Abstract
Low diversity ecosystems are expected to be more vulnerable to global changes although they have received less attention than high diversity ecosystems. Addressing the present state of the Antarctic Dry Valley region by focusing on the potential global changes that may alter the coupling of above- and below-ground species and ecosystem processes is a realistic and critical need that has value beyond the Antarctic community. Presented here are suggested implications of global change on the Dry Valley terrestrial systems and how these effects might be manifested in the future.
Keywords: soil, biodiversity, Antarctica, Dry Valleys, climate change
1. Introduction
Antarctic scientists are increasingly being asked to explain the relevance of their investigations in regions with low biodiversity, such as the McMurdo Dry Valleys (approx. 78° S), to those regions having higher biodiversity and experiencing unprecedented and rapid transformations. Antarctica's terrestrial biodiversity is not immune to global changes affecting biodiversity and ecosystem functioning elsewhere (Kennedy 1995; Freckman & Virginia 1997; Sinclair 2002; Cowan & Tow 2004; Frenot et al. 2005; Turner et al. 2005; Wall 2005). However, less attention is given to changes affecting these Antarctic ecosystems, possibly because the ice-free land mass is less than 0.5% (332 000 km2) of the continent (Drewry et al. 1982) and the role of the few species in the functioning of these cold desert terrestrial ecosystems seems minor compared with physical controls (Campbell & Claridge 1987; Fountain et al. 1999) or compared with the role of less cryptic biota in the maritime Antarctic or Arctic regions (Convey 2003; Callaghan et al. 2004).
In the Arctic, there are profound and obvious global changes affecting biodiversity, ecosystems and humans at local and regional scales (Walker et al. 2001). The Antarctic, in contrast, has no indigenous human population and terrestrial natural resource use has been negligible, as have opportunities to investigate effects of changes on resource distribution and availability. Therefore, it is still unclear whether similar changes occurring to low diversity terrestrial ecosystems such as in the McMurdo Dry Valleys are of consequence, and if they extend to and influence biodiversity and ecosystem functioning and, thus, human well-being, elsewhere (Wall 2004, 2005).
Globally, humans are causing major perturbations and significant shifts in the functioning of ecosystems. For example, there is a rapid acceleration of species extinctions, increased exchange of species, losses of populations of species and multiple changes to global nitrogen, carbon and hydrological cycles (Vitousek et al. 1997; Millennium Ecosystem Assessment 2005). A large proportion of the Earth's terrestrial vascular plant and vertebrate species are concentrated in warmer, tropical and temperate ecosystems and have become a priority for conservation, due to the high species richness (number of species), concentration of endemic species and the role of these species in ecosystem functioning (Dobson et al. 1997; Reid 1998; Myers et al. 2000; Araujo 2002; Brooks et al. 2002; Hobohm 2003). Areas of poor plant and animal species diversity, or ‘coldspots’, have also gained attention as a high priority for conservation because loss of a few species in these ecosystems due to global changes may more quickly result in loss of ecosystem function (Kareiva & Marvier 2003a,b). Although Antarctica is managed for scientific research (Waterhouse 1997) and could be considered as protected as agreed by nations signing the Antarctic Treaty, it is not isolated from human-related, global changes (land use change, pollution, invasive species and atmospheric change). Local to regional responses that occur in this polar desert may be magnified and have nonlinear feedbacks to the Dry Valleys and other distant ecosystems.
Global changes have tremendous significance for human well-being (Millennium Ecosystem Assessment 2005). People are dependent on benefits, such as clean air, water, fertile soils and nutrient cycles, which are provided by nature's ecosystems. It is now apparent (Millennium Ecosystem Assessment 2005) that the future ability of the Earth's biodiversity and ecosystems to provide these services is endangered. As governments, businesses and the public are making international efforts to address the enormity of these problems over the next 20–50 years, it is appropriate for the Antarctic scientific community to assess the state of knowledge of low diversity ecosystems and with data from more diverse systems develop scenarios of how biodiversity and ecosystems may change in the next 50 years. Compilation of information such as species distributions, vulnerabilities and potential global changes, which may shift the functioning of species-poor ecosystems to a new state, is highly relevant and needed for understanding and sustaining more diverse ecosystems and our global environment.
This paper does not attempt to compile information on global changes (Kennedy 1995; Sinclair 2002; Frenot et al. 2005), species diversity or distributions and functioning for all terrestrial habitats in Antarctica (but see Chown & Convey 2007). Instead, using one region of low diversity, the McMurdo Dry Valleys, I suggest ways in which biodiversity and ecosystem functioning in above- and below-ground coupled systems may be affected by a few of these global changes and the potential implications for the region. The relatively minimal anthropogenic activity compared with the level seen in complex systems, combined with the low biodiversity and relatively simple terrestrial habitats, helps us understand that disturbances in the Dry Valleys influencing species and ecosystem function can be extrapolated to more complex ecosystems (Freckman & Virginia 1997).
2. The McMurdo dry valley region
Known as one of the most extreme environments in the world, the McMurdo Dry Valleys (76°30′–78°00′ S, 160°00′–1645°00′ E) of southern Victoria Land, are the largest (4800 km2) ice-free region on the Antarctic continent, and are composed of multiple valleys formed by the Transantarctic mountain range near the Ross Sea. They are thought to have the lowest species diversity on Earth (Procter 1984; Adams et al. 2006). These valleys vary in size, elevation, geological age and orientation to the polar plateau, all of which contribute to each valley having unique local environments (Moorhead & Priscu 1998; Bockheim 2002). Valleys are typically characterized by a mosaic of glaciers, perennially ice-covered lakes, ephemeral meltstreams and wide expanses of exposed soil underlain with ice and permafrost at 10–50 cm depth (Pastor & Bockheim 1980; Bockheim 1997). Each of these habitats contains viable life, including invertebrates that are capable of surviving severe climatic conditions and returning to activity when liquid water becomes available. For information on species diversity, abundance and role in ecosystem functioning of glaciers see Fountain et al. 1998, 1999, 2004a,b; Porazinska et al. 2004; Ebnet et al. 2005 and for streams see Utermohl 1958; Broady 1982; Vincent & Howard-Williams 1986; Alger et al. 1997; McKnight et al. 1998; Barrett et al. 2004. A synthesis of lake biodiversity is yet to be compiled (but see Fritsen & Priscu 1999; Priscu et al. 1999; Roberts & Laybourn-Parry 1999; Laybourn-Parry & Pearce 2007). For soils, reviews of Victoria Land terrestrial ecosystems include information on the McMurdo Dry Valley biodiversity and distributions (Adams et al. 2006), biotic interactions (Hogg et al. 2006) and ecosystem functioning (Barrett et al. 2006a,b). The emphasis of this paper will be the large land portion of the McMurdo Dry Valleys, which is heterogeneous: bare ground with sand to soil that can be free of, or patterned with, small pebbles to large-size boulders and intersected by moist to aquatic habitats, such as ponds, snow patches, lake margins and meltstreams.
3. Above- and below-ground interactions
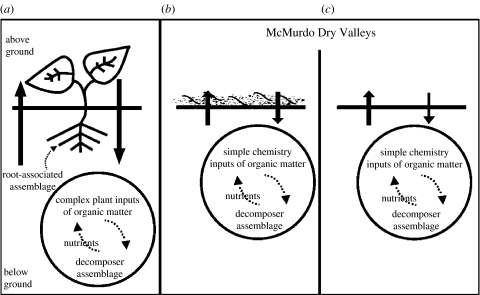
To understand how the McMurdo Dry Valley systems may change, consider the linked above- and below-ground subsystem approach (figure 1a) employed to examine the regulation and functional significance of biodiversity in all plant-dominated terrestrial ecosystems (Porazinska et al. 2003; Wardle et al. 2004; Bardgett et al. 2005a; De Deyn & Van der Putten 2005). A common feature of terrestrial ecosystems with vascular plants is coupled above- and below-ground systems that interact through positive and negative feedbacks to influence and regulate community and ecosystem functioning (Wardle et al. 2004; Bardgett et al. 2005a). Above-ground plant species communities differ in the quantity of biomass and quality—or chemical complexity and structural composition—of the organic matter they produce (Swift et al. 1979). As the above-ground plant parts and below-ground roots die, the quantity and quality of the organic matter becomes the basis for the energy and functioning of the below-ground community and contributes to soil habitat heterogeneity.
Figure 1.
Above-ground and below-ground interactions in (a) vascular plant-dominated ecosystems, compared to the terrestrial landscape of the McMurdo Dry Valleys. In the Dry Valleys, the highest productivity and diversity are found in the smallest area of the landscape, (b) the algal, moss and cyanobacterial-dominated above- and below-ground systems, and the lowest diversity in the largest area of the landscape (see text), (c) the dry soil-dominated ecosystems. Note the vascular plant dominated system (a) has greater depth compared with the shallow Dry Valley systems (b,c). Vertical arrows indicate the feedbacks and contributions to ecosystem processes above and below ground.
The below-ground system, in turn, responds by breaking down dead organic matter (Wall et al. 2004, 2005), altering plant metabolism, regulating the availability of soil nutrients for plant growth (Hunt et al. 1987; Moore et al. 2004), affecting plant community composition and, possibly, influencing plant distributions (Van der Putten et al. 2001; De Deyn et al. 2003; De Deyn & Van der Putten 2005). Soil, climate and the variation in quantity and quality (e.g. C : N and lignin) of organic matter inputs from the plant can drive the species composition of the below-ground community (Swift et al. 1979; Wardle 2002; Bardgett et al. 2005b). In the world's terrestrial soils, for example, the numbers or types of species (or OTUs—operational taxonomic units) may be in the millions of genomes of microbes per 10 g soil (Gans et al. 2005) and there may be hundreds of species of microscopic animals, rotifers, tardigrades, nematodes, mites, Collembola, and many species of larger animals such as isopods, millipedes, earthworms, termites and ants (Wardle et al. 2004). Each of these has roles in ecosystems (table 1) through their interactions within complex food webs.
Table 1.
Examples of some of the taxa below ground, the functional groups within soil food webs and their role in ecosystem processes in plant-dominated ecosystems. (The biodiversity of each functional group regulates or influences various ecosystem processes and thus provides benefits or ecosystem service for human well-being. See Wall (2004) and the Millennium Ecosystem Assessment (2005).)
| biodiversity | functional groups | ecosystem processes |
|---|---|---|
| vertebrates, invertebrates (oligochaetaes, polychaetaes, crustaceans, molluscs, ants, termites), plant roots | bioturbators, ecosystem engineers | soil alteration, soil structure, organic matter and microbes mixing laterally and vertical depths |
| plant roots, algae | primary producers | create biomass, stabilize soils and sediments, provide C and N at depth |
| millipedes, centipedes | shredders | fragment, rip and tear organic matter |
| bacteria and fungi, Collembola, mites, nematodes, rotifers, tardigrades, protozoa | primary decomposers and secondary decomposers | recycle nutrients, increase nutrient availability for primary production |
| symbiotic (e.g. Rhizobium) and asymbiotic bacteria (e.g. Cyanobacter, Azobacter) | nitrogen fixers | biologically fix atmospheric N2 |
| methanogenic bacteria, denitrifying bacteria | trace gas producers | C, N2, N2O, CH4 transfer and denitrification |
| roots, soil organisms | CO2 producers | respiration, emission of CO2 |
There are two soil food webs, root-associated and decomposer, with different functions. Root-associated webs affect plant architecture, metabolism and growth, while decomposer-based webs influence organic matter breakdown, nutrient availability for roots, and soil morphology by modifying soil porosity, soil structure and hydrology (Anderson 1988; Crossley et al. 1989; Bardgett et al. 2001; Hunt & Wall 2002; Wall 2004). Macroinvertebrates (termites, earthworms, millipedes, etc.) rip and shred complex organic matter. Smaller aquatic animal species such as rotifers, tardigrades and nematodes living in water films around soil particles have less obvious but numerous roles in the soil food webs. Two of the five nematode functional groups, fungivores and bacterivores, consume and regulate populations of primary decomposers (bacteria, yeast and fungi) and the release of inorganic nutrients (Ingham et al. 1985; Overhoff et al. 1993; Hunt & Wall 2002). Species in many functional groups of microarthropods living in air-filled pores in soils also feed on bacteria and fungi, adding to the complexity of the below-ground food web (Stevens & Hogg 2003; St. John et al. 2006). At the top of the food webs are predators, represented by species of macro- and microfauna, which feed on smaller invertebrates and protists. The complex interactions within a soil food web are notably confounded by the sheer abundance and types of soil biota (Wall et al. 2005).
How might the above- and below-ground interdependence in a diverse vegetated system (figure 1a) contrast with that of a cold desert ecosystem, such as the McMurdo Dry Valleys where many of these components are absent (figure 1b,c)? Major differences between these linked subsystems include:
the primary producer phototrophs are cyanobacteria, eukaryotic algae, mosses or lichens, not communities of vascular plants,
the Dry Valley system is horizontal and microbial, with nearly microscopic to invisible surface ‘plant-autotroph’ cover, no roots and only microscopic biota (figure 1b,c) compared with the vertical plant system (figure 1a) where plant cover extends upwards and roots penetrate to depths transporting large amounts of carbon and nutrients below ground,
the quality of organic inputs to the soil from surface algae is higher (greater C : N ratio) and has less variation in chemical composition than in vascular plant systems, and
the below-ground assemblage is depauperate in functional groups and diversity compared to vascular, plant-dominated systems (figure 1c).
Therefore, the analogue for a coupled above- and below-ground system in the Dry Valleys has to be adjusted because the components interact at a different resolution than in vascular, plant-dominated ecosystems.
In the McMurdo Dry Valley landscapes, there are two basic types of habitats for above- and below-ground biotic interactions: algal and moss diverse habitats (figure 1b) located in moist to wet soils that are analogues to plant-dominated ecosystems; or less diverse, very dry soil habitats (figure 1c). Across the Dry Valleys, gradients of above- and below-ground coupling occur where the above-ground component disappears as distance increases from the algal and moss–below-ground coupled habitats (figure 1b) to drier soils of only a below-ground dominated subsystem (figure 1c). The surface soil area in both types of habitats is thus a critical zone in the Dry Valleys and sensitive to disturbance. Biotic populations that establish in suitable above- and below-ground linked habitats (higher soil moisture and carbon and lower salinity) would have more diversity and stable populations compared with inhospitable habitats (desiccated, lower carbon and higher salinity soils) with no mesofauna or only a single invertebrate taxa (Schwarz et al. 1992, 1993; Powers et al. 1995, 1998; Virginia & Wall 1999; Courtright et al. 2001).
Across the Dry Valleys, the landscape shows little visible evidence of any above-ground primary producers. Knowledge of the distribution of phototrophs (cyanobacteria) and estimates of primary production in soils remains undetermined. However, within valleys in depressions where snow collects, edges of melt pools associated with glaciers, or stream channels formed over years from periodic melting of glaciers during the summer, there are visible mosses, algae and mats of cyanobacteria (figures 1b and 2; Gooseff et al. 2003). These are typically the areas of greatest diversity and functioning above the surface in the Dry Valleys and represent the highest complexity among terrestrial ecosystems in the region. Although this vegetation is technically considered aquatic and above sediment, not soil, it is most closely related to the above-ground component of vascular plant ecosystems.
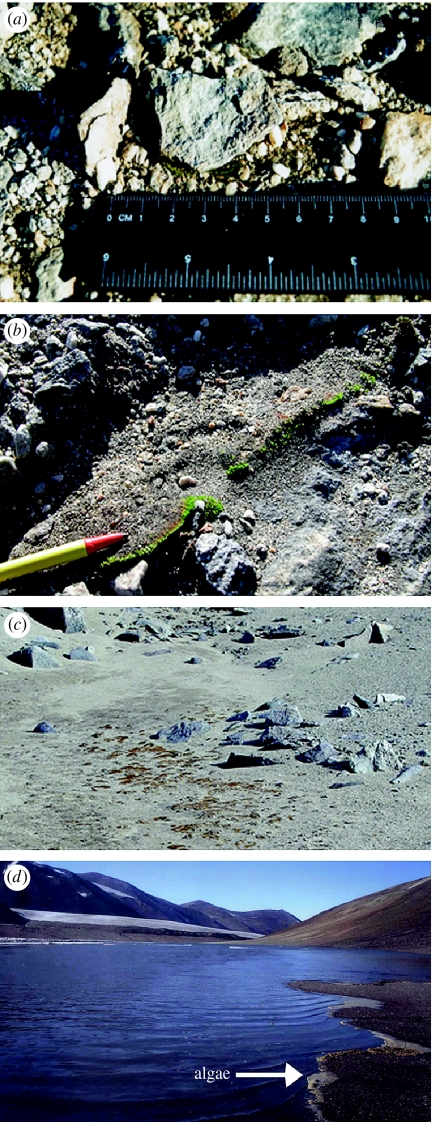
Figure 2.
The Dry Valley above-ground surface (a–d) at small-to-landscape scales: (a) surface pavement of rocks and pebbles, (b) visible green algae amidst a larger, drier surface, (c) patchy clumps of algae occurring in moist areas near rocks and (d) landscape of soils, glacier and lake with lake algae that can be blown to soil surfaces. (Photos courtesy of D. W. Hopkins.)
Species richness (number of species) of the algal above-ground component in streambeds is higher than might be expected: the Dry Valleys have about 80 algal species, with 50 confirmed species of freshwater diatoms, many of which are endemic (Alger et al. 1997; Esposito et al. 2006). Distribution of species varies depending on the composition of the streambed and velocity of stream flow with some species submerged only a few hours a day and others for longer periods in faster-flowing channels. These surface microbial mats form a diverse assemblage of species that contribute a narrow range of chemical quality as below-ground inputs to the sediments (figure 2c,d; Esposito et al. 2006). Organic compounds comprising mostly of simple mucopolysaccharides or slime and silica are degraded in soils to carbon and inorganic nutrients primarily by bacterial-dominated pathways. The cyanobacterial and algal mat biomass C : N ratios are consistently near the Redfield ratio of 6 : 1 (Barrett et al. 2007) compared to a wide C : N range of 12 : 100 for vascular plants (Hunt et al. 1999).
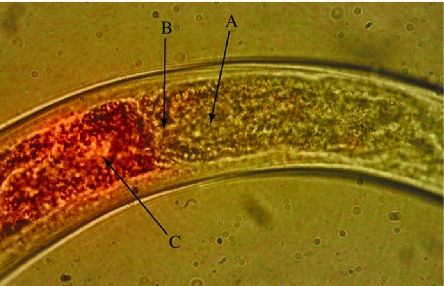
Intermingled and beneath the phototrophs, the sediments can have a complex assemblage of Collembola, mites, nematodes, tardigrades, rotifers, protozoa and microbes. The majority of these taxonomic groups can live in moist soils and sediments without visible algae, lending support to the linkage between sediments and soils across this landscape (Wall 2004). For example, Collembola are patchily distributed in moist soil areas under rocks to several centimetres depth, and can be dispersed in moving water sweeping across dry streambeds although they are not aquatic inhabitants (Stevens & Hogg 2003). The species of nematodes found in these productive moist areas are consumers (Plectus spp.) of bacteria, or as recently seen for Eudorylaimus (figure 3), consumers of algae (B. J. Adams & D. H. Wall 2004, unpublished data; Treonis et al. 1999; Gooseff et al. 2003). Many Eudorylaimus spp. in other ecosystems are predators of small invertebrates, but this has not been documented in the Dry Valley soils. The level of feeding specificity by invertebrates has not been determined for the Dry Valley invertebrates, but data on mouthparts, gut contents and diets indicate tardigrades and rotifers graze on algae and bacteria, Collembola are fungal and algal feeders (Davidson & Broady 1996) and mites are fungal feeders (Stevens & Hogg 2003; Joaquim-Justo et al. 2004; Hogg et al. 2006). Compared with the wide variety of functional groups in plant-dominated ecosystems (figure 1a), the functional groups in the two types of Dry Valley habitats (figure 1b,c) are remarkably few compared with temperate ecosystems, consisting of microbial consumers (fungivores, bacterial feeders, omnivores or microbial feeders) and ‘herbivores’ or algal feeders. Predation is apparently absent or unobserved for species of mites, nematodes or tardigrades (Freckman & Virginia 1998; Adams et al. 2006; Hogg et al. 2006). The predatory tardigrade, Macrobiotus, observed elsewhere in Antarctica (Convey & McInnes 2005) has not been found in the Dry Valley soil samples.
Figure 3.
Picture of the nematode Eudorylaimus spp. depicting algal ingestion. Image is a composite of two separate images generated by transmitted white light microscopy, and fluorescence microscopy. An acridine orange filter was used to observe refraction by chloroplasts, which appear at the base of the oesophagus as a red mass (100×, oil immersion). (Photo courtesy of Byron Adams.)
The biotic contribution to the functioning of this above- and below-ground subsystem is activated in the austral summer when increased temperatures melt ice and affect soil relative humidity. The limiting factor for activity and production of cyanobacteria and algae in streams and lake margins is this brief pulse of liquid water (McKnight et al. 1998). When there is no available moisture, algae and mosses desiccate. The invertebrates have survival strategies that enable them to respond quickly to changes in the environment (temperature, moisture and corresponding changes in soil chemistry) even during Antarctic summers, by ceasing feeding, movement and metabolism until, as with algae and mosses, they are reactivated by liquid water (McKnight et al. 1998; Treonis & Wall 2005). When desiccated or frozen, cyanobacteria, algal mats, invertebrates and microbes are windblown, and thus are important sources of organic matter and biotic inocula for dry soils across the region (Gressitt et al. 1960; Nkem et al. 2006).
Extending from obvious aquatic areas of ponds, streams and lakes, the landscape has large areas of barren, dry soils mostly formed into polygons (Pastor & Bockheim 1980; Virginia & Wall 1999; Bockheim 2002; Barrett et al. 2004). In Taylor Valley, the site of the US National Science Foundation McMurdo Long Term Ecological Research, arid soil occupies >95% of ice-free areas (105 km2) below 1000 m, compared with streams (0.2 km2) where productive cyanobacteria and algae (figure 1b) communities occur, and ice-covered lakes (4.7 km2; Virginia & Wall 1999; Burkins et al. 2001). Factors such as elevation, high salinity, low carbon (0.01–0.5 mg C g soil−1; Burkins et al. 2001) and moisture, limit life above and below the soil surface (figure 1c), resulting in biotic communities that are heterogeneously distributed across the valley. This vast area represents a unique extreme for the world: no apparent above-ground component coupled with the active below-ground.
Biotic carbon, primary productivity for the Dry Valleys, is supplied to soils primarily by above-surface algae and cyanobacteria. Carbon inputs include ‘invisible soil algae’ or cyanobacteria and algae, which are sparse on soil surfaces, windblown cyanobacterial and algal mats from streams, ponds and lakes (Eberling et al. 2006; Hopkins et al. 2006), cyptoendolithic rocks, marine inputs, and algae or mosses beneath quartzite rocks (Friedmann 1982; Wynn-Williams 1990; Burkins et al. 2001; Moorhead et al. 2003; Barrett et al. 2006a,b; Eberling et al. 2006; Hopkins et al. 2006). The below-ground system also contributes legacy carbon that has been stored during previous geological periods (Burkins et al. 2000; Barrett et al. 2006a,b). Legacy carbon is an important source of organic matter for drier soils over the long term, because there are no vascular plants above-ground contributing to the accumulation of below-ground storage of organic C (figure 1a), and above-ground algal subsidies are reduced and supplied on a short-term annual basis (Powers et al. 1995, 1998; Virginia & Wall 1999; Courtright et al. 2001). The persistence of this C pool in soils is a function of the original amount and turnover rates.
In contrast to the few algal and moss-dominated moist soils and streams where invertebrates live intertwined above and below ground (figure 1b), the arid soils (figure 1c) have no above-surface animals. However, data collected in the past 15 years show that vast areas of these dry soils once considered nearly sterile have a surprising biotic diversity. The richness (number of species) of the dry below-ground system (figure 1c) is still unknown, but can range from 1 to 10 metazoan taxa for the Dry Valleys (Adams et al. 2006). This is an order of magnitude less than the hundreds of invertebrates in temperate soil (Wardle 2002), although a coordinated taxonomic analysis (combining molecular and classical morphological techniques) of the Dry Valley soils would most probably discover additional species. While molecular techniques are crucial to delineating species of mites, Collembola and other invertebrates (Adams et al. 2006), ecological investigations are also important to assure that the species exist as active inhabitants of communities.
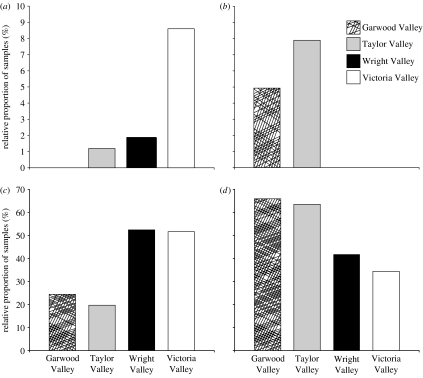
The distribution of mesofauna is more heterogeneous across the Dry Valley landscape than that in other ecosystems (Freckman & Virginia 1998; Virginia & Wall 1999; Barrett et al. 2004). For example, Beacon Valley has a high elevation, high soil salinity and no invertebrate soil species. Across the four other valleys sampled (figure 4; 415 soil samples), less than 5% of the samples had all three (rotifers, tardigrades and nematodes) groups of invertebrates represented (Freckman & Virginia 1998). A high percentage (approx. 35%) of these soils had no invertebrates (figure 4a–d) attesting to how geology, habitat, climate and dispersal dynamics all contribute to assembly of species communities. Of the 60% of Dry Valley soils with invertebrates, the most inhabitable valleys appeared to be Wright and Victoria (figure 4b–d). Taylor Valley had a higher proportion of three-invertebrate communities (figure 4b), and nematodes occurred in a higher proportion of soils in the Taylor and Garwood Valleys (figure 4d). Peak densities of nematodes (4000 kg dry soil−1) were comparable to plant-dominated hot desert soils (Freckman & Mankau 1986). Nematode species distributions in drier soils are limited by salinity, carbon and moisture, with the abundance and biomass of one species, Scottnema lindsayae, dominating the drier, salty soils (Freckman & Virginia 1997; Courtright et al. 2001). Soil protozoa, as measured by culture techniques, can occur in soils with or without nematodes (Bamforth et al. 2005).
Figure 4.
Relative proportion (%) of soil samples collected from four McMurdo Dry Valleys, Garwood Valley (n=41), Taylor Valley (n=178), Wright Valley (n=103) and Victoria Valley (n=93), which contained 0–3 invertebrate phyla: (a) rotifers and tardigrades, (b) three-taxa communities, (c) soils without invertebrates and (d) nematodes only. Other two-taxa communities represented less than 8% of all samples in all valleys and are not shown. Note the difference in scale on the y-axes.
Much less is known about microbial diversity and distribution, but as with recent studies of all ecosystems, molecular evidence is revealing greater diversity. In the Dry Valleys, yeasts are more widely distributed (Connell et al. 2006) and diverse than that previously thought (Atlas et al. 1978). Cowan et al. (2002), using luminometry to estimate ATP levels, also concluded that the richness of bacterial taxa in Taylor Valley soils is higher than that previously believed. An unexpectedly high diversity in soils of Arctic tundra compared with boreal forests was recently noted, but whether species are transient and/or inactive remains to be resolved (Cowan et al. 2002; Neufeld & Mohn 2005).
4. The functioning of low diversity systems
The relationship of below-ground biodiversity (species richness) to an ecosystem function such as primary production or decomposition is still ambiguous for high diversity terrestrial ecosystems (Loreau & Thébault 2005; Setälä 2005). This is partially because designing realistic experiments to include the numbers of invertebrates and microbes in soil assemblages becomes complex and the role of each species in an ecosystem process is seldom known (see Mikola et al. 2002 for review). A consensus arising from the results of laboratory microcosm experiments (Liiri et al. 2002; Setälä 2002), in which invertebrate species richness was related to an ecosystem process, decomposition, is that in high diversity, complex systems, functional diversity is more important than increased numbers of species (Heemsbergen et al. 2004; Roscher et al. 2004). The number of species appears more critical to ecosystem functioning in low diversity (1–10 species) systems, because if disturbed, there is less redundancy, i.e. fewer ‘back-up’ species to take on lost functional roles. In other words, if the amount of primary productivity and physico-chemical environment were held constant in all ecosystems, fewer species would be responsible for a greater amount of a particular ecosystem function in low diversity systems. This suggests that the few species involved in ecosystem functioning in the McMurdo Dry Valley ecosystems are crucial and may play a larger proportional role than that previously thought (Barrett et al. 2006b). In turn, they and related ecosystem processes may be more vulnerable to global changes than those in high diversity, more complex ecosystems.
5. Implications of global changes on coupled above- and below-ground subsystems
What are tipping points (Kemp 2005; Schellnhuber et al. 2006; http://kron1.eng.ox.ac.uk/climate/) or disturbances that might take this low diversity region to a different equilibrium state? Schellnhuber et al. in a workshop, which identified regions where the balance of a system has reached the critical point due to climate change, defined a tipping point as a point at which potentially irreversible change is about to occur, or is already occurring. The tipping point for the Dry Valley region may be climatic but could also include other significant human-induced changes that feedback to climate. Global changes that directly impact ecosystem processes and services in plant-dominated systems, such as land use change, increased urbanization, slash and burn of tropical forests or inputs of agricultural chemicals, become indirect drivers affecting climatic, atmospheric and hydrological cycles elsewhere, including the Dry Valleys, and might be potential tipping points. Direct human impacts on the Dry Valleys, which could be candidates for tipping points, might be invasive species, pollution and/or human trampling.
Jiang & Morin (2004) noted from laboratory experiments that climate change effects on species interactions within complex communities would be extremely difficult to predict. This suggests that it may be easier to forecast change in simple, low diversity communities. The questions are as follows: do we presently understand the role of species in these low diversity systems and the pattern of their responses to disturbance? Can observations of simple community-level responses to perturbations be used as a basis for extrapolating how species, processes and ecosystems may respond to more realistic multiple, rather than single, global changes in the future? Here, I use the ‘press’ and ‘pulse’ concepts of Bender et al. (1984) to frame disturbances that may alter responses of the species in above- and below-ground subsystems in the Dry Valleys. When an ecosystem is disturbed, it may respond by altering species composition and then return to its previous equilibrium. In this case, the system has been simply ‘pulsed’. Or, it may respond by being ‘pressed’ to a new equilibrium state due to sustained alteration to species composition or extinction of species. A pulse disturbance will alter the system perhaps notably within the boundaries of a stable ecosystem, but a press will be a type of change that brings the Dry Valleys to a tipping point (Kemp 2005) resulting in a new organization of species and a potentially irreversible change and new state for the ecosystem (figure 4). The disturbances discussed below are examples of global changes (climatic or land use) that may have direct and measurable impacts on the above- and below-ground coupling at local and regional scales, and perhaps become tipping points for the Dry Valleys over the next 50 years. While increased atmospheric CO2 concentration will certainly have an effect on biological communities, this has not been explicitly examined for the Antarctic Dry Valley terrestrial biota and is not discussed here (see Chown & Convey 2007 for a discussion of changes in maritime Antarctica).
(a) Temperature change: warming
The effect of a temperature increase in Antarctica during the austral summer would be more liquid water (Kennedy 1993), but these differences would vary across local, landscape and regional scales. In the Dry Valleys, for example, liquid water is mostly unavailable to biota. Fifty per cent of the arid soils are underlain with subsurface ice, either ice-cemented soil, or large, massive ice (Bockheim 2002). Because of low precipitation and air temperatures, snow sublimates and only a small amount melts (Gooseff et al. 2003). This moisture moves as a vapour, with limited migration of melt water from snow (Campbell & Claridge 1982). Most biological activity appears to occur in thin water films (Kennedy 1993; Treonis et al. 2000). The liquid water might be localized such as run-off from a north-facing glacier, melt of a shallow frozen melt pond, or non-uniform, patchy melt seen in normally dry soils due to variations in depth of permafrost.
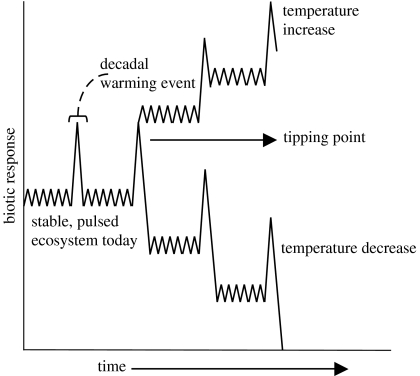
At regional scales, evidence accumulating from lake levels in Wright and Taylor Valleys (Taylor Valley's record began with R. F. Scott in 1903), with models (Bomblies et al. 2001; Ebnet et al. 2005) and more recent temperature and Onyx River flow data from New Zealand and US scientists, suggests that decadal events of warming have occurred in the past 100 years, even though overall, the Dry Valley region is cooling (Doran et al. 2002; Lyons et al. 2005). Decadal warming would be considered a pulse disturbance (figure 5) and an example of a stable, resilient system that oscillates back and forth in response to a periodic temperature event. Species diversity is not altered significantly and species are not lost, because the system rebounds to the dynamics of the previous condition. Experience gained from observations in Taylor Valley in the 2001–2002 austral summer on the pulse effects of the warmest temperature since continuous records were maintained, provides a case study for projecting how the two types of above- and below-ground ecosystems (figure 1b,c) may respond in the future if pressed to a new equilibrium state (figure 5).
Figure 5.
Conceptual figure of the response of terrestrial biota to alterations to climate over time. Biotic individual species and populations respond differently from year to year and with some large events such as a decadal warming event, but rebound to their previous stable, ecosystem state. When a tipping point occurs (see text), communities may reorganize, causing changes in ecosystem properties and rates, and move to a new ecosystem state with unknown effects within a region or globally.
In Taylor Valley, the Lake Bonney weather station recorded an average air temperature increase from −3.9°C in the previous year to a very warm −1.7°C. The temperature increase and corresponding hydrological melt response (glaciers, frozen lake ice, permafrost, snowmelt, etc.) altered the dry appearance of the soils to a wet surface flow and puddles of water in areas across the landscape. As would be expected in the annual ephemeral melt ponds and streams (algal and cyanobacterial-dominated systems, figure 1b), the response was more liquid water: increased glacial run-off; wider streambeds; more rapid stream flow; and greater water volume. For the stream phototrophs, this translated to altered species composition, and decreases in abundance and biomass due to water volume and scouring (Alger et al. 1997). The above-ground response to increased temperature and moisture would potentially produce a different quantity of organic matter below-ground to the soil–sediment biota and, in turn, perhaps alter composition of the microbes and invertebrate consumers and the bacterial or fungal (or physical) pathway of decomposition. For example, an increase in the proportion of biomass of diatoms in streams might change the percentage of silica. Modification in the uptake of dissolved silica and transformation into biogenic silica during diatom blooms in rivers could occur, as noted by Conley (1997). In other more vegetated ecosystems, the composition of the below-ground food web shifts to a bacterial pathway when the source of organic matter is more labile, and to a fungal-dominated food web when the organic matter is more complex and recalcitrant. Invertebrates and microbes respond by increasing their populations and altering the rate of nutrient turnover.
Surprisingly, in previously unwetted areas of the below-ground dominated (figure 1c) Taylor Valley landscape, two unusual but distinct features appeared in response to increased temperature: subsurface seeps of flowing water of unknown origin, which surfaced and spread much like a stream over areas of dried soil, and small circular patches that were wetted possibly due to snow or permafrost melt. Flows from a seep near Lake Bonney, which surfaced in Taylor Valley, differed considerably in geochemistry from nearby ephemeral annual streams during that year (Harris et al. 2007). Lyons et al. (2005) discovered a seep flow surfacing in soils near Lake Bonny that originated from a nearby snow plateau. They suggested that the seep flows dissolved and transported soluble salts in the soils, thereby making the soils a more suitable habitat for biota (Lyons et al. 2005). Across the Taylor Valley landscape, the many wet patches from seeps and melting permafrost showed an instantaneous above-ground response to moisture, but a delayed, ‘uncoupled’ below-ground response. The presence of algae, mosses and lichens in the wetted areas became a thin visible light green layer of contrast against the drier surrounding soil, but the below-ground response of invertebrates was inconspicuous and lagged by a year. In the following 2002–2003 season, when air temperatures and soil temperatures returned to colder, drier, unwetted norm for Taylor Valley, nematode assemblages located the prior year in wet areas had altered composition: Eudorylaimus, the rarer species in dry soils in previous cold years, and limited by water and carbon, increased; while Scottnema, the species widespread in drier, saltier soils, declined. Long life cycles (approx. 218 days at 10°C) have been shown for Scottnema in laboratory conditions (Overhoff et al. 1993), suggesting delayed and prolonged responses to environmental changes that affect life-history traits or distribution ecology.
Based on this case study of a short-term (2 years) response to a decadal warming event, how might a climate change of increased temperatures in the next 50 years impact above- and below-ground biodiversity and functioning and tip the ecosystem to a new equilibrium state? Convey et al. (2003) suggested that warming might lead to increased desiccation stress, while Harris et al. (2007) suggested that warming soils approximately 1°C would be sufficient to increase the thaw depth and result in permafrost melt as well as alter changes in geochemistry of the Dry Valley lakes and soils. If the frequency of decadal warming events increased over the next 50 years, reversing the current cooling trend, or if there was a smaller average temperature increase, this would possibly enlarge and meld the wetted areas (seeps and patches) and in effect be a tipping point (figure 5) moving this desert to a more homogeneous landscape with less patchiness, similar to warmer, moister ecosystems. More numerous suitable habitats for algal and moss-dominated systems would increase the sources of biota, which are dispersed by water and wind across the region and improve the chances that communities would establish. Increased variety and biomass of carbon inputs above (algal and moss production) and below ground (dead organic matter and invertebrates) would affect species diversity and feedbacks above and below ground. Soil assemblages would homogenize, reducing the number of areas that were once single-taxa communities, and change species composition through competition, parasitism, etc. Species distributions would contract or expand depending on habitat suitability and population and community dynamics. Collembolan, mites, tardigrades, rotifers, and the nematodes Eudorylaimus and Plectus, which favour moist soils, would expand their distribution. Scottnema, presently the nematode species with the greatest biomass and density and distribution across the McMurdo Dry Valleys, would become more restricted and a rare, less abundant species in wetter soils. With fewer, less abundant populations as a source to be distributed by wind, and possibly because Scottnema evolved slowly over time, a sudden or greater intensity of disturbance might switch this more stable, dominant animal population to a new equilibrium. Invasive species could possibly have a greater competitive advantage over the many endemic species from the valleys following perturbations (Adams et al. 2006; Hogg et al. 2006). The temporal lag below ground in response to above-ground algal and moss production noted in Taylor Valley might be shortened with long-term or more frequent decadal warming, altering the rates of decomposition and primary production.
Thus, the potential impact of temperature warming could dramatically change the Dry Valley landscape and press the system beyond the present decadal-pulsed (figure 5) and heterogeneous landscape (figure 1b,c) towards a wet algal and moss-dominated system and a homogeneous landscape (figure 1b). The fundamental properties, interactions of species, e.g. mutualism, parasitism and symbioses, and population processes, which are typical of all ecosystems, will become a characteristic of the biotic dynamics in the Dry Valleys.
(b) Temperature change: cooling
Continental climate trends remain the subject of much study, but data showing the Dry Valley region of Antarctica have demonstrated a cooling trend over the past 15–20 years is incontrovertible (Comiso 2000; Doran et al. 2002; Kwok & Comiso 2002; Thompson & Solomon 2002; Walsh et al. 2002; Vaughan et al. 2003; Bertler et al. 2004; Shindell & Schmidt 2004). The effects of cooling on biodiversity and ecosystem functioning on below-ground dominated systems (figure 1c) appear to have driven the system to a different state of declining soil moistures, decreasing population sizes of nematodes, decline in relative abundance of Plectus and Eudorylaimus and changes in species assemblages. What might be the effect of continued cooling in this region in the next 50 years?
A major result of continued decreasing temperatures in the Dry Valleys would be a press disturbance resulting in a shift to fewer moist, highly productive, coupled systems and increased drier below-ground systems (figure 1c). The present arid-dominated landscape would expand in area because the frequency of wetted patches and number of annual ephemeral streams would be reduced. The quantity of biotic inocula dispersed from the reduced algal and moss-dominated systems across the dry landscape would be constrained, even during decadal warming events. The scarcity or loss of wet soil patches and species (tardigrades, rotifers, mites, Collembola and some nematode species) would drive the above- and below-ground system into lower diversity below ground and potentially affect rates or losses of ecosystem functions. The cooling would keep soil water frozen and biota potentially in a survival state uncoupled from ecosystem processes for a longer period of time, thus altering rates of functioning.
Climate changes will drive the low diversity systems to a new ecosystem state. How the mechanisms of species interactions will operate, and over what time period, are subjects for modelling and a terrestrial above- and below-ground assessment of this region.
(c) UV radiation
Antarctic communities may experience alterations in the surface conditions of temperature, moisture and ultraviolet (UV) radiation caused by humans and changes in atmospheric chemistry (Antarctic ozone hole; Farman et al. 1985; Solomon et al. 2005). Concerns have mounted about the deleterious effects of increased UV-B (280–320 nm) and UV-A (320–400 nm) radiation reaching the surface of Antarctic ecosystems (Solomon 1990; Weiler & Penhale 1994; Callaghan et al. 2004). Thirty years of ozone depletion has occurred (Farman et al. 1985; Solomon 1990; Solomon et al. 2005) and communities may already be undergoing adaptation, species selection and changes in species assemblages, communities and distribution with effects on ecosystem function. For the Antarctic terrestrial communities, a significant change associated with the ozone hole is not in the magnitude of UV-B exposure, but in the timing of the exposure in early spring when organisms may be in a physiologically inactive state and unaccustomed to these levels. UV-B levels at the Antarctic soil surface in spring if there is snow cover may be similar to those normally received in mid-summer at the same locations (Cockell & Córdoba-Jabonero 2004). The UV-B effects considered most harmful to life include mutagenic interactions with DNA and RNA and absorption by proteins, and damage to membranes and pigments with negative effects on photosynthesis and primary productivity (Karentz 1994).
Wynn-Williams (1994) suggested that UV-B levels in Antarctica are deleterious to terrestrial surface cyanobacteria and the below-ground short food chains (Freckman & Virginia 1997) they support. Organisms most susceptible to UV-B damage will either be at or near the surface of soils and waters, or as they are dispersed by wind. Thus, UV-B exposure may alter the ability of both above-ground and below-ground organisms to successfully disperse and colonize new soil habitats (Wynn-Williams 1994). UV effects on Antarctic communities have been studied primarily in aquatic systems (Neale et al. 1994; Vincent & Quesada 1994) and in maritime terrestrial systems (Convey 2003; Newsham 2003; Frenot et al. 2005; Newsham et al. 2005). In Victoria Land, UV resistance has been shown in mosses (Green et al. 2005) and Tosi et al. (2005) found UV radiation-limited soil fungal assemblages and diversity.
The response of soil communities to UV-B is poorly studied in the Dry Valleys. In cryptoendolithic sandstone, UV radiation apparently attenuates and biota living in the rocks are relatively protected (Nienow et al. 1988; Onofri et al. 2004). In Antarctic regions that are warming, Cockell & Córdoba-Jabonero (2004) found that a thin layer of snow can protect underlying terrestrial communities from UV radiation and suggested that in regions that are cooling, such as the Dry Valleys, snow covers would be prolonged and provide protection. While this scenario is possible, it is also likely that in the future, the high rates of sublimation and strong katabatic winds that have been occurring with cooling in recent years, would continue to reduce available snow cover in early spring and leave terrestrial communities exposed to UV. At present, there is insufficient research on the response of terrestrial soil and sediment species in the Dry Valleys to UV radiation.
(d) Direct human influence: trampling
Effects of human trampling on biodiversity as a type of land use change have been examined primarily in forests and recreational areas (figure 1a) in order to provide information on recovery and restoration. Much of this research shows that plant sensitivity to trampling is dependent on the species (Cole & Schreiner 1981; Kuss & Hall 1991), with some plant communities experiencing reductions in vegetation height, cover and species richness, and others increasing species diversity (Grime 1979). Effects of trampling on plants were similarly noted above ground in the Antarctic on Marion Island (Gremmen et al. 2003) and Macquaire Island (Scott & Kirkpatrick 1994). Studies below ground in both temperate and Antarctic environments have addressed soil physical properties and showed trampling increased soil temperatures, compaction and decreased soil porosity and moisture (Kobayashi et al. 1997; Campbell et al. 1998). In alpine habitats, soil moisture levels were instrumental in plant resilience (Bell & Bliss 1973; Holmes & Dobson 1976). In other habitats, compaction increased over five years of trampling, changes in bulk density and compaction influenced seedling establishment and recovery (Sun & Liddle 1993), and changes noted in soil organic matter from trampling varied with habitat and vegetation (see Young & Gilmore 1976 and reviewed in Sun & Liddle 1993). This and other research on humans walking on soils illustrate that ecosystems sustaining damage above ground, also exhibit impacts below ground which, in turn, feedback to ecosystem recovery and restoration.
In contrast to these more complex ecosystems of high diversity, the terrestrial Dry Valley ecosystem has large areas that are characterized as essentially barren, absence of any protective vegetation except in algal and moss above- and below-ground systems. Campbell et al. (1998) presented data on three categories of human activities that impacted Antarctic Victoria Land soils: major disturbance (land surface change—Earth moving equipment, buildings, helicopter pads and re-contouring of land); moderate disturbance (camping, soil sampling and vehicles); and low disturbance (walking). Major disturbances were shown to have significant impacts on permafrost, soil physical properties and algal and moss-dominated communities, with some recovery of soil algae depending on the soil type (Balks et al. 1995; Campbell et al. 1998). Moderate impacts such as removal of small cobble size stones, and tents left impressions that remained for years. Even minor impacts of a few footsteps can be seen years later, although on covered stony surfaces, footprints are less visible. The scientific community has been proactive in addressing mechanisms to reduce impacts of trampling and other human activities on the Dry Valleys such as the use of paths, but consequences on the interdependence of biodiversity and functioning of the terrestrial ecosystem as a result of trampling and other activities are as yet unknown.
With the growth of human presence, including tourists, in the Dry Valleys since the R. F. Scott expedition into Taylor Valley in 1903, and relative growth in appreciation for the environment and biodiversity (Adams et al. 2006; Barrett et al. 2006a,b; Hopkins et al. 2006), the cumulative effects of human trampling on the interactions of the coupled above-ground and subsurface system must be considered as a significant press disturbance (not a pulse disturbance) that potentially will have a long-term effect. What changes to biodiversity and ecosystem functioning might result from this press disturbance of direct human impact on soils?
Above-ground algal-dominated systems associated with streams, ponds and moist soil areas might be altered in species composition and biomass due to changed soil and sediment physical and structural morphology. At the local level, walking could dislodge soil particles and pebbles and rocks that protect the soil interface. This could increase the potential for erosion, exposing deeper soil, changing soil temperatures, moistures and freeze–thaw patterns, and effectively modify the habitat for biota. This may fracture any microscopic biotic connections above and below ground affecting primary production, and potentially affect the return of biotic carbon inputs to soil organisms, as well as soil respiration, decomposition rates and availability of nutrients for above-ground algal and moss communities. Deeper below-surface, compression and compaction in streams and soils could further convert soil habitat and hydrology. As soil and sediment habitats are modified, species abundance, survival and competition for resources could occur, transforming species assemblages. J. N. Nkem et al. (2005, unpublished data) conducted a long-term biodiversity study (1994–2004) on the cumulative effects of trampling in an arid below-ground dominated system (figure 1c) in Taylor Valley and documented a severe decline in the abundance of two nematode species, particularly in highly disturbed surface (0–2.5 cm depth) soils compared with controls. This and other work (Campbell et al. 1998) suggest that paths reduce disturbance to the rest of the landscape.
These local changes may be magnified at regional scales. Over time, local scale reduction in both abundance and types of biota and suitable habitats could affect the quality of sources for species dispersal and the ability of species to establish across the landscape. Based on data from plant-dominated ecosystems, the cumulative result might be reduced biodiversity, changes in community composition and alterations in rates of ecosystem processes. Whether this effect would be minor or major compared to regional climatic and physical controls on biodiversity and ecosystem functioning is unknown. However, for this low diversity system, increased human presence and the expansion of trampling, like temperature change, over several years, may be a tipping point that would significantly drive the Dry Valleys into a different state of functioning.
6. Concluding remarks
There is sufficient evidence that the unusually low biotic diversity and low functional redundancy of the tightly coupled above-ground–below-ground Dry Valley system is both essential to ecosystem functioning and vulnerable to perturbations. Because the few faunal species are below ground and their response to global changes may operate at a different temporal scale than above-ground species, there is some uncertainty as to the detailed implications for the overall functioning of the Dry Valleys. In addition, climate change and land use changes (invasive species, pollution and human trampling) are occurring as multiple, cumulative interactions, not isolated events.
On other continents with plant-dominated systems, developing global change scenarios of how ecosystems may be tipped to a new state in the future is often complicated by responses and feedbacks from the higher biodiversity and number of colossal interactions. The above- and below-ground dependency is often overlooked, but in the Dry Valley region, developing predictions for responses of the whole system (biodiversity and ecosystem functioning) to single and multiple global changes for the next 50 years could be more easily accomplished. A short-term, field experiment as part of a polar network could be used to compare the reaction of soil biodiversity and ecosystem processes to increased temperature and UV across Antarctica regions. A scientific assessment of the geographic distribution of the few animal and ‘plant’ (phototrophs—cyanobacteria, algae, mosses and lichens) species present in the Dry Valleys, would be high priority as a baseline for noting future change.
Learning how the tightly linked, above- and below-ground species will cope under increasing global changes has value beyond the Antarctic. Because the Dry Valley biota are interlinked northwards to coastal Victoria Land, further inland to the ice-free areas of the terrestrial Antarctic interior and to surrounding marine systems, global changes affecting species in the Dry Valley food webs could have profound feedbacks beyond this most extreme ecosystem on Earth. Similarly, changes occurring elsewhere in the continent will influence the Dry Valley biota. Climate warming, for example in the interior, may increase ice-free surface area, thus revealing new soil habitat for species invasions and expansion of species range. Focusing on the tipping points of global change that press the low biodiversity Dry Valley system to a different ecosystem state is important for understanding overall change on the Antarctic continent, and globally. Perhaps as important for ecologists, knowing the vulnerabilities of species and whether the loss or change to faunal species composition in a low diversity system will have a significant and rippling impact on ecosystem functioning is a fundamental scientific question. This, I believe, can be answered most appropriately, only in the Dry Valleys of Antarctica where all the faunal and phototrophic species of the whole ecosystem are close to being known. Providing the urgent attention needed now, to the global changes affecting disruptions to the coupling of above- and below-ground biodiversity and related ecosystem processes in the Dry Valleys, can provide a rigorous basis for determining how the changes in Antarctica in the next 50 years will also affect global human well-being.
Acknowledgments
I acknowledge support from NSF (OPP-0423595, OPP-9813061 and OPP-0229836). I am grateful to three anonymous reviewers, and especially to W. B. Lyons, B. J. Adams, E. Ayres, D. W. Hopkins, H. W. Hunt and R. A. Virginia for their critical review of this article. S. Collins is especially thanked for his suggestions and advice. R. Esposito, J. E. Barrett, P. Doran, J. Priscu, D. McKnight, W. Parton and S. Spaulding contributed helpful comments. L. Hoffman and S. Advani are thanked for technical support.
Footnotes
One contribution of 10 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. Part 2: evolution, diversity and function’.
References
- Adams B.J, et al. Diversity and distribution of Victoria Land biota. Soil Biol. Biochem. 2006;38:3003–3018. doi:10.1016/j.soilbio.2006.04.030 [Google Scholar]
- Alger A.S, McKnight D.M, Spaulding S.A, Tate C.M, Shupe G.H, Welsh K.A, Edwards R, Andrews E.D, House H.R. Institute of Arctic and Alpine Research; Boulder, CO: 1997. Ecological processes in a cold desert ecosystem: the abundance and species distribution of algal mats in glacial meltwater streams in Taylor Valley, Antarctica. [Google Scholar]
- Anderson J.M. Invertebrate-mediated transport processes in soils. Agr. Ecosyst. Environ. 1988;24:5–19. doi:10.1016/0167-8809(88)90052-7 [Google Scholar]
- Araujo M.B. Biodiversity hotspots and zones of ecological transition. Conserv. Biol. 2002;16:1662–1663. doi:10.1046/j.1523-1739.2002.02068.x [Google Scholar]
- Atlas R.M, DiMenna M.E, Cameron R.E. Ecological investigations of yeasts in Antarctic soils. In: Parker B.C, editor. Terrestrial biology III. Antarctic research series. vol. 30. The William Byrd Press; Richmond, VA: 1978. pp. 27–34. [Google Scholar]
- Balks, M. R., Campbell, D. I., Campbell, I. B. & Claridge, G. G. C. 1995 Interim results of 1993/94 soil climate, active layer and permafrost investigations at Scott Base, Vanda and Beacon Heights, Antarctica. Antarctic research unit special report number 1. Hamilton: University of Waikato.
- Bamforth S.S, Wall D.H, Virginia R.A. Distribution and diversity of soil protozoa in the McMurdo Dry Valleys of Antarctica. Polar Biol. 2005;28:756–762. doi:10.1007/s00300-005-0006-4 [Google Scholar]
- Bardgett R.D, Anderson J.M, Behan-Pelletier V, Brussaard L, Coleman D.C, Ettema C, Moldenke A, Schimel J.P, Wall D.H. The influence of soil biodiversity on hydrological pathways and the transfer of materials between terrestrial and aquatic ecosystems. Ecosystems. 2001;4:421–429. doi:10.1007/s10021-001-0020-5 [Google Scholar]
- Bardgett R.D, Bowman W.D, Kaufmann R, Schmidt S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005a;20:634–641. doi: 10.1016/j.tree.2005.08.005. doi:10.1016/j.tree.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Bardgett R.D, Yeates G.W, Anderson J.M. Patterns and determinants of soil biological diversity. In: Bardgett R.D, Usher M.B, Hopkins D.W, editors. Biological diversity and function in soils. Cambridge University Press; Cambridge, UK: 2005b. pp. 100–118. [Google Scholar]
- Barrett J.E, Virginia R.A, Wall D.H, Parsons A.N, Powers L.E, Burkins M.B. Variation in biogeochemistry and soil biodiversity across spatial scales in a polar desert ecosystem. Ecology. 2004;85:3105–3118. [Google Scholar]
- Barrett J.E, et al. Terrestrial ecosystem processes of Victoria Land, Antarctica. Soil Biol. Biochem. 2006a;38:3019–3034. doi:10.1016/j.soilbio.2006.04.041 [Google Scholar]
- Barrett J.E, Virginia R.A, Parsons A.N, Wall D.H. A soil carbon turnover model for the McMurdo Dry Valleys, Antarctica. Soil Biol. Biochem. 2006b;38:3065–3082. doi:10.1016/j.soilbio.2006.03.025 [Google Scholar]
- Barrett J.E, Virginia R.A, Lyons W.B, McKnight D.M, Priscu J.C, Doran P.T, Fountain A.G, Wall D.H, Moorhead D.L. Stoichiometric evolution of Antarctic Dry Valley ecosystems. J. Geophys. Res. Biogeosci. 2007;112:G01 010. doi:10.1029/2005JG000141 [Google Scholar]
- Bell K, Bliss L.C. Alpine disturbance studies: Olympic National Park, USA. Biol. Conserv. 1973;5:25–32. doi:10.1016/0006-3207(73)90051-7 [Google Scholar]
- Bender E.A, Case T.J, Gilpin M.E. Perturbation experiments in community ecology—theory and practice. Ecology. 1984;65:1–13. doi:10.2307/1939452 [Google Scholar]
- Bertler N.A.N, Barrett P.J, Mayewski P.A, Fogt R.L, Kreutz K.J, Shulmeister J. El Niño suppresses Antarctic warming. Geophys. Res. Lett. 2004;31:L15 207. doi:10.1029/2004GL020749 [Google Scholar]
- Bockheim J.G. Properties and classification of cold desert soils from Antarctica. Soil Sci. Soc. Am. J. 1997;61:224–231. [Google Scholar]
- Bockheim J.G. Landform and soil development in the McMurdo Dry Valleys, Antarctica: a regional synthesis. Arct. Antarct. Alp. Res. 2002;34:308–317. doi:10.2307/1552489 [Google Scholar]
- Bomblies A, McKnight D.M, Andrews E.D. Retrospective simulation of lake-level rise in Lake Bonney based on recent 21-year record: indication of recent climate change in the McMurdo Dry Valleys, Antarctica. J. Paleolimnol. 2001;25:447–492. doi:10.1023/A:1011131419261 [Google Scholar]
- Broady P.A. Taxonomy and ecology of algae in a freshwater stream in Taylor Valley, Victoria Land. Arch. Hydrobiol. 1982;32:331–349. [Google Scholar]
- Brooks T.M, et al. Habitat loss and extinction in the hotspots of biodiversity. Conserv. Biol. 2002;16:909–923. doi:10.1046/j.1523-1739.2002.00530.x [Google Scholar]
- Burkins M.B, Virginia R.A, Chamberlain C.P, Wall D.H. Origin and distribution of soil organic matter in Taylor Valley, Antarctica. Ecology. 2000;81:2377–2391. doi:10.2307/177461 [Google Scholar]
- Burkins M.B, Virginia R.A, Wall D.H. Organic carbon cycling in Taylor Valley, Antarctica: quantifying soil reservoirs and soil respiration. Glob. Change Biol. 2001;7:113–125. doi:10.1046/j.1365-2486.2001.00393.x [Google Scholar]
- Callaghan T.V, et al. Responses to projected changes in climate and UV-B at the species level. Ambio. 2004;33:418–435. doi: 10.1579/0044-7447-33.7.418. doi:10.1639/0044-7447(2004)033[0418:RTPCIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Campbell I.B, Claridge G.G.C. The influence of moisture on the development of soils of the cold deserts of Antarctica. Geoderma. 1982;28:221–238. doi:10.1016/0016-7061(82)90004-0 [Google Scholar]
- Campbell I.B, Claridge G.G.C. Elsevier; New York, NY: 1987. Antarctica: soils, weathering processes, and environment. [Google Scholar]
- Campbell I.B, Claridge G.G.C, Campbell D.I, Balks M.R. The soil environment of the McMurdo Dry Valleys, Antarctica. In: Priscu J.C, editor. Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. American Geophysical Union; Washington, DC: 1998. pp. 297–322. [Google Scholar]
- Chown S.L, Convey P. Spatial and temporal variability across life's hierarchies in the terrestrial Antarctic. Phil. Trans. R. Soc. B. 2007;362:2307–2331. doi: 10.1098/rstb.2006.1949. doi:10.1098/rstb.2007.1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell C.S, Córdoba-Jabonero C. Coupling of climate change and biotic UV exposure through changing snow-ice covers in terrestrial habitats. Photochem. Photobiol. 2004;79:26–31. doi:10.1562/0031-8655(2004)79<26:COCCAB>2.0.CO;2 [PubMed] [Google Scholar]
- Cole, D. N. & Schreiner, E. G. 1981 Impacts of backcountry recreation: site management and rehabilitation—an annotated bibliography. Forest Service general technical report. Ogden, UT: USDA.
- Comiso J.C. Variability and trends in Antarctic surface temperatures from in situ and satellite infrared measurements. J. Climate. 2000;13:1674–1696. doi:10.1175/1520-0442(2000)013<1674:VATIAS>2.0.CO;2 [Google Scholar]
- Conley D.J. Riverine contribution of biogenic silica to the oceanic silica budget. Limnol. Oceanogr. 1997;42:774–777. [Google Scholar]
- Connell L, Redman R, Craig S, Rodriguez R. Distribution and abundance of fungi in the soils of Taylor Valley, Antarctica. Soil Biol. Biochem. 2006;38:3083–3094. doi:10.1016/j.soilbio.2006.02.016 [Google Scholar]
- Convey P. Maritime Antarctic climate change: signals from terrestrial biology. In: Domack E, Burnett A, Leventer A, Convey P, Kirby M, Bindschadler R, editors. Antarctic peninsula climate variability: historical and paleoenvironmental perspectives. American Geophysical Union; Washington, DC: 2003. pp. 145–158. [Google Scholar]
- Convey P, McInnes S.J. Exceptional tardigrade-dominated ecosystems in Ellsworth Land, Antarctica. Ecology. 2005;86:519–527. [Google Scholar]
- Convey P, Block W, Peat W.J. Soil arthropods as indicators of water stress in Antarctic terrestrial habitats? Global Change Biol. 2003;9:1718–1730. doi:10.1046/j.1365-2486.2003.00691.x [Google Scholar]
- Courtright E.M, Wall D.H, Virginia R.A. Determining habitat suitability for soil invertebrates in an extreme environment: the McMurdo Dry Valleys, Antarctica. Antarct. Sci. 2001;13:9–17. doi:10.1017/S0954102001000037 [Google Scholar]
- Cowan D.A, Tow L.A. Endangered Antarctic environments. Annu. Rev. Microbiol. 2004;58:649–690. doi: 10.1146/annurev.micro.57.030502.090811. doi:10.1146/annurev.micro.57.030502.090811 [DOI] [PubMed] [Google Scholar]
- Cowan D, Russell N, Mamais A, Sheppard D. Antarctic Dry Valley mineral soils contain unexpectedly high levels of microbial biomass. Extremophiles. 2002;6:431–436. doi: 10.1007/s00792-002-0276-5. doi:10.1007/s00792-002-0276-5 [DOI] [PubMed] [Google Scholar]
- Crossley D.A.J, Coleman D.C, Hendrix P.F. The importance of the fauna in agricultural soils: research approaches and perspectives. Agr. Ecosyst. Environ. 1989;27:47–55. doi:10.1016/0167-8809(89)90071-6 [Google Scholar]
- Davidson M.M, Broady P.A. Analysis of gut contents of Gomphiocephalus hodgsoni Carpenter (Collembola: Hypogastruridae) at Cape Geology, Antarctica. Polar Biol. 1996;16:463–467. [Google Scholar]
- De Deyn G.B, Van der Putten W.H. Linking aboveground and belowground diversity. Trends Ecol. Evol. 2005;20:625–633. doi: 10.1016/j.tree.2005.08.009. doi:10.1016/j.tree.2005.08.009 [DOI] [PubMed] [Google Scholar]
- De Deyn G.B, Raaijmakers C.E, Zoomer H.R, Berg M.P, de Ruiter P.C, Verhoef H.A, Bezemer T.M, Van der Putten W.H. Soil invertebrate fauna enhances grassland succession and diversity. Nature. 2003;422:711–713. doi: 10.1038/nature01548. doi:10.1038/nature01548 [DOI] [PubMed] [Google Scholar]
- Dobson A.P, Bradshaw A.D, Baker A.J.M. Hopes for the future: restoration ecology and conservation biology. Science. 1997;277:515–522. doi:10.1126/science.277.5325.515 [Google Scholar]
- Doran P.T, et al. Antarctic climate cooling and terrestrial ecosystem response. Nature. 2002;415:517–520. doi: 10.1038/nature710. doi:10.1038/nature710 [DOI] [PubMed] [Google Scholar]
- Drewry D.J, Jordan S.R, Jankowski E. Measured properties of the Antarctic ice sheet: surface configuration, ice thickness, volume and bedrock characteristics. Annu. Glaciol. 1982;3:83–91. [Google Scholar]
- Eberling B, Gregorich E.G, Hopkins D.W, Sparrow A.D, Novis P.M, Greenfield L.G. Distribution and dynamics of soil organic matter in an Antarctic Dry Valley. Soil Biol. Biochem. 2006;38:3095–3106. doi: 10.1098/rspb.2006.3595. doi:10.1016/j.soilbio.2005.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet A.F, Fountain A.G, Nylen T.H. A temperature-index model of stream flow at below-freezing temperatures in Taylor Valley, Antarctica. Annu. Glaciol. 2005;40:76–82. [Google Scholar]
- Esposito R.M.M, Horn S.L, McKnight D.M, Cox M.J, Grant M.C, Spaulding S.A, Doran P.T, Cozzetto K.D. Antarctic climate cooling and response of diatoms in glacial meltwater streams. Geophys. Res. Lett. 2006;33:L07 406. doi:10.1029/2006GL025903 [Google Scholar]
- Farman J.C, Murgatroyd R.J, Silnickas A.M, Thrush B.A. Ozone photochemistry in the Antarctic stratosphere in summer. Q. J. R. Meteor. Soc. 1985;111:1013–1025. doi:10.1256/smsqj.47005 [Google Scholar]
- Fountain A.G, Dana G.L, Lewis K.J, Vaughn B.H, McKnight D.M. Glaciers of the McMurdo Dry Valleys, southern Victoria Land, Antarctica. In: Priscu J.C, editor. Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. American Geophysical Union; Washington, DC: 1998. pp. 65–76. [Google Scholar]
- Fountain A.G, et al. Physical controls on the Taylor Valley ecosystem, Antarctica. Bioscience. 1999;49:961–971. doi:10.2307/1313730 [Google Scholar]
- Fountain A.G, Neumann T.A, Glenn P.L, Chinn T. Can climate warming induce glacier advance in Taylor Valley, Antarctica? J. Glaciol. 2004a;50:556–564. [Google Scholar]
- Fountain A.G, Tranter M, Nylen T.H, Lewis K.J, Mueller D.R. Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. J. Glaciol. 2004b;50:35–45. [Google Scholar]
- Freckman D.W, Mankau R. Abundance, distribution, biomass, and energetics of soil nematodes in a northern Mojave Desert ecosystem. Pedobiologia. 1986;29:129–142. [Google Scholar]
- Freckman D.W, Virginia R.A. Low diversity Antarctic soil nematode communities: distribution and response to disturbance. Ecology. 1997;78:363–369. doi:10.2307/2266013 [Google Scholar]
- Freckman D.W, Virginia R.A. Soil biodiversity and community structure in the McMurdo Dry Valleys, Antarctica. In: Priscu J.C, editor. Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. vol. 72. American Geophysical Union; Washington, DC: 1998. pp. 323–335. [Google Scholar]
- Frenot Y, Chown S.L, Whinam J, Selkirk P.M, Convey P, Skotnicki M, Bergstrom D.M. Biological invasions in the Antarctic: extent, impacts and implications. Biol. Rev. 2005;80:45–72. doi: 10.1017/s1464793104006542. doi:10.1017/S1464793104006542 [DOI] [PubMed] [Google Scholar]
- Friedmann E.I. Endolithic microorganisms in the Antarctic cold desert. Science. 1982;215:1045–1053. doi: 10.1126/science.215.4536.1045. doi:10.1126/science.215.4536.1045 [DOI] [PubMed] [Google Scholar]
- Fritsen C.H, Priscu J.C. Seasonal change in the optical properties of the permanent ice cover on Lake Bonney, Antarctica: consequences for lake productivity and phytoplankton dynamics. Limnol. Oceanogr. 1999;44:447–454. [Google Scholar]
- Gans J, Wolinsky M, Dunbar J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science. 2005;309:1387–1390. doi: 10.1126/science.1112665. doi:10.1126/science.1112665 [DOI] [PubMed] [Google Scholar]
- Gooseff M, Barrett J.E, Doran P.T, Fountain A.G, Lyons W.B, Parsons A.N, Porazinska D, Virginia R.A, Wall D.H. Snow-patch influence on soil biogeochemical processes and invertebrate distribution in the McMurdo Dry Valleys, Antarctica. Arct. Antarct. Alp. Res. 2003;35:91–99. doi:10.1657/1523-0430(2003)035[0091:SPIOSB]2.0.CO;2 [Google Scholar]
- Green T.G.A, Kulle D, Pannewitz S, Sancho L.G, Schroeter B. UV-A protection in mosses growing in continental Antarctica. Polar Biol. 2005;28:822–827. doi:10.1007/s00300-005-0011-7 [Google Scholar]
- Gremmen N.J.M, Smith V.R, van Tongeren O.F.R. Impact of trampling on vegetation of subantarctic Marion Island. Arct. Antarct. Alp. Res. 2003;35:442–446. doi:10.1657/1523-0430(2003)035[0442:IOTOTV]2.0.CO;2 [Google Scholar]
- Gressitt J.L, Leech R.E, O'Brien C.W. Trapping of air-borne insects in the Antarctic area. Pac. Insects. 1960;2:245–250. [Google Scholar]
- Grime J.P. Wiley; Chichester, UK: 1979. Plant strategies and vegetation processes. [Google Scholar]
- Harris K, Carey A, Lyons W.B, Welch K, Fountain A.G. Solute and isotope geochemistry of subsurface ice melt seeps in Taylor Valley, Antarctica. Geol. Soc. Am. Bull. 2007;119:548–555. [Google Scholar]
- Heemsbergen D.A, Berg M.P, Loreau M, van Haj J.R, Faber J.H, Verhoef H.A. Biodiversity effects on soil processes explained by interspecific functional dissimilarity. Science. 2004;306:1019–1020. doi: 10.1126/science.1101865. doi:10.1126/science.1101865 [DOI] [PubMed] [Google Scholar]
- Hobohm C. Characterization and ranking of biodiversity hotspots: centres of species richness and endemism. Biodivers. Conserv. 2003;12:279–287. doi:10.1023/A:1021934910722 [Google Scholar]
- Hogg I, et al. Biotic interactions in Antarctic terrestrial ecosystems: are they a factor? Soil Biol. Biochem. 2006;38:3035–3040. doi:10.1016/j.soilbio.2006.04.026 [Google Scholar]
- Holmes, D. O. & Dobson, H. E. M. 1976 Ecological carrying capacity research: Yosemite National Park: part 1: the effects of human trampling and urine on subalpine vegetation—a survey of past and present backcountry use and the ecological carrying capacity of wilderness. (ed. Department of Commerce). National Technical Information Service.
- Hopkins D.W, Sparrow A.D, Elberling B, Gregorich E.G, Novis P.M, Greenfield L.G, Tilston E.L. Carbon, nitrogen and temperature controls on microbial activity in soils from an Antarctic Dry Valley. Soil Biol. Biochem. 2006;38:3130–3140. doi:10.1016/j.soilbio.2006.01.012 [Google Scholar]
- Hunt H.W, Wall D.H. Modeling the effects of loss of soil biodiversity on ecosystem function. Glob. Change Biol. 2002;8:33–50. doi:10.1046/j.1365-2486.2002.00425.x [Google Scholar]
- Hunt H.W, Coleman D.C, Ingham E.R, Ingham R.E, Elliott E.T, Moore J.C, Rose S.L, Reid C.P.P, Morley C.R. The detrital food web in a shortgrass prairie. Biol. Fert. Soils. 1987;3:57–68. doi:10.1007/BF00260580 [Google Scholar]
- Hunt H.W, Reuss D.E, Elliott E.T. Correcting estimates of root chemical composition for soil contamination. Ecology. 1999;80:702–707. doi:10.2307/176645 [Google Scholar]
- Ingham R.E, Trofymow J.A, Ingham E.R, Coleman D.C. Interactions of bacteria, fungi, and their nematode grazers—effects on nutrient cycling and plant-growth. Ecol. Monogr. 1985;55:119–140. doi:10.2307/1942528 [Google Scholar]
- Jiang L, Morin P.J. Temperature-dependent interactions explain unexpected responses to environmental warming in communities of competitors. J. Anim. Ecol. 2004;73:569–576. doi:10.1111/j.0021-8790.2004.00830.x [Google Scholar]
- Joaquim-Justo C, Detry C, Caufman F, Thomé J.-P. Feeding of planktonic rotifers on ciliates: a method using natural ciliate assemblages labeled with fluorescent microparticles. J. Plankt. Res. 2004;26:1289–1299. doi:10.1093/plankt/fbh120 [Google Scholar]
- Kareiva P, Marvier M. Conserving biodiversity coldspots. Am. Sci. 2003a;91:344–351. doi:10.1511/2003.4.344 [Google Scholar]
- Kareiva P, Marvier M. Hotspots and coldspots—reply. Am. Sci. 2003b;91:385–386. doi:10.1511/2003.4.344 [Google Scholar]
- Karentz D. Ultraviolet tolerance mechanisms in Antarctic marine organisms. In: Weiler C.S, Penhale P.A, editors. Ultraviolet radiation in Antarctica: measurements and biological effects. vol. 62. American Geophysical Union; Washington, DC: 1994. pp. 93–110. [Google Scholar]
- Kemp M. Science in culture: inventing an icon. Nature. 2005;437:1238. doi:10.1038/4371238a [Google Scholar]
- Kennedy A.D. Water as a limiting factor in the Antarctic terrestrial environment: a biogeographical synthesis. Arct. Alp. Res. 1993;25:308–315. doi:10.2307/1551914 [Google Scholar]
- Kennedy A.D. Antarctic terrestrial ecosystem response to global environmental change. Annu. Rev. Ecol. Syst. 1995;26:683–704. doi:10.1146/annurev.es.26.110195.003343 [Google Scholar]
- Kobayashi T, Hori Y, Nomoto N. Effects of trampling and vegetation removal on species diversity and micro-environment under different shade conditions. J. Veg. Sci. 1997;8:873–880. doi:10.2307/3237032 [Google Scholar]
- Kuss F.R, Hall C.N. Ground flora trampling studies: five years after closure. Environ. Manage. 1991;15:715–727. doi:10.1007/BF02589629 [Google Scholar]
- Kwok R, Comiso J.C. Southern Ocean climate and sea ice anomalies associated with the Southern Oscillation. J. Climate. 2002;15:487–501. doi:10.1175/1520-0442(2002)015<0487:SOCASI>2.0.CO;2 [Google Scholar]
- Laybourn-Parry J, Pearce D.A. The biodiversity and ecology of Antarctic lakes—models for evolution. Phil. Trans. R. Soc. B. 2007;362:2273–2289. doi: 10.1098/rstb.2006.1945. doi:10.1098/rstb.2007.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liiri M, Setälä H, Haimi J, Pennanen T, Fritze H. Soil processes are not influenced by the functional complexity of soil decomposer food webs under disturbance. Soil Biol. Biochem. 2002;34:1009–1020. doi:10.1016/S0038-0717(02)00034-2 [Google Scholar]
- Loreau M, Thébault E. Food webs and the relationship between biodiversity and ecosystem functioning. In: de Ruiter P.C, Wolters V, Moore J.C, editors. Dynamic food webs: multispecies assemblages, ecosystem development and environmental change. Academic Press; Amsterdam, The Netherlands: 2005. pp. 270–294. [Google Scholar]
- Lyons W.B, et al. Groundwater seeps in Taylor Valley Antarctica: an example of a subsurface melt event. Annu. Glaciol. 2005;40:200–206. [Google Scholar]
- McKnight D.M, Alger A, Tate C.M, Shupe G, Spaulding S. Longitudinal patterns in algal abundance and species distribution in meltwater streams in Taylor Valley, Southern Victoria Island, Antarctica. In: Priscu J.C, editor. Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. American Geophysical Union; Washington, DC: 1998. pp. 109–128. [Google Scholar]
- Mikola J, Bardgett R.D, Hedlund K. Biodiversity, ecosystem functioning and soil decomposer food webs. In: Loreau M, Naeem S, Inchausti P, editors. Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press; Oxford, UK: 2002. pp. 169–180. [Google Scholar]
- Millennium Ecosystem Assessment. World Resources Institute; Washington, DC: 2005. Our human planet: summary for decision makers. [Google Scholar]
- Moore J.C, et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004;7:584–600. doi:10.1111/j.1461-0248.2004.00606.x [Google Scholar]
- Moorhead D.L, Priscu J.C. The McMurdo Dry Valley ecosystem: organization, controls, and linkages. In: Priscu J.C, editor. Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys. American Geophysical Union; Washington, DC: 1998. pp. 351–363. [Google Scholar]
- Moorhead D.L, Barrett J.E, Virginia R.A, Wall D.H, Porazinska D. Organic matter and soil biota of upland wetlands in Taylor Valley, Antarctica. Polar Biol. 2003;26:567–576. doi:10.1007/s00300-003-0524-x [Google Scholar]
- Myers N, Mittermeier R.A, Mittermeier C.G, da Fonseca G.A.B, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. doi:10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Neale P.J, Lesser M.P, Cullen J.J. Effects of ultraviolet radiation on the photosynthesis of phytoplankton in the vicinity of McMurdo Station, Antarctica. In: Weiler C.S, Penhale P.A, editors. Ultraviolet radiation in Antarctica: measurements and biological effects. American Geophysical Union; Washington, DC: 1994. pp. 93–110. [Google Scholar]
- Neufeld J.D, Mohn W.W. Unexpectedly high bacterial diversity in arctic tundra relative to boreal forest soils, revealed by serial analysis of ribosomal sequence tags. Appl. Environ. Microbiol. 2005;71:5710–5718. doi: 10.1128/AEM.71.10.5710-5718.2005. doi:10.1128/AEM.71.10.5710-5718.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsham K.K. UV-B radiation arising from stratospheric ozone depletion influences the pigmentation of the Antarctic moss Andreaea regularis. Oecologia. 2003;135:327–331. doi: 10.1007/s00442-003-1191-x. [DOI] [PubMed] [Google Scholar]
- Newsham K.K, Geissler P.A, Nicolson M.J, Peat H.J, Lewis-Smith R.I. Sequential reduction of UV-B radiation in the field alters the pigmentation of an Antarctic leafy liverwort. Environ. Exp. Bot. 2005;54:22–32. doi:10.1016/j.envexpbot.2004.05.006 [Google Scholar]
- Nienow J.A, McKay C.P, Friedmann E.I. The cryptoendolithic microbial environment in the Ross Desert of Antarctica—light in the photosynthetically active region. Microb. Ecol. 1988;16:271–289. doi:10.1007/BF02011700 [PubMed] [Google Scholar]
- Nkem J.N, Wall D.H, Virginia R.A, Barrett J.E, Broos E.J, Porazinska D.L, Adams B.J. Wind dispersal of soil invertebrates in the McMurdo Dry Valleys, Antarctica. Polar Biol. 2006;29:346–352. doi:10.1007/s00300-005-0061-x [Google Scholar]
- Onofri S, Selbmann L, Zucconi L, Pagano S. Antarctic microfungi as models for exobiology. Planet. Space Sci. 2004;52:229–237. doi:10.1016/j.pss.2003.08.019 [Google Scholar]
- Overhoff A, Freckman D.W, Virginia R.A. Life cycle of the microbivorous Antarctic Dry Valley nematode Scottnema lindsayae (Timm 1971) Polar Biol. 1993;13:151–156. doi:10.1007/BF00238924 [Google Scholar]
- Pastor J, Bockheim J.G. Soil development on moraines of Taylor Glacier, lower Taylor Valley, Antarctica. Soil Sci. Soc. Am. J. 1980;44:341–348. [Google Scholar]
- Porazinska D.L, Bardgett R.D, Blaauw M.B, Hunt H.W, Parsons A.N, Seastedt T.R, Wall D.H. Relationships at the aboveground—belowground interface: plants, soil microflora and microfauna, and soil processes. Ecol. Monogr. 2003;73:377–395. [Google Scholar]
- Porazinska D.L, Fountain A.G, Nylen T.H, Tranter M, Virginia R.A, Wall D.H. The biodiversity and biogeochemistry of cryoconite holes from McMurdo Dry Valley glaciers, Antarctica. Arct. Antarct. Alp. Res. 2004;36:84–91. doi:10.1657/1523-0430(2004)036[0084:TBABOC]2.0.CO;2 [Google Scholar]
- Powers L.E, Freckman D.W, Virginia R.A. Spatial distribution of nematodes in polar desert soils in Antarctica. Polar Biol. 1995;15:325–334. doi:10.1007/BF00238482 [Google Scholar]
- Powers L.E, Ho M.C, Freckman D.W, Virginia R.A. Distribution, community structure, and microhabitats of soil invertebrates along an elevational gradient in Taylor Valley, Antarctica. Arct. Alp. Res. 1998;30:133–141. doi:10.2307/1552128 [Google Scholar]
- Priscu J.C, Wolf C.F, Takacs C.D, Fritsen C.H, Laybourn-Parry J, Roberts E.C, Sattler B, Lyons W.B. Carbon transformations in a perennially ice-covered Antarctic lake. Bioscience. 1999;49:997–1008. doi:10.2307/1313733 [Google Scholar]
- Procter D.L.C. Towards a biogeography of free-living soil nematodes. 1. Changing species richness, diversity and densities with changing latitude. J. Biogeogr. 1984;11:103–117. doi:10.2307/2844684 [Google Scholar]
- Reid W. Biodiversity hotspots. Trends Ecol. Evol. 1998;13:275–280. doi: 10.1016/s0169-5347(98)01363-9. doi:10.1016/S0169-5347(98)01363-9 [DOI] [PubMed] [Google Scholar]
- Roberts E.C, Laybourn-Parry J. Mixotrophic cryptophytes and their predators in the Dry Valley lakes of Antarctica. Freshw. Biol. 1999;41:737–746. doi:10.1046/j.1365-2427.1999.00401.x [Google Scholar]
- Roscher C, Schumacher J, Baade J, Wilcke W, Gleixner G, Weisser W.W, Schmid B, Schulze E.D. The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl. Ecol. 2004;5:107–121. doi:10.1078/1439-1791-00216 [Google Scholar]
- Schnellnhuber H.J, Cramer W, Nakicenovic N, Wigley T, Yohe G. Avoiding dangerous climate change. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Schwarz A.M.J, Green T.G.A, Seppelt R.D. Terrestrial vegetation at Canada Glacier, Southern Victoria Land, Antarctica. Polar Biol. 1992;12:397–404. doi:10.1007/BF00243110 [Google Scholar]
- Schwarz A.M.J, Green J.D, Green T.G.A, Seppelt R.D. Invertebrates associated with moss communities at Canada Glacier, Southern Victoria Land, Antarctica. Polar Biol. 1993;13:157–162. doi:10.1007/BF00238925 [Google Scholar]
- Scott J.J, Kirkpatrick J.B. Effects of human trampling on the sub-Antarctic vegetation of Macquarie Island. Polar Rec. 1994;30:207–220. [Google Scholar]
- Setälä H. Sensitivity of ecosystem functioning to changes in trophic structure, functional group composition and species diversity in belowground food webs. Ecol. Res. 2002;17:207–215. doi:10.1046/j.1440-1703.2002.00480.x [Google Scholar]
- Setälä H. Does biological complexity relate to functional attributes of soil food webs? In: de Ruiter P.C, Wolters V, Moore J.C, editors. Dynamic food webs: multispecies assemblages, ecosystem development and environmental change. Academic Press; Amsterdam, The Netherlands: 2005. pp. 308–320. [Google Scholar]
- Shindell D.T, Schmidt G.A. Southern Hemisphere climate response to ozone changes and greenhouse gas increases. Geophys. Res. Lett. 2004;31:1–4. [Google Scholar]
- Sinclair B.J. Effects of increased temperatures simulating climate change on terrestrial invertebrates on Ross Island, Antarctica. Pedobiologia. 2002;46:150–160. doi:10.1078/0031-4056-00121 [Google Scholar]
- Solomon S. Progress towards a quantitative understanding of Antarctic ozone depletion. Nature. 1990;347:347–354. doi:10.1038/347347a0 [Google Scholar]
- Solomon S, Portmann R.W, Sasaki T, Hofmann D.J, Thompson D.W.J. Four decades of ozonesonde measurements over Antarctica. J. Geophys. Res.-Atmos. 2005;110:D21 311. doi:10.1029/2005JD005917 [Google Scholar]
- St. John M.G, Wall D.H, Behan-Pelletier V.M. Does plant species co-occurrence influence soil mite diversity? Ecology. 2006;87:625–633. doi: 10.1890/05-0380. [DOI] [PubMed] [Google Scholar]
- Stevens M.I, Hogg I.D. Long-term isolation and recent range expansion from glacial refugia revealed for the endemic springtail Gomphiocephalus hodgsoni from Victoria Land. Antarctica Mol. Ecol. 2003;12:2357–2369. doi: 10.1046/j.1365-294x.2003.01907.x. doi:10.1046/j.1365-294X.2003.01907.x [DOI] [PubMed] [Google Scholar]
- Sun D, Liddle M.J. A survey of trampling effects on vegetation and soil in eight tropical and subtropical sites. Environ. Manage. 1993;17:497–510. doi:10.1007/BF02394665 [Google Scholar]
- Swift M.J, Heal O.W, Anderson J.M. Blackwell; Oxford, UK: 1979. Decomposition in terrestrial ecosystems. [Google Scholar]
- Thompson D.W.J, Solomon S. Interpretation of recent Southern Hemisphere climate change. Science. 2002;296:895–899. doi: 10.1126/science.1069270. doi:10.1126/science.1069270 [DOI] [PubMed] [Google Scholar]
- Tosi S, Onofri S, Brusoni M, Zucconi L, Vishniac H. Response of Antarctic soil fungal assemblages to experimental warming and reduction of UV radiation. Polar Biol. 2005;28:470–482. [Google Scholar]
- Treonis A.M, Wall D.H. Soil nematodes and desiccation survival in the extreme arid environment of the Antarctic Dry Valleys. Integr. Comp. Biol. 2005;45:741–750. doi: 10.1093/icb/45.5.741. doi:10.1093/icb/45.5.741 [DOI] [PubMed] [Google Scholar]
- Treonis A.M, Wall D.H, Virginia R.A. Invertebrate biodiversity in Antarctic Dry Valley soils and sediments. Ecosystems. 1999;2:482–492. doi:10.1007/s100219900096 [Google Scholar]
- Treonis A.M, Wall D.H, Virginia R.A. The use of anhydrobiosis by soil nematodes in the Antarctic Dry Valleys. Funct. Ecol. 2000;14:460–467. doi:10.1046/j.1365-2435.2000.00442.x [Google Scholar]
- Turner J, Colwell S.R, Marshall G.J, Lachlan-Cope T.A, Carleton A.M, Jones P.D, Lagun V, Reid P.A, Iagovkina S. Antarctic climate change during the last 50 years. Int. J. Climatol. 2005;25:279–294. doi:10.1002/joc.1130 [Google Scholar]
- Utermohl H. Toward the improvement of the quantitative phytoplankton method. Mitteilungen-Interntionale Veriningung fur Limnologie. 1958;9:1–38. [Google Scholar]
- Van der Putten W.H, Vet L.E.M, Harvey J.A, Wackers F.L. Linking above- and belowground miltitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol. Evol. 2001;16:547–554. doi:10.1016/S0169-5347(01)02265-0 [Google Scholar]
- Vaughan D.G, Marshall G.J, Connolley W.M, Parkinson C, Mulvaney R, Hodgson D.A, King J.C, Pudsey C.J, Turner J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Change. 2003;60:243–274. doi:10.1023/A:1026021217991 [Google Scholar]
- Vincent W.F, Howard-Williams C. Antarctic stream ecosystems—physiological ecology of a blue-green-algal epilithon. Freshw. Biol. 1986;16:219–233. doi:10.1111/j.1365-2427.1986.tb00966.x [Google Scholar]
- Vincent W.F, Quesada A. Ultraviolet radiation effects on cyanobacteria: implications for Antarctic microbial ecosystems. In: Weiler C.S, Penhale P.A, editors. Ultraviolet radiation in Antarctica: measurements and biological effects. American Geophysical Union; Washington, DC: 1994. pp. 111–124. [Google Scholar]
- Virginia R.A, Wall D.H. How soils structure communities in the Antarctic Dry Valleys. BioScience. 1999;49:973–983. doi:10.2307/1313731 [Google Scholar]
- Vitousek P.M, Mooney H.A, Lubchenco J, Melillo J.M. Human domination of Earth's ecosystems. Science. 1997;277:494–499. doi:10.1126/science.277.5325.494 [Google Scholar]
- Walker M.D, Gould W.A, Chapin F.S. Scenarios of biodiversity changes in Arctic and Alpine Tundra. In: Chapin F.S, Sala O, Janetos A, editors. Global biodiversity in a changing environment: scenarios for the 21st century. Springer; New York, NY: 2001. pp. 83–100. [Google Scholar]
- Wall D.H. Island Press; Washington, DC: 2004. Sustaining biodiversity and ecosystem services in soil and sediments. [Google Scholar]
- Wall D.H. Biodiversity and ecosystem functioning in terrestrial habitats of Antarctica. Antarct. Sci. 2005;17:523–531. doi:10.1017/S0954102005002944 [Google Scholar]
- Wall D.H, Bardgett R.D, Covich A.P, Snelgrove P.V.R. The need for understanding how biodiversity and ecosystem functioning affect ecosystem services in soil and sediments. In: Wall D.H, editor. Sustaining biodiversity and ecosystem services in soils and sediments. Island Press; Washington, DC: 2004. pp. 1–12. [Google Scholar]
- Wall D.H, Fitter A.H, Paul E.A. Developing new perspectives from advances in soil biodiversity research. In: Bardgett R.D, Usher M.B, Hopkins D.W, editors. Biological diversity and function in soils. Cambridge University Press; Cambridge, UK: 2005. pp. 3–27. [Google Scholar]
- Walsh J.E, et al. Climate change—recent temperature trends in the Antarctic. Nature. 2002;418:292. doi: 10.1038/418291b. doi:10.1038/418292a [DOI] [PubMed] [Google Scholar]
- Wardle D.A. Princeton University Press; Princeton, NJ: 2002. Communities and ecosystems: linking the aboveground and belowground components. [Google Scholar]
- Wardle D.A, Bardgett R.D, Klironomos J.N, Setälä H, Van der Putten W.H, Wall D.H. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. doi:10.1126/science.1094875 [DOI] [PubMed] [Google Scholar]
- Waterhouse E.J. Implementing the protocol on ice free land: the New Zealand experience at Vanda Station. In: Lyons W.B, Howard-Williams C, Hawes I, editors. Ecosystem processes in Antarctic ice-free landscapes. A. A. Balkema; Rotterdam, The Netherlands: 1997. pp. 265–274. [Google Scholar]
- Weiler C.S, Penhale P.A. Antarctic research series. American Geophysical Union; Washington, DC: 1994. Ultraviolet radiation in Antarctica: measurements and biological effects. [Google Scholar]
- Wynn-Williams D.D. Ecological aspects of Antarctic microbiology. In: Marshall K.C, editor. Advances in microbial ecology. Plenum Press; New York, NY: 1990. pp. 71–146. [Google Scholar]
- Wynn-Williams D.D. Potential effects of ultraviolet radiation on Antarctic primary terrestrial colonizers: cyanobacteria, algae, and cryptogams. In: Weiler C.S, Penhale P.A, editors. Ultraviolet radiation in Antarctica: measurements and biological effects. vol. 62. American Geophysical Union; Washington, DC: 1994. pp. 243–257. [Google Scholar]
- Young V.B, Gilmore A.R. Effects of various camping intensities on soil properties in Illinois campgrounds. Soil Sci. Soc. Am. Pro. 1976;40:908–911. [Google Scholar]