Abstract
The Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) is bound by its Article II, 3 to follow an ecosystem approach to management. This approach has been extended to the application of a precautionary approach in the late 1980s. In our review, we deal primarily with the science-related aspects of CCAMLR and its development towards an ecosystem approach to the management of the living resources of the Southern Ocean. To assist the Commission in meeting its objectives, as set out in Article II, 3, the Scientific Committee established the CCAMLR Ecosystem Monitoring Programme to detect possible effects of krill fishing on the performance of top-level predators, such as albatrosses, penguins, petrels and fur seals. Fisheries in the Southern Ocean followed the fate of other fisheries worldwide in which target species were depleted to low level one after the other. Currently, two types of fisheries are open: the longline fisheries on Patagonian toothfish (Dissostichus eleginoides) and Antarctic toothfish (Dissostichus mawsoni) and the trawl fisheries on mackerel icefish (Champsocephalus gunnari). Both fisheries are managed in a single-species context, however, with conservation measures in place to protect by-catch species, such as rattails (Macrouridae) and skates and rays (Rajidae). Two major problems still exist in fisheries in the Southern Ocean: the by-catch of birds in longline fisheries primarily in the Indian Ocean and the high level of IUU fishing again in the Indian Ocean. Both, the by-catch of birds and high IUU catches undermine the credibility of CCAMLR to safeguard the marine living resources in the Southern Ocean.
Keywords: fisheries, Southern Ocean, CCAMLR, ecosystem approach
1. Introduction
Until recently, assessments of the effects of most harvesting of fishes and invertebrate stocks were based on single-species/stock approaches, avoiding considerations of interactions between prey, predators and the environment (Gulland 1988). Scientists had argued since the 1950s that ‘processes regulating fish stocks were much more complex than assumed in single-species models’ and that ecological ‘relationships could only be determined from quantitative empirical findings’ (Watt 1956). However, models needed for management on a multi-stock basis were too complex to be handled at that time.
One of the first major scientific papers to put multi-species and ecosystem considerations into the context of sustainable use of marine living resources was Holt and Talbot's paper published in 1978 (Holt & Talbot 1978). Overcapitalization of many fisheries and oversupply of fishing vessels delayed the introduction of multi-species approaches and single stock models prevailed as a basis for predicting and maximizing yield and setting total allowable catches (Smith 1994). As one of the consequences, scientists were unable to provide advice to the required extent to halt the severe decline of many fish stocks worldwide. It was not until the early 1990s that more scientific papers addressed the issue, and national and international meetings were held to foster the ecosystem approach to fisheries management. The United Nations World Summit on Sustainable Development held in Johannesburg in 2002 ‘encouraged the application by 2010 of the ecosystem approach’ (UN 2002). It is important to recognize that, ‘the ecosystem approach’ is not well defined and it may be more appropriate to consider a range of ecosystem-based approaches to fisheries management that take into account the broader impacts of specific fisheries on the host ecosystem.
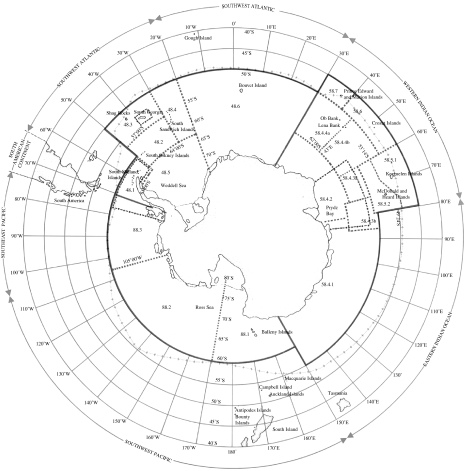
In 1980, the ‘Convention on the Conservation of Antarctic Marine Living Resources’ was signed (‘hereafter referred to as the Convention’). It came into force in 1982 concluding negotiations by the Antarctic Treaty Consultative Parties commenced in 1977 (Constable et al. 2000; Miller et al. 2004). The Convention area (figure 1) includes waters under international jurisdiction and areas around subantarctic islands such as South Georgia, Bouvetøya, Prince Edward Islands, Iles Crozet, Iles Kerguelen and Heard and McDonald Islands that form part of the exclusive economic zone (EEZ) of member nations. The Convention established a Commission for the Conservation of Antarctic Marine Living Resources, which is often referred to by its initials CCAMLR (pronounced ‘Kammelar’). CCAMLR is primarily concerned with krill and its dependant predators, and finfisheries, and with other marine living resources and represents the fourth convention regulating resources in Antarctica. The other conventions are:
the International Convention for the regulation of Whaling (in force since 1948)
the Antarctic Treaty (ATS, in force since 1959), and
the Convention for the Conservation of Antarctic Seals (CCAS, in force since 1978).
Figure 1.
The area of the Convention of the Conservation of Antarctic Marine Living Resources.
A fifth agreement protecting wildlife in the Southern Ocean is ‘The Agreement on the Conservation of Albatrosses and Petrels’ (ACAP) under the ‘Convention on the Conservation of Migratory Species of Wild Animals’. ACAP came into force in February 2004.
CCAMLR conservation measures regulating fishing in these latter areas require legislation by the relevant member. Such members may also opt not to be bound by a particular conservation measure suggested by CCAMLR. However, they are, in theory, obliged to impose alternative measures at least as effective as the CCAMLR ones. Management decisions are taken by CCAMLR which is mandated to act on the ‘best scientific advice available’ provided by the Scientific Committee and its subsidiary bodies. Decision-making in CCAMLR is based on consensus among all member states.
Here, we describe the development of fisheries in the Southern Ocean from a scientific point of view, how the fishery was and is regulated by CCAMLR and the extent to which CCAMLR has been able to implement the ecosystem approach set out in the Convention.
2. Early days of fishing in the Southern Ocean
(a) The early days of finfishing (until 1985)
(i) Atlantic Ocean sector
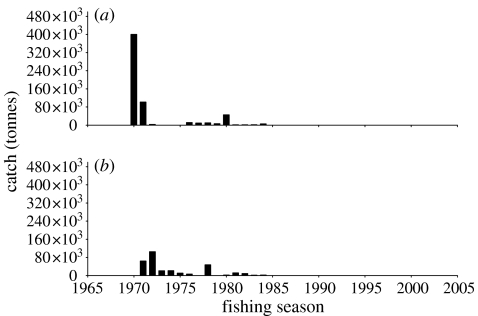
Fishing in the Southern Ocean was started at South Georgia in 1969/1970 and at Iles Kerguelen in the season thereafter. The early days of finfishing followed the same patterns as did commercial finfishing elsewhere: the first target species, the marbled notothenia (Notothenia rossii) became heavily depleted following large catches of several hundred thousand tonnes in the first few seasons and the fishery switched to other target species. Stocks of N. rossii in other areas of the Scotia Arc were much smaller and yielded catches of only 20 000 tonnes in a single season before they were depleted (figure 2a,b). The prohibition of the directed fishery on the species was one of the first conservation measures which CCAMLR established in 1985.
Figure 2.
Annual catch of the marbled notothenia (N. rossii) in (a) the Atlantic Ocean sector (Area 48) and (b) the Indian Ocean sector (Area 58).
The second target species, mackerel icefish (Champsocephalus gunnari) has a shorter life cycle and was more resilient to large-scale fishing. Mackerel icefish formed the backbone of the fisheries for 15 years with considerable variation in catches between years. From the second half of the 1970s, the fishery extended to fishing grounds further south (South Orkney Islands, South Shetland Islands) targeting mackerel icefish. Again, catches collapsed after a few seasons of large catches. The fishery reached the Antarctic continent by the end of the 1970s. Catches in most parts of the Antarctic continent, however, remained comparatively low and never went beyond an exploratory stage.
(ii) Indian Ocean sector
Fisheries in the Indian Ocean sector which started at Iles Kerguelen (Statistical Division 58.5.1) in 1970/1971 targeted marbled notothenia, grey notothenia (Lepidonotothen squamifrons) and mackerel icefish. They yielded annual catches of more than 50 000 tonnes per species in some years (figures 2b and 3b; Duhamel 1987, 1991). Catches of C. gunnari until 1978 (figure 3b) included those taken around Heard and McDonald Islands (Statistical Division 58.5.2). France and Australia created 200 nautical mile EEZs around Iles Kerguelen and Heard and McDonald Islands in 1978 and closed the fishery. The fishery was re-opened around Iles Kerguelen in 1979 (Duhamel 1991) but remained closed around Heard and McDonald Islands until 1995/1996 (Williams & de la Mare 1995).
Figure 3.
Annual catch of mackerel icefish (C. gunnari) in (a) the Atlantic Ocean sector area 48 (i) subarea 48.3, (ii) subarea 48.2 and (iii) subarea 48.1) and (b) and in the Indian Ocean sector area 58 ((i) Division 58.5.1 and (ii) Division 58.5.2). The two regions are presented separately to accommodate the different y axis scaling.
Detailed accounts on the first 15–20 years of finfishing in the Southern Ocean are provided in Duhamel (1987, 1991), Kock (1992, 1994), Agnew (2004) and in the annual statistical bulletins provided by CCAMLR.
(b) Krill fisheries
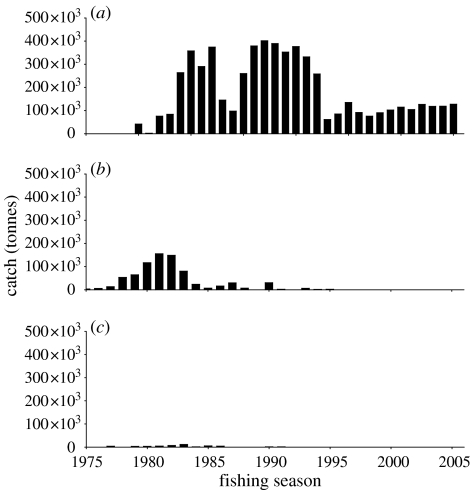
Experimental krill fishing began in 1973/1974. Annual catches soon increased and had reached more than 350 000 tonnes by 1978/1979 (Nicol & Endo 1997). In the early 1980s, catches further increased to 480–550 000 tonnes before declining to less than 200 000 tonnes in the mid-1980s which increased again to 350–400 000 tonnes until 1991 (Nicol & Endo 1997; figure 6).
Figure 6.
Annual catch of krill in the Southern Ocean from (a) the Atlantic Ocean sector (Area 48), (b) the Indian Ocean sector (Area 58) and (c) the Ross Sea (Area 88).
Most of the krill catch has been taken from the Atlantic Ocean sector (figure 6). Catches in the Indian Ocean sector peaked in the mid-1980s, but annual catches declined thereafter and no krill has been reported caught there since 1994/1995. A total of 750 000 tonnes has been harvested from the Indian Ocean (Area 58). Catches in the Pacific Ocean sector were small (a total of 40 000 tonnes) and restricted to the Western Ross Sea (Division 88.1; fig. 9, Ichii 2000).
3. The CCAMLR Scientific Committee and its working groups commenced their work
(a) Introduction
Article II, 3 of the Convention provides the basis for the work of the Scientific Committee by
balancing conservation with the needs for rational use,
providing protection for dependent and related species and restoring depleted populations and/or stocks to previous levels, and
avoiding changes that are potentially irreversible within two or three decades (Croxall & Nicol 2004).
(b) Management of the fish stocks
Article IX of the convention provides the means by which the Commission can manage fisheries. Decisions pertaining to management of fisheries are embodied in conservation measures. Non-binding but agreed decisions are often contained in resolutions (Constable et al. 2000). When the Commission commenced its work in 1982, it was soon faced with the severe effects of almost 15 years of unregulated exploitation of some finfish stocks (Kock 1992) and with a krill fishery with annual catches as high as 500 000 tonnes (Constable et al. 2000; Hewitt & Linen Low 2000; Ichii 2000).
The first two sessions of the Scientific Committee in 1982 and 1983 were mostly concerned with procedural matters. Until then, evaluation of the status of Antarctic exploited fish stocks had been undertaken by the Scientific Committee on Antarctic Research's Working Group on Fish Biology (later Ecology; Kock et al. 1985; Constable et al. 2000). The Scientific Committee established its first working groups, the ‘Working Group on Fish Stock Assessment’ (WG-FSA) in 1984 and the ‘Working Group on the Ecosystem Monitoring Programme’ (WG-CEMP) in 1985. WG-FSA and WG-CEMP interacted little with each other. WG-FSA had to embark on reactive management based on single-species approaches and insufficient data in an attempt to control the over-exploitation of fish stocks (Kock 1992; Constable et al. 2000).
Uncertainties arose from natural variation in stock abundance, statistical errors in stock assessment, uncertainties in estimates of model parameters, incomplete historical records, the imprecise submission of recent data and the novelty of a decision-making process in general (Constable et al. 2000). In the first years, catch restrictions suggested were opposed by a few fishing nations on the basis that the information and the analyses were incomplete. Conservation measures often had to be postponed by a year or two until agreement could be reached (Constable et al. 2000).
Insufficient data and the opposing standpoints of the few fishing nations and the much larger group of non-fishing nations set the scene subsequently for the implementation of important measures to protect depleted stocks and an intensive discussion on how stocks should be protected under uncertain advice by the Scientific Committee from the late 1980s onwards. In its deliberations, WG-FSA took more and more multi-species considerations into account, especially when by-catch species and protection of benthos became of particular concern (Kock 2001). Such considerations led to the prohibition of finfishing in the southern Scotia Arc region after the 1989/1990 season (CCAMLR 1990) and the prohibition of bottom trawling around South Georgia after 1992 (CCAMLR 1992). The reporting of catch and effort data became mandatory at this time.
(c) Establishing the CCAMLR Ecosystem Monitoring Programme
The krill fishery has consistently operated in same areas in the South Orkney Islands, the South Shetland Islands and South Georgia which are feeding grounds for land-breeding krill-eating penguins, other birds and seals. Therefore, the possibility that excessive quantities of krill could be removed from local areas is clearly a significant risk (Croxall & Nicol 2004). Given the central role of krill in the foodweb of the Southern Ocean, this potential effect of krill fishing on krill predators was of particular concern in the early years of CCAMLR. To address this concern, a monitoring programme was developed to provide information on the potential effects of krill exploitation on dependent species. In accordance with the approach set out in Article II, 3 of the Convention (Everson 2002), and based on studies undertaken by Bengtson (1984) and Lubimova et al. (1985), an Ecosystem Monitoring Programme (CEMP) was established (SC-CAMLR 1985).
The aims of the CEMP were to:
detect and record significant changes in critical components of the marine ecosystem within the Convention Area, to serve as a basis for the conservation of Antarctic marine living resources, and
distinguish between changes due to harvesting of commercial species and changes due to environmental variability, both physical and biological.
CCAMLR elaborated and refined a scheme to assimilate existing research on the following krill predators as well as identifying species, sites and parameters suitable for monitoring:
chinstrap penguin (Pygoscelis antarctica),
gentoo penguin (Pygoscelis papua),
Adelie penguin (Pygoscelis adeliae),
macaroni penguin (Eudyptes chrysolophus),
black-browed albatross (Thalassarche melanophrys),
Antarctic petrel (Thalassoica antarctica),
Cape pigeon (Daption capense),
Antarctic fur seal (Arctocephalus gazella), and
crabeater seal (Lobodon carcinophagus).
CEMP data are collected following a set of standard methods and submitted by members on a voluntary basis. With respect to penguins, parameters to be measured, which operated at various time and space scales, were: adult arrival weight; duration of first incubation shift; breeding population size; age-specific survival; foraging trip duration; breeding success; chick fledgling weight; and chick diet. Breeding population size, breeding success and age-specific survival are measured in black-browed albatross and foraging trip duration and pup growth in fur seals (Everson 2002). Main sites for CEMP were Bird Island (South Georgia), the islands of the southern Scotia Arc, the Antarctic Peninsula and the Prydz Bay.
The list of species given above represents the initial list (after minke whales Balaenoptera acutorostrata and the Antarctic silverfish, Pleuragramma antarcticum had already been excluded) compiled by CCAMLR. However, the logistic and financial constraints of working in the Antarctic mean that there is very limited data for some species and none for others. For instance, the crabeater seal is a krill-dependent predator and has many attributes that would make it an excellent monitoring species, with the exception of accessibility, since it breeds in the pack-ice where indices of its population and reproductive performance cannot readily be assessed on an annual basis.
Changes in predator performance can be evaluated as a function of krill availability and/or environmental variability (Miller 2002; Reid et al. 2005). Body mass variables had the lowest variability (CVs<10%) whereas those measuring breeding success showed the highest variability (CVs>50%; Reid et al. 2005). The key objectives which needed to be addressed through the programme are:
effects of fishing needed to be detected in sufficient time for decisions to be taken before irreversible damage to the ecosystem occurs, and
do changes in the environment require re-assessment of the controls of fishing? (Constable et al. 2000).
CEMP seeks to monitor the intrinsic impacts of fishing. However, extrinsic factors, such as climate change, provide an important context for the interpretation of CEMP. A detailed account of CEMP and its background has been provided by Agnew (1997) and a review of its first 15 years of operation is available in SC-CAMLR (2003a), Annex 3, Appendix D.
4. Towards the development of an ecosystem approach to management (from the late 1980s onwards)
(a) Introduction
The inability of the Commission to take account of the uncertainties in management advice from the Scientific Committee led to considerable concern among the scientists working in CCAMLR in the second half of the 1980s. Initial attempts to identify optimal rates of harvesting from fish stock production models, involving target fishing mortalities of F0.1, were considered by many scientists to be either too high or inappropriate for certain species, such as mackerel icefish and lanternfish (myctophids; SC-CAMLR 1991; Constable et al. 2000). Subsequent advice by scientists on the feasibility of providing unequivocal advice on catch limits (SC-CAMLR 1990) signalled a change towards a precautionary approach to management by CCAMLR (Constable et al. 2000). Operational issues such as marine debris and other similar environmental protection measures and maritime safety (CCAMLR 1996; SC-CAMLR 2000b; Kock 2001; Miller et al. 2004) have not been dealt with here.
(b) The development of the Krill Yield Model as a basis to safely harvest krill
(i) Krill fishing since 1990
Krill catches declined to approximately 100 000 tonnes from 1992/1993 onwards when economic considerations caused the states of the former Soviet Union to largely discontinue krill fishing. Since then, annual catches have fluctuated around that level (fig. 6, Ichii 2000; SC-CCAMLR 2004). There has been increasing interest among both CCAMLR member countries and non-signatories in the krill fishery in recent years (SC-CAMLR 2003a, 2004). Increased interest in the krill fishery has come about largely through requirements for increased supplies of aquaculture feed (Nicol & Foster 2003).
Since 1990, Japanese vessels have been taking 50–60% of the annual catch of krill. Other nations which have been fishing krill since 1990 include: Argentina, Chile, India, Latvia, South Africa, Ukraine, Russia, Uruguay, Poland, Republic of Korea, USA, Panama, Vanuatu and the UK (Ichii 2000; CCAMLR 2005a,b).
(ii) The development of a Krill Yield Model
Krill forms large aggregations in the surface waters. These aggregations are targeted by a large number of predator species. Given its complex relationship with a variety of predators, it was felt that krill should not be managed in a single-species context. Krill interactions were not only relevant to species at similar or lower trophic levels (i.e. ‘related species’, sensu Article II, 3) but also applied to higher trophic levels (‘dependant species’, sensu Article II, 3; Miller 2002). Concomitant with the development of the Ecosystem Monitoring Programme, CCAMLR foresaw the need to manage the effects of the krill fishery. Such approaches were to include considerations of spatial and temporal overlaps between fishing activities and those of land-based predators (Miller 2002). WG-Krill (established in 1988) thus had a direct and close link to WG-CEMP and the two groups were merged into the ‘Working Group on Ecosystem Monitoring and Management’ (WG-EMM) in 1994.
To make progress with implementing the objectives of Article II, the Commission was presented in 1985 with a framework for evaluating management procedures based on simulations that tested whether particular procedures are likely to achieve their objectives (de la Mare 1986). It took 5 years for the Commission in their ‘Working Group on the Development of Approaches to Conservation’ to agree on feedback management in order to minimize the over-exploitation of the resource with potentially serious effects on krill predators. This approach would involve the continuous adjustment of management measures in response to new and/or additional information. In the meantime, the setting of precautionary annual catch limits would be necessary (Constable et al. 2000). The Commission endorsed the general concept for setting catch limits for krill in 1990. A first precautionary catch limit for krill was set in 1991 (Nicol & de la Mare 1993).
Butterworth et al. (1991, 1994) and Constable & de la Mare (1996) developed the Krill Yield Model (KYM) modified from an approach by Beddington & Cooke (1983). Based on krill biomass estimates from the FIBEX surveys in 1980/1981 (Trathan et al. 1995), the first conservation measure with respect to krill limited its catch to 1.5 million tonnes. A proviso was included that catch limits by subarea should be introduced if the overall annual catch in statistical subareas 48.3, 48.2 and 48.1 exceeded 620 000 tonnes (CCAMLR 1991). Such a subdivision was essential to take account of the potential effects of localized fishing on land-based predators (Miller 2002). Predator requirements were also taken into account through a decision rule as part of the KYM process. This three-part rule was developed for determining the proportion of the krill population (γ) which could be harvested each year with minimal risk, thus addressing Article II, 3 of the Convention (Constable et al. 2000; Miller 2002; Croxall & Nicol 2004).
Choose γ1 such that the probability of the median krill spawning stock biomass declining below 20% of its pre-exploitation median level over a 20-year harvesting period is 10%.
Choose γ2 so that the median krill spawning stock biomass after over 20 years of fishing is 75% of the pre-exploitation median level.
Select the lower value of γ1 and γ2 as the level for γ for the calculation of krill yield.
Although the decision rules took explicit account of the needs of predators by allowing a krill escapement of 75%, this is an arbitrary level and there is no empirical evidence to suggest that this level is sufficient to meet those needs (Miller 2002).
Further input parameters to set precautionary catch limits for krill were:
an estimate of krill biomass with confidence intervals in an area usually obtained from a hydro-acoustic survey,
rate of natural mortality of krill,
individual krill growth in terms of weight, and
estimates of the inter-annual variability in recruitment.
The revised yield model, revised B0 estimates and the three-part decision rule were adopted by the Commission in 1994.
The intention was that that estimate of yield was to be revised as new information on improved methodology, biomass estimates or biological parameters, such as growth and recruitment, became available. It was envisaged that catch limits calculated for krill would be returned for a number of years until sufficient new information was available to revise catch figures. These rules provided the first example of specifying the objectives of CCAMLR in scientifically interpretable and measurable terms (Constable et al. 2000).
The subdivision of the krill catch into subareas has been hampered by uncertainty over the degree to which krill can be considered as effective stocks in some areas (Siegel 2005). Krill is thought to migrate actively between offshore and shelf areas in the course of a year in some regions (Siegel 1988, 2005). Passive transport is likely to play a larger role in the transport from west to east across larger regions such as from the South Shetland Islands/Antarctic Peninsula region through the Scotia Sea to South Georgia (Ichii & Naganobu 1996; Siegel 2005). However, the extent to which krill is exchanged between areas by passive transport or stays resident permanently and/or at times in highly productive areas remains unresolved (Miller 2002). The uncertainty associated with active krill movement, passive flux and the extremely patchy nature of the krill distribution is relevant to any management approach, in particular for the setting of precautionary catch limits for contiguous subareas, divisions or parts thereof (Everson 2002; Miller 2002). Where krill flux rates are not constant between areas, a simple pro rata allocation of some total precautionary catch for Area 48 to subareas could lead to inappropriate high catch levels in some smaller areas (Miller 2002).
(c) The fishery for mackerel icefish
Mackerel icefish is both a predator of krill (Permitin & Tarverdiyeva 1972, 1978; Kock 1981; Duhamel 1991; Kock et al. 1994; Barrera-Oro et al. 1998; Flores et al. 2004) and an important prey component for predators, such as Antarctic fur seals and gentoo penguins (Everson et al. 1999; Reid et al. 2005). Its high recruitment variability, of up to a factor of 20, and the consequential high variability in stock size means that the use of the same decision rules as used for krill would make γ1 a low value even without fishing. It makes it impractical to use the same management approach (Constable et al. 2000). Therefore, the catch limits for icefish are set according to a 2-year projection based on survey estimates of stock size and population structure. The effects of highly variable recruitment are compounded by sporadic years with apparently higher natural mortality than normal, such as in 1990/1991, possibly due to poor feeding conditions and increased predation by Antarctic fur seals and other predators when the abundance of their preferred diet (krill) is low (Constable et al. 2000). Modifications to the decision rule have been proposed to overcome the variation in recruitment and the occasional high natural mortality but have not yet been suitably implemented (Agnew et al. 1998).
(d) The fishery for Patagonian toothfish and Antarctic toothfish and its management
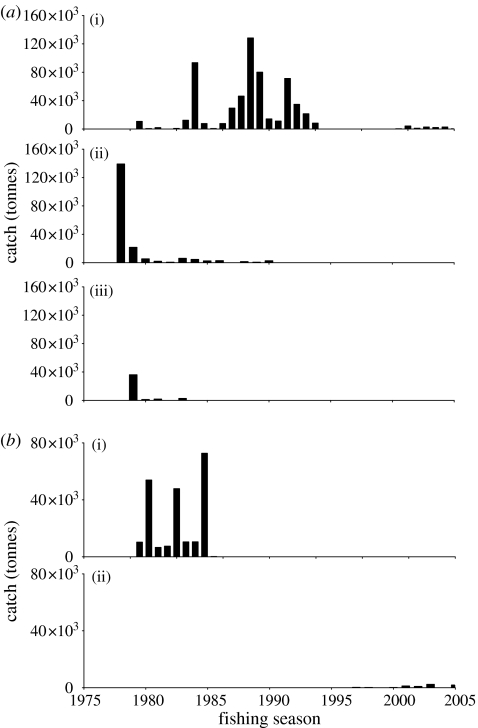
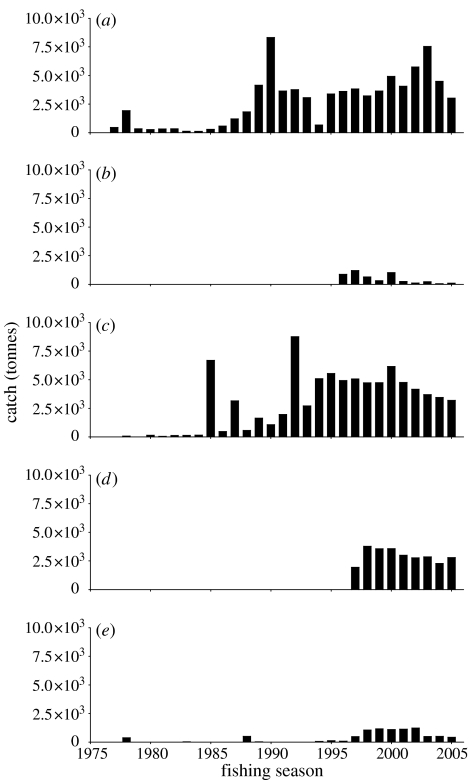
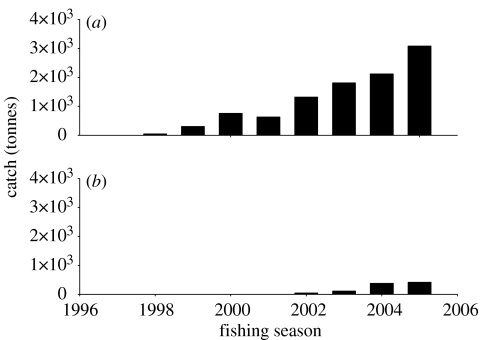
A longline fishery for Patagonian toothfish had been pioneered by the ex-Soviet Union at South Georgia in the season 1985/1986 (figure 4a). The fishery remained unnoticed for a few years. By the beginning of the 1990s, the Russian longline activities had declined due to the restructuring of their domestic fishery. The fishery had been largely taken over by Chilean and Argentinean longline vessels. At the same time, a longline fishery on Patagonian toothfish developed around Iles Kerguelen largely conducted by French vessels (figure 4d). Other fishing grounds such as the Prince Edward Islands were exploited after the mid-1990s (figure 4b). A fishery on Antarctic toothfish (Dissostichus mawsoni) was started in the Ross Sea in 1997 by New Zealand (figure 5).
Figure 4.
Annual catch of Patagonian toothfish (Dissostichus eleginoides) around (a) South Georgia (Subarea 48.3), (b) the Prince Edward Islands (Subarea 58.7), (c) around Iles Crozet (Subarea 58.6), (d) Iles Kerguelen (Division 58.5.1) and (e) Heard and McDonald Islands (Division 58.5.2).
Figure 5.
Annual catch of Antarctic toothfish (D. mawsoni) in the Ross Sea in (a) Area 88.1 and (b) 88.2.
After using conventional methods to assess and manage stocks of toothfish for several years with little success, CCAMLR recognized that:
data from the fishery were insufficient for conventional fish stock assessment methods and
adult stocks of toothfish were partly inaccessible to the fishery. Adult toothfish were known to occur as deep as 2500–3000 m. Fishing at that depth was unprofitable.
Toothfish are large predators which grow to more than 220 cm in size (Kock et al. 1985). They are known to constitute a small part of the diet of male sperm whales (Physeter macrocephalus; Yukhov 1970, 1971) and Weddell seals (Leptonychotes weddellii; Testa et al. 1985, Plötz 1986) but are hardly known from the diet of other abundant predators. Given this weak link to dependent species (in contrast to krill), CCAMLR has set catch limits for toothfish using a single-species approach. The same approach which led to the development of the KYM was used to develop a Generalized Yield Model (GYM) to be used for Patagonian toothfish (Constable & de la Mare 1996). The second part of the decision rule was applied at an escapement rate of 50% rather than at the 75% used for krill (Constable et al. 2000).
5. New challenges to CCAMLR (from the mid-1990s onwards)
(a) Introduction
By the mid-1990s, CCAMLR had been able to establish conservation measures or at least make major progress with respect to the protection and rational use of fish stocks and the protection of stocks of dependent species, through:
its Ecosystem Monitoring Programme,
the calculation of a precautionary catch limit for krill and Patagonian toothfish,
a 100% observer scheme in longline fisheries, and
the regulation of the development of new and exploratory fisheries.
However, a variety of problems were either continuing or were newly recognized and addressed from the mid-1990s onwards. Those were:
illegal, unreported and unregulated (IUU) fishing,
new and exploratory fisheries,
data acquisition through an observer scheme,
the incidental mortality of seabirds, and
the incidental mortality of marine mammals, and the by-catch of rays, skates and grenadiers in longline fisheries. These are dealt with in turn below.
(b) Illegal, unreported and unregulated fishing
Illegal, unreported and unregulated (IUU) fishing is a global problem. In the Southern Ocean, however, it arose with the establishment of the longline fisheries for Patagonian toothfish in the early 1990s. It was confined to South Georgia until 1996, but spread throughout the Southern Ocean toothfish fishing grounds within one or two seasons thereafter. IUU fishing had significant adverse effects on a number of Patagonian toothfish stocks, in particular, those around the Prince Edward Islands and Iles Kerguelen. It was probably responsible for large by-catches of albatrosses and larger petrels (Agnew 2000; Constable et al. 2000; Croxall & Nicol 2004). In order to address the problem, CCAMLR
collaborated operationally and through exchange of information with other RFMOs to police its area more effectively. This resulted in numerous arrests, fines and catch confiscation, for example, in the Kerguelen and Heard Island fishing zones (Croxall & Nicol 2004),
the use of satellite-linked vessel monitoring systems (VMS) became mandatory for longline vessels fishing in the Convention Area in 2003. However, to be fully effective, the system needs to be operated centrally in real time rather than through Flag States (Croxall & Nicol 2004), and
a catch documentation scheme (CDS) was implemented in May 2000 whereby all legitimate vessels declare their catch of toothfish and have it verified by their national authorities. The document travels with every shipment of toothfish and is required before toothfish can be imported (Agnew 2004).
Despite these initiatives around the Prince Edward Islands the size of the toothfish stock became only a small fraction of its initial size due to IUU activities (Croxall & Nicol 2004). In other areas, such as South Georgia, Iles Kerguelen and Heard and McDonald Islands, Member countries have increased their surveillance in order to reduce IUU fishing. However, it is of particular concern that certain members of CCAMLR are unable to regulate the activity of their flag vessels and/or to take appropriate action against them, such as through port control to prevent their apparent complicity in IUU activity (CCAMLR 2003).
(c) New and exploratory fisheries
New fisheries are usually developed by the fishing industry without prior notice to managers and scientists. Thus, management of these fisheries was often not implementable before a fishery was fully developed or the resource was already over-exploited (Miller et al. 2004). In light of this CCAMLR decided that:
the development of any fishery should not occur at a rate faster than the Commission is able to evaluate its potential consequences and
whether the objectives of Article II, 3 would be met (CCAMLR 1989, 1990).
Members who wish to develop or participate in a new fishery have to notify the Commission in advance (CCAMLR 1991) of their intention to undertake further exploratory fishing following the initiation of a fishery (CCAMLR 1993). These measures ensure that new fishing activities in the Convention Area remain sustainable (Constable et al. 2000; Miller et al. 2004).
However, the annual assessment of applications to continue exploratory fisheries places a great burden on CCAMLR working groups. The conversion of exploratory fisheries into fully assessed ones (which would in theory be much simpler to manage) has been much slower than expected, largely due to the difficulties in collecting and analysing appropriate data and of designing appropriate regulations to address issues such as by-catch of other fish species.
Thus, immediate priorities for research in new areas being fished for toothfish include recruitment surveys and the determination and evaluation of demographic and growth parameters (Constable et al. 2000). Until such data exists on which a proper assessment can be based, the GYM used estimates of recruitment from areas already exploited. They are extrapolated to new areas in proportion to the ratios of area of seabed. They are discounted by 60% to take account of uncertainties. Catches in new areas are restricted to 100 tonnes per rectangle of 1° longitude by 0.5° latitude in order to spread the fishing effort over a large area and avoid over-exploitation. This leads to conservative catch limits as can be seen from catch limits set by CCAMLR for island shelves, such as the Bouvetøya shelf (Subarea 48.6), or banks, such as Banzare Bank (Division 58.4.1). However, the development of an overall management procedure with prospective evaluation of different methodologies remains to be undertaken (Constable et al. 2000).
(d) The CCAMLR System of Scientific Observation
The CCAMLR System of Scientific Observation was established in 1992 (CCAMLR 1992). Scientific observers have been of great value to the Scientific Committee in collecting:
data needed from finfish fisheries on target species, such as Dissostichus ssp., which help managing a fishery,
estimates of the number of birds being caught in longline fisheries,
data on by-catch composition,
data on interactions with marine mammals, such as killer whales and sperm whales, and
data on operational practice.
It is greatly to the credit of all parties to CCAMLR and the fishing industry that scientific observers are accepted on all vessels fishing for finfish in the Convention Area. Both, the ‘Ad hoc Working Group on Incidental Mortality’ (IMAF) and WG-FSA depend heavily on data collected by scientific observers on board. It is hoped that the reluctance by some members to host international observers' onboard krill fishing vessels will be soon overcome (Croxall & Nicol 2004).
(e) Incidental mortality of seabirds
The Southern Ocean is fairly isolated with respect to its fishing in that most species fished do not occur outside the Antarctic. The only straddling stocks are those of the Patagonian toothfish. Another aspect which clearly impacts on resources in the Southern Ocean is the by-catch of albatrosses and petrels which breed inside the convention area, but are caught in large numbers in longline fisheries outside the Southern Ocean. Article II, 3c of CCAMLR clearly indicates the importance of assessing and avoiding incidental mortality of Antarctic marine living resources. Since 1984, the Commission requested members to document the number, species and further biological information on any bird or marine mammal taken incidentally during fishing operations (Miller et al. 2004).
By the early 1990s, it was clear that by-catch in tuna (Thunnus sp.) and billfish fisheries of albatrosses and some large petrel species breeding within the CCAMLR Convention Area was potentially the most serious cause of observed declines in their populations. At this time, the first reports were received of albatrosses and petrels killed by longline fishing for toothfish around South Georgia. As observer coverage improved (and fishing effort increased), the estimated levels of mortality reached several thousand birds annually, levels likely to be unsustainable for the populations of the most affected species (especially black-browed albatrosses and white-chinned petrels Procellaria aequinoctialis). The population recovery would probably be irreversible over the next 20–30 years (in potential contravention of Article II, 3 of the Convention) without rapid action (Croxall et al. 1998). CCAMLR immediately addressed this problem, both by improving observer coverage (to 100%) in longline fisheries and by researching and implementing measures to reduce seabird by-catch: The ‘Ad hoc Working Group on Incidental Mortality Associated with Longline Fisheries’ established in 1994 recommended the immediate use of streamer lines to deter birds, introduce heavier weights to sink lines more rapidly and the ban of offal discharge (to avoid attracting birds). These, together with some other measures, were immediately adopted as a conservation measure. However, compliance with the conservation measure was poor initially. The working group advised CCAMLR that until full compliance was achieved, longline fisheries should be closed during the main breeding season of albatrosses and petrels.
Seasonal restrictions in longline fishing were not introduced in the South African EEZ around the Prince Edward Islands and the French EEZ around Iles Kerguelen and Crozet. Reduction of fishing effort and fishing further away from breeding colonies, however, reduced the by-catch of birds in the Prince Edward Islands EEZ to similar low levels as around South Georgia (Croxall & Nicol 2004). Bird by-catch remained very high in the Kerguelen EEZ (Weimerskirch 1998) until the stricter use of CCAMLR procedures resulted in a 75% reduction in by-catch (mainly of white-chinned petrels) in 2004. Despite the success in minimizing seabird by-catch in most CCAMLR subareas, high levels of mortality continue in fisheries for tuna and allied species outside the Convention Area, directly putting those seabird species breeding in the CCAMLR area at risk (Neves & Olmos 1998; Schiavini et al. 1998; Stagi et al. 1998).
In contrast to longline fisheries, seabirds are seldom killed in either krill or most finfish trawl fisheries in CCAMLR waters. The only exception is the pelagic trawl fishery on mackerel icefish at South Georgia where 50–100 albatrosses and petrels have been killed in each of the last few years. The problem, mainly caused by birds diving into the net to obtain food and becoming entangled, is proving difficult to resolve, given that the net stays unduly long at the surface when problems during shooting or hauling of the net arise. However, recent gear trials devised to minimize entanglement risk appear promising and a mandatory conservation measure to this effect within the next year or two seems probable.
(f) Incidental mortality of marine mammals with longline and trawl fisheries
Incidental mortality of seals in longline fisheries in the convention area is rare, with occasional reports of fur seals and elephant seals (Mirounga leonina) only (e.g. SC-CAMLR 2000a, 2001, 2003b). Reports involving cetaceans (including humpback whales Megaptera novaeangliae, small baleen whales and dolphins) are even rarer and even fewer resulting in mortality (Kock et al. 2005). Summaries of relevant details from comprehensive reports by scientific observers are presented each year to the Scientific Committee.
The main interaction involving marine mammals and longline fisheries relates to loss of gear (by sperm whales becoming entangled in longlines) and fish (mainly from killer whale Orcinus orca predation; e.g. Ashford et al. 1996; Capdeville 1997). Fishing masters will not usually set lines when killer whales are present. They often use noise deterrents when killer whales appear at the time of hauling.
Very few reports of mammal fatalities have been received from krill fisheries until recently. However, in 2003, it became clear that krill fisheries have probably been killing at approximately 100 fur seals annually (SC-CAMLR 2003b), a situation that became apparent with the deployment of international observers on krill vessels around South Georgia. Japanese and Ukrainian operators have developed simple net modifications that allow seals to escape. Initial reports of their use suggest large reduced mortality (Hooper et al. 2005). The use of such devices is now mandatory in the Convention Area (CCAMLR 2003).
Another problem for fur seals has been entanglement in discarded fishing gear and plastic packaging bands from bait boxes, which may have involved several thousand individuals when the problem was first recognized in the late 1980s (Croxall et al. 1990). Long-term monitoring of such entanglement of seals at South Georgia suggest that CCAMLR measures introduced to prohibit the use of packaging bands in the Convention Area has reduced entanglement very substantially.
(g) The by-catch of rays, skates and grenadiers in longline fisheries
By-catch of rays, skates and grenadiers had occurred since longlining on Patagonian toothfish was introduced. Their by-catch levels vary considerably between longliners. Autoliners appear to have larger by-catches than those using the Spanish system where hooks tend to be further off the bottom. The by-catch may reach more than 15% of the catch of the target species toothfish (SC-CAMLR 1999).
It is difficult to assess how large the by-catch of rays, skates and grenadiers actually is in certain longline fisheries and to what extent longlining affects their stocks. Whenever possible, rays and skates are cut from the line by the fishermen before they are hauled on deck. Hooks are retained in their mouth. It is unknown to what level the rays and skate survive the release but some mortality is probable. Grenadiers do not survive the hauling process. They are discarded to an unknown extent after they have come on deck. Thus, catch levels estimated for grenadiers if not taken from scientific observer data are likely to be underestimates of the true catch level.
Given that grenadiers and rays and skates are presumably long lived and CCAMLR wishes to avoid the depletion stories of other long-lived species, CCAMLR follows a mixed strategy with respect to limiting the by-catch of rays, skates and grenadiers. The strategy has two components:
total removals by each by-catch species are limited by estimates of potential yield and
haul-specific by-catch limits are set at levels that permit prospecting but are not likely to cause the potential yield to be exceeded (SC-CAMLR 1997, 1998).
By-catch levels set by the Commission for specific subareas and divisions and for species groups (rays) and skates (combined) have been in place since 1999. Annual allowable catches vary between areas: they were 50–126 tonnes in rays and skates and 26–529 tonnes in grenadiers (CCAMLR 2004a).
6. Links between krill and fish and environmental conditions
(a) Introduction
Ecosystems in the Southern Ocean are dynamic on various scales. Variability in the physical environment can have direct and indirect short- and long-term effects on the abundance of krill, other prey, predators and fisheries (Croxall & Nicol 2004). To name the most important:
the oceanographic variability, its impact on the abundance of krill and predator performance at South Georgia (Boyd & Roberts 1993; Trathan et al. 2003),
changing sea-ice conditions have altered the winter fishery on krill and may have an effect on krill in the whole Antarctic Peninsula–Scotia Arc region (Croxall & Nicol 2004; Hofman & Murphy 2004; Siegel 2005), and
the El Niño–Southern Ocean Oscillation (ENSO) affects the demography and abundance of marine top predators (including penguins and mackerel icefish) and their impact on krill (Kock & Everson 2003; Croxall & Trathan 2004).
(b) Changes in sea-ice and oceanographic conditions and its relation to krill
Sea-ice, oceanic conditions and oceanographic boundaries have a marked effect in structuring Antarctic marine ecosystems (Nicol et al. 2000; Trathan et al. 2003; Hofman & Murphy 2004). The location of the ice edge is highly seasonal and inter-annually variable. The long-term ice edge location is influenced by thermodynamic processes and may be influenced by changes in ocean temperature and dynamics and long-term changes in atmospheric circulation on time-scales of years to decades (Worby & Comiso 2004). Sea-ice has a number of ecological roles which act as a primary forcing function for some and as a covariant for others (Constable et al. 2003).
Sea-ice acts as a habitat for ice algae and as a water column stratifying agent during the melt season in spring which facilitates the spring bloom (Croxall & Nicol 2004). The abundance of krill is a function of production and survival as well as retention and export (Hofman & Murphy 2004; Siegel 2005). Sea-ice algal communities provide the vital nutrient resource for krill (Brierley & Thomas 2002; Hofman & Murphy 2004). A close link exists between the abundance of adult krill, sea-ice extent in winter and the variability in krill recruitment (Siegel & Loeb 1995; Constable et al. 2003; Siegel 2005). Winters with more extensive and prolonged sea-ice cover are thought to result in a greater spatial extent and temporal duration of ice algae and extracellular polymerase substances in the brine channels (Brierley & Thomas 2002). Sea-ice microbial communities are released from the melting ice and can enhance feeding conditions for krill in spring leading to earlier maturation and spawning which could enhance the survival and growth of krill recruits. Krill larvae, in contrast to adult krill, are unable to survive long periods of starvation. They must feed during autumn and winter with more developed larvae better equipped to find food (Siegel & Loeb 1995; Quetin et al. 1996; Brierley & Thomas 2002). A much reduced ice coverage during winter and a much more extended ice-free zone removes habitat for krill and appears to favour salps as a competitor for food in the following summer (Loeb et al. 1997).
Data from whaling records indicate that the Antarctic sea-ice as a whole has retreated by up to 2.8° latitude since the 1950s and prior to the era of satellite remote sensing (de la Mare 1997). Such a retreat has been observed in the South Orkney Islands (Murphy et al. 1995), but it has been suggested that the observed change falls within the observed variation of the ice edge location from remote sensing data (Ackley et al. 2003, Worby & Comiso 2004). If such changes have occurred, they would represent a major alteration to the Southern Ocean ecosystem and would have an impact on krill populations. Observed changes in krill abundance may represent high inter-annual variability or the beginning of a downward trend, but consistent long-term data series are lacking to confirm either notion (Siegel 2000; Atkinson et al. 2004).
Advection is thought to be a dominant process which affects the distribution and abundance of krill. Although some active offshore/onshore migration of krill is known, there is no evidence that krill can undertake active migration against open ocean currents (Hofman & Murphy 2004). Interactions between krill and the prevailing current systems of the Southern Ocean may, however, allow persistent populations to exist in specific areas (Constable et al. 2003). Modelling studies suggest that climate-related changes in the intrusion of Upper Circumpolar Deep Water onto the shelf may have a long-term effect on the composition and abundance of the krill stock (s) (Hofman & Murphy 2004).
Recruitment of krill is one of the key components in the GYM which CCAMLR uses to manage the fishery, so changes that occur with time in response to climate variability are critical to ensuring sustainable harvest of krill. The current version of the GYM includes stochastic recruitment variation rather than incorporating a function which is driven by physical variables, partly because the degree of predictability for the effects of physical variation on krill recruitment is low (Croxall & Nicol 2004).
The lower winter sea-ice coverage in the last two decades may already have had an effect on the behaviour of the krill fishing fleet. The winter fishery on krill used to be confined to the ice-free waters of South Georgia. The trend in median catch date and the extension of the fishing season in the South Orkney Islands and the South Shetland Islands into June may have been either a response to the increasing availability of ice-free areas late in the season allowing fishing to continue until June (Croxall & Nicol 2004) or simply reflect different fleets using a variety of fishing behaviours (Miller & Agnew 2000).
(c) Changes in the relationship of krill abundance to predator feeding demands at South Georgia
Indices of population size of four important krill predators (Antarctic fur seals, black-browed albatrosses, gentoo penguins and macaroni penguins) at South Georgia showed declines from 1980 to 2000 and an increase in the frequency of years with low reproductive success. Abundance of krill was apparently large enough to support predator demand in the 1980s but not in the 1990s (Reid & Croxall 2001). The krill biomass index from the Antarctic Peninsula–South Shetland Islands region over the period 1977–1997 demonstrated a change from positive values until the mid-1980s to consistent negative values during the period 1988–1995 (Siegel et al. 1998). There is evidence of concordance in the pattern of inter-annual changes in krill biomass at South Georgia and the Antarctic Peninsula–South Shetland Islands (Brierley et al. 1999). The increase in the frequency of negative predator-breeding performance over the predator time-series suggests an increase in the frequency of periods of low krill biomass (Reid & Croxall 2001).
These changes in the abundance of krill at South Georgia in relation to predator demands cannot be explained by the impact of fishing. Catches were of the order of 80 000–250 000 tonnes until 1991/1992 (CCAMLR 1995) but declined to 4000–76 000 tonnes thereafter (CCAMLR 2005a,b). This reduction in catch reflects a decline in krill fishing effort rather than changes in the availability of krill. In addition, the demand for krill by two of its main predators, fur seals and macaroni penguins, may have declined in some areas. Unfortunately, changes in demand from other krill predators, such as fish, squid or baleen whales, remain unclear. This limits the ability to estimate the relative magnitude in changes of overall krill supply and/or predator demands (Reid & Croxall 2001). They concluded that:
there has been a change from a situation with a relatively large krill supply when compared with the predator demand (with the exception of a few krill-poor years), linked to a krill population structure that effectively buffered predators against the underlying variability in krill recruitment and krill transport to South Georgia,
the krill supply changed from the 1990s onwards, since when the krill supply has been sufficiently close to predator demands to cause the local mortality rate of krill to be substantially altered, and
predator-induced mortality of krill has effectively removed any surplus that previously existed and may have contributed to an increase in the frequency of years where the amount of krill around the island is insufficient to support predator demands (Reid & Croxall 2001).
(d) ENSO and its potential effect on mackerel icefish
Mackerel icefish is a low-Antarctic species which lives at a fairly narrow temperature range of −0.5 to 2.5°C. Fish living at Shag Rocks and South Georgia and further to the north in the Indian Ocean are or will soon be challenged by water temperatures at their upper limit of tolerance.
It is extremely difficult to distinguish between fishery-induced impacts on mackerel icefish which had been substantial from the mid-1970s to the early 1990s and environmental effects (Bakun 1990). There are several indications that negative effects of an increase in water temperature may have occurred. The sudden increase of surface water temperatures in the Kerguelen area over several months in 1998 (Duhamel & Claudet 2002) was one example of an ENSO associated effect recorded in one area of the northern part of the Southern Ocean. Nevertheless, the effects of this warming may have been less pronounced in near-bottom layers inhabited by mackerel icefish throughout most of the day.
The low stock size of mackerel icefish on the Kerguelen shelf after 1995 and in particular after the ENSO in 1998, and the absence of recolonization of Pike, Discovery and Shell Bank and possibly other banks to the north of Heard Island in addition to the decline in biomass (since the 1970s) around Heard Island may be another example of a subtle biological response of mackerel icefish to the changing environmental conditions. Banks at the northern margin of the distributional range of C. gunnari were used for fishing in years when temperatures were below average in the 1970s and early 1980s. If water temperature has increased beyond the average temperature permanently in the past 15 years, it will reduce the likelihood of recolonization of the aforementioned banks. However, the apparent absence of C. gunnari in the late 1990s and early 2000s at Shell Bank, where water temperatures are colder than around Heard Island, was neither linked to the effect of fishing, which did not occur at that time, nor a rise in water temperature.
7. Into the future of fisheries and management of the resources in the Southern Ocean
(a) Krill
The level of fishing on Antarctic krill is low when compared with the 1980s and is still very small when compared with the allowable catch. Indeed, it is the only fishery in the world with a catch limit (5 million tonnes: 4 million tonnes in the Atlantic Ocean and 1 million tonnes in the Indian Ocean sector) which exceeds the existing catch by 50 times. As such, it has a potential for large expansion in the future. The importance of this capacity for expansion means that the krill fishery is one of the only 5% of fisheries globally that is not either fully or over-exploited. Hence, there is a high likelihood that the krill fishery will expand in the future.
New products using krill and other crustaceans are constantly under development. There is a rapidly growing need to provide fishmeal and marine oils to supply the burgeoning aquaculture industry (Croxall & Nicol 2004). It is the development and marketing of new products that is likely to enhance the demand for krill in various industries from aquaculture and pet food markets to nutraceutical, cosmetic and pharmaceutical fields (Croxall & Nicol 2004).
CCAMLR has established compulsory schemes for the notification of intended entrance into a number of its finfisheries but not for krill. Thus, there has been no formal mechanism for the submission of information a priori on the probable market interest and hence the krill fishery trends (Croxall & Nicol 2004). Relying on voluntary submission of Members of plans for the following season has been found to be inadequate to predict future trends in the development of the fishery. However, a scheme for the voluntary notification of detailed information on Member's krill fishing plans was adopted by CCAMLR in 2003 (CCAMLR 2003)1. Nevertheless, a lack of economic data makes it difficult to forecast how the krill fishery will develop in the future. Although limited economic and technological data have been made available (Jones & Hull 2002), repeated requests by CCAMLR for these data have remained largely unanswered.
CCAMLR has begun the complex task of managing krill fisheries at the scales most relevant to many of the environment–prey–predator interactions. The first step is to define the size of smaller and more ecologically realistic and relevant small scale management units (SSMUs; Constable 2002). The geographical boundaries of 15 SSMU's have been agreed by CCAMLR in the Scotia Arc region and the Antarctic Peninsula and a number of candidate methods for subdividing the catch limit into SSMUs have been proposed (Hewitt et al. 2004). The concept of setting of catch limits for SSMUs based on historic catch information was developed by the International Whaling Commission (IWC) in their Revised Management Procedure. The IWC concept spreads effort over a larger area. However, in the CCAMLR situation this would tend to concentrate fishing in a few areas. Nevertheless, within the remit of the IWC, studies are underway modelling predator–prey interactions of krill, baleen whales and seals (Mori & Butterworth 2005). This calls for a closer collaboration between the CCAMLR and the IWC.
The evaluation of the ability of each if these catch allocation options to achieve the aims of CCAMLR is to develop a simulation framework that reflects the operation of the krill population, krill predators and the krill fishery. Clearly, there will be considerable uncertainty associated with each of these elements and it is essential that any process used to provide advice on which option should be used takes these uncertainties into account (Croxall & Nicol 2004).
A number of general Conservation Measures adopted by CCAMLR has specifically excluded the krill fishery and this may impede future regulation of an expanding krill fishery. Scientific observers are mandatory in all CCAMLR finfisheries but not on krill fishing vessels. The use of VMS to monitor the movements of fishing vessels in the CCAMLR area has become binding on all finfishing vessels in 2003. However, krill fishing were excluded from this measure. CCAMLR needs to develop a mechanism to increase the data reporting and observer coverage from the krill fishery in order to provide a structured framework into which an expanding krill fishery can develop in an orderly fashion.
(b) The fishery for toothfish and the associated by-catch of albatrosses and large petrels
Problems still exist with the assessment of toothfish stocks. The only stocks which appear to be reasonably well assessed with annual catch levels based on the GYM are the stocks around South Georgia and Heard Island (e.g. CCAMLR 2003, 2004b). Owing to the extensive control of the fishing grounds, IUU fishing has virtually come to an end at South Georgia while IUU fishing is still occurring at Heard Island. Catch levels are currently of the order of 3000 tonnes in both areas (CCAMLR 2004b). If Australia is able to control IUU fishing at Heard Island, it is probable that yield levels are long-term and sustainable over many years.
The stock of toothfish around the Prince Edward Islands, inside and outside the South African EEZ, is likely to be less than 10% of its initial size in the mid-1990s (Brandão et al. 2002). The annual yield is now unlikely to exceed a few hundred tonnes. The state of the stock of toothfish around Iles Kerguelen is currently unknown but requires urgent assessment. The stock has probably declined substantially due to substantial IUU activities in the area over the last 8–10 years.
The CDS has been considered to be a major achievement in combating IUU fishing. Initial evaluations of its effectiveness are encouraging. Its overall coverage now extends to more than 90% of the global toothfish trade. Using the CDS, parties have been able to deny toothfish landings and/or shipments in the absence of the required documents. Trading of IUU-caught toothfish appears to have become less profitable (Miller et al. 2004).
CCAMLR has been successful in reducing the number of birds taken in legal toothfish harvesting in some parts of the Southern Ocean, such as South Georgia and the Prince Edward Islands over the past 5 years. These successes were largely attributed to a combination of improved compliance with by-catch measures and delaying commencement of fishing until the end of the breeding season for most albatrosses and petrels (Miller et al. 2004).
A number of other problems still need to be resolved:
the large numbers of Convention Area birds are still taken in longline fisheries on tuna and allied species outside the Convention Area,
large numbers of birds are still being taken around Iles Kerguelen and in all IUU fisheries in the Southern Ocean,
adequate regulation of the activities of flag of convenience vessels,
more efficient survey and policing of the fishing areas,
lack of control on toothfish fisheries (legal and IUU) outside the Convention Area. Some toothfish stocks may be ‘straddling’ or migratory stocks from inside the Convention Area,
a more comprehensive approach to the management of toothfish fisheries, including additional trade-related measures may need to be developed,
by-catch species are still inadequately reported, and
VMS needs to be centralized and used in real time (Croxall & Nicol 2004).
(c) The participation of non-Members in CCAMLR fisheries
The FAO recognizes CCAMLR as the regional body responsible for fisheries in the Southern Ocean. In principle, CCAMLR should be able to accommodate all parties with a genuine interest in fishing and conservation in the Southern Ocean now and in the future (Constable 2005). There are no impediments for States not party to CCAMLR to exploit marine living resources of the Southern Ocean as non-contacting parties (NCPs). A typical example is the recently established krill fishery by Vanuatu (CCAMLR 2004b) and an application of the Cook Islands for krill fishing in the Southern Ocean in 2005/2006.
It might be useful to adopt guidelines for non-contracting parties who wish to cooperate with CCAMLR with a view to give guidance to those who might wish to join the CAMLR Commission. Such guidelines have already been adopted in various Regional Fisheries Organizations and to Article 8 of the UN Straddling Stocks Agreement (CCAMLR 2005b). Consensus was not reached on this matter at the 2005 Meeting of CCAMLR. Some parties consider it necessary when given the successful cooperation with non-contracting parties so far. Furthermore, some parties of the Convention or are not party the UN Straddling Stocks Agreement. It needs to be borne in mind, however, that non-cooperative NCPs can undermine CCAMLR by choosing to ignore conservation measures set by CCAMLR and/or by not contributing to the costs of CCAMLR.
8. Has CCAMLR been able to meet the expectations set out in Article II, 3 of the Convention in developing ecosystem-based management procedures?
CCAMLR's efforts to develop a management approach that is both precautionary and ecosystem-based have gone farther than in any other RFMO (Miller et al. 2004). The ecosystem approaches so far focuses mainly on the krill fishery and predators either directly or indirectly dependant on krill (Constable 2002). This approach has allowed krill decision rules to be extended to other species, such as toothfish and provided annual long-term yields for this species by accounting for uncertainty and applying additional precaution through management action (Constable 2002). Finfisheries, such as on toothfish and mackerel icefish, are managed in a single-species context with respect to the setting of catch limits in those situations. However, CCAMLR has managed the operation of those fisheries in such a way as to be able to address by-catch (especially of seabirds) in a more effective way than any other RFMO (Small 2005; Croxall et al. in press).
One of the key issues highlighted by CCAMLR as early as 1984 was the problem of distinguishing between the effects of fishing on dependant or related species and natural variation in their abundance and reproductive performance. Separating these two effects could in principle be tackled by establishing a series of fishing areas and closed areas that both contained monitoring of dependent species. This proposal was linked to a feedback management procedure in 1992. However, CCAMLR has made no commitment to an experimental approach to resolve this question (Constable et al. 2000). This may simply reflect the difficulties of replicating monitoring schemes at an appropriate spatial scale. The establishment of small-scale management units for the krill fishery may provide a potential means to direct the activities of the fishery in a way that maximizes information on the ecosystem effects of the fishery. In the context of the Southern Ocean, the ability to study the natural variability of dependent species, in the absence of a fully developed krill fishery, has allowed the nature of the relationship between environmental variability and reproductive performance to be investigated (Trathan et al. 2003; Reid et al. 2005). With a greater understanding of the relationship between environmental variability and indices of predator (dependent species) performance becomes the potential to attribute deviations from modelled expectations to the effects of fisheries (in those areas/times when fishing does occur).
With the monitoring in place and the ability to detect the effects of fisheries at what might be described as sub-lethal levels, a major task remains to determine the magnitude of change or a trigger level in the indices which would signal that action is needed by the Commission. Simply detecting a change in the ecosystem that is attributable to harvesting is not necessarily sufficient to require management action in order to satisfy the objectives of the Convention. Any fishery will have an effect on the host ecosystem, however, if those effects are such that they may not be reversible over a relevant time-scale (e.g. two to three decades as in stated in Article II, 3), then action is required in sufficient time for harvesting activities to be modified to avoid irreversible change. Applying indices of the performance of dependent species in management require determining how sensitive they are to assumptions about the functional relationship between species, between species and the physical environment, and between the fishery and krill. Ideally, such an index must be sensitive only to those factors which assist in managing the system and robust against others (Constable et al. 2000). The important task will be to develop models with sufficient structural complexity to capture the important dynamics of the CEMP species and how they might react to direct and indirect changes in the ecosystem (Constable 2002). A detailed account of the models envisaged, the model structure and the spatial and temporal configuration of the monitoring programmes underlying these models which are required to detect effects of krill fishing on the ecosystem are provided by Constable (2002).
CCAMLR is ahead of other conventions in developing ecosystem based-management procedures both in terms of the science that is conducted and the willingness to implement the broader ecosystem consequences and recommendations arising from that science. To be fully effective, this approach involves:
the development of plausible models of the ecosystem that reflect the operation of harvested, dependent and related species,
to determine the mechanisms and, where appropriate, the experimental design required to provide assessments on which robust management decisions can be based on the results of ecosystem monitoring, and
to evaluate proposed management procedures aimed at achieving the ecosystem objectives in particular with respect to dependant and related species prior to an expansion of the krill fishery (Constable 2002; Miller et al. 2004).
The next step is to extend this work into decision-making processes. Of utmost importance will always be the consideration that harvesting should not affect predators in unforeseen ways, in particular those for which CCAMLR is either unable to develop effective monitoring programmes, such as whales, or CCAMLR is unable to set up extensive monitoring due to the lack of resources, such as pack-ice seals and many seabirds (Constable et al. 2003). Fostering closer collaboration between those conventions responsible for the management of marine living resources in the Southern Ocean may help to fill in some of these gaps.
Further aspects into which CCAMLR has started to look more intensively into are the establishment of marine protected areas, although the idea of comparing areas fished with areas closed to any fishing dates back to the third Annual Meeting of CCAMLR in 1984. Not-withstanding natural causes impacting on diversity of Antarctic benthos caused, for example by grounding icebergs (Gutt & Piepenburg 2003), the destruction that trawling gear can cause to benthic communities has become a topic of concern not only in the Southern Ocean. The UN call for action against destructive fishing practices, the intensive discussion on the destruction of benthos and the change of benthic communities caused by the impact of bottom trawling gear in the North Sea and the danger to cold water corals brought upon by bottom trawling in areas, such as Rockall Banks and other banks at the continental slope of Europe are only a few examples of the recent discussion. The damage done to benthic communities by bottom trawling and the unsustainable level of by-catch of certain fish species led CCAMLR to prohibit fishing in the South Orkney Islands and the South Shetland Islands/Antarctic Peninsula regions and the use of bottom trawls in the fishery on mackerel icefish at South Georgia (CCAMLR 1990).
The ecosystem approach, as articulated by CCAMLR, is to manage fisheries with respect to the target species, dependent species and related species. Thus, while catch limits for certain species may be set using models that only include information on the target species, i.e. a single-species approach, the impact of the fishery on non-target (dependent and/or by-catch) has a considerable impact on the operation of the fisheries. To this effect, CCAMLR has implemented a number of ecosystem-based approaches which when taken together would represent an ecosystem approach to the management of Southern Ocean fisheries.
One contribution of 10 to a Theme Issue ‘Antarctic ecology: from genes to ecosystems. Part 2: evolution, diversity and function’.
Endnote
Note added in proof. CCAMLR introduced a mandatory notification of intention to fish for krill in 2006.
References
- Ackley S, Wadhams P, Comiso J.C, Worby A.P. Decadal decrease of Antarctic sea ice extent inferred from whaling records revisited on the basis of historical and modern sea ice records. Polar Res. 2003;22:19–25. doi:10.1111/j.1751-8369.2003.tb00091.x [Google Scholar]
- Agnew D.J. Review: the CCAMLR ecosystem monitoring program. Antarct. Sci. 1997;9:235–242. [Google Scholar]
- Agnew D.J. The illegal and unregulated fishery for toothfish in the Southern Ocean, and the CCAMLR catch documentation scheme. Mar. Policy. 2000;24:361–374. doi:10.1016/S0308-597X(00)00012-9 [Google Scholar]
- Agnew, D. J. 2004 Fishing south—the history and management of South Georgia Fisheries St Albans, UK: The Penna Press.
- Agnew D.J, Everson I, Kirkwood G.P, Parkes G.B. Towards the development of a management plan for the mackerel icefish (Champsocephalus gunnari) in Subarea 48.3. CCAMLR Sci. 1998;5:63–77. [Google Scholar]
- Ashford J.R, Rubilar P.S, Martin A.R. Interactions between cetaceans and longlining operations for Patagonian toothfish Dissostichus eleginoides at South Georgia. Mar. Mamm. Sci. 1996;12:452–457. doi:10.1111/j.1748-7692.1996.tb00598.x [Google Scholar]
- Atkinson A, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. doi:10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Bakun A. Global climate change and intensification of coastal upwelling. Science. 1990;247:198–201. doi: 10.1126/science.247.4939.198. doi:10.1126/science.247.4939.198 [DOI] [PubMed] [Google Scholar]
- Barrera-Oro E, Casaux R, Marschoff E. Analysis of the diet of Champsocephalus gunnari at South Georgia in late summer from 1994 to 1997, Dr Eduardo L. Holmberg surveys. CCAMLR Sci. 1998;5:103–123. [Google Scholar]
- Beddington J.R, Cooke J.G. FAO fisheries technical paper. vol. 242. 1983. The potential yield of fish stocks. [Google Scholar]
- Bengtson J.L. Selected scientific papers 1982–1984. Part II. CCAMLR; Hobart, Australia: 1984. Monitoring indicators of possible ecological changes in the Antarctic marine ecosystem. pp. 43–153. [Google Scholar]
- Boyd I.L, Roberts P.J. Tooth growth in male Antarctic fur seals (Arctocephalus gazella) from South Georgia: an indicator of long-term growth history. J. Zool. Lond. 1993;229:177–190. [Google Scholar]
- Brandão A, Butterworth D.S, Watkins B.P, Staverees L. WG-FSA-02/76. CCAMLR; Hobart, Australia: 2002. An updated assessment of the toothfish resources in the Prince Edward Islands vicinity and extensions taking commercial catch-at-length data into account. p. 24 (mimeogr) [Google Scholar]
- Brierley A.S, Thomas D.N. Ecology of the Southern Ocean pack-ice. Adv. Mar. Biol. 2002;43:173–278. doi: 10.1016/s0065-2881(02)43005-2. [DOI] [PubMed] [Google Scholar]
- Brierley A.S, Demer D.A, Hewitt R.P, Watkins J.L. Concordance of interannual fluctuations in densities of krill around South Georgia and Elephant Island: biological evidence of same—year teleconnections across the Scotia Sea. Mar. Biol. 1999;134:675–681. doi:10.1007/s002270050583 [Google Scholar]
- Butterworth D.S, Punt A.E, Basson M. Selected science papers 1991(SC-CAMLR-SSP/8) CCAMLR; Hobart, Australia: 1991. A simple approach for calculating the potential yield of krill from biomass survey results. pp. 207–217. [Google Scholar]
- Butterworth D.S, Gluckman G.R, Thomson R.B, Chalis S, Hiramatsu K, Agnew D. Further computations of the consequences of setting the annual krill catch limit to a fixed fraction of the estimate of krill biomass from a survey. CCAMLR Sci. 1994;1:81–106. [Google Scholar]
- Capdeville D. Interactions of marine mammals with the longline fishery around the Kerguelen Islands (Division 58.5.1) during the 1995/96 cruise. CCAMLR Sci. 1997;4:171–174. [Google Scholar]
- CCAMLR 1989 Report of the 8th meeting of the commission, Hobart, Australia, 6–17 November, p. 41.
- CCAMLR 1990 Report of the 9th meeting of the commission, Hobart, Australia, 22 October–2 November 1990.
- CCAMLR 1991 Report of the 10th meeting of the commission, Hobart, Australia, 21 October–1 November 1991.
- CCAMLR 1992 Report of the 11th meeting of the commission, Hobart, Australia, 26 October–6 November 1992.
- CCAMLR 1993 Report of the 12th meeting of the commission, Hobart, Australia, 25 October–5 November 1993.
- CCAMLR. Statistical bulletin. vol. 7. CCAMLR; Hobart, Australia: 1995. [Google Scholar]
- CCAMLR 1996 Report of the 15th meeting of the commission, Hobart, Australia, 21 October–1 November 1996.
- CCAMLR 2003 Report of the 22nd meeting of the commission, Hobart, Australia, 27 October–7 November 2003.
- CCAMLR. CCAMLR; Hobart, Australia: 2004a. Schedule of conservation measures in force 2004/05. [Google Scholar]
- CCAMLR 2004b Report of the 23rd meeting of the commission, Hobart, Australia, 26 October–6 November 2004.
- CCAMLR. Statistical bulletin. vol. 17. CCAMLR; Hobart, Australia: 2005a. [Google Scholar]
- CCAMLR 2005b Report of the 24th meeting of the commission, Hobart, Australia, 24 October–4 November 2005.
- Constable A.J. CCAMLR ecosystem monitoring and management: future work. CCAMLR Sci. 2002;9:233–253. [Google Scholar]
- Constable, A. J. 2005 Future actions for ensureing the resilience of CCAMLR in a changing world. CCAMLR Symp. 25 Years of CCAMLR, Valdivia 5–8 April 2005
- Constable A, de la Mare W.K. A generalized yield model for evaluating yield and the long-term status of fish stocks under conditions of uncertainty. CCAMLR Sci. 1996;3:31–54. [Google Scholar]
- Constable A.J, de la Mare W.K, Agnew D.J, Everson I, Miller D. Managing fisheries to conserve the Antarctic marine ecosystem: practical implementation of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR) ICES J. Mar. Sci. 2000;57:778–791. doi:10.1006/jmsc.2000.0725 [Google Scholar]
- Constable A.J, Nicol S, Strutton P.G. Southern Ocean production in relation to spatial and temporal variation in the physical environment. J. Geophys. Res. 2003;108:1–10. doi:10.1029/2001JC001270 [Google Scholar]
- Croxall J.P, Nicol S. Management of Southern Ocean fisheries: global forces and future sustainability. Antarct. Sci. 2004;16:569–584. doi:10.1017/S0954102004002330 [Google Scholar]
- Croxall J.P, Trathan P.N. The Southern Ocean: a model system for conserving resources? In: Glover L.K, Earle S.A, editors. Defying Ocean's end. Island Press; Washington, DC: 2004. pp. 71–87. [Google Scholar]
- Croxall J.P, Rodwell S, Boyd I.L. Entanglement in man-made debris of Antarctic fur seals at Bird Island, South Georgia. Mar. Mamm. Sci. 1990;6:221–233. doi:10.1111/j.1748-7692.1990.tb00246.x [Google Scholar]
- Croxall J.P, Prince P.A, Rothery P, Wood A.G. Population changes in albatrosses at South Georgia. In: Robertson G, Gales R, editors. Albatross—biology and conservation. Surrey Beatty & Sons; Chipping Norton, Australia: 1998. pp. 69–83. [Google Scholar]
- Croxall, J. P., Rivera, P. & Moreno, C. In press. Seabird by-catch mitigation: The Southern Ocean (CCAMLR) experience. In By-catch mitigation in long-lived marine vertebrates (ed. S. Kennelly).
- de la Mare W.K. Selected science papers 1986. CCAMLR; Hobart, Australia: 1986. Some principles for fisheries regulation from an ecosystem perspective. pp. 323–340. [Google Scholar]
- de la Mare W.K. Abrupt mid-twentieth-century decline in Antarctic sea-ice extent from whaling records. Nature. 1997;389:57–60. doi:10.1038/37956 [Google Scholar]
- Duhamel, G. 1987 Ichtyofaune des secteurs indien occidental et atlantique oriental de l'océan austral: biogeography, cycles biologiques et dynamique des d'etat. Université Pierre et Marie Curie, Paris.
- Duhamel G. The biological and demographic peculiarities of the icefish Champsocephalus gunnari Lönnberg 1905 from the Kerguelen Plateau. In: di Prisco G, Maresca B, Tota B, editors. Biology of Antarctic fish. Springer; Berlin, Germany: 1991. pp. 40–53. [Google Scholar]
- Duhamel G, Claudet J. WG-FSA-02/65. CCAMLR; Hobart, Australia: 2002. Preliminary analysis on the Kerguelen shelf icefish Champsocephalus gunnari stock from 1996/97 to 2000/01: no evidence in the recovery. [Google Scholar]
- Everson I. Considerations of major issues in ecosystem monitoring and management. CCAMLR Sci. 2002;9:213–232. [Google Scholar]
- Everson I, Parkes G, Kock K.-H, Boyd I. Variations in standing stock of the mackerel icefish Champsocephalus gunnari at South Georgia. J. Appl. Ecol. 1999;36:591–603. doi:10.1046/j.1365-2664.1999.00425.x [Google Scholar]
- Flores H, Kock K.-H, Wilhelms S, Jones C.D. Diet of two icefish species from the South Shetland Islands and Elephant Island, Champsocephalus gunnari and Chaenocephalus aceratus. Polar Biol. 2004;27:119–129. doi:10.1007/s00300-003-0570-4 [Google Scholar]
- Gulland J.A. 2nd edn. Wiley; Chichester, UK; New York, NY: 1988. Fish population dynamics. [Google Scholar]
- Gutt J, Piepenburg D. Scale-dependenct impact on diversity of Antarctic benthos caused by grounding of icebergs. Mar. Ecol. Prog. Ser. 2003;253:77–83. [Google Scholar]
- Hewitt R.P, Linen Low E.H. The fishery on Antarctic krill: defining an ecosystem approach to management. Rev. Fish. Sci. 2000;8:235–298. doi:10.1080/10641260091129224 [Google Scholar]
- Hewitt R.P, Everson I, Jones C.D. WG-EMM-04/48. CCAMLR; Hobart, Australia: 2004. Reconciling fisheries with conservation: three examples from the Southern Ocean. p. 20 (mimeogr) [Google Scholar]
- Hofman E.E, Murphy E. Advection, krill, and Antarctic marine ecoystems. Antarct. Sci. 2004;16:205–213. doi:10.1017/S0954102004002275 [Google Scholar]
- Holt S.J, Talbot L.M. New principles for the conservation of wild living resources. Wildl. Monogr. 1978;59:1–33. [Google Scholar]
- Hooper J, Clark J.M, Charman C, Agnew D. Seal mitigation measures on trawl vessels fishing for krill in Subarea 48.3. Incidental seal entanglements on trawl vessels fishing for krill in CCAMLR Subarea 48.3. CCAMLR Sci. 2005;12:195–205. [Google Scholar]
- Ichii T. Krill harvesting. In: Everson I, editor. Krill—biology, ecology and fisheries. Blackwell Science Ltd; Oxford, UK: 2000. pp. 228–261. [Google Scholar]
- Ichii T, Naganobu M. Surface water circulation in krill fishing areas near the South Shetland Islands. CCAMLR Sci. 1996;3:125–136. [Google Scholar]
- Jones C.D, Hull M. WG-EMM-02/18. CCAMLR; Hobart, Australia: 2002. The US commercial krill fishery in Area 48: development, fishing patters and decisions making. [Google Scholar]
- Kock K.-H. Fischereibiologische Untersuchungen an drei antarktischen Fischarten: Champsocephalus gunnari Lönnberg, 1905, Chaenocephalus aceratus (Lönnberg, 1906) und Pseudochaenichthys georgianus Norman, 1937 (Notothenioidei, Channichthyidae) Mitt. Inst. Seefisch. Hamb. 1981;32:1–226. [Google Scholar]
- Kock K.-H. Cambridge University Press; Cambridge, UK; New York, NY: 1992. Antarctic fish and fisheries. [Google Scholar]
- Kock K.-H. Fishing and conservation in southern waters. Polar Rec. 1994;30:3–22. [Google Scholar]
- Kock K.-H. The direct influence of fishing and fishery-related activities on non-target species in the Southern Ocean with particular emphasis on longline fishing and its impact on albatrosses and petrels—a review. Rev. Fish Biol. Fish. 2001;11:31–56. doi:10.1023/A:1014207719529 [Google Scholar]
- Kock K.-H, Everson I. Shedding new light on the life cycle of mackerel icefish in the Southern Ocean. J. Fish Biol. 2003;63:1–21. doi:10.1046/j.1095-8649.2003.00150.x [Google Scholar]
- Kock K.-H, Duhamel G, Hureau J.-C. The biology and status of exploited Antarctic fish stocks. BIOMASS Sci. Ser. 1985;6:1–143. [Google Scholar]
- Kock K.-H, Wilhelms S, Everson I, Gröger J. Variations in the diet composition and feeding intensity of mackerel icefish Champsocephalus gunnari at South Georgia (Antarctica) Mar. Ecol. Prog. Ser. 1994;108:43–57. [Google Scholar]
- Kock K.-H, Purves M, Duhamel G. Interactions between cetaceans and fisheries in the Southern Ocean. Polar Biol. 2005;28:379–388. [Google Scholar]
- Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S. Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature. 1997;387:897–900. doi:10.1038/43174 [Google Scholar]
- Lubimova T.G, Makarov R.R, Maslennikov V.V, Shetsov T, Shust K.V. Selected science papers 1982–1984, Part II, (SC-CAMLR-SSP/1) CCAMLR; Hobart, Australia: 1985. The ecological peculiarities, stocks and role of E. superba in the trophic structure of the Antarctic ecosystem. pp. 391–505. [Google Scholar]
- Miller D.G.M. Antarctic krill and ecosystem management—from Seattle to Siena. CCAMLR Sci. 2002;9:175–2002. [Google Scholar]
- Miller D, Agnew D. Management of krill fisheries in the Southern Ocean. In: Everson I, editor. Krill—biology, ecology and fisheries. Blackwell Science Ltd; Oxford, UK: 2000. pp. 300–337. [Google Scholar]
- Miller D.G.M, Sabourenkov E.N, Ramm D.C. Managing Antarctic marine living resources: the CCAMLR approach. Int. J. Mar. Coast. Law. 2004;19:317–363. doi:10.1163/1571808042886075 [Google Scholar]
- Mori M, Butterworth D.S. IWC SC/57/021. International Whaling Commission; Cambridge, UK: 2005. Modelling the predator–prey interactions of krill, baleen whales and seals in the Antarctic. [Google Scholar]
- Murphy E.J, Clarke A, Symon C, Priddle J. Temporal variation in Antarctic sea-ice: analysis of a long-term fast-ice record from the South Orkney Islands. Deep-Sea Res. I. 1995;42:1045–1062. doi:10.1016/0967-0637(95)00057-D [Google Scholar]
- Neves T, Olmos F. Albatross mortality in fisheries off the coast of Brazil. In: Robertson G, Gales R, editors. Albatross—biology and conservation. Surrey Beatty & Sons; Chipping Norton, Australia: 1998. pp. 214–219. [Google Scholar]
- Nicol S, de la Mare W.K. Ecosystem management and the Antarctic krill. Am. Sci. 1993;81:36–47. [Google Scholar]
- Nicol S, Endo Y. FAO fisheries technical paper. vol. 367. 1997. Krill fisheries of the world. [Google Scholar]
- Nicol S, Foster J. Recent trends in the fishery for Antarctic krill. Aquat. Living Resour. 2003;16:42–45. doi:10.1016/S0990-7440(03)00004-4 [Google Scholar]
- Nicol S, Pauly T, Bindoff N.L, Wright S, Thiele D, Hosie G.W, Strutton P.G, Woehler E. Ocean circulation off East Antarctica affects ecosystem structure and sea-ice extent. Nature. 2000;406:504–507. doi: 10.1038/35020053. doi:10.1038/35020053 [DOI] [PubMed] [Google Scholar]
- Permitin Y.Y, Tarverdiyeva M.I. The food of some Antarctic fish in the South Georgia area. Vopr. Iktiol. 1972;12:120–132. [Transl. as J. Ichthyol. 12, 104–114.] [Google Scholar]
- Permitin Y.Y, Tarverdiyeva M.I. Feeding of Antarctic cods (Nototheniidae) and ice-fishes (Chaenichthyidae) near the South Orkney Islands. Bio. Mor. 1978;2:75–81. [In Russian.] [Google Scholar]
- Plötz J. Summer diet of Weddell Seals (Leptonychotes weddelli) in the Eastern and Southern Weddell Sea, Antarctica. Polar Biol. 1986;6:97–102. [Google Scholar]
- Quetin L.B, Ross R.M, Frazer T.K, Haberman K.L. Factors affecting distribution and abundance of zooplankton, with an emphasis on Antarctic krill, Euphausia superba. Antarct. Res. Ser. 1996;70:357–371. [Google Scholar]
- Reid K, Croxall J.P. Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc. R. Soc. B. 2001;268:377–384. doi: 10.1098/rspb.2000.1371. doi:10.1098/rspb.2000.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K, Forcada J. Causes of offspring mortality in the Antarctic fur seal, Arctocephalus gazella: the interaction of density dependence and ecosystem variability. Can. J. Zool. 2005;83:604–609. doi:10.1139/z05-045 [Google Scholar]
- Reid K, Croxall J.P, Briggs D.R, Murphy E.J. Antarctic ecosystem montoring: quantifying the response of ecosystem indicators to variability in Antarctic krill. ICES J. Mar. Sci. 2005;62:366–373. doi:10.1016/j.icesjms.2004.11.003 [Google Scholar]
- SC-CAMLR 1985 Report of the 4th meeting of the scientific committee, Hobart, Australia, 2–9 September 1985. In Report of the 4th meeting of the scientific committee, Hobart, Australia, pp. 1–54.
- SC-CAMLR 1990 Report of the 9th meeting of the scientific committee, Hobart, Australia, 22–26 October 1990. In Report of the 9th meeting of the scientific committee, Hobart, Australia, pp. 1–66.
- SC-CAMLR 1991 Report of the 10th meeting of the scientific committee, Hobart, Australia, 21–25 October 1991. In Report of the 10th meeting of the scientific committee, Hobart, Australia, pp. 1–77.
- SC-CAMLR 1997 Report of the working group on fish stock assessment, Hobart, Australia, 13–22 October In Report of the 17th meeting of the scientific committee, Hobart, Australia, pp. 241–425.
- SC-CAMLR 1998 Report of the 18th meeting of the scientific committee, Hobart, Australia, 26–30 October 1998. In Report of the 18th meeting of the scientific committee, Hobart, Australia, pp. 1–83.
- SC-CAMLR 1999 Report of the working group on fish stock assessment, Hobart, Australia, 11–20 October 1999. In Report of the 18th meeting of the scientific committee, Hobart, Australia, pp. 229–445.
- SC-CAMLR 2000a Report of the working group on fish stock assessment, Hobart, Australia 9–19 October 2000. In Report of the 19th meeting of the scientific committee, Hobart, Australia, pp. 283–499.
- SC-CAMLR. SC-CAMLR-XIX/BG/28. CCAMLR; Hobart, Australia: 2000b. CCAMLR activities on monitoring marine debris in the Convention Area in 1999/2000. (mimeogr) [Google Scholar]
- SC-CAMLR 2001 Report of the working group on fish stock assessment 9–19 October, 22–31 October 2001. In Report of the 20th meeting of the scientific committee, Hobart, Australia, pp. 203–558.
- SC-CAMLR 2003a Report of the 22nd meeting of the scientific committee, Hobart, Australia, 27–31 October 2003. In Report of the 22nd meeting of the scientific committee, Hobart, Australia, pp. 1–98.
- SC-CAMLR 2003b Report of the working group on fish stock assessment 13–23 October, 2003. In Report of the 22th meeting of the scientific committee, Hobart, Australia, pp. 295–547.
- SC-CAMLR 2004 Report of the 23rd meeting of the scientific committee, Hobart, Australia, 25–29 October 2004. In Report of the 23rd meeting of the scientific committee, Hobart, Australia, pp. 1–101.
- Schiavini A, Frere E, Gandini P, Garcia N, Crespo E. Albatross–fisheries interactions in Patagonian shelf waters. In: Robertson G, Gales R, editors. Albatross—biology and conservation. Surrey Beatty & Sons; Chipping Norton, Australia: 1998. pp. 208–213. [Google Scholar]
- Siegel V. A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sahrhage D, editor. Antarctic Ocean and resources variability. Springer; Berlin, Germany: 1988. pp. 219–230. [Google Scholar]
- Siegel V. Krill (Euphausiaceae) life history and aspects of population dynamics. Can. J. Fish. Aquat. Sci. 2000;57:130–150. doi:10.1139/cjfas-57-S3-130 [Google Scholar]
- Siegel V. Distribution and population dynamics of Euphausia superba. Summary of recent findings. Polar Biol. 2005;29:1–22. doi:10.1007/s00300-005-0058-5 [Google Scholar]
- Siegel V, Loeb V. Recruitment of Antarctic krill Euphausia superba and possible causes for its variability. Mar. Ecol. Prog. Ser. 1995;123:45–56. [Google Scholar]
- Siegel V, Loeb V, Groeger J. Krill Euphausia superba density, proportional and absolute recruitment and biomass in the Elephant Island region (Antarctic Peninsula) during the period 1977–1997. Polar Biol. 1998;16:321–330. [Google Scholar]
- Small C. Their duties and performance in reducing incidental mortality of albatrosses. BirdLife International Cambridge; Cambridge, UK: 2005. Regional fisheries management organisations. [Google Scholar]
- Smith T.D. Cambridge University Press; Cambridge, UK; New York, NY: 1994. Scaling fisheries: the science of measuring the effect of fishing 1855–1955. [Google Scholar]
- Stagi A, Vaz-Ferreira R, Marin Y, Joseph L. The conservation of albatrosses in Uruguayan waters. In: Robertson G, Gales R, editors. Albatross—biology and conservation. Surrey Beatty & Sons; Chipping Norton, Australia: 1998. pp. 220–224. [Google Scholar]
- Testa J, Siniff D, Ross M, Winter J. Weddell seal–Antarctic cod interactions in McMurdo Sound, Antarctica. In: Siegfried W.R, Condy P.R, Laws R.M, editors. Antarctic nutrient cycles and food webs. Springer; Berlin, Germany: 1985. pp. 561–565. [Google Scholar]
- Trathan P.N, Everson I, Miller D.G.M, Watkins J.L, Murphy E.J. Krill biomass in the Atlantic. Nature. 1995;373:201–202. doi:10.1038/373201b0 [Google Scholar]
- Trathan P.N, Brierley A.S, Brandon M.A, Bone D.G, Goss C, Grant S.A, Murphy E.J, Watkins J.L. Oceanographic variability and changes in Antarctic krill (Euphausia superba) abundance at South Georgia. Fish. Oceanogr. 2003;12:569–583. doi:10.1046/j.1365-2419.2003.00268.x [Google Scholar]
- United Nations, 2002 Report of the world summit on sustainable development, Johannesburg, South Africa, 26 August–4 September 2002.
- Watt K.E.F. The choice and solution of mathematical models for predicting and maximizing the yield of a fishery. J. Fish. Res. Board Can. 1956;13:613–645. [Google Scholar]
- Weimerskirch H. Foraging strategies of Indian Ocean albatrosses and their relationships with fisheries. In: Robertson G, Gales R, editors. Albatross—biology and conservation. Surrey Beatty & Sons; Chipping Norton, Australia: 1998. pp. 168–179. [Google Scholar]
- Williams R, de la Mare W.K. Fish distribution and biomass in the Heard Island zone (Division 58.5.2) CCAMLR Sci. 1995;2:1–20. [Google Scholar]
- Worby A.P, Comiso J.C. Studies of the Antarctic sea-ice edge and ice extent from satellite and ship observations. Remote Sens. Environ. 2004;92:98–111. doi:10.1016/j.rse.2004.05.007 [Google Scholar]
- Yukhov V.L. The range of fish of the genus Dissotichus (Nototheniidae) in Antarctic waters of the Indian Ocean. Vopr. Ikthiol. 1970;12:384–385. [Transl. as J. Ichthyol. 12, 346–347.] [Google Scholar]
- Yukhov V.L. Some data on the feeding of sperm whales in the high latitudes of Antarctica. Rybn. Khoz. 1971;39:54–59. [In Russian.] [Google Scholar]