Abstract
Background
An interaction between lectins from marine algae and PLA2 from rattlesnake was suggested some years ago. We, herein, studied the effects elicited by a small isolectin (BTL-2), isolated from Bryothamnion triquetrum, on the pharmacological and biological activities of a PLA2 isolated from rattlesnake venom (Crotalus durissus cascavella), to better understand the enzymatic and pharmacological mechanisms of the PLA2 and its complex.
Results
This PLA2 consisted of 122 amino acids (approximate molecular mass of 14 kDa), its pI was estimated to be 8.3, and its amino acid sequence shared a high degree of similarity with that of other neurotoxic and enzymatically-active PLA2s. BTL-2 had a molecular mass estimated in approximately 9 kDa and was characterized as a basic protein. In addition, BTL-2 did not exhibit any enzymatic activity.
The PLA2 and BTL-2 formed a stable heterodimer with a molecular mass of approximately 24–26 kDa, estimated by molecular exclusion HPLC. In the presence of BTL-2, we observed a significant increase in PLA2 activity, 23% higher than that of PLA2 alone. BTL-2 demonstrated an inhibition of 98% in the growth of the Gram-positive bacterial strain, Clavibacter michiganensis michiganensis (Cmm), but only 9.8% inhibition of the Gram-negative bacterial strain, Xanthomonas axonopodis pv passiflorae (Xap). PLA2 decreased bacterial growth by 27.3% and 98.5% for Xap and Cmm, respectively, while incubating these two proteins with PLA2-BTL-2 inhibited their growths by 36.2% for Xap and 98.5% for Cmm.
PLA2 significantly induced platelet aggregation in washed platelets, whereas BTL-2 did not induce significant platelet aggregation in any assay. However, BTL-2 significantly inhibited platelet aggregation induced by PLA2. In addition, PLA2 exhibited strong oedematogenic activity, which was decreased in the presence of BTL-2. BTL-2 alone did not induce oedema and did not decrease or abolish the oedema induced by the 48/80 compound.
Conclusion
The unexpected results observed for the PLA2-BTL-2 complex strongly suggest that the pharmacological activity of this PLA2 is not solely dependent on the presence of enzymatic activity, and that other pharmacological regions may also be involved. In addition, we describe for the first time an interaction between two different molecules, which form a stable complex with significant changes in their original biological action. This opens new possibilities for understanding the function and action of crude venom, an extremely complex mixture of different molecules.
Background
Lectins are carbohydrate-binding proteins of non-immune origin found in a wide variety of living organisms that decipher glycocodes in the structure of glycans attached to soluble and integral cell membrane glycoconjugates [1]. Mechanisms for sugar recognition in microorganisms, plants, and animals are independently involved in several protein frameworks [2]. Protein-carbohydrate interactions play biological roles in many cellular processes, such as cell communication, host defence, fertilization, development, parasitic infection and tumour metastasis. Although, in many instances, their exact biological roles remain unknown, many lectins have been extensively studied as biochemical tools in biotechnology and biomedical research. Marine algal lectins, however, have been described at a low pace, since the first report on their haemagglutinating activity 40 years ago [3]. Marine organisms are recognized as rich sources of diverse and biologically-active molecules, many lectins from red and green marine algae have been isolated and characterised to date from more than 40 species. Amongst activities of potential therapeutic and diagnostic interest are the inhibition of fungal growth [4], induction and inhibition of human lymphocyte transformation [5], identification of methacillin-resistant Streptococcus aureus [6], induction of mitogenic activity [7], antibiotic activity against marine vibrios [8], endothelium-dependent relaxation of the rat aorta [9], inhibition of platelet aggregation [10], and as an anti-HIV protein [11].
In each family of lectins, the structure of their carbohydrate recognition domains (CRDs) is essentially conserved. Similar domains have been found in some phospholipase A2 (PLA2) receptors named M and N-type and play an important role in the pharmacological activity of PLA2 [12]. Phospholipases A2 (PLA2) (EC 3.1.1.4) from snake venom are very small proteins that catalyse the hydrolysis of glycerophospholipids at the sn-2 position in a Ca+2-dependent reaction, releasing lysophospholipids and fatty acids [13]. Snake PLA2s have many pharmacological effects, such as: neurotoxicity, myotoxicity, haemolytic activity, haemorrhagic and oedematogenic activities [13,14]. PLA2s that have lysine at the position 49 (Lys 49 or K49) [15] structurally keep the same motifs of PLA2s that have aspartate at the position 49 (Asp 49 or D49, catalytically active), but their enzymatic activity is lost. Despite this, these proteins display many biological activities, regardless of arachidonic acid release [16-18].
In 2000, Hori et al [19] developed the haemagglutinating activity assay, combining algal lectins (hypnin A) and PLA2s from the snakes, Naja naja and Crotalus adamanteus, and found an interaction between proteins, demonstrated by the inhibition of haemagglutination at a relatively low concentration [19]; it was suggested that this effect probably was dependent on a protein-recognition site present in the lectin surface where the PLA2 would bind. Thus, some algae lectin-like proteins may play other important biological functions, such as providing an alternative source of a novel class of PLA2 inhibitors. In this article, we investigate the effects induced by a new isolectin from the red marine alga, Bryothamnion triquetrum, on some of the biological activities of PLA2 and the interaction with catalytically-active PLA2 from snake venom.
Results
After the first purification step, on a preparative DEAE column, the main fraction, named Bryothamnion triquetrum lectin (BTL), was obtained and then dialyzed and lyophilised. The chromatography of BTL showed the presence of two main fractions, further named BTL-1 and BTL-2, which is the most abundant isoform isolated (Fig. 1a). After Reverse Phase HPLC, lectins were obtained with a high degree of purity (Fig. 1b). SDS-PAGE of BTL-2 revealed the presence of one main protein band with an estimated molecular mass of 9.0 kDa (Fig. 1c). The N-terminal sequences of both lectins were deduced and showed a high homology with BTL [20] and hypnins [19] (Fig. 2a). In addition, BTL-1 and BTL-2 had a pI of around 8.6. Circular dichroism spectra of BTL-2 were obtained in the wavelength region of 190 – 250 nm, revealing mostly random coil structures and a low content of α-helices or β-sheets (Fig. 2b).
Figure 1.
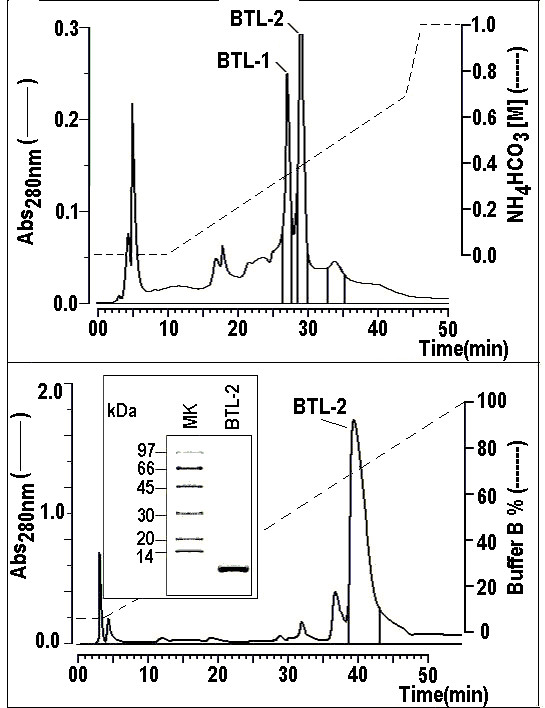
1a) HPLC profile of BTL-2 using an ion exchange column, protein Pack SP 5PW (Waters). Approximately 5 mg of the sample, obtained from the DEAE column, were dissolved in 0.05 M ammonium bicarbonate and applied onto the chromatographic column. The chromatography showed the presence of two main fractions containing haemagglutinating activity, named BTL-1 and BTL-2. The run was monitored at 280 nm and the elution was conducted using a discontinuous linear gradient of ammonium bicarbonate at a concentration of 1.0 M, pH 8.0, constant flow rate of 1 mL/min. 1b) The BTL-2 fraction was subjected to a final chromatography in a Reverse phase HPLC fractionation using a μ-Bondapack C18 column. The elution of samples was carried out using a linear gradient concentration of an aqueous solution of 66% acetonitrile and was monitored at 280 nm at a constant flow rate of 1 mL/min. Samples were previously dissolved in buffer A (0.1% TFA) that was used to equilibrate the column. 1c) Following the method [43], a discontinuous electrophoresis was performed with a final acrylamide concentration of 12% in the running gel. All the samples and the molecular markers were treated with SDS and DTT 1 M and the run was conducted at 40 mA. The samples were stained with Coomassie brilliant blue R-250. The SDS-PAGE profile of BTL-2 showed high molecular homogeneity in a single band with estimated molecular mass of approximately 9.0 kDa.
Figure 2.
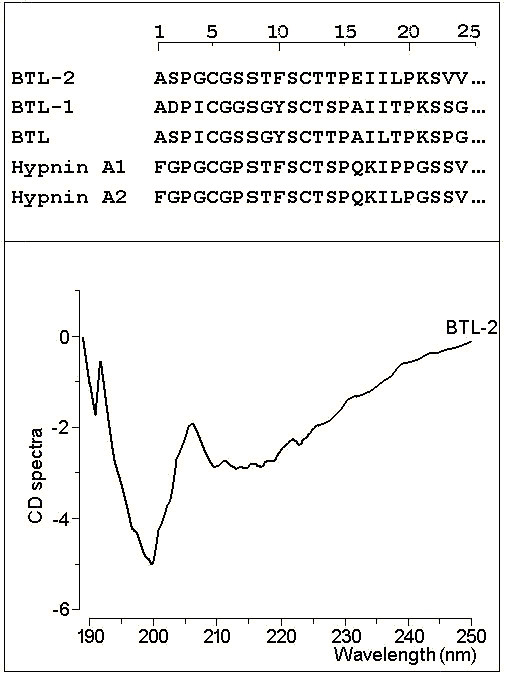
2a) BTL-1 and BTL-2 were previously reduced and carboxymethylated and later sequenced in an automatic gas-liquid sequencer Procise f (Applied Biosytem). 2b) The CD spectra were obtained using a J715 spectropolarimeter (Jarco Cop., Japan). BTL-2 was dissolved in water and the solution was adjusted to a final concentration of 10 mM of protein.
PLA2 was purified to high molecular homogeneity by reverse phase HPLC (Figure 3a), which resulted in only one electrophoretic band (Figure 3b). The estimated pl value of this purified protein was 8.3 (Figure 3c). Molecular exclusion chromatography indicated a molecular mass of approximately 10.0 kDa for the isolated BTL-2; 15.0 kDa for the isolated PLA2, while the PLA2 and BTL-2, when incubated for 30 minutes, had a single peak with a molecular mass of approximately 24.0 – 26.0 kDa (Figure 3d).
Figure 3.
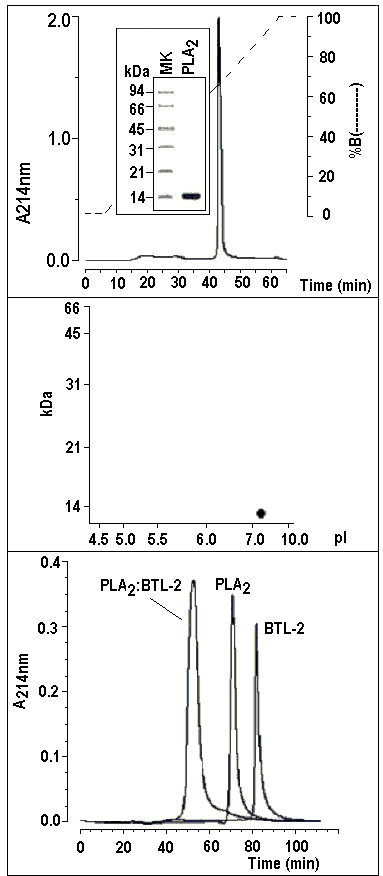
3a) The venom from the Crotalus durissus cascavella rattlesnake was fractionated by HPLC molecular exclusion chromatography (Superdex 75 column 1 × 60 Cmm) and the active fraction was subjected to Reverse Phase HPLC (μ-Bondapack C18 column) to obtain a high purity protein. 3b) Following the Laemmli method [43], a discontinuous electrophoresis was performed with a final acrylamide concentration of 12% in the running gel. All the samples and the molecular markers were treated with SDS and 1 M DTT and the run was conducted at 40 mA. The samples were stained with Coomassie brilliant blue R-250. The SDS-PAGE profile of PLA2 showed high molecular homogeneity in a single band with an estimated molecular mass of approximately 14.0 kDa. 3c) Two dimensional electrophoresis showing one protein spot with molecular mass of around 14 kDa and pI of approximately 8.3. 3d) One milligram of sample was dissolved in 0.5 mL of 0.05 M phosphate buffer, pH 7.5, and applied to a Protein Pack TSK gel 3000 column (0.8 Cmm 30 Cmm) equilibrated with this same buffer. This procedure was performed with one milligram of each protein, incubated together and with all molecular mass markers to estimate the molecular weight of the samples. It is possible to observe a peak with an estimated molecular mass of 10 kDa from BTL-2, another with 15 kDa from PLA2 and another of approximately 24–26 kDa that corresponds to both incubated proteins.
The PLA2 was characterized as an enzymatically-active D49 PLA2 with moderate enzymatic activity in comparison with PLA2 from Naja naja. In the presence of BTL-2, we observed a significant increase of 23% in the enzymatic activity of PLA2, when compared with the PLA2 alone (Figure 4a).
Figure 4.
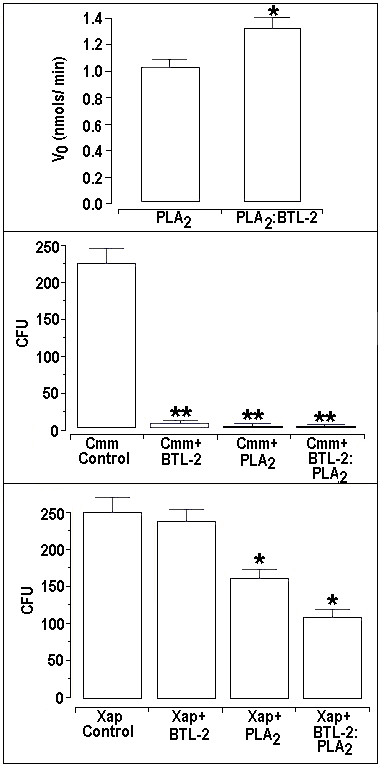
4a) The enzymatic activity assay [45] was analyzed using an Elisa plate containing 200 μL of buffer (10 mM Tris-HCl, 10 mM CaCl2, 100 mM NaCl, pH 8.0), 20 μL of substrate (3 mM, 4-nitro-3-octanoyloxy-bencoic acid), 20 μL of water and 20 μL of the sample at a concentration 1 μg/μL or 2 μg/μL when both proteins were incubated together. After 20 minutes from the beginning of the reaction, the absorbance was measured at 425 nm using a SpectraMax 340 multiwell plate reader (Molecular Devices). It is possible to observe that the enzymatic activity of the PLA2 was increased in the presence of BTL-2. 4b) and 4c) Clavibacter michiganensis subsp. michiganensis and Xanthomonas axonopodis pv.passiflorae were harvested from fresh agar plates and suspended in distilled and sterilized water (A600 nm = absorbance). BTL-2 demonstrated an inhibition of 98% of the growth of the Gram-positive bacterial strain, Clavibacter michiganensis subsp. michiganensis (Cmm), but only a 9.8% inhibition of the Gram-negative bacterial strain, Xanthomonas axonopodis pv passiflorae (Xap). PLA2 decreased Xap bacterial growth by 28.3% and Cmm growth by 98.5%, whilst incubation of these two proteins PLA2-BTL-2 inhibited their growths by 36.2% for Xap and 98.5% for Cmm.
BTL-2 demonstrated an inhibition of 98% in the growth of the Gram-positive bacterial strain, Clavibacter michiganensis subsp. michiganensis (Cmm), but only a 9.8% inhibition of the Gram-negative bacterial strain, Xanthomonas axonopodis pv passiflorae (Xap). PLA2 decreased Xap bacterial growth by 28.3% and Cmm growth by 98.5%, whilst incubation of these two proteins PLA2-BTL-2 inhibited their growths by 36.2% for Xap and 98.5% for Cmm (Figures 4b and 4c).
The PLA2 isolated from Crotalus durissus cascavella showed a strong platelet aggregation activity in washed platelets (Figures 5 and 6). The aggregation induced by PLA2 was very effective (Figure 5b), while BTL-2, at same concentration, did not produce any effect (Figure 5a). When PLA2 was incubated with BTL-2, we observed a significant decrease in the effect of PLA2 on platelet aggregation in the concentrations of 1 μg and 3 μg, respectivaly, 72.7% and 48.8%, when compared to PLA2 alone (Figure 5c). The averaged platelet aggregation assay data is depicted in Figure 6.
Figure 5.
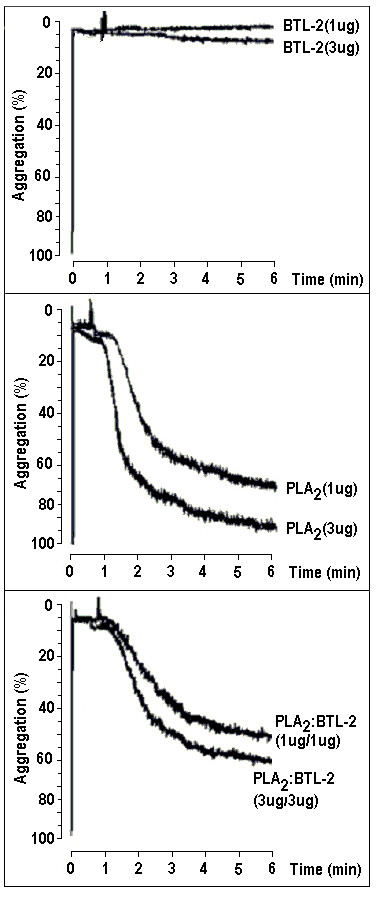
The washed platelets assay was performed with venous blood collected from healthy volunteers. The blood was centrifuged in 3.8% trisodium-citrate for 10 minutes at 200 × g and the residue was centrifuged again for 20 minutes at 1500 × g. Aggregations were performed in 1 μg and 3 μg concentrations in 20 μL of BTL-2, PLA2 and the two proteins (v/v). 5a) BTL-2 induced low aggregation, however the PLA2 induced high aggregation at these concentrations, and both proteins induced a lower aggregation when compared with the PLA2 alone.
Figure 6.
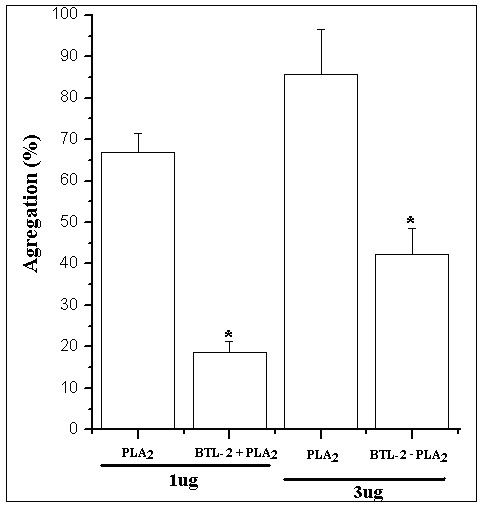
6a) For the concentrations 1 μg and 3 μg, the PLA2 induced high aggregations. When PLA2 was incubated for 30 minutes with BTL-2 at the same concentrations of 1 μg/1 μg and 3 μg/3 μg (v/v) in 20 μL volume it was possible to observe that the PLA2 induced a lower aggregation when compared to the PLA2 alone (n = 5; p < 0.05).
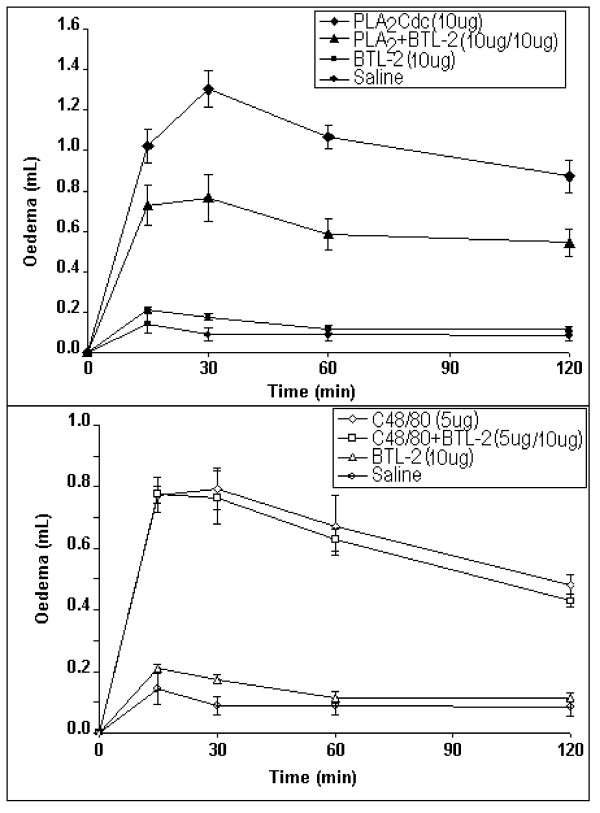
BTL-2, injected in the paw of rats, did not induce an evident inflammation and its oedematogenic effect was similar to that of the saline control (Figure 7a). PLA2 from C. durissus cascavella venom induced a strong oedema response at 15 minutes following injection. The maximum inflammatory response was observed at 30 min following injection of PLA2. Under the same experimental conditions, PLA2 incubated with BTL-2, exhibited a lower oedematogenic effect, compared to non-incubated PLA2 (Figure 7a). In addition, BTL-2 did not inhibit the oedema induced by compound 48/80 (C48/80), a potent oedematogenic agent that induces the release of inflammatory mediators (Figure 7b).
Figure 7.
Male Wistar rats (120 g–150 g) were anaesthetised with halothane (inhaled). The paw oedema was induced by a single subplantar injection of 100 μL of sample 100 μg/paw). Paw volume was measured immediately before the injection and the intervals thereafter (15, 30, 60 and 120 minutes) using a hydroplethysmometer (model 7150, Ugo Basile, Italy). 7a) BTL-2 induced similar oedema simihat of the saline control. PLA2 showed the highest oedema at 30 minutes before the injection. The presence of BTL-2 decreased the oedema induced by the enzyme. 7b) The compound 48/80 (C48/80) was used, incubated with BTL-2, to show that the lectin was not acting on the tissues to decrease the action of PLA2, but was probably binding to the enzyme. Thus, the lectin did not decrease the action of C48/80 act.
PLA2 from Crotalus durissus cascavella shared a high degree of amino acid similarity with other rattlesnake venom PLA2s, such as the PLA2s isolated from Crotalus durissus terrificus isoforms 15, 16 and 17 (Cdt F15, F16 and F17), CROTOX B and MOJAVE B (Figure 8). Furthermore, the amino acid sequence of PLA2 was highly conserved at the N-terminal region, calcium-binding region, active site, α-helical and β-wing. Based on its three dimensional structure, Crotalus durissus cascavella PLA2 exhibited a high number of random coil structures, mainly in the calcium binding and the C-terminal loops (Figure 9a and 9b). The 3D model also suggests the presence of an extensive loop that connects helix 1 to helix 2 and other significantly long random coil structures on the C-terminal region (Figure 9a) in this PLA2.
Figure 8.
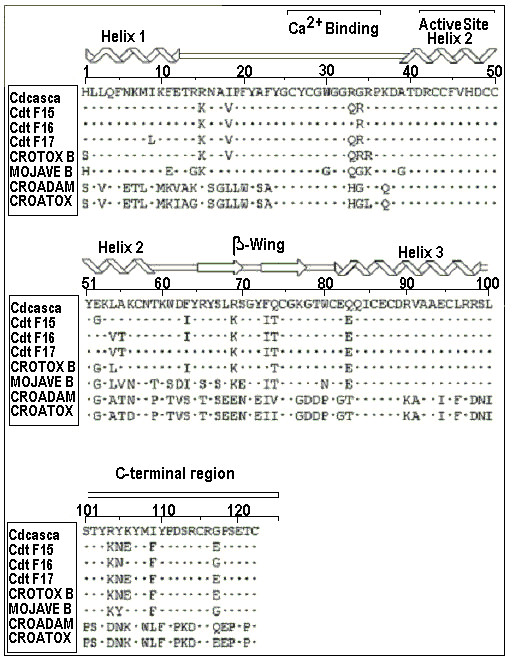
Amino acid sequence of PLA2 isolated from Crotalus durissus cascavella and its estimated secondary structure. The calcium binding loop, active site and C-terminal region are present in the sequence. Note the highly conserved calcium binding loop and active site, and an extensive portion of basic amino acid residues, such as Arg and Lys, in the C-terminal. This PLA2 showed high homology with other PLA2s, such as the isoforms from Crotalus durissus terrificus (Cdt F15, F16 and F17). Crotoxin B (cloned basic subunit of crotoxin) and mojave b (PLA2 subunit of Crotalus scutulatus scutulatus).
Figure 9.
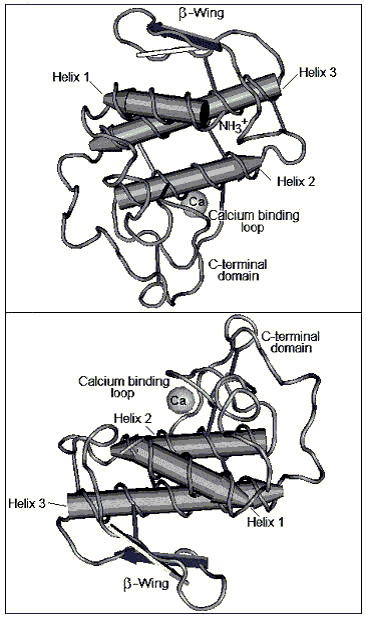
The three dimensional model of PLA2 from Crotalus durissus cascavella venom was determined by comparative homology modelling using BLASTP in Protein Data Bank. The model shows the β-wing, α-helix, calcium binding loop, and C-terminal loop.
Discussion and Conclusion
The interaction between a marine alga lectin and a PLA2 from a rattlesnake was first detected by Hori et al 2000 [19]. Herein, we report the marine alga extract purification of BTL-2, one of the lectin isoforms isolated from the red marine alga Bryothamnion triquetrum. Lectins from B. triquetrum, with low molecular weight, share similar structural features with other low molecular weight lectins isolated from red algae, such as Hypnea japonica [19]. These structural similarities found suggest the importance of this protein for the development of these organisms and define a low molecular weight class of lectin.
Hori et al (2000) [19] suggested that the interaction between marine alga lectin and PLA2 was correlated to the presence of a specific interaction domain. Experiments conducted by these authors showed that the haemagglutination activity of hypnins was strongly inhibited by the addition of PLA2, which strongly suggests that the interaction between hypnins and PLA2 involves specific molecular recognition. Accordingly [19], hypnins have two distinct binding sites; a carbohydrate-recognition site(s) (probably containing a C-type CRD motif) and a protein-recognition site(s) (phospholipase A2-binding site). Interestingly, the interaction between PLA2 with hypnins did not affect the hemolytic activity induced by PLA2.
The N-terminal amino acid sequence of BTL-2 has a high homology with the lectins of Hypnea japonica, which probably possess the C-type CDR motif [19], as such BTL-2 probably possesses these same sites in its structure. The CD spectra analysis revealed that both BTL-2 and BTL-1 have a predominance of random coils, stabilized by disulfide bridges, conferring some interesting properties to these proteins such as resistance to high temperatures, very similar to hypnins which possess thermostability [19].
The catalysis of enzymatically-active PLA2 can be affected by factors such as substrate, and PLA2 activity is strongly enhanced by aggregated substrates that include micelles or monolayers [21]. Another important factor is substrate concentration, when the Critical Micelle Concentration (CMC) is reached, this enhances the enzymatic activity by several orders of magnitude [22,23]. Conformational changes in the structure of PLA2 are also an important factors that increase enzymatic activity [24]; these changes are located in the N-terminal residue and in the surface binding loop in which the calcium binding loop is located. The main conformation change, located in the N-terminal helix, has a small shift towards the calcium ion of PLA2. The role and structural relevance of the N-terminal region have also been reported [25-27]. In addition, the suppression or the chemical modification of the N-terminal region results in a strong reduction in the enzymatic activity of catalytically-active PLA2.
It has been reported that highly-purified heparin sodium salt is able to increase the enzymatic activity of catalytically-isolated PLA2 from Crotalus durissus terrificus venom [28,29]. The potentialization of the enzymatic activity of heparin has been observed in the treatment of some vascular disorders in clinical trials. On the other hand, the inhibitory effects of the neurotoxic or other pharmacological activities of PLA2 are well known. The mechanism of action of PLA2 towards heparin involves inducible conformation changes to the active site, the N-terminal region, and strong binding with the cationic site located in the C-terminal region [30-32], which leads to the modifications in the biological effects induced by the catalytically-active PLA2.
Our results showed that this novel basic hypnin-like lectin, isolated from Bryothamnion triquetrum, seems to form a heterodimeric structure with PLA2 from the Crotalus durissus cascavella venom and that its molecular binding strongly increases the enzymatic activity of PLA2. Furthermore, our data suggest that the complex that is established between PLA2, from Crotalus durissus cascavella, and lectin, from Bryothamnion triquetrum, probably involves a hydrophobic contact and indirectly affects the catalytic site of PLA2. Thus, our results show that some pharmacological activities, induced by PLA2 isolated from Crotalus durissus cascavella, did not involve its solely enzymatic activity, indicating that the specific actions induced by this PLA2 are due to the presence of pharmacological sites on the protein surface, which are distinct from catalytic sites, allowing PLA2 to bind specifically to soluble proteins or membrane-bound proteins that participate in the mechanism of action and this is not an isolated case, as observed for other PLA2 from other sources [33,34]. The antibacterial activity, however, is very dependent on the ability of this PLA2 to destroy the bacterial membrane. These facts are supported by the results of the bacterial growth rate of Xap, which was strongly inhibited by a complex formed by PLA2 and BTL-2.
Conversely, the platelet aggregation assay showed that the presence of the lectin significantly decreased the PLA2 aggregation effect, showing that the pharmacological activity of the enzyme was affected by BTL-2. This same effect occurred in the oedema assay, where in the presence of the lectin, the pharmacological activity of the PLA2 was decreased, inducing a lower oedema. The hydrolysis of arachidonic acid (AA) by the PLA2 generates free AA and lysophospholipids that are the precursors of eicosanoids and platelet-activating factor, respectively [35]. At first, it was thought that secretory PLA2s served to provide substrates for the biosynthesis of proinflammatory lipid mediators; however, it was found that not all PLA2 hydrolyze AA from intact mammalian cells, suggesting that the generation of lipid mediators is not a general function of this enzyme [36,37]. A great breakthrough was the identification of other biological effects of PLA2 in cells involved in inflammatory and immune responses. As such, these results support the idea of the presence of another pharmacological site in the PLA2, isolated from the Crotalus durissus terrificus, which is distinct from the enzymatic site. Something similar is observed in some other PLA2 that interact with target cells via membrane peptidoglycans and with specific or promiscuous receptors [11] and, in some cases, lead to activation of some intracellular cytoplasmic PLA2 and several proteins including PKA and PKC [37-39]. Some specific mutation studies carried out with PLA2 showed that the binding of secretory PLA2 to the receptor involves a specific recognition of the CRD region of this receptor [34,36,37], which is located near the catalytic site of PLA2. Our results, however, suggest that the PLA2 region involved in the binding with BTL-2 produces a structural change that results in the reduction of its pharmacological action, compared to untreated PLA2. The C48/80 experiment supports this argument, since it shows that the lectin was not binding to the tissues to hinder the C48/80 or PLA2 activity, but that it was acting in the PLA2.
Class I and II secretory PLA2s possess an antiparallel, poorly conserved β-wing. The structural conformation, though variable in relation to the rest of the enzyme, is internally consistent across species. The wing extends outwards into the solvent, and the 'wingtip' is poorly anchored. It has been suggested that biological properties such as neurotoxicity, myotoxicity or anticoagulation may be associated with the β-wing [40-42]. The presence of a similar β-wing motif in the 3D model of the PLA2, isolated from Crotalus durissus cascavella venom, may explain its remaining pharmacological activity, which probably has two distinct pharmacological sites, one located in the random coil that links the N-terminal helix with the helix 2 and the β-wing.
The main conclusions of this study are that; 1) the pharmacological activity of PLA2 from Crotalus durissus cascavella is completely dissociated from its enzymatic activity and also some of its biological activity. 2) The PLA2 model revealed that the presence of an extensive hydrophobic random coiled region may be involved in the interaction with BLT-2, forming a random-coil based structure.
Methods
Source of algae
Specimens of the red marine alga Bryothamnion triquetrum were collected from the Atlantic Coast of Brazil (Pacheco Beach, Ceará) and kept in plastic bags at -20°C until processed.
Lectin purification
Algae were thawed, rinsed with distilled water, cleaned of epiphytes, and ground to a fine powder under liquid nitrogen. The powder was stirred for 4 hours with 0.02 M phosphate buffer (pH 7.0), with 0.15 M NaCl, filtered through nylon tissue, and then centrifuged at 7,000 × g, for 30 min, at 4°C. The supernatant was acidified and remained under refrigeration for 4 hours. The precipitated pigments were removed by centrifugation and the supernatant (crude extract) was adjusted to pH 7.0, followed by ammonium sulphate precipitation (0–60%). Precipitated proteins were recovered by centrifugation, dialyzed in distilled water and applied onto a DEAE-cellulose column, equilibrated and eluted with 0.02 M phosphate buffer (pH 7.6), followed by elution with 1.0 M NaCl solution in the same buffer, at a flow rate of 30 mL/h. The unabsorbed fractions with haemagglutinating activity were pooled and rechromatographed in the same column. Active fractions containing lectins were dialyzed and lyophilised (DEAE fraction).
After that, approximately 5 mg of freeze-dried DEAE-fraction was dissolved in 250 μL of ammonium bicarbonate buffer (0.05 M; pH 7.9) and homogenised until complete dissolution, followed by clarification by centrifugation (4,500 × g for 2 min, at 27°C). The supernatant was recovered and injected onto an ion exchange HPLC column (Protein Pack SP 5PW, 0.75 × 10 cm, Waters), previously equilibrated with the same buffer used for dissolving the fraction. The elution was conducted using a discontinuous linear gradient of ammonium bicarbonate at a concentration of 1.0 M, pH 8.0, at constant flow rate of 1 mL/min.
The chromatography was monitored at 280 nm and the peaks were collected and the two fractions containing haemagglutinanting activity were lyophilised and than subjected to the final chromatographic steps of the Reverse Phase (RP-HPLC). The elution of samples was carried out using a linear gradient concentration of an aqueous solution of 66% acetonitrile. Samples were previously dissolved in buffer A of RP-HPLC (0.1% TFA) that was used to equilibrate the column. The fractions were immediately concentrated by ultrafiltration (AMICON), followed by dialysis with 5.0 mM ammonium bicarbonate solution (pH 7.8), sterilized by microfiltration (0.22 μm) and stored at 4°C for further use.
Electrophoresis
The electrophoresis was carried out following the Laemmli method [43]. The degree of purity of fractions was assessed by discontinuous electrophoresis using a final acrylamide concentration of 12% in the running gels (1.0 M Tris-HCl, pH 8.8) and 5% in the stack gel (0.5 M Tris-HCl, pH 6.8). The electrophoretic run was carried out using a BIORAD electrophoretic system for SDS-PAGE. All samples and the molecular marker were treated with SDS and 1.0 M DTT and the run was conducted at 40 mA for both plates. After electrophoresis, samples were stained with Coomassie brilliant blue R-250.
Circular dichroism
The purified BTL-2 was dissolved in water and the solution was adjusted to a final concentration of 10 mM of protein. The sample was transferred to a 1 mm path-length quartz cuvette. Circular dichroism spectra in the wavelength range of 185–300 nm were obtained using a J715 spectropolarimeter (Jasco Corp., Japan) using a bandwidth of 1 nm and a response time of 1s. Data collection was performed at room temperature with a scanning speed of 100 nm/min; a total of nine scans were accumulated for the sample.
PLA2 purification
PLA2 from the rattlesnake, Crotalus durissussus casvella, was purified by a combination of chromatographic procedures and its molecular homogeneity was determined by SDS-PAGE. The whole venom was fractionated by HPLC molecular exclusion chromatography using a Superdex 75 column (1 × 60 cm). The active PLA2 fraction was then subjected to Reverse Phase HPLC in a C18 μ-Bondapack column (0.78 cm × 30 cm) (Waters 991-PDA system). The column was eluted with a linear gradient (0–66.5%, v/v) of acetonitrile (solvent B) at a flow rate of 2 mL/min, and absorbance was monitored at 280 nm. The fractions were collected manually, lyophilized and stored at -20°C.
Amino acid sequence
Three milligrams of purified protein were dissolved in 200 μl of 6 M guanidine chloride (Merck, Darmstadt, Germany) containing 0.4 mM Tris-HCl and 2 mM EDTA (final pH 8.15). Nitrogen was flushed over the top of the protein solution for 15 min, which was then reduced with DTT (6 M, 200 μl) and carboxymethylated with 14C-iodoacetic acid and cold iodoacetic acid. Nitrogen was again flushed over the surface of the solution and the reaction tube sealed. This solution was incubated in the dark at 37°C for 1 h and desalting was performed on a Sephadex G 25 column (0.7 × 12 cm) in 1 mM acetic acid buffer. The eluted reduced and carboxymethylated (RC) protein was lyophilised and stored at -20°C. The RC lectins were then purified and the N-terminal region sequenced.
For the RC-PLA2, we determined the complete amino acid sequence of the protein by sequencing the N-terminal region and the peptides from enzyme-treated PLA2. One sample of RC-protein was digested with Staphylococcus aureus protease V8 for 16 hours at 37°C, using an enzyme -substrate ratio of 1:30. The reaction was stopped by lyophilisation. A second sample of RC-PLA2 was digested with Clostripain for 8 hours at 37°C and then lyophilised [44]; the products were separated by Reverse Phase HPLC using a Waters PDA 991 system with a C-18 μ-Bondapack column.
Peptide peaks were isolated using a linear gradient (0–100% of acetonitrile in 0.1% TFA (V/V)). A third sample (2 mg) was cleaved with a 15-fold molar excess of cyanogens bromide (CNBr) over methionine residues in 70% formic acid (4 ml) under nitrogen for 24 hours at room temperature. The reaction mixture was then diluted with 40 mL of water and lyophilised. Excess reagents were removed by gel filtration on a Sephadex G-25 column (1 × 20 cm) equilibrated with 10% acetic acid. The CNBr peptide fragments were separated by Reverse Phase HPLC, using an analytical μ-Bondapack C18 column (0.39 × 30 cm; Waters) with 0.1% TFA as solvent A and acetonitrile containing 30% of solvent A (solvent B). The elution profile was monitored at 214 nm. Analysis of the amino acid sequence of the RC-protein, as well as that of the enzymatically-digested fragments were performed with an Applied Biosystems model Procise f gas-liquid protein sequencer. The phenylthiohydantoin (PTH) derivatives of the amino acids were identified with an Applied Biosystems model 450 microgradient PTH-analyser.
PLA2 activity
PLA2 activity was measured [45]. The standard assay mixture contained 200 μL of buffer (10 mM Tris-HCl, 10 mM CaCl2, 100 mM NaCl, pH 8.0), 20 μL of 2.98 mM substrate (4-nitro-3-octanoyloxy-benzoic acid; 4N3OBA), 20 μL of water, and 20 μL of PLA2 (1 mg/mL) in a final volume of 260 μL. After the addition of PLA2, the mixture was incubated for 20 min at 37°C. The increase in absorbance at 425 nm, due to product formation, was monitored during this period and the activity was expressed as nmols of product formed nmols/min/mg. The inhibitory effect of BTL-2 (1 mg/mL) on PLA2 activity was investigated by incubating the two proteins at 37°C for 30 min prior to assaying the enzyme activity using the SpectraMax 340 multiwell plate reader (Molecular Devices).
HPLC molecular exclusion chromatography of BTL-2 andBTL-2-PLA2
One milligram of PLA2 from C. d. cascavella was dissolved in 0.5 mL of 0.05 M phosphate buffer, pH 7.5, and applied to a Protein-Pack TSK gel 3000 column (0.8 cm × 30 cm), previously equilibrated with the same buffer before use. BTL-2 and PLA2 (1:1) were preincubated together for 30 minutes in phosphate buffer. Separately, molecular mass markers (bovine serum albumin, 66 kDa; egg album, 45 kDa; carbonic anhydrase, 29 kDa, and lysozyme 14 kDa) were used to estimate the molecular weight of PLA2, BTL-2 and PLA2:BTL-2. All molecular weight markers were dissolved in phosphate buffer at a concentration of 2 mg/mL. The proteins were eluted at a flow rate of 0.2 mL/min and the absorbance profile was monitored at 214 nm.
Platelet aggregation studies
Sample collection and aggregation studies
Venous blood was collected with informed consent from healthy volunteers who denied taking any medication in the previous 14 days. Blood was collected by a two-syringe technique using polypropylene syringes and 19-gauge needles, and immediately transferred into polypropylene tubes containing 1/10 of final volume of 3.8% trisodium citrate.
After removing the platelet-rich plasma (PRP), the remaining blood was prepared by centrifugation of citrated blood at 200 × g for 10 min and washed platelets (WP) were obtained from the residue by centrifugation of citrated blood at 1500 × g for 20 min. The platelets were left for 1 h at room temperature to recover their sensitivity to aggregating agents. Platelet counts were performed on a Coulter S Plus (Coulter Electronics, Hialeah, FL) or by phase-contrast microscopy. Platelet aggregation was carried out using 400 μL of the washed platelets solution in a cuvette and kept at 37°C with constant stirring.
The desired concentration of protein was added 3 minutes prior to the addition of platelet aggregation inducers (thrombin for washed platelets). Subsequently, the aggregation was recorded for 5–10 min by using an aggregometer (Payton Scientific Instruments, Inc, Buffalo, NY). Aggregation experiments were performed in two concentrations (1 μg and 3 μg) of BTL-2, PLA2, and PLA2 incubated with BTL-2 (v/v) in a final volume of 20 μL, which was previously incubated (30 min) at room temperature.
Paw oedema assay
Male Wistar rats (120–150 g) were anaesthetised with halothane (inhaled). The rat paw oedema was induced by a single subplantar injection of 100 μL of BTL-2 (10 μg/paw), PLA2 (10 μg/paw), and PLA2 incubated with BTL-2 (v/v). Paw volume was measured immediately before the injection of the samples and at selected time intervals thereafter (15, 30, 60, and 120 minutes) using a hydroplethysmometer (model 7150, Ugo Basile, Italy). The compound 48/80 (C48/80) was incubated with BTL-D2 to show that the lectin was not acting on the tissues to decrease the action of the PLA2. All samples were dissolved in sterile saline solution (0.9%). Results were expressed as the increase in paw volume (mL) and calculated by subtracting the basal volume.
Antibacterial assay
Xanthomonas axonopodis pv.passiflorae (Xap) and Clavibacter michiganensis subsp. michiganensis (Cmm) were harvested from fresh agar plates and suspended in distilled and sterilized water (A600 nm = absorbance, corresponding to 3 × 108 CFU/mL). Aliquots of this suspension were diluted to 3 × 103 CFU/mL and incubated with 100 μL of BTL-2 (1 mg/mL) for 30 min at 37°C. We then determined the survival on Agar Nutrient (Difco) Plate (n = 5).
Prediction of the three-dimensional structure of PLA2
Amino acid sequence similarity and alignment searches were carried out to compare the PLA2 isolated from Crotalus durissus cascavella venom against the Protein Data Bank using BLASTP. The 3D structure of PLA2 was determined by comparative homology modelling. Using computer graphics, the amino acid sequence of PLA2 was fitted to the high-resolution X-ray structure of the homologous 1BJJ_A. This allowed the construction of a model, which was then subjected to energy minimization and molecular dynamic simulations.
Statistical analyses
The results were reported as the means ± SEM of n experiments. The significance of differences between means was assessed by analysis of variance, followed by a Dunnett's test when several experimental groups were compared with the control group. The confidence limit for significance was 5% (p > 0.05).
Authors' contributions
SCBO -carried out all the purifications, activity of the PLA2, eletrophoresis, participated in all the assays, coordination of the study, helped to draft the manuscript and performed the statistical analysis. FVF; EA; EAC; RPM – carried out the pharmacological assays e.g. platelet aggregation studies and the paw oedema assay. RA – carried out the circular dichroism.
DOT; LOSB – carried out the antibacterial assays. EVN; BSC; CSN; AHS; KSN -collected the marine alga and performed the initial purification of the alga lectin. MHT – carried out the amino acid sequencing, prediction of the three-dimensional structure of PLA2, coordination of the study, helped to draft the manuscript and performed the statistical analysis. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support.
Contributor Information
Simone CB Oliveira, Email: simonebuzzo@hotmail.com.
Fabiana V Fonseca, Email: fvmacieira@hotmail.com.
Edson Antunes, Email: edson.antunes@uol.com.br.
Enilton A Camargo, Email: enicamargo@yahoo.com.br.
Rafael P Morganti, Email: rafaelprada@bol.com.br.
Ricardo Aparício, Email: aparicio@iqm.unicamp.br.
Daniela O Toyama, Email: gaveira@yahoo.com.br.
Luís OS Beriam, Email: beriam@biologico.sp.gov.br.
Eudismar V Nunes, Email: eudismar888@hotmail.com.
Benildo S Cavada, Email: bscavada@ufc.br.
Celso S Nagano, Email: naganocs@ibv.csic.es.
Alexandre H Sampaio, Email: sampaioa@ufc.br.
Kyria S Nascimento, Email: kyriasantiago@gmail.com.
Marcos H Toyama, Email: mhtjpn@yahoo.com.
References
- Gabius HJ, Gabius S. Glycoscience: Status and Perspectives. 1. Weinheim: Chapman and Hall; 1997. [Google Scholar]
- Elgavish S, Shaanan B. Lectin-carbohydrate interactions: different folds, common recognition principles. Trends Biochemistry Science. 1997;22:462–467. doi: 10.1016/S0968-0004(97)01146-8. [DOI] [PubMed] [Google Scholar]
- Boyd WC, Almodovar LR, Boyd LG. Agglutinin in marine algae from human erythrocytes. Transfusion. 1966;6:82–83. [Google Scholar]
- Melov V, Medeiros D, Rio F, Casterlar L, Carvalho F. Antifungal properties of proteins from the red alga Hypnea musciformis (Wulfen) Botanica Marina. 1997;40:281–284. [Google Scholar]
- Lima HC, Costa FHF, Sampaio AH, Neves SA, Bevebides NMB, Teixeira DIA, Rogers DJ, Freitas ALP. Induction and inhibition of human lymphocyte transformation by the lectin from the red marine alga Amansia multifida. Journal Applied Phycology. 1998;10:153–162. doi: 10.1023/A:1008016731752. [DOI] [Google Scholar]
- Munoz A, Alvarez O, Alonso B, Lovo J. Lectin typing of methicilin-resistant Staphyloccocus aureus. Journal of Medical Microbiology. 1999;48:495–498. doi: 10.1099/00222615-48-5-495. [DOI] [PubMed] [Google Scholar]
- Ashraf MT, Khan RH. Mytogenic lectins. Med Sci Monit. 2003;9:RA265–269. [PubMed] [Google Scholar]
- Liao W-R, Lin J-Y, Shieh W-Y, Jeng W-L, Huang R. Antibiotic activity of lectins from marine algae against marine vibrios. Journal of Ind Microbiology and Biotechnology. 2003;30:433–439. doi: 10.1007/s10295-003-0068-7. [DOI] [PubMed] [Google Scholar]
- Lima RF, Criddle DN, Souza EP, Sampaio AH, Nascimento KS, Cavada BS, Assreuy AM. Red marine alga Bryothamnion triquetrum lectin induces endothelium-dependent relaxation of the rat aorta via release of nitric oxide. J Pharm Pharmacol. 2004;56:1415–1421. doi: 10.1211/0022357044616. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Sumi H, Hori K. Platelet aggregation is inhibited by phycolectins. Experientia. 1996;52:540–543. doi: 10.1007/BF01969724. [DOI] [PubMed] [Google Scholar]
- Mori T, O'Keefe BR, Sowder RC, 2nd, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, MCmmahon JB, Boyd R. Isolation and Characterization of Griffithsin, a Novel HIV-inactivating Protein, from the red alga Griffithsia sp. The Journal of biological chemistry. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Valentin E, Lambeau G. What can venom phospholipases A(2) tell us about the functional diversity of mammalian secreted phospholipases A(2)? Biochimie. 2000;82:815–831. doi: 10.1016/S0300-9084(00)01168-8. [DOI] [PubMed] [Google Scholar]
- Toyama MH, Mancuso LC, Giglio JR, Novello JC, Oliveira B, Marangoni S. A quick procedure for the isolation of dimeric piratoxins-I and II, two myotoxins from Bothrops pirajai snake venom. N-terminal sequencing. Biochem Mol Biol Int. 1995;37:1047–1055. [PubMed] [Google Scholar]
- Kini RK, Iwanaga S. Structure-function relationships of phospholipases. II: Charge density distribution and the myotoxicity of presynaptically neurotoxic phospholipases. Toxicon. 1986;24:895–905. doi: 10.1016/0041-0101(86)90090-5. [DOI] [PubMed] [Google Scholar]
- Kini RM, Evans HJ. Structure-function relationships of phospholipases. The anticoagulant region of phospholipases A2. Journal of Biological Chemistry. 1987;262:14402–14407. [PubMed] [Google Scholar]
- Maraganore JM, Merutka G, Cho W, Welches W, Kezdy FJ, Heinrikson RL. A new class of phospholipases A2 with lysine in place of aspartate 49. Functional consequences for calcium and substrate binding. J Biol Chem. 1984;259:13839–13843. [PubMed] [Google Scholar]
- Gutierrez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33:1405–1424. doi: 10.1016/0041-0101(95)00085-Z. [DOI] [PubMed] [Google Scholar]
- Arni RK, Ward RJ. Phospholipase A2- a structural review. Toxicon. 1996;34:827–841. doi: 10.1016/0041-0101(96)00036-0. [DOI] [PubMed] [Google Scholar]
- Hori K, Matsubara K, Miyazawa K. Primary structure of two hemagglutinins from the marine red alga Hypnea japonica. Biochemica et Biophysica Acta. 2000;1474:226–236. doi: 10.1016/s0304-4165(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Costa FH, Saker-Sampaio S, Murciano MP, Nagano CS, Cavada BS, Grangeiro TB, Ramos MV, Bloch C, Jr, Silveira SB, Freitas BT, Sampaio AH. The amino acid sequence of the agglutinin isolated from the red marine alga Bryothamnion triquetrum defines a novel lectin structure. Cellular and Molecular Life Science. 2000;57:343–350. doi: 10.1007/PL00000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterson WA, Vidal JC, Volwerk JJ, de Haas GH. Zymogen-catalyzed hydrolysis of monomeric substrates and the presence of a recognition site for lipid-water interfaces in phospholipase A2. Biochemistry. 1974;13:1455–1460. doi: 10.1021/bi00704a021. [DOI] [PubMed] [Google Scholar]
- Volwerk JJ, de Haas GH. In: In: Molecular Biology of Lipid-Protein Interactions. Griffith OH, Jost PC, editor. Wiley New York; 1982. [Google Scholar]
- Berg B Van den, Tessari M, de Haas GH, Verheij HM, Boelens R, Kaptein R. Solution structure of porcine pancreatic phospholipase A2. EMBO Journal. 1995;14:4123–4131. doi: 10.1002/j.1460-2075.1995.tb00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares AM, Andrião-Escarso SH, Bortoleto RK, Rodrigues-Simioni L, Arni RK, Ward RJ, Gutiérrez JM, Giglio JR. Dissociation of enzymatic and pharmacological properties of piratoxins-I and -III, two myotoxic phospholipases A2 from Bothrops pirajai snake venom. Arrchives of Biochemistry and Biophysics. 2001;387:188–196. doi: 10.1006/abbi.2000.2244. [DOI] [PubMed] [Google Scholar]
- Soares AM, Rodrigues VM, Homsi-Brandeburgo MI, Toyama MH, Lombardi FR, Arni RK, Giglio JR. A rapid procedure for the isolation of the Lys-49 myotoxin II from Bothrops moojeni (Caissaca) venom: Biochemical characterization, crystallization, myotoxic and edematogenic activity. Toxicon. 1998;36:503–514. doi: 10.1016/S0041-0101(97)00133-5. [DOI] [PubMed] [Google Scholar]
- Lee WH, Giotto MTS, Marangoni S, Toyama MH, Polikarpov I, Garrat R. Structural Basis for Low Catalytic Activity in Lys49 Phospholipases A2sA Hypothesis: The Crystal Structure of Piratoxin II Complexed to Fatty Acid. Biochemistry. 2001;40:28–36. doi: 10.1021/bi0010470. [DOI] [PubMed] [Google Scholar]
- Rigden DJ, Hwa LW, Marangoni S, Toyama MH, Polikarpov I. The structure of the D49 phospholipase A2 piratoxin III from Bothrops pirajai reveals unprecedented structural displacement of the calcium-binding loop: possible relationship to cooperative substrate binding. Acta Crystallization. 2003;59:255–262. doi: 10.1107/s0907444902021467. [DOI] [PubMed] [Google Scholar]
- Toyama MH, de Oliveira DG, Beriam LOS, Novello JC, Rodrigues-Simioni L, Marangoni S. Structural, enzymatic and biological properties of new PLA(2) isoform from Crotalus durissus terrificus venom. Toxicon. 2003;41:1033–1038. doi: 10.1016/S0041-0101(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Hernandez-Oliveira S, Toyama MH, Toyama DO, Marangoni S, Hyslop S, Rodrigues-Simioni L. Biochemical, pharmacological and structural characterization of a new PLA2 from Crotalus durissus terrificus (South American rattlesnake) venom. Protein Journal. 2005;24:233–42. doi: 10.1007/s10930-005-6718-z. [DOI] [PubMed] [Google Scholar]
- Landucci EC, de Castro RC, Toyama MH, Giglio JR, Marangoni S, de Nucci G, Antunes E. Inflammatory edema induced by the lys-49 phospholipase A(2) homologue piratoxin-i in the rat and rabbit. Effect of polyanions and p-bromophenacyl bromide. Biochemical Pharmacology. 2000;59:1289–1294. doi: 10.1016/S0006-2952(00)00248-3. [DOI] [PubMed] [Google Scholar]
- Boechat AL, Paiva CS, Franca FO, Dos-Santos MC. Heparin-antivenom association: differential neutralization effectiveness in Bothrops atrox and Bothrops erythromelas envenoming. Rev Inst Med Trop Sao Paulo. 2001;43:7–14. doi: 10.1590/s0036-46652001000100002. [DOI] [PubMed] [Google Scholar]
- Bugs MR, Bortoleto-Bugs RK, Comelio ML. The interaction between heparin and Lys49 phospholipase A2 reveals the natural binding of heparin on the enzyme. International Journal of Biol Macromolecules. 2005;37:21–27. doi: 10.1016/j.ijbiomac.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kini RM, Evans HJ. A model to explain the pharmacological effects of snake venom phospholipases A2. Toxicon. 1989;27:613–635. doi: 10.1016/0041-0101(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Lambeau G, Lazdunski M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacology Science. 1999;20:162–70. doi: 10.1016/S0165-6147(99)01300-0. [DOI] [PubMed] [Google Scholar]
- Yedgar S, Cohen Y, Shoseyov D. Control of phospholipase A2 activities for the treatment of inflammatory conditions. Biochim Biophys Acta. 2006;11:1373–1382. doi: 10.1016/j.bbalip.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Triggiani M, Granata F, Oriente A, Gentile M, Petraroli A, Balestrieri B. Secretory phospholipases A2 induce cytokine release from blood and synovial fluid monocytes. Eur J Immunol. 2002;32:67–76. doi: 10.1002/1521-4141(200201)32:1<67::AID-IMMU67>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Granata F, Petraroli A, Boilard E, Bezzine S, Bollinger J, Del Vecchio L, Gelb MH, Lambeau G, Marone G, Triggiani M. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J Immunol. 2005;174:464–474. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
- Hanasaki K, Arita K. Phospholipase A2 receptor: a regulator of biological functions of secretory phospholipase A2. Prostaglandins Other Lipid Mediat. 2002;69:71–82. doi: 10.1016/S0090-6980(02)00022-9. [DOI] [PubMed] [Google Scholar]
- Hanasaki K. Mammalian phospholipase A2: phospholipase A2 receptor. Biology Pharmaceuthic Bulletin. 2004;27:1165–1167. doi: 10.1248/bpb.27.1165. [DOI] [PubMed] [Google Scholar]
- Harris LK, Franson RC. [1-14C]oleate-labeled autoclaved yeast: a membranous substrate for measuring phospholipase A2 activity in vitro. Anal Biochemistry. 1991;193:191–196. doi: 10.1016/0003-2697(91)90007-G. [DOI] [PubMed] [Google Scholar]
- Yang CC. Structure-function relationship of phospholipase A2 from snake venoms. J Toxicol. 1994;13:125–177. doi: 10.1093/toxsci/23.1.125. [DOI] [Google Scholar]
- Soares AM, Andriao-Escaso SH, Angulo Y, Lomonte B, Gutierrez JM, Marangoni S, Toyama MH, Arni RK, Giglio JR. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A(2) homologue from Bothrops moojeni (Caissaca) snake venom. Arch Biochemistry and Biophysica. 2000;373:7–15. doi: 10.1006/abbi.1999.1492. [DOI] [PubMed] [Google Scholar]
- Hames BD, Rickwood D. Gel Electhophoresis of Proteins. A practical Approach. Washington. IRL Press; 1983. pp. 27–33. [Google Scholar]
- Toyama MH, Soares AM, Wen-Hwa L, Polikarpov I, Giglio JR, Marangoni S. Amino acid sequence of piratoxin-II, a myotoxic lys49 phospholipase A(2) homologue from Bothrops pirajai venom. Biochimie. 2000;82:245–250. doi: 10.1016/S0300-9084(00)00202-9. [DOI] [PubMed] [Google Scholar]
- Holzer M, Mackessy SP. An aqueous endpoint assay of snake venom PLA2. Toxicon. 1996;34:1149–1155. doi: 10.1016/0041-0101(96)00057-8. [DOI] [PubMed] [Google Scholar]