Abstract
Antarctica and its surrounding islands lie at one extreme of global variation in diversity. Typically, these regions are characterized as being species poor and having simple food webs. Here, we show that terrestrial systems in the region are nonetheless characterized by substantial spatial and temporal variations at virtually all of the levels of the genealogical and ecological hierarchies which have been thoroughly investigated. Spatial variation at the individual and population levels has been documented in a variety of genetic studies, and in mosses it appears that UV-B radiation might be responsible for within-clump mutagenesis. At the species level, modern molecular methods have revealed considerable endemism of the Antarctic biota, questioning ideas that small organisms are likely to be ubiquitous and the taxa to which they belong species poor. At the biogeographic level, much of the relatively small ice-free area of Antarctica remains unsurveyed making analyses difficult. Nonetheless, it is clear that a major biogeographic discontinuity separates the Antarctic Peninsula and continental Antarctica, here named the ‘Gressitt Line’. Across the Southern Ocean islands, patterns are clearer, and energy availability is an important correlate of indigenous and exotic species richness, while human visitor numbers explain much of the variation in the latter too. Temporal variation at the individual level has much to do with phenotypic plasticity, and considerable life-history and physiological plasticity seems to be a characteristic of Antarctic terrestrial species. Environmental unpredictability is an important driver of this trait and has significantly influenced life histories across the region and probably throughout much of the temperate Southern Hemisphere. Rapid climate change-related alterations in the range and abundance of several Antarctic and sub-Antarctic populations have taken place over the past several decades. In many sub-Antarctic locations, these have been exacerbated by direct and indirect effects of invasive alien species. Interactions between climate change and invasion seem set to become one of the most significant conservation problems in the Antarctic. We conclude that despite the substantial body of work on the terrestrial biodiversity of the Antarctic, investigations of interactions between hierarchical levels remain scarce. Moreover, little of the available information is being integrated into terrestrial conservation planning, which lags far behind in this region by comparison with most others.
Keywords: biogeography, biological invasions, climate change, conservation planning, extinction, phenotypic plasticity
1. Introduction
Of all the characteristics of biodiversity, the most noteworthy is its variability. Recognition that the significance of the mechanisms underlying this variation changes as the scale of interest is altered, and that variation at one level may cascade up (or down) to affect many others in the ecological and genealogical hierarchies, are hallmarks of modern ecology (Wiens 1989). For example, it is clear that both local and regional scale processes affect the identity and richness of species at any given site (Ricklefs 1987, 2004; Hawkins & Porter 2003; Witman et al. 2004) and that local–regional interactions can profoundly affect the properties of assemblages (Gaston 2000; Blackburn & Gaston 2001a; Leibold et al. 2004; Rangel & Diniz-Filho 2005), even in circumstances where life-history characteristics have little influence over the demographic rates of their constituent species (Hubbell 2001; He 2005). Likewise, genetic-level variation in primary producers can cascade up through individuals to affect the functioning of whole ecosystems, including feedback loops to plant performance (Treseder & Vitousek 2001; Whitham et al. 2003). In consequence, understanding the determinants of biodiversity requires investigation of processes at a variety of spatial and temporal scales, and as a first step, the identification of the patterns which are the reflection, though sometimes beguiling, of these processes (Gaston & Blackburn 1999). Doing so is essential not only because of the insight into the natural world that such understanding brings, but also because it is only in this way that appropriate interventions can be recommended to slow the extraordinary impact humans are having on regional and global diversities (Brooks et al. 2002; Thomas et al. 2004; Balmford et al. 2005; Gaston 2005).
In the terrestrial ecosystems of the Antarctic (including the outlying sub-Antarctic islands), these impacts are smaller than they have been elsewhere. Humans first sighted the Antarctic Peninsula in 1820, with the first landing probably in 1821, and the first landing on East Antarctica (at Cape Adare) in 1895. Many of the sub-Antarctic islands have equally short human histories (Headland 1989; Chown et al. 2005). Early human impacts were mostly restricted to marine systems as a consequence of sealing and whaling (Knox 1994), with changes to the terrestrial environment being localized in their extent and nature. Now, the situation is quite different, and both the direct local and indirect influences of humans are increasing across the region. For example, invasive alien species have profoundly altered species assemblages and ecosystem functioning on most sub-Antarctic islands, and their direct effects are starting to be felt on the continent itself (Frenot et al. 2005), often in ways that are not immediately obvious (Kerry 1990; Wynn-Williams 1996; Hughes 2003). Indirect human influences include the long-range transport to and the presence of persistent organic and inorganic pollutants in Antarctic systems (Corsolini et al. 2002; Bargagli 2005; Dickhut et al. 2005), and the substantial alterations to terrestrial communities as a consequence of changing climates associated with global warming (Lewis Smith 1994; Bergstrom & Chown 1999; Walther et al. 2002; Convey 2003a). The significance of these impacts, and their scope for increase, given ongoing global change (Watson 2002) and growing human use of the Antarctic (Naveen et al. 2001; Frenot et al. 2005), have been recognized by the Committee for Environmental Protection of the Antarctic Treaty System, and by those nations that have responsibility for the sub-Antarctic islands (e.g. Anonymous 1996; McIntosh & Walton 2000). Both the requirements for conservation of Antarctic systems and the ways in which the probable impacts of increasing human travel to the Antarctic can be mitigated are major issues of political concern (http://www.cep.ats.aq/cep/). However, these issues can only be adequately addressed with a sound understanding of the spatial and temporal variability of Antarctic terrestrial biodiversity, the processes underlying it, and the ways in which humans are currently affecting Antarctic environments and are likely to do so in the future.
Antarctic terrestrial diversity lies at the low end of the global spectrum for many, if not most organisms (Convey 2001; Clarke 2003), food webs are typically simple (Block 1984, 1985, 1994; Burger 1985; Freckman & Virginia 1997; Wall & Virginia 1999), and life histories tend to be dominated by responses to a seasonally variable, ‘stressful’ environment (Lewis Smith 1984; Convey 1996a; Vernon et al. 1998). Moreover, very little of the largely ice-covered Antarctic continent (0.32% ice-free, British Antarctic survey 2004) is available to the terrestrial biota. Even in the areas that can be used, substantial spatial variation in abundance and occupancy exists (Janetschek 1970; Lewis Smith 1984; Kennedy 1993). Indeed, it has been clear ever since extensive work on Antarctic terrestrial systems commenced that they are highly variable through both time and space, and this theme continues to permeate recent work (Frati et al. 2001; Sinclair 2001; Hugo et al. 2004; Lawley et al. 2004). However, how and why this variation changes with spatial and temporal scales across the range of ecosystems and species found in the terrestrial Antarctic has perhaps been less well appreciated. This is partly due to the fact that wide recognition of the significance of scale is relatively recent, and partly because data collection (both in the past and in the present) has tended to focus on certain areas, species and scales. For example, while Antarctic terrestrial biodiversity and the biogeography thereof is thought to be well known, many ice-free areas have yet to be systematically explored and investigations of several areas are surprisingly recent (Broady & Weinstein 1998; Convey et al. 2000a,b; Marshall & Chown 2002; Stevens & Hogg 2002; Bargagli et al. 2004; Convey & McInnes 2005; Peat et al. 2007). Moreover, no comprehensive database of the distributions of Antarctic and sub-Antarctic species yet exists (see Griffiths et al. 2003 for a marine example), although several non-digital compilations are now becoming available (e.g. Pugh 1993; Bednarek-Ochyra et al. 2000; Øvstedal & Lewis Smith 2001; Pugh & Scott 2002; Pugh et al. 2002; Ochyra et al. in press). Likewise, quantitative ecological work was, until relatively recently, restricted largely to several maritime and sub-Antarctic Islands (see Block 1984; Lewis Smith 1984; Hänel & Chown 1999 for access to this literature), although early work had commenced, but has not been systematically continued, elsewhere (Janetschek 1967). In a similar vein, although more than 27 springtail and 60 mite species have been recorded from the Antarctic continent, comprehensive investigations of the autecology, life histories and environmental responses of these groups have, until recently, been restricted to just a few species, most notably the springtail Cryptopygus antarcticus and the mite Alaskozetes antarcticus (Block 1984; Block & Convey 1995; Convey 1996a).
Over the past several years, however, this useful early work has been integrated into a broader picture of variation across a variety of spatial and temporal scales in both the genealogical and ecological hierarchies (Eldredge 1986). Indeed, it is now clear that Antarctic terrestrial biodiversity, while certainly poor from a global perspective, is characterized by substantial variability across a range of spatial and temporal scales, and that, as is the case elsewhere, the significance of the mechanisms underlying this variability varies from scale to scale. Here, we review what is presently known of spatial and temporal variability in Antarctic and terrestrial biodiversity and the mechanisms underlying this variation. In doing so, we discuss the implications of these findings for understanding the evolution of this diversity, and for its conservation at a time when Antarctica and its surrounding islands are experiencing considerable regional variation in their responses to global environmental change and the extent of human interest therein.
2. Variation across space
Antarctic ice-free areas and the surrounding sub-Antarctic islands are isolated pockets of land in an area dominated by ice and ocean. Presently, of the Antarctic continent's 14 million km2, only approximately 0.32% is ice-free (British Antarctic survey 2004) and available for use by terrestrial organisms, although even these areas are not fully used owing to extremes of the local environments (Janetschek 1970; Kennedy 1993; Convey & Lewis Smith 1997; Frati et al. 2001; Sinclair & Sjursen 2001). Moreover, the geological and glaciological histories of these ice-free areas differ substantially, which is not surprising given the size of the continent. Thus, both local and regional environmental variation determines the spatial variation of current diversity across the continent (Peck et al. 2006). Similarly, between 30 and 60° S the ratio of land to water is 1 : 1, whereas across the same latitudes in the Northern Hemisphere, the ratio is 16 : 1 (Chown et al. 2004). The influence of the Southern Ocean on the sub-Antarctic islands and the Antarctic continent, by way of climate and isolation, is therefore considerable. The islands also differ markedly in their glaciological and geological histories, including the extent of current glaciation (LeMasurier & Thomson 1990; Hall 2002). Again, this multi-scale spatial variation in the environment has profoundly affected the distribution of diversity on the islands (Chown et al. 1998, 2002; Greve et al. 2005).
(a) Individual and population levels
Spatial variation in individuals is more typical of plants and fungi than that of animals given that the latter do not tend to form spatially extensive patches, at least not in terrestrial systems. Nonetheless, a form of individual spatial variation is found in migratory birds and mammals that have significantly different diets and physiologies in their breeding and wintering grounds and during the transitions between them (though such variation is temporal too; Bearhop et al. 2003; Landys-Ciannelli et al. 2003). In the Antarctic, several migratory species, including seabirds, whales and seals move in and out of the region on a seasonal basis. These are all typically pelagic species, with the greater sheathbill (Chionis alba) representing the only terrestrial one. Little is known about the change in ecology of individuals during movements of this species.
In plants, the most extensive spatial work has concerned individual- and population-level genetic variations of mosses, typically from Victoria Land and mostly using random-amplified polymorphic DNA (RAPD; reviewed by Skotnicki et al. 2000), which is not without methodological problems (Rogers 2007). In several species, substantial variation occurs within clumps, such that although paired shoots are typically (though not always) identical, the distance between shoots increases with the decrease in relatedness (Selkirk et al. 1998; Skotnicki et al. 1998a,b, 1999a,b, 2002, 2004; Dale et al. 1999). This within-clump variation has been attributed to high rates of somatic mutation. Most mosses on the continent reproduce vegetatively—sporophyte maturation is rare, but not absent, and increases northwards along the Peninsula and to the sub-Antarctic (Convey & Lewis Smith 1993; Convey 1994; Lewis Smith & Convey 2002). Skotnicki et al. (2002) have noted that shoot tips growing even a few millimetres apart within a clump are separated by many years of growth and considerable UV-B exposure, which seems a probable cause of elevated levels of somatic mutation characteristic of all of the mosses examined to date, given the known effects of UV-B radiation on mutagenesis (Ries et al. 2000, but see also Robinson et al. 2003). As a consequence of this somatic mutagenesis, genetic variability in Antarctic mosses is equal to or higher than that of sexually reproducing species found elsewhere (Skotnicki et al. 2000, 2004). Antarctic mosses may therefore provide an excellent model system for examining the importance of UV-B-associated somatic mutation for diversity in plants, although additional work is required to verify the extent of and reasons for this mutational change (Rogers 2007).
The significance of exploiting this model system might not seem obvious given that enhanced ultraviolet radiation typically has small physiological effects on Antarctic plants, and the plants also appear to be capable of rapid responses to changes in UV radiation (Huiskes et al. 2000; Newsham et al. 2002; Karentz 2003; Lud et al. 2003; Robinson et al. 2003). However, it has been suggested that ultraviolet-induced mutations and variation in the extent of UV radiation globally might be a cause of variation in speciation rates (Evans & Gaston 2005): one explanation for the global variation in species richness (the evolutionary-rates hypothesis; Rohde 1992; Willig et al. 2003). That both animals (Orme et al. 2005) and plants (Linder 2003) often show high local diversities in areas that have both high-incident UV and high water availability, but are not necessarily tropical (e.g. southwestern South Africa and Australia; high elevation areas in South America and Asia), perhaps deserves further exploration, especially given the evidence for UV effects on mutation rates in work on Antarctic species.
Genetic differences among moss populations have typically formed a major component of the RAPD analyses which have been undertaken. Variation among clumps varies significantly with quality of habitat; those areas with greater water supply typically have greater genetic variability (Skotnicki et al. 2000), though methodological issues might confound these conclusions. Patterns of variation also suggest substantially different colonization histories, with some sites showing evidence of multiple colonization events and little subsequent spread, and others indicating a single colonization and either substantial subsequent spread or pronounced antiquity of the population (Selkirk et al. 1997). The population-level analyses have also led these authors to suggest that long-distance dispersal is not uncommon, though the very fact that long-distance migrants can be detected suggests that the events might be rare (Rogers 2007). Wind is reputedly the primary agent responsible for dispersal over significant distances (Skotnicki et al. 1998b, 2001), while overland water flow during periods of elevated temperatures, when melt water is available, is responsible for local dispersal (Skotnicki et al. 2000).
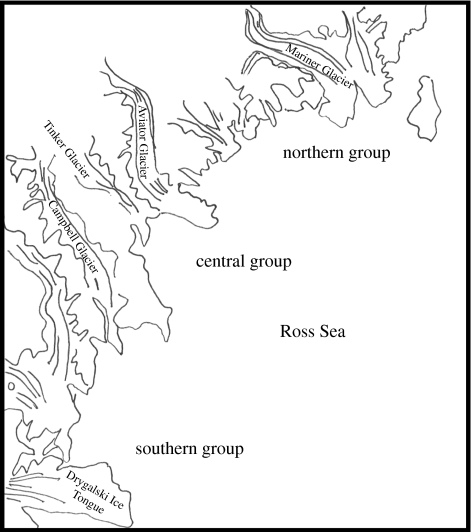
Substantial differentiation of populations even at fine spatial scales has also been found in the few studies that have examined springtail genetic diversity. Allozyme investigations have revealed significant differentiation of Gressittacantha terranova associated with major glacial barriers in Victoria Land (Fanciulli et al. 2001; figure 1). In both this species and Isotoma klovstadi (assessed using the mitochondrial cytochrome c oxidase subunit II gene), differences among populations are substantial. Although gene flow might have been higher in the past in I. klovstadi, it is now much reduced and dispersal rates in the population are very low (Frati et al. 2001). In Gomphiocephalus hodgsoni, another springtail species from Victoria Land, genetic isolation by distance is substantial, and extensive Pleistocene glaciations and limited dispersal capability have promoted isolation and divergence among its fragmented populations, which must once have been more widespread (though one recent dispersal event, by birds or humans, has taken place; Stevens & Hogg 2003). Low dispersal of springtails across what are essentially water-free landscapes (either ice or the dry terrestrial environments of the Dry Valleys and elsewhere) is not surprising given their limited desiccation resistance and tolerance (Harrisson et al. 1991; Hopkin 1997). However, dispersal by water is more probable (Coulson et al. 2002), but has not been investigated in Antarctic springtails other than on a very local scale (Hayward et al. 2004). The low tolerance of desiccation by springtails sets them apart from many other Antarctic organisms for which wind is a significant agent of dispersal (Marshall 1996, 1997; Marshall & Chalmers 1997; Marshall & Convey 1997; Muñoz et al. 2004).
Figure 1.
Division of populations of G. terranovae into northern, central and southern populations in Victoria Land on the basis of allozyme data. Adapted with permission from Fanciulli et al. (2001).
Population genetic structure of indigenous sub-Antarctic springtail species is quite different from that of the Antarctic endemics. On Marion Island, indigenous species tend to show little isolation by distance, but rather structure indicative of recent expansion from glacial refugia (Myburgh et al. 2007). These species also show evidence of a more ancient pattern of population differentiation which in C. a. travei suggests that it colonized the islands shortly after they became subaerial some 500 000 years ago (McDougall et al. 2001). The same rapid colonization of the Prince Edward Islands (of which Marion is one) seems to have taken place in at least one of the species of indigenous ectemnorhine weevils (Grobler et al. 2006), although the absence of a comparison with this species' closest putative ancestor means that the date of colonization must remain speculative. By contrast, in more recent invasive species, haplotype diversity is minimal, indicating a single colonization event. Analysis of the COI gene of many individuals of Isotomurus cf. palustris and Ceratophysella denticulata from across the island revealed that each species was represented by a single haplotype (Myburgh et al. 2007). Similarly, in the carabid beetle Trechisibus antarcticus, a relatively recent invader of South Georgia, apparently from the Falkland Islands, rare alleles are absent in the former population (Ernsting et al. 1995).
Variation among Antarctic populations is not restricted to the genetic level, as might be expected from knowledge of widespread species elsewhere (Spicer & Gaston 1999). Reproductive characteristics show substantial variation, with the extent of successful sexual reproduction in bryophytes declining with increasing latitude, though the signal can be spatially complex owing to variation in microclimates (Convey & Lewis Smith 1993; Convey 1996a; Lewis Smith & Convey 2002). The proportion of species (as distinct from individuals) occasionally recorded with sporophytes actually increases at higher latitudes (Lewis Smith & Convey 2002). Investment in reproduction in the mite A. antarcticus is also greater in northern (sub-Antarctic) than in southern (maritime Antarctic) populations, but little variation is found among the latter (Convey 1998). Physiological inertia has also been documented in the host-specific lepidopteran Embryonopsis halticella in the sub-Antarctic. Larvae on Marion Island and on Heard Island (separated by more than 1000 km) have statistically indistinguishable freezing points (−17 to −20°C), upper lethal temperatures (approx. 38°C) and survival times of dry conditions (Klok & Chown 2005). It is thought that larval physiological tolerances may have evolved as a response to cold dry conditions on the older Heard Island and have subsequently been retained in populations that colonized the younger Marion Island, which has a milder climate. However, not all groups show such inertia. For example, significant variation in critical thermal minima is found among populations of the freeze intolerant weevil Palirhoeus eatoni inhabiting Heard and Marion Islands, with populations on the former having lower values (Klok & Chown 2003).
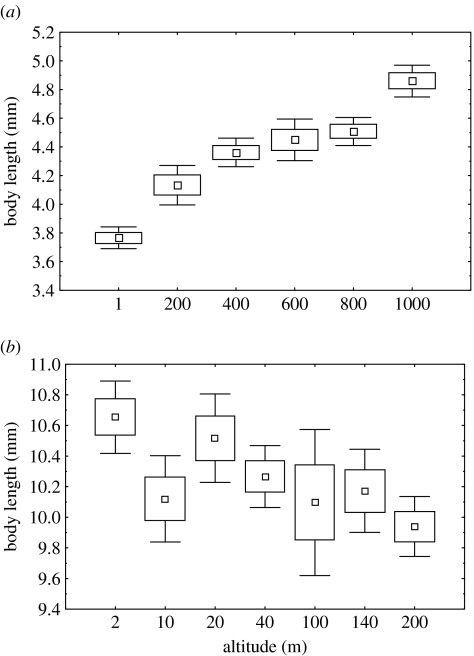
Complex, among-population, altitudinal differences in body size of invertebrates have also been recorded in the region. On Marion Island, body size increases with altitude in all of the eurytopic weevil species (figure 2a), while on Heard Island the opposite pattern has been found (figure 2b; Chown & Klok 2003). Opposing latitudinal and altitudinal size clines have frequently been recorded in insects (Chown & Gaston 1999; Blanckenhorn & Demont 2004; Kozłowski et al. 2004), and can be explained in an adaptive context of body size optimization given seasonal constraints on growth and development and life cycle duration (Roff 1980; Kozłowski et al. 2004). Differences in seasonality of Heard and Marion Islands, which lie below and above the Antarctic Polar Frontal Zone, respectively, are thought to be a major reason for the differences in size clines (Chown & Klok 2003).
Figure 2.
Body length variation across an altitudinal gradient in (a) Bothrometopus parvulus at Marion Island and (b) Ectemnorhinus viridis at Heard Island. Data are presented as mean, s.e. and 95% CI. Adapted with permission from Chown & Klok (2003).
(b) Species level
How long particular species have occurred in Antarctica, or on the sub-Antarctic islands, and what determines species incidences and abundances, are questions that have long occupied ecologists and systematists working in the region (Skottsberg 1960; Gressitt 1965, 1971; Janetschek 1967, 1970; Lewis Smith 1984). Very low species endemism in some groups, particularly the mosses (Bednarek-Ochyra et al. 2000; Peat et al. 2007; Ochyra et al. in press), combined with substantially more extensive glaciation of the Antarctic during the last glacial maximum than at present (Peck et al. 2006), has encouraged the view that the majority of Antarctic species are relatively recent arrivals, with perhaps a few microbial or protozoan taxa being substantially older. However, recent work has altered this perspective. It now seems that mosses may have an atypical pattern of endemism compared with most other major groups of Antarctic flora and fauna, and that the generalization of an assumption of recent origin may be little more than dogma. In the continental Antarctic, it is clear that several areas (e.g. parts of the Victoria Land Dry Valleys, Transantarctic Mountains, some inland nunatak groups) have remained ice-free since at least the end of the Miocene (Boyer 1979; Prentice et al. 1993). Careful reassessment of some continental Antarctic mite distributions has indicated that the majority of species are probably pre-Pleistocene endemics, and that speciation in some groups, such as the endemic oribatid genus Maudheimia, is in keeping with models of the development of the East Antarctic ice sheet (Marshall & Pugh 1996; Marshall & Coetzee 2000). The West Antarctic remains problematic, because the large majority of biota are present in coastal and low-altitude locations, which glacial and ice sheet reconstructions indicate would have been obliterated at glacial maximum by ice sheets extending out to the point of continental shelf drop-off. Thus, the existence and location of potential refuge regions remain hypothetical (Convey 2003a). Nevertheless, evidence is increasing for the presence of an ancient and vicariant biota, such as species of endemic midges (Diptera: Chironomidae) whose age of evolutionary separation has been estimated using a ‘molecular clock’ approach at 20–40 Myr, in keeping with the opening of the Drake Passage (Allegrucci et al. 2006). It is also clear that many Antarctic nematodes are endemic to either the continental or maritime Antarctic, strongly suggesting that they are glacial survivors rather than post-glacial colonists (Andrássy 1998; Maslen & Convey 2006). Recent work on springtails has demonstrated ancient (11–21 Myr) divergence associated with glaciation of Antarctica, but much younger colonization events among sub-Antarctic islands (less than 2 Myr; Stevens et al. 2006).
For small organisms, such as microbes and ciliates, it has been suggested that global diversity is low because substantial global dispersal of the propagules of these species hampers isolation and consequently speciation (Fenchel 1993; Finlay et al. 1996; Wilkinson 2001; Finlay 2002). In other words, most species in these groups should be virtually cosmopolitan, and should include the Antarctic within their ranges, with their distribution limited by their ecophysiological abilities to survive the challenges of the Antarctic environment, rather than by the process of dispersal per se. However, modern investigations of both prokaryote and eukaryote diversities in Antarctica provide little support for the idea, given low relatedness of Antarctic taxa to those in existing sequence databases (Franzmann 1996; Lawley et al. 2004). Although Vincent (2000) cautioned that lack of sequence data from elsewhere and insufficient taxonomic resolution might compromise conclusions of substantial endemicity in Antarctica, other taxa show similar levels of endemicity (see above). Work in temperate regions is also starting to show that the ecological biogeography of microbes and other small organisms is similar to that of the macrobiota (Green et al. 2004; Horner-Devine et al. 2004; Smith et al. 2005).
Endemism can also be high across the sub-Antarctic, although this differs among taxa. In vagile taxa, such as the bryophytes and lichens, endemism tends to be low (acknowledging that further survey and taxonomic work might change the situation; Øvstedal & Gremmen 2001; Muñoz et al. 2004), while in insects, it is more variable. In islands close to continental or other landmasses genera and sometimes species are shared with what appears to be the source areas (Kuschel 1971; Vogel 1985; Marris 2000), while in more distant archipelagos, endemism is high and relationships are enigmatic. Indeed, the origin and closest relatives of many of the insect taxa from the South Indian Ocean Province Islands (Prince Edward Islands, Crozet Islands, Kerguelen Islands, Heard and McDonald Islands) remain a subject of vigorous contention, as they have been since the discovery of these species (see Jeannel 1964 for early discussion and Greve et al. 2005 for recent partial review).
Although regional species pools and biogeographic history might determine what species can potentially occur at a given site, actual occurrence is determined by site suitability. Investigations of site suitability at the species level (e.g. incidence functions across a range of habitable patches; Ovaskainen & Hanski 2003) are not typical of the Antarctic literature (but see Usher & Booth 1984, 1986). Nonetheless, the determinants of local scale occupancy and abundance have been extensively investigated across a range of sites. Water availability, temperature (which also influences water availability), protection from wind, the availability of nutrients (often nitrogen and also carbon—many continental Antarctic systems are poor in carbon), the extent of lateral water movement, and the extent of soil movement and ice formation all have a pronounced effect on the suitability of sites for colonization, growth and reproduction (Janetschek 1970; Lewis Smith 1984; Ryan & Watkins 1989; Kennedy 1993; Convey 1996a; Freckman & Virginia 1997; Convey et al. 2000a; Sinclair 2001; Smith et al. 2001). Of these, water availability (and the elevated temperatures that drive it) is thought to be most significant on the Antarctic continent and peninsula, while nutrient availability, soil water movement and temperature are most significant in the sub-Antarctic. Most authors have concluded that, at least on the continent, extreme abiotic conditions preclude life in many ice-free areas and that, unlike the situation across most of the planet, abiotic rather than biotic stressors exert a controlling influence on life histories (Janetschek 1970; Convey 1996a; Wall & Virginia 1999).
If spatially aggregated, suitable abiotic conditions are major drivers of the incidence and abundance of many Antarctic organisms, it might be expected that a few key environmental and spatial terms would explain a large proportion of the variance in incidence or abundance (depending on the scale of the study; Usher & Booth 1986). Such an approach has rarely been applied to modelling the abundance and distribution of Antarctic species (most investigations focus on the assemblage level). Where this has been done, spatial structure has typically not been considered despite wide acceptance of its importance in ecology (Legendre 1993; Thomson et al. 1996; Liebhold & Gurevitch 2002). Notable exceptions are the studies by Usher & Booth (1984, 1986), which appear to have been limited only by the availability of appropriate analytical techniques. Other Antarctic studies have either examined the relationship between abundances and environmental variables directly (Freckman & Virginia 1997; Courtright et al. 2001; Sinclair & Sjursen 2001), or have sought to assess the habitat specificity and fidelity of species without explicit reference to underlying environmental variables (Mercer et al. 2000; Barendse et al. 2002). However, in a recent investigation of the abundance structure of several arthropod species at Cape Hallett (Northern Victoria Land; Sinclair et al. 2006), spatially explicit analytical methods were used. Although temperature and chlorophyll-a availability were not included as variables in the models (despite their importance in determining arthropod abundance; Sinclair & Sjursen 2001), the models including spatial and environmental terms explained between 60 and 86% of the variation in abundance of each of three springtail species (table 1), which is high by comparison with invertebrates elsewhere (Brewer & Gaston 2002; McGeoch & Price 2004). Soil properties (excluding assessments of spatial pattern) explain 40–50% of spatial abundance variation in nematodes in Taylor Valley (Powers et al. 1998). Therefore, it appears that spatial and environmental variables do explain much of the variation in the abundance of individual species. However, explanatory power depends on both the scale of investigation and the taxa considered (Usher & Booth 1986). Further, explicit modelling work is required to determine whether the abundance structure of Antarctic arthropods can be more readily explained by a few environmental and spatial variables than is the case for species from more temperate environments.
Table 1.
Variance partitioning (Legendre & Legendre 1998) from generalized linear models of the effects of environmental and spatial variables on the abundance of springtails at Cape Hallett, Northern Victoria Land. Values are percentage deviance explained (Sinclair et al. 2006).
| model | space | spatially structured environmental | environmental | unexplained |
|---|---|---|---|---|
| soil | ||||
| Cryptopygus cisantarcticus | 56.3 | 4.1 | 19.6 | 20.0 |
| Isotoma klovstadi | 39.4 | 27.3 | 16.5 | 16.8 |
| stones | ||||
| Cryptopygus cisantarcticus | 8.4 | 19.2 | 33.9 | 38.5 |
| Friesea grisea | 16.4 | 17.8 | 51.1 | 14.7 |
| Isotoma klovstadi | 39.0 | 14.5 | 0.5 | 54.0 |
| Stereotydeus belli | 0.0 | 0.0 | 40.1 | 59.9 |
| Tydeus setsukoae | 11.8 | 15.7 | 16.3 | 56.2 |
Qualitative work on species incidences has mostly focused on determinants of habitat specificity. In the sub-Antarctic, it appears that species occupying the epilithic biotope (fellfield, coastal rockfaces) have greater habitat specificity and have occurred in these habitats for much longer, surviving glaciations in epilithic refugia, than species from the typically post-glacial vegetated biotope (Chown 1990a, 1994; Barendse et al. 2002). A global analysis of habitat specificity in ameronothroid mites has revealed a similar pattern in south and north polar species (Marshall & Convey 2004). Habitat specificity tends to decline towards the poles. In the Antarctic region, species such as A. antarcticus and Halozetes belgicae occupy a range of terrestrial habitats at high latitudes, but are almost exclusively supralittoral in the sub-Antarctic. The most plausible explanation for these patterns is recent post-glacial colonization of the ice-free areas (Marshall & Convey 2004). Phylogeographic analysis (Avise et al. 1987) across a range of sites could be used to test these ideas, but to date such work is rare in the Antarctic region (§2a).
(c) Assemblage and ecosystem levels
Unlike Antarctic marine systems (Clarke & Johnston 2003), and terrestrial environments elsewhere, the terrestrial ecosystems of the continental and maritime Antarctic are species poor (Convey 2001). Some sites are characterized by less than a dozen invertebrate and plant species, in others, life is restricted to depauperate endolithic communities in sandstone rocks or gypsum crusts, and at the limit many ice-free areas seem totally devoid of life (Broady & Weinstein 1998; Wall & Virginia 1999; Hughes & Lawley 2003; Cockell & Stokes 2004; Convey & McInnes 2005). Nonetheless, substantial spatial complexity in the richness and identity of species is found across Antarctica. At a large scale, species richness increases with a decline in latitude (Block 1984; Broady 1996; Smith 1996), but the pattern is spatially complex rather than monotonic (Clarke 2003). For example, in the maritime Antarctic, soil eukaryote microbial diversity is as high at approximately 71–72° S as it is at approximately 60–67° S, and only declines steeply beyond 74° S (Lawley et al. 2004). Likewise, in West Antarctic Alexander Island and East Antarctic Dronning Maud Land, the metazoan microfauna shows evidence of a complex pattern of richness across ice-free areas (Sohlenius et al. 1996; Sohlenius & Boström 2005; Maslen & Convey 2006). Similar patterns of complexity are emerging for mosses and lichens (Clarke 2003; Peat et al. 2007), as taxonomic resolution and sampling coverage improves. Complex spatial variation in richness is not unexpected, given that it is characteristic of life elsewhere on the planet (Gaston 2000). However, owing to relatively poor sampling across the ice-free areas of the Antarctic continent, it is not possible to discern whether any systematic trends characterize this large-scale richness variation. Therefore, the identification of any underlying mechanisms (beyond local scale factors influencing site suitability, see §2b above) is still some way off.
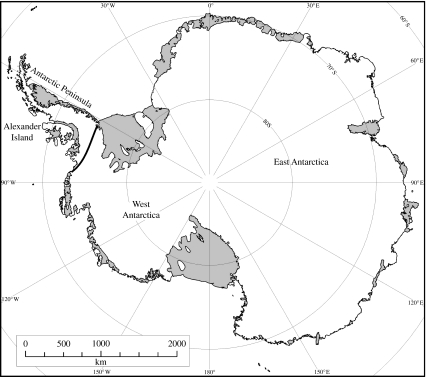
Early biogeographic work divided the Antarctic region into continental and maritime Antarctic, and sub-Antarctic (reviewed in Lewis Smith 1984). Recent work has identified a much clearer distinction between the Antarctic Peninsula and the remainder of continental Antarctica than previously recognized. Indeed, there is a particularly striking lack of overlap at species level between the representatives of several higher-order taxonomic groups. Thus, the most recent taxonomic treatments indicate that there is no overlap at species level between these regions for nematodes (Andrássy 1998) and free-living mites (Pugh 1993), and that only a single springtail species is shared (Greenslade 1995). Other groups, such as tardigrades, show an intermediate level of species overlap (approx. 50%; Convey & McInnes 2005), while bryophytes, as mentioned earlier, appear to show a completely different pattern of biodiversity, with very few (less than five species) or possibly even no endemism across the entire continent. Although the Antarctic terrestrial biota are individually less spectacular, the strength of this separation between the two regions is reminiscent of the much better known ‘Wallace Line’ of Southeast Asia (Brown & Lomolino 1998), and is likewise indicative of ancient and different evolutionary origins of their biota. Such is its biogeographic significance that we propose the adoption of a similarly named feature, the Gressitt Line, to emphasize the boundary between these two regions. It is named in recognition of the fundamental contribution of J. L. Gressitt to the early development of understanding of Antarctic and Southern Ocean terrestrial biogeography during the 1960s and 1970s (figure 3). Although, as with much of Antarctica, there remain many specific unsurveyed locations in the region of this boundary, it lies (i) south of Alexander Island and the English and Bryan Coasts, whose biota includes maritime rather than continental affinities (Peat et al. 2007), (ii) north of the inland Ellsworth Land nunatak ranges (Sky Hi and Sweeney Nunataks, Hauberg Mountains), whose fauna has continental affinities (Convey & McInnes 2005), and (iii) on the east coast of the Antarctic Peninsula south of the Wakefield Mountains, whose very limited known mite fauna is maritime in origin (P. Convey & P. J. A. Pugh 2005, unpublished data).
Figure 3.
Map of the ‘Gressitt Line’, a strong biogeographic region of separation between the biota of the Antarctic Peninsula and continental Antarctica.
By comparison with the continental and maritime Antarctic, the Southern Ocean islands are species rich and well surveyed, at least for taxa such as insects and vascular plants (Vernon & Voisin 1990; Dreux & Voisin 1993; Chown et al. 1998, 2006; Gremmen & Smith 1999; Frenot et al. 2001; Jones et al. 2003). Moreover, patterns in species richness and the mechanisms underlying these patterns have been comprehensively investigated (Chown et al. 1998, 2001, 2005; Greve et al. 2005). Across the islands, species richness of indigenous and alien vascular plants covaries significantly with available energy and the same is true of indigenous and alien insect species richness. The richness of alien insects and vascular plants also covaries with human visitor frequency to the islands. Positive relationships between energy availability and species richness have been documented in many systems and at a wide variety of spatial scales (Waide et al. 1999; Hawkins et al. 2003), and are considered one of only a few ecological laws (Rosenzweig 1995). However, the mechanisms underlying positive species–energy relationships remain contentious (Evans & Gaston 2005; Evans et al. 2005), as do explanations for spatial variation in species richness generally (Willig et al. 2003), despite the antiquity of the pattern. Current data for the Southern Ocean islands preclude identification of a mechanism underlying these positive species–energy relationships. Nonetheless, that strong positive relationships exist for both alien and indigenous species on islands which are characterized by relatively high levels of endemicity provides some insight into probable mechanisms.
The species richness of an area is determined by four processes: speciation; extinction; immigration; and emigration. For isolated islands such as those of the Southern Ocean, and in indigenous taxa such as vascular plants and insects, immigration has probably been low owing to the considerable distance of the islands from source areas (Chown et al. 1998, but see also Greenslade et al. 1999). Moreover, high endemicity (Chown 1990a; Greve et al. 2005) indicates substantial in situ speciation. Little data exist for emigration and extinction (but see Convey et al. (2000b) for examples of proposed short-term colonization and then extinction associated with ephemeral geothermally active locations on the maritime Antarctic South Sandwich Islands). Indigenous species are often found in the aerial plankton (Marshall & Convey 1997; Peck et al. 2006) and palynological analyses of interglacial peat lenses and lake sediments indicate the presence of taxa that are now absent (Scott & Hall 1983; Barnes et al. 2006). Thus, for indigenous taxa, mechanisms that both generate species and cause their removal, and which are related to energy variation, are likely to be responsible for the positive relationship between richness and energy availability. These mechanisms include enhanced evolutionary rates (Rohde 1992; Evans & Gaston 2005) and reduced extinction rates as a consequence of increased population size (Chown & Gaston 2000; Evans et al. 2005) under conditions of higher energy availability. However, it is difficult to envisage any mechanism associated with elevated speciation rates causing the relationship between alien species richness and energy availability. After all, the alien species have immigrated to the islands as a consequence of direct or indirect human intervention (Chown et al. 1998; Frenot et al. 2005). Thus, only two mechanisms and their interaction can explain a positive alien species–energy relationship: the ecological processes that enable large numbers of species to coexist (Gaston 2000; Evans et al. 2005), and the historical processes that have meant enhanced propagule pressure as a consequence of high visitor frequency (Lonsdale 1999; McKinney 2002). Both sets of processes are largely to do with the ways in which increases in abundance reduce the chances of extinction typically associated with small populations. Therefore, it would seem that, at least in alien species, positive species–energy relationships are established rapidly owing to spatial variation in extinction rates associated with spatial variation in energy availability. Indeed, it is clear that the majority of the phases of invasion identified for plants (Richardson & van Wilgen 2004) typically are to do with avoidance of rarity and extinction. The sole exception is arrival, which is associated with transport success and the survival of individuals during the process.
Ultimately, variation in extinction rates cannot be the only process determining spatial variation in richness for all species: new species must arise at some point if richness is not to show a long-term temporal decline (which is what happens in neutral models with no speciation or immigration from the metacommunity; Hubbell 2001). However, the rapid development of alien richness–energy relationships (most introductions to the Southern Ocean islands have taken place over the past 200 years; Gaston et al. 2003; Chown et al. 2005) suggests that spatial variation in speciation rate need not be spatially concordant with variation in richness, but might show a more complex pattern. By contrast, spatial variation in extinction rate should follow richness more closely.
Richness is only one property of assemblages, others being the subdivision of individuals and biomass among each of the species found in the assemblages, the body size and range size of each of the species, and the trophic interactions among the species and their constituent individuals (Blackburn & Gaston 2001b; Bell 2003; Cohen et al. 2003, 2005). Composite assemblage properties and their underlying correlates have been examined for a wide range of Antarctic and sub-Antarctic ecosystems, typically using multivariate techniques, and spatial variation in the abundances of individual species has also been investigated across a range of sites (Goddard 1979; Block 1982; Smith & French 1988; Ryan & Watkins 1989; Courtright et al. 2001; Gabriel et al. 2001; Sinclair & Sjursen 2001). Assemblage properties that are the stuff of modern macroecological analyses have enjoyed less attention, despite the fact that these species-poor systems lend themselves to this kind of analysis. For example, Gaston et al. (2001) provided the most complete animal species–body size frequency distribution for any system globally by compiling and analysing these data for the well-surveyed Marion Island. Likewise, although early work was concerned with food webs in the Antarctic, largely as a consequence of the International Biosphere Programme (Block 1984, 1985), little subsequent work has taken place (though see Wall & Virginia 1999). Nonetheless, Antarctic systems are remarkable from a food web perspective because predation and parasitism are either low or non-existent, herbivory is uncommon, and biological invasions, which are being documented more frequently across the Antarctic (Frenot et al. 2005), have probably significantly altered food web structure, leading to trophic cascades (Crafford & Scholtz 1987; Bergstrom & Chown 1999).
3. Variation through time
Over geological time, biodiversity in Antarctica and on the sub-Antarctic islands has varied considerably. The continent was once home to a diverse flora (Quilty 1990) and a fauna that included dinosaurs (Hammer & Hickerson 1994), the earliest representatives of the globe's modern avifauna (Clarke et al. 2005), and presumably a wide variety of insects (Ashworth & Kuschel 2003). Likewise, extensive fossil floras characterized the oldest of the sub-Antarctic islands on the Kerguelen Plateau (Chastain 1958; Quilty & Wheller 2000). Over shorter time-scales, palynological evidence has demonstrated compositional change in the floras of many sites (Scott 1985), and lake sediment cores have also revealed substantial variation in the abundances of terrestrial and freshwater invertebrates such as mites and crustaceans (Cromer et al. 2006; Hodgson & Convey 2006). Interest in temporal variation in Antarctic terrestrial systems was initially focused on seasonal changes in the abundances, phenology and life histories of plants and animals, and on successional changes in plant communities (reviewed in Block 1984; Lewis Smith 1984; Convey 1996a). More recently, the realized and probable impacts of global environmental change have come to dominate research interest (Smith & Steenkamp 1990; Lewis Smith 1994; Block & Harrison 1995; Kennedy 1995a; Bergstrom & Chown 1999; Walther et al. 2002; Convey 2003a; Robinson et al. 2003), especially owing to the rapid rates of climate change along parts of the Antarctic Peninsula and at the sub-Antarctic islands (King et al. 2003), increasing numbers of biological invasions (Frenot et al. 2005), and the rise in human traffic to the region (Naveen et al. 2001). Nonetheless, temporal variation in diversity has been documented across a broad range of scales in the Antarctic region.
(a) Individual level
Individuals vary across a range of temporal scales. Various physiological, life-history and morphological traits (all form part of biodiversity; Spicer & Gaston 1999; Roy et al. 2001) change with ontogeny, ageing, nutrient availability, diurnal cycle and season. Such change is often discussed under the rubric of phenotypic plasticity, and the literature on this topic is large (West-Eberhard 2003; De Witt & Scheiner 2004; Chown & Terblanche 2007). Much of it is concerned with the contentious question of what conditions should promote adaptive phenotypic plasticity (De Witt & Scheiner 2004; Chown & Terblanche 2007). To some extent, the controversy has been fuelled by the difficulty of establishing a direct link between trait variation and fitness (Feder 1987; Angilletta et al. 2002), and by the absence of critical hypothesis testing (Huey & Berrigan 1996). Moreover, it has not always been appreciated that phenotypic plasticity (or its absence) in a given trait must be seen in the context of the character complex of which the trait is a part, and the overall contribution of the trait to fitness (Woods & Harrison 2002; Pigliucci 2003).
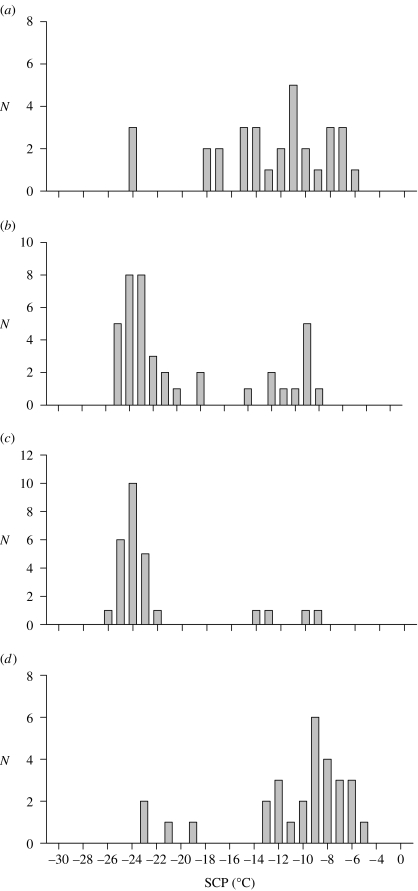
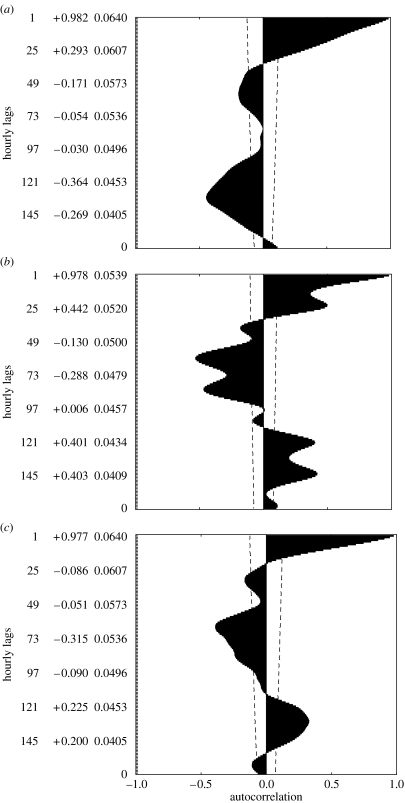
Antarctic plants and animals are typically characterized as having ‘very flexible’ life histories (Convey 1996a), which are thought to enable them to survive a low temperature, dry (at least on the continent) and seasonally variable environment. Seasonal variation in a wide range of physiological traits has been investigated in several species of plants and animals (Sømme & Block 1982; Convey 1996a; Convey et al. 2003; Slabber & Chown 2005). However, recent work has also demonstrated that responses to environmental variation can be even more rapid. For example, supercooling point (or freezing point) can vary within a matter of hours in response to decreasing or increasing temperatures in the springtail C. antarcticus and in the mites A. antarcticus and H. belgicae, although the mechanisms underlying such rapid responses have yet to be fully elucidated (Worland & Convey 2001). Similar responses have since been found in the East Antarctic springtail species Isotoma klovstadi and Cryptopygus cisantarcticus (but not in Friesea grisea; Sinclair et al. 2003a). Short-term variation in the moulting status of a springtail also has a dramatic effect on its freezing point, as has been demonstrated in population-level analyses of the indigenous Tullbergia antarctica (Worland 2005) and the invasive species Ceratophysella denticulata on sub-Antarctic Marion Island (figure 4; Worland et al. 2006). Moulting effects on supercooling points mean that previously adaptive interpretations of supercooling point variation might have to be reinterpreted. Rapid responses have also been reported in field studies of pigment biochemistry of species of moss and liverwort on maritime Antarctic Adelaide Island. Non-manipulated plants, exposed to naturally varying levels of ultraviolet B radiation associated with the dynamically changing depth of the Antarctic ozone hole, showed rapid changes in the concentrations of some screening pigments that were best correlated with the levels of UV-B experienced within the previous 24 h rather than any longer period (Newsham et al. 2002; Newsham 2003). It is recognized that any such rapid biochemical and ecophysiological responses carry clear implications in terms of the diversion or trade-off of energy resources, an important factor determining life-history strategies (Convey 1996a, 1998, 2003a), but to date little or no effort has been directed towards quantifying these shifts in energy budgets.
Figure 4.
Supercooling point (SCP) distributions in the springtail C. denticulata from (a) an ‘arbitrary’ field sample, (b) pre-moulting animals from the same main sample, (c) recently moulted animals and (d) recently moulted animals that had been fed for 1 day (10°C). Note the decline in SCP with moulting. Adapted with permission from Worland et al. (2006).
Antarctic terrestrial organisms are not only thought to be flexible in their strategies, but also appear to be especially capable of responding to unpredictable environmental variation (Convey 1996a). Unpredictability over short time-scales is thought to be a major feature of Antarctic terrestrial environments (Peck et al. 2006). Rapid responses in supercooling points of Antarctic springtails are clearly one form of response to this unpredictability. Another is the preponderance of freezing tolerance (i.e. the capability of surviving extracellular and perhaps partial intracellular ice-formation; Sinclair et al. 2003b) in sub-Antarctic insects (excluding springtails). The large majority of the species that have been investigated are not only able to survive freezing, but do so typically only to a few degrees below their freezing points, making them moderately freezing tolerant (Klok & Chown 1997; Van der Merwe et al. 1997). Subsequent work has demonstrated that this is true of Southern Hemisphere insects in general, by comparison with their freeze intolerant Northern Hemisphere counterparts (Sinclair et al. 2003c; Sinclair & Chown 2005a), though the complexities of phylogenetic inertia and key innovations in certain groups require further investigation. In much of the temperate to cold Southern Hemisphere, winter temperatures fluctuate around 0°C, the freezing point of water. Thus, insects have to be capable of responding to rapid and unpredictable freezing events. Freeze intolerance typically requires substantial preparation by individual insects, including gut clearance and the production of various anti-freeze compounds (Chown & Nicolson 2004). Substantial preparatory change is also typical of strong freezing tolerance (where death takes place tens of degree below the freezing point), though some Antarctic nematodes can do this with apparent ease under laboratory conditions (Convey & Worland 2000). By contrast, moderate freezing tolerance does not appear to require substantial preparatory change and ice nucleation from gut material would have no deleterious effects. Thus, insects can simply freeze during cold snaps (which are typically not severe) and then return to their normal feeding activities (though see also Sinclair & Chown 2005b). Selection to survive an environment that fluctuates around 0°C either for most of winter or for much of the year appears to have favoured moderate freezing tolerance (Sinclair & Chown 2005a). Moreover, in many species from the sub-Antarctic islands, seasonal variation in cold hardiness strategy is not nearly as marked as it is elsewhere, in keeping with a strategy tuned to coping with an unpredictable environment.
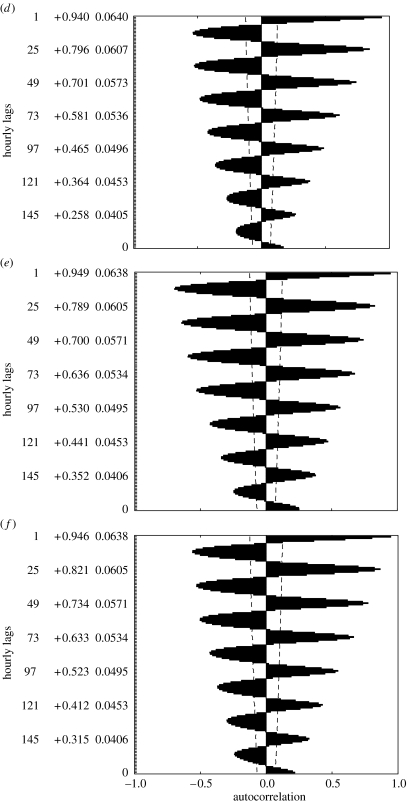
Environmental unpredictability (i.e. creating the risk of inappropriate responses to misleading environmental cues) is also thought to be a primary reason why phenotypic plasticity in some traits might not be favoured (Chown & Terblanche 2007), and has been suggested as underlying the absence of ‘true’ diapause within the life cycles of Antarctic invertebrates (Convey 1996b). This certainly seems to be the case for several traits in mites and insects on sub-Antarctic Marion Island. By comparison with more temperate sites, temperatures at Marion Island are highly unpredictable from day-to-day (figure 5). This seems to be a major reason why acclimation responses in many traits are virtually non-existent, including locomotion traits and supercooling points in amerononthroid mites (Deere & Chown 2006; Deere et al. 2006), supercooling points in a flightless moth (Sinclair & Chown 2003) and rapid cold hardening in a kelp fly (Terblanche et al. 2007). However, responses to acclimation are present in other traits, notably critical thermal minima in beetles, and this trait and supercooling points in another flightless moth species (Klok & Chown 1998, 2003), and several thermal tolerance traits in springtails (Slabber et al. 2007). Unfortunately, it is not yet known why traits vary in their response to acclimation, although some difference between indigenous and invasive species exists. However, studies elsewhere have demonstrated that even closely related tolerance traits can be controlled by different genes and respond quite differently to acclimation or selection (reviewed in Hoffmann et al. 2003; Chown & Nicolson 2004). Unravelling the relationships between environmental predictability, trait variation and fitness would be a useful avenue for future terrestrial research in the Antarctic (Peck et al. 2006).
Figure 5.
Autocorrelation plots for hourly microclimate temperatures recorded on Marion Island during (a) August 2002, (b) November 2002 and (c) June 2003, and for (d) August 2002, (e) November 2002 and (f) June 2003, for Lambert's Bay, South Africa. The dashed lines on each figure represent the 95% confidence intervals, while the values reported to the right of the lags on the y-axis are the autocorrelation coefficients and their standard errors. These figures indicate that microclimate temperatures are predictable from day-to-day in South Africa, but not at Marion Island. Adapted with permission from Deere & Chown (2006).
(b) Population level
To some extent, population-level analyses have been used as a means to identify individual-level variation in traits such as the influence of moulting on supercooling points (Worland 2005; Worland et al. 2006), so blurring the distinction between the two. Nonetheless, substantial population-level work on temporal variation has been undertaken in its own right. Much of this work has focused on the reproductive phenology of both plants and animals and changes in the abundances and activities of invertebrate populations over one or more seasons (Lewis Smith 1984; Convey 1996a). The latter work has demonstrated that the extent to which abundance fluctuates with season, and the months in which peak abundances may occur, vary significantly between different species at different sites (Block 1982; West 1982; Barendse & Chown 2001). Moreover, the three-dimensional spatial distribution of individuals also changes substantively with season (Goddard 1979).
Over the longer term, it is clear that at least on the Antarctic Peninsula and on the sub-Antarctic Islands, many populations are responding rapidly to climate change. The most widely reported example of such a response relates to the local colonization and large increases in population numbers and extents of the two native Antarctic flowering plants (Deschampsia antarctica and Colobanthus quitensis) over the past four to five decades in the region of the Antarctic Peninsula (Fowbert & Lewis Smith 1994; Lewis Smith 1994; Grobe et al. 1997; Gerighausen et al. 2003). Some local populations have increased by two orders of magnitude in as little as 30 years, interpreted as a combination of increased temperature encouraging growth and vegetative expansion, and increased probability of establishment of seedlings. Regional warming is also thought to increase the frequency of mature seed production (Convey 1996c), and to stimulate growth of dormant seeds in soil propagule banks (McGraw & Day 1997).
Rapid expansions of plant populations on sub-Antarctic Heard Island have been ascribed to warming (Scott 1990), and this has been implied for changes in the distributions of invasive alien plant species on Marion Island too (Chown et al. 2000; see also Frenot et al. 2005). Recent manipulative experiments have demonstrated that if the warming and drying trend continues at Marion Island, significant impacts on the keystone cushion plant of fellfields, Azorella selago, are likely to be felt (Le Roux et al. 2005). Drying increases stem mortality and accelerates autumn senescence. Moreover, shading has a pronounced effect on the plants, reducing their surface integrity, leading to increases in the impacts of mechanical wind stress and a reduction in the capacity of plants to buffer thermal stress and moisture loss. One prediction of the increase in temperatures at the sub-Antarctic islands is that several plant species would overtop the cushions, which often happens in low-altitude areas, so leading to long-term population declines. This kind of effect might be exacerbated by the simultaneous increase in CO2 levels that are being experienced globally. Elevated CO2 can offset declining precipitation (stomata need to open less frequently), and is also thought to be responsible for an increase in the abundance of woody plants in many fire-prone areas globally owing to the CO2-enhanced growth rates of these woody species (Bond et al. 2003). Although interactions between CO2 and tropospheric ozone effects (Karnosky et al. 2003) make predictions of outcomes complex, an increase in woody growth on the islands (woody plants are typically absent from the sub-Antarctic, Lewis Smith 1984) would certainly have significant long-term consequences for A. selago and many other species. In this regard, the arrival and establishment of an unidentified woody shrub, far from any sites of human activity, but in the area predicted to receive new natural colonists on Marion Island (Chown & Avenant 1992), is noteworthy, as is the preponderance of woody species on other more temperate Southern Ocean islands such as Gough Island and several of the New Zealand sub-Antarctic islands.
The effects of warming and drying, associated with global climate change, can also be significantly influenced by the presence of invasive alien species. In the case of the sedge, Uncinia compacta, a species typical of drier mire vegetation on the Prince Edward Islands, little expansion in response to pronounced declines in precipitation has been found on Marion Island, but considerable expansion has been found on the neighbouring Prince Edward Island (Chown & Smith 1993). This difference is a consequence of invasive alien house mice (Mus musculus sensu lato), which are present on Marion Island, but absent from Prince Edward Island. Mice remove seed from individual U. compacta plants, so reducing the rate of spread of the population. At the Kerguelen archipelago, eradication of invasive rabbits (Oryctolagus cuniculus) has not led to the expected rapid recovery of indigenous plant species favoured by the rabbits largely owing to the effects of drought on the plants (Chapuis et al. 2004). By contrast, invasive alien plant species, such as the dandelion Taraxacum officinale, have shown substantial increases in abundance.
Increases in house mouse populations, associated with the warming climate at Marion Island (Van Aarde et al. 1996), have also significantly impacted indigenous invertebrates, especially the flightless moth, P. marioni and several weevil species which are among the preferred prey of the mice (Gleeson & van Rensburg 1982). Thus, between 1976/1977 and 1996/1997, the biomass of the caterpillars of the moth declined significantly, from 802±305 to 47±316 mg m−2, in tussock grassland. Over the same period and in the same habitat, biomass of larvae of the weevil E. similis declined from 375±83 to 45±34 mg m−2 (Chown et al. 2002). These impacts have indirectly influenced the abundance of lesser sheathbills (Chionis minor), which rely on invertebrates for overwinter survival (Huyser et al. 2000). Mice have also led to a long-term change in weevil body size on the island, whereas body sizes and populations appear to be more stable on neighbouring Prince Edward Island. Similar interactions between indigenous and invasive species characterize many other sub-Antarctic islands (Chevrier et al. 1997; Chown & Block 1997; Ernsting et al. 1999), and changes in climates seem set to exacerbate them (Kennedy 1995a; Walther et al. 2002).
(c) Species level
Temporal variation at the species level amounts either to anagenetic change in one or more populations, to cladogenesis, to hybridization or to some combination of these processes through time (Erwin & Anstey 1995). They can all lead to speciation, and hybridization might also result in the merging of lineages that were becoming distinct so leading to reticulate evolution. Nonetheless, it is also important to recognize that at the species level, large intervals might pass where stasis, rather than change, is the norm, despite complex geographical structure in phenotypic variation (Eldredge et al. 2005).
By contrast with Antarctic marine species (Bargelloni et al. 2000; Page & Linse 2002), stasis and change in terrestrial taxa have not been well investigated. On the continent, phylogenetic work based on morphological characters has suggested patterns of speciation for paleoendemic taxa such as the continental ameronothroid genus Maudheimia (Marshall & Coetzee 2000). On the sub-Antarctic islands, investigations of patterns of speciation have mostly been restricted to the Ectemnorhinus group weevils, again largely based on morphological studies, though complemented by ecological and molecular investigations in a few cases (Chown 1990b; Grobler et al. 2006). One recent study has also investigated patterns in speciation in the springtail genus Cryptopygus (Stevens et al. 2006). Comprehensive phylogenetic assessments of taxa, using modern molecular methods, and incorporating ecological and biogeographic information, are curiously rare in the literature on Antarctic terrestrial biodiversity. Springtail sequences have been used to inform broader systematic questions (Frati & Dell'Ampio 2000; D'Haese 2002), and only two recent studies have sought to investigate patterns of speciation across the Antarctic (Allegrucci et al. 2006; Stevens et al. 2006; §2b)
(d) Assemblage and ecosystem levels
Antarctic and sub-Antarctic assemblages have changed dramatically over the long term as is clear from the fossil record, and from palynological analyses that provide information on change at scales of several thousand years (Barnes et al. 2006; Cromer et al. 2006; Hodgson & Convey 2006). In both the maritime Antarctic and sub-Antarctic, clear successional changes associated with glacial retreat, both in the past and in the present, have been documented, and form among the clearest examples of temporal variation in assemblages (Frenot et al. 1998; Lewis Smith 2000). Over the shorter term, changes in the distribution of biogeographically unusual plant assemblages on heated ground provide further evidence that assemblages in the Antarctic are temporally dynamic (Convey et al. 2000a). Perhaps of most interest recently are the outcomes of experiments designed to assess the probable impacts of forecast regional climate change on Antarctic communities.
Early studies, using passive greenhouses (or cloches) demonstrated a remarkable effect of warming, including the development of substantial bryophyte assemblages and invertebrate communities on what were previously either unoccupied sites, or sites characterized by low abundance and species richness (Kennedy 1994; Convey 2003a,b). These experiments showed that viable propagules are available in the Antarctic soil, and that strong responses to temperature take place. However, not all treatments have resulted in changes to local assemblages (Sinclair 2002), and passive greenhouse methods have also been criticized for introducing confounding effects and for not replicating the predictions of general circulation models (GCMs) particularly well (Kennedy 1995b). That the GCMs are unable to predict current warming in the Antarctic Peninsula region (King et al. 2003) perhaps somewhat ameliorates this critique, but the latter has drawn attention to the need to carefully evaluate the effects of passive greenhouses.
Subsequent manipulations have become more elegant, and have altered temperature, water availability and ultraviolet radiation, and examined their direct effects as well as interactions between them (Day et al. 1999). For example, the effects of climate alteration on microarthropod assemblages were investigated in a sophisticated manipulation of vegetation dominated by D. antarctica and C. quitensis at Anvers Island (Convey et al. 2002). Elevated temperatures typically reduced arthropod abundances, which are thought to be a result of increases in water stress owing to increased evaporation. By contrast, enhanced water availability elevated abundances. Microarthropods are known to be especially sensitive to desiccation and this is true also of Antarctic species (Harrisson et al. 1991). Filtering of UV-B radiation resulted in an increase in arthropod abundances, and it is thought that the negative effects of UV are associated with indirect effects on the species via changes in resource quality and habitat structure. Similar negative responses to warming and drying have been reported for arthropod communities occupying A. selago on Marion Island (McGeoch et al. 2006).
4. Conclusions and implications
Variation over several spatial and temporal scales is as much a major characteristic of biodiversity in the Antarctic as it is of biodiversity elsewhere. This is clearly one of the primary messages that has emerged from both recent and earlier work in the region. Nonetheless, while patterns and mechanisms at some scales (e.g. the plot scale over a few years) are reasonably well understood, and information on variation in biodiversity is becoming available at several other scales, interactions between levels in the genealogical and ecological hierarchies, and by implication different spatial and temporal scales, have received little attention. How processes at different scales cascade up or down the hierarchies to affect each other in terrestrial Antarctic systems is not yet clear. Even so, recent work provides a tantalizing glimpse of the kinds of interactions that might be taking place. Two examples serve to illustrate the point.
On Marion Island, it has long been known that caterpillars of the flightless moth Pringleophaga marioni are abundant in recently abandoned (and presumably in occupied) albatross nests, and elevated nutrient availability was typically considered the cause thereof (Joly et al. 1987). However, what has now emerged is that these caterpillars are susceptible to repeated low-temperature stresses (i.e. temperatures below their chill coma temperature of −0.6°C), and show reduced growth probably as a consequence of reversible gut injury (Sinclair & Chown 2005b). In consequence, any circumstance that reduces this stress should either favour caterpillars or be favoured by them. Occupied wandering albatross (Diomedea exulans) nests have thermal regimes approximately 5°C warmer than the surrounding soil environment. Thus, the birds not only reduce the chances of low-temperature stress for the caterpillars, but also provide an environment close to the optimum for caterpillar feeding. They are, in effect, thermal ecosystem engineers (Sinclair & Chown 2006). In consequence, caterpillar biomasses are much higher and much less variable in wandering albatross nests than in surrounding habitats. What this means is that caterpillar physiology and albatross abundance are interacting to provide spatially complex patterns in caterpillar biomass that might well indicate an underlying system of source–sink dynamics.
Physiological and life-history investigations have also demonstrated that invasive alien species have shorter life cycles, steeper rate–temperature relationships and improved physiological performance under warm conditions by comparison with their indigenous counterparts (Barendse & Chown 2000; Chown et al. 2002; Slabber et al. 2007). In consequence, regional changes in climate, a consequence of global scale change, are likely to have pronounced effects on the relative abundances of invasive alien and indigenous species via differential thermal responses of these two groups of organisms (Frenot et al. 2005). Given that similar interactions have been recorded in other systems (Stachowicz et al. 2002), it seems probable that interactions between climate change and invasion, often mediated through differing physiological responses, will have major influences on terrestrial systems.
These examples illustrate the significance of investigating variation in diversity across a range of spatial and temporal scales. Only by understanding how processes operating at a variety of scales interact can variation in biodiversity be fully comprehended. In this context, it is concerning that at several scales even the patterns of variation, let alone their probable underlying mechanisms and interactions, are poorly understood. At the broadest scale, much of the ice-free land that constitutes only 0.32% of the Antarctic continent has yet to be surveyed. Moreover, even in well-known accessible areas, long-term assessments of changes in terrestrial biodiversity (or some component thereof) are either typically not systematically conducted or coordinated, or in some instances where this has taken place (Quayle et al. 2002, 2003), have since been abandoned. Without spatially and temporally explicit information on biodiversity, the conservation thereof is simply not possible.
The benefits of spatially explicit conservation planning are widely appreciated (Margules & Pressey 2000) and several conservation programmes are now reaping substantial rewards from their implementation (Younge & Fowkes 2003). Moreover, the importance of temporally explicit information has been highlighted by the discovery of population declines in what were otherwise thought to be abundant British bird species (Vickery et al. 2004), and by the establishment of the 2010 biodiversity targets (Balmford et al. 2005). In the Antarctic, modern conservation practice has profited taxa such as land-breeding pelagic birds (Woehler et al. 2001). However, for some reason, the value of explicit conservation planning and monitoring, and the need for sound species and population-level data to do so have not been fully realized for terrestrial systems, despite early demonstrations of the utility thereof (Usher & Edwards 1986). Two examples, among many, serve to re-emphasize and demonstrate the value of explicit conservation planning in the region.
First, it has been shown that the use of spatially explicit biodiversity data and optimization software can readily identify a set of sub-Antarctic islands that maximize the representation of indigenous species, while minimizing the occurrence of alien species, so improving the longer-term prospects for conservation (Chown et al. 2001). Second, recent surveys of Cape Hallett have demonstrated that the diverse arthropod fauna and the Adélie penguin (Pygoscelis adeliae) colony are spatially separated, with the algal flats at the site representing primary habitat for many of the species, and the penguin colony being totally devoid of arthropods (Sinclair et al. 2006). However, recent changes to the protected area at Cape Hallett have moved the emphasis to protection of breeding birds at the site, and shifts in the recommended campsite are also likely to mean significant disturbance to the flats (http://www.cep.aq/apa/aspa/sites/aspa106/index.html). Explicit conservation planning, incorporating all biodiversity elements, would have circumvented the present situation, which now contradicts the original purpose of the protected area.
In conclusion, it is clear that if conservation is to be successful in the Antarctic, then the bodies that guide conservation actions in the region are going to have to keep pace with conservation best practice, and to that end substantial investment in biodiversity research will be required. By comparison with the overall costs of Antarctic research and logistics, this work is inexpensive (Balmford & Gaston 1999). Moreover, the outcomes thereof will not only inform global understanding of biodiversity, but also contribute to the long-term conservation of Antarctica as a continent for peace and science.
Acknowledgments
We are grateful to Alex Rogers for inviting us to contribute to this special issue and for his comments on the manuscripts. Melodie McGeoch and two anonymous referees provided useful comments on a previous version of the work. SLC was partially supported by a grant made by the South African National Antarctic Programme to B. J. Jansen van Vuuren. P.C. is funded through the core BAS science project BIOPEARL. This is a contribution to the SCAR Research Programme: Evolution and Biodiversity in the Antarctic.
Footnotes
One contribution of 10 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. Part 2: evolution, diversity and function’.
References
- Allegrucci G, Carchini G, Todisco V, Convey P, Sbordoni V. A molecular phylogeny of Antarctic Chironomidae and its implications for biogeographical history. Polar Biol. 2006;29:320–326. doi:10.1007/s00300-005-0056-7 [Google Scholar]
- Andrássy I. Nematodes in the sixth continent. J. Nemat. Morphol. Syst. 1998;1:107–186. [Google Scholar]
- Angilletta M.J, Niewiarowski P.H, Navas C.A. The evolution of thermal physiology in ectotherms. J. Therm. Biol. 2002;27:249–268. doi:10.1016/S0306-4565(01)00094-8 [Google Scholar]
- Anonymous. Department of Environmental Affairs and Tourism; Pretoria, South Africa: 1996. Prince Edward Islands management plan. [Google Scholar]
- Ashworth A.C, Kuschel G. Fossil weevils (Coleoptera: Curculionidae) from latitude 85° S Antarctica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003;191:191–202. doi:10.1016/S0031-0182(02)00712-5 [Google Scholar]
- Avise J.C, Arnold J, Ball R.M, Bermingham E, Lamb T, Neigel J.E, Reeb C.A, Saunders N.C. Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. Annu. Rev. Ecol. Syst. 1987;18:489–522. [Google Scholar]
- Balmford A, Gaston K.J. Why biodiversity surveys are good value. Nature. 1999;398:204–205. doi:10.1038/18339 [Google Scholar]
- Balmford A, Crane P, Dobson A, Green R.E, Mace G.M. The 2010 challenge: data availability, information needs and extraterrestrial insights. Phil. Trans. R. Soc. B. 2005;360:221–228. doi: 10.1098/rstb.2004.1599. doi:10.1098/rstb.2004.1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse J, Chown S.L. The biology of Bothrometopus elongatus (Coleoptera, Curculionidae) in a mid-altitude fellfield on sub-Antarctic Marion Island. Polar Biol. 2000;23:346–351. doi:10.1007/s003000050454 [Google Scholar]
- Barendse J, Chown S.L. Abundance and seasonality of mid-altitude fellfield arthropods from Marion Island. Polar Biol. 2001;24:73–82. doi:10.1007/s003000000172 [Google Scholar]
- Barendse J, Mercer R.D, Marshall D.J, Chown S.L. Habitat specificity of mites on sub-Antarctic Marion Island. Environ. Entomol. 2002;31:612–625. [Google Scholar]
- Bargagli R. Ecological Studies. vol. 175. Springer; Berlin, Germany: 2005. Antarctic ecosystems: environmental contamination, climate change and human impact. [Google Scholar]
- Bargagli R, Skotnicki M.L, Marri L, Pepi M, Mackenzie A, Agnorelli C. New record of moss and thermophilic bacteria species and physico-chemical properties of geothermal soils on the northwest slope of Mt. Melbourne (Antarctica) Polar Biol. 2004;27:423–431. doi:10.1007/s00300-004-0612-6 [Google Scholar]
- Bargelloni L, Zane L, Derome N, Lecointre G, Patarnello T. Molecular zoogeography of Antarctic euphausiids and notothenioids: from species phylogenies to intraspecific patterns of genetic variation. Antarct. Sci. 2000;12:259–268. [Google Scholar]
- Barnes D.K.A, Hodgson D.A, Convey P, Allen C, Clarke A. Incursion and excursion of Antarctic biota: past, present and future. Global Ecol. Biogeogr. 2006;15:121–142. doi:10.1111/j.1466-822X.2006.00216.x [Google Scholar]
- Bearhop S, Furness R.W, Hilton G.M, Votier S.C, Waldron S. A forensic approach to understanding diet and habitat use from stable isotope analysis of (avian) claw material. Funct. Ecol. 2003;17:270–275. doi:10.1046/j.1365-2435.2003.00725.x [Google Scholar]
- Bednarek-Ochyra H, Väňa J, Ochyra R, Lewis Smith R.I. Polish Academy of Sciences, Institute of Botany; Cracow, Poland: 2000. The liverwort flora of Antarctica. [Google Scholar]
- Bell G. The interpretation of biological surveys. Proc. R. Soc. B. 2003;270:2531–2542. doi: 10.1098/rspb.2003.2550. doi:10.1098/rspb.2003.2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom D, Chown S.L. Life at the front: history, ecology and change on Southern Ocean islands. Trends Ecol. Evol. 1999;14:472–477. doi: 10.1016/s0169-5347(99)01688-2. doi:10.1016/S0169-5347(99)01688-2 [DOI] [PubMed] [Google Scholar]
- Blackburn T.M, Gaston K.J. Local avian assemblages as random draws from regional pools. Ecography. 2001a;24:50–58. doi:10.1034/j.1600-0587.2001.240107.x [Google Scholar]
- Blackburn T.M, Gaston K.J. Linking patterns in macroecology. J. Anim. Ecol. 2001b;70:338–352. doi:10.1046/j.1365-2656.2001.00484.x [Google Scholar]
- Blanckenhorn W.U, Demont M. Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr. Comp. Biol. 2004;44:413–424. doi: 10.1093/icb/44.6.413. doi:10.1093/icb/44.6.413 [DOI] [PubMed] [Google Scholar]
- Block W. The Signy Island terrestrial reference sites: XIV. Population studies on the Collembola. Br. Antarct. Surv. Bull. 1982;55:33–49. [Google Scholar]
- Block W. Terrestrial microbiology, invertebrates and ecosystems. In: Laws R.M, editor. Antarctic ecology. vol. 1. Academic Press; London, UK: 1984. pp. 163–236. [Google Scholar]
- Block W. Arthropod interactions in an Antarctic terrestrial community. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Antarctic nutrient cycles and food webs. Springer; Berlin, Germany: 1985. pp. 614–619. [Google Scholar]
- Block W. Terrestrial ecosystems: Antarctica. Polar Biol. 1994;14:293–300. doi:10.1007/BF00238443 [Google Scholar]
- Block W, Convey P. The biology, life cycle and ecophysiology of the Antarctic mite Alaskozetes antarcticus. J. Zool. 1995;236:431–449. [Google Scholar]
- Block W, Harrison P.M. Collembolan water relations and environmental change in the maritime Antarctic. Global Change Biol. 1995;1:347–359. doi:10.1111/j.1365-2486.1995.tb00033.x [Google Scholar]
- Bond W.J, Midgley G.G, Woodward F.I. The importance of low atmospheric CO2 and fires in promoting the spread of grasslands and savannas. Global Change Biol. 2003;9:973–982. doi:10.1046/j.1365-2486.2003.00577.x [Google Scholar]
- Boyer S.J. Glacial geologic observations in the Dufek Massif and Forrestal Range, 1978–79. Antarct. J. US. 1979;14:46–48. [Google Scholar]
- Brewer A.M, Gaston K.J. The geographical range structure of the holly leaf-miner. I. Population density. J. Anim. Ecol. 2002;71:99–111. doi:10.1046/j.0021-8790.2001.00578.x [Google Scholar]
- British Antarctic survey. British Antarctic Survey; Cambridge, UK: 2004. Antarctica, 1 : 10 000 000 scale map. BAS (Misc) 11. [Google Scholar]
- Broady P.A. Diversity, distribution and dispersal of Antarctic terrestrial algae. Biodivers. Conserv. 1996;5:1307–1335. doi:10.1007/BF00051981 [Google Scholar]
- Broady P.A, Weinstein R.N. Algae, lichens and fungi in la Gorce Mountains, Antarctica. Antarct. Sci. 1998;10:376–385. [Google Scholar]
- Brooks T.M, et al. Habitat loss and extinction in the hotspots of biodiversity. Conserv. Biol. 2002;16:909–923. doi:10.1046/j.1523-1739.2002.00530.x [Google Scholar]
- Brown J.H, Lomolino M.V. 2nd edn. Sinauer Associates; Sunderland, MA: 1998. Biogeography. [Google Scholar]
- Burger A.E. Terrestrial food webs in the sub-Antarctic: island effects. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Antarctic nutrient cycles and food webs. Springer; Berlin, Germany: 1985. pp. 582–591. [Google Scholar]
- Chapuis J.L, Frenot Y, Lebouvier M. Recovery of native plant communities after eradication of rabbits from the subantarctic Iles Kerguelen, and influence of climate change. Biol. Conserv. 2004;117:167–179. doi:10.1016/S0006-3207(03)00290-8 [Google Scholar]
- Chastain A. La flore et la vegetation des Îles de Kerguelen. Polymorphisme des espèces australes. Mem. Mus. Nat. Hist. Nat. B. 1958;11:1–136. [Google Scholar]
- Chevrier M, Vernon P, Frenot Y. Potential effects of two alien insects on a sub-Antarctic wingless fly in the Kerguelen islands. In: Battaglia B, Valencia J, Walton D.W.H, editors. Antarctic communities. species, structure and survival. Cambridge University Press; Cambridge, UK: 1997. pp. 424–431. [Google Scholar]
- Chown S.L. Possible effects of Quaternary climatic change on the composition of insect communities of the South Indian Ocean Province Islands. S. Afr. J. Sci. 1990a;86:386–391. [Google Scholar]
- Chown S.L. Speciation in the sub-Antarctic weevil genus Dusmoecetes Jeannel (Coleoptera: Curculionidae) Syst. Entomol. 1990b;15:283–296. [Google Scholar]
- Chown S.L. Historical ecology of sub-Antarctic weevils (Coleoptera: Curculionidae): patterns and processes on isolated islands. J. Nat. Hist. 1994;28:411–433. [Google Scholar]
- Chown S.L, Avenant N. Status of Plutella xylostella at Marion Island six years after its colonization. S. Afr. J. Antarct. Res. 1992;22:37–40. [Google Scholar]
- Chown S.L, Block W. Comparative nutritional ecology of grass-feeding in a sub-Antarctic beetle: the impact of introduced species on Hydromedion sparsutum from South Georgia. Oecologia. 1997;111:216–224. doi: 10.1007/s004420050228. doi:10.1007/s004420050228 [DOI] [PubMed] [Google Scholar]
- Chown S.L, Gaston K.J. Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol. Rev. 1999;74:87–120. doi:10.1017/S000632319800526X [Google Scholar]
- Chown S.L, Gaston K.J. Areas, cradles and museums: the latitudinal gradient in species richness. Trends Ecol. Evol. 2000;15:311–315. doi: 10.1016/s0169-5347(00)01910-8. doi:10.1016/S0169-5347(00)01910-8 [DOI] [PubMed] [Google Scholar]
- Chown S.L, Klok C.J. Altitudinal body size clines: latitudinal effects associated with changing seasonality. Ecography. 2003;26:445–455. doi:10.1034/j.1600-0587.2003.03479.x [Google Scholar]
- Chown S.L, Nicolson S.W. Oxford University Press; Oxford, UK: 2004. Insect physiological ecology. Mechanisms and patterns. [Google Scholar]
- Chown S.L, Smith V.R. Climate change and the short-term impact of feral house mice at the sub-Antarctic Prince Edward Islands. Oecologia. 1993;96:508–516. doi: 10.1007/BF00320508. doi:10.1007/BF00320508 [DOI] [PubMed] [Google Scholar]
- Chown S.L, Terblanche J.S. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 2007;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. doi:10.1016/S0065-2806(06)33002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L, Gremmen N.J.M, Gaston K.J. Ecological biogeography of Southern Ocean islands: species–area relationships, human impacts, and conservation. Am. Nat. 1998;152:562–575. doi: 10.1086/286190. doi:10.1086/286190 [DOI] [PubMed] [Google Scholar]
- Chown S.L, Gaston K.J, Gremmen N.J.M. Including the Antarctic: insights for ecologists everywhere. In: Davison W, Howard-Williams C, Broady P, editors. Antarctic ecosystems: models for wider ecological understanding. New Zealand Natural Sciences; Christchurch, New Zealand: 2000. pp. 1–15. [Google Scholar]
- Chown S.L, Rodrigues A.S.L, Gremmen N.J.M, Gaston K.J. World heritage status and the conservation of Southern Ocean islands. Conserv. Biol. 2001;15:550–557. doi:10.1046/j.1523-1739.2001.015003550.x [Google Scholar]
- Chown S.L, McGeoch M.A, Marshall D.J. Diversity and conservation of invertebrates on the sub-Antarctic Prince Edward Islands. Afr. Entomol. 2002;10:67–82. [Google Scholar]
- Chown S.L, Sinclair B.J, Leinaas H.P, Gaston K.J. Hemispheric asymmetries in biodiversity—a serious matter for ecology. PLoS Biol. 2004;2:1701–1707. doi: 10.1371/journal.pbio.0020406. doi:10.1371/journal.pbio.0020406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L, Hull B, Gaston K.J. Human impacts, energy availability, and invasion across Southern Ocean islands. Global Ecol. Biogeogr. 2005;14:521–528. doi:10.1111/j.1466-822x.2005.00173.x [Google Scholar]
- Chown S.L, Greenslade P, Marshall D.J. Terrestrial invertebrates of Heard Island. In: Green K, Woehler E.J, editors. Heard Island: Southern Ocean sentinel. Surrey & Beatty; Chipping Norton, UK: 2006. pp. 91–104. [Google Scholar]
- Clarke A. Evolution, adaptation and diversity: global ecology in an Antarctic context. In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vies S.S, Wolff W.J, editors. Antarctic biology in a global context. Backhuys Publishers; Leiden, The Netherlands: 2003. pp. 3–17. [Google Scholar]
- Clarke A, Johnston N.M. Antarctic marine benthic diversity. Oceanogr. Mar. Biol. Annu. Rev. 2003;41:47–114. [Google Scholar]
- Clarke J.A, Tambussi C.P, Noriega J.I, Erickson G.M, Ketcham R.A. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005;433:305–308. doi: 10.1038/nature03150. doi:10.1038/nature03150 [DOI] [PubMed] [Google Scholar]
- Cockell C.S, Stokes M.D. Widespread colonization by polar hypoliths. Nature. 2004;431:414. doi: 10.1038/431414a. doi:10.1038/431414a [DOI] [PubMed] [Google Scholar]
- Cohen J.E, Jonsson T, Carpenter S.R. Ecological community description using the food web, species abundance, and body size. Proc. Natl Acad. Sci. USA. 2003;100:1781–1786. doi: 10.1073/pnas.232715699. doi:10.1073/pnas.232715699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.E, Jonsson T, Müller C.B, Godfray H.C.J, Savage V.M. Body sizes of hosts and parasitoids in individual feeding relationships. Proc. Natl Acad. Sci. USA. 2005;102:684–689. doi: 10.1073/pnas.0408780102. doi:10.1073/pnas.0408780102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convey P. Modelling reproductive effort in sub- and maritime Antarctic mosses. Oecologia. 1994;100:45–53. doi: 10.1007/BF00317129. doi:10.1007/BF00317129 [DOI] [PubMed] [Google Scholar]
- Convey P. The influence of environmental characteristics on life history attributes of Antarctic terrestrial biota. Biol. Rev. 1996a;71:191–225. [Google Scholar]
- Convey P. Overwintering strategies of terrestrial invertebrates from Antarctica—the significance of flexibility in extremely seasonal environments. Eur. J. Entomol. 1996b;93:489–505. [Google Scholar]
- Convey P. Reproduction of Antarctic flowering plants. Antarct. Sci. 1996c;8:127–134. [Google Scholar]
- Convey P. Latitudinal variation in allocation to reproduction by the Antarctic oribatid mite, Alaskozetes antarcticus. Appl. Soil Ecol. 1998;9:93–99. doi:10.1016/S0929-1393(98)00060-2 [Google Scholar]
- Convey P. Antarctic ecosystems. In: Levin S.A, editor. Encyclopedia of biodiversity. vol. 1. Academic Press; San Diego, CA: 2001. pp. 171–184. [Google Scholar]
- Convey P. Maritime Antarctic climate change: signals from terrestrial biology. In: Domack E, Burnett A, Leventer A, Convey P, Kirby M, Bindschadler R, editors. Antarctic Peninsula climate variability: historical and palaeoenvironmental perspectives. vol. 79. American Geophysical Union; Washington, DC: 2003a. pp. 145–158. [Google Scholar]
- Convey P. Soil faunal community response to environmental manipulation on Alexander Islands, southern maritime Antarctic. In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vies S.S, Wolff W.J, editors. Antarctic biology in a global context. Backhuys Publishers; Leiden, The Netherlands: 2003b. pp. 74–78. [Google Scholar]
- Convey P, Lewis Smith R.I. Investment in sexual reproduction by Antarctic mosses. Oikos. 1993;68:293–302. doi:10.2307/3544842 [Google Scholar]
- Convey P, Lewis Smith R.I. The terrestrial arthropod fauna and its habitats in northern Marguerite Bay and Alexander Island, maritime Antarctic. Antarct. Sci. 1997;9:12–26. [Google Scholar]
- Convey P, McInnes S.J. Exceptional tardigrade-dominated ecosystems in Ellsworth Land. Antarct. Ecol. 2005;86:519–527. [Google Scholar]
- Convey P, Worland M.R. Survival of freezing by free-living Antarctic soil nematodes. Cryo Lett. 2000;21:327–332. [PubMed] [Google Scholar]
- Convey P, Lewis Smith R.I, Hodgson D.A, Peat H.J. The flora of the South Sandwich Islands, with particular preference to the influence of geothermal heating. J. Biogeogr. 2000a;27:1279–1296. doi:10.1046/j.1365-2699.2000.00512.x [Google Scholar]
- Convey P, Greenslade P, Pugh P.J.A. Terrestrial fauna of the South Sandwich islands. J. Nat. Hist. 2000b;34:597–609. doi:10.1080/002229300299462 [Google Scholar]
- Convey P, Pugh P.J.A, Jackson C, Murray A.W, Ruhland C.T, Xiong F.S, Day T.A. Response of Antarctic terrestrial microarthropods to long-term climate manipulations. Ecology. 2002;83:3130–3140. doi:10.2307/3071848 [Google Scholar]
- Convey P, Block W, Peat H.J. Soil arthropods as indicators of water stress in Antarctic terrestrial habitats? Global Change Biol. 2003;9:1718–1730. doi:10.1046/j.1365-2486.2003.00691.x [Google Scholar]
- Corsolini S, Romeo T, Ademolla N, Focardi S. POPs in key species of marine Antarctic ecosystem. Microchem. J. 2002;73:187–193. doi:10.1016/S0026-265X(02)00063-2 [Google Scholar]
- Coulson S.J, Hodkinson I.D, Webb N.R, Harrison J.A. Survival of terrestrial soil-dwelling arthropods on and in seawater: implications for trans-oceanic dispersal. Funct. Ecol. 2002;16:353–356. doi:10.1046/j.1365-2435.2002.00636.x [Google Scholar]
- Courtright E.M, Wall D.H, Virginia R.A. Determining habitat suitability for soil invertebrates in an extreme environment: the McMurdo Dry valleys, Antarctica. Antarct. Sci. 2001;13:9–17. doi:10.1017/S0954102001000037 [Google Scholar]
- Crafford J.E, Scholtz C.H. Quantitative differences between the insect faunas of sub-Antarctic Marion and Prince Edward Islands: a result of human intervention? Biol. Conserv. 1987;40:255–262. doi:10.1016/0006-3207(87)90119-4 [Google Scholar]
- Cromer L, Gibson J.A.E, Swadling K.M, Hodgson D.A. Evidence for a lacustrine faunal refuge in the Larsemann Hills, East Antarctica, during the Last Glacial Maximum. J. Biogeogr. 2006;33:1314–1323. doi:10.1111/j.1365-2699.2006.01490.x [Google Scholar]
- Dale T.M, Skotnicki M.L, Adam K.D, Selkirk P.M. Genetic diversity in the moss Hennediella heimii in Miers Valley, southern Victoria Land, Antarctica. Polar Biol. 1999;21:228–233. doi:10.1007/s003000050357 [Google Scholar]
- Day T.A, Ruhland C.T, Grobe C.W, Xiong F. Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia. 1999;119:24–35. doi: 10.1007/s004420050757. doi:10.1007/s004420050757 [DOI] [PubMed] [Google Scholar]
- Deere J.A, Chown S.L. Testing the beneficial acclimation hypothesis and its alternatives for locomotor performance. Am. Nat. 2006;168:630–644. doi: 10.1086/508026. doi:10.1086/508026 [DOI] [PubMed] [Google Scholar]
- Deere J.A, Sinclair B.J, Marshall D.J, Chown S.L. Phenotypic plasticity of thermal tolerances in five oribatid mite species from sub-Antarctic Marion Island. J. Insect Physiol. 2006;52:693–700. doi: 10.1016/j.jinsphys.2006.03.009. doi:10.1016/j.jinsphys.2006.03.009 [DOI] [PubMed] [Google Scholar]
- De Witt T.J, Scheiner S.M. Oxford University Press; Oxford, UK: 2004. Phenotypic plasticity: functional and conceptual approaches. [Google Scholar]
- D'Haese C.A. Were the first springtails semi-aquatic? A phylogenetic approach by means of 28S rDNA and optimization alignment. Proc. R. Soc. B. 2002;269:1143–1151. doi: 10.1098/rspb.2002.1981. doi:10.1098/rspb.2002.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickhut R.M, Cincinelli A, Cochran M, Ducklow H.W. Atmospheric concentrations and air–water flux of organochlorine pesticides along the western Antarctic Peninsula. Environ. Sci. Technol. 2005;39:465–470. doi: 10.1021/es048648p. doi:10.1021/es048648p [DOI] [PubMed] [Google Scholar]
- Dreux P, Voisin J. Faune entomologique de l'ile des Pingouins (archipel Crozet) Bull. Soc. Entomol. Fr. 1993;97:453–464. [Google Scholar]
- Eldredge N. Information, economics, and evolution. Annu. Rev. Ecol. Syst. 1986;17:351–369. doi:10.1146/annurev.es.17.110186.002031 [Google Scholar]
- Eldredge N, et al. The dynamics of evolutionary stasis. Paleobiology. 2005;31:133–145. doi:10.1666/0094-8373(2005)031[0133:TDOES]2.0.CO;2 [Google Scholar]
- Ernsting G, van Ginkel W, Menken S.B.J. Genetical population structure of Trechisibus antarcticus (Coleoptera; Carabidae) on South Georgia and on the Falkland islands. Polar Biol. 1995;15:523–525. doi:10.1007/BF00237467 [Google Scholar]
- Ernsting G, Brandjes G.J, Block W, Isaaks J.A. Life-history consequences of predation for a subantarctic beetle: evaluating the contribution of direct and indirect effects. J. Anim. Ecol. 1999;68:741–752. doi:10.1046/j.1365-2656.1999.00322.x [Google Scholar]
- Erwin D.H, Anstey R.L, editors. New approaches to speciation in the fossil record. Columbia University Press; New York, NY: 1995. [Google Scholar]
- Evans K.L, Gaston K.J. Can the evolutionary-rates hypothesis explain species–energy relationships? Funct. Ecol. 2005;19:899–915. doi:10.1111/j.1365-2435.2005.01046.x [Google Scholar]
- Evans K.L, Gaston K.J, Warren P.H. Species–energy relationships at the macroecological scale: a review of the mechanisms. Biol. Rev. 2005;80:1–25. doi: 10.1017/s1464793104006517. doi:10.1017/S1464793104006517 [DOI] [PubMed] [Google Scholar]
- Fanciulli P.F, Summa D, Dallai R, Frati F. High levels of genetic variability and population differentiation in Gressitacantha terranova (Collembola, Hexapoda) from Victoria Land, Antarctica. Antarct. Sci. 2001;13:246–254. doi:10.1017/S0954102001000360 [Google Scholar]
- Fenchel T. There are more small than large species? Oikos. 1993;68:375–378. doi:10.2307/3544855 [Google Scholar]
- Feder M.E. The analysis of physiological diversity: the prospects for pattern documentation and general questions in ecological physiology. In: Feder M.E, Bennett A.F, Burggren W, Huey R.B, editors. New directions in ecological physiology. Cambridge University Press; Cambridge, UK: 1987. pp. 38–75. [Google Scholar]
- Finlay B.J. Global dispersal of free-living microbial eukaryote species. Science. 2002;296:1061–1063. doi: 10.1126/science.1070710. doi:10.1126/science.1070710 [DOI] [PubMed] [Google Scholar]
- Finlay B.J, Esteban G.F, Fenchel T. Global diversity and body size. Nature. 1996;383:132–133. doi:10.1038/383132a0 [Google Scholar]
- Fowbert J.A, Lewis Smith R.I. Rapid population increase in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct. Alpine Res. 1994;26:290–296. doi:10.2307/1551941 [Google Scholar]
- Franzmann P.D. Examination of Antarctic prokaryotic diversity through molecular comparisons. Biodivers. Conserv. 1996;5:1295–1305. doi:10.1007/BF00051980 [Google Scholar]
- Frati F, Dell'Ampio E. Molecular phylogeny of three subfamilies of the Neanuridae (Insecta, Collembola) and the position of the Antarctic species Friesea grisea Schäffer. Pedobiologia. 2000;44:342–360. doi:10.1078/S0031-4056(04)70054-2 [Google Scholar]
- Frati F, Spinsanti G, Dallai R. Genetic variation of mtCOII gene sequences in the collembolan Isotoma klovstadi from Victoria Land, Antarctica: evidence for population differentiation. Polar Biol. 2001;24:934–940. doi:10.1007/s003000100302 [Google Scholar]
- Freckman D.W, Virginia R.A. Low-diversity Antarctic soil nematode communities: distribution and response to disturbance. Ecology. 1997;78:363–369. doi:10.2307/2266013 [Google Scholar]
- Frenot Y, Gloaguen J.-C, Cannavacciuolo M, Bellido A. Primary succession on glacier forelands in the subantarctic Kerguelen Islands. J. Veg. Sci. 1998;9:75–84. doi:10.2307/3237225 [Google Scholar]
- Frenot Y, Gloaguen J.C, Masse L, Lebouvier M. Human activities, ecosystem disturbance and plant invasions in subantarctic Crozet, Kerguelen and Amsterdam Islands. Biol. Conserv. 2001;101:33–50. doi:10.1016/S0006-3207(01)00052-0 [Google Scholar]
- Frenot Y, Chown S.L, Whinam J, Selkirk P.M, Convey P, Skotnici M, Bergstrom D.M. Biological invasions in the Antarctic: extent, impacts and implications. Biol. Rev. 2005;80:45–72. doi: 10.1017/s1464793104006542. doi:10.1017/S1464793104006542 [DOI] [PubMed] [Google Scholar]
- Gabriel A.G.A, Chown S.L, Barendse J, Marshall D.J, Mercer R.D, Pugh P.J.A, Smith V.R. Biological invasions on Southern Ocean islands: the Collembola of Marion Island as a test of generalities. Ecography. 2001;24:421–430. doi:10.1034/j.1600-0587.2001.d01-198.x [Google Scholar]
- Gaston K.J. Global patterns in biodiversity. Nature. 2000;405:220–227. doi: 10.1038/35012228. doi:10.1038/35012228 [DOI] [PubMed] [Google Scholar]
- Gaston K.J. Biodiversity and extinction: species and people. Prog. Phys. Geogr. 2005;29:239–247. doi:10.1191/0309133305pp445pr [Google Scholar]
- Gaston K.J, Blackburn T.M. A critique for macroecology. Oikos. 1999;84:353–368. doi:10.2307/3546417 [Google Scholar]
- Gaston K.J, Chown S.L, Mercer R.D. The animal species–body size distribution of Marion Island. Proc. Natl Acad. Sci. USA. 2001;98:14 493–14 496. doi: 10.1073/pnas.251332098. doi:10.1073/pnas.251332098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston K.J, Jones A.G, Hänel C, Chown S.L. Rates of species introduction to a remote oceanic island. Proc. R. Soc. B. 2003;270:1091–1098. doi: 10.1098/rspb.2003.2332. doi:10.1098/rspb.2003.2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerighausen U, Bräutigam K, Mustafa O, Peter H.-U. Expansion of vascular plants on an Antarctic island—a consequence of climate change? In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vies S.M, Wolff W.J, editors. Antarctic biology in a global context. Backhuys; Leiden, The Netherlands: 2003. pp. 79–83. [Google Scholar]
- Gleeson J.P, van Rensburg P.J.J. Feeding ecology of the house mouse Mus musculus on Marion Island. S. Afr. J. Antarct. Res. 1982;12:34–39. [Google Scholar]
- Goddard D.G. The Signy Island terrestrial reference sites: XI. Population studies on the Acari. Br. Antarct. Surv. Bull. 1979;48:71–92. [Google Scholar]
- Green J, Holmes A.J, Westoby M, Oliver I, Briscoe D, Dangerfield M, Gillings M, Beattie A.J. Spatial scaling of microbial eukaryote diversity. Nature. 2004;432:747–750. doi: 10.1038/nature03034. doi:10.1038/nature03034 [DOI] [PubMed] [Google Scholar]
- Greenslade P. Collembola from the Scotia Arc and Antarctic Peninsula including descriptions of two new species and notes on biogeography. Polskie Pismo Entomologiczne. 1995;64:305–319. [Google Scholar]
- Greenslade P, Farrow R.A, Smith J.M.B. Long distance migration of insects to a subantarctic island. J. Biogeogr. 1999;26:1161–1167. doi:10.1046/j.1365-2699.1999.00356.x [Google Scholar]
- Gremmen N.J.M, Smith V.R. New records of alien vascular plants from Marion and Prince Edward Islands, sub-Antarctic. Polar Biol. 1999;21:401–409. doi:10.1007/s003000050380 [Google Scholar]
- Gressitt J.L. Biogeography and ecology of land arthropods of Antarctica. In: Van Mieghem J, Van Oye P, Schell J, editors. Biogeography and ecology in Antarctica. Junk; The Hague, The Netherlands: 1965. pp. 431–490. [Google Scholar]
- Gressitt J.L. Antarctic entomology with emphasis on biogeographical aspects. Pac. Insects Monogr. 1971;25:167–178. [Google Scholar]
- Greve M, Gremmen N.J.M, Gaston K.J, Chown S.L. Nestedness of South Ocean island biotas: ecological perspectives on a biogeographical conundrum. J. Biogeogr. 2005;32:155–168. doi:10.1111/j.1365-2699.2004.01169.x [Google Scholar]
- Griffiths H.J, Linse K, Crame A. SOMBASE—Southern Ocean Molluscan Database: a tool for biogeographic analysis in diversity and ecology. Org. Divers. Evol. 2003;3:207–213. doi:10.1078/1439-6092-00079 [Google Scholar]
- Grobe C.W, Ruhland C.T, Day T.A. A new population of Colobanthus quitensis near Arthur Harbor, Antarctica: correlating recruitment with warmer summer temperatures. Arct. Alpine Res. 1997;29:217–221. doi:10.2307/1552049 [Google Scholar]
- Grobler G.C, Janse van Rensburg L, Bastos A.D.S, Chimimba C.T, Chown S.L. Molecular and morphometric assessment of Ectemnorhinus weevil species (Coleoptera, Curculionidae, Brachycerinae) from the sub-Antarctic Prince Edward Islands. J. Zool. Syst. Evol. Res. 2006;44:200–211. doi:10.1111/j.1439-0469.2006.00358.x [Google Scholar]
- Hall K. Review of Present and Quaternary periglacial processes and landforms of the maritime and sub-Antarctic region. S. Afr. J. Sci. 2002;98:71–81. [Google Scholar]
- Hammer W.R, Hickerson W.J. A crested theropod dinosaur from Antarctica. Science. 1994;264:828–830. doi: 10.1126/science.264.5160.828. doi:10.1126/science.264.5160.828 [DOI] [PubMed] [Google Scholar]
- Hänel C, Chown S.L. Fifty years at Marion and Prince Edward Islands: a bibliography of scientific and popular literature. S. Afr. J. Sci. 1999;95:87–112. [Google Scholar]
- Harrisson P.M, Rothery P, Block W. Drying processes in the Antarctic collembolan Cryptopygus antarcticus (Willem) J. Insect Physiol. 1991;37:883–890. doi:10.1016/0022-1910(91)90003-I [Google Scholar]
- Hawkins B.A, Porter E.E. Relative influences of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Global Ecol. Biogeogr. 2003;12:475–481. doi:10.1046/j.1466-822X.2003.00060.x [Google Scholar]
- Hawkins B.A, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- Hayward S.A.L, Worland M.R, Convey P, Bale J.S. Effects of moisture on the local distribution of the Antarctic Collembola Cryptopygus antarcticus and Friesea grisea. Soil Biol. Biochem. 2004;36:927–934. doi:10.1016/j.soilbio.2004.02.007 [Google Scholar]
- He F. Deriving a neutral model of species abundance from fundamental mechanisms of population dynamics. Funct. Ecol. 2005;19:187–193. doi:10.1111/j.0269-8463.2005.00944.x [Google Scholar]
- Headland R.K. Cambridge University Press; Cambridge, UK: 1989. Chronological list of Antarctic expeditions and related historical events. [Google Scholar]
- Hodgson D.A, Convey P. A 7000-year record of oribatid mite communities on a maritime Antarctic island: responses to climate change. Arct. Antarct. Alpine Res. 2006;37:239–245. doi:10.1657/1523-0430(2005)037[0239:AYROOM]2.0.CO;2 [Google Scholar]
- Hoffmann A.A, Sørensen J.G, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 2003;28:175–216. doi:10.1016/S0306-4565(02)00057-8 [Google Scholar]
- Hopkin S.P. Oxford University Press; Oxford, UK: 1997. Biology of the springtails (Insecta: Collembola) [Google Scholar]
- Horner-Devine M.C, Lage M, Hughes J.B, Bohannan B.J.M. A taxa–area relationship for bacteria. Nature. 2004;432:750–753. doi: 10.1038/nature03073. doi:10.1038/nature03073 [DOI] [PubMed] [Google Scholar]
- Hubbell S.P. Princeton University Press; Princeton, NJ: 2001. The unified neutral theory of biodiversity and biogeography. [DOI] [PubMed] [Google Scholar]
- Huey R.B, Berrigan D. Testing evolutionary hypotheses of acclimation. In: Johnston I.A, Bennett A.F, editors. Animals and temperature. Phenotypic and evolutionary adaptation. Cambridge University Press; Cambridge, UK: 1996. pp. 205–237. [Google Scholar]
- Hughes K.A. Aerial dispersal and survival of sewage-derived faecal coliforms in Antarctica. Atmos. Environ. 2003;37:3147–3155. doi:10.1016/S1352-2310(03)00207-3 [Google Scholar]
- Hughes K.A, Lawley B. A novel Antarctic microbial endolithic community within gypsum crusts. Environ. Micorbiol. 2003;5:555–565. doi: 10.1046/j.1462-2920.2003.00439.x. doi:10.1046/j.1462-2920.2003.00439.x [DOI] [PubMed] [Google Scholar]
- Hugo E.A, McGeoch M.A, Marshall D.J. Fine scale variation in microarthropod communities inhabiting the keystone species Azorella selago on Marion Island. Polar Biol. 2004;27:466–473. doi:10.1007/s00300-004-0614-4 [Google Scholar]
- Huiskes A.H.L, Moerdijk-Poortvliet T.C.W, Lud D. Responses to UV-B radiation in terrestrial Antarctic vegetation. In: Davison W, Howard-Williams C, Broady P, editors. Antarctic ecosystems. Models for wider ecological understanding. New Zealand Natural Sciences; Christchurch, New Zealand: 2000. pp. 252–257. [Google Scholar]
- Huyser O, Ryan P.G, Cooper J. Changes in population size, habitat use and breeding biology of lesser sheathbills (Chionis minor) at Marion Island: impacts of cats, mice and climate change? Biol. Conserv. 2000;92:299–310. doi:10.1016/S0006-3207(99)00096-8 [Google Scholar]
- Janetschek H. Arthropod ecology of South Victoria Land. In: Gressitt J.L, editor. Entomology of Antarctica. Geophysical Union; Washington, DC: 1967. pp. 205–293. [Google Scholar]
- Janetschek H. Environments and ecology of terrestrial arthropods in the high Antarctic. In: Holdgate M.W, editor. Antarctic ecology. vol. 2. Academic Press; London, UK: 1970. pp. 871–885. [Google Scholar]
- Jeannel R. Biogéographie des terres Australes de l'Océan Indien. Rev. Fr. Entomol. 1964;31:319–417. [Google Scholar]
- Joly Y, Frenot Y, Vernon P. Environmental modifications of a subantarctic peat-bog by the Wandering Albatross (Diomedea exulans): a preliminary study. Polar Biol. 1987;8:61–72. doi:10.1007/BF00297166 [Google Scholar]
- Jones A.G, Chown S.L, Webb T.J, Gaston K.J. The free-living pterygote insects of Gough Island, South Atlantic Ocean. Syst. Biodivers. 2003;1:213–273. doi:10.1017/S1477200003001142 [Google Scholar]
- Karentz D. Environmental change in Antarctica: ecological impacts and responses. In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vies S.S, Wolff W.J, editors. Antarctic biology in a global context. Backhuys Publishers; Leiden, The Netherlands: 2003. pp. 45–55. [Google Scholar]
- Karnosky D.F, Zak D.R, Pregitzer K.S, Awmack C.S, Bockheim J.G, et al. Tropospheric O3 moderates responses of temperate hardwood forests to elevated CO2: a synthesis of molecular to ecosystem results from the Aspen FACE project. Funct. Ecol. 2003;17:289–304. doi:10.1046/j.1365-2435.2003.00733.x [Google Scholar]
- Kennedy A.D. Water as a limiting factor in the Antarctic terrestrial environment: a biogeographical synthesis. Arct. Alpine Res. 1993;25:308–315. doi:10.2307/1551914 [Google Scholar]
- Kennedy A.D. Simulated climate change: a field manipulation study of polar microarthropod community response to global warming. Ecography. 1994;17:131–140. doi:10.1111/j.1600-0587.1994.tb00085.x [Google Scholar]
- Kennedy A.D. Antarctic terrestrial ecosystem response to global environmental change. Annu. Rev. Ecol. Syst. 1995a;26:683–704. doi:10.1146/annurev.es.26.110195.003343 [Google Scholar]
- Kennedy A.D. Simulated climate change: are passive greenhouses a valid microcosm for testing the biological effects of environmental perturbations? Global Change Biol. 1995b;1:29–42. doi:10.1111/j.1365-2486.1995.tb00004.x [Google Scholar]
- Kerry E. Microorganisms colonising plants and soil subjected to different degrees of human activity, including petroleum contamination, in the Vestfold Hills and MacRobertson land. Polar Biol. 1990;10:423–430. [Google Scholar]
- King J.C, Turner J, Marshall G.J, Connally W.M, Lachlan-Cope T.A. Antarctic Peninsula climate variability and its causes as revealed by analysis of instrumental records. In: Domack E, Burnett A, Leventer A, Convey P, Kirby M, Bindschadler R, editors. Antarctic Peninsula climate variability: historical and palaeoenvironmental perspectives. Antarctic research series. vol. 79. American Geophysical Union; Washington, DC: 2003. pp. 17–30. [Google Scholar]
- Klok C.J, Chown S.L. Critical thermal limits, temperature tolerance and water balance of a sub-Antarctic caterpillar, Pringleophaga marioni Viette (Lepidoptera: Tineidae) J. Insect Physiol. 1997;43:685–694. doi: 10.1016/s0022-1910(97)00001-2. doi:10.1016/S0022-1910(97)00001-2 [DOI] [PubMed] [Google Scholar]
- Klok C.J, Chown S.L. Interactions between desiccation resistance, host–plant contact and the thermal biology of a leaf-dwelling sub-antarctic caterpillar, Embryonopsis halticella (Lepidoptera: Yponomeutidae) J. Insect Physiol. 1998;44:615–628. doi: 10.1016/s0022-1910(98)00052-3. doi:10.1016/S0022-1910(98)00052-3 [DOI] [PubMed] [Google Scholar]
- Klok C.J, Chown S.L. Resistance to temperature extremes in sub-Antarctic weevils: interspecific variation, population differentiation and acclimation. Biol. J. Linn. Soc. 2003;78:401–414. doi:10.1046/j.1095-8312.2003.00154.x [Google Scholar]
- Klok C.J, Chown S.L. Inertia in physiological traits: Embryonopsis halticella caterpillars (Yponomeutidae) across the Antarctic Polar Frontal Zone. J. Insect Physiol. 2005;51:87–97. doi: 10.1016/j.jinsphys.2004.11.011. doi:10.1016/j.jinsphys.2004.11.011 [DOI] [PubMed] [Google Scholar]
- Knox G.A. Cambridge University Press; Cambridge, UK: 1994. The biology of the Southern Ocean. [Google Scholar]
- Kozłowski J, Czarnoleski M, Dańko M. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr. Comp. Biol. 2004;44:480–493. doi: 10.1093/icb/44.6.480. doi:10.1093/icb/44.6.480 [DOI] [PubMed] [Google Scholar]
- Kuschel G. Entomology of the Aucklands and other islands south of New Zealand: Coleoptera: Curculionidae. Pac. Insect Monogr. 1971;27:225–259. [Google Scholar]
- Landys-Ciannelli M.M, Piersma T, Jukema J. Strategic size changes of internal organs and muscle tissue in the Bar-tailed Godwit during fat storage on a spring stopover site. Funct. Ecol. 2003;17:151–159. doi:10.1046/j.1365-2435.2003.00715.x [Google Scholar]
- Lawley B, Ripley S, Bridge P, Convey P. Molecular analysis of geographic patterns of eukaryotic diversity in Antarctic soils. Appl. Environ. Microbiol. 2004;70:5963–5972. doi: 10.1128/AEM.70.10.5963-5972.2004. doi:10.1128/AEM.70.10.5963-5972.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. Spatial autocorrelation: trouble or new paradigm? Ecology. 1993;74:1659–1673. doi:10.2307/1939924 [Google Scholar]
- Legendre P, Legendre L. Elsevier; Amsterdam, The Netherlands: 1998. Numerical ecology. [Google Scholar]
- Leibold M.A, et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 2004;7:601–613. doi:10.1111/j.1461-0248.2004.00608.x [Google Scholar]
- LeMasurier W.E, Thomson J.W. American Geophysical Union; Washington, DC: 1990. Volcanoes of the Antarctic plate and southern oceans. [Google Scholar]
- Le Roux P.C, McGeoch M.A, Nyakatya M.J, Chown S.L. Effects of a short-term climate change experiment on a keystone plant species in the sub-Antarctic. Global Change Biol. 2005;11:1628–1639. doi:10.1111/j.1365-2486.2005.001022.x [Google Scholar]
- Lewis Smith R.I. Terrestrial plant biology of the sub-Antarctic and Antarctic. In: Laws R.M, editor. Antarctic ecology. vol. 1. Academic Press; London, UK: 1984. pp. 61–162. [Google Scholar]
- Lewis Smith R.I. Vascular plants as bioindicators of regional warming in Antarctica. Oecologia. 1994;99:322–328. doi: 10.1007/BF00627745. doi:10.1007/BF00627745 [DOI] [PubMed] [Google Scholar]
- Lewis Smith R.I. Plant colonisation on a 45-year sequence of annual micromoraines on a South Georgia glacier foreland. In: Davison W, Howard-Williams C, Broady P, editors. Antarctic ecosystems: models for wider ecological understanding. New Zealand Natural Sciences; Christchurch, New Zealand: 2000. pp. 1–15. [Google Scholar]
- Lewis Smith R.I, Convey P. Enhanced sexual reproduction in bryophytes at high latitudes in the maritime Antarctic. J. Bryol. 2002;24:107–117. doi:10.1179/037366802125000962 [Google Scholar]
- Liebhold A.M, Gurevitch J. Integrating the statistical analysis of spatial data in ecology. Ecography. 2002;25:553–557. doi:10.1034/j.1600-0587.2002.250505.x [Google Scholar]
- Linder H.P. The radiation of the Cape flora, southern Africa. Biol. Rev. 2003;78:597–638. doi: 10.1017/s1464793103006171. doi:10.1017/S1464793103006171 [DOI] [PubMed] [Google Scholar]
- Lonsdale W.M. Global patterns of plant invasions and the concept of invasibility. Ecology. 1999;80:1522–1536. doi:10.2307/176544 [Google Scholar]
- Lud D, Buma A.G.J, Moerdijk T.C.W, Huiskes A.H.L. DNA damage and photosynthesis in Prasiola crispa ssp. Antarctica and Sanionia uncinata in response to manipulated UV-B radiation. In: Huiskes A.H.L, Gieskes W.W.C, Rozema J, Schorno R.M.L, van der Vies S.S, Wolff W.J, editors. Antarctic biology in a global context. Backhuys Publishers; Leiden, The Netherlands: 2003. pp. 69–73. [Google Scholar]
- Margules C.R, Pressey R.L. Systematic conservation planning. Nature. 2000;405:243–253. doi: 10.1038/35012251. doi:10.1038/35012251 [DOI] [PubMed] [Google Scholar]
- Marris J.W.M. The beetle (Coleoptera) fauna of the Antipodes Islands, with comments on the impact of mice; and an annotated checklist of the insect and arachnic fauna. J. R. Soc. N. Zeal. 2000;30:169–195. [Google Scholar]
- Marshall W.A. Aerial dispersal of lichen soredia in the maritime Antarctic. New Phytol. 1996;134:523–530. doi:10.1111/j.1469-8137.1996.tb04370.x [Google Scholar]
- Marshall W.A. Seasonality in Antarctic airborne fungal spores. Appl. Environ. Microbiol. 1997;63:2240–2245. doi: 10.1128/aem.63.6.2240-2245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D.J, Chown S.L. The acarine fauna of Heard Island. Polar Biol. 2002;25:688–695. [Google Scholar]
- Marshall D.J, Coetzee L. Historical biogeography and ecology of a Continental Antarctic mite genus, Maudheimia (Acari, Oribatida): evidence for a Gondwanan origin and Pliocene–Pleistocene speciation. Zoo. J. Linn. Soc. 2000;129:111–128. doi:10.1006/zjls.1999.0209 [Google Scholar]
- Marshall D.J, Convey P. Latitudinal variation in habitat specificity of ameronothroid mites (Oribatida) Exp. Appl. Acarol. 2004;34:21–35. doi: 10.1023/b:appa.0000044437.17333.82. doi:10.1023/B:APPA.0000044437.17333.82 [DOI] [PubMed] [Google Scholar]
- Marshall D.J, Pugh P.J.A. Origin of the inland Acari of Continental Antarctica, with particular reference to Dronning Maud Land. Zoo. J. Linn. Soc. 1996;118:101–118. doi:10.1006/zjls.1996.0051 [Google Scholar]
- Marshall W.A, Chalmers M.O. Airborne dispersal of Antarctic terrestrial algae and cyanobacteria. Ecography. 1997;20:585–594. doi:10.1111/j.1600-0587.1997.tb00427.x [Google Scholar]
- Marshall W.A, Convey P. Dispersal of moss propagules on Signy Island, maritime Antarctic. Polar Biol. 1997;18:376–383. doi:10.1007/s003000050203 [Google Scholar]
- Maslen N.R, Convey P. Nematode diversity and distribution in the southern maritime Antarctic—clues to history? Soil Biol. Biochem. 2006;38:3141–3151. doi:10.1016/j.soilbio.2005.12.007 [Google Scholar]
- McDougall I, Verwoerd W, Chevallier L. K–Ar geochronology of Marion Island, Southern Ocean. Geol. Mag. 2001;138:1–17. doi:10.1017/S0016756801005039 [Google Scholar]
- McGeoch M.A, Price P.W. Spatial abundance structures in an assemblage of gall-forming sawflies. J. Anim. Ecol. 2004;73:506–516. doi:10.1111/j.0021-8790.2004.00825.x [Google Scholar]
- McGeoch M.A, le Roux P.C, Hugo A.E, Chown S.L. Species and community responses to short-term climate manipulation: microarthropods in the sub-Antarctic. Aust. Ecol. 2006;31:719–731. doi:10.1111/j.1442-9993.2006.01614.x [Google Scholar]
- McGraw J.B, Day T.A. Size and characteristics of a natural seed bank in Antarctica. Arct. Alpine Res. 1997;29:213–216. doi:10.2307/1552048 [Google Scholar]
- McIntosh E, Walton D.W.H. Government of South Georgia and South Sandwich Islands; South Georgia, UK: 2000. Environmental management plan for South Georgia. [Google Scholar]
- McKinney M.L. Influence of settlement time, human population, park shape and age, visitation and roads on the number of alien plant species in protected areas in the USA. Divers. Distrib. 2002;8:311–318. doi:10.1046/j.1472-4642.2002.00153.x [Google Scholar]
- Mercer R.D, Chown S.L, Marshall D.J. Mite and insect zonation on a Marion Island rocky shore: a quantitative approach. Polar Biol. 2000;23:775–784. doi:10.1007/s003000000151 [Google Scholar]
- Muñoz J, Felicísimo A.M, Cabezas F, Burgaz A.R, Martínez I. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science. 2004;304:1144–1147. doi: 10.1126/science.1095210. doi:10.1126/science.1095210 [DOI] [PubMed] [Google Scholar]
- Myburgh M, Chown S.L, Daniels S.R, Jansen van Vuuren B. Population structure, propagule pressure, and conservation biogeography in the sub-Antarctic: lessons from indigenous and invasive springtails. Divers. Distrib. 2007;13:143–154. [Google Scholar]
- Naveen R, Forrest S.C, Dagit R.G, Blight L.K, Trivelpiece W.Z, Trivelpiece S.G. Zodiac landings by tourist ships in the Antarctic Peninsula region, 1989–99. Polar Rec. 2001;37:121–132. [Google Scholar]
- Newsham K.K. UV-B radiation arising from stratospheric ozone depletion influences the pigmentation of the Antarctic moss Andreaea regularis. Oecologia. 2003;135:327–331. doi: 10.1007/s00442-003-1191-x. [DOI] [PubMed] [Google Scholar]
- Newsham K.K, Hodgson D.A, Murray A.W.A, Peat H.J, Lewis Smith R.I. Response of two Antarctic bryophytes to stratospheric ozone depletion. Global Change Biol. 2002;8:972–983. doi:10.1046/j.1365-2486.2002.00509.x [Google Scholar]
- Ochyra, R., Bednarek-Ochyra, H. & Lewis Smith, R. I. In press. The moss flora of Antarctica Cambridge, UK: Cambridge University Press.
- Orme C.D, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. doi:10.1038/nature03850 [DOI] [PubMed] [Google Scholar]
- Ovaskainen O, Hanski I. The species–area relationship derived from species-specific incidence functions. Ecol. Lett. 2003;6:903–909. doi:10.1046/j.1461-0248.2003.00517.x [Google Scholar]
- Øvstedal D.O, Gremmen N.J.M. The lichen flora of Marion and Prince Edward islands. S. Afr. J. Bot. 2001;67:552–572. [Google Scholar]
- Øvstedal D.O, Lewis Smith R.I. Cambridge University Press; Cambridge, UK: 2001. Lichens of Antarctica and South Georgia. A guide to their identification and ecology. [Google Scholar]
- Page T.J, Linse K. More evidence of speciation and dispersal across the Antarctic Polar Front through molecular systematics of Southern Ocean Limatula (Bivalvia: Limidae) Polar Biol. 2002;25:818–826. [Google Scholar]
- Peat H.J, Clarke A, Convey P. Diversity and biogeography of the Antarctic flora. J. Biogeogr. 2007;34:132–146. doi:10.1111/j.1365-2699.2006.01565.x [Google Scholar]
- Peck L, Convey P, Barnes D.K.A. Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol. Rev. 2006;81:75–109. doi: 10.1017/S1464793105006871. doi:10.1017/S1464793105006871 [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Ecol. Lett. 2003;6:265–272. doi:10.1046/j.1461-0248.2003.00428.x [Google Scholar]
- Powers L.E, Ho M, Freckman D.W, Virginia R.A. Distribution, community structure, and microhabitats of soil invertebrates along an elevational gradient in Taylor Valley, Antarctica. Arct. Alpine Res. 1998;30:133–141. doi:10.2307/1552128 [Google Scholar]
- Prentice M.L, Bockheim J.G, Wilson S.C, Burckle L.H, Hodell D.A, Schlüchter C, Kellogg D.E. Late Neogene Antarctic glacial history: evidence from central Wright Valley. In: Kennett J, Warnke D, editors. The Antarctic paleoenvironment: a perspective on global change. Antarctic reseach series. vol. 60. American Geophysical Union; Washington, DC: 1993. pp. 207–250. [Google Scholar]
- Pugh P.J.A. A synonymic catalogue of the Acari from Antarctica, the sub-Antarctic Islands and the Southern Ocean. J. Nat. Hist. 1993;27:323–421. [Google Scholar]
- Pugh P.J.A, Scott B. Biodiversity and biogeography of non-marine Mollusca on the islands of the Southern Ocean. J. Nat. Hist. 2002;36:927–952. doi:10.1080/00222930110034562 [Google Scholar]
- Pugh P.J.A, Dartnall H.J.G, McInnes S.J. The non-marine Crustacea of Antarctica and the islands of the Southern Ocean: biodiversity and biogeography. J. Nat. Hist. 2002;36:1047–1103. doi:10.1080/00222930110039602 [Google Scholar]
- Quayle W.C, Peck L.S, Peat H, Ellis-Evans J.C, Harrigan P.R. Extreme responses to climate change in Antarctic lakes. Science. 2002;295:645. doi: 10.1126/science.1064074. doi:10.1126/science.1064074 [DOI] [PubMed] [Google Scholar]
- Quayle W.C, Convey P, Peck L.S, Ellis-Evans J.C, Butler H.G, Peat H.J. Ecological responses of maritime Antarctic lakes to regional climate change. In: Domack E, Burnett A, Leventer A, Convey P, Kirby M, Bindschadler R, editors. Antarctic Peninsula climate variability: historical and palaeoenvironmental perspectives. American Geophysical Union; Washington, DC: 2003. pp. 159–170. [Google Scholar]
- Quilty P.G. Significance of evidence for changes in the Antarctic marine environment over the last 5 million years. In: Kerry K.R, Hempel G, editors. Antarctic ecosystems. Ecological change and conservation. Springer; Berlin, Germany: 1990. pp. 3–8. [Google Scholar]
- Quilty P.G, Wheller G. Heard Island and the McDonald Islands: a window into the Kerguelen Plateau. Pap. Proc. R. Soc. Tasman. 2000;133:1–12. [Google Scholar]
- Rangel T.F.L.V.B, Diniz-Filho J.A.F. Neutral community dynamics, the mid-domain effect and spatial patterns in species richness. Ecol. Lett. 2005;8:783–790. doi:10.1111/j.1461-0248.2005.00786.x [Google Scholar]
- Richardson D.M, van Wilgen B.W. Invasive alien plants in South Africa: how well do we understand the ecological impacts? S. Afr. J. Sci. 2004;100:45–52. [Google Scholar]
- Ricklefs R.E. Community diversity: relative roles of local and regional processes. Science. 1987;235:167–171. doi: 10.1126/science.235.4785.167. doi:10.1126/science.235.4785.167 [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 2004;7:1–15. doi:10.1046/j.1461-0248.2003.00554.x [Google Scholar]
- Ries G, Heller W, Puchta H, Sandermann H, Seidlitz B.K, Hohn B. Elevated UV-B radiation reduces genome stability in plants. Nature. 2000;406:98–101. doi: 10.1038/35017595. doi:10.1038/35017595 [DOI] [PubMed] [Google Scholar]
- Robinson S.A, Wasley J, Tobin A.K. Living on the edge—plants and global change in continental and maritime Antarctica. Global Change Biol. 2003;9:1681–1717. doi:10.1046/j.1365-2486.2003.00693.x [Google Scholar]
- Roff D. Optimizing development time in a seasonal environment: the ‘ups and downs’ of clinal variation. Oecologia. 1980;45:202–208. doi: 10.1007/BF00346461. doi:10.1007/BF00346461 [DOI] [PubMed] [Google Scholar]
- Rogers A D. Evolution and biodiversity of Antarctic organisms: a molecular perspective. Phil. Trans. R. Soc. B. 2007;362:2191–2214. doi: 10.1098/rstb.2006.1948. doi:10.1098/rstb.2006.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde K. Latitudinal gradients in species diversity: the search for the primary cause. Oikos. 1992;65:514–527. doi:10.2307/3545569 [Google Scholar]
- Rosenzweig M.L. Cambridge University Press; Cambridge, UK: 1995. Species diversity in space and time. [Google Scholar]
- Roy K, Balch D.P, Hellberg M.E. Spatial patterns of morphological diversity across the Indo-Pacific: analyses using strombid gastropods. Proc. R. Soc. B. 2001;268:2503–2508. doi: 10.1098/rspb.2000.1428. doi:10.1098/rspb.2000.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P.G, Watkins B.P. The influence of physical factors and ornithogenic products on plant and arthropod abundance at an inland nunatak group in Antarctica. Polar Biol. 1989;10:151–160. [Google Scholar]
- Scott L. Palynological indications of the Quaternary vegetation history of Marion Island (sub-Antarctic) J. Biogeogr. 1985;12:413–431. doi:10.2307/2844951 [Google Scholar]
- Scott J.J. Changes in vegetation on Heard Island 1947–1987. In: Kerry K.R, Hempel G, editors. Antarctic ecosystems. Ecological change and conservation. Springer; Berlin, Germany: 1990. pp. 61–76. [Google Scholar]
- Scott L, Hall K.J. Palynological evidence for interglacial vegetation cover on Marion Island, subantarctic. Paleogeog. Paleoclimatol. Paleoecol. 1983;41:35–43. doi:10.1016/0031-0182(83)90074-3 [Google Scholar]
- Selkirk P.M, Skotnicki M.L, Adam K.D, Connett M.B, Dale T, Joe T.W, Armstrong J. Genetic variation in Antarctic populations of the moss Sarconeurum glaciale. Polar Biol. 1997;18:344–350. doi:10.1007/s003000050198 [Google Scholar]
- Selkirk P.M, Skotnicki M.L, Ninham J.A, Connett M.B, Armstrong J. Genetic variation and dispersal of Bryum argenteum and Hennediella heimii populations in the Garwood Valley, southern Victoria Land, Antarctica. Antarct. Sci. 1998;10:423–430. [Google Scholar]
- Sinclair B.J. On the distribution of terrestrial invertebrates at Cape Bird, Ross Island, Antarctica. Polar Biol. 2001;24:394–400. doi:10.1007/s003000000223 [Google Scholar]
- Sinclair B.J. Effects of increased temperatures simulating climate change on terrestrial invertebrates on Ross Island, Antarctica. Pedobiologia. 2002;46:150–160. doi:10.1078/0031-4056-00121 [Google Scholar]
- Sinclair B.J, Chown S.L. Rapid responses to high temperature and desiccation but not to low temperature in the freeze tolerant sub-Antarctic caterpillar Pringleophaga marioni (Lepidoptera, Tineidae) J. Insect Physiol. 2003;49:45–52. doi: 10.1016/s0022-1910(02)00225-1. doi:10.1016/S0022-1910(02)00225-1 [DOI] [PubMed] [Google Scholar]
- Sinclair B.J, Chown S.L. Climatic variability and hemispheric differences in insect cold tolerance: support from southern Africa. Funct. Ecol. 2005a;19:214–221. doi:10.1111/j.1365-2435.2005.00962.x [Google Scholar]
- Sinclair B.J, Chown S.L. Deleterious effects of repeated cold exposure in a freeze-tolerant sub-Antarctic caterpillar. J. Exp. Biol. 2005b;208:969–879. doi: 10.1242/jeb.01455. doi:10.1242/jeb.01455 [DOI] [PubMed] [Google Scholar]
- Sinclair B.J, Chown S.L. Caterpillars benefit from thermal ecosystem engineering by wandering albatrosses on sub-Antarctic Marion Island. Biol. Lett. 2006;2:51–54. doi: 10.1098/rsbl.2005.0384. doi:10.1098/rsbl.2005.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair B.J, Sjursen H. Terrestrial invertebrate abundance across a habitat transect in Kebble Valley, Ross Island, Antarctica. Pedobiologia. 2001;45:134–145. doi:10.1078/0031-4056-00075 [Google Scholar]
- Sinclair B.J, Klok C.J, Scott M.B, Terblanche J.S, Chown S.L. Diurnal variation in supercooling points of three species of Collembola from Cape Hallett, Antarctica. J. Insect Physiol. 2003a;49:1049–1061. doi: 10.1016/j.jinsphys.2003.08.002. doi:10.1016/j.jinsphys.2003.08.002 [DOI] [PubMed] [Google Scholar]
- Sinclair B.J, Vernon P, Klok C.J, Chown S.L. Insects at low temperatures: an ecological perspective. Trends Ecol. Evol. 2003b;18:257–262. doi:10.1016/S0169-5347(03)00014-4 [Google Scholar]
- Sinclair B.J, Addo-Bediako A, Chown S.L. Climatic variability and the evolution of insect freeze tolerance. Biol. Rev. 2003c;78:181–195. doi: 10.1017/s1464793102006024. doi:10.1017/S1464793102006024 [DOI] [PubMed] [Google Scholar]
- Sinclair B.J, Scott M.B, Klok C.J, Terblanche J.S, Marshall D.J, Reyers B, Chown S.L. A survey of terrestrial arthropods at Cape Hallett, North Victoria Land, Antarctica. Antarct. Sci. 2006;18:303–312. [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Beard C. RAPD profiling of genetic diversity in two populations of the moss Ceratodon purpureus in Victoria Land, Antarctica. Polar Biol. 1998a;20:172–176. doi:10.1007/s003000050231 [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Ninham J.A. RAPD analysis of genetic variation and dispersal of the moss Bryum pseudotriquetrum from Southern Victoria Land, Antarctica. Polar Biol. 1998b;20:121–126. doi:10.1007/s003000050285 [Google Scholar]
- Skotnicki M.L, Ninham J.A, Selkirk P.M. Genetic diversity and dispersal of the moss Sarconeurum glaciale on Ross Island, East Antarctica. Mol. Ecol. 1999a;8:753–762. doi:10.1046/j.1365-294X.1999.00619.x [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Ninham J.A. RAPD analysis of genetic variation and dispersal of the moss Bryum argenteum in Ross Island and Victoria Land, Antarctica. Polar Biol. 1999b;21:417–422. doi:10.1007/s003000050382 [Google Scholar]
- Skotnicki M.L, Ninham J.A, Selkirk P.M. Genetic diversity, mutagenesis and dispersal of Antarctic mosses—a review of progress with molecular studies. Antarct. Sci. 2000;12:363–373. [Google Scholar]
- Skotnicki M.L, Selkirk P.M, Broady P, Adam K.D, Ninham J.A. Dispersal of the moss Campylopus pyriformis on geothermal ground near the summits of Mount Erebus and Mount Melbourne, Victoria Land, Antarctica. Antarct. Sci. 2001;13:280–285. doi:10.1017/S0954102001000396 [Google Scholar]
- Skotnicki M.L, Bargagli R, Ninham J.A. Genetic diversity in the moss Pohlia nutans on geothermal ground of Mount Rittmann, Victoria Land, Antarctica. Polar Biol. 2002;25:771–777. [Google Scholar]
- Skotnicki M.L, Mackenzie A.M, Ninham J.A, Selkirk P.M. High levels of genetic variability in the moss Ceratodon purpureus from continental Antarctica, subAntarctic Heard and Macquarie Islands, and Australasia. Polar Biol. 2004;27:687–698. doi:10.1007/s00300-004-0640-2 [Google Scholar]
- Skottsberg C. Remarks on plant geography of the southern cold temperate zone. Proc. R. Soc. B. 1960;152:447–457. doi:10.1098/rspb.1960.0053 [Google Scholar]
- Slabber S, Chown S.L. Differential responses of thermal tolerance to acclimation in the sub-Antarctic rove beetle Halmaeusa atriceps. Physiol. Entomol. 2005;30:195–204. doi:10.1111/j.1365-3032.2005.00448.x [Google Scholar]
- Slabber S, Worland M.R, Leinaas H.P, Chown S.L. Acclimation effects on thermal tolerances of springtails from sub-Antarctic Marion Island: indigenous and invasive species. J. Insect Physiol. 2007;53:113–125. doi: 10.1016/j.jinsphys.2006.10.010. doi:10.1016/j.jinsphys.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Smith H.G. Diversity of Antarctic terrestrial protozoa. Biodivers. Conserv. 1996;5:1379–1394. doi:10.1007/BF00051984 [Google Scholar]
- Smith V.R, French D.D. Patterns of variation in the climates, soils and vegetation of some subantarctic and Antarctic islands. S. Afr. J. Bot. 1988;54:35–46. [Google Scholar]
- Smith V.R, Steenkamp M. Climatic change and its ecological implications at a subantarctic island. Oecologia. 1990;85:14–24. doi: 10.1007/BF00317338. doi:10.1007/BF00317338 [DOI] [PubMed] [Google Scholar]
- Smith V.R, Steenkamp M, Gremmen N.J.M. Terrestrial habitats on sub-Antarctic Marion Island: their vegetation, edaphic attributes, distribution and response to climate change. S. Afr. J. Bot. 2001;67:641–654. [Google Scholar]
- Smith V.H, Foster B.L, Grover J.P, Holt R.D, Leibold M.A, deNoyelles F. Phytoplankton species richness scales consistently from laboratory microcosms to the world's oceans. Proc. Natl Acad. Sci. USA. 2005;102:4393–4396. doi: 10.1073/pnas.0500094102. doi:10.1073/pnas.0500094102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlenius B, Boström S. The geographic distribution of metazoan microfauna on East Antarctic nunataks. Polar Biol. 2005;28:439–448. doi:10.1007/s00300-004-0708-z [Google Scholar]
- Sohlenius B, Boström S, Hirshcfelder A. Distribution patterns of microfauna (nematodes, rotifers and tardigrades) on nunataks in Dronning Maud land, East Antarctica. Polar Biol. 1996;16:191–200. [Google Scholar]
- Sømme L, Block W. Cold hardiness of Collembola at Signy Island, maritime Antarctic. Oikos. 1982;38:168–176. doi:10.2307/3544016 [Google Scholar]
- Spicer J.I, Gaston K.J. Blackwell Science; Oxford, UK: 1999. Physiological diversity and its ecological implications. [Google Scholar]
- Stachowicz J.J, Terwin J.R, Whitlatch R.B, Osman R.W. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc. Natl Acad. Sci. USA. 2002;99:15 497–15 500. doi: 10.1073/pnas.242437499. doi:10.1073/pnas.242437499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.I, Hogg I.D. Expanded distributional records of Collembola and Acari in southern Victoria Land, Antarctica. Pedobiologia. 2002;46:485–495. doi:10.1078/0031-4056-00154 [Google Scholar]
- Stevens M.I, Hogg I.D. Long-term isolation and recent range expansion from glacial refugia revealed for the endemic springtail Gomphiocephalus hodgsoni from Victoria Land, Antarctica. Mol. Ecol. 2003;12:2357–2369. doi: 10.1046/j.1365-294x.2003.01907.x. doi:10.1046/j.1365-294X.2003.01907.x [DOI] [PubMed] [Google Scholar]
- Stevens M.I, Greenslade P, Hogg I.D, Sunnucks P. Southern hemisphere springtails: could any have survived glaciation of Antarctica? Mol. Biol. Evol. 2006;23:874–882. doi: 10.1093/molbev/msj073. doi:10.1093/molbev/msj073 [DOI] [PubMed] [Google Scholar]
- Terblanche J.S, Marais E, Chown S.L. Stage-related variation in rapid cold hardening as a test of the environmental predictability hypothesis. J. lnsect. Physiol. 2007;53:455–462. doi: 10.1016/j.jinsphys.2007.01.006. doi:10.1016/j.jinsphys.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Thomson J.D, Weiblen G, Thomson B.A, Alfaro S, Legendre P. Untangling multiple factors in spatial distributions: lilies, gophers, and rocks. Ecology. 1996;77:1698–1715. doi:10.2307/2265776 [Google Scholar]
- Thomas C.D, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. doi:10.1038/nature02121 [DOI] [PubMed] [Google Scholar]
- Treseder K.K, Vitousek P.M. Potential ecosystem-level effects of genetic variation among populations of Metrosideros polymorpha from a soil fertility gradient in Hawaii. Oecologia. 2001;126:266–275. doi: 10.1007/s004420000523. doi:10.1007/s004420000523 [DOI] [PubMed] [Google Scholar]
- Usher M.B, Booth R.G. Arthropod communities in a maritime Antarctic moss-turf habitat: three-dimensional distribution of mites and collembola. J. Anim. Ecol. 1984;53:427–441. doi:10.2307/4526 [Google Scholar]
- Usher M.B, Booth R.G. Arthropod communities in a maritime Antarctic moss-turf habitat: multiple scales of pattern in the mites and Collembola. J. Anim. Ecol. 1986;55:155–170. doi:10.2307/4699 [Google Scholar]
- Usher M.B, Edwards M. The selection of conservation areas in Antarctica: an example using the arthropod fauna of Antarctic islands. Environ. Conserv. 1986;13:115–122. [Google Scholar]
- Van Aarde R.J, Ferreira S, Wassenaar T, Erasmus D.G. With the cats away the mice may play. S. Afr. J. Sci. 1996;92:357–358. [Google Scholar]
- Van der Merwe M, Chown S.L, Smith V.R. Thermal tolerance limits in six weevil species (Coleoptera, Curculionidae) from sub-Antarctic Marion Island. Polar Biol. 1997;18:331–336. doi:10.1007/s003000050196 [Google Scholar]
- Vernon P, Voisin J.-F. Faune entomologique de la Grande Ile des Apôtres (Archipel Crozet, Océan Indien Austral) Bull. Soc. Entomol. Fr. 1990;95:263–268. [Google Scholar]
- Vernon P, Vannier G, Trehen P. A comparative approach to the entomological diversity of polar regions. Acta Oecol. 1998;19:303–308. doi:10.1016/S1146-609X(98)80034-9 [Google Scholar]
- Vickery J.A, Bradbury R.B, Henderson I.G, Eaton M.A, Grice P.V. The role of agri-environment schemes and farm management practices in reversing the decline of farmland birds in England. Biol. Conserv. 2004;119:19–39. doi:10.1016/j.biocon.2003.06.004 [Google Scholar]
- Vincent W.F. Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct. Sci. 2000;12:374–385. [Google Scholar]
- Vogel M. The distribution and ecology of epigeic invertebrates on the sub-Antarctic Island of South Georgia. Spixiana. 1985;8:153–163. [Google Scholar]
- Waide R.B, Willig M.R, Steiner C.F, Mittelbach G, Gough L, Dodson S.I, Juday G.P, Parmenter R. The relationship between productivity and species richness. Annu. Rev. Ecol. Syst. 1999;30:257–300. doi:10.1146/annurev.ecolsys.30.1.257 [Google Scholar]
- Wall D.H, Virginia R.A. Controls on soil biodiversity: insights from extreme environments. Appl. Soil Ecol. 1999;13:137–150. doi:10.1016/S0929-1393(99)00029-3 [Google Scholar]
- Walther G.-R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.-M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Watson R.T. Cambridge University Press; Cambridge, UK: 2002. Climate change 2001: synthesis report. [Google Scholar]
- West C. Life histories of three species of sub-Antarctic oribatid mite. Pedobiologia. 1982;23:59–67. [Google Scholar]
- West-Eberhard M.J. Oxford University Press; Oxford, UK: 2003. Developmental plasticity and evolution. [Google Scholar]
- Whitham T.G, et al. Community and ecosystem genetics: a consequence of the extended phenotype. Ecology. 2003;84:559–573. [Google Scholar]
- Wiens J.A. Spatial scaling in ecology. Funct. Ecol. 1989;3:385–397. doi:10.2307/2389612 [Google Scholar]
- Wilkinson D.M. What is the upper size limit for cosmopolitan distribution in free-living microorganisms? J. Biogeogr. 2001;28:285–291. doi:10.1046/j.1365-2699.2001.00518.x [Google Scholar]
- Willig M.R, Kaufman D.M, Stevens R.D. Latitudinal gradients of biodiversity: pattern, process, scale and synthesis. Annu. Rev. Ecol. Syst. 2003;34:273–309. doi:10.1146/annurev.ecolsys.34.012103.144032 [Google Scholar]
- Witman J.D, Etter R.J, Smith F. The relationship between regional and local species diversity in marine benthic communities: a global perspective. Proc. Natl Acad. Sci. USA. 2004;101:15 664–15 669. doi: 10.1073/pnas.0404300101. doi:10.1073/pnas.0404300101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehler E.J, et al. Scientific Committee on Antarctic Research; Cambridge, UK: 2001. A statistical assessment of the stautus and trends of Antarctic and subantarctic seabirds. [Google Scholar]
- Woods H.A, Harrison J.F. Interpreting rejections of the beneficial acclimation hypothesis: when is physiological plasticity adaptive? Evolution. 2002;56:1863–1866. doi: 10.1111/j.0014-3820.2002.tb00201.x. doi:10.1554/0014-3820(2002)056[1863:IROTBA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Worland M.R. Factors that influence freezing in the sub-Antarctic springtail Tullbergia antarctica. J. Insect Physiol. 2005;51:881–894. doi: 10.1016/j.jinsphys.2005.04.004. doi:10.1016/j.jinsphys.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Worland M.R, Convey P. Rapid cold hardening in Antarctic microarthropods. Funct. Ecol. 2001;15:515–524. doi:10.1046/j.0269-8463.2001.00547.x [Google Scholar]
- Worland M.R, Leinaas H.-P, Chown S.L. Supercooling point frequency distributions in Collembola are affected by moulting. Funct. Ecol. 2006;20:323–329. doi:10.1111/j.1365-2435.2006.01089.x [Google Scholar]
- Wynn-Williams D.D. Antarctic microbial diversity: the basis of polar ecosystem processes. Biodivers. Conserv. 1996;5:1271–1293. doi:10.1007/BF00051979 [Google Scholar]
- Younge A, Fowkes S. The Cape Action Plan for the environment: overview of an ecoregional planning process. Biol. Conserv. 2003;112:15–28. doi:10.1016/S0006-3207(02)00393-2 [Google Scholar]