Abstract
Percutaneous vertebroplasty is widely discussed in the management of osteoporotic spinal compression fracture, but few reports are available concerning salvage procedures after failure of this technique. We studied 22 percutaneous vertebroplasty patients who required revision surgery upon presentation of new symptoms postoperatively. The indications for revision surgery included recurrent intractable back pain with no response to medical treatment, infectious spondylitis, cement leakage with neurologic deficit, and cement dislodgement and/or fragmentation. Five patients underwent repeated percutaneous vertebroplasty of the initially cemented vertebrae. Seventeen patients underwent anterior, posterior, or combined anterior and posterior surgery. Four patients required a third surgical procedure because of poor augmentation with cement, subsidence of the anterior bone graft, or pullout of the instrumentation. Finally, four (18%) patients underwent repeat vertebroplasty, two (9%) patients underwent anterior surgery only, one (5%) patient underwent posterior surgery only, and 15 (68%) patients underwent combined anterior and posterior surgery; all but one regained ambulatory status equivalent to that prior to surgery. In conclusion, percutaneous vertebroplasty is a simple and effective, but not risk- or complication-free procedure for the treatment of osteoporotic spinal compression fracture. The spine surgeon should be familiar with varied approaches and techniques for revision surgery. Combined anterior and posterior surgery seems to be the most secure salvage method to treat severely osteoporotic patients in whom percutaneous vertebroplasty initially failed.
Keywords: Revision surgery, Percutaneous vertebroplasty, Osteoporotic spinal compression fracture, Bone cement
Introduction
Percutaneous vertebroplasty is a minimally invasive therapeutic procedure initially described by Galibert and Deramond in 1987 [13]. The technique involves a posterior transpedicular approach using a spinal needle under fluoroscopic guidance and then, injection of polymethylmethacrylate (PMMA) cement into the collapsed vertebral body [12]. Thereafter, the procedure became popular for the management of pain associated with benign compression fractures, multiple myelomas, lymphomas, vertebral metastatic lesions, and hemangiomas [24, 29]. Outstanding results are achieved using percutaneous vertebroplasty for the treatment of osteoporotic spinal compression fractures [2, 6, 18, 20, 38]. Nonetheless, acute complications are reported, such as bleeding at the puncture site, local infection, leakage of cement into the spinal canal, adjacent discs, paravertebral soft tissues, or perivertebral venous system, and pulmonary embolism. Delayed sequelae are also reported, such as adjacent vertebral fracture, cement dislodgement or fragmentation, and pyogenic spondylitis [1, 7, 25, 27, 33, 34]. Most of these complications can be resolved by conservative treatment, but in some circumstances, surgical intervention is indicated. To our knowledge, few reports are available concerning salvage procedures after the failure of percutaneous vertebroplasty. We present our clinical experience and offer a strategy for patients needing revision surgery after complications and failure of vertebroplasties.
Materials and methods
The study population comprised 1,523 consecutive patients and two referred patients who underwent percutaneous vertebroplasty for the treatment of osteoporotic spinal compression fractures from 2000 through 2006 at our institutions. One level vertebroplasty was performed in 1,137 patients, two levels in 263 patients, three levels in 91 patients, four levels in 27 patients, five levels in five patients, and six levels in two patients. All surgeries were performed by the same team, who obtained a detailed and standardized medical history. Preoperative and postoperative clinical course and research data were gathered retrospectively from the case notes and assembled in a database by one of the authors.
Inclusion criteria were presentation of new symptoms caused by the same level after percutaneous vertebroplasty and subsequent revision surgery. The indications for revision surgery included recurrent intractable back pain of the cemented vertebral body with no response to medical treatment, pyogenic spondylitis, cement leakage with neurologic deficit, and cement dislodgement or fragmentation. Patients who underwent operations for adjacent or new fractures were excluded.
After a comprehensive review of the medical records, 22 patients who underwent revision surgery for failure of vertebroplasty were enrolled in the study. There were three men and 19 women with a mean age of 69.2 years (range 57–85 years). Of the 22 patients, 20 patients had histories of recurrent back pain, with a short pain relief period after first-time vertebroplasty, while two patients had neurological deficits after first-time vertebroplasty and were referred from the division hospitals. All patients underwent diagnostic radiography and magnetic resonance imaging (MRI). Imaging showed residual vacuum cleft or poor augmentation in seven patients, pyogenic spondylitis in eight patients, and cement dislodgement and/or fragmentation in five patients.
Five patients with residual vacuum cleft or poor augmentation underwent repeat vertebroplasty through the contralateral pedicle. Two patients with poor augmentation and progressive kyphosis and instability underwent posterior instrumentation and fusion. Three patients underwent anterior interbody fusion with iliac autografting and instrumentation for cement dislodgement or fragmentation repair. The remaining 12 patients with infections and cement problems underwent combined anterior and posterior surgery. In severely osteoporotic patients, pedicle screw augmentation with PMMA was used to improve the initial fixation and fatigue strength of the instrumentation.
Most dislodged or fragmented cement was removed through the anterior approach. Pyogenic spondylitis with infected cement could also be easily retrieved because of the non-interdigital and liquiform interface between the cement and vertebral bone. After extensive debridement, anterior reconstruction with autogenous or allogeneic bone grafting was performed. Additional posterior instrumentation and stabilization was usually secured before or after anterior surgery according to the patient’s situation.
Between the two patients with neurological deficits, laminectomies had been performed at transferring hospitals. Because extremely unstable and postlaminectomy kyphotic deformities progress after destruction of posterior complexes, posterior stabilization with instrumentation was performed first. Corpectomy and retrieval of encroached cement in the spinal canal, followed by bone graft reconstruction was then completed through the anterior approach.
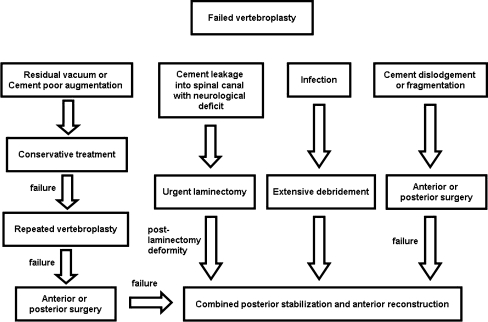
Table 1 shows patient demographic data. Figure 1 presents the strategy for approaching patients who sustained failure of vertebroplasty for osteoporotic compression fractures.
Table 1.
Patient demographic data
| Case no | Age (years) | Gender | Level of vertebroplasty | Complication | Revision surgery | Third surgery |
|---|---|---|---|---|---|---|
| 1 | 78 | Female | T12 | Residual vacuum | Repeat vertebroplasty | |
| 2 | 71 | Female | L1 | Cement poor augmentation | Posterior surgery | Anterior and posterior surgery |
| 3 | 67 | Female | L3 | Cement fragmentation | Anterior surgery | |
| 4 | 61 | Female | T12 | Infection | Anterior and posterior surgery | |
| 5 | 62 | Male | L1 | Infection | Anterior and posterior surgery | |
| 6 | 65 | Female | L2 | Cement dislodgement | Anterior surgery | Anterior and posterior surgery |
| 7 | 68 | Female | T12 | Infection | Anterior and posterior surgery | |
| 8 | 80 | Female | T12 and L1 | Neurological deficit | Anterior and posterior surgery | |
| 9 | 63 | Female | L1 | Infection | Anterior and posterior surgery | |
| 10 | 77 | Female | L1 and L2 | Cement dislodgement | Anterior and posterior surgery | |
| 11 | 66 | Female | L1 | Residual vacuum | Repeat vertebroplasty | |
| 12 | 72 | Female | T12 | Residual vacuum | Repeat vertebroplasty | Anterior and posterior surgery |
| 13 | 71 | Female | L1 | Infection | Anterior and posterior surgery | |
| 14 | 57 | Female | L1 | Cement fragmentation | Anterior surgery | |
| 15 | 69 | Female | T12 | Neurological deficit | Anterior and posterior surgery | |
| 16 | 69 | Female | L1 | Cement poor augmentation | Posterior surgery | Posterior surgery |
| 17 | 60 | Female | L2 | Infection | Anterior and posterior surgery | |
| 18 | 72 | Male | L1 | Infection | Anterior and posterior surgery | |
| 19 | 85 | Female | L3 | Residual vacuum | Repeat vertebroplasty | |
| 20 | 62 | Female | L1 | Residual vacuum | Repeat vertebroplasty | |
| 21 | 69 | Male | T12 | Cement dislodgement | Anterior and posterior surgery | |
| 22 | 78 | Female | T12 | Infection | Anterior and posterior surgery |
Fig. 1.
A strategic approach to patients needing revision surgery for failure of vertebroplasty
Results
Of the five patients who underwent repeat vertebroplasty, four patients had immediate pain relief with satisfactory outcomes. Two patients with posterior instrumentation and fusion had recurrent back pain after short periods of pain relief. Of the three patients who underwent anterior surgery, two had acceptable lordotic alignment with pain relief by protection of orthoses. Patients with combined anterior and posterior surgery all recovered smoothly (Figs. 2, 3).
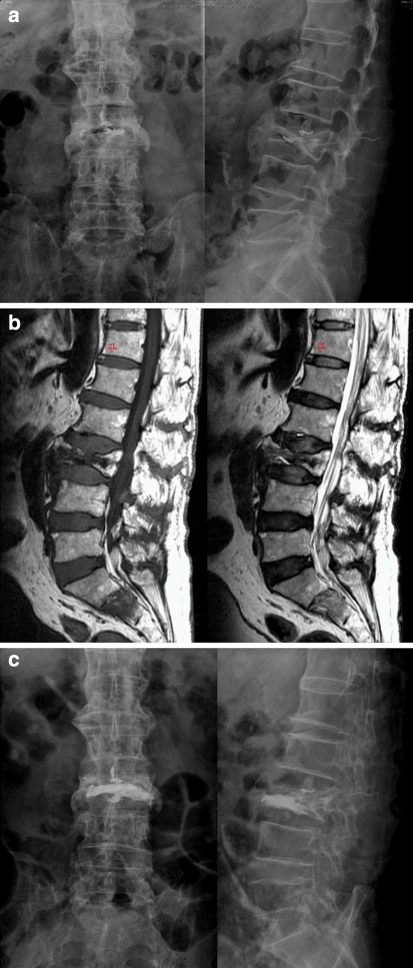
Fig. 2.
An 85-year-old woman sustained recurrent back pain after a short period of pain relief. a Radiography showed a L3 osteoporotic compression fracture after vertebroplasty with bone cement. b Sagittal MRI demonstrated residual vacuum in the L3 vertebral body. c Postoperative radiography revealed good augmentation of the L3 vertebral body after repeat vertebroplasty
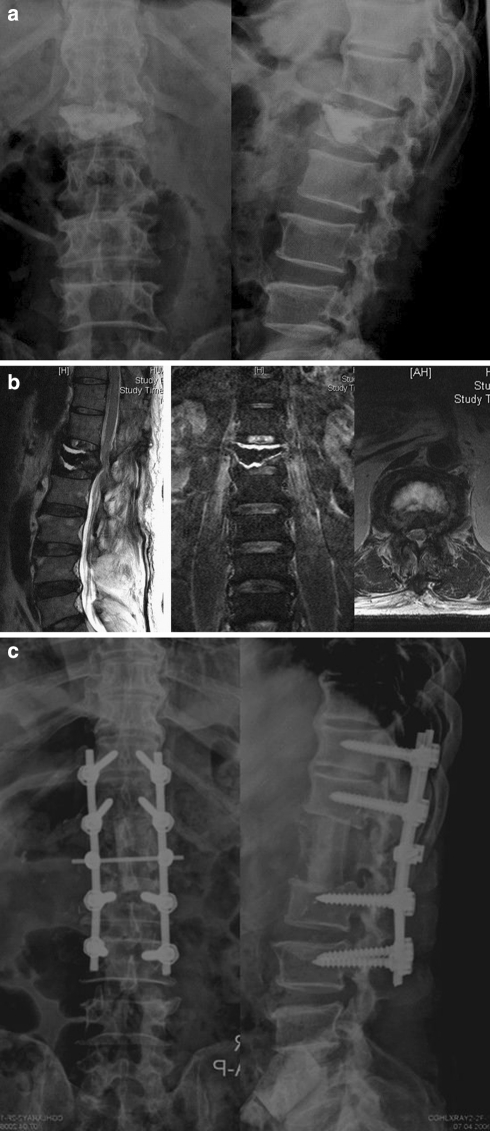
Fig. 3.
A 62-year-old man was diagnosed as infectious spondylitis clinically in conjunction with high values of erythrocyte sedimentation rate and C-reactive protein tests after vertebroplasty. a Radiography showed radiolucent lines between the bone and cement interface and L1 superior endplate erosion. b Sagittal and axial MRI demonstrated fluid (abscesses) surrounding the bone cement. c Anterior extensive debridement and reconstruction using a strut autograft, followed by posterior instrumentation was performed to control infection and restore spinal alignment and stability
Four patients required a third surgical procedure because of poor augmentation of the PMMA in one patient, subsidence of the anterior bone graft in one patient, or pullout and displacement of the instrumentation in two patients. Of these four patients, one patient who had undergone repeated vertebroplasty underwent combined anterior and posterior surgery. One patient who had undergone anterior surgery only required posterior instrumentation for secure fixation and stabilization to prevent progressive kyphotic deformity and further subsidence of anterior bone grafting. Of the remaining two patients who had undergone posterior surgery only, one underwent anterior fusion surgery, and the other underwent extension of posterior fixation due to a history of several other abdominal surgeries.
No major surgery related complications occurred. One superficial wound infection responded to early management. One patient experienced postoperative peptic ulcer, which recovered smoothly after upper gastrointestinal panendoscopic treatment and proton pump inhibitors (pantoprazole) medication. Most patients were discharged from the hospital within two weeks except the patients with infections, who required longer hospitalization because of at least a six-week course of parenteral antibiotics therapy.
In the end, four (18%) patients underwent repeated vertebroplasty, two (9%) patients underwent anterior surgery only, one (5%) patient underwent posterior surgery only, and 15 (68%) patients underwent combined anterior and posterior surgery; all patients except one regained their prior ambulatory status. Only one patient had a neurological disorder, although urgent decompression surgery was done for cement leakage into spinal canal.
Discussion
Management of symptomatic osteoporotic spinal compression fractures using percutaneous vertebroplasty with PMMA gained widespread use because of its simplicity and effectiveness. Although percutaneous vertebroplasty is considered a minimally invasive procedure, several dreadful complications are reported [1, 7, 25, 27, 33, 34]. We retrospectively reviewed the medical records of patients who underwent vertebroplasty in our institutions and provided the different surgical methods used for patients who required revision surgery to deal with various complications after vertebroplasty failure.
Percutaneous vertebroplasty with bipedicular cement injection is advocated to treat osteoporotic compression fractures in most circumstances [3, 28, 32]. In patients with vertebral cleft, a unipedicle approach is used because the bone cement generally can distribute into the entire cleft. Using the unipedicle approach saves time and reduces costs and is considered less traumatic for patients [11, 32]. This practice is widely used in our institutions. When vertebroplasty fails, repeat vertebroplasty can be considered as the first choice surgery, especially for patients in whom the unipedicle approach was initially used. Bone cement is introduced via a virgin pedicle to repair the fracture nonunion and fill the residual space. For patients in whom the bipedicle approach was initially used, repeat vertebroplasty is more difficult. Because a normal-sized pedicle is relatively larger than the diameter of the spinal needle, repeat vertebroplasty can still be performed by experienced spine surgeons. Otherwise, a parapedicular approach can be used if the pedicles are no longer accessible. We always consider repeat vertebroplasty to treat patients with failed initial vertebroplasty because of its characteristic simplicity and effectiveness. However, not all failed vertebroplasties can be resolved by repeat vertebroplasty. Therefore, MRI should be performed for each patient to determine if repeated injection of bone cement will be effective.
The reported incidence of cement extrusion is common, including leakage into the vertebral venous system, adjacent discs, lateral or anterior vertebrae, and leakage leading to pulmonary embolism [1, 7, 23, 36]. Most authors have described that cement extrusion presented as asymptomatic in the majority of cases and could be treated conservatively. The most serious complication is cement leakage into the spinal canal and compression of the neural elements that results in neurological injury necessitating urgent surgical intervention. Until this review, a total of 18 patients receiving either vertebroplasty or kyphoplasty with neurological complications have been reported [14, 27, 30]. Sixteen of 18 patients underwent a revision operative procedure, through anterior, posterior, or combined approaches. Two patients were treated with observation because the underlying medical diseases contraindicated surgical intervention.
The most common salvage procedure is laminectomy for urgent spinal cord decompression. The hard bone cement can compress and even adhere to the dura, but it is not necessary to remove all of the cement due to the high risk of producing additional neurological complications. The combined anterior and posterior approach is usually mandatory for postlaminectomy reconstruction because of the anterior lesion, posterior complex destruction after laminectomy, and the underlying osteoporosis in this group of patients. Two patients with neurological deficits due to cement leakage were included in our study. Although urgent laminectomy for decompression had been performed at the original hospitals, one patient still had low-grade motor dysfunction below the level of the injury. Combined posterior stabilization and anterior reconstruction allowed these patients early mobilization, rehabilitation, and family care regardless of their neurological status, thereby decreasing overall morbidity and mortality.
Infection of vertebrae resulted from vertebroplasty with cement is reported, but rarely. Olmos et al. [26] suggested postvertebroplasty infection is predisposed by external factors, such as iatrogenic inoculation due to poor sterile procedures, inappropriate use of antibiotic prophylaxis, or by internal factors, such as immunosuppression by steroid or chemotherapy, contiguous spread from neighboring infected sources, or concomitant systemic infections. Vats and McKiernan [34] found the infection in a vertebroplasty site can present either early or late after the procedure. Skin colonizing and hospital-acquired organisms cause early infections, while organisms for which the patients have a predisposition due to their comorbidities cause late infections. The proposed mechanism of infection at the vertebroplasty site is the dynamic nature of vertebral fractures with clefts [21, 22, 37]. Clefted vertebral fractures can be lined by fibrocartilage forming intervertebral pseudoarthroses, providing a potential space for infection. Intervertebral deep infection is very difficult to eradicate due to its poor vascularity and hence inaccessibility to systemic antibiotics. Therefore, anterior extensive debridement and associated autogenous bone grafting, combined with additional anterior or posterior instrumentation is necessary for infection control and spinal stabilization.
Bone cement dislodgement or fragmentation is another complication that occurred in our clinical practice. In order to minimize the risk of direct cement extrusion into the spinal canal through cortical defects or enlarged venous sinusoid, most authors who are experienced in performing vertebroplasty recommend that the PMMA be injected in a viscous or partially polymerized consistency through a large-bore needle [9]. In a biomechanical study, Bohner et al. [8] used a theoretical model to determine the distribution of a PMMA cement after its injection into a porous structure, and then compared with experimental results. The model predicted that the extravasation risk was decreased when the cement viscosity, the bone pore size, the bone permeability and the bone porosity were increased, and when the diameter of the extravasation path and the viscosity of the marrow were decreased. The experimental results demonstrated that the best way to decrease the risk of extravasation is to increase the cement viscosity. Baroud and Steffen [5] designed a new cannula with a larger internal diameter in the proximal section. Both the experimental and analytical findings confirmed the redesigned cannula reduces the delivery pressure significantly and has the potential to improve vertebroplasty.
However, injection of viscous cement with low pressure decreases the penetration of the cement into the microstructure of cancellous bone [4]. Krause et al. [16] reported that doughy cement on an unclean surface resulted in a very low interface strength compared to a low-viscosity cement made to penetrate a cleaned bone surface. Additionally, thermal necrosis of the surrounding tissue caused by the high polymerization temperature and the nonunion of fibrous tissue on the surface of the fractured cancellous bone intercept the interdigitation of PMMA to achieve a mechanical interlock. In a case control study, Huang et al. [15] found thermal osteonecrosis, foreign body reaction, and fibrotic wall formation in the cement–bone interface; the lack of revascularization and repair process within the vertebral bodies are the characteristic histopathologic findings of retrieved specimens from vertebroplasty with PMMA cement. The authors concluded these interface problems were associated with subsequent failure of PMMA vertebroplasty.
Several patients in the current study had delayed posttraumatic vertebral collapse with an intravertebral vacuum cleft, which is considered as pathognomonic for avascular necrosis [17, 19]. Injection of PMMA into a cystic cavity is expected to have far less interdigitation with the surrounding bone than would injection into partially intact trabecular bone. The above-mentioned reasons make PMMA cement in vertebroplasty merely a space occupying material without mechanical interlock and biocompatibility and, therefore, increase the potential for dislodgment or fragmentation. Anterolateral displacement of the bone cement and associated micro-motion would result in breakdown of the anterior and middle column of the vertebral body, leading further kyphotic deformity. Thus, combined anterior and posterior surgery is still the most secure method for treating this kind of complication. Otherwise, augmentation of pedicle screws fixation with various bone cements is recommended to provide initial and fatigue strength of instrumentation among severely osteoporotic patients [10, 31, 35].
No major perioperative and postoperative complications were observed in this study. However, advancing age increased the surgical and anesthetic risks for these combined interventions because of an increase in the number of medical comorbidities in this elderly patient population. Thus, a detailed preoperative survey and preparation, close cooperative surgical teamwork, and intensive postoperative care is mandatory to avoid undesired complications.
In conclusion, percutaneous vertebroplasty is a safe and effective, but not risk- or complication-free operation for the treatment of osteoporotic spinal compression fractures. In addition to careful preoperative diagnostic surveys, the performance of vertebroplasty requires some technical learning and experience. Once the catastrophic complications take place, either acute or delayed, the spine surgeon should be familiar with varied approaches and techniques for revision surgery. What would be the most secure salvage method in the treatment of failed vertebroplasty among severely osteoporotic patients? The results of this study imply that the appropriate targeted treatment taking into account the biomechanics of the spine can lead to a good outcome.
Footnotes
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Abdul-Jalil Y, Bartels J, Alberti O, Becker R. Delayed presentation of pulmonary polymethylmethacrylate emboli after percutaneous vertebroplasty. Spine. 2007;32(20):E589–E593. doi: 10.1097/BRS.0b013e31814b84ba. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez L, Alcaraz M, Perez-Higueras A, Granizo JJ, Miguel I, Rossi RE, Quinones D. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine. 2006;31(10):1113–1118. doi: 10.1097/01.brs.0000216487.97965.38. [DOI] [PubMed] [Google Scholar]
- 3.Amar AP, Larsen DW, Esnaashari N, Albuquerque FC, Lavine SD, Teitelbaum GP. Percutaneous transpedicular polymethylmethacrylate vertebroplasty for the treatment of spinal compression fractures. Neurosurgery. 2001;49(5):1105–1114. doi: 10.1097/00006123-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Askew MJ, Steege JW, Lewis JL, Ranieri JR, Wixson RL. Effect of cement pressure and bone strength on polymethylmethacrylate fixation. J Orthop Res. 1984;1(4):412–420. doi: 10.1002/jor.1100010410. [DOI] [PubMed] [Google Scholar]
- 5.Baroud G, Steffen T. A new cannula to ease cement injection during vertebroplasty. Eur Spine J. 2005;14:474–479. doi: 10.1007/s00586-004-0822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine. 2000;25(8):923–928. doi: 10.1097/00007632-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Baumann C, Fuchs H, Kiwit J, Westphalen K, Hierholzer J. Complications in percutaneous vertebroplasty associated with puncture or cement leakage. Cardiovasc Intervent Radiol. 2007;30(2):161–168. doi: 10.1007/s00270-006-0133-5. [DOI] [PubMed] [Google Scholar]
- 8.Bohner M, Gasser B, Baroud G, Heini P. Theoretical and experimental model to describe the injection of a polymethylmethacrylate cement into a porous structure. Biomaterials. 2003;24:2721–2730. doi: 10.1016/S0142-9612(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 9.Bostrom MP, Lane JM. Future directions. Augmentation of osteoporotic vertebral bodies. Spine. 1997;22(24):S38–S42. doi: 10.1097/00007632-199712151-00007. [DOI] [PubMed] [Google Scholar]
- 10.Burval DJ, McLain RF, Milks R, Inceoglu S. Primary pedicle screw augmentation in osteoporotic lumbar vertebrae: biomechanical analysis of pedicle fixation strength. Spine. 2007;32(10):1077–1083. doi: 10.1097/01.brs.0000261566.38422.40. [DOI] [PubMed] [Google Scholar]
- 11.Chen LH, Lai PL, Chen WJ. Unipedicle percutaneous vertebroplasty for spinal intraosseous vacuum cleft. Clin Orthop Relat Res. 2005;435:148–153. doi: 10.1097/01.blo.0000155346.12405.70. [DOI] [PubMed] [Google Scholar]
- 12.Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36(3):533–546. doi: 10.1016/S0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 13.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. [PubMed] [Google Scholar]
- 14.Harrington KD. Major neurological complications following percutaneous vertebroplasty with polymethylmethacrylate: a case report. J Bone Joint Surg Am. 2001;83(7):1070–1073. doi: 10.2106/00004623-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Huang KY, Yan JJ, Lin RM. Histopathologic findings of retrieved specimens of vertebroplasty with polymethylmethacrylate cement: case control study. Spine. 2005;30(19):E585–E588. doi: 10.1097/01.brs.0000182226.56498.55. [DOI] [PubMed] [Google Scholar]
- 16.Krause WR, Krug W, Miller J. Strength of the cement–bone interface. Clin Orthop Relat Res. 1982;163:290–299. [PubMed] [Google Scholar]
- 17.Lane JI, Maus TP, Wald JT, Thielen KR, Bobra S, Luetmer PH. Intravertebral clefts opacified during vertebroplasty: pathogenesis, technical implications, and prognostic significance. AJNR Am J Neuroradiol. 2002;23(10):1642–1646. [PMC free article] [PubMed] [Google Scholar]
- 18.Layton KF, Thielen KR, Koch CA, Luetmer PH, Lane JI, Wald JT, Kallmes DF. Vertebroplasty, first 1000 levels of a single center: evaluation of the outcomes and complications. AJNR Am J Neuroradiol. 2007;28(4):683–689. [PMC free article] [PubMed] [Google Scholar]
- 19.Maldague BE, Noel HM, Malghem JJ. The intravertebral vacuum cleft: a sign of ischemic vertebral collapse. Radiology. 1978;129(1):23–29. doi: 10.1148/129.1.23. [DOI] [PubMed] [Google Scholar]
- 20.Martin JB, Jean B, Sugiu K, San Millan Ruiz D, Piotin M, Murphy K, Rufenacht B, Muster M, Rufenacht DA. Vertebroplasty: clinical experience and follow-up results. Bone. 1999;25(2):S11–S15. doi: 10.1016/S8756-3282(99)00126-X. [DOI] [PubMed] [Google Scholar]
- 21.McKiernan F, Faciszewski T. Intravertebral clefts in osteoporotic vertebral compression fractures. Arthritis Rheum. 2003;48(5):1414–1419. doi: 10.1002/art.10984. [DOI] [PubMed] [Google Scholar]
- 22.McKiernan F, Jensen R, Faciszewski T. The dynamic mobility of vertebral compression fractures. J Bone Miner Res. 2003;18(1):24–29. doi: 10.1359/jbmr.2003.18.1.24. [DOI] [PubMed] [Google Scholar]
- 23.Mirovsky Y, Anekstein Y, Shalmon E, Blankstein A, Peer A. Intradiscal cement leak following percutaneous vertebroplasty. Spine. 2006;31(10):1120–1124. doi: 10.1097/01.brs.0000216461.48751.d6. [DOI] [PubMed] [Google Scholar]
- 24.Murphy KJ, Deramond H. Percutaneous vertebroplasty in benign and malignant disease. Neuroimaging Clin N Am. 2000;10(3):535–545. [PubMed] [Google Scholar]
- 25.Nussbaum DA, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol. 2004;15(11):1185–1192. doi: 10.1097/01.RVI.0000144757.14780.E0. [DOI] [PubMed] [Google Scholar]
- 26.Olmos MA, Gonzalez AS, Clemente JD, Tome CV. Infected vertebroplasty due to uncommon bacteria solved surgically: a rare and threatening life complication of a common procedure. Report of a case and a review of the literature. Spine. 2006;31(20):E770–E773. doi: 10.1097/01.brs.0000240202.91336.99. [DOI] [PubMed] [Google Scholar]
- 27.Patel AA, Vaccaro AR, Martyak GG, Harrop JS, Albert TJ, Ludwig SC, Youssef JA, Gelb DE, Mathews HH, Chapman JR, Chung EH, Grabowski G, Kuklo TR, Hilibrand AS, Anderson DG. Neurologic deficit following percutaneous vertebral stabilization. Spine. 2007;32(16):1728–1734. doi: 10.1097/BRS.0b013e3180dc9c36. [DOI] [PubMed] [Google Scholar]
- 28.Peters KR, Guiot BH, Martin PA, Fessler RG. Vertebroplasty for osteoporotic compression fractures: current practice and evolving techniques. Neurosurgery. 2002;51(5):S96–S103. [PubMed] [Google Scholar]
- 29.Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J. 2006;15(12):1749–1758. doi: 10.1007/s00586-006-0159-z. [DOI] [PubMed] [Google Scholar]
- 30.Ratliff J, Nguyen T, Heiss J. Root and spinal cord compression from methylmethacrylate vertebroplasty. Spine. 2001;26(13):E300–E302. doi: 10.1097/00007632-200107010-00021. [DOI] [PubMed] [Google Scholar]
- 31.Renner SM, Lim TH, Kim WJ, Katolik L, An HS, Andersson GB. Augmentation of pedicle screw fixation strength using an injectable calcium phosphate cement as a function of injection timing and method. Spine. 2004;29(11):E212–E216. doi: 10.1097/00007632-200406010-00020. [DOI] [PubMed] [Google Scholar]
- 32.Tohmeh AG, Mathis JM, Fenton DC, Levine AM, Belkoff SM. Biomechanical efficacy of unipedicular versus bipedicular vertebroplasty for the management of osteoporotic compression fractures. Spine. 1999;24(17):1772–1776. doi: 10.1097/00007632-199909010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Tsai TT, Chen WJ, Lai PL, Chen LH, Niu CC, Fu TS, Wong CB. Polymethylmethacrylate cement dislodgment following percutaneous vertebroplasty: a case report. Spine. 2003;28(22):E457–E460. doi: 10.1097/01.BRS.0000096668.54378.25. [DOI] [PubMed] [Google Scholar]
- 34.Vats HS, McKiernan FE. Infected vertebroplasty: case report and review of literature. Spine. 2006;31(22):E859–E862. doi: 10.1097/01.brs.0000240665.56414.88. [DOI] [PubMed] [Google Scholar]
- 35.Wuisman PI, Dijk M, Staal H, Royen BJ. Augmentation of (pedicle) screws with calcium apatite cement in patients with severe progressive osteoporotic spinal deformities: an innovative technique. Eur Spine J. 2000;9(6):528–533. doi: 10.1007/s005860000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br. 2003;85(1):83–89. doi: 10.1302/0301-620X.85B1.13026. [DOI] [PubMed] [Google Scholar]
- 37.Yu SW, Chen WJ, Lin WC, Chen YJ, Tu YK. Serious pyogenic spondylitis following vertebroplasty—a case report. Spine. 2004;29(10):E209–E211. doi: 10.1097/00007632-200405150-00023. [DOI] [PubMed] [Google Scholar]
- 38.Yu SW, Lee PC, Ma CH, Chuang TY, Chen YJ. Vertebroplasty for the treatment of osteoporotic compression spinal fracture: comparison of remedial action at different stages of injury. J Trauma. 2004;56(3):629–632. doi: 10.1097/01.TA.0000053471.73514.2E. [DOI] [PubMed] [Google Scholar]