Abstract
A cause and effect understanding of thermal limitation and adaptation at various levels of biological organization is crucial in the elaboration of how the Antarctic climate has shaped the functional properties of extant Antarctic fauna. At the same time, this understanding requires an integrative view of how the various levels of biological organization may be intertwined. At all levels analysed, the functional specialization to permanently low temperatures implies reduced tolerance of high temperatures, as a trade-off. Maintenance of membrane fluidity, enzyme kinetic properties (Km and kcat) and protein structural flexibility in the cold supports metabolic flux and regulation as well as cellular functioning overall. Gene expression patterns and, even more so, loss of genetic information, especially for myoglobin (Mb) and haemoglobin (Hb) in notothenioid fishes, reflect the specialization of Antarctic organisms to a narrow range of low temperatures. The loss of Mb and Hb in icefish, together with enhanced lipid membrane densities (e.g. higher concentrations of mitochondria), becomes explicable by the exploitation of high oxygen solubility at low metabolic rates in the cold, where an enhanced fraction of oxygen supply occurs through diffusive oxygen flux. Conversely, limited oxygen supply to tissues upon warming is an early cause of functional limitation. Low standard metabolic rates may be linked to extreme stenothermy. The evolutionary forces causing low metabolic rates as a uniform character of life in Antarctic ectothermal animals may be linked to the requirement for high energetic efficiency as required to support higher organismic functioning in the cold. This requirement may result from partial compensation for the thermal limitation of growth, while other functions like hatching, development, reproduction and ageing are largely delayed. As a perspective, the integrative approach suggests that the patterns of oxygen- and capacity-limited thermal tolerance are linked, on one hand, with the capacity and design of molecules and membranes, and, on the other hand, with life-history consequences and lifestyles typically seen in the permanent cold. Future research needs to address the detailed aspects of these interrelationships.
Keywords: Antarctic, thermal adaptation, global warming, life history evolution, notothenioid fishes, marine invertebrates
1. Introduction: climate-dependent evolution of Antarctic fauna
It is widely accepted that marine biogeography is largely shaped by direct effects of temperature (Angel 1991; Murawski 1993). Temperature also influences biodiversity patterns at various latitudes (e.g. Roy et al. 1998; Astorga et al. 2003). Climate-induced effects on marine organisms are thus mostly attributed to a changing temperature regime. These effects may be direct, through effects of temperature itself, or indirect, through thermally induced changes in the composition of the food chain or through fluctuating food availability in time and space. Such changes may start with fluctuations in phytoplankton availability influenced by temperature, changing ocean currents and stratification or changing levels of nutrients like iron (Gowen et al. 1995; Reid et al. 1998; Wiltshire & Manly 2004; Wang et al. 2005). These patterns, combined with direct temperature effects on higher-level food-chain components may lead to changing availability of, for example, zooplankton for fish (e.g. Beaugrand & Reid 2003; Beaugrand et al. 2003; Platt et al. 2003). However, direct effects of temperature on the physiology of the marine organisms involved may be the overarching process behind these ecological phenomena. In a wide range of terrestrial organisms (plants to birds), such direct temperature effects proved to be most relevant for setting the biogeography and biodiversity of species, regardless of their position in the food chain (Huntley et al. 2004). The respective analyses have yet to be carried out for aquatic organisms. Nonetheless, available evidence indicates that thermal windows relate to biogeography. The ‘climate envelope’ of a species needs to be known (Pearson & Dawson 2003) in order to elaborate the background of changes in the abundance and distribution of marine ectotherms upon climate change. This includes knowledge of the upper and lower limits of thermal tolerance as key features of this envelope. ‘Climate envelope models’ have proven useful in analyses of terrestrial biogeography and biodiversity (Huntley et al. 2004), but have not been tested in the marine realm. Application and credibility of such concepts would largely benefit from a profound cause and effect understanding based on knowledge of the physiological mechanisms setting ecologically relevant thermal envelopes in marine organisms.
Such considerations should also be applicable to Antarctic organisms, which live permanently at the low end of the temperature scale in the oceans. In a global comparison of marine temperate and cold environments, temperature variability is currently lowest in the marine Antarctic, with temperature maintained close to freezing in several areas (Clarke 1998; Cheng & Detrich 2007). Current evidence suggests a long evolutionary history in situ for much of the Southern Ocean fauna, with a large degree of endemism and some exchange of fauna via the deep sea (Clarke & Crame 1989). Global climate change close to the end of the Eocene (approx. 34 Myr ago), associated with a continued repositioning of the continents, characterized the transition from a cool temperate climate in Antarctica to the polar climate that exists there today (for review, see Crame 1993; Clarke 1996). The loss of the warm water supply to the Antarctic coastline during separation of Antarctica and Australia, and the formation of the circum-Antarctic current have traditionally been seen as the processes initiating cooling. This view has recently been challenged and it has been conjectured that a rapid drop in atmospheric carbon dioxide has contributed greatly to the sudden climate shift and, then, continental ice formation at the close of the Eocene (Huber et al. 2004, Tripati et al. 2005). Water temperatures first remained close to 4 or 5°C, and only during the last 4–5 Myr did cooling continue and reach the low temperatures that characterize extant Antarctic waters, and shaped modern Antarctic marine communities. Cooling probably reduced the abundance and diversity of fishes, crabs, gastropods and bivalves, which, in turn, reduced skeleton-crushing predation on invertebrates. Reduced predation allowed dense populations of ophiuroids and crinoids to dominate Antarctic (shallow) marine communities until today (Aronson et al. 1997). Nonetheless, temperature oscillations have occurred repeatedly on time-scales of several thousands of years during recent Antarctic climate history (Hodell et al. 2001).
Present physiological characters and the loss of functional groups from the marine ecosystems of Antarctica must be interpreted in the context of climate history and Antarctic climate variability as well as by considering the evolutionary and geographical origin of Antarctic fauna. Antarctic marine ectotherms live at the low end of the temperature continuum found in marine environments, and some species are considered highly stenothermal, i.e. they are highly specialized in their low and narrow temperature window (Somero & De Vries 1967; Somero et al. 1996; Pörtner et al. 1999a, 2000; Peck & Conway 2000; Peck et al. 2002). Most species die in experiments when temperatures are raised short term to between 5 and 10°C. The most temperature-sensitive species to date, the Weddell Sea bivalve Limopsis marionensis, displays an upper critical temperature of around 2°C (Pörtner et al. 1999a). The brachiopod, Liothyrella uva (Peck 1989), is unable to survive long term at 4°C. Recent analyses of metabolic features in various groups of Antarctic species indicate, however, that some species may be more tolerant of high temperatures than would appear to be necessary under the cold and stable temperature conditions of the marine Antarctic (cf. Pörtner et al. 2000; Lannig et al. 2005; Seebacher et al. 2005). Thus, brachiopods, the bivalve L. marionensis and the scallop Adamussium colbecki, are all affected by temperature elevations of only 1 or 2°C above current summer maxima. Other species, however, such as the eelpout Pachycara brachycephalum (Van Dijk et al. 1999), the starfish Odontaster validus (K. Webb 2004, personal communication) and the limpet Nacella concinna (Peck 1989; Pörtner et al. 1999a) can tolerate temperatures 4–5°C higher than this. This flexibility allows N. concinna to dwell in the intertidal zone. The predatory nemertean, Parborlasia corrugatus, is also tolerant of elevated temperatures up to 10°C. The width of the thermal tolerance window and the level of motor activity and mobility may be interdependent (Pörtner 2002b) due to the dependence of thermal tolerance on aerobic scope (see below). More mobile organisms, for example predatory species, have higher aerobic scopes and will therefore cope with changing environments better than some secondary consumers. Moreover, while thermal acclimation capacity still exists among Antarctic fishes, as verified in a zoarcid (Lannig et al. 2005) and a notothenioid (Seebacher et al. 2005), marine invertebrates may, on average, be more thermally sensitive than fishes. The question has very recently been addressed in epifaunal scallops (A. colbecki), where 50% mortality of specimens was obtained in 19 days at 4°C (D. Bailey 2001, personal communication), infaunal clams (Laternula elliptica), where 50% mortality was obtained in two months at 3°C and the brittle star (Ophionotus victoriae), where 50% mortality occurred in less than one month at 3°C (L. Peck 2006, personal observation).
The question clearly arises whether and how thermal tolerances of Antarctic ectotherms might be related to their other functional characteristics, and whether these physiological characters bear importance at the ecological level, by explaining why and how species can survive in the Antarctic through the shaping of Antarctic ecosystem functioning. This essentially implies asking for the unifying trade-offs and constraints involved in adaptation to Antarctic conditions, with a focus on the key role of temperature. In addition, there is a need for a unifying functional concept that is able to integrate information from the various molecular to systemic levels of biological organization and to provide the basis for an integrative view of the links between them. Such a concept should also be able to provide a framework for the development of functional hypotheses and for testing the validity of alternative interpretations of individual phenomena. Conversely, the validity of the concept can be tested against its ability to fully integrate individual findings. Such an integrative approach would provide answers to the question of how Antarctic organisms will respond to, and whether and which Antarctic organisms are able to adjust to, ongoing climate change. Global warming has in fact started to impact Antarctic oceans. This has been documented by a warming trend of 0.17°C seen at water depths between 700 and 1100 m from the Fifties to the Eighties of last century (Gille 2002). During the last 20–30 years, continued warming caused a rise in ocean average temperatures by about another 0.2°C between 50 and 90° S (Chapman & Walsh 2005). The surface layers of the Bellingshausen Sea close to the Western Antarctic Peninsula warmed by approximately 0.5°C between the mid-Sixties and mid-Nineties of the last century (Meredith & King 2005). On top of direct and indirect effects of temperature, such continued warming will also lead to the retreat of specific polar habitats as associated with the shrinking ice coverage of some of the Antarctic oceans (Loeb et al. 1997).
While the present work focuses on marine Antarctic fauna undergoing more extreme thermal specialization, it should not be forgotten that intertidal fauna in the Antarctic experience relatively wide temperature fluctuations (reaching well beyond +10°C) due to air and sun exposure during Antarctic summers. In shallow waters at 10–20 m depths, temperature variability rises with falling latitudes. It amounts to between less than 1 and 2°C in Ellis Fjord and McMurdo Sound, but can reach up to 4.5°C (South Georgia) towards the Antarctic polar front (Barnes et al. 2006).
2. Phenomena of thermal specialization and limitation
Features of thermal specialization and limitation are found at various levels of biological organization, from molecules and membranes to whole organisms and ecosystems. The study of these various features has a long history in Antarctic biological research, but studies at each of the various functional levels have frequently been carried out in isolation, so functional links have rarely been established. Studies of thermal adaptation during the last 30–40 years have largely focused on mechanisms providing tolerance. Only recently have mechanisms come into focus that shape the temperature-dependent performance of Antarctic species and the functional optimum of a species. At the same time, awareness increases that the molecular findings need to be interpreted in a whole organism context. The question arises how disturbance and modification of individual molecular processes contribute to whole-organism thermal stress and adaptation or vice versa. To achieve this multilevel analysis, we shall start with investigating molecular and membrane aspects of thermal specialization and limitation and, then, move sequentially to the cellular, whole-organism and ecosystem levels of biological organization. Finally, we explore how the various functional levels may be intertwined.
With respect to the thermal specialization and thermal limits of molecular function, three questions are especially pertinent focal points, whether the species under consideration are cold-adapted Antarctic stenotherms or more eurythermal species from temperate or tropical habitats. The first question asks at what temperature a particular molecular system, such as an enzyme or a membrane, is specialized through adaptation to work best. To answer this question, it is necessary to identify quantitatively the optimal values for a temperature-dependent trait. Here, optimal values are defined as those values for a trait that, as a result of evolutionary adaptation or acclimatization, are strongly conserved among differently thermally adapted species or differently acclimatized conspecifics at their normal body temperatures.
Due to trade-offs involved in the adjustment of molecular properties to a limited temperature range, a second question follows closely from this first question and asks how the temperature sensitivities of molecular-level structures and processes contribute to the thermal limitation of whole-organism performance and then to organismic thermal tolerance limits. How far can the values for a temperature-sensitive trait deviate from the optimal range before function is impacted, eventually to a lethal extent? In fact, as discussed in later sections of this article, one can reasonably inquire as to whether functional decrements and, then, death due to high or low extremes of temperature can be attributed to loss of performance or to failures at the molecular level, which may contribute or lead to collapse of higher-order physiological processes such as oxygen transport.
A third question concerns the actual rates at which physiological processes take place in organisms adapted or acclimated to different temperatures. This question asks to what extent molecular adaptations have achieved compensation to temperature in the rates of physiological activity and how this compensation supports the setting of performance capacity of the whole organism such that warm- and cold-adapted species manifest rates of function that are more similar than would be predicted on the basis of acute (Q10) effects on biochemical reactions. This question is of special relevance in the context of Antarctic species, for which a long-standing debate has existed about the extent—and even the very existence—of metabolic compensation to temperature (see Clarke 1993, 2003 and below).
Answers to these three general questions will not only assist us in defining the range of temperatures over which an organism functions best, but also, in the context of climate change, may provide insights into the consequences that global warming may have on species' physiological functions and biogeographic distributions. In the case of Antarctic ectotherms, defining the thermal limits of molecular function may provide unique insights into the magnitude of threat posed by climate change, especially when put into the context of whole organism phenomena.
(a) Molecular and membrane aspects
We address the issues of thermal optima, thermal limits and temperature compensation in three distinct types of biochemical systems: individual proteins, including enzymes, ion-binding proteins and oxygen transport proteins; membranes, which comprise approximately equal amounts of lipids and proteins; and nucleic acids, whose temperature sensitivity of structure and function is coming to be increasingly appreciated as a critical component of thermal relationships. The elaborate three-dimensional structures of proteins, nucleic acids and the lipid–protein assemblages of cellular membranes are dependent for their integrity on non-covalent (‘weak’) chemical bonds whose energies are of the order of the thermal energy of the cell. These critical molecular constituents of organisms and the processes they support therefore can be strongly perturbed by changes in temperature. It follows that molecular-level adaptation to temperature has played a key role throughout evolution and has enabled life to succeed in the remarkably diverse thermal conditions found throughout the biosphere. We show that, due to trade-offs involved in thermal specialization, common principles of thermal perturbation and temperature adaptation apply in all cases. Notably, the conservation of a critical balance between stability and flexibility of higher-order, weak-bond-dependent structures, a balance that is a requisite for effective function by proteins, lipids and nucleic acids, is a primary end result of adaptive processes.
(i) Proteins
Changes in temperature have two primary effects on most proteins. First, because the rates of biochemical processes are determined by the kinetic energy of the reactants, rates of protein-based activity, e.g. enzymatic catalysis, are altered by changes in temperature. Second, changes in temperature alter the three-dimensional structures of proteins, including the conformation of an individual protein and the assembly states of multiprotein complexes. At the extreme, this may lead to denaturation and loss of activity. However, within the normal temperature range of an organism, subtle changes in structure have as a primary consequence a perturbation of molecular recognition events, such as those involved in substrate binding by enzymes and oxygen by respiratory proteins.
The optimal thermal ranges for protein function can most readily be delineated by comparing temperature-sensitive characteristics of orthologous proteins (orthologues) isolated from species evolutionarily adapted to different temperatures. Temperature-sensitive kinetic properties like substrate binding typically show a strong conservation among orthologous enzymes, when compared at the respective habitat temperatures (Hochachka & Somero 2002). For A4-lactate dehydrogenase (A4-LDH) from vertebrates adapted to an approximately 45°C range of body temperatures, the binding of the substrate pyruvate, as measured by the apparent Michaelis–Menten constant, KmPYR, is highly conserved between approximately 0.12 and 0.32 mM pyruvate (Fields & Somero 1998). For A4-LDHs of Antarctic notothenioids, KmPYR rises beyond this optimal range when temperatures reach approximately 15°C. Cold temperate notothenioids from South America have A4-LDHs that are less cold adapted than those of their Antarctic relatives; KmPYR values indicate enzymes that are well poised to function at temperatures of 5°C to at least 20°C.
Baldwin (1971) showed that the neurotransmitter-degrading enzyme, acetylcholine esterase (AChE), has an even sharper response to temperature than A4-LDH. The Km of acetylcholine for the AChE of the Antarctic notothenioid, Pagothenia borchgrevinki, rises so rapidly with increasing temperature that by 5°C its value is well above those found for temperate and tropical fishes at their normal body temperatures. Such a rapid loss in neurotransmitter-binding ability, coupled with an accelerated release of acetylcholine at the synapses of P. borchgrevinki as temperature rises above 6°C (Macdonald et al. 1988), could contribute to setting the low thermal limits of this and other notothenioid species of McMurdo Sound, which appear unable to withstand acclimation to temperatures higher than approximately 5–6°C (Somero & DeVries 1967; Hofmann et al. 2000). Such an extreme collapse in the function of a critical neurotransmitter-degrading enzyme might contribute to the rapid loss of swimming ability and, within several minutes, thermal death, which is seen for these fish at temperatures above approximately 10°C (Somero & DeVries 1967).
Conservation of ligand-binding ability is also found for non-enzymatic proteins. For instance, the calcium-binding protein, parvalbumin (PV), isolated from two Antarctic notothenioids (Gobionotothen gibberifrons and Chaenocephalus aceratus) exhibited dissociation constants (KDs) for calcium at 0–5°C, which were similar to those of PVs from two temperate fishes (Cyprinus carpio; carp and Micropterus salmoides; bass) at 20–25°C (6–8 nm; Erickson et al. 2005). Owing to the important role that PVs play in binding calcium ions during excitation/contraction cycles in muscle, evolutionary adaptation of KD values for Ca2+ appears critical for supporting effective locomotory function in differently thermally adapted species. Oxygen transport, which may play a highly important role in setting thermal limits (see below), also reflects temperature-adaptive patterning of ligand binding. For the oxygen-binding protein, myoglobin (Mb), the oxygen dissociation rates of Mbs from Antarctic notothenioids at 0°C were similar to those of mammalian Mbs at their much higher body temperatures (Cashon et al. 1997). The oxygen binding of circulating haemoglobin (Hb) also manifests pronounced adaptation in oxygen affinity and regulatory functions, e.g. Bohr effects (reviewed by Di Prisco et al. 1991).
Two important and closely linked questions about protein evolution and protein structure–function relationships arise: how much change in amino acid sequence is needed to achieve adaptation to temperature and where in the sequence and in the folded three-dimensional structure of the protein amino acid substitutions are most effective in bringing about adaptation of function. In the case of A4-LDH, the amino acid substitutions that are responsible for conservation of KmPYR have been identified for several species (Holland et al. 1997; Johns & Somero 2004; Fields & Houseman 2005). Using the A4-LDH of the Antarctic notothenioid C. aceratus as a template for site-directed mutagenesis, Fields & Houseman (2005) showed that only a single amino acid substitution was sufficient to shift the KmPYR from the high value of the native enzyme to a lower value typical of a temperate zone fish. This finding is consistent with other studies of A4-LDH by Holland et al. (1997) and Johns & Somero (2004), who also found that a single amino acid substitution was adequate to interconvert KmPYR values of tropical and temperate orthologues of the enzyme.
In all of the cases where adaptive substitutions occurred, the sites and the nature of the amino acids indicate a common mechanism of adaptation. This mechanism involves adjustments in the conformational stability of the regions of the enzyme that undergo large displacements during substrate binding. These structural changes are the rate-limiting step in LDH function, and thus determine the speed with which the enzyme acts (see below; Dunn et al. 1991). Temperature-adaptive substitutions are proposed to achieve the retention of a consistent balance between stability and flexibility of these ‘moving parts’ of the enzyme (Fields 2001; Hochachka & Somero 2002). Thus, during adaptation to colder (warmer) temperatures, amino acid substitutions in those regions of a protein that affect the energetics of functionally important conformational changes tend to reduce (increase) the rigidity of the molecule (Fields 2001; Somero 2003).
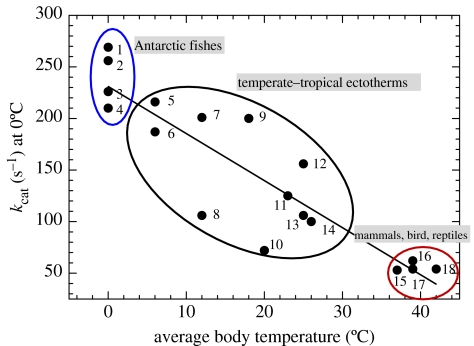
To adequately understand the significance of adaptive changes in the conformational mobility of the moving parts of an enzyme, it is necessary to consider a second functional property, the catalytic rate constant (kcat), measured in terms of the number of substrate molecules converted to product per unit time. Differences in the intrinsic stabilities of the moving parts of orthologues of proteins also affect kcat. For A4-LDH at a common temperature of measurement (0°C), the orthologues of cold-adapted species like Antarctic notothenioid fishes operate at four to five times higher kcat values than the orthologues from warm-adapted mammals, birds and reptiles (figure 1). Weaker binding in the less rigid enzymes thus supports a higher rate of catalysis due to lower energy costs of catalytic conformational changes (Fields & Somero 1998). Further evidence for this linkage among structural stability/flexibility, binding and rate of function is provided by thermodynamic analyses of activation parameters: the activation enthalpy changes during catalysis for many cold-adapted enzymes are lower than those for warm-adapted orthologues, and the activation entropy changes tend to be more negative as well (Low et al. 1973; Fields & Houseman 2005). The lower activation enthalpies for cold-adapted enzymes reflect reduced energy barriers for catalytic conformational changes, and the less favourable changes in activation entropy reflect the less-ordered structures of these molecules: more order must be generated for these flexible molecules to bring their conformations into the correct geometry for binding and catalysis.
Figure 1.
Temperature-adaptive variation in catalytic rate constant (kcat) values for A4-LDH. At a common temperature of measurement (0°C), orthologues of cold-adapted species like Antarctic notothenioid fishes operate at four to five times higher kcat values than orthologues from warm-adapted mammals, birds and reptiles. Species studied were: (1) Parachaenichthys charcoti (Antarctic fish), (2) Lepidonotothen nudifrons (Antarctic fish), (3) Champsocephalus gunnari (Antarctic fish), (4) Harpagifer antarcticus (Antarctic fish), (5) Patagonotothen tessellate (South American notothenioid fish), (6) Eleginops maclovinus (South American notothenioid fish), (7) Sebastes mystinus (rockfish), (8) Hippoglossus stenolepis (halibut), (9) Sphyraena argentea (temperate barracuda), (10) Squalus acanthias (dogfish), (11) Sphyraena lucasana (subtropical barracuda), (12) Gillichthys mirabilis (temperate goby), (13) Thunnus thynnus (tuna), (14) Sphyraena ensis (tropical barracuda), (15) Bos taurus (cow), (16) Gallus gallus (chicken), (17) Meleagris gallopavo (turkey) and (18) Dipsosaurus dorsalis (desert iguana). (figure modified after Fields & Somero, 1998).
However, there are exceptions to the general rule of reduced activation enthalpies (mirrored by Arrhenius activation energies, Ea) of enzymatic activity in the cold. Some enzymes such as glyceraldehyde phosphate dehydrogenase (GAPDH), phosphofructokinase (PFK) and isocitrate dehydrogenase (IDH) displayed higher Ea values after cold adaptation (cf. Pörtner et al. 2000). Other enzymes, including some that contribute to the control of metabolic flux like cytochrome c oxidase (seen in sub-polar populations of Arenicola marina) or some that include energy-dependent functions which support overall tissue function in the cold like Na+/K+-ATPase (seen in the Antarctic P. brachycephalum), demonstrated the expected reduction in Ea values compared with those found in their temperate conspecifics or confamilials (Sommer & Pörtner 2002; Lucassen et al. 2006). The latter category also includes a drop in Ea of myofibrillar ATPase (Johnston et al. 1975). To explain these contrasting patterns, the hypothesis was developed that the level of Ea reflects a trade-off between the enzyme concentration required to minimize diffusional limitations and the required facilitation or restriction of flux through specific reactions or pathways (Pörtner et al. 2000). A drop in Ea should be found mainly when flux is to be cold compensated. This may predominantly be the case in equilibrium enzymes with a lower contribution to metabolic control, as in LDH. Conversely, a rise in Ea might be found when flux is to be reduced at maintained or elevated levels of the respective enzymes.
The variation in kcat found among orthologous proteins from species adapted to different temperatures speaks to the controversial issue of how fully the metabolic processes of cold-adapted species are compensated for temperature (Clarke 1993, 2003). Assuming equal concentrations of a particular type of enzyme in a given cell type among all species and a Q10 value for the reaction of approximately 2, one would predict that kcat values of the enzyme would need to double with each 10°C decrease in evolutionary adaptation temperature, if temperature compensation in enzymatic activity were to be complete, i.e. if Q10 effects were to be fully offset by differences in kcat among orthologues. This is clearly not the case: for A4-LDH, the enzyme for which the most data on kcat are available, orthologues of species adapted to temperatures differing by approximately 40°C differ in kcat by only four- to fivefold, not the predicted 16-fold difference needed to fully offset Q10 effects. Thus, in terms of activity per active site (equal to kcat), temperature compensation is far from complete.
Because the amount of enzymatic activity in a cell is the product of kcat X [enzyme], temperature compensation might be achieved by adjusting enzyme concentrations. If one knows the kcat for an enzyme, measurement of the total enzymatic activity (units of activity per unit wet mass) in a tissue allows estimates to be made of the enzyme's concentration. In only a few cases has this type of analysis been performed in the context of temperature compensation. The one interspecific comparison we are aware of is the work of Kawall et al. (2002), who measured activities of LDH in brains of Antarctic notothenioids and several tropical fishes. At a measurement temperature of 10°C, brain LDH activity was 2.5-fold greater in Antarctic notothenioids than in tropical fishes. However, at their respective habitat temperatures near 0 and 25°C, respectively, LDH activity in brain of the Antarctic notothenioids was only 48% that of activity in the tropical species. The differences in LDH activity observed at a common measurement temperature of 10°C appear to be fully explained by the observed differences in kcat (figure 1). It follows that for brain LDH activity in fishes that have evolved at different temperatures, there is no indication of temperature-compensatory adjustments in [LDH].
In contrast to this study of differently adapted species, studies of differently adapted populations of a single species, the eurythermal killifish Fundulus heteroclitus, have revealed temperature-compensatory adjustments in enzyme concentration and specific activity (Place & Powers 1979; Crawford & Powers 1989). Populations of F. heteroclitus found in the northern extent of this species' biogeographic range along the East Coast of North America possess a LDH-B allozyme with significantly higher specific activity and a higher level of expression than the allozyme found in southern populations. The differences in LDH-B expression between populations has been linked to a stress-responsive gene regulatory element (Schulte et al. 2000). It remains to be determined whether this type of dual mechanism for adjusting enzymatic activities in temperature-compensatory fashion, using more of a ‘better’ enzyme, as it were, is important in interspecific compensation to temperature.
The occurrence of temperature-compensatory adjustments in enzyme concentration thus merits broader investigation. There is some evidence that the capacity for aerobic respiration in the swimming muscle of Antarctic fishes is enhanced by increased densities of mitochondria (Crockett & Sidell 1990, cf. Cheng & Detrich 2007), while the capacity for anaerobic metabolism is reduced. In terms of ATP-generating capacity normalized to mitochondrial mass, however, no evidence for temperature compensation was seen (Johnston et al. 1994). Thus, for mitochondrial respiration, ‘more’ rather than better (equal to higher specific activity) appears to be the strategy of choice for achieving some degree of compensation to cold temperatures. In contrast to the respective findings in Antarctic stenotherms, increased capacities (more activity per mitochondrial protein) of mitochondrial respiration were, however, seen in cold-adapted populations of invertebrate and vertebrate eurytherms in a Northern Hemisphere (East Atlantic) temperature cline (for review, see Pörtner et al. 2000, 2005a). Ambiguities also remain about the role that locomotory mode might play in selecting for enzymatic activities in muscle (see Kawall et al. 2002), so rigorous phylogenetically controlled studies will be required to resolve the more versus better issue. Furthermore, the width of the thermal tolerance range and the aerobic scope for locomotion are probably interdependent (Pörtner 2002b, 2004), making this a complex issue to address.
(ii) Membranes
The effects of temperature on membranes and the adaptive modifications made by cells to offset this perturbation have been well characterized in many species (for review, see Hazel 1995; Hochachka & Somero 2002). According to Hazel's (1995) dynamic phase behaviour model, adjustments in the lipid compositions of membranes in response to a change in temperature involve two principal types of changes. First, a consistent state of static order (sometimes referred to as ‘fluidity’ or ‘viscosity’) in the liquid crystalline bilayer is maintained. This is termed ‘homeoviscous adaptation’, and is achieved through adjustments in head group (membrane class), fatty acyl chain composition (membrane species) and, in some cases, cholesterol concentration. Most commonly, the saturation level (double bond content) of acyl chains is modified to retain a consistent fluidity or viscosity of the bilayer (Hazel 1995; Logue et al. 2000). Second, the capacity for forming non-lamellar phases, which are needed for the processes of exocytosis and endocytosis, is conserved. Low temperatures disfavour non-lamellar phases, whereas high temperatures tend to favour formation of non-lamellar phases such as the hexagonal II phase. Changes in phospholipid head groups are the primary mechanism for modulating the tendency for non-lamellar phases to occur; the ratio of phosphatidyl ethanolamine head groups to phosphatidyl choline head groups is increased during adaptation to cold (Hazel 1995). Therefore, as in the case of adaptation in protein structure, modifications of lipids involve a strategy of conserving the appropriate balance between opposing tendencies, between rigidity and flexibility in the liquid crystalline bilayer and between formation of lamellar and non-lamellar phases.
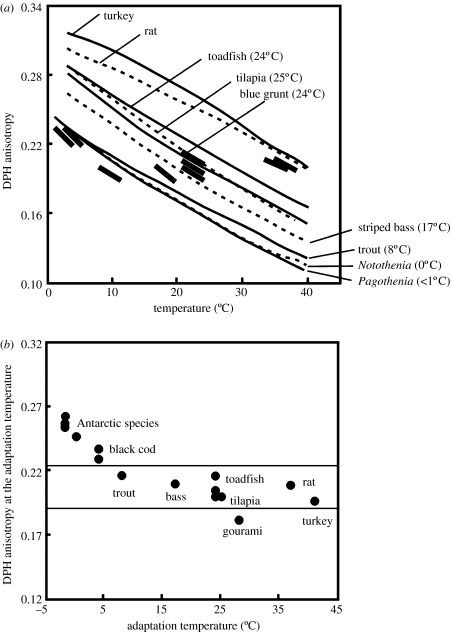
Homeoviscous adaptation in Antarctic notothenioids has been examined in detail by Logue et al. (2000), who studied the properties of brain synaptosomal membranes in these species as well as in more warm-adapted animals (figure 2). They found a high degree of compensation to temperature in membrane static order, as indexed by the fluorescence anisotropy of the probe molecule 1,6-diphenyl-1,3,5-hexatriene (DPH; figure 2a). However, compensation was not complete, as shown more directly in figure 2b: at their low adaptation temperature, Antarctic notothenioids had brain synaptosomal membranes that were less fluid than predicted on the basis of full compensation. The same observation was made for a cold temperate (New Zealand) notothenioid, the black cod (Logue et al. 2000). The significance of this apparent ‘failure’ to fully compensate membrane static order to sub-zero temperatures is not clear. Logue et al. (2000) conjecture that there may be limits to the extent to which membrane physical properties can be compensated for the effects of very low temperatures. If this is the case, then important consequences may result for membrane-based processes such as transport. Because activities of membrane-localized enzymes may depend strongly on the physical state of the lipid milieu in which they function—the so-called ‘viscotropic’ effects—the inability of Antarctic fishes to achieve complete homeoviscous adaptation could have an impact on the extent to which activities of membrane-localized enzymes can compensate for temperature. Such relationships have been elaborated for temperate fishes, for example, the dependence of cytochrome c oxidase activity on the degree of homeoviscous adaptation (Wodtke 1981). This may be a reason why cytochrome c oxidase experiences less cold compensation than the matrix enzyme, citrate synthase (Hardewig et al. 1999a; Lucassen et al. 2006).
Figure 2.
Homeoviscous adaptation of membrane structure. A high degree of compensation to temperature in membrane static order (‘fluidity’), as indexed by the fluorescence anisotropy of the probe molecule 1,6-diphenyl-1,3,5-hexatriene (DPH), is found in the comparisons of synaptosomal membranes from brain tissues of differently thermally adapted vertebrates. (a) Acute effects of measurement of temperature on DPH fluorescence anisotropy. Thick line segments show approximate body temperatures of the species. (b) DPH fluorescence anisotropy at each species' adaptation temperature. (figure modified after Logue et al. 2000).
Another distinctive feature of the thermal responses of membranes of Antarctic notothenioids is the absence of acclimatory adjustment in lipid composition (Gonzalez-Cabrera et al. 1995). Two McMurdo Sound notothenioids, Trematomus bernacchii and Trematomus newnesi, were acclimated to 4°C for several weeks. Although changes were noted in serum osmolality and activity of the Na+/K+-ATPase in gill and kidney, no changes were found in the fatty acid saturation of cellular membranes. As discussed below under the heading ‘Genomic aspects’, this lack of an acclimatory response could be a reflection of the loss of one or more of the enzyme systems required for lipid restructuring in the face of changes in body temperature.
One of the consequences of possessing highly fluid membranes is that tolerance of high temperatures may be extremely limited. Above, we indicated that in P. borchgrevinki, synaptic vesicles containing acetylcholine tended to release massive amounts of this transmitter when temperature rose above 6°C (Macdonald et al. 1988). This breakdown of membrane integrity is possibly due to an unusually strong propensity for the formation of non-lamellar phases in this species' synaptosomes, which would facilitate massive exocytosis and transmitter release at high temperatures.
Collapse of mitochondrial function has also been observed at lower temperatures in an Antarctic notothenioid, T. bernacchii, than in temperate species (Weinstein & Somero 1998). The temperature at which a sharp discontinuity occurs in the slope of an Arrhenius plot of oxygen consumption rate versus temperature, the Arrhenius Break Temperature (ABT), provides an index of thermal tolerance of mitochondrial function, reflected in phase transition of the inner mitochondrial membrane. For T. bernacchii, the ABT was approximately 17°C. In contrast to mitochondria of notothenioid fish (T. bernacchii, Weinstein & Somero 1998; Lepidonotothen nudifrons, Hardewig et al. 1999b), mitochondria from the Antarctic clam L. elliptica showed an ABT as low as 9°C (Pörtner et al. 1999b). This is the lowest ABT ever recorded for mitochondrial function, a finding that suggests that Antarctic fish are more tolerant towards high temperatures than Antarctic invertebrates (see above). The collapse of mitochondrial function could be a reflection of the high intrinsic fluidity and the strong propensity for formation of non-lamellar structures of the cold-adapted membranes of Antarctic organisms.
In summary, the membrane-based systems of Antarctic organisms may serve as critical sites of thermal perturbation and be at least indirectly involved in both acute thermal death and longer-term physiological failure (see below). The rapid loss of binding ability noted for AChE could contribute to rapid heat death at temperatures near 10°C in some fish species. The apparent lack of an ability to adjust membrane lipid composition in a temperature-compensatory manner could explain the very limited, albeit demonstrable (Seebacher et al. 2005), abilities of McMurdo Sound notothenioids to acclimate to elevated temperatures.
(iii) Nucleic acids (RNA and DNA)
The nucleic acids of Antarctic organisms must conduct their diverse functions under thermal conditions that favour rigidity of higher-order structure, due to the increased stabilities of ionic interactions and hydrogen bonds at low temperatures. The double helical structure of DNA is stabilized in the cold, and the formation of secondary structures in single-stranded RNA molecules, likewise, is favoured at low temperatures. The latter effects represent a threat to such key RNA-dependent functions as protein synthesis. Cold-shock proteins, RNA chaperones that prevent formation of inappropriate secondary structures in newly transcribed RNAs at cold temperatures, are an important means for reducing this type of cold stress (for review, see Hochachka & Somero 2002).
The possible ways in which DNA structure is adapted to compensate for the effects of temperature have enjoyed a lively debate for several decades. The fact that guanine (G): cytosine (C) base pairs are stabilized by three hydrogen bonds whereas adenine (A): thymine (T) base pairs form with only two bridging hydrogen bonds has led to the hypothesis that the G+C percentages of genomes should increase with rising evolutionary adaptation temperature (for review, see Bernardi 2004). This hypothesis has been subjected to rigorous testing in Bacteria and Archaea where substantial numbers of genome sequences are available (Hurst & Merchant 2001). Except for genes encoding structural ribosomal RNAs (rRNAs), no evidence for genome-wide adjustment in G+C content has been found. The discovery that rRNAs of warm-adapted prokaryotes do contain higher G+C contents does indicate that this mode of modifying RNA thermal stability is employed in some instances. However, the absence of a relationship between adaptation temperature and G+C content of total genomic DNA in prokaryotes and the finding that the protein-coding regions of genes for A4-LDH and alpha-actin from vertebrates differing in adaptation by approximately 45°C (Antarctic notothenioid fishes to desert reptiles) showed no relationship between G+C percentage and adaptation temperature (Ream et al. 2003) argue against the general (genome-wide) validity of the G+C percentage model of thermal adaptation of nucleic acids.
This negative conclusion should not be taken as a sign that the effects of temperature on the stability of DNA structure are not problematic, however. There is at least one line of evidence showing that cells closely modulate the stability of DNA, such that the capacity for regulating gene transcription is highly conserved at different temperatures. For a gene to be transcribed, the DNA must be adequately ‘relaxed’ or ‘open’ for transcription factors to interact with gene regulatory regions and carry out their highly specific regulatory roles. In this regard, the effects of proteins that influence the openness of DNA structure may be critical in compensating for temperature effects. One such protein is high-mobility group B1 protein (HMGB1), which is known to play a role in regulating transcription by increasing the openness of DNA structure (Thomas & Travers 2001). HMGB1 does not target specific genes, but rather leads to a global stimulation of transcription (Aizawa et al. 1994). In a study of temperature-dependent gene expression in a eurythermal teleost fish, Austrofundulus limnaeus, Podrabsky & Somero (2004) discovered significant temperature-dependent variation in the expression of the hmgb1 gene. In this study, fish were acclimated to constant temperatures of 37 and 20°C or were cycled on a daily rhythm between these two temperature extremes. In the fish held at constant 20°C, transcript levels for HMGB1 were significantly higher than in the 37°C-acclimated fish. In specimens in the cycling thermal regime, the level of transcript for HMGB1 cycled regularly with diurnal variation in temperature: transcript rose rapidly as temperature was reduced (simulating cooler night time conditions) and fell equally rapidly when temperature was increased (simulating daytime heating). If we assume that the level of HMGB1 protein tracked the variation seen for its mRNA, then modulation in the levels of a DNA structure-modifying protein could lead to a high degree of temperature compensation in the openness, i.e. the transcriptional poise, of DNA throughout the genome. A recent study of thermal acclimation in carp (Gracey et al. 2005) also found temperature-compensatory shifts in the transcript for HMGB1 protein. These studies of thermal acclimation suggest that evolutionary adaptation, too, could involve elevated expression of HMGB1 proteins in cold-adapted species like Antarctic animals. The non-specific ‘global’ effects of HMGB1 on DNA structure could achieve genome-wide temperature compensation in the transcriptional poise of DNA without a need to alter the intrinsic stability of DNA, e.g. through adjusting G+C percentages.
To summarize briefly the primary conclusions from this analysis of molecular-level adaptations that influence thermal optima and thermal limits, it is seen that proteins, lipids and nucleic acids are subject to the same basic types of weak-bond-based perturbations by temperature. Compensating for these effects entails the acquisition of the appropriate balance between stability and instability of structure. Only when this balance is achieved can physiological performance occur optimally. When this balance is upset, physiological systems face constraints that, if severe enough, can prove to be lethal. Lethal effects stemming from molecular-level perturbations may arise from the failures of proteins to bind ligands; the breakdown of membrane structures, which abolishes permeability barriers or leads to excessive levels of exocytosis (e.g. of transmitters); and, as hypothesized immediately above, the loss of control of gene transcription when DNA structure becomes either too rigid or too open. The importance of these effects seen in vitro in the real-world thermal relationships of organisms remains to be more fully established, of course. Thus, one can ask whether thermal perturbations at the molecular level commonly contribute to whole-organism thermal stress and mortality in the lab and the field, or whether these severe molecular perturbations occur only at temperatures well beyond those at which the onset of whole-organism limits occurs, as hypothesized elsewhere (cf. Pörtner 2002a, cf. figure 3). It also needs to be shown which of these molecular-level effects contribute to acute mortality; others may take a considerable period of time to exert their effects and may work through or in conjunction with system-level effects like oxygen transport, to establish thermal tolerance limits.
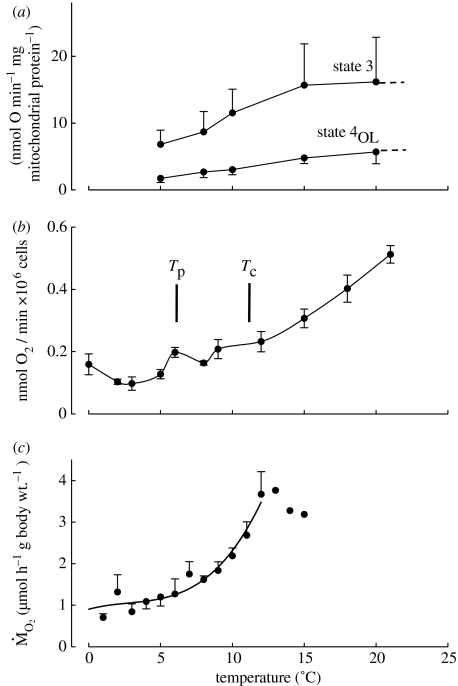
Figure 3.
Comparison of metabolic rate data obtained in (a) isolated mitochondria (Lannig et al. 2005), (b) isolated hepatocytes (Mark et al. 2005) and (c) intact specimens (Mark et al. 2002) of the Antarctic eelpout (P. brachycephalum). High thermal limits are apparent in whole-organism oxygen consumption where it levels off close to critical temperatures, characterized by the onset of anaerobic metabolism (cf. figure 5). Pejus and critical temperatures (Tp and Tc) seen in vivo (according to Van Dijk et al. 1999; Mark et al. 2002) occur within a temperature range, where functional integrity of mitochondria and cells is still undisturbed. The pattern of hepatocyte respiration rates as studied in the Antarctic eelpout, P. brachycephalum, acclimated to 0°C reveals an energetic minimum that matches the putative thermal window of the species.
(b) Genomic aspects: gene expression and loss of genetic information
One of the most exciting frontiers in Antarctic biology concerns the information content of the genomes of the cold-adapted stenothermal organisms that populate the Southern Ocean. The long evolutionary histories of these species in a thermally stable ‘ice bath’ have involved many critically important changes in the genome that facilitate life in the cold. These adaptations range from the ‘invention’ of new types of proteins, such as macromolecular antifreezes, to the improvement of pre-existing proteins, e.g. the evolution of enzymes with high kcat values, which partially offset the decelerating effects of cold on enzymatic activity. Changes in gene expression to alter the quantities of proteins could also contribute to adaptation to cold, e.g. through upregulation of enzymes for aerobic metabolism (e.g. Hardewig et al. 1999a; Lucassen et al. 2003). As in the case of the other molecular and biochemical adaptations discussed in this review, integrating information on the genomes of Antarctic organisms with whole-organism function remains a challenge.
Genetic changes in Antarctic organisms involve more than the acquisition of adaptations that are essential for life at cold temperatures; other genetic changes may underlie the losses of certain traits. These losses could potentially reflect the disappearance of a trait that was no longer required under the thermal conditions of the Southern Ocean. Mutations in the coding or regulatory regions of the genes encoding these no-longer-required traits may have had no negative selective effects on the organism. The scope of this sort of loss remains to be revealed, although the examples discussed below show that a number of biochemical characteristics may be involved in a ‘loss-without-penalty’ scenario.
A striking example of the loss of a molecular system that is usually essential in vertebrates is the absence of Hb in all 15 species of the notothenioid family Channichthyidae (icefish). Molecular analysis suggests that a single mutational event led to loss from the channichthyid genome of the gene encoding the Hb beta chain plus a portion of the 5′ end of the contiguous Hb alpha chain (Cocca et al. 1997). The loss of the ability to synthesize Hb in channichthyids is associated with a suite of compensatory changes that appear to function to restore oxygen transport capacity in the cold: an enlarged heart; a large blood volume (approx. two to four times that of similar-sized teleosts possessing Hb); and low systemic resistance of the circulatory system (Montgomery & Clements 2000). The latter trait is due, in part, to the absence of erythrocytes in channichthyids. The muscle ultrastructure of channichthyids also reflects characteristics that would seem to foster improved diffusion and usage of oxygen. O'Brien & Sidell (2000) showed that the percentage of cell volume occupied by mitochondria in the heart ventricle was greater in a channichthyid (C. aceratus) that lacked heart Mb than in an Mb-containing channichthyid (Chionodraco rastrospinosus) or in an Hb- and Mb-containing nototheniid (G. gibberifrons). They proposed that a high mitochondrial volume enhances diffusion from the lumen of the ventricle to mitochondria. Since membranes are approximately one-half lipid, oxygen diffusion would be favoured due to the higher solubility of oxygen in lipid than in aqueous solutions. The high lipid concentrations found in the muscle tissue of many notothenioids can also be viewed as a mechanism for facilitating oxygen movement (reviewed by Sidell 1998). At the same time, the high lipid content of muscle of many notothenioids, especially pelagic species, relates to buoyancy regulation in these secondarily pelagic species (Eastman 1993; Montgomery & Clements 2000). Enhanced lipid anabolism as probably triggered by cold temperature (Pörtner 2002c) secondarily supports various organismic functions in the cold. In that sense, high lipid levels become an exaptation, which is the adoption of a character that had one use in an ancestral form into a new different use in a descendant form (Gould & Vrba 1982).
Myoglobin, too, has been lost in many notothenioids, at least in certain tissues. No notothenioid has Mb in its skeletal locomotory muscle, a loss of tissue-specific gene expression that must have occurred early in the notothenioid radiation some 7–15 Myr ago (Sidell et al. 1997). In channichthyids, the loss of Mb has occurred at least three times and has involved at least two different molecular mechanisms (Sidell et al. 1997). Unlike the case for Hb, where a gene has disappeared from the genome, the gene encoding Mb is present in all icefish so far examined. However, in some cases, the reading frame is disrupted, while in other cases the gene is transcribed but the message is not translated into protein (Grove et al. 2004). Loss of Mb in heart muscle of channichthyids may not be an instance of loss-without-penalty. The basis of this conclusion is that, in those channichthyids that have retained a functional Mb gene and express the protein in heart, poisoning of Mb binding of oxygen leads to reduction in cardiac performance (Acierno et al. 1997). In those forms lacking the Mb, compensation then occurred through the development of a high mitochondrial volume as outlined above (cf. Cheng & Detrich (2007) for a recent treatment of regulatory aspects).
From an integrative point of view, the loss of Mb and Hb should be viewed not only in the context of enhanced oxygen solubility in body fluids and dense mitochondrial membrane networks of lipid-rich tissues (Sidell 1998), but the respective interpretation must also take into account observations of large cell sizes, of a low mitochondrial capacity, as well as of the concomitant reduction of metabolic energy and thus oxygen demands (cf. Pörtner et al. 2005a). All of these characters are, in fact, indicative of excessive O2 supply at low cellular costs and oxygen demand in the cold. Rather than indicating oxygen limitation, these patterns imply structural overcompensation of oxygen supply reductions at the cellular and tissue level, thereby reducing the selective pressure on retaining Hb or Mb functions. This, in turn, gave room for the development of an alternative physiological design, Hb-free blood and Mb-free muscle cells (cf. Cheng & Detrich 2007), that could perform similarly well in the cold. With respect to the loss of Mb from muscle tissue, these conclusions are valid for the notothenioids in general (see below). Functional trade-offs that indicate benefits from this loss include reduced costs for protein synthesis and simplified regulatory networks. The loss of Hb in icefish may have supported reduced overall costs of circulation, together with a shift to enhanced diffusive oxygen supply. The benefits thus involve a reduction in the cost of oxygen transport and a reduction of oxygen radical formation which is usually associated with the use of ferrous proteins. All of these trade-offs, especially those linked to cost reductions (see below), might have been crucial to support the success of the mutation such that it became a species character. However, the shift to more diffusive oxygen pathways in the permanent cold may also have contributed to enhance the sensitivity of the icefish and the notothenioids to warmer temperatures (see below).
Another loss of genetic capacity in notothenioids is the disappearance of the heat-shock response (HSR; Hofmann et al. 2000). In McMurdo Sound notothenioids, no type of heat shock protein (Hsp) could be induced by thermal stress. Constitutively expressed chaperones are present in these fish, however, as would be expected on the basis of the essential role of these molecular chaperones in protein biosynthesis and proteolysis. It is also noteworthy that two cold temperate New Zealand notothenioids do display the HSR. The loss of the HSR in notothenioids of the Southern Ocean is not a reflection of the loss of functional genes for Hsps. Place and collaborators (Place & Hofmann 2004; Place et al. 2004) demonstrated that a commonly occurring heat-induced molecular chaperone, Hsp70, is produced at relatively high rates in Antarctic notothenioid fishes. However, neither the rate of hsp70 transcription nor the concentration of Hsp70 itself is enhanced by increases in temperature. Thus, the genetic lesion that has occurred in these fish involves some type of regulatory mechanism, not the structural gene encoding Hsp70.
This discovery immediately raises the question as to why a cold-adapted stenothermal Antarctic fish should benefit from a high level of production of a molecular chaperone that is normally induced only under conditions of stress. One potential answer to this paradoxical question involves the phenomenon of cold denaturation. Protein denaturation is caused by low extremes of temperature, as well as by high temperatures (Place et al. 2004 and references therein). The discovery that substantial amounts of ubiquitin-tagged proteins slated for proteolysis were found in tissues of Antarctic fish is consistent with, albeit does not prove, the possibility that protein synthesis at sub-zero temperatures represents severe challenges to the protein folding and assembly apparatus of the cell. High levels of chaperones to offset inappropriate folding or, should this fail, to assist in proteolysis might be one of the ‘costs of living’ of Antarctic species. The high levels of protein turnover estimated for Antarctic animals could be due, in part, to the high costs of protein folding and assembly in the cold (Fraser et al. 2002). In any event, the loss of the HSR in Antarctic notothenioids and in an Antarctic ciliate (La Terza et al. 2001) may be a reflection of an adaptive change, i.e. the establishment of a high constitutive level of synthesis of a much-needed protein. The conjecture that a high level of constitutive expression of a normally heat-induced chaperone like Hsp70 is beneficial at low temperatures is supported by the discovery that an Antarctic zoarcid (Lycodichthys dearborni), which has retained the HSR, nonetheless constitutively expresses Hsp70 at high levels (Place & Hofmann 2004).
(c) From molecular to systemic aspects: thermal limitation
The principles of thermal optimization and limitation can also be applied to higher levels of biological organization. In various phyla of marine invertebrates and in fish, transition to internal (systemic) hypoxia (hypoxaemia) and a limitation of functional capacity of oxygen supply systems like ventilation or circulation were found to characterize the borders of the organismic thermal tolerance window. At more extreme temperatures, both cold and warm, anaerobic metabolism sets in, especially involving mitochondrial pathways. These observations led to the development of the concept that animals are characterized by an oxygen- and capacity-limited thermal tolerance (for review, see Pörtner et al. 2000, 2004; Pörtner 2001, 2002a,b). This concept suggests that animals, when near the low and high edges of the thermal envelope, first lose whole organism aerobic scope at the so-called pejus thresholds. This loss is the first level of thermal intolerance and occurs in fully oxygenated waters through the onset of a mismatch between oxygen supply and demand. With continued cooling or warming, aerobic scope finally vanishes at low or high critical threshold temperatures (Tc), as cellular energy levels become progressively insufficient. These transitions may occur prior to the onset of severe molecular damage, such as protein denaturation or membrane disruption.
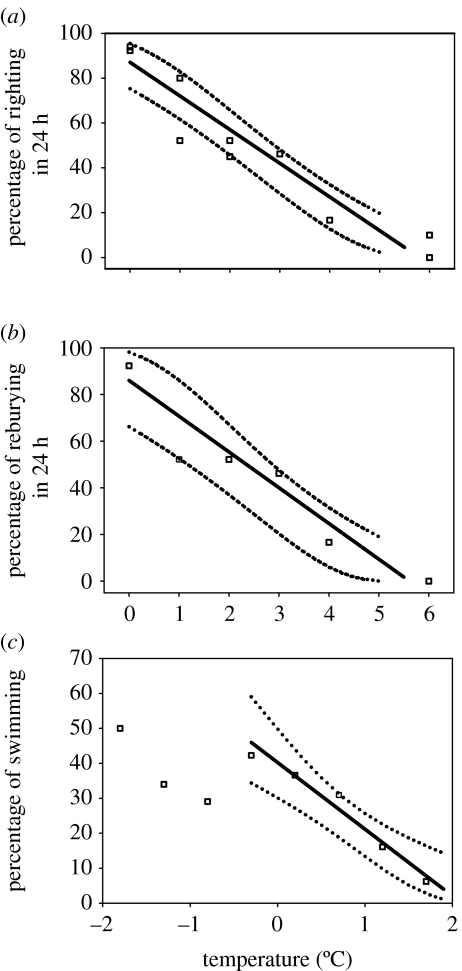
Figure 3 shows an example for the Antarctic eelpout (P. brachycephalum). This species is one of the deep-sea immigrants to Antarctica (Brodte et al. 2006). It has a circum-Antarctic distribution and lives in waters close to 0°C, but is most frequently found in ‘warmer’ water bodies of the Antarctic Ocean, from the peninsula to the bottom waters of the high Antarctic Weddell Sea. In the eelpout, the level of aerobic energy expenditure increases beyond a pejus temperature of 7°C, while blood flow becomes thermally independent and indicates the onset of a mismatch between oxygen supply and demand (Mark et al. 2002). The critical temperature is reached at approximately 12°C, where the rate of oxygen consumption levels off and mitochondrial succinate accumulation sets in (Van Dijk et al. 1999). Among Antarctic marine invertebrates, upper critical temperatures characterized by succinate accumulation have been identified in the bivalves, L. marionensis, at approximately 2°C and in L. elliptica at approximately 6°C (Pörtner et al. 1999a, Peck et al. 2002). Before critical temperatures are reached, systemic hypoxia (hypoxaemia) develops within the pejus range and reduces whole-organism performance. That the poor temperature tolerances of Antarctic ectotherms are brought about by the limited capacity of oxygen supply pathways is reflected in rapid reductions in aerobic scope. This results in a dramatic loss of functional or behavioural capability with a very small rise in temperature. Thus, the fish, Pagothenia borchgrevincki, can survive for at least limited periods at temperatures up to 11°C, but its fastest sustained swimming speed (2.7 body lengths per second) is maintained between -1.0 and +2.0°C (Wilson et al. 2002). Above this, there is a rapid and monotonic decline in performance, such that the maximum sustainable speed is halved at 7°C. However, P. borchgrevincki may be one of the less stenothermal Antarctic species so far studied (Seebacher et al. 2005). More recently, Peck et al. (2004a) showed a complete loss of ability to burrow in L. elliptica or to right in the limpet N. concinna at 5°C, and a 50% loss of capability at temperatures between 2 and 3°C (figure 4). The scallop, A. colbecki, was even more thermally constrained, being totally incapable of swimming at 2°C. Based on an early loss of performance and a progressive reduction in haemolymph oxygenation, pejus thresholds close to 0°C result for L. elliptica (Peck et al. 2004a; Pörtner et al. 2006). As a corollary, Antarctic stenotherms, especially among the invertebrates, live close to their thermal optimum as reflected in figure 5.
Figure 4.
(a) Righting responses in the Antarctic limpet, N. concinna, with temperature. Data shown are the proportion of limpets righting in 24 h. For each point, n=20–31. All regressions were made following square root and arcsin transforms of percentage data (arcsin(√%righting) =1.20 −0.180T°C; r2=0.90, F=77.9, p<0.001, d.f.=9). (b) Reburying in the bivalve mollusc, L. elliptica, with temperature. Data show the proportion of animals reburying in 24 h (n=18–26 for each point). Regression line: arcsin(√%burying) =0.95−0.173T°C (r2=0.85, F=22.4, p=0.009, d.f.=5). (c) The proportion of Antarctic scallops, A. colbecki, swimming in response to freshwater stimulation. Each point is the proportion swimming at that temperature (n=57–175 for each point). A regression was fitted to data for temperatures above −0.3°C, where a clear temperature effect was apparent. This regression was fitted to square root- and arcsin-transformed percentage values. Regression line: arcsin(√%swimming) =0.682–0.230T°C (r2=0.93, F=51.5, p=0.006, d.f.=4). In all figures, dotted lines indicate 95% CIs for regressions. For all plots, lines and CIs shown were plotted following sine and square back transforms. (figure modified from Peck et al. 2004b.)
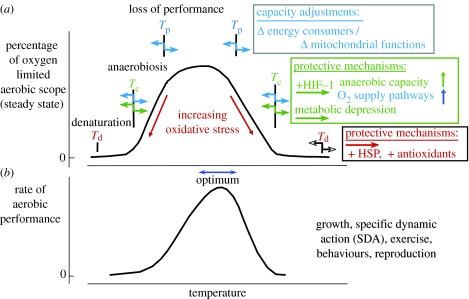
Figure 5.
Conceptual model of thermal limitation and functional optima (modified after Pörtner 2002a; Pörtner et al. 2005a). (a) Progressively enhanced thermal limitation occurs through the consecutive onset of a loss in aerobic scope beyond Tp, the onset of anaerobic metabolism at Tc and of molecular denaturation at Td. Antarctic stenotherms, especially among invertebrates, live close to their thermal optimum. The parallel shift of low and high thermal tolerance thresholds (Tp and Tc) during temperature adaptation occurs by adjustments of capacity at various functional levels. Td probably shifts with molecular modifications as well as the adjustment of molecular protection mechanisms like heat shock proteins or antioxidants (see text). Maximum scope in ATP generation at the upper Tp supports maximum capacity of organismic oxygen supply by circulatory and ventilatory muscles. It may also support (b) an asymmetric performance curve of the whole organism with optimal performance (e.g. growth, exercise) close to upper pejus temperature Tp.
Available data thus indicate that performance capacity falls within the pejus range. At the same time, the level of molecular damage may also rise progressively as indicated by enhanced oxidative stress, seen in the Antarctic limpet, N. concinna (Abele et al. 1998), the North Sea eelpout, Zoarces viviparus (Heise et al. 2006a,b), and the Antarctic eelpout, P. brachycephalum (Heise 2005). These data support the hypothesis that oxidative stress is elicited through temperature-induced hypoxaemia at the edges of the thermal window and indicate a role for hypoxia-inducible factor 1 (HIF-1) in thermal adaptation (figure 5; Pörtner 2002a). Sensitivity to oxidative stress may be enhanced in those Antarctic ectotherms which display enhanced unsaturation levels in membrane lipids, as seen in a comparison of temperate and Antarctic eelpout (Heise 2005). Resistance to oxidative stress is improved by very high glutathione levels found in an Antarctic bivalve, L. elliptica (Philipp et al. 2005a), and Antarctic eelpout, P. brachycephalum (Heise 2005). Anaerobic metabolism, combined with molecular protection mechanisms, would thus enable the animals to survive temporary periods of exposure to temperature extremes.
Figure 3 illustrates for the Antarctic eelpout, P. brachycephalum, that the limits of thermal tolerance are set narrower at the organismic level than at lower levels of biological organization, namely at cellular and mitochondrial levels. Similarly, the mitochondrial ABTs given above for L. elliptica (Pörtner et al. 1999b) and also those for Antarctic fishes (Weinstein & Somero 1998; Hardewig et al. 1999b) are found beyond whole organism thermal limits. These findings suggest that oxygen limits occur earlier in the whole organism than damage to cellular and subcellular structures. Conversely, the temperature-dependent oxygen demand of isolated cells and mitochondria can still be covered and their functional integrity maintained in fully oxygenated media, at temperatures when whole-animal oxygen supply is limited and transition to anaerobic metabolism occurs. In addition, the wider thermal range of functioning in mitochondria and cells means that molecular disturbance is either delayed in isolated systems or occurs earlier in the whole organism. The latter would imply that an organismic process feeds back on molecular functions and causes disturbance and disruption. The onset of enhanced oxidative stress (see above) does, in fact, indicate that thermally induced systemic hypoxia not only elicits limitations in tissue functional capacity due to insufficient aerobic energy but may also trigger membrane or molecular damage. Such interpretation is supported by multiple studies in temperate zone fishes and invertebrates and resulted in a generalized model of thermal tolerance (figure 5). There is no evidence available that molecular damage sets in prior to the onset of temperature-induced hypoxaemia and reduction in aerobic scope.
In similar ways as discussed above for the specialization of molecules and membranes in limited temperature windows, high and low limits of thermal tolerance are interdependent at the cellular and systemic levels, due to trade-offs involved in thermal adaptation. From an integrative point of view, they can be interpreted as resulting from trade-offs between optimized tissue functional capacities, on one hand, and associated baseline oxygen and energy demand, on the other hand. The biochemical factors that are involved and modified during thermal adaptation support temperature-dependent functional capacity and include components of the glycolytic pathway and of mitochondrial metabolism, such as the respiratory chain and Krebs cycle, as well as interactions of membrane-bound and catabolic processes. They also define demand as does, for example, mitochondrial proton leakage (cf. Pörtner et al. 2005a for review). The thermal responses of such fundamental biochemical mechanisms should contribute to setting whole-organism functional capacity and performance levels including the overall capacity of oxygen supply and delivery (ventilation and circulation), which matches the scope of demand only within a limited window of thermal tolerance (figure 5).
As a corollary, the earliest limits of thermal tolerance are set at the highest level of organizational complexity, namely the ability of oxygen supply to match demand. A progressive mismatch will affect all higher organismic functions beyond those relevant for basic maintenance. Such higher functions include motor activity, behaviour, feeding, digestion, growth, reproduction and development. Inadequate O2 supply will thus affect the long-term fate of organisms, populations and species in various climates. Only when an organism is within the optimum range of the window, where maximum aerobic scope is available, is there adequate flexibility of aerobic metabolic rate and associated functional capacity. Adequate functional capacity defines how animals exert their ecological functions through their mode of life and behavioural traits within a limited thermal window. Hence, under the concept of oxygen- and capacity-limited thermal tolerance, there are links between the different levels of functional organization from ecosystem via whole animal to molecules, which have to be qualified and quantified in an integrative approach. These generalizations form a suitable framework for integrating and interpreting species-specific information, discussed in the following sections of this review.
(d) From molecular to systemic aspects: thermal adaptation of performance capacity
While the consideration of structural trade-offs and thermal sensitivities at molecular to whole-organism levels may help to understand the onset of thermal limitation in whole organisms, they do not completely explain optimality patterns with respect to temperature or the patterns of energy turnover and lifestyle characteristics commonly observed in Antarctic ectotherms. An emerging and crucial pattern that supports life in cold oceans is that of high cost efficiency and energy savings at cellular and organismal levels. Mark et al. (2005) studied the temperature dependence of cellular metabolic rates and the patterns of energy allocation to various cellular energy consumers in isolated hepatocytes from high- and sub-Antarctic notothenioids and zoarcids. Key findings are that cellular oxygen consumption is minimal close to the habitat temperature of the species and rises at temperatures below and above this metabolic minimum. The fraction of oxygen consumption allocated to various energy consumers remained relatively constant regardless of temperature. From the point of view of energy efficiency, the temperature where oxygen consumption is lowest would be the energetic optimum. Such an optimum is also visible in the cellular oxygen consumption rates of the Antarctic eelpout, P. brachycephalum (figure 3). The temperature with minimum costs in isolated cells might be equivalent or related to the temperature with minimum costs of tissue maintenance. According to available data on thermal tolerance and species distribution patterns, this temperature would most likely be close to optimum temperatures in figure 5. The cost increment at temperatures below the optimum would decrease aerobic scope. The cost increment above may be first paralleled by a temperature-dependent rise in functional capacity until oxygen supply becomes limiting for that at the upper pejus temperature. The range between lower and upper limits is narrow in stenothermal invertebrates but may be wider in the Antarctic eelpout.
Energy efficiency and savings as overarching principles are also reflected in the low level of standard metabolism seen in Antarctic marine compared with temperate or tropical species (e.g. Holeton 1974). Some studies have claimed to show relatively high metabolic rates in polar species and have suggested that metabolism could be compensated for the low temperature (e.g. Wells 1987). However, the highest standard metabolic rates from polar species are only on a par with the lowest temperate species rates and are still around an order of magnitude lower than the highest rates from lower latitudes. Comparison of literature data for many fish species within a given taxon (Clarke & Johnston 1999) showed convincingly that standard or resting oxygen consumption varied consistently with environmental temperature regime across latitudes. The slope of the relationship between standard oxygen consumption and temperature did not vary across the temperature range of 0–30°C, and there was no temperature compensation of metabolism in Antarctic species. This was also shown for bivalve molluscs by Peck & Conway (2000). The low temperature regime with little seasonal variation, combined with intense seasonality of light, ice and phytoplankton production, has thus resulted in Antarctic species that are characterized by low physiological rates.
While these analyses have clearly shown that the old paradigm of metabolic cold adaptation can no longer be maintained for polar ectotherms in general, it should not be replaced with the opposite paradigm that metabolic cold adaptation does not exist at all. It has recently been emphasized that ambient temperature variability needs to be taken into account in evaluating the cost and level of cold compensation. Comparison of populations of marine invertebrate and fish species in the Northern Hemisphere, mostly East Atlantic latitudinal cline, has shown a rise in temperature-specific metabolic rate towards high Northern latitudes. The conclusion is that cold-adapted eurytherms (which cover a wide thermal range including cold temperatures) display wide windows of thermal tolerance at the expense of high baseline metabolic costs and high levels of aerobic metabolic capacity (Pörtner et al. 2000, 2005a). These energetic costs can be reduced by compensatory strategies like hibernation or the use of crucial activities in summer only (Pörtner 2004). Eurythermy is supported by small body sizes due to reduced diffusion distances and high body surface to volume ratios. In contrast, large body sizes as seen among Antarctic marine ectotherms not only reflect the exploitation of high oxygen concentrations of cold waters (Chapelle & Peck 1999; see below) but are also supported by low rates of metabolism at stable cold temperatures (Pörtner 2002c). As a simple reason, low metabolic rates require lower oxygen gradients and tolerate larger distances for diffusion. Thereby, it becomes possible to extract sufficient oxygen from oxygen-rich water despite large body sizes. These points suggest that an integrated adjustment of the functional capacity of oxygen supply systems and body size in relation to energy turnover and mode of life and to a limited thermal window was determined or co-determined by ambient temperature variability.
In line with, on average, reduced metabolic rates in Antarctic stenotherms, the past environmental conditions in the Southern Ocean have produced animals that exhibit markedly reduced rates of activity compared with non-Antarctic species. Activities such as walking in limpets, swimming in scallops, drilling in predatory trophonid snails, walking in isopods and burrowing in anemones and bivalve molluscs are all carried out at rates between two and five times slower when compared with similar temperate species (Peck et al. 2004b; reviewed in Peck 2005). The only activity so far measured in Antarctic ectotherms that appears compensated for temperature is sustained swimming in fishes (Van Dijk et al. 1998). However, more recent results have suggested that although there is thermal compensation in sustained swimming in fishes, this is far from complete (Wilson et al. 2002).
From an integrative point of view, the question is how the low metabolic rates of Antarctic stenotherms have evolved and how they are linked to the narrow windows of thermal tolerance. These low metabolic rates are paralleled by high mitochondrial densities, which should, in principle, cause elevated costs due to mitochondrial maintenance and proton leakage. The reduction in standard metabolism among cold-adapted stenotherms, in contrast to cold-adapted eurytherms, is reflected in reduced mitochondrial capacities, as described by Johnston et al. (1998) for Antarctic fishes. It was hypothesized that these patterns are paralleled by increased activation enthalpies of baseline mitochondrial oxygen demand, especially through proton leakage, and of flux-limiting enzymes in metabolism like IDH (Pörtner et al. 2000). Such high kinetic barriers may support low metabolic flux in cold-adapted stenotherms, despite mitochondrial proliferation. While Ea of overall metabolism appears to be reduced in active winter-acclimated animals with cold-compensated SMR (cf. Van Dijk et al. 1999; Pörtner 2002a; Zakhartsev et al. 2003), normal or high Ea values of baseline oxygen demand in Antarctic species would reflect a higher temperature dependence of metabolism and, in consequence, reduced heat tolerance, i.e. cold stenothermy of the whole organism. However, this high level of oxygen demand may not always become visible in whole animal standard metabolic rate, due to limited capacity and flexibility of ventilation and circulation. Conversely, a rapid rise in baseline oxygen demand would contribute to the rapid loss in aerobic scope, by absorbing a larger fraction of the oxygen available to the organism.
The rising baseline cost during warming is mirrored not only in the level of baseline mitochondrial and other cellular costs but also in the cost of enhanced rates of ventilation and circulation. The thermal increment in these costs can be alleviated by exposure to hyperoxia (Mark et al. 2002). In hypometabolic animals, ventilation and circulation systems are low-cost, low-capacity systems. Therefore, the cost increment per degree temperature change will be higher than in high-capacity, high-flow systems as in tuna. Such mechanistic links between the various levels of function and organization, however, are only just emerging and should be elaborated in future research.
As a corollary, further studying the changing kinetic and thermodynamic properties of individual enzymes, metabolic complexes, transmembrane ion exchange, and tissue and whole-organism functions with temperature adaptation will be relevant in understanding the response of whole organisms to temperature. The specific and contrasting strategies observed emphasize that molecular modifications must be interpreted in the light of their functional consequences from the enzyme to the whole-organism level. In the light of whole-organism adaptations to cold, enzymes that share control in metabolic flux are the most suitable candidates for studying structural and kinetic modifications, including changes in activation enthalpy, and their role in cellular and whole-organism metabolic control. Furthermore, the temperature-dependent capacities of higher whole-organism functions, the underlying molecular functions and their associated oxygen demand will also play a role in shaping thermal tolerance and temperature-dependent performance.
(e) Ecological implications
The great sensitivity of high-order physiological functions to temperature exhibited by Antarctic marine ectotherms has significant consequences for the survival of populations and species in a changing environment. The loss in aerobic scope due to temperature can be expected not only to reduce the ability to perform activities such as burrowing, swimming or righting but also to compromise the rates of growth, feeding or reproductive investment or other behaviours. Summer maximum temperatures in the maritime Antarctic range (in shallow waters) between 0.5°C and more than 3°C (South Georgia), and in the high Antarctic often do not exceed 0.5°C (Hunt et al. 2003, Barnes et al. 2007). The recent studies on temperature effects of whole-animal physiological capacities would indicate that with as little as a 2°C rise over current summer maxima in much of the maritime Antarctic, and 3°C in the high Antarctic, biological function could become compromised in a wide range of species. However, these sensitivities may shift depending on latitude and with acclimation. The phenomena of acclimation are only just starting to be studied in Antarctic ectotherms (see §1).
Another physiological rate that appears temperature limited is embryonic and larval development. Although there is still uncertainty whether either temperature or seasonal restrictions to food supply are more important in restricting growth in juveniles and adults (e.g. Clarke 1991), it appears that development is constrained by temperature. Several studies have shown that development is much slower in Antarctic species than their temperate or tropical relatives. The benchmark work for this was for echinoids, where Bosch et al. (1987) showed that species from McMurdo Sound take 85–140 h to hatch following fertilization compared with 15–30 h for species living at 8–25°C (figure 6). Similar results were obtained for echinoids from Signy Island by Stanwell-Smith & Peck (1998), for bivalve molluscs by Powell (2001) and for brooding periods in gastropod molluscs (L. Peck, personal observation). The surprising phenomenon here is that the effect of temperature on development rate is not constant across latitudes. Thus, a change in temperature has a much larger effect on development rate at polar than at tropical temperatures. Calculating Q10 values for temperature effects has been criticized as being poor when looking at the temperature effects on complex systems, but they are still valuable in identifying when there is a major change. Such a major change is clear for development when Q10 values are calculated for different temperature steps across the range in figure 6 (table 1). The Q10 values across the temperate and tropical range vary between 2.01 and 2.76, all well within the expected range for biological systems. At the low temperature end of the range, these values become 4.06 and 33.65 and are thus extraordinarily high. Clearly, there are additional factors affecting development rate in polar species on top of straight temperature effects. Further to this, Hoegh-Guldberg & Pearse (1995) analysed development rate across latitudes and showed that development rates were near their maximum for a given temperature at any site worldwide, and that temperature was the main regulator of development rate. Development thus appears thermally constrained in Antarctic species, an observation that requires mechanistic explanation (see below).
Figure 6.
Duration from embryonic development to hatching as a function of temperature for several species of echinoids from different latitudes. Time to hatching is plotted against mean experimental or environmental temperature for that species (adapted from Bosch et al. 1987; Stanwell-Smith & Peck 1998). Data for tropical and temperate species (filled triangles), Sterechinus neumayeri from McMurdo Sound (open triangles; Bosch et al. 1987) and S. neumayeri from Signy Island (open circles; Stanwell-Smith & Peck 1998).
Table 1.
Effects of different temperature steps on the development rate of echinoid embryos. Data shown are Q10 values for different temperature steps for rates shown in figure 6.
| temperature step | Q10 values for difference in development time |
|---|---|
| −1.8 to 0°C | 33.65 |
| 0 to 5°C | 4.06 |
| 5 to 10°C | 2.60 |
| 10 to 15°C | 2.76 |
| 15 to 20°C | 2.77 |
| 20 to 25°C | 2.01 |
Embryonic and larval forms are often thought of as more sensitive than adult stages. Studies in Antarctic species generally show decreased viability of embryos and larvae as temperatures go outside the normal environmental range in echinoderms (Stanwell-Smith & Peck 1998) and bivalve molluscs (Powell 2001). The ability of sperm to fertilize eggs also declines markedly between 0 and 2°C in the bivalve, L. elliptica (Powell 2001). Elevated environmental temperature could have two opposing effects, firstly to markedly enhance development rate and secondly to increase embryonic and larval mortality. These observations suggest that fertilization and development are further high-order processes, which may be among the first to be affected by ambient warming.
How temperature limitations on physiological capacities such as those described above affect ecological characters such as competitive interactions, predator/prey relationships, migration and dispersal or community structure and disturbance is an area that has generally received little or no attention. One way in which ecological balance would be affected is if some species are more temperature sensitive than others as outlined above in §1, with higher functional capacities, aerobic scopes and thus wider thermal windows, especially in pelagic predatory species. We would predict that the differences in temperature tolerance between species will be one of the first effects to come into play in a changing environment, because it will alter the balance of success between species and will change community composition and structure. These effects may become apparent very early on in the change process. Different species sensitivities to climate change may also be a key to an understanding of changes in ecosystem structure in other latitudes.
As already outlined for developmental rate, other life-history characters such as growth, longevity, reproduction, and sizes at birth and maturity, and how these characters interact with each other (e.g. Lessells 1991; Stearns 1992), will be influenced by temperature. Several models have been developed with the aim to link life-history characters to environmental factors, such as the ‘r–K–A’ system (Southwood 1977; Greenslade 1983), and the C-S-R triangle initially proposed by Grime (1974) and modified by Hodgson et al. (1999). Both models have competition, adversity or stress and colonization or disturbance elements. Fitness of individuals is dictated by selective forces that are defined by biotic and abiotic habitat characteristics. Life histories evolve in response to these selective forces and involve high-order processes that integrate various levels of biological organization and are thus prone to be affected early by temperature change. Life-history analyses have been used in Antarctic terrestrial groups (e.g. Convey 1996, 2000), but they are rare in marine studies. Antarctic terrestrial groups generally exhibit life histories that fit the main features of adversity selection (after Southwood 1977; Greenslade 1983). Thus, they have long life cycles, slow annual growth rates, deferred maturity, low reproductive output, low dispersal ability and high investment in survival adaptations.
Marine species in Antarctica are also characterized by generally slow growth rates, compared with temperate relatives. This has been known since the 1970s (Dayton et al. 1974; Everson 1977). Growth rates are usually two to five times slower than in temperate species (Arntz et al. 1994; Peck et al. 2000; Peck 2002), however, with some considerable inter-species variability (Barnes et al. 2007). Owing to variations in animal size and methods used to measure growth, widespread comparisons are often difficult to make. One way around this is to compare parameters of growth equations such as k from the von Bertalanffy equation, which is an estimate of the rate at which size approaches the maximum or asymptotic size for that species. When this was done for echinoids by Brockington (2001), an exclusion of high growth rates at high latitudes was apparent. When the parameter overall growth performance P (=log K+log M∞) is used instead, the analysis corrects for body size and describes the maximum growth rate reached at the point of inflection of the size (mass) growth curve (Pauly 1979). Brey & Clarke (1993) compiled data for 28 Antarctic and 141 non-Antarctic marine benthic species and showed that overall growth performance in Antarctic species was significantly lower, an analysis extended and confirmed by Brey (1999).
Slower growth of cold-adapted species has also been confirmed among pectinids in a latitudinal cline (Heilmayer et al. 2004). In this comparison, Q10 for annual overall growth was 1.12 across latitudes (figure 7), with A. colbecki showing higher growth than other Antarctic bivalves and invertebrates (Heilmayer et al. 2003), indicating some countergradient compensation of growth, i.e. higher than expected growth rates at high latitude. Other species have also been reported to show growth faster than expected, e.g. some sponges (Dayton et al. 1974), ascidians (Rauschert 1991) and bryozoans (Barnes 1995), and this has been used to argue that seasonality of food supply and not low temperature per se is the constraint on growth (Clarke 1991; Clarke & Peck 1991). However, these fast growth rates are still significantly slower than the fastest growth rates from lower latitude sites, and it is clear there is an overall decrease in growth rate at high latitude, even within the Antarctic (Clarke et al. 2004). In most studies outside Antarctica, growth is tied to periods of food supply. However, some species in the Southern Ocean appear to have much less seasonal growth than would be expected from such a highly seasonal environment, and this has been interpreted as a mechanism for minimizing overall annual costs (Peck et al. 2006).
Figure 7.
Arrhenius plots for the comparison of (a) average standard metabolic rates (SMR; (lnSMRavg)=30.116–8874.24 1/T, 82 measurements, 13 species, r2=0.725, p<0.001, Q10=2.28) with their annual rates of growth defined by (b) the overall growth performance (P, ln(OGP P) =4.22–958.466 1/T, 198 studies, 25 species, r2=0.132, p<0.001, Q10=1.12) in pectinid species from various temperature regimes (modified after Heilmayer et al. 2004). Note that growth rates in polar species (on the right) result higher than expected parallel decrease in SMR with temperature, thereby indicating high growth efficiency.
Interestingly, a recent analysis of daily summer growth of pectinids in a latitudinal cline yielded a higher Q10 of 2.7–3.2 for the growth of juvenile Antarctic scallops, A. colbecki (Heilmayer et al. 2005), compared with a lower Q10 of 1.12 seen in annual overall growth comparisons (figure 7). Furthermore, juvenile Antarctic scallops displayed slow summer growth at 0°C, but an extreme rise in growth rate at 3°C with a Q10 of 71 (Heilmayer et al. 2005). These patterns clearly suggest that comparisons of growth performance need to consider the life stage and season at which such growth is analysed. In fact, the difference between Antarctic and temperate growth rates may be much larger for juveniles than during periods of adult growth performance. Constraints on juvenile performance are discussed below. Overall, countergradient compensation may occur but does not appear as a unifying principle for all species or processes. Variations may depend on climate and climate variability.
At the same time, growth efficiency rises at high latitudes. Metabolic rate data available for pectinids across latitudinal clines show a much steeper fall with increasing latitude and associated temperature than the reduction in overall growth (Heilmayer et al. 2004). These observations clearly indicate less overall energy expenditure per unit growth in the cold and, thereby, match the observations of enhanced energy efficiency in metabolism discussed above.
In life-history analyses, slow growth rates are usually accompanied with long age and deferred maturity. Studies of longevity in polar marine benthos are rare, but those that exist have revealed markedly extended lifespans compared with temperate species in almost all cases, similar to the observations in deep sea species (Cailliet et al. 2001). The Antarctic bivalve, Yoldia eightsi, lives for well over 50 years (Nolan & Clarke 1993; Peck & Bullough 1993), two to ten times longer than temperate protobranch bivalves. The Antarctic clam, L. elliptica, lives for ca 36 years, longer than the 13-year-old temperate clam, Mya arenaria, which displays a similar ecotype (Philipp et al. 2005a,b). The pectinid, A. colbecki, lives for ca 30–40 years, compared with 8–10 years in the related temperate, Aequipecten opercularis (Philipp et al. 2006). The sea urchin, Sterechinus neumayeri, lives for over 35 years at Adelaide Island (Brockington 2001) and over 50 years at McMurdo Sound (Brey et al. 1995a), and the brachiopods L. uva and Magellania fragilis live for over 55 years (Peck & Brey 1996) and 49 years (Brey et al. 1995b), respectively. The starfish, O. validus, may live for more than 100 years (Pearse 1969). Polar amphipods also live longer than non-polar amphipods (Bluhm et al. 2001). In colonial species, bryozoans also have extended lifespans, with some Antarctic species living for up to 25 years (Barnes 1995). Data on deferred maturity are even scarcer. However, species that live for at least 15–20 years, such as the common Notothenia coriiceps, do not spawn until they are 6–8 years old, and species that live for over 25 years, such as Dissostichus eleginoides, do not spawn until they are 8–10 years old (Kock 1992). Most temperate fish of similar size begin spawning at 2–4 years of age. The brachiopod, L. uva, begins reproduction at just over 30 mm length (Peck & Holmes 1989), when they are at least 20 years old and the bivalve, Y. eightsi, becomes fully mature at 20 mm length, by at least 15 years of age (Peck et al. 2000). These ages at maturity are older than the maximum ages for most temperate protobranch bivalve molluscs and brachiopods. The question arises as to what extent these patterns of long age and delayed maturity relate not only to low temperature but also to the energy efficient mode of life at Antarctic or deep-sea temperatures.
Similarly, there are many studies showing that reproductive output in Antarctic marine species is low compared with temperate species (Arntz et al. 1994), and clines of decreasing egg number per female with latitude have been demonstrated across large latitudinal ranges for amphipods (Bregazzi 1972), and even within Antarctic waters for caridean shrimps (Clarke & Gore 1992; Gorny et al. 1992) and isopods (Wägele 1987). Studies showing reproductive output in Antarctic species of similar levels to temperate animals are rare, but it does appear that in echinoids, this is the case (McClintock 1989; Brockington 2001). A general reduction in reproductive output in the Antarctic marine benthos is accompanied by an increase in egg size (Clarke 1987; Kock 1992; Arntz et al. 1994). Larger egg size at higher latitude may account for some of the slowing of embryonic development rate described earlier, as there is a positive correlation between development rate and egg size, although it does not account for the major part of the slowing of development in Antarctic species.
In life-history terms, the number and size of offspring per year may not be the most important criteria, and it has been suggested that extended lifetimes and reproducing over many years may compensate for reduced numbers of offspring per year (Clarke 1987). Thus, lifetime reproductive effort appears similar across latitudes in caridean shrimps (Clarke 1987), and it may even be higher in Antarctic echinoids compared with temperate and tropical species on this basis (Brockington 2001). Although comparisons are difficult, the low or very low metabolic and growth rates of Antarctic species also mean that when reproductive effort is calculated as a proportion of energy available, or energy assimilated, a different picture emerges. On this basis, reproductive effort in echinoids is higher in Antarctic species than for those from lower latitudes, even though annual reproductive output is lower (Brockington 2001).
Another life-history character of importance is body size. The Southern Ocean holds many species viewed as giants, including decolopodid sea spiders over 30 cm in diameter. However, there are few detailed analyses of body size variation with latitude in marine groups. One that does exist, however, is for amphipod crustaceans. This study showed that the maximum size of any species of amphipod increases with latitude, and that the largest Antarctic species are over five times longer than the largest tropical species (Chapelle & Peck 1999). Size was investigated by looking at the spectrum of sizes for all species at a given site from tropical to polar habitats and, interestingly, they showed that size was related to water oxygen content and not temperature (figure 8). In a later study, Chapelle & Peck (2004) showed that all aspects of the size range, largest species, mean size, modal size and smallest size increased with latitude. Thus, at least in amphipods animal size is greater at polar latitudes.
Figure 8.
Effects of (a) temperature and (b) oxygen availability on the largest amphipod crustacean sizes for nine marine (filled circle) and three reduced salinity sites (open circle). (a) 95%/5% threshold size (TS95/5, TS95/5 is the cut-off point between the 95% smallest species and the 5% largest species in the size distribution) versus mean annual water temperature (inverted scale). This threshold size is used as a proxy for maximum size to allow for occasions where large, but very rare, species may not have been sampled. (b) TS95/5 versus calculated dissolved oxygen content at saturation (O2 μmol kg−1), based on mean surface water temperature and salinity. Although not every habitat in a site will experience permanently high oxygen saturation, this 100% value represents optimal conditions for species to attain large size (adapted from Chapelle & Peck, 1999).
All of the life-history characters described above are consistent with a K-selected environment in the r–K–A selection system. Thus, slow growth, large size, deferred maturity and large egg size are all consistent with adaptation to a predictable environment, maximizing investment in offspring survivorship through extended longevity and despite slow development. It is interesting that life-history selection would produce the characters seen in many Antarctic benthic species on the basis of adaptation to a constant or predictable but harsh environment, whereas many of these characters have been interpreted by ecophysiologists as products of low temperature and limited resource availability. From an integrative point of view, mechanistic links exist between the two, and their identification would support the requested cause and effect understanding (Pörtner et al. 2005b). Potential links and the trade-offs involved are elaborated in more detail below.
(f) Integration of phenomena: concepts, results and perspectives
While the previous discussion strongly suggests that the phenomena of thermal specialization at the various molecular to ecological levels are linked and interdependent, the question arises how these interdependences can be defined and quantified. Which parameters would limit phenotypic plasticity or acclimatization capacity? At the highest organizational levels, which mechanisms or trade-offs are shaping the typical life-history traits like behaviours, reproductive patterns, growth and development. While the trade-offs involved in thermal specialization at each functional level would contribute to explaining thermal limitation and also the linkage between low and high thermal limits, the interdependence of these trade-offs at various levels remains mostly unclear. The relevant processes linking the various levels of biological organization, the signalling mechanisms triggering acclimatization or the driving forces of temperature-dependent evolutionary adaptation are only vaguely known. Although the interpretation of findings in Antarctic species already benefits largely from present knowledge of animals living in different climate zones, the qualitative and quantitive picture of how shifts in thermal windows are elicited and of the associated transitions at molecular to whole-animal performance levels between various climates is incomplete.
Furthermore, explaining ecological patterns as in Antarctic ecosystems meets the great difficulty of how to transfer experimental laboratory-derived information, including physiological data, to the field and how to interpret them correctly with respect to their ecological meanings, both conceptually and numerically. In general, the variety of experimental laboratory data needs to be integrated into a larger context, starting from reductions of aerobic scope and performance in response to temperature change, over experimental analyses of mortality at critical thermal maxima (Lutterschmidt & Hutchison 1997a,b), over analyses of HSPs and their relevance in thermal protection (Feder & Hofmann 1999; Tomanek 2005) to timely analyses of modified gene expression patterns during thermal acclimatization (Hardewig et al. 1999a, Lucassen et al. 2003; Podrabsky & Somero 2004; Gracey et al. 2005). Previous efforts were focused on understanding passive tolerance rather than performance, although maintenance of performance is a requirement for long-term tolerance and probably the most crucial link between adaptation and ecological processes and among, if not the first to be affected by thermal stress. This approach goes beyond analyses of lethal thermal stress, where the physiological processes that collapse are likely to differ between acute extreme thermal exposures that elicit death within minutes and more prolonged lethal exposures that cause slow deterioration of critical physiological functions like oxygen transport, and thereby, eventually, lead to the death of the organism. An integrative concept, which would link the various levels of biological organization and support a more holistic view of adaptation, has clearly been lacking. The need for such a concept is evident from the requirement for a cause and effect understanding in explaining climate influences on ecosystems at all levels.
Among the approaches discussed in this chapter, the concept of oxygen- and capacity-limited thermal tolerance (Pörtner 2001, 2002a) might provide a suitable framework for integrative studies. It is supported by comparative analyses of species and their populations in marine phyla such as Sipunculida, Annelida, Crustacea, Mollusca and Vertebrata from various climate zones (see above). The applicability of this concept for small zooplankton (Lamkemeyer et al. 2003; Seidl et al. 2005) was also demonstrated, indicating its validity across body sizes. The experimental data supporting this concept show oxygen supply of an animal and associated aerobic performance capacity to occur within a limited thermal window specific for the species or its population. Aerobic performance capacity builds on the capacity of molecular and cellular functions. Aerobic performance also reflects the ability to enhance oxygen turnover for muscular activity, behaviours, growth or reproduction and is essential for many life functions in animals. The concept thus considers the relationships between the various levels of biological organization. Owing to trade-offs in thermal adaptation at all functional levels, the processes and limits of thermal tolerance are set through the specialization of aerobic performance to a limited thermal window, with a narrower performance optimum within this window. Currently, the following elements appear crucial (cf. figure 5).
When comparing molecular, mitochondrial, cellular and organismal levels of biological organization, thermal windows are narrowest at the highest levels of biological organization, as a result of integration.
At the edges of thermal windows, an early process to become constrained is aerobic performance capacity. Cellular and molecular stress phenomena are elicited by systemic hypoxia (hypoxaemia) or set in beyond the whole-organism window of temperature-dependent performance.
The optimization of functional capacity to within a thermal window is associated with trade-offs in adaptation. Energy efficiency defines performance levels in growth, activity, reproduction, development or, secondarily, longevity in various climate zones.
These principles explain why species in a Northern Hemisphere latitudinal and climatic cline form separate populations, which display different physiological and genetic characteristics. These probably result from selection for different thermal windows (Hummel et al. 1997; Nielsen et al. 2001; Sommer et al. 1997; Sommer & Pörtner 2002; Lannig et al. 2003) and, thereby, specialization on climate. Selection may occur long term or among offspring due to genetic variation or polymorphisms present among populations. The widths of the windows are dictated by ambient temperature variability and also influenced by activity capacity and mode of life. Molecular protection mechanisms (chaperones like Hsps or antioxidants) may play a lesser role (on short time-scales) in species that live at constant or only moderate fluctuations of temperatures. They are probably most relevant in species dwelling in the intertidal zone, when these are passively and short-term exposed to extreme temperatures beyond aerobic limits (Pörtner 2002a, Sokolova & Pörtner 2003; Tomanek 2005; cf. figure 5). However, in Antarctic species, they may also be relevant on long time-scales, e.g. due to extended longevity (cf. Pörtner 2006). High longevity and low numbers of generations per unit time in Antarctic species will also influence the rate of fixation of favourable mutations, although this will also be influenced by population size.
As already pointed out, the contrasting patterns of no or minimal metabolic cold adaptation in Antarctic stenotherms versus significant metabolic cold adaptation in (sub)-Arctic eurytherms suggest that one reason for the specialization to a very limited thermal window as in (some) Antarctic stenotherms lies in the associated energy savings. In Antarctic fauna, permanently cold and stable temperatures have permitted reduced rates of energy turnover. Nonetheless, they still needed to adjust functions to cold temperatures. The reptant decapod crustaceans (Anomura, Brachyura) were unable to do so, due to high levels of Mg2+ in the haemolymph, which constrain cold-adaptation capacity by acting as a relaxant on neuromuscular junctions. This evolutionary cul-de-sac would exclude the whole group from areas with water temperatures below 0°C in the Antarctic and the Arctic (Frederich et al. 2001). This effect, together with a cold-induced discrimination against active pelagic larvae (see below), might in fact contribute to the progressive reduction of anomuran and brachyuran biodiversity with increasing latitudes. Astorga et al. (2003) demonstrated such a reduction in biodiversity for the Southern Hemisphere. The observation of 54 Brachyuran species in the English Channel versus only two species in Svalbard (Hiscock et al. 2004) would be in line with these findings for the Northern Hemisphere, where such an analysis is not yet available.
The question arises as to what are the overarching reasons that have led to energy savings in virtually all Antarctic marine ectotherms, when compared with temperate species? A recent review of functional characteristics of Antarctic ectotherms came to the conclusion that these energy savings are enforced because all life functions are to be carried out at cold temperatures. One key component within an energy budget needs to be protected from reduced energy availability and that is growth (Pörtner 2006). Ectotherms grow fastest when they can keep other costs low, as reflected in low SMRs. In fact, reduced rates of aerobic energy turnover are not paralleled by similar reductions in growth rates in invertebrates (see above; figure 7). This effect of temperature is continuous across latitudinal clines leading to progressively enhanced growth efficiency with falling temperatures as seen in pectinids (Heilmayer et al. 2004). Growth efficiency is also higher in demersal than in pelagic Antarctic fish (Pörtner et al. 2005b). High growth efficiency at cold temperatures indicates cold-compensated growth in relation to SMR. This observation suggests that factors like variable food availability may not be crucial in expressing the key trade-off between growth performance, temperature and energy demands. In support of (partially) cold-compensated growth rates, the capacity for protein synthesis is found to be cold compensated in Antarctic fish and invertebrates (Storch et al. 2003, 2005). Largely cold-compensated protein synthesis capacities are complemented by cold-compensated cellular proliferation capacities as seen in Antarctic fish (Brodeur et al. 2003). Both processes are crucial for growth, and their cold compensation is in line with the suggested trend in polar ectotherms to maintain growth performance elevated in relation to largely decreased baseline metabolic costs, and also with the potential need to replace unstable protein at a higher rate than in warmer water species (see above).
These relationships lead to the hypothesis that increased growth efficiency imposed by the permanent cold probably occurs at the expense of other functions. This will bear specific life-history consequences, as other higher functions may suffer from reduced energy allocation and are, accordingly, slowed down at low energy costs over time. At all stages of life history, reduced overall energy turnover for the sake of (partially) cold-compensated growth may be responsible for late maturity, late and reduced fecundity, and late and extended offspring release. Overall reduction in the pace of Antarctic life may also be supported by a parallel reduction in the rate of natural predation (Aronson et al. 1997). In fact, the brachiopod L. uva becomes mature only when aged at least 20 years. Numbers of offspring produced per year are lower in Antarctica than at lower latitudes. The numbers of eggs produced by Antarctic species are often around two to five times less than the numbers produced by warmer water species. At the same time, successful fertilization of eggs in Antarctic marine species requires extra cost by 10–100 times as many sperm as in temperate species, and this may be because sperm swims more slowly at polar temperatures (Powell 2001). This extra cost may slow down reproductive activity even further. The trade-offs involved in high growth efficiency may also explain extraordinarily high Q10 levels as seen in echinoid development in extreme cold (figure 6). Furthermore, these relationships are probably emphasized by the effects of allometry, which predict that energy savings for the sake of growth are exacerbated at small body size (Pörtner 2006). These constraints would favour not only slow development but also passive lecithotrophic larvae. As a consequence of delayed or extended life-history phases, extended lifespans evolved, not as a result of hypometabolism or of enhanced antioxidative protection per se, but probably due to life-history requirements, e.g. for sufficient reproductive output (Clarke 1987; Pörtner 2006). Along the same line of thought, polar gigantism is enabled by a combination of elevated oxygen levels in cold waters with reduced metabolism and associated low activity levels and by extended periods of cold-compensated growth at slow developmental rates.
As a corollary, the concept of oxygen- and capacity-limited thermal tolerance is able to integrate information from molecular to systemic levels and provide consistent lines of explanation for molecular to ecological phenomena. We are not aware of other integrative concepts with the same power in developing integrative mechanistic explanations. This concept should thus provide a useful framework for testing hypotheses of functional adaptation and limitation in changing climates. The recent observation that early limits in oxygen supply coincide with the onset of decreased abundance of fish in the field (Pörtner & Knust 2007) emphasizes the validity of such an approach. This concept is also suitable to identify gaps in our understanding or develop functional hypotheses for future work in areas where no comprehensive dataset yet exists, e.g. in gene expression studies during thermal adaptation.
In summary, Antarctic organisms are sensitive to small degrees of acute warming, due to loss of aerobic scope and associated loss of performance. The life-history characters exhibited by Antarctic marine species are related to the low level of standard metabolism and the trade-offs for the sake of maximized growth rate in the cold. These life-history characters are all likely to make them slow in responding to change, and vulnerable to temperature change. Organism abilities to adapt to changing conditions are a product of rates of substitution in genetic code, levels of mixing of genetic material at reproductive events and numbers of offspring produced over time. Since maturity is deferred and numbers of offspring produced per year are thus lower in Antarctica than at lower latitudes, and since growth is slow, the Antarctic marine fauna is especially vulnerable to change. The brachiopod, L. uva, that does not become mature until aged at least 20 years has five to ten times fewer generations in 100 years than temperate brachiopods that become mature in 2–4 years. With two to five times fewer numbers of eggs, these rough calculations would suggest that the ability of Antarctic marine species to produce a novel genetic adaptation is around 10 times slower than in temperate marine species. The overall conclusion must be that the Antarctic marine fauna is more vulnerable to changing conditions than faunas from lower latitudes. Owing to species-specific thermal sensitivities even in the Antarctic, warming trends will affect species differently and thereby modulate interaction between them. Such effects have not yet been investigated in Antarctic fauna.
Acknowledgments
The authors wish to thank Dave Barnes for critically commenting on the manuscript. The work was supported by the Mar Co Pol I programme of the AWI (H.O.P.) and National Science Foundation grant IBN-0133184 (to G.N.S.).
Footnotes
One contribution of 10 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. Part 2: Evolution, diversity and function’.
References
- Abele D, Burlando B, Viarengo A, Pörtner H.O. Exposure to elevated temperatures and hydrogen peroxide elicits oxidative stress and antioxidant response in the Antarctic intertidal limpet Nacella concinna. Comp. Biochem. Physiol. B. 1998;120:425–435. [Google Scholar]
- Acierno R, Agnisola C, Tota B, Sidell B.D. Myoglobin enhances cardiac performance in Antarctic fish species that express the protein. Am. J. Physiol. 1997;273:R100–R106. doi: 10.1152/ajpregu.1997.273.1.R100. [DOI] [PubMed] [Google Scholar]
- Aizawa A.S, Nishino H, Saito K, Kimura K, Shirakawa H, Yoshida M. Stimulation of transcription in cultured cells by high mobility group protein 1: essential role of the acidic carboxyl-terminal region. Biochemistry. 1994;33:14 690–14 695. doi: 10.1021/bi00253a006. [DOI] [PubMed] [Google Scholar]
- Angel M.V. Variations in time and space: is biogeography relevant to studies of long-time scale change? J. Mar. Biol. Assoc. UK. 1991;71:191–206. [Google Scholar]
- Arntz W.E, Brey T, Gallardo V.A. Antarctic zoobenthos. Oceangr. Mar. Biol.: Annu. Rev. 1994;32:241–304. [Google Scholar]
- Aronson R.B, Blake D.B, Oji T. Retrograde community structure in the Late Eocene of Antarctica. Geology. 1997;15:903–906. [Google Scholar]
- Astorga A, Fernández M, Boschi E.E, Lagos N. Two oceans, two taxa and one mode of development: latitudinal diversity patterns of South American crabs and test for possible causal processes. Ecol. Lett. 2003;6:420–427. [Google Scholar]
- Baldwin J. Adaptation of enzymes to temperature: acetylcholinesterases in the central nervous systems of fishes. Comp. Biochem. Physiol. 1971;40:181–187. doi: 10.1016/0305-0491(71)90074-5. [DOI] [PubMed] [Google Scholar]
- Barnes D.K.A. Seasonal and annual growth in erect species of Antarctic bryozoans. J. Exp. Mar. Biol. Ecol. 1995;188:181–198. [Google Scholar]
- Barnes D.K.A, Fuentes V, Clarke A, Schloss I.R, Wallace M.I. Spatial and temporal variation in shallow seawater temperatures around Antarctica. Deep-Sea Res. II. 2006;53:853–865. [Google Scholar]
- Barnes D.K.A, Webb K.E, Linse K. Growth rate and its variability in erect Antarctic bryozoans. Polar Biol. 2007:xx. doi:10.1007/s00300-007-0266-2 [Google Scholar]
- Beaugrand G, Reid P.C. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Global Change Biol. 2003;9:801–817. [Google Scholar]
- Beaugrand G, Brander K.M, Lindley J.A, Souissi S, Reid P.C. Plankton effect on cod recruitment in the North Sea. Nature. 2003;426:661–664. doi: 10.1038/nature02164. [DOI] [PubMed] [Google Scholar]
- Bernardi G. Elsevier; Amsterdam, The Netherlands: 2004. Structural and evolutionary genomics: natural selection in genome evolution. [Google Scholar]
- Bluhm B.A, Brey T, Klages M. The autofluorescent age pigment lipofuscin: key to age, growth and productivity of the Antarctic amphipod Waldeckia obesa (Chevreux, 1905) J. Exp. Mar. Biol. Ecol. 2001;258:215–235. doi: 10.1016/s0022-0981(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Bosch I, Beauchamp K.A, Steele M.E, Pearse J.S. Development, metamorphosis and seasonal abundance of embryos and larvae of the Antarctic sea urchin Sterechinus neumayeri. Biol. Bull. 1987;173:126–135. doi: 10.2307/1541867. [DOI] [PubMed] [Google Scholar]
- Bregazzi P.K. Life cycles and seasonal movements of Cheirimedon femoratus (Pfeffer) and Tryphosella kergueleni (Miers)(Crustacea: Amphipoda) Br. Antarct. Surv. Bull. 1972;30:1–34. [Google Scholar]
- Brey T. Growth performance and mortality in aquatic macrobenthic invertebrates. Adv. Mar. Biol. 1999;35:153–223. [Google Scholar]
- Brey T, Clarke A. Population dynamics of marine benthic invertebrates in Antarctic and subantarctic environments: are there unique adaptations? Antarct. Sci. 1993;5:253–266. [Google Scholar]
- Brey T, Pearse J.S, Basch L, McLintock J.B, Slattery M. Growth and production of Sterechinus neumayeri (Echinoidea: Echinodermata) in McMurdo Sound. Antarct. Mar. Biol. 1995a;124:279–292. [Google Scholar]
- Brey T, Peck L.S, Gutt J, Hain S, Arntz W. Population dynamics of Magellania fragilis, a brachiopod dominating a mixed-bottom macrobenthic assemblage on the Antarctic shelf. J. Mar. Biol. Ass. UK. 1995b;75:857–870. [Google Scholar]
- Brockington, S. 2001 The seasonal ecology and physiology of Sterechinus neumayeri (Echinodermata: Echinoidea) at Adelaide Island, Antarctica. PhD Thesis, British Antarctic Survey, Cambridge, UK.
- Brodeur J.C, Calvo J, Clarke A, Johnston I.A. Myogenic cell cycle duration in Harpagifer species with sub-Antarctic and Antarctic distributions: evidence for cold compensation. J. Exp. Biol. 2003;206:1011–1016. doi: 10.1242/jeb.00204. [DOI] [PubMed] [Google Scholar]
- Brodte E, Knust R, Pörtner H.O, Arntz W.E. Pachycara brachycephalum—tracing the life history of an Antarctic eelpout. Deep Sea Res. II. 2006;53:1131–1140. [Google Scholar]
- Cailliet G.M, Andrews A.H, Burton E.J, Watters D.L, Kline D.E, Ferry-Graham L.A. Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp. Gerontol. 2001;36:739–764. doi: 10.1016/s0531-5565(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Cashon R.E, Vada M.E, Sidell B.D. Kinetic characterization of myoglobins from vertebrates with vastly different body temperatures. Comp. Biochem. Physiol. 1997;118B:613–620. doi: 10.1016/s0305-0491(97)00011-4. [DOI] [PubMed] [Google Scholar]
- Chapelle G, Peck L.S. Polar gigantism dictated by oxygen availability. Nature. 1999;399:114–115. [Google Scholar]
- Chapelle G, Peck L. Amphipod crustacean size spectra: new insights in the relationship between size and oxygen. Oikos. 2004;106:167–175. [Google Scholar]
- Chapman, W. L. & Walsh, J. E. 2005 A synthesis of Antarctic temperatures. Manuscript. See http://arctic.atmos.uiuc.edu
- Cheng C.C.-H, Detrich III H.W. Molecular ecophysiology of Antarctic notothenioid fishes. Phil. Trans. R. Soc. B. 2007;362:2215–2232. doi: 10.1098/rstb.2006.1946. doi:10.1098/rstb.2006.1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. Temperature, latitude and reproductive output. Mar. Ecol. Prog. Ser. 1987;38:89–99. [Google Scholar]
- Clarke A. What is cold adaptation and how should we measure it? Am. Zool. 1991;31:81–92. [Google Scholar]
- Clarke A. Seasonal acclimatisation and latitudinal compensation in metabolism: do they exist? Funct. Ecol. 1993;7:139–149. [Google Scholar]
- Clarke A. The influence of climate change on the distribution and evolution of organisms. In: Johnston I.A, Bennett A.F, editors. Animals and temperature: phenotypic and evolutionary adaptation. Cambridge University Press; Cambridge, UK: 1996. pp. 377–407. [Google Scholar]
- Clarke A. Seasonality in the Antarctic marine environment. Comp. Biochem. Physiol. 1998;90B:461–473. [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 2003;18:573–581. [Google Scholar]
- Clarke A, Crame J.A. The origin of the southern ocean marine fauna. In: Crame J.A, editor. Origins and evolution of the Antarctic Biota. Special publications. vol. 47. Geological Society; London, UK: 1989. pp. 253–268. [Google Scholar]
- Clarke A, Gore D.J. Egg size and composition in Ceratoserolis (Crustacea: Isopoda) from the Weddell Sea. Polar Biol. 1992;12:129–134. [Google Scholar]
- Clarke A, Johnston N.M. Scaling of metabolic rate and temperature in teleost fish. J. Anim. Ecol. 1999;68:893–905. [Google Scholar]
- Clarke A, Peck L.S. The physiology of polar marine zooplankton. In: Sakshaug E, Hopkins C, Oritsland N, editors. Proc. Pro Mare Symp. on Polar Ecology, Trondheim. Polar Research. vol. 10. Norwegian Polar Research Institute; Oslo, Norway: 1991. pp. 355–369. [Google Scholar]
- Clarke A, Prothero-Thomas E, Beaumont J.C, Chapman A.L, Brey T. Growth in the limpet Nacella concinna from contrasting sites in Antarctica. Polar Biol. 2004;28:62–71. [Google Scholar]
- Cocca E, Ratnayake-Lecamwasam M, Parker S.K, Camardella L, DiPrisco G, Detrich H.W. Do the hemoglobinless icefishes have globin genes? Comp. Biochem. Physiol. A. 1997;118:1027–1030. [Google Scholar]
- Convey P. The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biol. Rev. 1996;71:191–225. [Google Scholar]
- Convey P. How does cold constrain life cycles of terrestrial plants and animals? Cryo Lett. 2000;21:73–82. [PubMed] [Google Scholar]
- Crame J.A. Latitudinal range fluctuations in the marine realm through geological time. Trends Ecol. Evol. 1993;8:162–166. doi: 10.1016/0169-5347(93)90141-B. [DOI] [PubMed] [Google Scholar]
- Crawford D.L, Powers D.A. Molecular basis of evolutionary adaptation at the lactate dehydrogenase-B locus in the fish Fundulus heteroclitus. Proc. Natl Acad. Sci. USA. 1989;86:9365–9369. doi: 10.1073/pnas.86.23.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett E, Sidell B.D. Some pathways of energy metabolism are cold adapted in Antarctic fishes. Physiol. Zool. 1990;63:472–488. [Google Scholar]
- Dayton P.K, Robilliard G.A, Paine R.T, Dayton L.B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol. Monogr. 1974;44:105–128. [Google Scholar]
- Di Prisco G, D'Avino R, Caruso C, Tamburrini M, Camardella L, Rutigliano B, Carratore V, Romano M. The biochemistry of oxygen transport in red-blooded Antarctic fishes. In: di Prisco G, Maresca B, Tota B, editors. Biology of Antarctic fish. Springer; Berlin, Germany: 1991. pp. 263–281. [Google Scholar]
- Dunn C.R, Wilks H.M, Halsall D.J, Atkinson T, Clarke A.R, Muirhead H, Holbrook J.J. Design and synthesis of new enzymes based on the lactate dehydrogenase framework. Phil. Trans. R. Soc. B. 1991;332:177–184. doi: 10.1098/rstb.1991.0047. doi:10.1098/rstb.1991.0047 [DOI] [PubMed] [Google Scholar]
- Eastman J.T. Academic Press; San Diego, CA: 1993. Antarctic fish biology: evolution in a unique environment. [Google Scholar]
- Erickson J.R, Sidell B.D, Moerland T.S. Temperature sensitivity of calcium binding for parvalbumins from Antarctic and temperate zone teleost fishes. Comp. Biochem. Physiol. A. 2005;140:179–185. doi: 10.1016/j.cbpb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Everson I. Antarctic marine secondary production and the phenomenon of cold adaptation. Phil. Trans. R. Soc. B. 1977;279:55–66. doi:10.1098/rstb.1977.0070 [Google Scholar]
- Feder M.E, Hofmann G.E. Heat-shock proteins, molecular chaperones and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Fields P.A. Review: protein function at thermal extremes: balancing stability and flexibility. Comp. Biochem. Physiol. A. 2001;129:417–431. doi: 10.1016/s1095-6433(00)00359-7. [DOI] [PubMed] [Google Scholar]
- Fields P.A, Houseman D.E. Decreases in activation energy and substrate affinity in cold-adapted A4 -lactate dehydrogenase: evidence from the Antarctic notothenioid fish Chaenocephalus aceratus. Mol. Biol. Evol. 2005;21:2246–2255. doi: 10.1093/molbev/msh237. [DOI] [PubMed] [Google Scholar]
- Fields P.A, Somero G.N. Hot spots in cold adaptation: localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc. Natl Acad. Sci. USA. 1998;95:11 476–11 481. doi: 10.1073/pnas.95.19.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser K.P.P, Clarke A, Peck L.S. Low-temperature protein metabolism: seasonal changes in protein synthesis and RNA dynamics in the Antarctic limpet Nacella concinna Strebel 1908. J. Exp. Biol. 2002;205:3077–3086. doi: 10.1242/jeb.205.19.3077. [DOI] [PubMed] [Google Scholar]
- Frederich M, Sartoris F.J, Pörtner H.O. Distribution patterns of decapod crustaceans in polar areas: a result of magnesium regulation? Polar Biol. 2001;24:719–723. [Google Scholar]
- Gille S.T. Warming of the Southern Ocean since the 1950s. Science. 2002;295:1275–1277. doi: 10.1126/science.1065863. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera P.J, Dowd F, Pedibhotla V.K, Rosario R, Stanley-Samuelson D, Petzel D. Enhanced hypo-osmoregulation induced by warm-acclimation in antarctic fish is mediated by increased gill and kidney Na+/K+-ATPase activities. J. Exp. Biol. 1995;198:2279–2291. doi: 10.1242/jeb.198.11.2279. [DOI] [PubMed] [Google Scholar]
- Gorny M, Arntz W.E, Clarke A, Gore D.J. Reproductive biology of caridean decapods from the Weddell Sea. Polar Biol. 1992;12:111–120. [Google Scholar]
- Gould S.J, Vrba E.S. Exaptation—a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- Gowen R.J, Stewart B.M, Mills D.K, Elliott P. Regional differences in stratification and its effect on phytoplankton production and biomass in the northwestern Irish Sea. J. Plankton Res. 1995;17:753–769. [Google Scholar]
- Gracey A.Y, Fraser E.J, Li W, Fang Y, Taylor R.R, Rogers J, Brass A, Cossins A.R. Coping with cold: an integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc. Natl Acad. Sci. USA. 2005;101:16 970–16 975. doi: 10.1073/pnas.0403627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenslade P.J.M. Adversity selection and the habitat templet. Am. Nat. 1983;122:352–365. [Google Scholar]
- Grime J.P. Vegetation classification by reference to strategies. Nature. 1974;250:26–31. [Google Scholar]
- Grove T.J, Hendrickson J.W, Sidell B.D. Two species of antarctic icefishes (genus Champsocephalus) share a common genetic lesion leading to the loss of myoglobin expression. Polar Biol. 2004;27:579–585. [Google Scholar]
- Hardewig I, van Dijk P.L.M, Moyes C.D, Pörtner H.O. Temperature-dependent expression of cytochrome c oxidase in fish: a comparison between temperate and Antarctic and eelpout. Am. J. Physiol. 1999a;277:R508–R516. doi: 10.1152/ajpregu.1999.277.2.R508. [DOI] [PubMed] [Google Scholar]
- Hardewig I, Peck L.S, Pörtner H.O. Thermal sensitivity of mitochondrial function in the Antarctic notothenioid, Lepidonotothen nudifrons. J. Comp. Physiol. B. 1999b;169:597–604. [Google Scholar]
- Hazel J.R. Thermal adaptation in biological membranes—is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- Heilmayer O, Brey T, Chiantore M, Cattaneo-Vietti R, Arntz W.E. Age and productivity of the Antarctic scallop, Adamussium colbecki, in Terra Nova Bay (Ross Sea, Antarctica) J. Exp. Mar. Biol. Ecol. 2003;288:239–256. [Google Scholar]
- Heilmayer O, Brey T, Pörtner H.O. Growth efficiency and temperature in scallops: a comparative analysis of species adapted to different temperatures. Funct. Ecol. 2004;18:641–647. [Google Scholar]
- Heilmayer O, Honnen C, Jacob U, Chiantore C, Cattaneo-Vietti R, Brey T. Temperature effects on summer growth rates in the Antarctic scallop, Adamussium colbecki. Polar Biol. 2005;28:523–527. [Google Scholar]
- Heise, K. 2005 Interaction of oxygen supply, oxidative stress, and molecular defence systems in fishes during cold and heat stress in a latitudinal cline. PhD thesis, Bremen University.
- Heise K, Puntarulo S, Nikinmaa M, Abele D, Pörtner H.O. Oxidative stress during stressful heat exposure and recovery in the North Sea eelpout (Zoarces viviparus) J. Exp. Biol. 2006a;209:353–363. doi: 10.1242/jeb.01977. [DOI] [PubMed] [Google Scholar]
- Heise K, Puntarulo S, Nikinmaa M, Pörtner H.O, Abele D. Functional hypoxia and reoxygenation upon cold exposure effects oxidative stress and hypoxic signaling in the North Sea eelpout (Zoarces viviparus) Comp. Biochem. Physiol. A. 2006b;143:494–503. doi: 10.1016/j.cbpa.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Hiscock K, Southward A, Tittley I, Hawkins S. Effects of changing temperature on benthic marine life in Britain and Ireland. Aquatic Conserv. Mar. Freshw. Ecosyst. 2004;14:333–362. [Google Scholar]
- Hochachka P.W, Somero G.N. Oxford University Press; Oxford, UK: 2002. Biochemical adaptation: mechanism and process in physiological evolution. [Google Scholar]
- Hodell D.A, Kanfoush S.L, Shemesh A, Crosta X, Charles C.D, Guilderson T.P. Abrupt cooling of Antarctic surface waters and sea ice expansion in the south atlantic sector of the Southern Ocean at 5000 cal yr B.P. Quatern. Res. 2001;56:191–198. [Google Scholar]
- Hodgson J.G, Wilson P.J, Hunt R, Grime J.P, Thompson K. Allocating C–S–R plant functional types: a soft approach. Oikos. 1999;85:282–294. [Google Scholar]
- Hoegh-Guldberg O, Pearse J.S. Temperature, food availability and the development of marine invertebrate larvae. Am. Zool. 1995;35:415–425. [Google Scholar]
- Hofmann G.E, Buckley B.A, Airaksinen S, Keen J.E, Somero G.N. Heat-shock protein expression is absent in the Antarctic fish Trematomus bernacchii (Family Nototheniidae) J. Exp. Biol. 2000;203:2331–2339. doi: 10.1242/jeb.203.15.2331. [DOI] [PubMed] [Google Scholar]
- Holeton G.F. Metabolic cold adaptation of polar fish: fact or artefact? Physiol. Zool. 1974;47:137–152. [Google Scholar]
- Holland L.Z, McFall-Ngai M, Somero G.N. Evolution of lactate dehydrogenase-A homologs of barracuda fishes (genus Sphyraena) from different thermal environments: differences in kinetic properties and thermal stability are due to amino acid substitutions outside the active site. Biochemistry. 1997;36:3207–3215. doi: 10.1021/bi962664k. [DOI] [PubMed] [Google Scholar]
- Huber M, Brinkhuis H, Stickley C.E, Döös K, Sluijs A, Warnaar J, Schellenberg S.A, Williams G.L. Eocene circulation of the Southern Ocean: was Antarctica kept warm by subtropical waters? Paleoceanography. 2004;19:PA4026. doi:10.1029/2004PA001014 [Google Scholar]
- Hummel H, Sommer A, Bogaards R.H, Pörtner H.O. Variation in genetic traits of the lugworm Arenicola marina: temperature related expression of mitochondrial allozymes? Mar. Ecol. Prog. Ser. 1997;159:189–195. [Google Scholar]
- Hunt B.M, Hoefling K, Cheng C.H.C. Annual warming episodes in seawater temperatures in McMurdo Sound in relation to endogenous ice in notothenioid fish. Antarct. Sci. 2003;15:333–338. [Google Scholar]
- Huntley B, Green R.E, Collingham Y.C, Hill J.K, Willis S.G, Bartlein P.J, Cramer W, Hagemeijer W.J.M, Thomas C.J. The performance of models relating species geographical distributions to climate is independent of trophic level. Ecol. Lett. 2004;7:417–426. [Google Scholar]
- Hurst L.D, Merchant A.R. High guanine–cytosine content is not an adaptation to high temperature: a comparative analysis amongst prokaryotes. Proc. R. Soc. B. 2001;268:493–497. doi: 10.1098/rspb.2000.1397. doi:10.1098/rspb.2000.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns G.C, Somero G.N. Evolutionary convergence in adaptation of proteins to temperature: A4-lactate dehydrogenases of Pacific damselfishes (Chromis spp.) Mol. Biol. Evol. 2004;21:314–320. doi: 10.1093/molbev/msh021. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Walesby N.J, Davison W, Goldspink G. Temperature adaptation in myosin of Antarctic fish. Nature. 1975;254:74–75. doi: 10.1038/254074a0. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Guderley H.E, Franklin C.E, Crockford T, Kamunde C. Are mitochondria subject to evolutionary temperature adaptation? J. Exp. Biol. 1994;195:293–306. doi: 10.1242/jeb.195.1.293. [DOI] [PubMed] [Google Scholar]
- Johnston I.A, Calvo J, Guderley H, Fernandez D, Palmer L. Latitudinal variation in the abundance and oxidative capacities of muscle mitochondria in perciform fishes. J. Exp. Biol. 1998;201:1–12. doi: 10.1242/jeb.201.1.1. [DOI] [PubMed] [Google Scholar]
- Kawall H.G, Torres J.J, Sidell B.D, Somero G.N. Metabolic cold adaptation in Antarctic fishes: evidence from enzymatic activities of brain. Mar. Biol. 2002;140:279–286. [Google Scholar]
- Kock K.-H. Cambridge University Press; Cambridge, UK: 1992. Antarctic fish and fisheries. [Google Scholar]
- La Terza A, Papa G, Miceli C, Leporini P. Divergence between two Antarctic species of the ciliate Euplotes, E. Forcardi and E. nobilii, in the expression of heat-shock protein 70 genes. Mol. Ecol. 2001;10:1061–1067. doi: 10.1046/j.1365-294x.2001.01242.x. [DOI] [PubMed] [Google Scholar]
- Lamkemeyer T, Zeis B, Paul R.J. Temperature acclimation influences temperature-related behaviour as well as oxygen transport physiology and biochemistry in the water flea Daphnia magna. Can. J. Zool. 2003;81:237–249. [Google Scholar]
- Lannig G, Eckerle L, Serendero I, Sartoris F.J, Fischer T, Knust R, Johansen T, Pörtner H.O. Temperature adaptation in eurythermal cod (Gadus morhua): comparison of mitochondrial enzyme capacities in Boreal & Arctic populations. Mar. Biol. 2003;142:589–599. [Google Scholar]
- Lannig G, Storch D, Pörtner H.O. Aerobic mitochondrial capacities in Antarctic and temperate eelpout (Zoarcidae) subjected to warm versus cold acclimation. Polar Biol. 2005;28:575–584. doi: 10.1007/s00300-005-0730-9 [Google Scholar]
- Lessells C.M. The evolution of life histories. In: Krebs J.R, Davies N.B, editors. Behavioural ecology, an evolutionary approach. Blackwell; Oxford, UK: 1991. pp. 32–68. [Google Scholar]
- Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. [Google Scholar]
- Logue J.A, DeVries A.L, Fodor E, Cossins A.R. Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J. Exp. Biol. 2000;203:2105–2115. doi: 10.1242/jeb.203.14.2105. [DOI] [PubMed] [Google Scholar]
- Low P.S, Bada J.L, Somero G.N. Temperature adaptation of enzymes: roles of the free energy, the enthalpy, and the entropy of activation. Proc. Natl Acad. Sci. USA. 1973;70:430–432. doi: 10.1073/pnas.70.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen M, Schmidt A, Pörtner H.O. Cold induced mitochondrial proliferation in Zoarces viviparus: changes in enzyme activities and mRNA levels. Am. J. Physiol. 2003;258:R1410–R1420. doi: 10.1152/ajpregu.00111.2003. [DOI] [PubMed] [Google Scholar]
- Lucassen M, Koschnick N, Eckerle L.G, Pörtner H.-O. Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J. Exp. Biol. 2006;209:2462–2471. doi: 10.1242/jeb.02268. doi:10.1242/jeb.02268 [DOI] [PubMed] [Google Scholar]
- Lutterschmidt W.I, Hutchison V.H. The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can. J. Zool. 1997a;75:1553–1560. [Google Scholar]
- Lutterschmidt W.I, Hutchison V.H. The critical thermal maximum: history and critique. Can. J. Zool. 1997b;75:1561–1574. [Google Scholar]
- Macdonald J.A, Montgomery J.C, Wells R.M.G. The physiology of McMurdo Sound fishes: current New Zealand research. Comp. Biochem. Physiol. B. 1988;90:567–578. [Google Scholar]
- Mark F.C, Bock C, Pörtner H.O. Oxygen limited thermal tolerance in Antarctic fish investigated by magnetic resonance imaging (MRI) and spectroscopy (31P-MRS) Am. J. Physiol. 2002;283:R1254–R1262. doi: 10.1152/ajpregu.00167.2002. [DOI] [PubMed] [Google Scholar]
- Mark F.C, Hirse T, Pörtner H.O. Thermal sensitivity of cellular energy budgets in some Antarctic fish hepatocytes. Polar Biol. 2005;28:805–814. doi: 10.1007/s00300-005-0018-0 [Google Scholar]
- McClintock J.B. Energetic composition, reproductive output and resource allocation of Antarctic asteroids. Polar Biol. 1989;9:147–153. [Google Scholar]
- Meredith M.P, King J.C. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys. Res. Lett. 2005;32:L19 604. doi:10.1029/2005GL024042 [Google Scholar]
- Montgomery J, Clements K. Disaptation and recovery in the evolution of Antarctic fishes. Trends Ecol. Evol. 2000;15:267–270. doi: 10.1016/s0169-5347(00)01896-6. [DOI] [PubMed] [Google Scholar]
- Murawski S.A. Climate change and marine fish distributions: forecasting from historical analogy. Trans. Am. Fish. Soc. 1993;122:647–658. [Google Scholar]
- Nielsen E.E, Hansen M.M, Schmidt C, Meldrup D, Groenkjaer P. Population of origin of Atlantic cod. Nature. 2001;413:272. doi: 10.1038/35095112. [DOI] [PubMed] [Google Scholar]
- Nolan C.P, Clarke A. Growth in the bivalve Yoldia eightsi at Signy Island, Antarctica, determined from internal shell increment and Calcium-45 incorporation. Mar. Biol. 1993;117:243–250. [Google Scholar]
- O'Brien K.M, Sidell B.D. The interplay among cardiac ultrastructure, metabolism and the expression of oxygen-binding proteins in Antarctic fishes. J. Exp. Biol. 2000;203:1287–1297. doi: 10.1242/jeb.203.8.1287. [DOI] [PubMed] [Google Scholar]
- Pauly D. Gill size and temperature as governing factors in fish growth: a generalization of von Bertalanffy's growth formula. Ber. Inst. Meereskd. Christian-Albrecht-Univ. Kiel. 1979;63:1–156. [Google Scholar]
- Pearse J.S. Antarctic sea star. Aust. Nat. History. 1969;16:234–238. [Google Scholar]
- Pearson R.G, Dawson T.P. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003;12:361–371. [Google Scholar]
- Peck L.S. Temperature and basal metabolism in two Antarctic marine herbivores. J. Exp. Mar. Biol. Ecol. 1989;127:1–12. [Google Scholar]
- Peck L.S. Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol. 2002;25:31–40. [Google Scholar]
- Peck L.S. Prospects for survival in the Southern ocean: extreme temperature sensitivity of benthic species. R. Soc. Spec. Issue Antarct. Sci. 2005;17:495–505. [Google Scholar]
- Peck L.S, Brey T. Bomb signals in old Antarctic brachiopods. Nature. 1996;380:207–208. [Google Scholar]
- Peck L.S, Bullough L.W. Growth and population structure in the infaunal bivalve Yoldia eightsi in relation to iceberg activity at Signy Island, Antarctica. Mar. Biol. 1993;117:235–241. [Google Scholar]
- Peck L.S, Conway L.Z. The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalves. In: Harper E.M, Taylor J.D, Crame J.A, editors. The evolutionary biology of the bivalvia. Special publications. vol. 177. Geological Society; London, UK: 2000. pp. 441–445. [Google Scholar]
- Peck L.S, Holmes L.J. Seasonal and ontogenetic changes in tissue size in the Antarctic brachiopod Liothyrella uva (Broderip, 1983) J. Exp. Mar. Biol. Ecol. 1989;134:25–36. [Google Scholar]
- Peck L.S, Colman J.G, Murray A.W.A. Growth and tissue mass cycles in the infaunal bivalve Yoldia eightsi at Signy Island, Antarctica. Polar Biol. 2000;23:420–428. [Google Scholar]
- Peck L.S, Pörtner H.O, Hardewig I. Metabolic demand, oxygen supply and critical temperatures in the Antarctic bivalve, Laternula elliptica. Physiol. Biochem. Zool. 2002;75:123–133. doi: 10.1086/340990. [DOI] [PubMed] [Google Scholar]
- Peck L.S, Webb K.E, Bailey D. Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct. Ecol. 2004a;18:625–630. [Google Scholar]
- Peck L.S, Ansell A.D, Webb K.E, Hepburn L, Burrows M. Burrowing in Antarctic bivalve molluscs. Polar Biol. 2004b;27:357–367. [Google Scholar]
- Peck L.S, Convey P, Barnes D.K.A. Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol. Rev. 2006;81:75–109. doi: 10.1017/S1464793105006871. [DOI] [PubMed] [Google Scholar]
- Philipp E, Brey T, Pörtner H.-O, Abele D. Chronological and physiological ageing in a polar and a temperate mud clam. Mech. Ageing Develop. 2005a;126:598–609. doi: 10.1016/j.mad.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Philipp E, Pörtner H.O, Abele D. Mitochondrial ageing of a polar and a temperate mud clam. Mech. Ageing Dev. 2005b;126:610–619. doi: 10.1016/j.mad.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Philipp E, Brey T, Heilmayer O, Abele D, Pörtner H.O. Physiological ageing in a polar and a temperate swimming scallop. Mar. Ecol. Prog. Ser. 2006;307:187–198. [Google Scholar]
- Place A.R, Powers D.A. Genetic variation and relative catalytic efficiencies: lactate dehydrogenase-B allozymes of Fundulus heteroclitus. Proc. Natl Acad. Sci. USA. 1979;76:2354–2358. doi: 10.1073/pnas.76.5.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place S.P, Hofmann G.E. Constitutive expression of a stress-inducible heat shock protein gene, hsp70, in phylogenetically distant Antarctic fish. Polar Biol. 2004;28:261–267. [Google Scholar]
- Place S.P, Zippay M.L, Hofmann G.E. Constitutive roles for inducible genes: evidence for the alteration in expression of the inducible hsp70 gene in Antarctic notothenioid fishes. Am. J. Physiol. 2004;287:R429–R436. doi: 10.1152/ajpregu.00223.2004. [DOI] [PubMed] [Google Scholar]
- Platt T, Fuentes-Yaco C, Frank K.T. Spring algal bloom and larval fish survival. Nature. 2003;423:398–399. doi: 10.1038/423398b. [DOI] [PubMed] [Google Scholar]
- Podrabsky J.E, Somero G.N. Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in an annual killifish Austrofundulus limnaeus. J. Exp. Biol. 2004;207:2237–2254. doi: 10.1242/jeb.01016. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A. 2002a;132:739–761. doi: 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Physiological basis of temperature dependent biogeography: tradeoffs in muscle design and performance in polar ectotherms. J. Exp. Biol. 2002b;205:2217–2230. doi: 10.1242/jeb.205.15.2217. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Environmental and functional limits to muscular exercise and body size in marine invertebrate athletes. Comp. Biochem. Physiol. A. 2002c;133:303–321. doi: 10.1016/s1095-6433(02)00162-9. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Climate variability and the energetic pathways of evolution: the origin of endothermy in mammals and birds. Physiol. Biochem. Zool. 2004;77:959–981. doi: 10.1086/423742. [DOI] [PubMed] [Google Scholar]
- Pörtner H.O. Climate dependent evolution of Antarctic ectotherms: an integrative analysis. Deep Sea Res. II. 2006;53:1071–1104. doi:10.1016/j.dsr2.2006.02.015 [Google Scholar]
- Pörtner H.O, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. doi:10.1126/science.1135471 [DOI] [PubMed] [Google Scholar]
- Pörtner H.O, Peck L.S, Zielinski S, Conway L.Z. Intracellular pH and energy metabolism in the highly stenothermal Antarctic bivalve Limopsis marionensis as a function of ambient temperature. Polar Biol. 1999a;22:17–30. [Google Scholar]
- Pörtner H.O, Hardewig I, Peck L.S. Mitochondrial function and critical temperature in the Antarctic bivalve, Laternula elliptica. Comp. Biochem. Physiol. A. 1999b;124:179–189. [Google Scholar]
- Pörtner H.O, Van Dijk P.L.M, Hardewig I, Sommer A. Levels of metabolic cold adaptation: tradeoffs in eurythermal and stenothermal ectotherms. In: Davison V, Williams C.W, editors. Antarctic ecosystems: models for a wider understanding. Caxton Press; Christchurch, New Zealand: 2000. pp. 109–122. [Google Scholar]
- Pörtner H.O, Mark F.C, Bock C. Oxygen limited thermal tolerance in fish? Answers obtained by nuclear magnetic resonance techniques. Resp. Physiol. Neurobiol. 2004;141:243–260. doi: 10.1016/j.resp.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Pörtner, H. O., Lucassen, M. & Storch, D. 2005a Metabolic biochemistry: its role in thermal tolerance and in the capacities of physiological and ecological function. In The physiology of polar fishes (eds A. P. Farrell & J. F. Steffensen) Fish Physiology 22, pp. 79–154 (Series eds W. S. Hoar, D. R. Randall & A. P. Farrell). San Diego, CA: Elsevier Academic Press.
- Pörtner H.O, Storch D, Heilmayer O. Constraints and trade-offs in climate dependent adaptation: energy budgets and growth in a latitudinal cline. Scientia Marina. 2005b;69:271–285. [Google Scholar]
- Pörtner H.O, Peck L.S, Hirse T. Hyperoxia alleviates thermal stress in the Antarctic bivalve, Laternula elliptica: evidence for oxygen limited thermal tolerance. Polar Biol. 2006;29:688–693. doi:10.1007/s00300-005-0106-1 [Google Scholar]
- Powell, D. 2001 The reproductive ecology of Antarctic free-spawning molluscs. PhD thesis, University of Southampton, p. 142.
- Rauschert M. Ergebnisse der faunistischen arbeiten im Benthal von King George Island (Südshetlandinseln Antarktis) Berichte für Polarforschung. 1991;76:1–75. [Google Scholar]
- Ream R, Johns G, Somero G.N. Base compositions of genes encoding α-actin and lactate adehydrogenase-A from differently adapted vertebrates show no temperature-adaptive variation in G+C content. Mol. Biol. Evol. 2003;20:105–110. doi: 10.1093/molbev/msg008. [DOI] [PubMed] [Google Scholar]
- Reid P.C, Edwards M, Hunt H.G, Warner A.J. Phytoplankton change in the North Atlantic. Nature. 1998;391:546. [Google Scholar]
- Roy K, Jablonski D, Valentine J.W, Rosenberg G. Marine latitudinal diversity gradients: tests of causal hypotheses. Proc. Natl Acad. Sci. USA. 1998;95:3699–3702. doi: 10.1073/pnas.95.7.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte P.M, Glémet H, Fiebig A.A, Powers D.A. Adaptive variation in lactate dehydrogenase-B gene expression: role of a stress-responsive regulatory element. Proc. Natl Acad. Sci. USA. 2000;97:6597–6602. doi: 10.1073/pnas.97.12.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebacher F, Davison W, Lowe C.J, Franklin C.E. A falsification of the thermal specialization paradigm: compensation for elevated temperatures in Antarctic fish. Biol. Lett. 2005;2:151–154. doi: 10.1098/rsbl.2004.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl M.D, Pirow R, Paul R.J. Acclimation of the microcrustacean Daphnia magna to warm temperatures is dependent on haemoglobin expression. J. Therm. Biol. 2005;30:532–544. [Google Scholar]
- Sidell B.D. Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J. Exp. Biol. 1998;201:1118–1127. doi: 10.1242/jeb.201.8.1119. [DOI] [PubMed] [Google Scholar]
- Sidell B.D, Vayda M.E, Small D.J, Moylan T.J, Londraville R.L, Yuan M.L, Rodnick K.J, Eppley Z.A, Costello L. Variable expression of myoglobin among the hemoglobinless Antarctic icefishes. Proc. Natl Acad. Sci. USA. 1997;94:3420–3524. doi: 10.1073/pnas.94.7.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova I.M, Pörtner H.O. Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J. Exp. Biol. 2003;206:195–207. doi: 10.1242/jeb.00054. [DOI] [PubMed] [Google Scholar]
- Somero G.N. Protein adaptations to temperature and pressure: complementary roles of adaptive changes in amino acid sequence and internal milieu. Comp. Biochem. Physiol. B. 2003;136:577–591. doi: 10.1016/s1096-4959(03)00215-x. [DOI] [PubMed] [Google Scholar]
- Somero G.N, DeVries A.L. Temperature tolerance of some Antarctic fishes. Science. 1967;156:257–258. doi: 10.1126/science.156.3772.257. [DOI] [PubMed] [Google Scholar]
- Sommer A.M, Pörtner H.O. Metabolic cold adaptation in the lugworm Arenicola marina: comparison of a North Sea and a White Sea population. Mar. Ecol. Prog. Ser. 2002;240:171–182. [Google Scholar]
- Somero G.N, Dahlhoff E, Lin J.J. Stenotherms and eurytherms: mechanisms establishing thermal optima and tolerance ranges. In: Johnston I.A, Bennett A.F, editors. Animals and temperature: phenotypic and evolutionary adaptation. Cambridge University Press; Cambridge, UK: 1996. pp. 53–57. [Google Scholar]
- Sommer A, Klein B, Pörtner H.O. Temperature induced anaerobiosis in two populations of the polychaete worm Arenicola marina. J. Comp. Physiol. B. 1997;167:25–35. [Google Scholar]
- Southwood T.R.E. Habitat, the templet for ecological strategies. J. Anim. Ecol. 1977;46:337–365. [Google Scholar]
- Stanwell-Smith D.P, Peck L.S. Temperature and embryonic development in relation to spawning and field occurrence of larvae of 3 Antarctic echinoderms. Biol. Bull. 1998;194:44–52. doi: 10.2307/1542512. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Storch D, Heilmayer O, Hardewig I, Pörtner H.O. In vitro protein synthesis capacities in a cold stenothermal and a temperate eurythermal pectinid. J. Comp. Physiol. B. 2003;173:611–620. doi: 10.1007/s00360-003-0371-7. [DOI] [PubMed] [Google Scholar]
- Storch D, Lannig G, Pörtner H.O. Temperature dependent protein synthesis capacities in Antarctic and temperate (North Sea) fish (Zoarcidae) J. Exp. Biol. 2005;208:2409–2420. doi: 10.1242/jeb.01632. [DOI] [PubMed] [Google Scholar]
- Thomas J.O, Travers A.A. MHG1 and 2 and related ‘architectural’ DNA-binding proteins. Trends Biochem. Sci. 2001;26:167–174. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- Tomanek L. Two-dimensional gel analysis of the heat-shock response in marine snails (genus Tegula): interspecific variation in protein expression and acclimation ability. J. Exp. Biol. 2005;208:3133–3143. doi: 10.1242/jeb.01748. [DOI] [PubMed] [Google Scholar]
- Tripati A, Backman J, Elderfield H, Ferretti P. Eocene bipolar glaciation associated with global carbon cycle changes. Nature. 2005;436:341–346. doi: 10.1038/nature03874. [DOI] [PubMed] [Google Scholar]
- Van Dijk P.M.L, Hardewig I, Pörtner H. Exercise in the cold: high energy turnover in Antarctic fish. In: di Prisco G, Pisano E, Clarke A, editors. Fishes of Antarctica: a biological overview. Springer; Berlin, Germany; New York, NY: 1998. pp. 225–236. [Google Scholar]
- Van Dijk P.L.M, Tesch C, Hardewig I, Pörtner H.O. Physiological disturbances at critically high temperatures: a comparison between stenothermal Antarctic and eurythermal temperate eelpouts (Zoarcidae) J. Exp. Biol. 1999;202:3611–3621. doi: 10.1242/jeb.202.24.3611. [DOI] [PubMed] [Google Scholar]
- Wägele J.W. On the reproductive biology of Ceratoserolis trilobitoides (Crustacea: Isopoda): latitudinal variation of fecundity and embryonic development. Polar Biol. 1987;7:11–24. [Google Scholar]
- Wang X, Christian J.R, Murtugudde R, Busalacchi A.J. Ecosystem dynamics and export production in the central and eastern equatorial Pacific: a modeling study of impact of ENSO. Geophys. Res. Lett. 2005;32:L02 608. doi:10.1029/2004GL021538 [Google Scholar]
- Weinstein R.B, Somero G.N. Effects of temperature on mitochondrial function in the Antarctic fish Trematomus bernacchii. J. Comp. Physiol. B. 1998;168:190–196. [Google Scholar]
- Wells R.M.G. Respiration of Antarctic fishes from McMurdo sound. Comp. Biochem. Physiol. A. 1987;88:417–424. doi: 10.1016/0300-9629(87)90056-9. [DOI] [PubMed] [Google Scholar]
- Wilson R.S, Kuchel L.J, Franklin C.E, Davison W. Turning up the heat on subzero fish: thermal dependence of sustained swimming in an Antarctic notothenioid. J. Therm. Biol. 2002;27:381–386. [Google Scholar]
- Wiltshire K.H, Manly B.F.J. The warming trend at Helgoland Roads, North Sea: phytoplankton response. Helgol. Mar. Res. 2004;58:269–273. [Google Scholar]
- Wodtke E. Temperature adaptation of biological membranes. Compensation of the molar activity of cytochrome c oxidase in the mitochondrial energy-transducing membrane during thermal acclimation of the carp (Cyprinus carpio L.) Biochim. Biophys. Acta. 1981;640:710–720. doi: 10.1016/0005-2736(81)90101-2. [DOI] [PubMed] [Google Scholar]
- Zakhartsev M.V, De Wachter B, Sartoris F.J, Pörtner H.O, Blust R. Thermal physiology of the common eelpout (Zoarces viviparus) J. Comp. Physiol. B. 2003;173:365–378. doi: 10.1007/s00360-003-0342-z. [DOI] [PubMed] [Google Scholar]