Abstract
The notothenioid fishes of the Southern Ocean surrounding Antarctica are remarkable examples of organismal adaptation to extreme cold. Their evolution since the mid-Miocene in geographical isolation and a chronically cold marine environment has resulted in extreme stenothermality of the extant species. Given the unique thermal history of the notothenioids, one may ask what traits have been gained, and conversely, what characters have been lost through change in the information content of their genomes. Two dramatic changes that epitomize such evolutionary transformations are the gain of novel antifreeze proteins, which are obligatory for survival in icy seawater, by most notothenioids and the paradoxical loss of respiratory haemoproteins and red blood cells, normally deemed indispensable for vertebrate life, by the species of a highly derived notothenioid family, the icefishes. Here, we review recent advances in our understanding of these traits and their evolution and suggest future avenues of investigation.
The formerly coherent paradigm of notothenioid freeze avoidance, developed from three decades of study of antifreeze glycoprotein (AFGP) based cold adaptation, now faces challenges stemming from the recent discovery of antifreeze-deficient, yet freeze-resistant, early notothenioid life stages and from definitive evidence that the liver is not the physiological source of AFGPs in notothenioid blood. The resolution of these intriguing observations is likely to reveal new physiological traits that are unique to the notothenioids. Similarly, the model of AFGP gene evolution from a notothenioid pancreatic trypsinogen-like gene precursor is being expanded and refined based on genome-level analyses of the linked AFGP loci and their ancestral precursors. Finally, the application of comparative genomics to study evolutionary change in the AFGP genotypes of cool-temperate notothenioids from sub-Antarctic habitats, where these genes are not necessary, will contribute to the mechanistic understanding of the dynamics of AFGP gene gain and loss.
In humans and most vertebrates, mutations in the α- or β-globin genes or defects in globin chain synthesis are causes of severe genetic disease. Thus, the 16 species of haemoglobinless, erythrocyte-null icefishes are surprising anomalies—in fact, they could only have evolved and thrived due to relaxed selection pressure for oxygen-binding proteins in the cold, oxygen-rich waters of the Southern Ocean. Fifteen of the sixteen icefish species have lost most of the adult αβ-globin locus and retain only a small 3′ fragment of the α-globin gene. The only exception to this pattern occurs in Neopagetopsis ionah, which possesses a disrupted αβ-globin gene complex that probably represents a non-functional intermediate on the evolutionary pathway to near total globin gene extinction. By contrast, six of the icefish species fail to express myoglobin. The absence of myoglobin expression has occurred by several independent mutations and distinct mechanisms. Haemoprotein loss is correlated with dramatic increases in cellular mitochondrial density, heart size, blood volume and capillary bed volume. Evolution of these compensatory traits was probably facilitated by the homeostatic activity of nitric oxide, a key modulator of angiogenesis and mitochondrial biogenesis. These natural knockouts of the red blood cell lineage are an excellent genomic resource for erythroid gene discovery by comparative genomics, as illustrated for the newly described gene, bloodthirsty.
Keywords: Antarctic water temperatures, stenothermal, teleost freeze avoidance paradigm shift, evolutionary genomics, haemoprotein loss, notothenioid fishes
1. Introduction
The opening of the Drake Passage (approx. 34–30 Myr ago) and the formation of the Antarctic Circumpolar Current commenced the thermal isolation of Antarctica (Livermore et al. 2005). Terrestrial and sea-level glaciation followed (Livermore et al. 2005; Tripati et al. 2005) and current frigid conditions were thought to be reached by the mid-Miocene (approx. 10–14 Myr ago; Kennett 1977). The climatic and geographical changes of Antarctica were accompanied by large changes in the Antarctic fish fauna. The late Eocene (38 Myr ago) shallow-water, benthic marine fossil assemblages of the La Meseta Formation on Seymour Island (Aronson & Blake 2001) included a diverse, cosmopolitan and temperate ichthyofauna that has largely disappeared in today's Southern Ocean (Eastman 1993; Eastman & McCune 2000). The modern fish fauna is taxonomically limited, highly endemic and singularly dominated by the fishes of the suborder Notothenioidei in species number and biomass (Eastman & McCune 2000; Eastman 2005). The dominance of notothenioid fishes is the result of some of the most interesting evolutionary biological responses to the advent of polar conditions in the Southern Ocean, at the organismal, physiological and molecular levels. The expansion of the ice sheet due to the abrupt cooling in the late Eocene scoured the continental margin, leading to shelf habitat loss and alterations in the marine fauna (Clarke & Crame 1992; Clarke 1993; Eastman & McCune 2000; Aronson & Blake 2001). Much of the Eocene fish fauna became extinct (Eastman 1993; Eastman & McCune 2000), which created vast ecological opportunities for the remaining fish that could adapt to habitat change. The ancestral notothenioid, presumably a shallow benthic taxon, must have been able to exploit the changing habitats, and in the absence of significant niche competition diversified into the dominant suborder that makes up almost half (46%) of today's Antarctic fish species (Eastman 2005). The dominance is particularly clear and remarkable at the icy, high Antarctic shelves and embayments where notothenioids comprise approximately 77% of species and greater than 90% of fish biomass (Eastman 2005).
The absence of niche competition alone would not have guaranteed the notothenioids' rise to dominance if evolutionary adaptations to the frigid polar environment had not occurred. Evolution in persistently cold and oxygen-rich Antarctic waters over 10–14 million years has forged molecular and genomic changes leading to dramatic outcomes in the physiological and biochemical capacities of these fishes (NRC 2003). Extant notothenioid fishes are cold stenotherms; high-latitude species succumb to thermal stress at approximately 4°C and this incipient lethal temperature appears not to be raised by acclimation in the laboratory (Somero & DeVries 1967). This stenothermal phenotype reflects evolutionary adaptive changes towards cold stability in the molecular and cellular machineries for optimal function in a perennially frigid environment. Examples include efficient microtubule assembly (Williams et al. 1985; Detrich et al. 1989; Detrich et al. 2000; Paluh et al. 2004) and protein translocation across endoplasmic reticulum (Römisch et al. 2003) at low temperatures, high-membrane lipid unsaturation for homeoviscous adaptation (Logue et al. 2000), highly cold-stable lens crystallin proteins to maintain lens transparency at temperatures far below that would induce cold-cataract in temperate fishes or mammals (Kiss et al. 2004), and the apparent loss of the inducibility of the heat shock response in constantly cold ambient temperatures (Hofmann et al. 2000; Buckley et al. 2004). The evolutionary tinkering is especially evident in two large-scale genetic changes, the acquisition of novel antifreeze proteins that are critical for survival in ice-laden freezing marine environments, and the loss of oxygen-binding haemoproteins necessary for survival of eurytherms in variable thermal environments.
‘To freeze or not to freeze’ is all or none for marine teleosts in icy, freezing seawater. Marine teleosts are hyposmotic to seawater (Black 1951; DeVries 1974) and thus have a higher colligative freezing point than seawater. When in contact with environmental ice, inoculative nucleation of the body fluids will rapidly occur, and the fish will freeze and die. The vast niches of the glaciated Southern Ocean left vacant by late Eocene ichthyofaunal extinction could not be exploited by the notothenioid ancestor if it had not evolved the ability to resist freezing. The evolutionary gain of the antifreeze gene and function (Chen et al. 1997a; Cheng & Chen 1999) was the key event to successful colonization of icy water niches, which in turn empowered the adaptive radiation of the Antarctic notothenioids.
The glaciated Antarctic waters, perennially at or near freezing, have much higher concentrations of dissolved oxygen than other marine waters. In this cold, oxygen-rich marine environment where the selection pressure for cellular oxygen-binding proteins was relaxed, haemoglobin expression was lost in the notothenioid predecessor to the icefishes (family Channichthyidae). All extant icefishes lack haemoglobin (Cocca et al. 1995; di Prisco et al. 2002) and some have also lost myoglobin expression (Sidell et al. 1997; Grove et al. 2004). The loss of the haemoproteins has been accompanied by a suite of compensatory cardiovascular and cellular adaptations, the origins of which are now becoming clear.
The requisite antifreeze protective function in all Antarctic notothenioids and the haemoprotein-null state in the icefishes arose from related effects of past climate change on the Antarctic marine environment. These two phenotypes are valuable systems for understanding the interplay of environment, organismal biochemistry and physiology, and evolutionary adaptation. This paper presents a concise review of our progress made in these two areas of Antarctic fish ecological and evolutionary physiology using tools ranging from molecular and genomic analyses to environmental monitoring.
2. Surviving the big chill—notothenioid freezing avoidance by antifreeze proteins
(a) Freezing challenge in frigid Antarctic marine environment
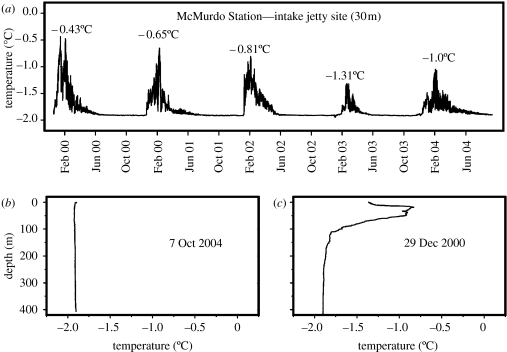
The isolated Antarctic marine environment is the coldest and harshest in the world for ectothermic teleost fishes, the combined result of perennial freezing seawater temperatures (−1.91°C) and the prevalence of ice in the water column (Littlepage 1965; Hunt et al. 2003). There are no other marine counterparts with equivalent extreme properties including the Arctic region (DeVries & Steffensen 2005). In terms of temperature, the cold, stable nature of the high Antarctic near-shore water was long appreciated since the periodic measurements of McMurdo Sound water temperatures by Littlepage (1965). To assess year-round water temperatures and iciness of notothenioid fish habitats, we have in recent consecutive years deployed high-resolution (±0.001°C), continuous (measurements at 15 s intervals) temperature recorders in McMurdo Sound, and the uninterrupted, finely resolved temperature records revealed steadfast freezing water temperatures from April to mid-December, and even with austral summer warming episodes, summer maxima of shallow water were well below 0°C, seldom above −0.5°C (figure 1a; Hunt et al. 2003). In addition, regardless of austral winter or summer, CTD (conductivity/temperature/depth) casts showed that water temperature is at freezing point with depth in the Sound where some deeper water notothenioid species reside (figure 1b,c; Hunt et al. 2003). In terms of the prevalence of ice crystals in the water column, it arises as a result of extensive sea ice cover, and in particular the presence of large floating ice shelves along much of the Antarctic continental margin. The ice shelves, some of which reach enormous thickness (such as the Ross and Filchner-Ronne Ice Shelves), act as a heat sink such that the water in contact at the shelf base may be as cold as −2.4°C (Nicholls & Makinson 1998). Our McMurdo Sound shallow water site temperature and pressure records through the winter months showed that daily tidal cycles were synchronized with below-freezing cold water temperature spikes, indicative of upward and seaward entrainments by the tides of cold shelf-base water from the nearby Ross Ice Shelf to the shallow sites, the habitats of many notothenioid fish species (Hunt et al. 2003). The cold shelf water is undercooled relative to the in situ equilibrium freezing point of seawater as it rises and could spontaneously nucleate producing numerous ice crystals in the water column. This process probably also contributes to the growth of abundant sub-ice platelets underneath surface fast ice, and mats of anchor ice on shallow bottoms (Dayton et al. 1969; Hunt et al. 2003). The tidal entrainment of cold shelf-base water to shallow fish habitats and ice nucleation in the water column would happen regardless of season, even though the transient daily temperature drops in shallow sites would not be measurable as they will be masked by the larger temperature fluctuations resulting from summer warming episodes in surface water. In sum, the notothenioid fish conceivably is exposed to ice for much of the year if not year round.
Figure 1.
Cold stable nature of McMurdo Sound seawater. (a) High resolution, continuous seawater temperature records from November 1999 to September 2004 at the McMurdo Station water intake jetty (30 m depth). Water temperatures remain steadfast at freezing (−1.91°C) from April through end of November each year. Peak water temperature from summer warming episodes is given for each of the five consecutive years. Maximum summer rise from freezing is as little as 0.6°C (2003), to less than 1.5°C (1999). (b) A CTD depth–temperature profile during austral spring (7 October 2004), showing no temperature variation from freezing from surface to below 400 m. (c) A CTD depth–temperature profile during austral summer (29 December 2000), showing slightly warmer temperature at surface, but little temperature variation from freezing at depths below approximately 100 m.
It is the combination of ambient seawater reaching its freezing point and ubiquitous ice crystals appearing in the water column as sea-level glaciation of the Southern Ocean progressed that created the most daunting freezing challenge for marine teleosts. The colligative or equilibrium freezing point (FP) of oceanic seawater (−1.91°C) results from depression of the FP of water by dissolved oceanic salts and solutes (osmotic concentration of approx. 1030 mOsm in McMurdo Sound). Marine teleost fishes are hyposmotic to seawater, and most temperate species have blood osmotic concentration of only approximately 300 mOsm, which translate into a FP of −0.6 to −0.7°C (Black 1951). The blood of the cold-adapted Antarctic notothenioid fishes has higher osmolarity (more salt), at approximately 550 mOsm, and therefore an equilibrium FP of −1.0°C. (DeVries 1983). The approximately 0.9°C numerical difference in FP between fish and seawater is deceptively small, because when the fish is seeded with environmental ice, which occurs at high probability for notothenioid fish in its icy habitats, inoculative nucleation of the fish blood and body fluids will nevertheless quickly occur leading to death. This is the survival challenge that the endemic Antarctic fish face in their icy freezing environment; it was overcome by the evolution of biological antifreeze proteins in the Antarctic notothenioid fish. Antifreeze proteins recognize ice crystals that enter the fish, bind to them and arrest ice growth by about 1.2–1.5°C below the colligative FP of −1.0°C due to blood osmolytes. The resultant FP is about −2.2 to −2.7°C, below the ambient temperature of −1.91°C, and thus the blood and body fluids are preserved in the liquid state. The 1.2–1.5°C of FP depression due to antifreeze activity is also known as thermal hysteresis (TH), reflecting the separation of the colligative FP from the actual temperature of ice growth in the presence of antifreeze protein. The evolution of the encoding gene for the novel ice-binding protein (antifreeze glycoprotein; AFGP) in notothenioid fish involved an innovative mechanism of new gene genesis—recruitment of portions of a structurally and functionally distinct pancreatic trypsinogen-like protease gene, and de novo amplification of a rudimentary tripeptide (ThrAlaAla) coding element to create an entirely new AFGP coding region (Chen et al. 1997a; Cheng 1998; Cheng & Chen 1999).
(b) Historical paradigm of teleost freezing avoidance
The prevailing physical principle that freezing point depression is governed by the concentration of dissolved small solutes formed the mindset behind the search for the responsible agent in the incipient years of fish freezing avoidance research (Scholander et al. 1957), which proved to be elusive. The discovery of the first biological antifreeze compound in fish, the AFGP in Antarctic notothenioids (DeVries 1970, 1971), and the elucidation of its ice-binding and ice-growth inhibition properties (Raymond & DeVries 1972, 1977) dispelled the dogma that only small colligative osmolytes serve as antifreeze agents, and established a macromolecular, non-colligative mechanism of freezing point depression, ushering in a new area of research in adaptation to extreme cold. A recent development in the antifreeze system of the Antarctic notothenioids is our discovery of a second and less abundant antifreeze protein in these species, named antifreeze potentiating protein (AFPP) for its ability to augment total antifreeze activity (AFGP and AFPP) above additive levels (Jin 2003; DeVries & Cheng 2005). This review will limit to discussions pertinent to the major component, the AFGPs, for which much more is known. Inclusive of the AFPP, six structural types of fish antifreeze proteins in diverse fish lineages are now known and their role in preventing freezing has been examined (Fletcher et al. 2001; DeVries & Cheng 2005). The paradigm of polar teleost freeze avoidance that emerged from the large body of literature that accrued over the past three or so decades encompasses two key physiological features: the ability to resist freezing in Antarctic notothenioids and other antifreeze-bearing cold-water fishes results from high levels of antifreeze proteins (10–35 mg ml−1) in their blood and extracellular fluids, and the liver is the site of synthesis and secretion of the abundant circulatory antifreeze proteins, similar to most other plasma proteins in vertebrate animals. Revisions to these long-held aspects of the teleost fish freeze avoidance paradigm have become necessary in light of new findings from our recent studies of Antarctic notothenioid fish.
(c) Paradigm shift I—the ‘larval paradox’
High circulatory levels of antifreeze proteins as the basis for freezing prevention was a conclusion drawn from adult fish data (DeVries & Lin 1977; DeVries 1983; Fletcher et al. 2001; DeVries & Cheng 2005) as teleost freeze avoidance research to-date almost exclusively investigated adult specimens for obvious logistic reasons (relative ease of capture). Eggs, larvae and juveniles are difficult to locate and acquire in the wild, and thus until recently almost nothing was known about the levels of antifreeze proteins in these early life stages and their role in surviving icy freezing seawater. Antarctic notothenioids are oviparous and the larvae are pelagic (North 1991; Loeb et al. 1993; Vacchi et al. 2003; Evans et al. 2005; Koch 2005), thus a priori it is reasonable to expect that these early life stages are also protected by a full complement of antifreeze proteins as they are exposed to the same frigid conditions as the adults. It was, therefore, a complete surprise when we found that maturing embryos and new hatchling larvae of two notothenioid species, the Antarctic naked dragonfish Gymnodraco acuticeps and the Antarctic silverfish Pleuragramma antarcticum, have drastically inadequate amounts of antifreeze proteins to avoid freezing (Cziko et al. 2005).
Adult dragonfish blood antifreeze proteins provide a TH of 1.55±0.04°C, and in conjunction with blood osmolytes effectuate a blood FP of −2.61±0.03°C, well below the FP of seawater (−1.91°C). In McMurdo Sound, dragonfish eggs are spawned as an adherent monolayer on rock surface in shallow water during austral spring (October), fully exposed to icy, freezing water (Evans et al. 2005). The eggs are hyposmotic to seawater and at spawning are maternally endowed with a considerable amount of antifreeze proteins (TH 1.02±0.05°C; FP of whole egg homogenate −2.31±0.04°C) and should be able to resist freezing. However, this amount dwindled to less than half (TH 0.44±0.03°C; FP −1.48±0.03°C) in the ready-to-hatch larvae during the protracted developmental time of approximately 10 months in ambient freezing water (Cziko et al. 2005; Evans et al. 2005). The ready-to-hatch larvae of the Antarctic silverfish P. antarcticum have even less antifreeze proteins, with TH of only 0.11±0.02°C, about 25% of the already antifreeze-deficient ready-to-hatch dragonfish larvae. Their FP is −0.99±0.08°C (whole egg homogenate), 0.92°C above the ambient temperature of −1.91°C. Clearly, these antifreeze levels are entirely insufficient to protect the embryonic larvae against freezing. The protection is probably afforded by the tough chorion acting as an effective physical barrier against ice transmission, and the perivitelline fluid which is essentially isosmotic with seawater providing no osmotic gradient for ice propagation. Remarkably, P. antarcticum eggs with intact chorion and fully developed larvae within can resist freezing even in contact with ice to −9.6°C, far below the ambient water temperature of −1.91°C (Cziko et al. 2005).
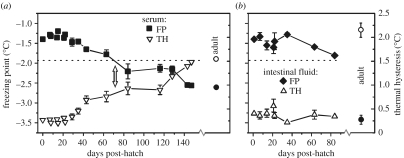
The nascent hatchlings that emerge from the protective chorion, however, are immediately confronted with the icy, frigid conditions that one would expect to cause certain death in their antifreeze-deficient state. Paradoxically, not only do they survive but also newly hatched dragonfish larvae were observed to seek out the coldest and iciest habitat (crevices in the sub-ice platelet layer) presumably to forage for food and avoid predation (Evans et al. 2005). We collected ready-to-hatch dragonfish eggs and reared the hatchlings in ambient water aquarium facilities at the McMurdo Station, and measured their antifreeze levels for five months post-hatching. Larval serum antifreeze protein concentration (TH value) did not increase for 30 days post-hatch (dph), thereafter rose slowly (approx. 0.008°C per day), and finally reaching a level that would depress the serum FP to below −1.91°C between 64 and 84 dph. In other words, larval and young juvenile (yolk reabsorbed by 15 dph) dragonfish are antifreeze-deficient and at risk of freezing for two to almost three months after hatching. It took another two-plus months for serum FP to reach adult levels (−2.56±0.05°C), at 147 dph (figure 2a; Cziko et al. 2005).
Figure 2.
Time course of antifreeze level and freezing points of body fluids of hatchling G. acuticeps larvae. Freezing point (FP, left ordinate) and antifreeze levels (TH, right ordinate). Larvae fluid samples with FP above the dashed line (−1.91°C, FP of seawater) are at risk of freezing if seeded with ice. (a) Serum TH (open triangle) and FP (closed squares) from hatching to 147 dph. Blood antifreeze did not reach sufficient protective levels until about 64–84 dph (arrow). (b) Intestinal fluid TH (open triangle) and FP (closed diamond) from hatching to 84 dph. Intestinal antifreeze levels are inadequate to prevent intestinal freezing for at the entire sampling period of 84 dph. Adult serum and intestinal TH and FP values (open and closed circle, respectively) are included for comparison. Data are re-plotted from Cziko et al. (2005).
This ‘larval paradox’—antifreeze-deficient but freeze-resistant larvae, is a baffling twist to the conventional paradigm that underscores the necessity of high antifreeze concentrations to prevent freezing in ice-laden water. We assessed the role of the larval integument as a physical barrier to ice entry, analogous to the chorion for the eggs. Antifreeze-deficient hatchling dragonfish larvae with intact skin can resist freezing while in contact with ice to −3.63±0.09°C, more than 2.3°C below the FP of their body fluids and well below the FP of seawater. When the skin was artificially breached and external ice applied, larvae froze at −1.54±0.01°C (comparable to serum FP) and 0.36°C higher than the ambient temperature of McMurdo Sound at the time of hatching (Cziko et al. 2005). Thus, it appears that dragonfish larvae must rely on intact surface integument to exclude ice entry in nature in their undercooled state until serum antifreeze reaches protective concentrations. In conjunction, the gills of hatchling larvae of G. acuticeps, P. antarcticum and a third species Pagothenia borchgrevinki are morphologically underdeveloped, lacking both filaments and lamella, which would serve to minimize the surface area of the delicate single-cell epithelium exposed to ambient seawater, reducing freezing susceptibility (Cziko et al. 2005). Pagothenia borchgrevinki hatchling larvae were the only one of the three species examined that appeared to have adequate serum antifreeze concentrations at hatch (Cziko et al. 2005). Collectively, these results from two nototheniids and one bathydraconid larval fish indicate that unlike adult fish, antifreeze fortification is not the only mechanism against freezing in early notothenioid life. Morphological features specific to larval fish—small surface area, undamaged nascent integument and delayed development of the gills may be absolute requirements for antifreeze-deficient larvae to survive in ice-laden waters.
An enduring aspect of the larval paradox is the antifreeze-deficient, hyposmotic intestinal fluid which is at risk of nucleation by imbibed ice from ingestion of ice-laden seawater or food stuff, but does not freeze. Adult notothenioids have high levels of antifreeze protein in their intestinal fluids, depressing their FP to below ambient freezing water temperature (O'Grady et al. 1982b, 1983; Cziko et al. 2005). Gymnodraco acuticeps larvae actively feed upon hatching, but their intestinal fluids have insufficient antifreeze (TH 0.38±0.02°C; FP−1.45±0.03°C) at hatch, which remained unchanged for at least 84 dph (approx. three months; figure 2b; Cziko et al. 2005). There is currently no plausible explanation to resolve this puzzle and further investigations are necessary.
(d) Paradigm shift II—liver is not the source of blood AFGP in notothenioids
Liver is well known as the major synthetic organ for secreted plasma proteins in vertebrate animals (Miller et al. 1951; Haschemeyer & Smith 1979). For non-notothenioid antifreeze-bearing fishes, liver expression of the mRNA for the respective type of antifreeze protein in each fish taxon was readily confirmed by cDNA cloning and Northern analysis (Hew & Yip 1976; Hew et al. 1988; Ewart & Fletcher 1993; Wang et al. 1995; Chen et al. 1997b), consistent with the liver being the major synthesis and secretory source of circulatory antifreeze. For Antarctic notothenioid fishes, early investigations of antifreeze biosynthesis logically adopted the hepatic origin mindset, since there were no apparent a priori reasons to question liver as the source of this abundant plasma protein. However, in recent years we repeatedly found evidence that contradicted a hepatic origin for notothenioid blood AFGP—a physiological peculiarity and a second twist to the long-held conventional teleost freeze avoidance paradigm.
Two key early studies on notothenioid AFGP synthesis (Hudson et al. 1979; Haschemeyer & Mathews 1980) using radioactive tracers yielded some major inconsistencies with liver as the main site of synthesis and secretion. First, the appearance of 14C-labelled AFGPs in blood lagged other labelled plasma proteins by 8–18 h. If both proteins were made in the liver, the time course of secretion should have been more synchronized. Second, the low level of liver synthesis of AFGP estimated from 14C incorporation was not commensurate with its high circulatory concentrations. Various possibilities were invoked to account for these discrepancies, including a possible non-hepatic synthesis site. Molecular detection of AFGP mRNA by Northern blot was first reported for Notothenia coriiceps (Hsiao et al. 1990). In retrospect, the amount of liver polyA+RNA used was extremely large 50 μg, equivalent to 5 mg of starting total RNA (assuming 1% mRNA content), and thus inconsistent with liver having high levels of AFGP expression. Highly expressed messages register intense hybridization signal with very little (≤10 μg) total RNA used, as shown for liver expression in other fish taxa of their respective type of AFP (Wang et al. 1995; Gong et al. 1996; Chen et al. 1997b).
In our work that uncovered the pancreatic TLP (trypsinogen-like protease) evolutionary ancestry of notothenioid AFGP gene, we also uncovered strong AFGP mRNA expression in the pancreas (Chen et al. 1997a; Cheng & Chen 1999). Unlike all other vertebrates, the pancreas of teleosts is a dispersed tissue. It is found on surfaces of the abdominal gastrointestinal tract components, non-GI organs, mesentery, blood vessels and ducts, on and in the gall bladder wall, and it also occasionally infiltrates the liver along hepatic portal blood vessels forming hepatopancreas islets (Hinton et al. 1972; Kurokawa & Suzuki 1995; Eastman & DeVries 1997). Some of the prior ‘positive’ indicators of liver AFGP synthesis could be the result of the dispersed distribution of strongly AFGP expressing pancreatic tissue. The large amounts of liver total RNA for polyA+RNA isolation in Hsiao et al. (1990) might have included pancreatic RNA leading to a positive Northern blot for AFGP mRNA. Similarly, tissue heterogeneity could account for the radiotracer-based AFGP synthesis shown in primary cell cultures derived from whole livers of P. borchgrevinki (O'Grady et al. 1982a).
The lack of AFGP synthesis in Antarctic notothenioid liver is now corroborated by recent evidence from ongoing large-scale Expressed Sequence Tags (EST) sequencing projects of Dissostichus mawsoni (a nototheniid) by us (L. Chen & C.-H. C. Cheng 2007, unpublished data) and Harpagifer antarcticus (a harpagiferid) by fellow biologists (Melody Clark, British Antarctic Survey, personal communication), two species that are endowed with AFGP genes and proteins (Cheng et al. 2003). No liver AFGP cDNA was found so far in either species, from over 30 000 ESTs sequenced in the case of D. mawsoni. While the highly repetitive AFGP polyprotein coding sequences are difficult templates for cDNA synthesis, one would expect reduced rather than non-existent frequency of AFGP clones in the cDNA library. The fact that no AFGP cDNA could be found among a large number of EST sequenced could only mean that AFGP mRNA was either not present or very rare in the first place. Thus, it is very clear that notothenioid liver cannot be the major output site of blood AFGPs.
The foregoing studies described all used adult fish. We have also recently examined hatchling larvae and juveniles of notothenioid species using molecular detection by Northern blots and protein detection by immunohistochemistry, and found no hepatic expression of AFGPs in these early life stages (Cheng et al. 2006). Thus, all available evidence indicates that the liver plays little if any role in AFGP synthesis or freezing avoidance throughout the life of notothenioid fish.
(e) Gut versus blood—importance of intestinal freeze avoidance
Marine teleost fishes actively drink seawater to compensate for their water loss to the hyperosmotic environment, and absorb the excess salt from the intestinal lumen for elimination through chloride cells at the gills (Smith 1930; Evans 1993; Loretz 2001). In the perennially ice-laden Antarctic waters, this obligatory teleost physiology engenders a steady avenue for ice entry into the hyposmotic intestinal fluid of the notothenioid fish. In addition, ice can enter the GI tract on a regular basis with ingestion of food stuff that harbours ice. The intestinal fluid is therefore at constant risk of freezing. A focal thesis of the conventional teleost freezing avoidance paradigm is the prevention of blood freezing by means of abundant blood AFGPs, and while exogenous ice does find its way into the circulation (DeVries & Cheng 1992, 2005), the frequency of ice reaching the GI fluids is expected to be far greater owing to ingestion of ice-laden seawater and food. Consistent with this hypothesis is the observation that intestinal fluid samples from environmental specimens caught in McMurdo Sound invariably test positive for ice (assayed by nucleation of undercooled saline), while the spleens (proxy for the blood volume because spleen filters blood and ice becomes lodged) are not always ice-positive. It is therefore no coincidence that adult notothenioid fish have high levels of AFGPs that can depress intestinal fluid FP below ambient to prevent intestinal freezing (O'Grady et al. 1982b, 1983; Cziko et al. 2005), as a frozen gut translates into a frozen fish and sure death. While it has been known since the 1980s that adult intestinal fluids are full of AFGPs (O'Grady et al. 1982b, 1983), the tissue source remained obscure. The past belief that liver is the major source of secreted AFGPs further complicated the picture as there is no direct anatomical route for delivery from the blood to the intestine lumen. Our recent discovery of high pancreatic AFGP expression solved the conundrum (Cheng et al. 2006). It is clear that intestinal AFGPs originate from pancreatic secretion, delivered via the pancreatic duct at the anterior of the intestine. In the giant nototheniid, the Antarctic toothfish D. mawsoni, a full complement of AFGP molecules are found in its pancreatic fluid sampled from a small reservoir that is part of the pancreatic duct (Cheng et al. 2006). AFGP expression by the pancreas makes eminent physiological sense for intestinal freezing avoidance. Pancreatic secretions of digestive enzymes and bicarbonate in vertebrate animals are triggered by the entry of food into the intestine, and in the case of the notothenioids, they would be temporally coordinated with pancreatic AFGP delivery to the hyposmotic intestinal milieu to inhibit the growth of ice that enters with the diet. In the constantly icy Antarctic marine environment, GI freezing avoidance is as critical as blood freezing avoidance.
(f) Non-hepatic source of plasma AFGP
Since liver is not the source of plasma AFGPs in notothenioids, we are left with the conundrum of where the high levels of plasma AFGPs come from. Are there other possible sites of synthesis that have direct anatomical connection to the systemic circulation? A Northern blot survey of tissues showed that in addition to the pancreas, the anterior stomach of notothenioids also shows strong AFGP mRNA expression, but no expression is detected in any other tissues or organs examined (DeVries & Cheng 2005; Cheng et al. 2006). This GI-only expression pattern again underscores the critical importance of preventing GI freezing in the constantly icy Antarctic marine environment. However, the source of blood AFGPs remains unresolved because there is no direct anatomical route for transport of the protein from these tissue sites to the blood circulation. It should be pointed out that AFGPs are extremely hardy molecules owing to their high carbohydrate content (approx. 60%), and are resistant to exposure to acid or alkali. In addition, their unique amino acid sequence, repeats of the tripeptide ThrAlaAla, do not include known sites for protease cleavage. AFGPs recovered from stomach and intestinal fluids show little degradation (Cheng et al. 2006). If large amounts of intestinal AFGPs are excreted with undigested material, it would be extremely energetically costly to replenish, especially considering the low temperature the notothenioids live in. Thus, the possibility exists that intact AFGPs in the intestinal lumen are reabsorbed, and eventually returned to the systemic circulation by way of the hepatic portal system. Whether this circuitous (and therefore slower) pathway exists remains to be experimentally determined, but in principle it can account for both past and recent experimental observations—the substantial lag of the appearance of labelled AFGP in blood relative to other labelled plasma proteins in the early tracer studies of adult notothenioids (Hudson et al. 1979; Haschemeyer & Mathews 1980), and the protracted time course for the blood antifreeze in G. acuticeps hatchling larvae to accumulate to adult levels in our recent study (Cziko et al. 2005). Investigations of AFGP synthesis and transport pathways may yet reveal interesting unique physiology associated with the notothenioid fishes that have evolved under geographical isolation in the Antarctic.
(g) Alterations in environments and dynamic evolutionary change in notothenioid AFGP gene families
Past Antarctic climate change clearly forged the evolutionary gain of the antifreeze gene and function in the Antarctic notothenioids, which in turned empowered their organismal diversification and radiation in an environment that is otherwise out of reach (Chen et al. 1997a; Cheng 1998). Under strong freezing selection, the AFGP gene has undergone repeated duplications such that contemporary notothenioids in frigid habitats have large AFGP gene families (figure 3; Cheng 1996; Cheng et al. 2003), commensurate with the need for the production of large amounts of the protective protein (20–35 mg ml−1 in the blood; DeVries 1983; DeVries & Cheng 2005). The continued maintenance of a large family of functional genes apparently relies on persistent selection pressure from Antarctic marine frigidity, because non-freezing environmental temperatures where the antifreeze function becomes unessential is correlated with reduction or degeneracy of the AFGP genes and function. The latter is seen in a number of taxa from the family Nototheniidae residing in non-freezing coasts of the southern landmasses north of the Antarctic Convergence.
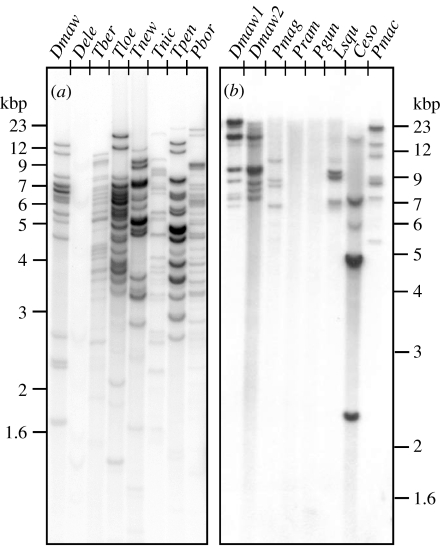
Figure 3.
Genomic DNA blots of notothenioid taxa hybridized with radio-labelled probe derived from a Notothenia coriiceps AFGP polyprotein gene. Genomic DNA was digested with (a) TaqI or (b) EcoRI, both known not to cleave within AFGP polyprotein coding sequences, thus each hybridized band represents one or more AFGP genes. (a) D. eleginoides (Dele) shows nearly undetectable hybridization signal indicating highly mutated or absence of AFGP sequences in its genome, in stark contrast to its fully AFGP-fortified sister species D. mawsoni (Dmaw), and related nototheniid relatives Trematomus bernacchii (Tber), T. loennbergii (Tloe), T. newnesi (Tnew), T. nicolai (Tnic), T. pennelli (Tpen) and Pagothenia borchgrevinki (Pbor). (b) The DNA of the two Patagonotothen species, P. ramsayi (Pram) from South America and P. guntheri (Pgun) from Shag Rocks, Antarctic Peninsula showed no hybridization, indicative of absence of functional AFGP sequences, in contrast to other nototheniids D. mawsoni (Dmaw1, Dmaw2), Paranotothenia magellanica (Pmag) and Lepidonotothen squamifrons (Lsqu). Like most of the Patagonotothen species, P. magellanica and the Channichthyid icefish Champsocephalus esox occur in South American waters, but show presence of AFGP sequences in their DNA, consistent with a species evolutionary origin in the Antarctic. Pmac is the icefish Pagetopsis macropterus.
The New Zealand nototheniids Notothenia angustata and N. microlepidota (commonly known as black cods) live in cool-temperate southern coastal waters where winter minimum does not drop below 2–4°C. They have an evolutionary origin in the Antarctic region based on molecular phylogenetic analyses and the presence of functional AFGP genes and protein (Cheng et al. 2003). In their current temperate habitats, their AFGP systems are much reduced—very low blood AFGP concentration (μg ml−1), and only two to three genes, with substitutions that result in amino acid changes to the functionally important repetitive tripeptide (ThrAlaAla) backbone (Cheng et al. 2003). Another example is the Patagonian toothfish Dissostichus eleginoides, sister species to the amply AFGP-fortified Antarctic toothfish D. mawsoni. D. eleginoides appears to have no functional AFGP sequences in its DNA based on almost non-existent hybridization signals on genomic Southern blot (figure 3a), consistent with its non-Antarctic distribution where freezing protection is irrelevant. In terms of species and AFGP evolutionary history, however, the apparent absence of detectable AFGP remnants in D. eleginoides is surprising. The AFGP gene was thought to have evolved once, before the Antarctic notothenioid radiation, at the base of the family Nototheniidae, because select endemic taxa from the five Antarctic families, including the presumed basal nototheniid D. mawsoni, all carry the AFGP genotype (figure 3; Cheng 1996; Cheng et al. 2003). Divergence of D. eleginoides before the evolutionary gain of the AFGP gene is inconsistent with its position in the notothenioid phylogeny; the alternative is that it had the primordial AFGP genotype, which was lost or became greatly mutated after it somehow arrived at its current non-Antarctic habitats. A third nototheniid group—the members of the genus Patagonotothen also appear to be AFGP-null. Patagonotothen is a large genus with 12 known species, all with South American distribution except for one, Patagonotothen guntheri that occurs at the tip of the Antarctic Peninsula. We have examined two South American species Patagonotothen tessellata (Cheng et al. 2003) and Patagonotothen ramsayi, as well as the sole Antarctic (near Shag Rocks) species P. guntheri, and found no detectable AFGP sequences in their genomes based on genomic Southern blot (figure 3b). Similar to the conundrum in the case of D. eleginoides, either the common ancestor of the Patagonotothen clade diverged before the evolution of AFGP gene, which again is counter to AFGP having evolved before the notothenioid radiation, or it diverged after the evolution of AFGP gene but went through a rapid loss. To distinguish these possibilities and the associated biogeographical factors involved leading to the current peculiar distribution of this genus will require further investigations.
Evolutionary histories unresolved notwithstanding, it is clear that the AFGP genotype was subject to dynamic changes resulting from alterations in the species' thermal environment over evolutionary time. Given this causal relationship and the availability of phylogenetically related nototheniid taxa in freezing and non-freezing habitats, we have an excellent model system and unparalleled opportunity for examining the molecular bases of environmentally driven gene birth and death. With large-insert DNA clones and high throughput sequencing, the syntenic genomic regions where AFGP genes or gene remnants reside in select nototheniid species can be reconstructed for comparative analyses to identify the molecular changes associated with the presence and absence of the AFGP phenotype as modulated by environmental change. As a basis for comparison, we have selected D. mawsoni as the cornerstone Antarctic species (Dissostichus is considered as the oldest nototheniid lineage) that is fully AFGP-endowed and subject to constant freezing selection. We constructed a large-insert DNA library using a BAC (bacterial artificial chromosome) vector and identified the positive clones. The AFGP genes and extant members of their evolutionary precursor, the TLP (trypsinogen-like protease) gene (Chen et al. 1997a; Cheng & Chen 1999) are found to be located in one major genomic region (approximately 720 kbp) based on fingerprinted contig analysis (Soderlund et al. 2000) and shotgun sequencing of AFGP/TLP-positive BAC clones, consistent with one single hybridized region we detected by fluorescence in situ hybridization of metaphase chromosomes. The results of shotgun sequence assembly thus far showed that the AFGP polyprotein genes are not only close neighbours to the TLP genes, but also to two other distinct families of trypsinogen genes, clearly establishing the trypsinogen-type serine protease evolutionary ancestry of the novel ice-binding protein. Putative coding remnants of trypsinogens and the AFGP tripeptide repeats (ThrAlaAla)n are found, indicating the region is potentially a recombinant hot spot. A surprising find is the presence of four other AFGP/TLP chimaeric genes, distinct from the homologue (also found) of the one we previously discovered in D. mawsoni that was considered an evolutionary transitional form (Cheng & Chen 1999). The persistence of multiple AFGP/TLP chimaeric genes among independent AFGP and TLP genes in the extant genome would only make sense if they are functionally necessary. Through cDNA cloning, we verified that these chimaeric genes are functional in that they are transcriptional active (Cheng & Chen 1999; C.-H. C. Cheng 2007, unpublished results), and it is reasonable to suggest that they are translated into protein. While the cellular fate of potential AFGP/TLP chimaeric pro-proteins remains to be determined, the possibility exists that they may undergo autocatalysis with the TLP portion cleaving the three-residue connectors of the AFGP polyprotein portion into individual mature antifreeze peptides.
We expect interesting evolutionary information of the AFGP genes and gene family that are under constant selection to be forthcoming when the reconstruction of the contiguous sequence of the AFGP/TLP genomic region is complete for D. mawsoni. The same experimental undertakings are underway to examine the AFGP/TLP region (or its remnant) of the putative AFGP-null Patagonian toothfish D. eleginoides and of the New Zealand black cod N. angustata that has reduced AFGP genotype and function in the absence of selection. For the Patagonian toothfish, the BAC clones encompassing the target region can be identified with TLP probes, since TLP genes are now verified to be closely clustered with the AFGP genes in its sister species D. mawsoni. The two Dissostichus species and the New Zealand black cod collectively cover the range of AFGP functional capacity. Comparative analyses of the syntenic AFGP/TLP regions in these three species are expected to produce a wealth of data that would advance our knowledge of dynamic genomic and evolutionary changes of a crucial protein function resulting from environmental change.
(h) Summary comments—antifreeze protein gain in Antarctic notothenioid fish
Evolutionary adaptation of organisms to changing environments is often subtle, and represents the culmination of changes in multiple traits that collectively contribute to a new phenotype.
In stark contrast, the evolutionary gain of the AFGP in Antarctic notothenioids singularly permitted continued existence of the notothenioid ancestor in the glaciated polar marine environment and enabled its subsequent diversification into icy niches. For this reason, AFGP is considered a key innovation (Eastman & McCune 2000). The causal linkages inherent in this novel form of cold adaptation had provided unparalleled opportunities for the studies of the interplay of geographical, molecular and organismal evolution. The novel mechanism of genesis of the AFGP gene has enlightened us of the breadth of creativity of the molecular evolutionary process. The recently commenced genomic scale analyses of AFGP/TLP loci in notothenioids in freezing and non-freezing environment promise to further our insight into the dynamics of evolutionary change in their genomes. And, over 30 years after the initial discovery of AFGP when the physiology of the production and distribution of this key survival protein, and its role in notothenioid freeze resistance were thought to be well understood, we encounter new experimental evidence that contradict long-held paradigms, invoking new directions of inquiry that may yet reveal unique physiological and adaptational features in Antarctic notothenioids that arose from evolution under isolation.
3. Haemoprotein loss and cardiovascular adaptation in icefishes—Dr NO to the rescue?
(a) Vertebrates without haemoglobins—you must be kidding!
Haemoglobinopathies and thalassemias are well known genetic diseases of humans that stem from structural defects in the α- or β-globin polypeptide chains and from defects in the synthesis of one or more of the globin chains, respectively (Forget & Pearson 1995; Nagel 1995; Platt 1995). The universally deleterious effects of these diseases, together with the results of numerous studies of the respiratory physiology of other animal species, strongly support the conclusion that possession of haemoglobin-bearing erythrocytes is a condicio sine qua non of the vertebrate condition. One can well imagine the startled reactions of biologists when they read J. T. Ruud's seminal Nature article on Antarctic icefishes, ‘Vertebrates without erythrocytes and blood pigment’ (Ruud 1954). Even today, conversations about icefishes with non-Antarctic biologists elicit questions such as, ‘How do they transport oxygen to their tissues?’, ‘How could this condition have evolved?’ and ‘Why has this apparently deleterious trait persisted?’ Based on approximately 40 years of biochemical and physiological research, we have partial answers to some of these questions. With the advent of molecular and genomic methods and strategies, we are now poised to test explicit evolutionary hypotheses. And in a remarkable turn-about, we can exploit the icefishes to advance our understanding of the genetic programme that controls erythropoiesis (formation of red blood cells or erythrocytes) in the vast majority of vertebrates that do have red blood.
Ruud himself was sceptical when, on a visit to South Georgia in 1929, he first heard about the blodlaus-fisk said to inhabit the surrounding waters. Twenty-four years were to elapse before he returned to South Georgia, caught several specimens of the ‘white crocodile fish’ Chaenocephalus aceratus (family Channichthyidae, suborder Notothenioidei), and characterized their blood. He reported that fresh C. aceratus blood is nearly transparent, lacks erythrocytes and haemoglobin, contains leucocytes at less than 1% by volume and is iron poor (Ruud 1954). Oxygen is carried in physical solution in the blood plasma, which provides the icefish with approximately 10% of the carrying capacity of red-blooded notothenioids. Ruud surmised that evolutionary loss of red cells and haemoglobin could only occur in well aerated and very cold waters, which typify the Southern Ocean.
(b) Haemoprotein loss in icefishes: an evolutionary perspective
Ruud (1954) recognized that the absence of erythrocytes and haemoglobin, a phenotype that is lethal for fishes living at high temperature, may be disadvantageous but non-lethal (‘disaptive’; Baum & Larson 1991; Montgomery & Clements 2000) at low temperature. The development in icefishes of compensatory readaptations that enhance oxygen delivery, including increases in weight-specific cardiac output and blood volume, cutaneous uptake of oxygen through a scaleless skin and modest decreases in metabolic oxygen demand (Hemmingsen et al. 1972; Hemmingsen 1991), indicates that the phenotype was indeed maladaptive (see below). How, then, does one explain the successful divergence and diversification of the icefishes over the past 8 million years (Near 2004)? The answer lies in the well documented collapse in species diversity in the Southern Ocean, mediated by glaciation and ice shelf scouring, that occurred during the mid-Tertiary (see §1; Eastman 1993, 2005), which created many ecological opportunities for surviving groups. The formation of ice-free marine embayments during the periodic recession of the glacial ice cover of the continent provided the cold refugia that facilitated notothenioid diversification (Bargelloni et al. 2000). Thus, the icefishes evolved and diversified as selection pressure for cellular oxygen-binding proteins was relaxed in a cold, stable and oxygen-rich marine environment.
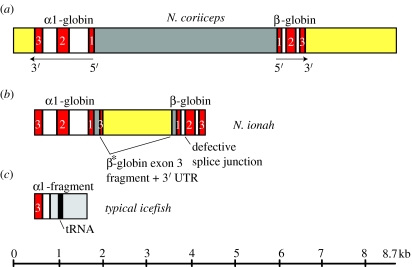
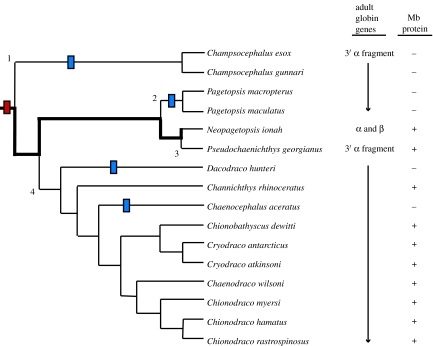
The absence of haemoglobin expression is a derived character (i.e. a synapomorphy) that is shared by all members of the Channichthyidae and probably arose at or near the divergence of icefishes from their sister lineage, the red-blooded Bathydraconidae (dragonfishes). Virtually all icefish genomes (15 of 16) lack most of the adult αβ-globin gene complex of their haemoglobin-expressing notothenioid relatives (figure 4a), retaining only a truncated, 3′ fragment of the α gene (figure 4c; Cocca et al. 1995, 2000; Zhao et al. 1998; Lau et al. 2001; di Prisco et al. 2002; NRC 2003). (There is strong evidence that the presumptive juvenile αβ-globin complexes are also deleted in icefishes (Cocca et al. 2000), but the survey of species is incomplete). Moreover, the breakpoint in intron 2 of the α-globin gene is identical for the 15 species (S. A. Parker & H. W. Detrich III 2005, unpublished results). The sole exception to this pattern occurs in Neopagetopsis ionah, which possesses a disrupted adult αβ-globin locus (figure 4b). The major mutation of the N. ionah complex is the replacement of most of the ‘red-blooded’ intergenic region (figure 4a, dark grey), which contains the promoter and enhancer elements necessary for α- and β-globin gene transcription (Lau et al. 2001), by part of exon 3 and the 3′-UTR (yellow) from a second β*-globin gene that is very closely related, but not identical to the adult β gene. The complete N. ionah β-globin gene also contains a splice site mutation at the junction of intron 1 and exon 2 (figure 4b, arrow). Together, these mutations almost certainly render the N. ionah globin gene complex non-functional. Determination of the recombinatorial event, or more likely events, that caused the disruption of the N. ionah globin complex undoubtedly will require careful comparison to representative globin loci from the haemoglobin-expressing dragonfishes.
Figure 4.
Cartoon depicting the loss of globin genes by the icefishes. (a) The adult αβ-globin gene complex of the red-blooded notothenioid N. coriiceps. The α- and β-globin genes are linked in 5′–5′ orientation. The exons (E1–E3) and introns (I1, I2) of the globin genes are represented by red and white rectangles, respectively. The intergenic region, defined as the sequence between the initiator codons of the two genes, is shown in dark grey, and the 3′-untranslated regions (3′-UTR) are shown in yellow. The direction of transcription (5′–3′) is indicated for each gene. (b) The corrupted αβ-globin gene complex of the icefish N. ionah. Note that most of the ‘red-blooded’ intergenic region (a, dark grey) is replaced by part of exon 3 from a related β*-globin gene and its associated 3′-untranslated region (3′-UTR, yellow). The N. ionah adult β-globin gene also contains a splice site mutation at the junction of intron 1 and exon 2. (c) The remaining 15 species of icefish possess only a 3′ fragment of the adult α-globin gene that is linked, presumably via non-homologous recombination, to a shared segment of genomic DNA (light grey; in 14 of 15 species a tRNA gene is present). Lengths of sequence components can be estimated from the scale at the bottom.
One may speculate that the mutated N. ionah complex represents the ancestral ‘smoking gun’ that eliminated expression of the α- and β-globins, thus making the globin genes superfluous and easily deleted at the start of icefish diversification. However, we now encounter a major conundrum. The consensus phylogeny of the icefishes (Near et al. 2003) places N. ionah deep in the tree, rather than at the basal position expected for possession of a nearly intact αβ-globin locus. So located, we could hypothesize that the adult αβ-globin gene complex was lost by icefishes on four independent occasions (figure 5, numbered branch points), that each deletion occurred at precisely the same location in the α-globin gene (presumably due to the presence of a recombinational ‘hot spot’), that the same non-globin DNA was recruited to the α-globin breakpoint, and that each deletion was sufficiently large to remove the entire β-globin locus! Suffice it to say that this series of contingent events is extremely unlikely. A more likely alternative is that the αβ-globin cluster in Neopagetopsis represents an intermediate condition between a fully functional globin cluster and the 5′-truncated α pseudogene (i.e. a 3′ remnant) seen in most icefishes. The presence of the complex pseudogene in the phylogenetically derived N. ionah could then be explained through the retention of ancestral polymorphism (Hudson 1990, 1992). These scenarios of globin gene loss are as of yet unresolved. Moreover, the evolutionary history of haemoproteins in icefishes becomes even more puzzling when we consider myoglobin.
Figure 5.
Haemoprotein loss in the icefish family. The loss of globin genes and the expression of myoglobin are mapped on a consensus phylogeny of the channichthyids (Near et al. 2003). The red bar represents the loss of the ability to express haemoglobin, which probably occurred in the ancestral channichthyid. The thick black line traces the retention of adult α- and β-globin genes by N. ionah, and the numbered branch points indicate the four independent deletions of most of the adult globin complex. The blue bars illustrate the four independent mutational events that explain the loss of myoglobin expression. Figure adapted from Sidell & O'Brien (2006).
Six of the sixteen icefish species do not express myoglobin in the heart (Sidell et al. 1997; Moylan & Sidell 2000; Grove et al. 2004), the only tissue in which this haemoprotein is found in the group (figure 5, blue bars). When present, myoglobin enhances cardiac performance relative to icefishes that lack it (Acierno et al. 1997), so loss of myoglobin expression is clearly disadvantageous. Failure to produce cardiac myoglobin by the six species has apparently occurred by four independent mutations and at least three distinct molecular mechanisms (Small et al. 1998, 2003; Grove et al. 2004). Moreover, the pattern of myoglobin loss does not coincide with that proposed for deletion of the globin gene complex. Reconciliation of the patterns of haemoprotein loss with the phylogeny of icefishes will clearly require further study.
(c) Cellular correlates of haemoprotein loss
The loss of myoglobin expression by a subset of icefishes is associated with quantitative changes in mitochondrial density and morphology. O'Brien et al. (2000) have shown that the mitochondrial density of cardiomyocytes from C. aceratus, a myoglobin non-expresser, is greatly increased (36% of cell volume) relative to those of the icefish expresser Chionodraco rastrospinosus (20%) and the red-blooded, myoglobin-expressing notothenioid Gobionotothen gibberifrons (16%). (However, the density of cristae within C. aceratus mitochondria is low compared with the densities found in the mitochondria of myoglobin expressers, which suggests that the former are less active in oxidative phosphorylation). If mitochondrial expansion is physiologically related to myoglobin loss, what role does the former play in oxygen delivery to cells?
The striking expansion of cellular mitochondrial density in the myoglobinless icefish C. aceratus is likely to enhance oxygen flux in the heart by two mechanisms (Sidell 1998; O'Brien et al. 2000). High densities of mitochondria reduce the mean diffusional path length for oxygen transfer from capillaries to these organelles. Furthermore, elaboration of the requisite membrane for mitochondrial expansion may provide a ‘lipid highway’ that enhances diffusion of oxygen through the cell due to the greater solubility of the gas in lipid than in aqueous media. Together, these mechanisms may well compensate for the absence of myoglobin-mediated intracellular oxygen diffusion (Merx et al. 2001; Wittenberg & Wittenberg 2003).
(d) The icefish cardiovascular system
The tissue- and organ-level properties of the icefish cardiovascular system have also been impacted by the loss of haemoproteins. The icefish heart is a spongy myocardium that functions as a high-volume, low-pressure pump. Compared with red-blooded notothenioids of comparable size, the icefishes have very large hearts that produce a weight-normalized cardiac output that is four- to fivefold larger, blood volumes that are two- to fourfold greater and dense beds of capillaries (Hemmingsen et al. 1972; Hemmingsen 1991). In addition, the mean capillary diameter in icefishes is 1.5 times larger than in red-blooded notothenioids (Egginton et al. 2002). Since resistance to laminar flow in a cylinder varies inversely with the fourth power of the radius and directly with viscosity, relatively small changes in capillary diameter are expected to contribute significantly to efficient cardiovascular performance.
Although flow resistance and arterial blood pressure are low, the cardiac work of C. aceratus and other icefishes is estimated to consume more than 22% of resting metabolic energy production (Hemmingsen & Douglas 1977). Using published performance data for icefish hearts, Sidell & O'Brien (2006) have calculated that the weight-specific power expenditure of an icefish heart is approximately twice that of a red-blooded notothenioid, primarily because it must distribute a far larger volume of blood per unit time to support a body of equivalent mass. Nevertheless, icefishes have a substantial reserve capacity for the uptake and transport of oxygen and are certainly able to perform non-resting activities such as vertical migration in the water column in search of prey (Hemmingsen 1991).
(e) Compensatory adjustment of the icefish cardiovascular system in a regime of reduced interspecific competition? Enter Dr NO
We have argued that haemoglobin-null phenotype of the icefish clade arose through a single evolutionary event and has persisted at the population level due to their unique habitat and the relaxation of interspecific niche competition. We have also explicitly assumed that haemoglobin loss by icefishes was evolutionarily antecedent to the appearance of secondary cardiovascular traits that compensate in part for this loss.1 If Darwinian selection pressure is truly relaxed, we must ask how these apparently re-adaptive traits could arise. Sidell & O'Brien (2006) have proposed that evolution of the secondary cardiovascular adaptations of icefishes was ‘jump-started’ by natural, homeostatic responses mediated by nitric oxide (NO).
NO, which is produced by three isoforms of nitric oxide synthase including neuronal NOS (nNOS or NOS I), inducible NOS (iNOS or NOS II) and endothelial NOS (eNOS or NOS III), functions as a modulator of vasodilation (Palmer et al. 1987) and as a signal that stimulates angiogenesis (reviewed by Conway et al. 2001). Over-expression of iNOS has been shown to induce cardiac hypertrophy in the myoglobin-knockout mouse (Gödecke et al. 2003). Recently, NO has been found to stimulate mitochondrial biogenesis (Nisoli et al. 2003, 2004). Thus, organisms subject to chronic systemic elevation of NO levels might experience expansion of tissue capillary density via angiogenesis, enlargement of the heart and increases in mitochondrial densities in heart muscle and other aerobic tissues. These predicted physiological responses correspond to three of the distinctive cardiovascular adaptations of Antarctic icefishes.
Because NO regulates physiological responses that are similar to the cardiovascular re-adaptations of icefishes, increasing attention is being focused on the pathways of NO production and degradation in Antarctic notothenioids. Mòrla et al. (2003) report that five icefish species express nNOS constitutively in skeletal muscle at levels that are dramatically higher than those for six red-blooded notothenioids. NO also regulates several cardiovascular activities in the heart of the icefish Chionodraco hamatus, and NOS activity is present in the endocardial endothelium (probably eNOS) and in cardiomyocytes (iNOS; Pellegrino et al. 2004). Though the data are limited, they do suggest that the metabolism of NO by icefishes differs substantially from that of red-blooded notothenioids.
(f) Haemoproteins, NO metabolism and icefish evolution
We now return to the haemoprotein status of the icefishes and its impact on NO metabolism. The oxygenated forms of haemoglobin and myoglobin are recognized as the major proteins responsible for the degradation of NO to nitrate (Kerwin et al. 1995; Flögel et al. 2001; Gardner 2005). Thus, Sidell & O'Brien (2006) have proposed that loss of the expression of haemoproteins triggered the initial physiological reconfiguration of the icefish cardiovascular system by elimination of the degradative pathways that modulate NO signalling. The resulting elevation of steady-state NO levels mediated the modification of icefish cardiovascular systems and the expansion of mitochondrial populations in oxidative tissues, and these characters then became fixed, most probably in the ancestral channichthyid prior to diversification within the clade. This hypothesis may be a simplification and the causality perhaps indirect, but it seems clear that the icefishes possessed the physiological tools to correct a deleterious, but non-lethal oxygen-transport phenotype.
(g) Icefishes and erythropoietic gene discovery
At first blush, one might question the use of icefishes, which do not produce red blood cells, to study the genetic pathway of erythrocyte formation. However, Yergeau et al. (2005) reasoned that the icefishes could be paired with closely related, but red-blooded, notothenioids to discover novel erythropoietic genes by cDNA-based representational difference analysis (cDNA RDA, also known as subtractive suppression hybridization, SSH; Hubank & Schatz 1994, 1999; Seta et al. 2004). The strategy of cDNA RDA entails the selective enrichment of gene fragments unique to, or heavily over-represented in, a ‘tester representation’ by favourable hybridization kinetics and exponential PCR amplification, and the concomitant removal of gene fragments shared by both ‘tester’ and ‘driver’ cDNA representations. In our study, we used cDNA from the haematopoietic kidney of the red-blooded species Notothenia coriiceps as tester and cDNA from the kidney of the icefish C. aceratus as the driver.
Using this subtractive technology, we obtained several DNA fragments that are expressed at significantly greater levels by the kidney of the red-blooded fish (Detrich & Yergeau 2004); these are candidates for genes involved in erythropoiesis.2 One of these encodes a protein domain called B30.2, which is thought to function as a protein–protein interaction motif (Henry et al. 1998). We used the B30.2 probe to clone two related genes from an N. coriiceps spleen cDNA library. The first to be studied, designated bloodthirsty (bty), belongs to the RBCC (RING-B box-coiled-coil) multigene family (Borden 1998), which is also known as the TRIM (tripartite motif) family (Reymond et al. 2001). The Bty protein of N. coriiceps contains 547 amino acids and is organized in sequential RING finger, B Box, coiled-coil, and B30.2 domains (Yergeau et al. 2005).
Due to their long generation times (many years to reproductive maturity), Antarctic fishes are not suitable subjects for functional analysis of the putative erythropoietic genes that we discovered by cDNA RDA. Therefore, we turned to the zebrafish Danio rerio, a model system that is widely used for analysis of gene function during vertebrate development (Eisen 1996; Grunwald 1996). We cloned the orthologous zebrafish bty gene from a kidney cDNA library and found that the 532-residue Bty protein was approximately 55% identical to N. coriiceps Bty (Yergeau et al. 2005).
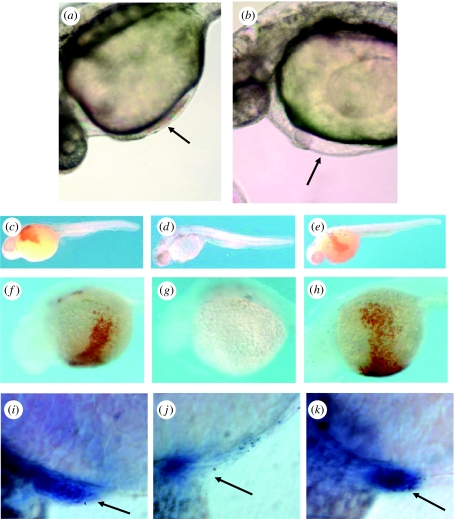
To assess the function of Bty during development, we employed a reverse-genetic strategy to suppress its expression. Zebrafish embryos were injected with antisense morpholino-modified oligonucleotides (MOs; Nasevicius & Ekker 2000) that were designed to bind to two contiguous sites at the 5′ end of the bty mRNA, thus preventing its translation. Control MOs covered the same target sequence but contained 4 or 5 bp mismatches to prevent binding to the mRNA. (For complete details, see Yergeau et al. 2005.) figure 6a,b compare the hearts of two living, 28-h embryos, one uninjected wild-type (figure 6a) and the second an ‘experimental’ embryo (wild-type injected with an antisense MO; figure 6b). The former developed a normal heart that circulated large numbers of erythrocytes (as did embryos injected with the control MOs; data not shown), whereas the latter developed a slightly edematous heart that beat normally but pumped an acorpuscular fluid. To gain further insight into the function of Bty, we stained MO-injected and uninjected embryos (32 h post-fertilization, hpf) with o-dianisidine to assess the production of haemoglobin or with α-globin antisense RNA to detect the corresponding mRNA. Figure 6d and g show that embryos injected with the antisense MO expressed almost no haemoglobin and exhibited few corpuscular elements, either in the embryo as a whole or on the yolk sinus. By contrast, both uninjected and control-MO-injected embryos (figure 6c,e,f,h, respectively) expressed wild-type levels of haemoglobin-rich erythrocytes, which in living embryos were observed circulating in the arteries, veins and capillary beds. Furthermore, embryos injected with antisense MOs (figure 6j) produced very few α-globin mRNA-positive erythrocytes compared with the uninjected (figure 6i) and control-MO-injected (figure 6k) embryos. We conclude that disruption of Bty synthesis suppresses both the production of erythrocytes and the synthesis of haemoglobin, as would be anticipated if Bty is required to express the programme of terminal erythroid differentiation from the proerythroblast progenitor. Our future studies will focus on the mechanism of action of the Bty protein in the production of red blood cells.
Figure 6.
Suppression of red blood cell formation in zebrafish embryos by antisense MOs targeted to the bty mRNA. (a,b) Differential interference contrast microscopy (DIC) of zebrafish embryos in vivo, 28 hpf (Nikon Eclipse 800 microscope). (a) Non-injected, wild-type embryo. The arrow shows circulating erythrocytes in the heart. (b) Wild-type embryo injected at the two cell stage with 10 ng of an antisense MO-1 targeted to the bty start codon and 21 bases downstream. Although the heart beats normally, circulating blood cells were absent (arrow). (c–e) Haemoglobin detection by o-dianisidine, whole embryo lateral view (32 hpf). Fixed and stained embryos were micrographed in 70% glycerol/PBS using a Nikon dissecting microscope. (c) Control wild-type embryo injected with MO buffer only. The circulation stained reddish brown when reacted with o-dianisidine, indicating the presence of haemoglobin-expressing red cells. (d) Antisense MO-2 directed to the 5′-UTR of the bty mRNA, 5 ng. Note the nearly complete absence of red blood cells. (e) MO-2 control with 5-bp mismatch, 5 ng. Red blood cells were present at wild-type levels. (f–h) Haemoglobin detection by o-dianisidine, view of venous circulation over yolk to heart. Embryos were micrographed as in (c–e). (f) Uninjected wild-type embryo. (g) Antisense MO-2, 10 ng. (h) Control 4-bp mismatch MO, 10 ng. (i–k) Whole-mount in situ hybridization of MO-treated zebrafish to α-globin antisense RNA (32 hpf). Micrographs were recorded in DIC mode (see a,b). (i) Uninjected embryo. (j) Embryo injected with 10 ng of antisense MO-1. (k) Embryo injected with 15 ng of control 4-bp mismatch MO. The arrows indicate the position of the heart. Globin-mRNA-positive red cells were greatly reduced in the experimental MO-injected embryo. Reprinted with permission from Yergeau et al. (2005). Copyright © 2005 Elsevier, Inc.
(h) Summary comments—haemoprotein loss in Antarctic icefishes
Antarctic icefishes are a uniquely valuable system for understanding the physiology and biochemistry of cardiovascular adaptation. Evolution of the erythrocyte-null condition of the Antarctic icefishes could have occurred, almost certainly, only in the Southern Ocean, which is characterized by extremely cold temperatures, high oxygen availability and low niche competition. Loss of haemoprotein expression in icefishes has been compensated by numerous cardiovascular adaptations. Recent evidence suggests that at least three of these adaptations, expansion of tissue capillary density, enlargement of the heart and increases in mitochondrial densities in heart muscle and other aerobic tissues, result from the physiological function of the NO/NOS system. Because the icefishes are, in effect, natural genetic ‘knockouts’ of the erythroid lineage, they are also a uniquely valuable resource for analysing the genetic programme of erythropoiesis by subtractive genomic strategies.
4. Concluding remarks
The Antarctic notothenioid fishes are remarkable creatures and outstanding models for understanding evolutionary responses to environmental change. Study of these extremophiles not only reveals their unique adaptational ‘secrets’ but also provides a unique perspective from which to examine the commonality of life processes, of cellular design and function, and of evolutionary change in essentially all species. We have reviewed two novel traits—the acquisition of AFGPs by fishes of the notothenioid suborder and the loss of haemoproteins of a subset of the notothenioids, the icefishes—that illustrate the fundamental and applied utility of the ecophysiology of this group. There are many more yet to be explored.
NOTE ADDED IN PROOF
Near et al. (2006) have recently found that the globin pseudogene complex of N. ionah is an ancestral intermediate on the pathway of globin gene deletion in the icefish clade.
Acknowledgments
C.-H.C.C. thanks Dr Arthur L. DeVries for his productive collaborations over many years. H.W.D. wishes to thank Dr Bruce Sidell and Dr Kristin O'Brien for sharing their insights into the relationship of haemoprotein loss to cardiovascular adaptation in Antarctic icefishes. The authors acknowledge the National Science Foundation for the support (grants OPP-0089451 and OPP-0336932 to H.W.D., and grants OPP-9909841 and OPP-0002654 to C.-H.C.C. and A.L.D.) of the work described.
5. Endnotes
It is conceivable that the cardiovascular changes evolved in the ancestral channichthyid before, or concomitant with, loss of haemoglobin expression, but the factor(s) that would drive this alternative path are not apparent.
Proof-of-principle was established by the recovery of adult α- and β-globin cDNAs, which cannot be expressed by the icefish.
One contribution of 10 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. Part 2: evolution, diversity and function’.
References
- Acierno R, Agnisola C, Tota B, Sidell B.D. Myoglobin enhances cardiac performance in Antarctic icefish species that express the protein. Am. J. Physiol. 1997;273:R100–R106. doi: 10.1152/ajpregu.1997.273.1.R100. [DOI] [PubMed] [Google Scholar]
- Aronson R.B, Blake D.B. Global climate change and the origin of modern benthic communities in Antarctica. Am. Zool. 2001;41:27–39. doi:10.1668/0003-1569(2001)041[0027:GCCATO]2.0.CO;2 [Google Scholar]
- Bargelloni L, Marcato S, Zane L, Patarnello T. Mitochondrial phylogeny of notothenioids: a molecular approach to Antarctic fish evolution and biogeography. Syst. Biol. 2000;49:114–129. doi: 10.1080/10635150050207429. doi:10.1080/10635150050207429 [DOI] [PubMed] [Google Scholar]
- Baum D.A, Larson A. Adaptation reviewed: a phylogenetic methodology for studying character macroevolution. Syst. Zool. 1991;40:1–18. doi:10.2307/2992218 [Google Scholar]
- Black V.S. Some aspects of the physiology of fish. Univ. Toronto Stud. Biol. Ser. 1951;71:53–89. [Google Scholar]
- Borden K.L. RING fingers and B-boxes: zinc-binding protein–protein interaction domains. Biochem. Cell Biol. 1998;76:351–358. doi: 10.1139/bcb-76-2-3-351. doi:10.1139/bcb-76-2-3-351 [DOI] [PubMed] [Google Scholar]
- Buckley B.A, Place S.P, Hofmann G.E. Regulation of heat shock genes in isolated hepatocytes from an Antarctic fish, Trematomus bernacchii. J. Exp. Biol. 2004;207:3649–3656. doi: 10.1242/jeb.01219. doi:10.1242/jeb.01219 [DOI] [PubMed] [Google Scholar]
- Chen L, DeVries A.L, Cheng C.C.-H. Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc. Natl Acad. Sci. USA. 1997a;94:3811–3816. doi: 10.1073/pnas.94.8.3811. doi:10.1073/pnas.94.8.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, DeVries A.L, Cheng C.-H.C. Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod. Proc. Natl Acad. Sci. USA. 1997b;94:3817–3822. doi: 10.1073/pnas.94.8.3817. doi:10.1073/pnas.94.8.3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C.-H.C. Genomic basis for antifreeze glycopeptide heterogeneity and abundance in Antarctic notothenioid fishes. In: Ennion S.J, Goldspink G, editors. Gene expression and manipulation in aquatic organisms. Cambridge University Press; Cambridge, UK: 1996. pp. 1–20. [Google Scholar]
- Cheng C.-H.C. Origin and mechanism of evolution of antifreeze glycoproteins in polar fish. In: di Prisco G, Pisano E, Clarke A, editors. Evolution of the Antarctic ichthyofauna. Springer; Milan, Italy: 1998. pp. 311–328. [Google Scholar]
- Cheng C.-H.C, Chen L. Evolution of an antifreeze glycoprotein. Nature. 1999;40:443–444. doi: 10.1038/46721. doi:10.1038/46721 [DOI] [PubMed] [Google Scholar]
- Cheng C.-H.C, Chen L, Near T.J, Jin Y. Functional antifreeze glycoprotein genes in temperate–water New Zealand nototheniid fish infer an Antarctic evolutionary origin. Mol. Biol. Evol. 2003;20:1897–1908. doi: 10.1093/molbev/msg208. doi:10.1093/molbev/msg208 [DOI] [PubMed] [Google Scholar]
- Cheng C.-H.C, Cziko P.A, Evans C.W. Non-hepatic origin of notothenioid antifreeze reveals pancreatic synthesis as common mechanism in polar fish freezing avoidance. Proc. Natl Acad. Sci. USA. 2006;103:10 491–10 496. doi: 10.1073/pnas.0603796103. doi:10.1073/pnas.0603796103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. Temperature and extinction in the sea: a physiologist's view. Paleobiology. 1993;19:499–518. [Google Scholar]
- Clarke A, Crame J.A. The Southern Ocean benthic fauna and climate change: a historical perspective. Phil. Trans. R. Soc. B. 1992;338:299–309. doi:10.1098/rstb.1992.0150 [Google Scholar]
- Cocca E, Ratnayake-Lecamwasam M, Parker S.K, Camardella L, Ciaramella M, Prisco G, Detrich III H.W. Genomic remnants of alpha-globin genes in the hemoglobinless Antarctic icefishes. Proc. Natl Acad. Sci. USA. 1995;92:1817–1821. doi: 10.1073/pnas.92.6.1817. doi:10.1073/pnas.92.6.1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocca E, Detrich H.W, III, Parker S.K, di Prisco G. A cluster of four globin genes from the Antarctic fish Notothenia coriiceps. J. Fish Biol. 2000;57:33–50. [Google Scholar]
- Conway E.M, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. doi:10.1016/S0008-6363(00)00281-9 [DOI] [PubMed] [Google Scholar]
- Cziko P, Evans C.W, Cheng C.-H.C, DeVries A.L. Freezing resistance of antifreeze-deficient larval Antarctic fish. J. Exp. Biol. 2005;209:407–420. doi: 10.1242/jeb.02008. doi:10.1242/jeb.02008 [DOI] [PubMed] [Google Scholar]
- Dayton P.K, Robilliard G.A, DeVries A.L. Anchor ice formation in McMurdo sound, Antarctica, and its biological effects. Science. 1969;163:273–274. doi: 10.1126/science.163.3864.273. doi:10.1126/science.163.3864.273 [DOI] [PubMed] [Google Scholar]
- Detrich III H.W, Yergeau D.A. Comparative genomics in erythropoietic gene discovery: synergisms between the Antarctic icefishes and the zebrafish. Methods Cell Biol. 2004;77:475–503. doi: 10.1016/s0091-679x(04)77026-0. [DOI] [PubMed] [Google Scholar]
- Detrich III H.W, Johnson K.A, Marchese-Ragona S.P. Polymerization of Antarctic fish tubulins at low temperatures: energetic aspects. Biochemistry. 1989;28:10 085–10 093. doi: 10.1021/bi00452a031. doi:10.1021/bi00452a031 [DOI] [PubMed] [Google Scholar]
- Detrich III H.W, Parker S.K, Williams R.C.J, Nogales E, Downing K.H. Cold adaptation of microtubule assembly and dynamics. Structural interpretation of primary sequence changes present in the alpha- and beta-tubulins of Antarctic fishes. J. Biol. Chem. 2000;275:37 038–37 047. doi: 10.1074/jbc.M005699200. doi:10.1074/jbc.M005699200 [DOI] [PubMed] [Google Scholar]
- DeVries A.L. Freezing resistance in Antarctic fishes. In: Holdgate M.W, editor. Antarctic ecology. Academic Press; New York, NY: 1970. pp. 320–328. [Google Scholar]
- DeVries A.L. Glycoproteins as biological antifreeze agents in Antarctic fishes. Science. 1971;172:1152–1155. doi: 10.1126/science.172.3988.1152. doi:10.1126/science.172.3988.1152 [DOI] [PubMed] [Google Scholar]
- DeVries A.L. Survival at freezing temperatures. In: Malins D.C, Sargent J.R, editors. Biochemical and biophysical perspectives in marine biology. Academic Press; London, UK: 1974. pp. 289–330. [Google Scholar]
- DeVries A.L. Biological antifreeze agents. Annu. Rev. Physiol. 1983;45:245–260. doi: 10.1146/annurev.ph.45.030183.001333. doi:10.1146/annurev.ph.45.030183.001333 [DOI] [PubMed] [Google Scholar]
- DeVries A.L, Cheng C.-H.C. The role of antifreeze glycopeptides and peptides in the survival of cold-water fishes. In: Somero G.N, Osmond C.B, Bolis C.L, editors. Water and life: comparative analysis of water relationships at the organismic, cellular and molecular levels. Springer; Berlin, Germany: 1992. pp. 301–315. [Google Scholar]
- De Vries A.L, Cheng C.-H.C. Antifreeze proteins and organismal freezing avoidance in polar fishes. In: Farrell A.P, Steffenson J.F, editors. The physiology of polar fishes. fish physiology series. vol. 22. Elsevier Academic Press; San Diego, CA: 2005. pp. 155–201. [Google Scholar]
- DeVries A.L, Lin Y. The role of glycoprotein antifreezes in the survival of Antarctic fishes. In: Llano G.A, editor. Adaptations within Antarctic ecosystems. Gulf; Houston, TX: 1977. pp. 439–458. [Google Scholar]
- DeVries A.L, Steffensen J.F. The Arctic and Antarctic polar marine environments. In: Farrell A.P, Steffensen J.F, editors. The physiology of polar fishes. fish physiology series. vol. 22. Elsevier Academic Press; San Diego, CA: 2005. pp. 1–24. [Google Scholar]
- di Prisco G, Cocca E, Parker S, Detrich III H.W. Tracking the evolutionary loss of hemoglobin expression by the white-blooded Antarctic icefishes. Gene. 2002;295:185–191. doi: 10.1016/s0378-1119(02)00691-1. doi:10.1016/S0378-1119(02)00691-1 [DOI] [PubMed] [Google Scholar]
- Eastman J.T. Academic Press; San Diego, CA: 1993. Antarctic fish biology: evolution in a unique environment. [Google Scholar]
- Eastman J.T. The nature of the diversity of Antarctic fishes. Polar Biol. 2005;28:94–107. doi:10.1007/s00300-004-0667-4 [Google Scholar]
- Eastman J.T, DeVries A.L. Morphology of the digestive system of Antarctic nototheniid fishes. Polar Biol. 1997;17:1–13. doi:10.1007/s003000050098 [Google Scholar]
- Eastman J.T, McCune A.R. Fishes on the Antarctic continental shelf: evolution of a marine species flock? J. Fish Biol. 2000;57(Suppl. A):84–102. [Google Scholar]
- Egginton S, Skilbeck C, Hoofd L, Calvo J, Johnston I.A. Peripheral oxygen transport in skeletal muscle of Antarctic and sub-Antarctic notothenioid fish. J. Exp. Biol. 2002;205:769–779. doi: 10.1242/jeb.205.6.769. [DOI] [PubMed] [Google Scholar]
- Eisen J.S. Zebrafish make a big splash. Cell. 1996;87:969–977. doi: 10.1016/s0092-8674(00)81792-4. doi:10.1016/S0092-8674(00)81792-4 [DOI] [PubMed] [Google Scholar]
- Evans D.H. Osmotic and ionic regulation. In: Evans D.H, editor. The physiology of fishes. CRC Press; Boca Raton, FL: 1993. [Google Scholar]
- Evans C.W, Cziko P.A, Cheng C.-H.C, DeVries A.L. Spawning behaviour and early development in the naked dragonfish Gymnodraco acuticeps. Antarct. Sci. 2005;17:319–327. doi:10.1017/S0954102005002749 [Google Scholar]
- Ewart K.V, Fletcher G.L. Herring antifreeze protein: primary structure and evidence for a C-type lectin evolutionary origin. Mol. Mar. Biol. Biotechnol. 1993;2:20–27. [PubMed] [Google Scholar]
- Fletcher G.L, Hew C.L, Davies P.L. Antifreeze proteins of teleost fishes. Annu. Rev. Physiol. 2001;63:359–390. doi: 10.1146/annurev.physiol.63.1.359. doi:10.1146/annurev.physiol.63.1.359 [DOI] [PubMed] [Google Scholar]
- Flögel U, Merx M.W, Gödecke A, Decking U.K, Schrader J. Myoglobin: a scavenger of bioactive NO. Proc. Natl Acad. Sci. USA. 2001;98:735–740. doi: 10.1073/pnas.011460298. doi:10.1073/pnas.011460298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B.G, Pearson H.A. Hemoglobin synthesis and the thalassemias. In: Handin R.I, Lux S.E, Stossel T.P, editors. BLOOD: principles and practice of hematology. J. B. Lippincott Company; Philadelphia, PA: 1995. pp. 1525–1590. [Google Scholar]
- Gardner P.R. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J. Inorg. Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. doi:10.1016/j.jinorgbio.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Gödecke A, Molojavyi A, Heger J, Flögel U, Ding Z, Jacoby C, Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J. Biol. Chem. 2003;278:21 761–21 766. doi: 10.1074/jbc.M302573200. doi:10.1074/jbc.M302573200 [DOI] [PubMed] [Google Scholar]
- Gong Z, Ewart K.V, Hu Z, Fletcher G.L, Hew C.L. Skin antifreeze protein genes of the winter flounder, Pleuronectes americanus, encode distinct and active polypeptides without the secretory signal and pro-sequences. J. Biol. Chem. 1996;271:4106–4112. doi: 10.1074/jbc.271.8.4106. doi:10.1074/jbc.271.32.19037 [DOI] [PubMed] [Google Scholar]
- Grove T.J, Hendrickson J.W, Sidell B.D. Two species of Antarctic icefishes (genus Champsocephalus) share a common genetic lesion leading to the loss of myoglobin expression. Polar Biol. 2004;27:579–585. doi:10.1007/s00300-004-0634-0 [Google Scholar]
- Grunwald D.J. A fin-de-siècle achievement: charting new waters in vertebrate biology. Science. 1996;274:1634–1635. doi: 10.1126/science.274.5293.1634. doi:10.1126/science.274.5293.1634 [DOI] [PubMed] [Google Scholar]
- Haschemeyer A.E.V, Mathews R.W. Antifreeze glycoprotein synthesis in the Antarctic fish Trematomus hansoni by constant infusion in vivo. Physiol. Zool. 1980;53:383–393. [Google Scholar]
- Haschemeyer A.E.V, Smith M.A.K. Protein synthesis in liver, muscle, and gill of mullet (Mugil cephalus L.) in vivo. Biol. Bull. 1979;156:93–102. doi: 10.2307/1541005. [DOI] [PubMed] [Google Scholar]
- Hemmingsen E.A. Respiratory and cardiovascular adaptations in hemoglobin-free fish: resolved and unresolved problems. In: di Prisco G, Maresca B, Tota B, editors. Biology of Antarctic fish. Springer; Berlin, Germany: 1991. pp. 191–203. [Google Scholar]
- Hemmingsen E.A, Douglas E.L. Respiratory and circulatory adaptations to the absence of hemoglobin in Chaenichthyid fishes. In: Llano G.A, editor. Adaptations within Antarctic ecosystems. Gulf; Houston, TX: 1977. pp. 479–487. [Google Scholar]
- Hemmingsen E.A, Douglas E.L, Johansen K, Millard R.W. Aortic blood flow and cardiac output in the hemoglobin-free fish Chaenocephalus aceratus. Comp. Biochem. Physiol A. 1972;43:1045–1051. doi: 10.1016/0300-9629(72)90176-4. doi:10.1016/0300-9629(72)90176-4 [DOI] [PubMed] [Google Scholar]
- Henry J, Mather I.H, McDermott M.F, Pontarotti P. B30.2-like domain proteins: update and new insights into a rapidly expanding family of proteins. Mol. Biol. Evol. 1998;15:1696–1705. doi: 10.1093/oxfordjournals.molbev.a025896. [DOI] [PubMed] [Google Scholar]
- Hew C.L, Yip C. The synthesis of freezing-point depression protein of the winter flounder Pseudopleuronectes americanus in Xenopus laevis oocytes. Biochim. Biophys. Res. Commun. 1976;71:845–850. doi: 10.1016/0006-291x(76)90908-6. [DOI] [PubMed] [Google Scholar]
- Hew C.L, Wang N.-C, Joshi S, Fletcher G.L, Scott G.K, Hayes P.H, Buettner B, Davies P.L. Multiple genes provide the basis for antifreeze protein diversity and dosage in the ocean pout, Macrozoarces americanus. J. Biol. Chem. 1988;263:12 049–12 055. [PubMed] [Google Scholar]
- Hinton D.E, Snipes R.L, Kendall M.W. Morphology and enzyme histochemistry in liver of largemouth bass (Micropterus salmoides) J. Fish Res. Board Can. 1972;29:531–534. [Google Scholar]
- Hofmann G.E, Buckley B.A, Airaksinen S, Keen J, Somero G.N. The Antarctic fish Trematomus bernacchii lacks heat-inducible heat shock protein synthesis. J. Exp. Biol. 2000;203:2331–2339. doi: 10.1242/jeb.203.15.2331. [DOI] [PubMed] [Google Scholar]
- Hsiao K.C, Cheng C.-H.C, Fernandes I.E, Detrich III H.W, DeVries A.L. An antifreeze glycopeptide gene from the Antarctic cod Notothenia coriiceps neglecta encodes a polyprotein of high peptide copy number. Proc. Natl Acad. Sci. USA. 1990;87:9265–9269. doi: 10.1073/pnas.87.23.9265. doi:10.1073/pnas.87.23.9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubank M, Schatz D.G. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucleic Acids Res. 1994;22:5640–5648. doi: 10.1093/nar/22.25.5640. doi:10.1093/nar/22.25.5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubank M, Schatz D.G. cDNA representational difference analysis: a sensitive and flexible method for identification of differentially expressed genes. Methods Enzymol. 1999;303:325–349. doi: 10.1016/s0076-6879(99)03021-9. [DOI] [PubMed] [Google Scholar]
- Hudson R.R. Gene genealogies and the coalescent process. In: Futuyma D, Antonovics J, editors. Oxford surveys in evolutionary biology. Oxford University Press; New York, Oxford: 1990. pp. 1–44. [Google Scholar]
- Hudson R.R. Gene trees, species trees and the segregation of ancestral alleles. Genetics. 1992;131:509–512. doi: 10.1093/genetics/131.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A.P, DeVries A.L, Haschemeyer A.E.V. Antifreeze glycoprotein biosynthesis in Antarctic fishes. Comp. Biochem. Physiol. B. 1979;62:179–183. doi:10.1016/0305-0491(79)90308-0 [Google Scholar]
- Hunt B.M, Hoefling K, Cheng C.-H.C. Annual warming episodes in seawater temperatures in McMurdo Sound in relationship to endogenous ice in notothenioid fish. Antarct. Sci. 2003;15:333–338. doi:10.1017/S0954102003001342 [Google Scholar]
- Jin Y. 2003. Freezing avoidance of Antarctic fishes: the role of a novel antifreeze potentiating protein and the antifreeze glycoproteins. PhD Thesis, University of Illinois. [Google Scholar]
- Kennett J.P. Cenozoic evolution of Antarctic glaciation, the circum-Antarctic Ocean, and their impact on global paleoceanography. J. Geophys. Res. 1977;82:3843–3860. [Google Scholar]
- Kerwin J.F, Jr, Lancaster J.R, Jr, Feldman P.L. Nitric oxide: a new paradigm for second messengers. J. Med. Chem. 1995;38:4343–4362. doi: 10.1021/jm00022a001. doi:10.1021/jm00022a001 [DOI] [PubMed] [Google Scholar]
- Kiss A.J, Mirarefi A.Y, Ramakrishnan S, Zukoski C, DeVries A.L, Cheng C.-H.C. Cold stable eye lens crystallins of the Antarctic nototheniid toothfish Dissostichus mawsoni (Norman) J. Exp. Biol. 2004;207:4633–4649. doi: 10.1242/jeb.01312. doi:10.1242/jeb.01312 [DOI] [PubMed] [Google Scholar]
- Koch K.-H. Antarctic icefishes (Channichthyidae): a unique family of fishes. A review, part I. Polar Biol. 2005;28:862–895. doi:10.1007/s00300-005-0019-z [Google Scholar]
- Kurokawa T, Suzuki T. Structure of the exocrine pancreas of flounder (Paralichthys olivaceus): immunological localization of zymogen granules in the digestive tract using anti-trypsinogen antibody. J. Fish Biol. 1995;46:292–301. doi:10.1111/j.1095-8649.1995.tb05970.x [Google Scholar]
- Lau D.T, Saeed A, Parker S.K, Detrich H.W., III Adaptive evolution of gene expression in Antarctic fishes: divergent transcription of the 5′-to-5′ linked adult α1- and β-globin genes of the Antarctic teleost Notothenia coriiceps is controlled by dual promoters and intergenic enhancers. Am. Zool. 2001;41:113–132. doi:10.1668/0003-1569(2001)041[0113:AEOGEI]2.0.CO;2 [Google Scholar]
- Littlepage J.L. Oceanographic investigations in McMurdo sound, Antarctica. In: Llano G.A, editor. Biology of the Antarctic Seas II. vol. 5. American Geophysical Union; Washington, DC: 1965. pp. 1–37. [Google Scholar]
- Livermore R, Nankivell A, Eagles G, Morris P. Paleogene opening of Drake Passage. Earth Planet. Sci. Lett. 2005;236:459–470. doi:10.1016/j.epsl.2005.03.027 [Google Scholar]
- Loeb V.J, Kellerman A.K, Koubbi P, North A.W, White M.G. Antarctic larval fish assemblages: a review. Bull. Mar. Sci. 1993;53:416–449. [Google Scholar]
- Logue J.A, DeVries A.L, Fodor E, Cossins A.R. Lipid compositional correlates of temperature-adaptive interspecific differences in membrane structure. J. Exp. Biol. 2000;230:2105–2113. doi: 10.1242/jeb.203.14.2105. [DOI] [PubMed] [Google Scholar]
- Loretz C.A. Drinking and alimentary transport in teleost osmoregulation. In: Goos H.J.T, Rastogi R.K, Vaudry H, Pierantoni R, editors. Perspective in comparative endocrinology: unity and diversity. Monduzzi Editore; Bologna, Italy: 2001. pp. 723–732. [Google Scholar]
- Merx M.W, Flögel U, Stumpe T, Gödecke A, Decking U.K, Schrader J. Myoglobin facilitates oxygen diffusion. FASEB J. 2001;15:1077–1079. doi: 10.1096/fj.00-0497fje. [DOI] [PubMed] [Google Scholar]
- Miller L.L, Bly C.G, Watson M.L, Bale W.F. The dominant role of the liver in plasma protein synthesis: a direct study of the isolated perfused rat liver with the aid of lysine-e-C14. J. Exp. Med. 1951;94:431–453. doi: 10.1084/jem.94.5.431. doi:10.1084/jem.94.5.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Clements K. Disaptation and recovery in the evolution of Antarctic fishes. Trends Ecol. Evol. 2000;15:267–271. doi: 10.1016/s0169-5347(00)01896-6. doi:10.1016/S0169-5347(00)01896-6 [DOI] [PubMed] [Google Scholar]
- Mòrla M, Agusti A.G.N, Rahman I, Motterlini R, Saus C, Morales-Nin B, Company J.B, Busquets X. Nitric oxide synthase type I (nNOS), vascular endothelial growth factor (VEGF) and myoglobin-like expression in skeletal muscle of Antarctic fishes (Notothenioidei: Channichthyidae) Polar Biol. 2003;26:458–462. [Google Scholar]
- Moylan T.J, Sidell B.D. Concentrations of myoglobin and myoglobin mRNA in heart ventricles from Antarctic fishes. J. Exp. Biol. 2000;203:1277–1286. doi: 10.1242/jeb.203.8.1277. [DOI] [PubMed] [Google Scholar]
- Nagel R.L. Disorders of hemoglobin function and stability. In: Handin R.I, Lux S.E, Stossel T.P, editors. BLOOD: principles and practice of hematology. J. B. Lippincott Company; Philadelphia, PA: 1995. pp. 1591–1644. [Google Scholar]
- Nasevicius A, Ekker S.C. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. doi:10.1038/79951 [DOI] [PubMed] [Google Scholar]
- Near T.J. Estimating divergence times of notothenioid fishes using a fossil-calibrated molecular clock. Antarct. Sci. 2004;16:37–44. doi:10.1017/S0954102004001798 [Google Scholar]
- Near T.J, Pesavento J.J, Cheng C.-H.C. Mitochondrial DNA, morphology, and the phylogenetic relationships of Antarctic icefishes (Notothenioidei: Channichthyidae) Mol. Phylogenet. Evol. 2003;28:87–98. doi: 10.1016/s1055-7903(03)00029-0. doi:10.1016/S1055-7903(03)00029-0 [DOI] [PubMed] [Google Scholar]
- Near T.J, Parker S.K, Detrich III H.W. A genomic fossil reveals key steps in hemoglobin loss by the Antarctic ice fishes. Mol. Biol. Evol. 2006;23:2008–2016. doi: 10.1093/molbev/msl071. doi:10.1093/molbev/ms1071 [DOI] [PubMed] [Google Scholar]
- Nicholls K.W, Makinson K. Ocean circulation beneath the western Ronne ice shelf, as derived from in situ measurements of water currents and properties. In: Jacobs S.S, Weiss R.F, editors. Ocean, ice and atmosphere: interactions at the Antarctic continental margin. vol. 75. American Geophysical Union; Washington, DC: 1998. pp. 301–318. [Google Scholar]
- Nisoli E, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. doi:10.1126/science.1079368 [DOI] [PubMed] [Google Scholar]
- Nisoli E, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl Acad. Sci. USA. 2004;101:16 507–16 512. doi: 10.1073/pnas.0405432101. doi:10.1073/pnas.0405432101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North A.W. Review of the early life history of Antarctic notothenioid fish. In: di Prisco G, Maresca B, Tota B, editors. Biology of Antarctic fish. Springer; Berlin, Germany; New York, NY: 1991. pp. 70–86. [Google Scholar]
- NRC. National Academy of Sciences; Washington, DC: 2003. Frontiers in polar biology in the genomic era. [Google Scholar]
- O'Brien K.M, Xue H, Sidell B.D. Quantification of diffusion distance within the spongy myocardium of hearts from Antarctic fishes. Resp. Physiol. 2000;122:71–80. doi: 10.1016/s0034-5687(00)00139-0. doi:10.1016/S0034-5687(00)00139-0 [DOI] [PubMed] [Google Scholar]
- O'Grady S.M, Clarke A, DeVries A.L. Characterization of glycoprotein antifreeze biosynthesis in isolated hepatocytes from Pagothenia borchgrevinki. J. Exp. Zool. 1982a;220:179–189. doi: 10.1002/jez.1402200207. doi:10.1002/jez.1402200207 [DOI] [PubMed] [Google Scholar]
- O'Grady S.M, Ellory J.C, DeVries A.L. Protein and glycoprotein antifreezes in intestinal fluid of polar fishes. J. Exp. Biol. 1982b;98:429–438. doi: 10.1242/jeb.98.1.429. [DOI] [PubMed] [Google Scholar]
- O'Grady S.M, Ellory J.C, DeVries A.L. The role of low molecular weight antifreeze glycopeptides in the bile and intestinal fluid of Antarctic fishes. J. Exp. Biol. 1983;104:149–162. [Google Scholar]
- Palmer R.M, Ferrige A.G, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. doi:10.1038/327524a0 [DOI] [PubMed] [Google Scholar]
- Paluh J.L, Killilea A.N, Detrich III H.W, Downing K.H. Meiosis-specific failure of cell cycle progression in fission yeast by mutation of a conserved β-tubulin residue. Mol. Biol. Cell. 2004;15:1160–1171. doi: 10.1091/mbc.E03-06-0389. doi:10.1091/mbc.E03-06-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino D, Palmerini C.A, Tota B. No hemoglobin but NO: the icefish (Chionodraco hamatus) heart as a paradigm. J. Exp. Biol. 2004;207:3855–3864. doi: 10.1242/jeb.01180. doi:10.1242/jeb.01180 [DOI] [PubMed] [Google Scholar]
- Platt O.S. The sickle syndromes. In: Handin R.I, Lux S.E, Stossel T.P, editors. BLOOD: principles and practice of hematology. J. B. Lippincott Company; Philadelphia, PA: 1995. pp. 1645–1700. [Google Scholar]
- Raymond J.A, DeVries A.L. Freezing behavior of fish blood glycoproteins with antifreeze properties. Cryobiology. 1972;9:541–547. doi: 10.1016/0011-2240(72)90176-9. doi:10.1016/0011-2240(72)90176-9 [DOI] [PubMed] [Google Scholar]
- Raymond J.A, DeVries A.L. Adsorption–inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl Acad. Sci. USA. 1977;74:2589–2593. doi: 10.1073/pnas.74.6.2589. doi:10.1073/pnas.74.6.2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond A, et al. The tripartite motif family identifies cell compartments. EMBO J. 2001;20:2140–2151. doi: 10.1093/emboj/20.9.2140. doi:10.1093/emboj/20.9.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römisch K, Collie N, Soto N, Logue J, Lindsay M, Scheper W, Cheng C.-H.C. Protein translocation across the endoplasmic reticulum membrane in cold-adapted organisms. J. Cell Sci. 2003;116:2875–2883. doi: 10.1242/jcs.00597. doi:10.1242/jcs.00597 [DOI] [PubMed] [Google Scholar]
- Ruud J.T. Vertebrates without erythrocytes and blood pigment. Nature. 1954;173:848–850. doi: 10.1038/173848a0. doi:10.1038/173848a0 [DOI] [PubMed] [Google Scholar]
- Scholander P.F, Van Dam L, Kanwisher J.W, Hammel H.T, Gordon M.S. Supercooling and osmoregulation in Arctic fish. J. Cell. Comp. Physiol. 1957;49:5–24. doi:10.1002/jcp.1030490103 [Google Scholar]
- Seta K.A, Ferguson T.K, Millhorn D.E. Discovery of oxygen-responsive genes in pheochromocytoma cells. Methods Enzymol. 2004;381:449–464. doi: 10.1016/S0076-6879(04)81030-9. [DOI] [PubMed] [Google Scholar]
- Sidell B.D. Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J. Exp. Biol. 1998;201:1118–1127. doi: 10.1242/jeb.201.8.1119. [DOI] [PubMed] [Google Scholar]
- Sidell B.D, O'Brien K.M. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J. Exp. Biol. 2006;209:1791–1802. doi: 10.1242/jeb.02091. [DOI] [PubMed] [Google Scholar]
- Sidell B.D, Vayda M.E, Small D.J, Moylan T.J, Londraville R.L, Yuan M.L, Rodnick K.J, Eppley Z.A, Costello L. Variable expression of myoglobin among the hemoglobinless Antarctic icefishes. Proc. Natl Acad. Sci. USA. 1997;94:3420–3424. doi: 10.1073/pnas.94.7.3420. doi:10.1073/pnas.94.7.3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.J, Vayda M.E, Sidell B.D. A novel vertebrate myoglobin gene containing three A+T-rich introns is conserved among Antarctic teleost species which differ in myoglobin expression. J. Mol. Evol. 1998;47:156–166. doi: 10.1007/pl00006372. doi:10.1007/PL00006372 [DOI] [PubMed] [Google Scholar]
- Small D.J, Moylan T, Vayda M.E, Sidell B.D. The myoglobin gene of the Antarctic icefish, Chaenocephalus aceratus, contains a duplicated TATAAAA sequence that interferes with transcription. J. Exp. Biol. 2003;206:131–139. doi: 10.1242/jeb.00067. doi:10.1242/jeb.00067 [DOI] [PubMed] [Google Scholar]
- Smith H.W. The absorption and excretion of water and salts by marine teleosts. Am. J. Physiol. 1930;93:480–505. [Google Scholar]
- Soderlund C, Humphrey S, Dunhum A, French L. Contigs built with fingerprints, markers and FPC V4.7. Genome Res. 2000;10:1772–1787. doi: 10.1101/gr.gr-1375r. doi:10.1101/gr.GR-1375R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero G.N, DeVries A.L. Temperature tolerance of some Antarctic fishes. Science. 1967;156:257–258. doi: 10.1126/science.156.3772.257. doi:10.1126/science.156.3772.257 [DOI] [PubMed] [Google Scholar]
- Tripati A, Backman J, Elderfield H, Ferretti P. Eocene bipolar glaciation associated with global carbon cycle changes. Nature. 2005;436:341–346. doi: 10.1038/nature03874. doi:10.1038/nature03874 [DOI] [PubMed] [Google Scholar]
- Vacchi M, La M.M, Dalu M, MacDonald J. Early life stages in the life cycle of Antarctic silverfish, Pleuragramma antarcticum in Terra Nova Bay, Ross Sea. Antarct. Sci. 2003;16:299–305. doi:10.1017/S0954102004002135 [Google Scholar]
- Wang X, DeVries A.L, Cheng C.-H.C. Genomic basis for antifreeze peptide heterogeneity and abundance in an Antarctic eel pout: gene structures and organization. Mol. Mar. Biol. Biotechnol. 1995;4:135–147. [PubMed] [Google Scholar]
- Williams R.C, Correia J.J, DeVries A.L. Formation of microtubules at low temperature by tubulin from Antarctic fishes. Biochem. 1985;24:2790–2798. doi: 10.1021/bi00332a029. doi:10.1021/bi00332a029 [DOI] [PubMed] [Google Scholar]
- Wittenberg J.B, Wittenberg B.A. Myoglobin function reassessed. J. Exp. Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. doi:10.1242/jeb.00243 [DOI] [PubMed] [Google Scholar]
- Yergeau D.A, Cornell C.N, Parker S.K, Zhou Y, Detrich III H.W. bloodthirsty, an RBCC/TRIM gene required for erythropoiesis in zebrafish. Dev. Biol. 2005;283:97–112. doi: 10.1016/j.ydbio.2005.04.006. doi:10.1016/j.ydbio.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ratnayake-Lecamwasam M, Parker S.K, Cocca E, Camardella L, di Prisco G, Detrich H.W., III The major adult α-globin gene of Antarctic teleosts and its remnants in the hemoglobinless icefishes: calibration of the mutational clock for nuclear genes. J. Biol. Chem. 1998;273:14 745–14 752. doi: 10.1074/jbc.273.24.14745. doi:10.1074/jbc.273.24.14745 [DOI] [PubMed] [Google Scholar]