion of tissues was ensured microscopically for each group. The cell densities were calculated as the number of released chondrocytes per gram wet weight of the dissected cartilages. It has been suggested that the yield of released cells, especially from human cartilage, is relatively low and perhaps depends on the amount of damage near sliced edges. Due to methodological differences, direct comparisons with other studies are difficult. However, to compare our results to previous studies, we calculated the ratio between the densities of the superficial zone and the deeper zones and found that it was comparable to those of other studies (Hunziker et al., 2002; Quinn et al., 2005; Temple et al., 2007).
On selected distal femora and human tali, cartilage were removed manually from the weight bearing areas of the articular surfaces, pinpricked and stained with Indian ink to reveal split-line patterns as previously described (Below et al., 2002; Meachim et al., 1974).
Statistical Analysis
All data is presented as mean ± SEM (standard error of the mean) of n. All data were subjected to one way ANOVA or ANOVA on Ranks, followed by Holm-Sidak or Dunn’s Test post-hoc-tests depending on normal or non-normal data distribution, respectively. A Spearman Rank Order Correlation was performed to test for significant correlations between the percentage of cells that were situated in a specific pattern and the diarthrodial joint type of the articular surface in which this particular pattern occurred. Differences were considered significant at p ≤ 0.05. All tests were performed using SigmaStat 3.1 software (Systat Software Inc., San Jose, CA, USA).
Results
Fluorescence Microscopy
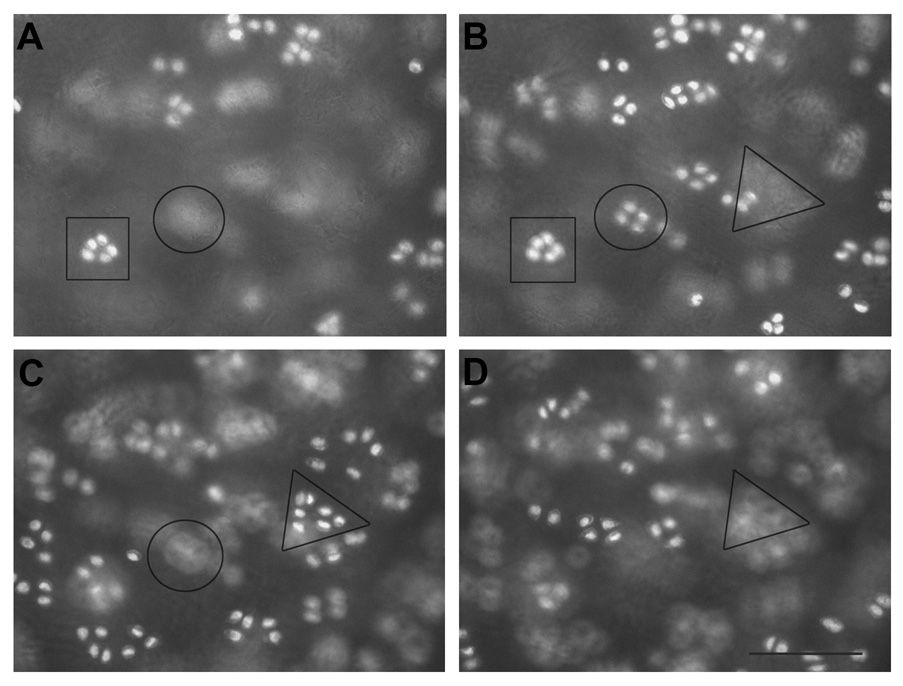
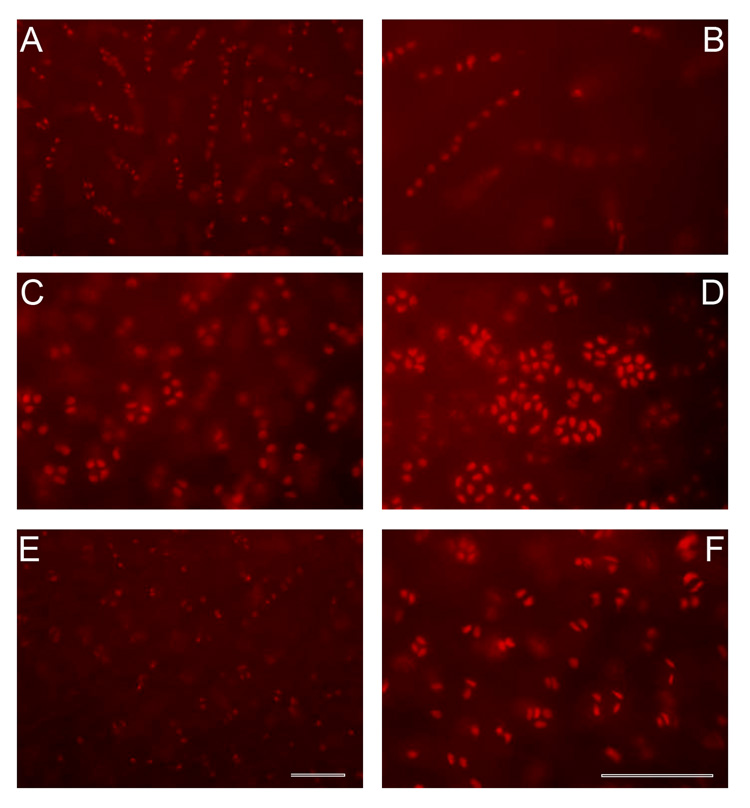
To visualize the spatial arrangement of chondrocytes of the superficial zone, fluorescent microscopic images of the entire depth of the uncut, intact superficial cartilage of human shoulder, elbow, knee, and ankle joints were photographed from top-down views. Chondrocytes of the superficial zone were neither homogeneously nor randomly distributed. As shown in Figure 1, they were organized into groups that were aligned parallel to the articular surface. Throughout the depth of the superficial zone, chondrocyte groups were situated in distinctly different focal planes. With increasing tissue depth, chondrocyte groups came in and out of focus and were separated from each other by extracellular matrix both vertically and horizontally. Superficial chondrocytes were organized in four distinctly different spatial patterns that we classified as strings, containing three or more cells in a straight line (Figure 2, A and B), clusters containing three or more cells in a circle or ellipse (Figure 2, C and D), pairs containing two cells in close proximity of one another (Figure 2, E and F), or single chondrocytes that were not in proximity to one another. The chondrocyte patterns were not aligned with the orientation of the cartilage sample within the joint surface.
Figure 1. Different focal planes of patellofemoral groove clusters.
Representative images of the most superficial (A, approximately a few micrometers beneath the tissue surface) and increased tissue depths (B–D, approximately 30 micrometer steps deeper into the tissue) within the superficial zone. The square, circle and triangle represent different groups of cells situated in different focal planes. Scale bar, 100 µm.
Figure 2. Chondrocyte cell patterns of the superficial zone.
Fluorescent microscopic representative images showing cells stained with propidium iodide of strings (A–B), clusters (C–D) and pairs (E–F). Single cells were dispersed among the other patterns. Scale bar, 100 µm.
Quantitative Image Analysis
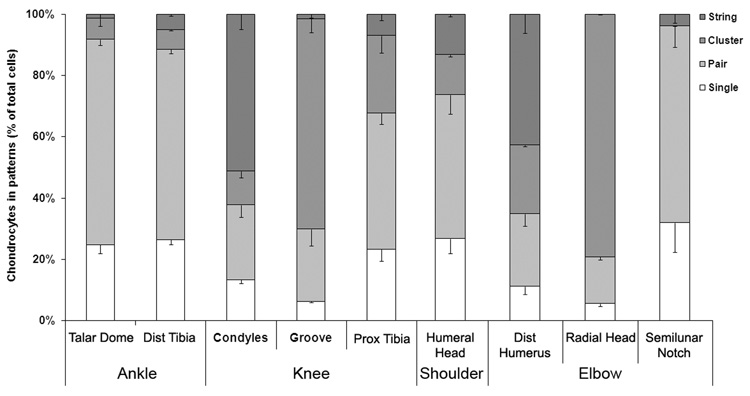
Although in all articular surfaces four chondrocyte patterns such as strings, clusters, pairs, and singles were found, most articular joint surfaces were dominated by only one of these four patterns. Most chondrocytes of the articular surfaces of the ankle joint were situated in pairs; 67.08 ± 1.96% of talus dome chondrocytes and 62.14 ± 1.32% of distal tibia chondrocytes were paired (Figure 3). In contrast, strings of chondrocytes were found in half of the superficial chondrocytes of the femoral condyles of the knee joint (51.25 ± 4.94 %), whereas circular clusters were observed in two thirds of the chondrocytes in the patellofemoral groove of the femur (68.72 ± 5.46 %). In the third articulating surface of the knee, the meniscus-covered medial tibia plateau, we found that 44.5 ± 3.81% of the chondrocytes were paired and 7.0 ± 2.1% had formed strings. In the shoulder and elbow joints, all articular surfaces were also dominated by a particular cellular pattern. Thus, almost half of all cells of the distal humerus were located in strings (42.83 ± 6.14 %), whereas more than two thirds of cells of the radial head were positioned in clusters (79.24 ± 1.02%). Half of the superficial chondrocytes of the humeral head (47.05 ± 6.33 %) and almost two thirds of the cells of the proximal ulna (64.11 ± 6.7 %) were situated in pairs. Overall, the predominant pattern varied across joints, and, depending on the surface, consisted either of pairs, clusters, or strings but not single chondrocytes. Furthermore, the percentage of cells situated in the predominant cell pattern of each given articular surface was consistently and significantly higher than the percentage of cells in the remaining, non-predominant cell patterns (p < 0.001). Significant differences between the various examined anatomical locations are summarized in Table I. In addition, we looked at the femoral border between condyles and patellofemoral groove and noticed a transitional zone, in which the predominant patterns of the two bordering regions were intertwined. However, we did not further quantify those images.
Figure 3. Percentage of lower and upper extremity chondrocytes that were grouped in strings, clusters, pairs or as singles.
Values are expressed as the mean ± standard error of the mean (SEM). p values are listed in Table I.
Table I.
Significant differences between articular surfaces
| Pattern | Comparison | p |
|---|---|---|
| Strings | Condyles vs. Patellofemoral Groove | <0.001 |
| Condyles vs. Talar Dome | <0.001 | |
| Condyles vs. Proximal Tibia | <0.002 | |
| Condyles vs. Humeral Head | <0.001 | |
| Condyles vs. Semilunar Notch | <0.001 | |
| Condyles vs. Radial Head | <0.001 | |
| Distal Humeral vs. Humeral Head | <0.002 | |
| Distal Humerus vs. Semilunar Notch | <0.002 | |
| Distal Humerus vs. Radial Head | <0.001 | |
| Distal Humerus vs. Patellofemoral Groove | <0.001 | |
| Distal Humerus vs. Talar Dome | <0.001 | |
| Distal Humerus vs. Proximal Tibia | <0.002 | |
| Cluster | Patellofemoral Groove vs. Condyles | <0.001 |
| Patellofemoral Groove vs. Talar Dome | <0.001 | |
| Patellofemoral Groove vs. Humeral Head | <0.002 | |
| Patellofemoral Groove vs. Semilunar Notch | <0.001 | |
| Patellofemoral Groove vs. Distal Humerus | <0.002 | |
| Radial Head vs. Talar Dome | <0.001 | |
| Radial Head vs. Condyles | <0.001 | |
| Radial Head vs. Humeral Head | <0.001 | |
| Radial Head vs. Semilunar Notch | <0.001 | |
| Radial Head vs. Distal Humerus | <0.002 | |
| Proximal Tibia vs. Talar Dome | <0.001 | |
| Proximal Tibia vs. Semilunar Notch | <0.001 | |
| Proximal Tibia vs. Condyles | <0.001 | |
| Proximal Tibia vs. Humeral Head | <0.002 | |
| Proximal Tibia vs. Distal Humerus | <0.002 | |
| Pairs | Talar Dome vs. Condyles | <0.001 |
| Talar Dome vs. Patellofemoral Groove | <0.001 | |
| Talar Dome vs. Proximal Tibia | <0.001 | |
| Talar Dome vs. Radial Head | <0.001 | |
| Talar Dome vs. Distal Humerus | <0.001 | |
| Semilunar Notch vs. Condyles | <0.002 | |
| Semilunar Notch vs. Patellofemoral Groove | <0.002 | |
| Semilunar Notch vs. Proximal Tibia | <0.001 | |
| Semilunar Notch vs. Radial Head | <0.001 | |
| Semilunar Notch vs. Distal Humerus | <0.002 | |
| Humeral Head vs. Proximal Tibia | <0.002 | |
| Humeral Head vs. Radial Head | <0.002 | |
| Singles | Talar Dome vs. Condyles | <0.05 |
| Distal Tibia vs. Patellofemoral Groove | <0.05 |
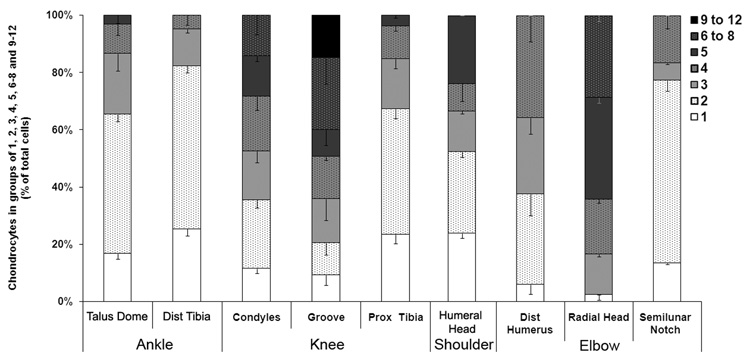
In some articular surfaces there was, in addition to a predominant pattern, a predominant number of superficial chondrocytes per group (Figure 4). In the patellofemoral groove, the groups consisted predominantly of 6 to 8 chondrocytes (25.12 ± 9.19 %), whereas the superficial zones of the talar dome and distal tibia consisted largely of groups of two cells (48.72 ± 2.47 % and 56.97 ± 2.34 % of cells were paired). In other articular surfaces such as the femoral condyles, there was no predominant number of chondrocytes per group (Figure 4). These findings were significantly different from those of the patellofemoral groove (p < 0.008) or the talus (p < 0.001). In the upper extremity, two predominant cell number groupings were found. Within the articular surface of the radial head, 35.71 ± 6.07 % of chondrocytes were situated in groups of five cells, whereas in the proximal ulna, the chondrocytes were primarily grouped in pairs (63.92 ± 3.72 %, p < 0.001). While single cells were never the predominant organization, the highest percentages of single cells were found in the proximal humerus and the distal tibia (Figure 4). The lowest percentage of single cells were found in the distal humerus (5.94 ± 1.62%) and the radial head (2.38 ± 1.86%). Significantly higher numbers of superficial chondrocytes per cell group were also found when data of the upper extremity was compared with data of the lower extremity (p = 0.002) and when the knee joint was compared with the ankle joint (p < 0.001). No differences in cell numbers per group between shoulder and elbow joints were noted.
Figure 4. Percentage of chondrocytes that appear as single cells or in groups of 2, 3, 4, 5, 6 to 8, or 9 to 12 cells.
Values are expressed as the mean ± SEM.
Point Pattern Analysis
To test if the spatial arrangement of cells was random, homogeneous or grouped, a point pattern analysis (Diggle et al., 2000) was performed by calculating the ratio (R) of observed values of the distance to the nearest neighbor and their expected values in an assumed homogeneous distribution. Briefly, a ratio = 1 describes a random distribution, a ratio < 1 describes a grouped distribution, while a ratio > 1 defines a homogeneous distribution. The ratio (R) was R = 0.794 for chondrocytes forming strings, R = 0.625 for clusters, and R = 0.836 for pairs, strongly indicating a significantly grouped and not a random or homogeneous cellular distribution within normal cartilage (strings, p < 0.001, clusters, p < 0.0001, pairs, p < 0.05).
To determine differences in the proximity of chondrocytes, the mean nearest neighbor distances of superficial chondrocytes were calculated. Those of knee cartilages were significantly smaller than those of the ankle joint (p < 0.01). This finding was based on the mean nearest neighbor distances between cells of the condyles and the talus (p < 0.05) but not on the patellofemoral groove and the talus, suggesting a closer proximity of condylar than talar superficial cells. No differences were noted when comparing the mean nearest neighbor distances of superficial chondrocytes of the two ankle joint surfaces (talus and tibia) or those of the upper with those of the lower extremity or of the shoulder compared with those of the elbow joint (data not shown).
Correlation of Chondrocyte Patterns with Joint Types
To determine if there was a significant correlation between specific chondrocyte patterns and the joint type of the articular surface in which they were situated, the articular surfaces were coded as convex surfaces of a uni-axial hinge joint or biaxial joint with a secondary rotational axis, or as concave surfaces. A Spearman Rank Order Correlation was performed, which revealed significant correlations between single chondrocytes and a uni-axial hinge joint type (p < 0.001, correlation coefficient = −0.829), between paired chondrocytes and a uni-axial hinge joint type (p < 0.001, correlation coefficient = −0.828), and between clusters and a concave articular surface shape (p < 0.001, correlation coefficient = 0.831). For chondrocyte strings, significant correlations were not found. However, the percentage of cells that were situated in strings of a joint type with a rotational axis was significantly higher than the percentage of cells that was located in strings of joints without a rotational axis (p < 0.001).
Cell Density and Split Lines
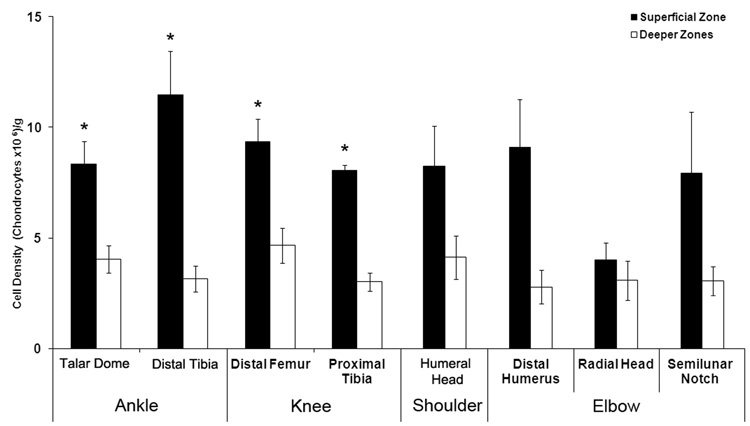
To determine if differences in the numerical, spatial and proximity based chondrocyte organization may or may not affect the bulk zonal cell density, for each surface the cell densities were analyzed. Interestingly, our results showed no differences between the superficial densities of the different articular surfaces that were analyzed (Figure 5), suggesting that the observed spatial, numerical and proximity based differences were local occurrences whose presence did not affect the bulk cell density. As shown previously for articular cartilage (Hunziker et al., 2002; Quinn et al., 2005; Stockwell, 1971), all cartilages of the knee and ankle joints showed significantly higher cell densities in their superficial zones than in their corresponding deeper zones (p < 0.05). In the shoulder and elbow joints, a trend to higher superficial than deeper zones cell densities was observed.
Figure 5. Cell densities of the superficial and the deeper zones of the upper and lower extremities.
Values are expressed as the mean ± SEM. *, p < 0.05 compared with the respective deeper zone. The cell density of the distal femur consisted of the groove and the condylar regions; separate densities for both regions were not calculated.
Split line patterns are well described in the distal femur and talar dome (Below et al., 2002; Meachim et al., 1974), however, their significance is not entirely clear. While some authors assume a correlation between split lines and collagen fibril orientation, ultrastructural studies revealed little evidence that the split-line direction correlated with any preferred alignment of fibrils (Kamalanathan and Broom, 1993). We observed split line patterns of the distal femur and the talar dome similar to those reported (Below et al., 2002; Meachim et al., 1974). However, we were unable to establish any relationship between the split lines and superficial chondrocyte groups, which were not aligned in any identifiable pattern with neighboring or distant cell groups (data not shown).
Discussion
The present study compared the organizational structure of superficial chondrocytes in non-degenerated articular cartilages obtained from different human joints in the upper and lower extremities. Perhaps the most important observation of the present study was that superficial chondrocytes, when viewed in a top-down view, were grouped in patterns that were oriented parallel to the articular surface. We identified four distinctly different patterns: strings, clusters, pairs and single chondrocytes. The observed patterns were not due to a random distribution of chondrocytes but rather represented a significant grouping of cells in the context of a statistical analysis of point patterns. Although a few studies of the superficial zone reported clusters in human cartilage (Schumacher et al., 2002), pairs in bovine cartilage (Jadin et al., 2005) and incidentally symmetrical rows in rabbit cartilage (Clark and Rudd, 1991), to the author’s knowledge, the present study is the first to identify complex patterns in the arrangement of human chondrocyte groups in the superficial zone of non-degenerated cartilage.
That chondrocytes of the middle and deep zones can be grouped in single, double or multiple chondron columns with interconnecting segments between adjacent chondrons has been reported for non-degenerative tibial cartilage (Poole, 1997). It has been suggested that chondrocyte groups may have formed during skeletal development (Morrison et al., 2000), and their pairing may have arisen after cell division followed by an incomplete separation within the chondron microenvironment (Chi et al., 2004). The position of daughter cells can be determined by positioning of the cell division axis and is influenced by the spatial distribution of the ECM (Thery et al., 2005). In fact, an electron microscopy study of the articular surface reported associations between ECM surface depressions and superficial cell groups in rabbit cartilage (Clark and Rudd, 1991). In addition, the local zonal collagen architecture appear to influence the chondron shape and orientation (Youn et al., 2006). Thus, specific patterns in the organization of superficial chondrocytes may be associated with the local articular surface structure.
In fact, data from the present study suggests that a specific chondrocyte pattern is correlated with the diarthrodial joint type of the articular surface in which they occur. All articular surfaces, which were predominated by chondrocyte pairs, such as both surfaces of the ankle joint and the humero-ulnar compartment of the elbow joint, function mainly as an uni-axial hinge joint (Gray, 2004). The surfaces in which chondrocyte strings were predominantly observed (the femoral condyles of the knee joint and the capitulum of the distal humerus) function in bi-axial joints with a secondary rotational axis (Gray, 2004). To a lesser extent, strings were found in the humeral head, which is part of a ball-and-socket joint that also allows rotatory movements (Gray, 2004). Surfaces with a concave anatomy were associated with chondrocyte clusters. Concave surfaces differ from a convex anatomy in terms of joint functioning; when a concave surface moves on a convex surface, rolling and sliding occur in the same direction (Gray, 2004), but when a convex surface moves on a concave surface, rolling and sliding movements occur in opposite directions (Gray, 2004). Clusters were found in the shallow concave patellofemoral groove of the distal femur and the concave depression of the radial head. Although we found significant correlations between chondrocyte patterns and diarthrodial joint types, further studies will be required to determine if specific chondrocyte patterns are causally linked to specific biomechanical functions.
The present study showed numerical, spatial and proximity based differences in the superficial cellular organization when comparing the articular surfaces of different joint types. Numerical differences may occur with alterations in the cellularity of human articular cartilage due to aging. Those age-dependent alterations occur in joints such as the hip and knee, but are less likely to occur in the humeral head (Meachim and Collins, 1962; Stockwell, 1967; Vignon et al., 1976). If present, the principal age-related changes are seen in the superficial zone (Stockwell, 1978). Therefore, it may be possible that age-related changes in the cellularity affect the observed superficial chondrocyte patterns, possibly by changing the pattern type or by decreasing the presence of a certain pattern. However, in the present study we did not observe such age-related changes, perhaps because young donors were not included in the present study. Our numerical, spatial and proximity based data may be relevant to tissue engineering with different seeding densities of chondrocytes that are used to increase material properties such as compressive and dynamic stiffness (Mauck et al., 2003). However, our data showed that the cell densities of the superficial zones were similar when comparing the articular surfaces examined. Thus, the observed differences in the numerical, spatial and proximity based chondrocyte organization did not affect the zonal cell density and appear to be local variations within the chondrocyte microcosm.
The question arises whether the presented cellular orientation was dependent on collagen fiber orientation or on the established split line pattern found in the distal femur and talar dome (Below et al., 2002; Meachim et al., 1974). We were unable to show a split line and cellular correlation. Because we examined only the cellular, but not the collagen organization, the question concerning the alignment of collagen and cells cannot be answered directly. However, an electron microscopy study showed that rows of rabbit chondrocytes run parallel to adjacent collagen fibrils within the same superficial plane (Clark and Rudd, 1991), suggesting a spatial relationship between collagen fibers and the chondrocyte organization. An ultrastructural study revealed little evidence that the split-line direction correlated with any preferred alignment of collagen fibrils (Kamalanathan and Broom, 1993). Our data excluded a close association between the presented chondrocyte orientation and the split lines of the femur and tibia.
Biomechanical forces and their mechanotransduction are an important modulator in the development (Heegaard et al., 1999; Wong and Carter, 2003), differentiation (Brama et al., 2000; Little and Ghosh, 1997), and maintenance of articular cartilage (Grodzinsky et al., 2000; Lane Smith et al., 2000; Smith et al., 1995). During skeletal development, the morphogenesis of articular cartilage is regulated by mechanical forces, resulting in the formation of the four cartilage zones (Heegaard et al., 1999; Wong and Carter, 2003). The biochemical content of these zones is further modulated by a functional adaptation of the extracellular matrix to weight bearing (Brama et al., 2000). Mechanical forces also modulate the chondrocyte phenotypic expression during development (Little and Ghosh, 1997). In adulthood, mechanical loading directly influences cartilage homeostasis, which is important for maintaining a functional matrix (Lane Smith et al., 2000; Smith et al., 1995). Biomechanical forces play a major role in the genesis of articular cartilage zonal heterogeneity. They may also contribute to the unique superficial zone heterogeneities in the organizational patterns of human chondrocytes across different joint types as observed in the present study. Although a functional explanation for these organizational chondrocyte patterns has not yet been established, their correlation with a specific joint type may suggest an association with the occurring biomechanical forces and perhaps a role in mechano-sensing and transduction. Additional bovine and human articular cartilage studies are ongoing in our laboratory to further elucidate a possible metabolic or biomechanical role of the superficial chondrocyte organization in articular functioning and repair. Although the hypothesis of such roles is clearly intriguing, it remains to be established whether these organizational patterns are a passive consequence of their surrounding anatomical and biomechanical environment or whether they actively serve a functional purpose. The present study has uncovered a new dimension of complexity to an already intricate tissue that has not been appreciated previously.
Acknowledgements
We would like to acknowledge the Gift of Hope Organ and Tissue Donor network and the donor families for their support. We gratefully thank Arcady Margulis, M.D., and Lev Rappoport, M.D., for procurement of human donor tissue, their continuous support and friendship. This work was funded in part by NIH grants P5O-AR39239 (K.E.K. and A.C.), R01-AR45779 (A.G.), and DFG grants RO 2511/1-1 und 2-1 (B.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect Tissue Res. 1988;18:205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- Below S, Arnoczky SP, Dodds J, Kooima C, Walter N. The split-line pattern of the distal femur: A consideration in the orientation of autologous cartilage grafts. Arthroscopy. 2002;18:613–617. doi: 10.1053/jars.2002.29877. [DOI] [PubMed] [Google Scholar]
- Brama PA, Tekoppele JM, Bank RA, Barneveld A, van Weeren PR. Functional adaptation of equine articular cartilage: the formation of regional biochemical characteristics up to age one year. Equine Vet J. 2000;32:217–221. doi: 10.2746/042516400776563626. [DOI] [PubMed] [Google Scholar]
- Brighton CT, Kitajima T, Hunt RM. Zonal analysis of cytoplasmic components of articular cartilage chondrocytes. Arthritis Rheum. 1984;27:1290–1299. doi: 10.1002/art.1780271112. [DOI] [PubMed] [Google Scholar]
- Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12 Suppl A:S20–S30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Bywaters E. The metabolism of joint tissues. J. Path. Bact. 1937:247–268. [Google Scholar]
- Chen AC, Bae WC, Schinagl RM, Sah RL. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech. 2001;34:1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- Chi SS, Rattner JB, Matyas JR. Communication between paired chondrocytes in the superficial zone of articular cartilage. J Anat. 2004;205:363–370. doi: 10.1111/j.0021-8782.2004.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Rudd E. Cell patterns in the surface of rabbit articular cartilage revealed by the backscatter mode of scanning electron microscopy. J Orthop Res. 1991;9:275–283. doi: 10.1002/jor.1100090216. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Evans FC. Distance to Nearest Neighbor as a Measure of Spatial Relationships in Populations. Ecology. 1954;35:445–453. [Google Scholar]
- Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22:1182–1187. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am J Epidemiol. 1989;130:278–288. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Mateu G, Clough HE. A comparison between parametric and nonparametric approaches to the analysis of replicated spatial point patterns. Adv. in Appl. Probab. 2000;32:331–343. [Google Scholar]
- Eger W, Schumacher BL, Mollenhauer J, Kuettner KE, Cole AA. Human knee and ankle cartilage explants: catabolic differences. J Orthop Res. 2002;20:526–534. doi: 10.1016/S0736-0266(01)00125-5. [DOI] [PubMed] [Google Scholar]
- Gray H. Gray's Anatomy: The Anatomical Basis of Medicine & Surgery. 38th edition. ed. New York: Churchill Livingstone; 1995. [Google Scholar]
- Gray H. Gray's Anatomy: The Anatomical Basis of Medicine & Surgery. British Edition, 39th Ed ed. 2004. [Google Scholar]
- Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- Heegaard JH, Beaupre GS, Carter DR. Mechanically modulated cartilage growth may regulate joint surface morphogenesis. J Orthop Res. 1999;17:509–517. doi: 10.1002/jor.1100170408. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564–572. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- Jadin KD, Bae WC, Schumacher BL, Sah RL. Three-dimensional (3-D) imaging of chondrocytes in articular cartilage: growth-associated changes in cell organization. Biomaterials. 2007;28:230–239. doi: 10.1016/j.biomaterials.2006.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadin KD, Wong BL, Bae WC, Li KW, Williamson AK, Schumacher BL, Price JH, Sah RL. Depth-varying density and organization of chondrocytes in immature and mature bovine articular cartilage assessed by 3d imaging and analysis. J Histochem Cytochem. 2005;53:1109–1119. doi: 10.1369/jhc.4A6511.2005. [DOI] [PubMed] [Google Scholar]
- Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19:677–687. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- Jurvelin JS, Muller DJ, Wong M, Studer D, Engel A, Hunziker EB. Surface and subsurface morphology of bovine humeral articular cartilage as assessed by atomic force and transmission electron microscopy. J Struct Biol. 1996;117:45–54. doi: 10.1006/jsbi.1996.0068. [DOI] [PubMed] [Google Scholar]
- Kamalanathan S, Broom ND. The biomechanical ambiguity of the articular surface. J Anat. 1993;183(Pt 3):567–578. [PMC free article] [PubMed] [Google Scholar]
- Khan IM, Salter DM, Bayliss MT, Thomson BM, Archer CW. Expression of clusterin in the superficial zone of bovine articular cartilage. Arthritis Rheum. 2001;44:1795–1799. doi: 10.1002/1529-0131(200108)44:8<1795::AID-ART316>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kim AC, Spector M. Distribution of chondrocytes containing alpha-smooth muscle actin in human articular cartilage. J Orthop Res. 2000;18:749–755. doi: 10.1002/jor.1100180511. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech. 2007;40:182–190. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Park S, Eckstein F, Ateshian GA. Inhomogeneous cartilage properties enhance superficial interstitial fluid support and frictional properties, but do not provide a homogeneous state of stress. J Biomech Eng. 2003;125:569–577. doi: 10.1115/1.1610018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuettner KE, Aydelotte MB, Thonar EJ. Articular cartilage matrix and structure: a minireview. J Rheumatol. 1991;Suppl 27:46–48. [PubMed] [Google Scholar]
- Lane Smith R, Trindade MC, Ikenoue T, Mohtai M, Das P, Carter DR, Goodman SB, Schurman DJ. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37:95–107. [PubMed] [Google Scholar]
- Lee DA, Noguchi T, Knight MM, O'Donnell L, Bentley G, Bader DL. Response of chondrocyte subpopulations cultured within unloaded and loaded agarose. J Orthop Res. 1998;16:726–733. doi: 10.1002/jor.1100160615. [DOI] [PubMed] [Google Scholar]
- Little CB, Ghosh P. Variation in proteoglycan metabolism by articular chondrocytes in different joint regions is determined by post-natal mechanical loading. Osteoarthritis Cartilage. 1997;5:49–62. doi: 10.1016/s1063-4584(97)80031-3. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II. Swelling. Ann Rheum Dis. 1977;36:399–406. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Meachim G, Collins DH. Cell counts of normal and osteoarthritic articular cartilage in relation to the uptake of sulphate (35SO4) in vitro. Ann Rheum Dis. 1962;21:45–50. doi: 10.1136/ard.21.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meachim G, Stockwell R. The matrix. In: MAR F, editor. Adult Articular Cartilage. Tunbridge Wells: Pitman Medical; 1979. pp. 1–67. [Google Scholar]
- Meachim G, Denham D, Emery IH, Wilkinson PH. Collagen alignments and artificial splits at the surface of human articular cartilage. J Anat. 1974;118:101–118. [PMC free article] [PubMed] [Google Scholar]
- Morrison SL, Campbell CK, Wright GM. Chondrogenesis of the branchial skeleton in embryonic sea lamprey, Petromyzon marinus. Anat Rec. 2000;260:252–267. doi: 10.1002/1097-0185(20001101)260:3<252::AID-AR50>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, Jurvelin JS. T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med. 2001;46:487–493. doi: 10.1002/mrm.1218. [DOI] [PubMed] [Google Scholar]
- Poole CA. Articular cartilage chondrons: form, function and failure. J Anat. 1997;191(Pt 1):1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TM, Hunziker EB, Hauselmann HJ. Variation of cell and matrix morphologies in articular cartilage among locations in the adult human knee. Osteoarthritis Cartilage. 2005;13:672–678. doi: 10.1016/j.joca.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Rousseeuw PJ. Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis. J. Comput. Appl. Math. 1987:53–65. [Google Scholar]
- Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Re. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- Schumacher BL, Su JL, Lindley KM, Kuettner KE, Cole AA. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat Rec. 2002;266:241–248. doi: 10.1002/ar.10063. [DOI] [PubMed] [Google Scholar]
- Skellam JG. Random dispersal in theoretical populations. Biometrika. 1951;38:196–218. [PubMed] [Google Scholar]
- Smith RL, Donlon BS, Gupta MK, Mohtai M, Das P, Carter DR, Cooke J, Gibbons G, Hutchinson N, Schurman DJ. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J Orthop Res. 1995;13:824–831. doi: 10.1002/jor.1100130604. [DOI] [PubMed] [Google Scholar]
- Stockwell RA. The cell density of human articular and costal cartilage. J Anat. 1967;101:753–763. [PMC free article] [PubMed] [Google Scholar]
- Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411–421. [PMC free article] [PubMed] [Google Scholar]
- Stockwell RA. Chondrocytes. J Clin Pathol Suppl (R Coll Pathol) 1978;12:7–13. [PMC free article] [PubMed] [Google Scholar]
- Temple MM, Bae WC, Chen MQ, Lotz M, Amiel D, Coutts RD, Sah RL. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2007;15:1042–1052. doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nat Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]
- Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignon E, Arlot M, Patricot LM, Vignon G. The cell density of human femoral head cartilage. Clin Orthop Relat Res. 1976:303–308. [PubMed] [Google Scholar]
- Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- Youn I, Choi JB, Cao L, Setton LA, Guilak F. Zonal variations in the threedimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage. 2006;14:889–897. doi: 10.1016/j.joca.2006.02.017. [DOI] [PubMed] [Google Scholar]