Abstract
The Southern Ocean is a major component within the global ocean and climate system and potentially the location where the most rapid climate change is most likely to happen, particularly in the high-latitude polar regions. In these regions, even small temperature changes can potentially lead to major environmental perturbations. Climate change is likely to be regional and may be expressed in various ways, including alterations to climate and weather patterns across a variety of time-scales that include changes to the long interdecadal background signals such as the development of the El Niño–Southern Oscillation (ENSO). Oscillating climate signals such as ENSO potentially provide a unique opportunity to explore how biological communities respond to change. This approach is based on the premise that biological responses to shorter-term sub-decadal climate variability signals are potentially the best predictor of biological responses over longer time-scales. Around the Southern Ocean, marine predator populations show periodicity in breeding performance and productivity, with relationships with the environment driven by physical forcing from the ENSO region in the Pacific. Wherever examined, these relationships are congruent with mid-trophic-level processes that are also correlated with environmental variability. The short-term changes to ecosystem structure and function observed during ENSO events herald potential long-term changes that may ensue following regional climate change. For example, in the South Atlantic, failure of Antarctic krill recruitment will inevitably foreshadow recruitment failures in a range of higher trophic-level marine predators. Where predator species are not able to accommodate by switching to other prey species, population-level changes will follow. The Southern Ocean, though oceanographically interconnected, is not a single ecosystem and different areas are dominated by different food webs. Where species occupy different positions in different regional food webs, there is the potential to make predictions about future change scenarios.
Keywords: climate change, recent, rapid, regional warming
1. Introduction
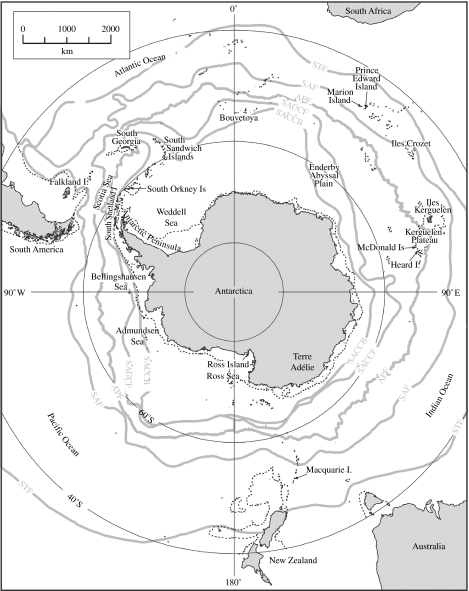
The Southern Ocean (figure 1) is a major component within the global ocean and climate system. It not only unites the Atlantic Ocean with the Indian and the Pacific Oceans, but also connects low tropical latitudes with high polar latitudes. In addition, the Southern Ocean is also the origin of important teleconnections that link around the globe and across the equator into the Northern Hemisphere. Consequently, and given this unique global situation, there is now considerable concern that significant changes to the Southern Ocean (resulting from recent rapid regional warming) have occurred over the past 50 years (King 1994; Smith et al. 1999; Levitus et al. 2000; Gille 2002).
Figure 1.
The Southern Ocean. The 1000 m bathymetric contour and the major fronts in the Antarctic Circumpolar Current are shown. Place names mentioned in the text are indicated.
Recently, The Intergovernmental Panel on Climate Change (IPCC 2001) noted that the location where rapid climate change is most likely to happen is in the polar regions, and that one of the locations where there has been the greatest recorded warming is to the west of the Antarctic Peninsula (King 1994). Over the past 50 years, this region has undergone warming that is unprecedented over the previous two millennia, and as such is unlikely to be a natural mode of variability (Vaughan et al. 2003). The Antarctic Peninsula region is also one of only three high-latitude areas that have warmed more rapidly than the global mean, and the only one that is almost entirely marine (Vaughan et al. 2003). Though warming to the west of the Antarctic Peninsula is rapid, evidence of substantial and sustained warming is also apparent elsewhere in the South Atlantic. For example, at Signy Island in the South Orkney Islands, air temperature rises of at least 0.8°C have occurred over the past 50 years (Quayle et al. 2002).
Beyond the South Atlantic, there are also strong indications of an increase in ocean temperatures (Gille 2002). For example, in both the Indian Ocean and the Pacific sector, surface temperature increases are now evident (Jacka & Budd 1998). The extent of this warming underlines the fact that at most sites in the Southern Ocean, indications exist of a major change in key properties of the physical environment.
In the polar regions, even small temperature changes can potentially lead to major environmental perturbations. Thus, there is now growing evidence that the maximum extent of winter sea ice is declining (Curran et al. 2003). However, these signals are spatially and temporally variable, with some regions (such as the Ross Sea) showing recent increases in sea-ice extent, while others (such as the Bellingshausen and Amundsen Seas) show decreases (Jacka & Budd 1998; Parkinson 2002, 2004). The observed patterns in sea-ice decline correspond well with trends in air temperature, but there is still a substantial amount of work to be undertaken before the patterns are fully understood and can be modelled in the context of global change (Parkinson 2004).
Long-term observations such as those above suggest that some areas in the Southern Ocean may be the most vulnerable parts of the global marine system with the potential for ecosystem change. Furthermore, given the interconnected nature of the Southern Ocean, impacts felt in one area may be transferred rapidly elsewhere (Trathan & Murphy 2002; Trathan et al. 2003; Murphy et al. submitted) and indeed even around the globe (Salinger 2005). Such vulnerability has the potential to disrupt the oceans of the world causing dramatic biological and economic impacts.
Regional climate change may be expressed in the physical environment in a number of different ways. The most obvious include the warming of both sea and air temperatures (King 1994; Smith et al. 1999; Levitus et al. 2000; Gille 2002), together with a number of impending consequences of this, such as changed sea-ice dynamics (Vaughan et al. 2003), including duration, thickness and extent of sea ice. However, less obvious changes may also be manifest (Salinger 2005). These include alterations to climate and weather patterns across a variety of time-scales that range from long interdecadal background signals to the frequency of short weekly or even daily events. For example, changes to the pattern of El Niño–Southern Oscillation (ENSO) development since the late 1970s has been emphasized by Trenberth & Hoar (1996), who related the more frequent occurrence of El Niño (warm) events and the less frequent occurrence of La Niña (cold) events to decadal changes in climate throughout the Pacific (see also Fedorov & Philander 2000). Similarly, mean annual typhoon activity is generally higher (lower) during an El Niño (La Niña) year (Chan & Liu 2004), and the altered frequency of such events is of potential importance to biological communities. Other global climate signals are also important in the Southern Ocean and may potentially have impacts upon biological communities and population-level processes. For example, the Antarctic Oscillation Index (AAO; Gong & Wang 1999) is thought to impact upon biological communities in the Ross Sea (Ainley et al. 2005), though the relationship between the AAO and other long-term processes currently remains largely unexplored. The emerging consequences of climate variability and change signals are potentially profound; population and ecosystem-level changes may be rapid, far-reaching and decadal-scale changes, or regime shifts (Steele 1998; Hunt et al. 2002) may be evident where ecosystem changes trigger population-level processes.
In this paper, we review recent Southern Ocean studies on marine predators to determine how the environment affects their populations. More specifically, we attempt to determine whether, given the regional impacts of climate change, there are common factors in different areas that may enable us to understand these recent population changes. Furthermore, we consider the role of climate variability in order to examine how these processes may operate and how populations may respond to future change.
2. Using oscillatory climate signals to predict future change in biological communities
At the moment, most Southern Ocean biological time-series are still too short in duration (in relation to non-stationary long-term climate change signals) to allow for confident predictions about the possible ecosystem consequences of climate change (Croxall et al. 2002). However, though evidence is slowly accumulating about some of the likely biological consequences, some recent studies suggest an alternative approach. This approach is based on the premise that biological responses to shorter-term sub-decadal signals in climate variability may be the best predictor of biological responses over longer time-scales. Clearly, not all such predictions will be correct; however, they potentially form a plausible component of predicting future change.
Thus, oscillating climate signals such as ENSO potentially provide a unique opportunity to explore how biological communities respond to change. For example, El Niño (warm) events sometimes lead to warm temperature anomalies of over 2.0°C at the South Orkney Islands (Forcada et al. 2006). These anomalies compare closely (being of a similar order of magnitude) with regional warming signals at the same location, where there have been increases of perhaps 2.0°C since 1903 (Forcada et al. 2006) and of at least 0.8°C over the past 50 years (Quayle et al. 2002). At the South Orkney Islands, impacts from ENSO warm anomalies are now known to be profound across a range of trophic levels (Forcada et al. 2006); however, the impacts of regional warming remain largely unexplored but are potentially comparable or of a similar magnitude (cf. Fraser et al. 1992; Trathan et al. 1996).
Elsewhere in the Southern Ocean, high sea surface temperature anomalies in response to El Niño (warm) events also occur over short periods (Trathan & Murphy 2002; Trathan et al. 2006). Consequently, extreme anomalous events in the oscillatory climate system potentially provide a telescope with which to examine the future consequences of warming. This is potentially very powerful as such oscillatory signals provide an opportunity to examine some of the probable ecosystem consequences of warming, prior to the consolidation of increased temperature into regional climatologies.
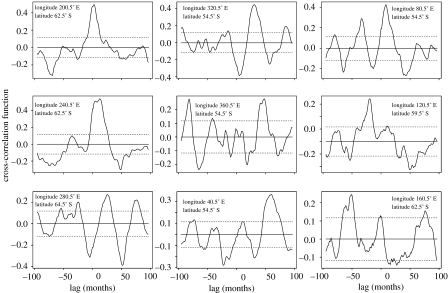
The impacts of El Niño are clearly evident around the globe (Diaz & Markgraf 2000) and, in the Southern Ocean, propagate westward at a rate consistent with oceanographic transport (Peterson & White 1998). Analyses (figure 2) show that correlations exist between sea surface temperature in the El Niño regions and temperatures in other locations around the Southern Ocean (see also Trathan & Murphy 2002). Such relationships remain significant even after spurious cross-correlations that arise from autocorrelation within each time-series have been converted to approximate white noise (Forcada et al. 2005; Trathan et al. 2006). Though sea surface temperatures in both the Atlantic and the Indian Oceans are related to the Pacific Ocean, the manner in which these teleconnections operate and structure the physical environment remains unclear (Connolley 2002; Trathan & Murphy 2002); certainly, coherent correlations decrease (figure 2) between the Indian Ocean (longitude 80° E) and the western Pacific Ocean (longitude 160° E).
Figure 2.
Plots of the cross-correlation function between the El Niño 4 region (5° N to 5° S, 160° E to 50° W; http://www.cpc.noaa.gov/data/indices/sstoi.indices) and sea surface temperatures (Reynolds et al. 2002) close to the Antarctic Polar Front at longitudes 40°, 80°, 120°, 160°, 200°, 240°, 280°, 320° and 360° E. The time-series at each longitude represents the mean temperature value from a box measuring 2° of latitude×2° of longitude. The 95% confidence limits (±1.96/√n where n=280) are shown to help indicate whether cross-correlations are significantly different from zero.
3. Potential for regional impacts on the biosphere
Though the world oceans have warmed over the past 50 years (Levitus et al. 2000), the biological consequences of this remain poorly described (Croxall 1992; Croxall et al. 2002). This is partly because long-term datasets with the potential to catalogue the biological response are rare, partly because signals vary between taxa, and partly because biological responses may be lagged by one or more generations (Saether et al. 2005). Furthermore, the biological response to climate change for any given species may be extremely complex and may vary between locations, between genders and between demographic categories (Stenseth et al. 2002), depending upon where animals breed or over-winter, and how local is any regional climate change.
Climate change is likely to have impacts at all trophic levels and will be manifest in a variety of ways (Chambers et al. 2005). For seabirds and marine mammals, most responses will be evident as changes in behaviour, phenotypic expression or in genotype that result in one or more of the following.
Changes in distribution. At its simplest, a pole-ward shift in ranges may be evident; however, given the complexity of ocean circulation in some regions (figure 1), changes in distribution may be much more complex.
Changed patterns of movement. If the distribution of important prey species is also altered by changes in the climate, then regular commuting between breeding sites and foraging grounds may be affected. Similarly, migration routes between summer and winter foraging grounds may be altered.
Changes in population density. Population sizes and densities may change due to the direct effects of extreme environments, through impacts on vital rates (such as survival, fertility and breeding success). Populations may fragment and genetic diversity may be reduced.
Changes in phenology. As a consequence of changed environmental conditions, a wide variety of processes could alter in their absolute timing over the course of a year, including for example dates of arrival at a breeding colony, dates of egg laying and dates of fledging in birds, or dates of pupping and weaning in mammals. If temperature is a direct cue and if the degree of regional warming varies between regions, then some processes may also change in their relative timing and relation to each other, and this could have further ecological implications.
Changes in behaviour. Diurnal patterns of activity and behaviour may also change as a consequence of altered environmental conditions. For example, if animals experience warmer temperatures, they may need to spend more time cooling themselves; this will potentially have important energetic consequences for individuals.
Change in community interactions. If climate change impacts upon a number of trophic levels, then wholesale community changes may become evident. This may be apparent as a ‘regime shift’ (Rodionov 2004) or as changes in the food web as new species interactions or predator–prey interactions develop.
Changes in morphology or physiology. As environmental conditions alter, animal populations may respond by changes in morphology, such as changes in body mass or breeding performance, either as a result of energetic constraints or because of altered physiological efficiencies.
4. Confounding issues in identifying a biological signal
In the Southern Ocean, some of these responses are now becoming apparent (Croxall et al. 2002; Fraser & Hofmann 2003; Weimerskirch et al. 2003; Ainley et al. 2005), yet they are not widespread and not always well documented. Further, some response signals are more readily detectable than others, while others are confounded. This is particularly the case where there have been levels of human harvesting that have caused ecosystem change. At times, historical levels of harvesting for fur seals, some penguin species, whales and more recently some fish and crustaceans have been such that ecosystem dynamics have been disrupted to a very great extent and community interactions greatly affected (e.g. the so called ‘krill surplus’ hypothesis of Laws 1977). For some areas, upper trophic-level predators were removed in a wholesale manner, particularly where they were of high economic value (Pauly et al. 1998). Removing these species has had fundamental impacts on species interactions in the marine food web and probably caused a major shift in food web structure with consequent impacts on ecosystem stability (Odum 1969). In addition to high economic value species, other upper trophic-level predators were sometimes also removed. For example, in some areas, apex predators such as killer whales (Orcinus orca) were removed, though documentation of such removals is often incomplete. Removing such predators has probably had important implications for some populations of penguins and seals that form important prey items (Guinet 1992). Thus, at St Andrews Bay, South Georgia, only 700 pairs of king penguins (Aptenodytes patagonicus) bred in 1928 (Kohl-Larsen 2002); today, the same colony is sufficiently large that it may comprise upwards of 150 000 pairs (P. N. Trathan 2004, personal observation). Such a rate of growth may be in part due to the removal of apex predators. More recently, other upper trophic-level predators have also been removed through incidental mortality associated with long-line fishing; this has impacted many species of Procellariiformes (Brothers 1991) and rajids (Endicott & Agnew 2004). Consequently, harvesting has had various impacts upon the Southern Ocean marine ecosystem such that many species interactions are now probably absent or reduced and some feedback mechanisms may be absent (Pauly & Maclean 2003). Thus, in the face of the Southern Ocean's recovery towards a new equilibrium after large-scale harvesting, understanding how recent rapid regional climate change has affected the biosphere is complex. Signals reflecting ecosystem recovery post-harvest are likely to confound signals of climate change; thus for a given species, patterns of distribution, movement, density, phenology, behaviour and community interaction will probably be difficult to disentangle.
5. Regional ecosystem responses as a consequence of regional food webs variation
Marine mammals and seabirds are some of the best-studied taxa in the Southern Ocean. Much of the reasoning behind this intensive study has been that these long-lived species are assumed to integrate across space and time and as such are thought to be good indicators of ecosystem status. This emphasis has in part been driven by the desire to manage and harvest marine resources in a sustainable way using dependent species as indicators (CCAMLR 1982). Thus, if top predators reflect ecosystem status (Thompson & Ollason 2001), it is precisely because they are dependent upon an extensive set of trophic links within the wider marine food web. As such, understanding how top predators respond to the environment requires that we also understand how the wider food web responds and which trophic interactions are key.
The Southern Ocean, though oceanographically interconnected, is not a single ecosystem. Different areas are dominated by different food webs. For example, different species of the crustacean genus Euphausia are commonly found within the Southern Ocean. These have distinct latitudinal gradients in their distribution, reflecting specific temperature tolerances (Everson 2000). For instance, ice krill (Euphausia crystallorophius) inhabit more southerly coastal and neritic regions of the Antarctic continent, whereas sub-Antarctic krill (Euphausia vallentini) inhabit more northerly latitudes to the north of the Antarctic Polar Front (APF). Antarctic krill (Euphausia superba), Euphausia frigida and Euphausia tricantha each occupy different intermediate regions.
The distribution of these species also varies meridionally, possibly because of their differing habitat preferences and the regional differences in bathymetry and sea-ice limits (Everson 2000). The rugged topography of the Southern Ocean is particularly important in this respect as submarine features sometimes constrain and steer the main water masses, thereby further modifying latitudinal temperature regimes (Orsi et al. 1995). Topography also impacts upon where animals can successfully spawn and larvae develop to form self-sustaining populations (Hofmann & Husrevoglu 2003).
Euphausids are part of distinct Southern Ocean zooplankton communities that vary spatially and temporally and which contribute towards food web structure critical to higher trophic levels. Consequently, given the observed levels of spatial variability in lower trophic levels, coupled with specific temperature tolerances, it is almost certain that the impacts of regional warming will be very different in different regions. The consequences for upper trophic levels will inevitably depend upon the regional food webs they reside within.
6. Where will biological signals be most apparent?
The areas where biological impacts are most likely to be evident will plausibly be where regional warming is most apparent. Thus, to the west of the Antarctic Peninsula, in the Amundsen Sea and across almost the entire Bellingshausen Sea, the duration of the sea-ice season has shortened by more than 1 day per year between 1979 and 2002 (Parkinson 2002, 2004). Similarly, to the east of the Antarctic Peninsula across the northwestern portion of the Weddell Sea, the sea-ice season has also decreased (Parkinson 2002, 2004). Consequently, the Southwest Atlantic region is potentially one of the most probable areas where the impacts of recent rapid regional warming should be observable over a range of trophic levels.
Elsewhere in the Southern Ocean, climate change signals may be evident in the marine ecosystem; however, as warming is less pronounced, such signals may be less clear. The area around the Île de Kerguelan is such an area where warming is evident (Xue et al. 2003), but to a lesser degree than to the west of the Antarctic Peninsula. Either side of the Kerguelan Plateau, the duration of the sea-ice season has reduced, both over the Enderby Abyssal Plain and over the South Indian Abyssal Plain (Parkinson 2004). Thus, in the Indian Ocean, and particularly at Île de Kerguelan and at Îles de Crozet, where large populations of land-based, air-breathing predators breed, the impacts of climate change may be evident (Weimerskirch et al. 2003).
Beyond the Atlantic and the Indian Oceans, positive trends in surface temperatures are less apparent. Generally, much of the Southern Ocean has experienced a lengthening of the sea-ice season by at least 1 day per year over the period 1979–1999 (Parkison 2004). These areas encompass some 5.6×106 km2, and lie mainly within the Pacific Ocean and the Ross Sea sector (Parkison 2002, 2004). In these regions, the impacts of climate change may be less evident or may even be the reverse of those observed in the Atlantic and the Indian Oceans.
Given the regional nature of climate warming, regional differences in marine food web communities, regional differences in both historical and current levels of harvesting, and the short nature of many biological time-series, it is not possible to expect consistent responses from upper trophic-level marine predators. Nevertheless, in each of the ocean basins, the impacts of climate are evident as driving forces in the regional communities. For example, the biological consequences of ENSO are manifest in each ocean and potentially provide some evidence of plausible future change scenarios.
7. The Southwest Atlantic
The food webs of the Southwest Atlantic have been studied since the Discovery Investigations (1925–1939) at the beginning of the last century (Marr 1962; Mackintosh 1972; see also Atkinson et al. 2004). Food webs here are dominated by E. superba, which is most commonly found in shelf/shelf slope locations (Everson 2000; Trathan et al. 2003). Thus, the distribution of Antarctic krill in the Southwest Atlantic potentially reflects the species temperature tolerance (latitudinal limits), modified by the extensive area of continental shelf (Everson 2000; Trathan et al. 2003).
One of the major nursery grounds for Antarctic krill lies to the north of the Antarctic Peninsula (Siegel 1988; Brinton 1991; Spiridonov 1995; Siegel et al. 2002), close to the area of recent rapid regional warming (King 1994). Ocean currents are thought to carry krill from this area to other areas of the Southwest Atlantic (Hofmann et al. 1998; Murphy et al. 1998; Thorpe et al. 2004). Consequently, changes in the environment close to the nursery grounds have the potential to have far-reaching impacts on both local and more distant marine communities. Changes in the abundance of krill are thought to have occurred over the latter part of the last century, with abundance levels approximately half of those found during the middle part of the century (Atkinson et al. 2004). This reduction overlies the well-documented levels of intra-annual (Brierley et al. 2002) and inter-annual (Brierley et al. 1999; Trathan et al. 2003) variability in abundance known to occur at some sites.
Periodic pulses in krill biomass have been observed since the early part of the last century when the Discovery Investigations were initiated in association with the shore-based whaling industry at South Georgia. Early analyses indicated that substantial levels of variability in krill abundance were potentially linked with changes in mean temperature (Harmer 1931; Kemp & Bennett 1932). Later, Mackintosh (1972) reviewed the available data and suggested that krill abundance was linked with cold and warm periods, which in turn were linked with variability in the extent and concentration of regional sea ice and oceanography. Other more recent ecosystem studies in the Southwest Atlantic have pointed to strong relationships between temperature and the abundance of Antarctic krill (Trathan et al. 2003).
Temporal patterns in krill recruitment have also been highlighted recently (Fraser & Hofmann 2003) and suggest that there is a direct causal relationship between variability in sea-ice cover, krill recruitment, prey availability and predator foraging ecology, and that large-scale forcing associated with climate variability may be governing ecological interactions between ice, krill and their predators in the western Antarctic Peninsula and Scotia Sea regions. Similar regional patterns and trends in krill recruitment have been observed elsewhere in the Southwest Atlantic, for example at the South Orkney Islands (Lynnes et al. 2004) and as far north as South Georgia (Reid et al. 1999). Strong age classes that emerge episodically every 4–5 years have sustained krill populations over the past few decades at these sites. This suggests the possibility that cohort senescence has become an additional ecosystem force in an environment where ice conditions favourable to good krill recruitment are deteriorating due to climate warming (Fraser & Hofmann 2003).
Murphy et al. (submitted) suggest that krill of age 2+ years recruit into the population 1 year after warm summers and that because of the oscillatory nature of the physical environment (ENSO), this tends to be 1 year before the system enters a cold phase and sea-ice extent is increasing. Krill biomass is therefore likely to peak during a cold period (Trathan et al. 2003) when krill are 3+ years of age. Strong recruitment is related to the timing of winter sea-ice advance, retreat, duration and extent (Quetin & Ross 2003). Strong connections between El Niño and sea surface temperature (Trathan & Murphy 2002; Trathan et al. 2006) and between El Niño and sea ice (Quetin & Ross 2003; Trathan et al. 2006) also result in strong connections between El Niño and krill recruitment (Quetin & Ross 2003). As a consequence of the oscillatory nature of the environment (ENSO), krill populations rely upon strong recruitment in only a few years, with subsequent senescence of the cohorts (Fraser & Hofmann 2003; Quetin & Ross 2003; Lynnes et al. 2004). Such periodic recruitment events have the potential to generate periodic pulses in krill biomass (Murphy et al. submitted).
Pulses in krill biomass are likely to impact upon other components in the marine ecosystem. Thus, Fraser & Hofmann (2003) reported that during the breeding season, Adélie penguin (Pygoscelis adeliae) foraging trip duration varied in a nonlinear manner but in accordance with sea-ice extent and changes in krill abundance; years with the lowest sea-ice extent were associated with the longest foraging trip duration and the lowest measures of krill abundance. Years with intermediate or extensive sea ice were associated with shorter foraging trip durations and higher measures of krill abundance. These relationships are evident during the breeding season.
Impacts of the link between El Niño, sea-surface temperature, sea ice and krill abundance are also evident at other times of year. For example, changes in the population processes of pygoscelid penguins are now evident at Signy Island in the South Orkney Islands where congeneric Adélie, chinstrap (Pygoscelis antarctica) and gentoo (Pygoscelis papua) penguins all now show population responses to the impacts of sea-ice reduction (Trathan et al. 1996; Forcada et al. 2006). These three penguin species breed sympatrically at Signy Island where both Adélie and chinstrap penguins have declined and the less ice-adapted gentoo penguin has increased significantly in numbers over the last 26 years. These trends have occurred in parallel with regional long-term warming and significant reductions in sea-ice extent. Periodic warm events, with teleconnections to the tropical El Niño region, have caused cycles in sea ice that have potentially led to reduced prey biomass, and concurrent inter-annual population decreases in the three penguin species. With the loss of sea ice, Adélie penguins have become less well buffered against the environment, so that their numbers have fluctuated greatly such that their populations have shown a strong linear decline. In contrast, chinstrap penguins (considered to be less pagophilic and better adapted to ice-free conditions) have been more affected by discrete events of locally increased sea-ice cover. As a consequence, chinstrap penguin population numbers have been less variable, though showing a nonlinear response to sea-ice loss. Similarly, gentoo penguins have been temporarily affected by negative anomalies in regional sea ice, but persistent sea-ice reductions are the probable cause of an increase in their available niche, which is likely to be largely separate from that of their more abundant congeners. Thus, the regional consequences of climate perturbations have affected the marine habitat at Signy Island, with repercussions for penguin food supply and competition for resources. Ultimately, variability in the population numbers of these three penguin species reflects the local balance between penguin adaptation to sea-ice conditions and trophic-mediated changes cascading from global climate forcing (Forcada et al. 2006).
Elsewhere in the Southwest Atlantic, other species of krill-dependent air-breathing predators show similar population responses; currently, those species investigated include gentoo penguins and Antarctic fur seals (Arctocephalus gazella) which breed at Bird Island, South Georgia (Trathan et al. 2006) and southern right whales (Eubaleana australis) which feed in the waters around South Georgia and congregate to breed in the waters surrounding Península Valdés, Argentina (Leaper et al. 2006). Of these three species, Antarctic fur seals have been examined in the greatest detail (Forcada et al. 2005).
The time-series for gentoo penguin breeding performance at Bird Island are among the longest available for the species, with data available from 1988/1989 to 2005/2006. Gentoo penguins are important predators of Antarctic krill with typically more than 50% krill in their diets (Williams 1991). In addition, they consume a number of fish (such as mackerel icefish, Champsocephalus gunneri), which in turn are also consumers of Antarctic krill (Everson 2000). Gentoo penguins are resident at South Georgia and rarely travel far from the island either during chick rearing (Croxall & Prince 1980; Croxall et al. 1988) or during winter (Tanton et al. 2004). Trathan et al. (2006) used information on the number of nests (that is the number of breeding pairs), the total number of chicks fledged and breeding success (defined as the average number of chicks fledged per nest or pair). The reproductive performance of gentoo penguins shows high levels of inter-annual variability, with some years of extremely low reproductive output. The years of lowest productivity (within the lowest quartile) for gentoo penguins were 1990/1991, 1993/1994, 1997/1998 and 1999/2000). Trathan et al. (2006) used lagged cross-correlation analyses between monthly sea surface temperature and gentoo penguin productivity (number of chicks fledged) to show that strong links exist between sea surface temperatures during the preceding winter period and gentoo penguin breeding performance. The strongest correlations were present during the January to March period prior to hatching.
The time-series for Antarctic fur seals are also among the longest available for the species, with data from 1984/1985 to 2005/2006. Antarctic fur seals are also important predators of both Antarctic krill and mackerel icefish (Staniland & Boyd 2003). Antarctic fur seals are not resident at South Georgia but are typically thought to migrate away from the region after the breeding season (Boyd et al. 2002). As with gentoo penguins, the years of lowest reproductive productivity for Antarctic fur seals were 1990/1991, 1993/1994, 1997/1998 and 1999/2000. Trathan et al. (2006) and more recently, Forcada et al. (2005) used lagged cross-correlation analyses to examine the reproductive performance of Antarctic fur seals; these studies showed that strong links exist between sea surface temperatures during the preceding winter period and fur seal breeding performance. Over 20 years, positive anomalies in sea surface temperature in the tropical Pacific and at South Georgia explained significant nonlinear reductions in Antarctic fur seal pup production. The strongest correlations were present during the January to March period prior to pupping. Simulation of sea surface temperature time-series and their transformation into pup production values indicated that environmental forcing was only detectable within a narrow range of extreme positive sea surface temperature values and this explained the nonlinearity of the observed responses (Forcada et al. 2005).
There are only limited data on the diet of southern right whales, but it seems probable that their diet is dominated by Antarctic krill, at least for whales feeding south of the APF. For example, historical data taken from stomachs of right whale sampled around South Georgia indicate that southern right whales feed on krill (Lönnberg 1906; Matthews 1938). More recently, it has also been reported that the stomachs of whales taken south of 50° S contained only euphausids, whereas those taken north of 40° S were feeding only on copepods. Between 40° and 50° S copepods dominated, but there were also euphausids in the sampled stomachs (Tormosov et al. 1998). In addition to uncertainties about diet, the main feeding grounds of the southern right whale are also relatively poorly known. Nevertheless, recent survey data indicate that the major feeding grounds in the Southwest Atlantic are probably in the area around South Georgia (Reid et al. 2004). In the absence of extensive data on feeding, information from ecosystem proxies is extremely valuable when trying to understand fluctuations in whale population processes. Thus, it is now known that such variability is related to variability in the marine ecosystem at South Georgia. For example, cross-correlations between sea surface temperatures at South Georgia and southern right whale breeding success (modelled from calving output from photo-identification studies; Cooke et al. 2003) show that poor calving output follows years with warm temperature anomalies and better calving output follows years with cold temperature anomalies (Leaper et al. 2006). A similar (but more direct) correlation between the breeding success of right whales and prey abundance, also related to climate signals (the North Atlantic Oscillation), has been demonstrated in the North Atlantic (Greene et al. 2003).
All of these relationships between the environment and predator productivity show a high level of periodicity, typically of 3–4 years; they are also all congruent at specific temporal scales. The patterns are potentially driven by physical forcing in the Pacific Ocean, leading to ecosystem changes in the Atlantic. Thus, sea surface temperature changes in the Pacific lead to similar changes in the Southwest Atlantic approximately 3 years later (Trathan & Murphy 2002). In addition, atmospheric links between the Pacific and the Atlantic also influence temperatures over a scale of months (Murphy et al. submitted). Both these oceanic and atmospheric links impact the environment in the Atlantic influencing both sea surface temperatures and sea-ice extent (Trathan et al. 2006; Murphy et al. submitted). Changes to the environment in the Atlantic affect krill recruitment and biomass (Fraser & Hofmann 2003; Trathan et al. 2003; Murphy et al. submitted) that in turn affects predator survival and breeding performance (Fraser & Hofmann 2003; Forcada et al. 2005, 2006; Leaper et al. 2006; Trathan et al. 2006). The substantial reductions in predator breeding performance following warm ENSO events also suggests that similar events may be evident following longer-term climate change.
8. The Indian Ocean
The marine food web in the Indian Ocean has been less intensively studied than that in the Southwest Atlantic. Nevertheless, much is still known about the food web and the upper trophic-level predators that breed there and about recent climate related changes in populations of a number of important species. Thus, air temperatures have increased steadily over the past 50 years (Weimerskirch et al. 2003), beginning in the mid 1960s and being particularly marked in the sub-Antarctic sector. At the same time, but lagged by between 2 and 9 years, the population size of most seabirds and seals monitored across several breeding sites, decreased severely, with only two monitored species, king penguin and Amsterdam fur seal (Arctocephalus tropicalis), showing population increases over the same period. These changes in environmental conditions potentially underlie profound functional changes in the ecosystem across other trophic levels, as shown by the decline in both chlorophyll a and zooplankton concentrations after the late 1970s (Hunt et al. 2001). These changes suggest that decreases in food availability at lower trophic levels may underlie the general decrease of top predator populations (Weimerskirch et al. 2003). Though less well understood than the trophic changes in the Southwest Atlantic, these changes, together with indications of a simultaneous decrease in secondary production in sub-Antarctic waters and the reduction of sea-ice extent further south, indicate that a major system shift has also probably occurred in the Indian Ocean (Weimerskirch et al. 2003; Jenouvrier et al. 2005a).
Ecosystems and populations are influenced by both long-term climatic trends (Thompson & Ollason 2001) as well as by other short-term climatic modes such as inter-annual and decadal-scale variability (e.g. ENSO; Trathan et al. 2003, 2006). However, interactions between climatic forcing, abiotic and biotic components of ecosystems are complex, and as such the analysis of long-term data series of both physical and biological factors are essential to any understanding of the interactions. In this context, Jenouvrier et al. (2005a) used wavelet analysis to understand long-term change in the environment of the Indian Ocean and populations and breeding success of three Antarctic seabirds, southern fulmars (Fulmarus glacialoides), snow petrels (Pagodroma nivea) and emperor penguins (Aptenodytes forsteri) breeding in Terre Adélie. Jenouvrier et al. (2005a) showed that over the past 40 years, populations and demographic parameters of these three species fluctuated with a periodicity of 3–5 years that was also detected in sea-ice extent and the Southern Oscillation Index (and hence ENSO). Though the major inter-annual variability frequency signals were different in the different species and environmental variables, the observed cyclical patterns revealed a significant change since 1980. Moreover, cross-correlation analysis suggested that a regime shift has probably occurred since that date, significantly affecting the southern Indian Ocean marine ecosystem, but with contrasted effects for different species.
For southern fulmars, adult survival varies little between years, and therefore does not explain the strong fluctuations observed in the number of breeders and chicks (Jenouvrier et al. 2005b). Indeed, the strongest impact on their population dynamics is probably the high level of temporal variability in the proportion of breeders and the variability in breeding success. Before the 1980s, population fluctuations were mainly explained by a direct impact of sea-ice extent anomalies during summer. After the 1980s, the observed 3-year periodic population fluctuations were best predicted by 3-year cyclic variation in the proportion of breeders. Sea-ice extent also showed a marked change of periodicity during the 1980s, with sea-ice extent during winter fluctuating with a 3-year periodicity over the period 1980–1995. The marked change in the population dynamics of southern fulmars, through a change of the variations of the proportion of breeders, may be explained in the light of a regime shift that probably occurred around the 1980s, and which affected the sea-ice environment, the availability of prey, and thus the demographic parameters and population dynamics.
Using time-series data from 1973 to 1999, Barbraud & Weimerskirch (2001a) examined the influence of regional sea-ice extent on a number of indices of breeding performance of snow petrels, one of the most pagophilic of avian predators in the Antarctic. The percentage of breeding pairs was highly variable and there were fewer birds breeding when sea-ice cover was extensive during July. By contrast, overall breeding success and fledgling body condition were improved during years with extensive sea-ice cover during the preceding November and July to September. Their results indicate that the same sea-ice conditions may have different effects on the breeding performance of a species. The overall increase in winter sea-ice extent during the last decade appears to have resulted in an overall improvement of the quality of fledglings produced, and thus probably of future recruitment.
Over the past 50 years, populations of emperor penguins breeding in Terre Adélie have declined by 50%, most probably because of a decrease in apparent adult survival during the late 1970s (Barbraud & Weimerskirch 2001b). At this time, there was a prolonged abnormally warm period with reduced sea-ice extent. Mortality and permanent emigration rates increased when warm sea-surface temperatures occurred in the foraging area and when annual sea-ice extent was reduced, and were higher for males than for females. In contrast with survival, emperor penguins hatched fewer eggs when winter sea ice was more extensive. These results indicate strong and contrasting effects of large-scale oceanographic processes and sea-ice extent on the demography of emperor penguins, and their potential high susceptibility to climate change.
In the Indian Ocean, relationships between the environment and predator productivity show a high level of periodicity, typically of 3–4 years. These patterns are potentially driven by physical forcing from the Pacific Ocean, leading to ecosystem changes in the Indian Ocean (e.g. Barbraud & Weimerskirch 2003). The links via the lower and mid-trophic levels are less well understood than in the Atlantic, but evidence is accumulating to indicate that these have been affected by sea surface temperature changes.
9. The Pacific Ocean
The food web in the Pacific Ocean and in the Ross Sea sector has been studied less extensively than elsewhere in the Southern Ocean; however, some studies of higher trophic-level predators have been ongoing for a number of decades. For example, potentially one of the most extensive long-term monitoring datasets in the Antarctic is the annual estimate of breeding population size for Adélie penguin colonies located on Ross Island, Ross Sea (Ainley 2002). Monitoring for these colonies first began in the late 1950s, since which time significant inter-annual variability has been recorded. Recently, Wilson et al. (2001) hypothesized that such changes in population numbers were related to natural physical environmental factors. Based on available satellite imagery for sea ice between 1973 and 1997, Wilson et al. (2001) concluded that population growth (measured annually during the summer) was best explained by the inverse of the sea-ice extent five winters earlier; and further, that population growth was also related to the Southern Oscillation Index (and hence ENSO). Using a demographic model to show how variation in survival of juveniles and subadults might account for the observed population variation, Wilson et al. (2001) suggested that the 5-year lag in population growth resulted from the interval that it takes for surviving juveniles and subadult birds to recruit to the breeding population.
In the Ross Sea, extensive sea ice during winter appears to reduce subadult survival, but is expressed only when cohorts subsequently reach maturation. Thus, Wilson et al. (2001) suggest that extensive (more northerly) sea-ice limits penguin access to productive waters that are known to occur south of the southern boundary of the Antarctic Circumpolar Current, with starvation or increased predation disproportionately affecting less experienced birds. Wilson et al. (2001) suggest that the observed patterns of penguin population change, including those preceding the satellite era, result from differences in sea-ice extent and that this has changed significantly over recent decades.
Also in the western Pacific and Ross Sea sector, Ainley et al. (2005) have shown decadal-scale changes in population trajectories for both Adélie and emperor penguins during the early 1970s and again during the late 1980s. These population changes corresponded to changes in weather and sea-ice patterns, which were related to shifts in the AAO (Ainley et al. 2005). The AAO (Gong & Wang 1999) is based on the zonal mean sea-level pressure between 40° and 65° S. It is sometimes known as the Southern Annular Mode (SAM; Hall & Visbeck 2002), and is the leading pattern of variability of the extratropical winds (Kushner et al. 2001). The observed patterns of variability also show that the AAO and ENSO interact and that there is evidence of recent change (Carleton 2003); however, the manner in which many such global climate signals relate to long-term change remains unexplored.
Both Wilson et al. (2001) and Ainley et al. (2005) highlight the demographic lags inherent in the response of long-lived species to habitat or environmental variation. The proximate mechanisms responsible for the population changes are undoubtedly mediated through the prey field of the upper trophic-level predator species, but may involve shifts in coastal wind strength and air and sea temperature changes, which in turn affect the seasonal formation and decay of sea ice and polynyas, and thus altered prey accessibility or availability (distribution or biomass). Environmental impacts upon the prey field potentially influence predators through various vital rates, such as the proportion of adults breeding and consequently the reproductive output of populations.
10. Similarities between the Atlantic, the Indian and the Pacific Oceans
In marine ecosystems, air-breathing predators, such as seabirds and seals, are the only components that can be easily monitored and for which long-term datasets exist (Croxall & Nicol 2004). In many situations, predators are dependent upon a relatively restricted range of prey species for food, particularly during their breeding season. Consequently, air-breathing predator species offer the potential to examine the way in which the marine system is responding to regional climate forcing.
Patterns of distribution, movement, density, phenology, behaviour and community interaction can be difficult to disentangle for most species. Consequently, the most reliable signals of change are likely to derive from systems where abiotic and biotic signals provide parallel information. The signals of most value are likely to be those where upper trophic-level responses are congruent (albeit lagged) with responses in low- to mid-trophic levels (Croxall et al. 1999), and where these in turn are congruent (again lagged) with physical forcing factors (Wilson et al. 2001; Trathan et al. 2003; Jenouvrier et al. 2005a; Murphy et al. submitted). Such a situation requires information about the demography and population abundances of predator species, their prey and the physical environment. Nevertheless, even where such data exist, some signals for some species of top predator can be difficult to interpret, due to previous levels of harvesting (Jackson et al. 2001).
Fundamental to any conclusions about climate impacts upon predators is a clear understanding of climate variability. At present, there is a very active debate about Southern Hemisphere atmospheric circulation and how it impacts upon Southern Ocean variability, and also how it may be related to the various climate indices; for example, ENSO, the Trans-Polar Index (TPI) or the Antarctic Circumpolar Wave (ACW; White & Peterson 1996; Peterson & White 1998). This debate is crucial to any understanding of how Southern Ocean marine ecosystems potentially respond to climate variability. Interactions between different climate signals exist (Carleton 2003). The AAO dominates patterns of low-frequency variability in Southern Hemisphere atmospheric circulation. This involves an alternation of atmospheric mass between middle and high southern latitudes and an oscillation in pressure between the Australian and South American sectors, the TPI; it also involves an oscillation in the coupled atmosphere/ocean/sea-ice system, the ACW. The AAO also has a similar periodicity to ENSO and is strongest in the Pacific and Southwest Atlantic sectors (Carleton 2003).
In the Atlantic, the Indian and the Pacific Oceans, studies linking biological responses (e.g. Trathan et al. 2003, 2006; Forcada et al. 2005, 2006; Leaper et al. 2006; Murphy et al. submitted) to long-term variability in environmental signals such as to the ACW (Loeb et al. 1997; Waluda et al. 1999), TPI (Waluda et al. 2004), ENSO (Trathan & Murphy 2002; Jenouvrier et al. 2003, 2005a,b) or the AAO (Ainley et al. 2005) indicate that the Southern Ocean marine ecosystem is very much under the control of strong physical forcing and that clear links with upper-trophic levels are evident. The lag, magnitude and sign of these correlations are species and site specific. For example, in some situations such as in the Southwest Atlantic, positive correlations exist between sea-ice extent and Adélie penguin population size (Fraser et al. 1992; Trathan et al. 1996; Fraser & Hofmann 2003; Forcada et al. 2006), while in others such as in the Ross Sea, negative correlations exist (Wilson et al. 2001). Currently, no unifying model exists that relates Adélie penguin population size to sea-ice extent. The most recent studies on this topic (Forcada et al. 2006) predict possible outcomes for Adélie penguins under different environmental conditions in the Southwest Atlantic.
The model of Forcada et al. (2006) assumes that predator responses to climate are mediated through the prey field, but does not include specific details of these trophic levels. Nevertheless, the balance between habitat availability, predator habitat preference and environmental impacts upon the prey field are conceptually included within the model. This balance is critical to any understanding of environmental change. As yet, very few Southern Ocean studies provide mechanistic details about how upper trophic-level responses and low- to mid-tropic level responses are congruent with physical forcing factors (Trathan et al. 2003; Murphy et al. submitted). Understanding habitat preference and exploring the mid-trophic-level responses to climate remains one of the key challenges for future studies.
11. What ENSO can tell us?
Short- to medium-term fluctuations in the climate, such as take place during ENSO events, occur frequently each decade (Trenberth & Hoar 1996; Fedorov & Philander 2000). During these periods, anomalous physical conditions occur that impact widely upon marine biological systems; both positive and negative anomalies affect the ecosystem. In the South Atlantic, warm and cold periods affect the recruitment and biomass of krill (Fraser & Hofmann 2003; Murphy et al. submitted) and consequently higher trophic levels (Trathan et al. 2006). In the Indian Ocean, anomalous temperatures are correlated with fluctuations in the population processes of a number of marine predators (Jenouvrier et al. 2005a). While in the Pacific Ocean changes in the populations of top predators, including both Adélie and emperor penguins, Weddell seals (Leptonychotes weddellii; Cameron 2001 cited in Ainley et al. 2005) and minke whales (Balaenoptera bonaerensis; Branch & Butterworth 2001) have been recorded; however, though thought to be linked to changes in sea ice, these studies do not explore links through the mid-trophic levels. Nevertheless, changes in some of these predator populations are certainly correlated with changes in climate signals (Wilson et al. 2001; Ainley et al. 2005).
The short-term changes to ecosystem structure and function observed during ENSO events herald potential, long-term changes that may ensue following regional climate change. For example, in the South Atlantic, failure of Antarctic krill recruitment will inevitably foreshadow recruitment failures in higher trophic levels for some species. Where these predator species are not able to accommodate by switching to other prey species (which may in turn already have been affected) population-level changes will probably follow. Speculation about how ecosystems will change must be cautious, as our understanding of the recovery dynamics and succession of species in marine systems is sparse, and similar starting points may result in different end points. Nevertheless, a few detailed examples should suffice to show how potential changes might ensue following regional change to the ecosystem.
In the Southern Ocean, those predator species that breed in each of the major ocean basins provide the potential to predict possible outcomes of regional change. This is because the same species may occupy a different niche or food web position in the different ocean basins, depending upon the composition of the food web in that region. Thus, if a change to the food web occurs in one region, we may be able to predict the future status in that region using information from other ocean basins; this is based on the premise that population responses potentially differ in relation to different types of prey and prey availability. Two examples suffice to explore how such changes may help future predictions.
Antarctic fur seals were harvested almost to extinction by commercial sealing in the eighteenth and nineteenth centuries. Although only a few hundred seals remained into the twentieth century, small-scale hunting continued until 1907. The species has now recovered with the main centre of population at South Georgia. Other populations also exist and show signs of recovery, though to a lesser extent than the population at South Georgia. Populations now exist at the South Shetland Islands, South Orkney Islands, South Sandwich Islands, Bouvetøya, Prince Edward Island, Marion Island, Îles de Crozet, Île de Kerguelen, Heard Island, McDonald Island and Macquarie Island. Populations at South Georgia increased from a few tens of individuals in the 1950s to over 1.5 million by the early 1990s (Boyd 1993). Elsewhere, recovery has been slower; for example, the first pups born post-sealing at Maquarie Island were reported in 1955 and at Heard Island in 1963. Nevertheless, despite over 40 years of post-sealing expansion, populations at Maquarie Island and at Heard Island remain very much smaller than at South Georgia. This is potentially a consequence of differing environmental conditions, differing founder effects, but importantly, differing food web interactions in the different regions.
At South Georgia in the South Atlantic, Antarctic krill is the main prey item of Antarctic fur seals, with fish also important. The myctophid Protomyctophum choriodon as well as mackerel icefish and the notothenid Lepidonotothen larseni agg. dominate the fish consumed (Reid & Arnould 1996). At Livingston Island in the South Shetland Islands, the most frequent prey species is also Antarctic krill, followed by several myctophid fish (Gymnoscopelus nicholsi, Electrona antarctica and Electrona carlsbergi), squid and penguins (Osman et al. 2004). At Bouvetøya, Antarctic krill also dominate the diet with squid and myctophid fish taken opportunistically (Kirkman et al. 2000). In contrast, at Île de Kerguelen in the Indian Ocean, diet analyses show that seals feed primarily on fish and squid; myctophid fish account for most fish consumed with three species, G. nicholsi, G. piabilis and Electrona subaspera, forming the core diet. At Macquarie Island in the west Pacific, the myctophid, E. subaspera, is the main prey species (Robinson et al. 2002).
Thus, if Antarctic fur seal populations in the South Atlantic are vulnerable to changes in the Antarctic krill-based food web (sensu Trathan et al. 2006), they may switch towards a core diet that is more fish based, particularly myctophid fish. As a consequence of the change in diet, critical population processes may be affected, and recruitment and consequently the number of animals in the population may be impacted, possibly severely.
Regional differences in the summer diet of Adélie penguins are also well documented (Ainley 2002). In the region around the Antarctic Peninsula and in the Southwest Atlantic, their diet is dominated by Antarctic krill (White & Conroy 1975; Volkman et al. 1980; Lynnes et al. 2004); in East Antarctica, the diet includes Antarctic krill, but also ice krill and Antarctic silverfish (Pleuragramma antarcticum; Watanuki et al. 1994); in the Ross Sea, the diet is mostly ice krill over shelf areas, but in the deeper shelf slope waters, Antarctic krill occur in the diet (Ainley et al. 1984, 1998). In the winter, at least in the Weddell Sea, Antarctic krill and even myctophids and squid are common in the diet (Ainley et al. 1992).
Changes in the populations of Adélie penguins are now evident in the Antarctic Peninsula region and in the Southwest Atlantic, where penguin populations are thought to be responding to environmental processes and to the potential impacts of sea-ice reduction (Fraser et al. 1992; Trathan et al. 1996; Forcada et al. 2006). With the loss of sea ice, Adélie penguins are less buffered against the environment, their numbers fluctuate greatly and their populations show a strong linear decline (Forcada et al. 2006). Ultimately, Adélie penguin populations reflect a local balance between trophic-mediated changes cascading from global climate forcing and adaptation to ice conditions. In these areas, Adélie penguins are at the northern extent of their range (Williams 1995) where they are potentially more vulnerable to changes to critical population processes that may affect recruitment and the number of animals in the population. Environmental conditions following ENSO events highlight the situation that Adélie penguin numbers may decline following changes in sea-ice extent and changes in diet (Fraser & Hofmann 2003; Forcada et al. 2006).
Elsewhere in the Antarctic, Adélie penguins occupy a more southerly ice-dominated habitat where the impacts of ENSO may be less obvious but where climatic forcing may still be evident (Wilson et al. 2001). In the Ross Sea, Adélie penguins show an inverse relationship with sea-ice extent, highlighting the complexity of interpreting regional climate change. Here, extensive sea ice potentially prevents birds from foraging in more productive waters, selectively discriminating against inexperienced juveniles and subadults. In the Ross Sea, the sea-ice season is now longer in duration than during the late 1970s, increasing in length by between 1 and 4 days a year over the past 24 years (Parkinson 2004).
Thus, if Adélie penguin populations are vulnerable to changes either in the marine food web (sensu Trathan et al. 2006) or to habitat availability (Wilson et al. 2001; Forcada et al. 2006), critical population processes may be affected and recruitment and consequently the number of animals in the population may be impacted. The recent study of Forcada et al. (2006) predicts possible outcomes for Adélie penguins under different environmental conditions in the Southwest Atlantic; it would therefore be instructive to develop this model with datasets from elsewhere including those from the Antarctic Peninsula and from the Ross Sea.
12. Future scenarios
In each basin within the Southern Ocean, environmental signals originating from the Pacific are evident in the populations of a range of air-breathing predators. Impacted species include both seabirds and marine mammals, each of which has very different foraging strategies, as well as diverse breeding strategies, with both resident and migratory species included. That sub-decadal ENSO signals are so evident in the populations of this range of species is testament to the importance of environmental variability and the power of environmental forcing on predator populations.
Based on the evidence from sub-decadal climate variability, it is inevitable that longer-term climate change (either through an altered frequency of ENSO events or more directly through the effects of warming) is also likely to be instrumental in predator population changes. Further, some of the documented responses to shorter-term, sub-decadal climate variability offer the best opportunities to understand some of the processes likely to be important in longer-term environmental change. However, predicting the outcomes of longer-term environmental forcing for populations of the Southern Ocean marine predators is still fraught with difficulties. Only with greater ecological understanding about the processes whereby the environment impacts upon predators (particularly during the winter period where current datasets are especially sparse), we will be in a position to make more robust and reliable predictions about future scenarios.
Footnotes
One contribution of 10 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. Part 2: evolution, diversity and function’.
References
- Ainley D.G. Columbia University Press; New York, NY: 2002. The Adélie penguin: bellwether of climate change. [Google Scholar]
- Ainley, D. G., O'Connor, E. F. & Boekelheide, R. J. 1984 The marine ecology of birds in the Ross Sea, Antarctica American Ornithologists Union Monograph 32.
- Ainley D.G, Ribic C.A, Fraser W.R. Does prey preference affect habitat choice in Antarctic seabirds? Mar. Ecol. Prog. Ser. 1992;90:207–221. [Google Scholar]
- Ainley D.G, Wilson P.R, Barton K.J, Ballard G, Nur N, Karl B. Diet and foraging effort of Adélie penguins in relation to pack-ice conditions in the southern Ross Sea. Polar Biol. 1998;20:311–319. doi:10.1007/s003000050308 [Google Scholar]
- Ainley D.G, Clarke E.D, Arrigo K, Fraser W.R, Kato A, Barton K.J, Wilson P.R. Decadal-scale changes in the climate and biota of the Pacific sector of the Southern Ocean, 1950s to the 1990s. Antarct. Sci. 2005;17:171–182. doi:10.1017/S0954102005002567 [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. doi:10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Barbraud C, Weimerskirch H. Contrasting effects of the extent of sea-ice on the breeding performance of an Antarctic top predator, the snow petrel Pagodroma nivea. J. Avian Biol. 2001a;32:297–302. doi:10.1111/j.0908-8857.2001.320402.x [Google Scholar]
- Barbraud C, Weimerskirch H. Emperor penguins and climate change. Nature. 2001b;411:183–186. doi: 10.1038/35075554. doi:10.1038/35075554 [DOI] [PubMed] [Google Scholar]
- Barbraud C, Weimerskirch H. Climate and density shape population dynamics of a marine top predator. Proc. R. Soc. B. 2003;1529:2111–2116. doi: 10.1098/rspb.2003.2488. doi:10.1098/rspb.2003.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I.L. Pup production and distribution of breeding Antarctic fur seals Arctocephalus gazella at South Georgia. Antarct. Sci. 1993;5:17–24. [Google Scholar]
- Boyd I.L, Staniland I.J, Martin A.R. Distribution of foraging by female Antarctic fur seals. Mar. Ecol. Prog. Ser. 2002;242:285–294. [Google Scholar]
- Branch T.A, Butterworth D.S. Estimates of abundance south of 60° S for cetacean species sighted frequently on the 1978/79 to 1997/98 IWC/IDCR-SOWER sightings surveys. J. Cetacean Res. Manage. 2001;3:143–174. [Google Scholar]
- Brierley A.S, Watkins J.L, Goss C, Wilkinson M.T, Everson I. Acoustic estimates of krill density at South Georgia, 1981 to 1998. CCAMLR Sci. 1999;6:47–57. [Google Scholar]
- Brierley A.S, et al. Significant intra-annual variability in krill distribution and abundance at South Georgia revealed by multiple acoustic surveys during 2000/01. CCAMLR Sci. 2002;9:71–82. [Google Scholar]
- Brinton E. Distribution and population structures of immature and adult Euphausia superba in the western Bransfield Strait region during the 1986–87 summer. Deep-Sea Res. Part A Oceanogr. Res. Pap. 1991;38:1169–1193. doi:10.1016/0198-0149(91)90101-K [Google Scholar]
- Brothers N. Albatross mortality and associated bait loss in the Japanese longline fishery in the Southern Ocean. Biol. Conserv. 1991;55:255–268. doi:10.1016/0006-3207(91)90031-4 [Google Scholar]
- Cameron, M. F. 2001 Dynamics of a Weddell seal (Leptonychotes weddellii) population in McMurdo Sound. Antarctica. PhD thesis, University of Minnesota, Minneapolis.
- Carleton A.M. Atmospheric teleconnections involving the Southern Ocean. J. Geophys. Res. 2003;108:8080. doi:10.1029/2000JC000379 [Google Scholar]
- CCAMLR (Convention for the Conservation of Antarctic Marine Living Resources) 1982 Understanding CCAMLR's approach to management. See http://www.ccamlr.org/pu/e/e_pubs/am/toc.htm
- Chambers L.E, Hughes L, Weston M.A. Climate change and its impact on Australia's avifauna. Emu. 2005;105:1–20. doi:10.1071/MU04033 [Google Scholar]
- Chan J.C.L, Liu K.S. Typhoon intensity in a warming world. Bull. Am. Meteorol. Soc. 2004;85:660–661. [Google Scholar]
- Connolley W.M. Long term variability of the Antarctic Circumpolar Wave. J. Geophys. Res. 2002;108:8076. doi:10.1029/2000JC000380 [Google Scholar]
- Cooke, J., Rowntree, V. & Payne, R. 2003 Analysis of inter-annual variation in reproductive success of South Atlantic right whales (Eubalaena australis) from photo-identifications of calving females observed off Península Valdés, Argentina, during 1971–2000. Paper SC/55/O23 presented to Scientific Committee of the International Whaling Commission, Berlin.
- Croxall J.P. Southern Ocean environmental changes—effects on seabird, seal and whale populations. Phil. Trans. R. Soc. B. 1992;338:319–328. doi:10.1098/rstb.1992.0152 [Google Scholar]
- Croxall J.P, Nicol S. Management of Southern Ocean fisheries: global forces and future sustainability. Antarct. Sci. 2004;16:569–584. doi:10.1017/S0954102004002330 [Google Scholar]
- Croxall J.P, Prince P.A. Food, feeding ecology and ecological segregation of seabirds at South Georgia. Biol. J. Linn. Soc. 1980;14:103–131. [Google Scholar]
- Croxall J.P, McCann T.S, Prince P.A, Rothery P. Reproductive performance of seabirds and seals at South Georgia and Signy Island, South Orkney Islands, 1976–1987: implications for Southern Ocean monitoring studies. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 261–285. [Google Scholar]
- Croxall J.P, Reid K, Prince P.A. Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar. Ecol. Prog. Ser. 1999;177:115–131. [Google Scholar]
- Croxall J.P, Trathan P.N, Murphy E.J. Environmental change and Antarctic seabird populations. Science. 2002;297:1510–1514. doi: 10.1126/science.1071987. doi:10.1126/science.1071987 [DOI] [PubMed] [Google Scholar]
- Curran M.A.J, Van Ommen T.D, Morgan V.I, Phillips K.L, Palmer A.S. Ice core evidence for Antarctic sea ice decline since the 1950s. Science. 2003;302:1203–1206. doi: 10.1126/science.1087888. doi:10.1126/science.1087888 [DOI] [PubMed] [Google Scholar]
- Diaz H.F, Markgraf V, editors. El Niño and the Southern Oscillation: multiscale variability and global and regional impacts. Cambridge University Press; Cambridge, UK: 2000. pp. 496. [Google Scholar]
- Endicott M, Agnew D.J. The survivorship of rays discarded from the South Georgia longline fishery. CCAMLR Sci. 2004;11:155–164. [Google Scholar]
- Everson I. Role of krill in marine food webs: the Southern Ocean. In: Everson I, editor. Krill: biology, ecology and fisheries. Blackwell Science; Oxford, UK: 2000. pp. 194–201. [Google Scholar]
- Fedorov V, Philander S.G. Is El Niño changing. Science. 2000;288:1997–2002. doi: 10.1126/science.288.5473.1997. doi:10.1126/science.288.5473.1997 [DOI] [PubMed] [Google Scholar]
- Forcada J, Trathan P.N, Reid K, Murphy E.J. The effects of global climate variability in pup production of Antarctic fur seals. Ecology. 2005;86:2408–2417. [Google Scholar]
- Forcada J, Trathan P.N, Reid K, Murphy E.J, Croxall J.P. Contrasting population changes in sympatric penguin species with climate warming. Global Change Biol. 2006;12:411–423. doi:10.1111/j.1365-2486.2006.01108.x [Google Scholar]
- Fraser W.R, Hofmann E.E. A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Mar. Ecol. Prog. Ser. 2003;265:1–15. [Google Scholar]
- Fraser W.R, Trivelpiece W.Z, Ainley D.G, Trivelpiece S. Increases in Antarctic penguin populations—reduced competition with whales or a loss of sea ice due to environmental warming. Polar Biol. 1992;11:525–531. doi:10.1007/BF00237945 [Google Scholar]
- Gille S.T. Warming of the southern ocean since the 1950s. Science. 2002;295:1275–1277. doi: 10.1126/science.1065863. doi:10.1126/science.1065863 [DOI] [PubMed] [Google Scholar]
- Gong D.Y, Wang S.W. Definition of Antarctic Oscillation Index. Geophys. Res. Lett. 1999;26:459–462. doi:10.1029/1999GL900003 [Google Scholar]
- Greene C.H, Pershing A.J, Kenney R.D, Jossi J.W. Impact of climate variability on the recovery of endangered north Atlantic right whales. Oceanography. 2003;16:96–101. [Google Scholar]
- Guinet C. Predation behavior of killer whales (Orcinus orca) around Crozet Islands. Can. J. Zool. 1992;70:1656–1667. [Google Scholar]
- Hall A, Visbeck M. Synchronous variability in the southern hemisphere atmosphere, sea-ice, and ocean resulting from the annular mode. J. Clim. 2002;15:3043–3057. doi:10.1175/1520-0442(2002)015<3043:SVITSH>2.0.CO;2 [Google Scholar]
- Harmer S.F. Southern whaling. Proc. Linn. Soc. Lond. 1931;142:85–163. [Google Scholar]
- Hofmann E.E, Husrevoglu Y.S. A circumpolar modeling study of habitat control of Antarctic krill (Euphausia Superba) reproductive success. Deep-Sea Res. Part II Topical Stud. Oceanogr. 2003;50:3121–3142. doi:10.1016/j.dsr2.2003.07.012 [Google Scholar]
- Hofmann E.E, Klinck J.M, Locarnini R.A, Fach B, Murphy E.J. Krill transport in the Scotia Sea and environs. Antarct. Sci. 1998;10:406–415. [Google Scholar]
- Hunt B.P.V, Pakhomov E.A, Mcquaid C.D. Community structure of mesozooplankton in the Antarctic Polar Frontal Zone in the vicinity of the Prince Edward Islands (Southern Ocean): Small-scale distribution patterns in relation to physical parameters. Deep-Sea Res. Part II Topical Stud. Oceanogr. 2001;49:3307–3325. doi:10.1016/S0967-0645(02)00085-1 [Google Scholar]
- Hunt G.L, Stabeno P, Walters G, Sinclair E, Brodeur R.D, Napp J.M, Bond N.A. Climate change and control of the southeastern Bering Sea pelagic ecosystem. Deep-Sea Res. Part II Topical Stud. Oceanogr. 2002;49:5821–5853. doi:10.1016/S0967-0645(02)00321-1 [Google Scholar]
- IPCC 2001 Climate change 2001: the scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (eds J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Dai, K. Maskell & C. A. Johnson). Cambridge, UK; New York, NY: Cambridge University Press. See http://www.ipcc.ch/
- Jacka T.H, Budd W.F. Detection of temperature and sea-ice extent changes in the Antarctic and Southern Ocean, 1949–96. Ann. Glaciol. 1998;27:553–559. [Google Scholar]
- Jackson J.B.C, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. doi:10.1126/science.1059199 [DOI] [PubMed] [Google Scholar]
- Jenouvrier S, Barbraud C, Weimerskirch H. Effects of climate variability on the temporal population dynamics of southern fulmars. J. Anim. Ecol. 2003;72:576–587. doi: 10.1046/j.1365-2656.2003.00727.x. doi:10.1046/j.1365-2656.2003.00727.x [DOI] [PubMed] [Google Scholar]
- Jenouvrier S, Weimerskirch H, Barbraud C, Park Y.H, Cazelles B. Evidence of a shift in the cyclicity of Antarctic seabird dynamics linked to climate. Proc. R. Soc. B. 2005a;272:887–895. doi: 10.1098/rspb.2004.2978. doi:10.1098/rspb.2004.2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenouvrier S, Barbraud C, Cazelles B, Weimerskirch H. Modelling population dynamics of seabirds: importance of the effects of climate fluctuations on breeding proportions. Oikos. 2005b;108:511–522. doi:10.1111/j.0030-1299.2005.13351.x [Google Scholar]
- Kemp S, Bennett A.G. On the distribution and movements of whales on the South Georgia and South Shetland whaling grounds. Discov. Rep. 1932;6:165–190. [Google Scholar]
- King J.C. Recent climate variability in the vicinity of the Antarctic Peninsula. Int. J. Climatol. 1994;14:357–369. doi:10.1002/joc.3370140402 [Google Scholar]
- Kirkman S.P, Wilson W, Klages N.T.W, Bester M.N, Isaksen K. Diet and estimated food consumption of Antarctic fur seals at Bouvetøya during summer. Polar Biol. 2000;23:745–752. doi:10.1007/s003000000145 [Google Scholar]
- Kohl-Larsen L. South Georgia: gateway to Antarctica. vol. 336. The Erskine Press; Norwich, UK: 2002. [Google Scholar]
- Kushner P.J, Held I.M, Delworth T.L. Southern Hemisphere atmospheric circulation response to global warming. J. Clim. 2001;14:2238–2249. doi:10.1175/1520-0442(2001)014<0001:SHACRT>2.0.CO;2 [Google Scholar]
- Laws R.M. Seals and whales of the Southern Ocean. Phil. Trans. R. Soc. B. 1977;279:81–96. doi:10.1098/rstb.1977.0073 [Google Scholar]
- Leaper R, Cooke J, Trathan P.N, Reid K, Rowntree V, Payne R. Global climate drives southern right whale (Eubalaena australis) population dynamics. Biol. Lett. 2006;2:289–292. doi: 10.1098/rsbl.2005.0431. doi:10.1098/rsbl.2005.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitus S, Antonov J.I, Boyer T.P, Stephens C. Warming of the world ocean. Science. 2000;287:2225–2229. doi:10.1126/science.287.5461.2225 [Google Scholar]
- Loeb V, Siegel V, Holmhansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. doi:10.1038/43174 [Google Scholar]
- Lönnberg, E. 1906 Contributions to the fauna of South Georgia. I. Taxonomic and biological notes on vertebrates. Kungliga Svenska Vetenskapsakademiens Handlingar 40.
- Lynnes A.S, Reid K, Croxall J.P. Diet and reproductive success of Adélie and chinstrap penguins: linking response of predators to prey population dynamics. Polar Biol. 2004;27:544–554. doi:10.1007/s00300-004-0617-1 [Google Scholar]
- Mackintosh N.A. Life cycle of Antarctic krill in relation to ice and water conditions. Discov. Rep. 1972;36:1–94. [Google Scholar]
- Marr J. The natural history and geography of the Antarctic krill Euphausia superba Dana. Discov. Rep. 1962;32:33–464. [Google Scholar]
- Matthews H.L. Notes on the southern right whale, Eubalaena australis. Discov. Rep. 1938;17:169. [Google Scholar]
- Murphy E.J, et al. Innterannual variability of the South Georgia marine ecosystem: biological and physical sources of variation in the abundance of krill. Fish. Oceanogr. 1998;7:381–390. doi:10.1046/j.1365-2419.1998.00081.x [Google Scholar]
- Murphy, E. J., Trathan, P. N., Watkins, J. L., Reid, K., Meredith, M. P., Forcada, J. & Thorpe, S. E. Submitted. Climate driven fluctuations in distant ocean ecosystems revealed in prey and predator responses to ENSO.
- Odum E.P. The strategy of ecosystem development. Science. 1969;164:262–270. doi: 10.1126/science.164.3877.262. doi:10.1126/science.164.3877.262 [DOI] [PubMed] [Google Scholar]
- Orsi A.H, Whitworth T, Nowlin W.D. On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res. Part I Oceanogr. Res. Pap. 1995;42:641–673. doi:10.1016/0967-0637(95)00021-W [Google Scholar]
- Osman L.P, Hucke-Gaete R, Moreno C.A, Torres D. Feeding ecology of Antarctic fur seals at Cape Shirreff, South Shetlands, Antarctica. Polar Biol. 2004;27:92–98. doi:10.1007/s00300-003-0555-3 [Google Scholar]
- Parkinson C.L. Trends in the length of the Southern Ocean sea-ice season, 1979–99. Ann. Glaciol. 2002;34:435–440. [Google Scholar]
- Parkinson C.L. Southern Ocean sea ice and its wider linkages: insights revealed from models and observations. Antarct. Sci. 2004;16:387–400. doi:10.1017/S0954102004002214 [Google Scholar]
- Pauly D, Maclean J. In a perfect ocean: the state of fisheries and ecosystems in the North Atlantic Ocean. vol. 160. Island Press; Washington, DC: 2003. [Google Scholar]
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F. Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. doi:10.1126/science.279.5352.860 [DOI] [PubMed] [Google Scholar]
- Peterson R.G, White W.B. Slow oceanic teleconnections linking the Antarctic Circumpolar Wave with the tropical El Niño–Southern Oscillation. J. Geophys. Res. 1998;103:24 573–24 583. doi:10.1029/98JC01947 [Google Scholar]
- Quayle W.C, Peck L.S, Peat H, Ellis-Evans J.C, Harrigan P.R. Extreme responses to climate change in Antarctic lakes. Science. 2002;295:645–645. doi: 10.1126/science.1064074. doi:10.1126/science.1064074 [DOI] [PubMed] [Google Scholar]
- Quetin L.B, Ross R.M. Episodic recruitment in Antarctic krill Euphausia Superba in the Palmer LTER study region. Mar. Ecol. Prog. Ser. 2003;259:185–200. [Google Scholar]
- Reid K, Arnould J.P.Y. The diet of Antarctic fur seals Arctocephalus gazella during the breeding season at South Georgia. Polar Biol. 1996;16:105–114. [Google Scholar]
- Reid K, Watkins J.L, Croxall J.P, Murphy E.J. Krill population dynamics at South Georgia 1991–1997, based on data from predators and nets. Mar. Ecol. Prog. Ser. 1999;177:103–114. [Google Scholar]
- Reid K, Sims M, White R.W, Gillon K.W. Spatial distribution of predator/prey interactions in the Scotia Sea: implications for measuring predator/fisheries overlap. Deep-Sea Res. Part II Topical Stud. Oceanogr. 2004;51:1383–1396. doi:10.1016/j.dsr2.2004.06.007 [Google Scholar]
- Reynolds R.W, Rayner N.A, Smith T.M, Stokes D.C, Wang W. An improved in situ and satellite SST analysis for climate. J. Clim. 2002;15:1609–1625. doi:10.1175/1520-0442(2002)015<1609:AIISAS>2.0.CO;2 [Google Scholar]
- Robinson S.A, Goldsworthy S.G, Van Den Hoff J, Hindell M.A. The foraging ecology of two sympatric fur seal species, Arctocephalus gazella and Arctocephalus tropicalis, at Macquarie Island during the Austral Summer. Mar. Freshw. Res. 2002;53:1071–1082. doi:10.1071/MF01218 [Google Scholar]
- Rodionov S.N. A sequential algorithm for testing climate regime shifts. Geophys. Res. Lett. 2004;31:L09204. doi:10.1029/2004GL019448 [Google Scholar]
- Saether B.E, et al. Generation time and temporal scaling of bird population dynamics. Nature. 2005;436:99–102. doi: 10.1038/nature03666. doi:10.1038/nature03666 [DOI] [PubMed] [Google Scholar]
- Salinger M. Climate variability and change: past, present and future—an overview. Clim. Change. 2005;70:9–29. doi:10.1007/s10584-005-5936-x [Google Scholar]
- Siegel V. A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 219–230. [Google Scholar]
- Siegel V, Bergstrom B, Muhlenhardt-Siegel U, Thomasson M. Demography of krill in the Elephant Island area during summer 2001 and its significance for stock recruitment. Antarct. Sci. 2002;14:162–170. doi:10.1017/S095410200200072X [Google Scholar]
- Smith R.C, et al. Marine ecosystem sensitivity to climate change. BioScience. 1999;49:393–404. doi:10.2307/1313632 [Google Scholar]
- Spiridonov V.A. Spatial and temporal variability in reproductive timing of Antarctic krill (Euphausia superba Dana) Polar Biol. 1995;15:161–174. doi:10.1007/BF00239056 [Google Scholar]
- Staniland I.J, Boyd I.L. Variation in the foraging location of Antarctic fur seals (Arctocephalus gazella) and the effects on diving behavior. Mar. Mamm. Sci. 2003;19:331–343. doi:10.1111/j.1748-7692.2003.tb01112.x [Google Scholar]
- Steele J.H. Regime shifts in marine ecosystems. Ecol. Appl. 1998;8:S33–S36. doi:10.2307/2641361 [Google Scholar]
- Stenseth N.C, Mysterud A, Ottersen G, Hurrell J.W, Chan K.S, Lima M. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. doi:10.1126/science.1071281 [DOI] [PubMed] [Google Scholar]
- Tanton J.L, Reid K, Croxall J.P, Trathan J.P. Winter distribution and behaviour of gentoo penguins Pygoscelis papua at South Georgia. Polar Biol. 2004;27:299–303. doi:10.1007/s00300-004-0592-6 [Google Scholar]
- Thompson P.M, Ollason J.C. Lagged effects of ocean climate change on fulmar population dynamics. Nature. 2001;413:417–420. doi: 10.1038/35096558. doi:10.1038/35096558 [DOI] [PubMed] [Google Scholar]
- Thorpe S.E, Heywood K.J, Stevens D.P, Brandon M.A. Tracking passive drifters in a high resolution ocean model: implications for interannual variability of larval krill transport to South Georgia. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2004;51:909–920. doi:10.1016/j.dsr.2004.02.008 [Google Scholar]
- Tormosov D.D, Mikhaliev Y.A, Best P.B, Zemsky V.A, Sekiguchi K, Brownell R.L. Soviet catches of southern right whales Eubalaena australis, 1951–1971 Biological data and conservation implications. Biol. Conserv. 1998;86:185–197. doi:10.1016/S0006-3207(98)00008-1 [Google Scholar]
- Trathan P.N, Murphy E.J. Sea surface temperature anomalies near South Georgia: relationships with the pacific El Niño regions. J. Geophys. Res. 2002;108:8075. doi:10.1029/2000JC000299 [Google Scholar]
- Trathan P.N, Croxall J.P, Murphy E.J. Dynamics of Antarctic penguin populations in relation to interannual variability in sea ice distribution. Polar Biol. 1996;16:321–330. [Google Scholar]
- Trathan P.N, Brierley A.S, Brandon M.A, Bone D.G, Goss C, Grant S.A, Murphy E.J, Watkins J.L. Oceanographic variability and changes in Antarctic krill (Euphausia superba) abundance at South Georgia. Fish. Oceanogr. 2003;12:569–583. doi:10.1046/j.1365-2419.2003.00268.x [Google Scholar]
- Trathan P.N, Murphy E.J, Forcada J, Croxall J.P, Reid K, Thorpe S.E. Physical forcing in the southwest Atlantic: ecosystem control. In: Boyd I.L, Wanless S, Camphuysen K, editors. Top predators in marine ecosystems: their role in monitoring and management. Cambridge University Press; Cambridge, UK: 2006. pp. 28–45. [Google Scholar]
- Trenberth K.E, Hoar T.L. The 1990–1995 El Niño–Southern Oscillation event: longest on record. Geophys. Res. Lett. 1996;57:60. [Google Scholar]
- Vaughan D.G, Marshall G.J, Connolley W.M, Parkinson C, Mulvaney R, Hodgson D.A, King J.C, Pudsey C.J, Turner J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Change. 2003;60:243–274. doi:10.1023/A:1026021217991 [Google Scholar]
- Volkman N.J, Presler P, Trivelpiece W. Diets of pygoscelid penguins at King George Island, Antarctica. Condor. 1980;82:373–378. doi:10.2307/1367558 [Google Scholar]
- Waluda C.M, Trathan P.N, Rodhouse P.G. Influence of oceanographic variability on recruitment in the Illex argentinus (Cephalopoda: Ommastrephidae) fishery in the South Atlantic. Mar. Ecol. Prog. Ser. 1999;183:159–167. [Google Scholar]
- Waluda C.M, Trathan P.N, Rodhouse P.G. Synchronicity in southern hemispere squid stocks and the influence of the Southern Oscillation and Trans Polar Index. Fish. Oceanogr. 2004;13:1–12. doi:10.1111/j.1365-2419.2004.00288.x [Google Scholar]
- Watanuki Y, Mori Y, Naito Y. Euphausia superba dominates in the diet of Adélie penguins feeding under fast sea-ice in the shelf areas of Enderby Land in summer. Polar Biol. 1994;14:429–432. doi:10.1007/BF00240264 [Google Scholar]
- Weimerskirch H, Inchausti P, Guinet C, Barbraud C. Trends in bird and seal populations as indicators of a system shift in the Southern Ocean. Antarct. Sci. 2003;15:249–256. doi:10.1017/S0954102003001202 [Google Scholar]
- White M.G, Conroy J.W.H. Aspects of competition between pygoscelid penguins at Signy Island, South Orkney Islands. Ibis. 1975;117:371–373. [Google Scholar]
- White W.B, Peterson R.G. An Antarctic Circumpolar Wave in surface pressure, wind, temperature and sea-ice extent. Nature. 1996;380:699–702. doi:10.1038/380699a0 [Google Scholar]
- Williams T.D. Foraging ecology and diet of gentoo penguins Pygoscelis papua at South Georgia during winter and an assessment of their winter prey consumption. Ibis. 1991;133:3–13. [Google Scholar]
- Williams T.D. Oxford University Press; Oxford, UK: 1995. The penguins. [Google Scholar]
- Wilson P.R, Ainley D.G, Nur N, Jacobs S.S, Barton K.J, Ballard G, Comiso J.C. Adélie penguin population change in the pacific sector of Antarctica: relation to sea-ice extent and the Antarctic Circumpolar Current. Mar. Ecol. Prog. Ser. 2001;213:301–309. [Google Scholar]
- Xue Y, Smith T.M, Reynolds R.W. Interdecadal changes of 30-yr SST normals during 1871–2000. J. Clim. 2003;16:1601–1612. [Google Scholar]