Abstract
A possible association between congenital scoliosis and low mental status has been recognized, but there are no reports describing the mental status or cerebral metabolism in patients with congenital scoliosis in detail. We investigated the mental status using a mini-mental status exam as well as the cerebral glucose metabolism using F-18 fluorodeoxyglucose brain positron emission tomography in 12 patients with congenital scoliosis and compared them with those of 14 age-matched patients with adolescent idiopathic scoliosis. The mean mini-mental status exam score in the congenital scoliosis group was significantly lower than that in the adolescent idiopathic scoliosis group. Group analysis found that various brain areas of patients with congenital scoliosis showed glucose hypometabolisms in the left prefrontal cortex (Brodmann area 10), right orbitofrontal cortex (Brodmann area 11), left dorsolateral prefrontal cortex (Brodmann area 9), left anterior cingulate gyrus (Brodmann area 24) and pulvinar of the left thalamus. From this study, we could find the metabolic abnormalities of brain in patients with congenital scoliosis and suggest the possible role of voxel-based analysis of brain fluorodeoxyglucose positron emission tomography.
Keywords: Congenital scoliosis, Mental status, Cerebral glucose metabolism, F-18 fluorodeoxyglucose, Positron emission tomography
Introduction
Congenital scoliosis (CS) can be associated with congenital abnormalities that can affect multiple organ systems or lead to acquired medical problems and morbidity as the child grows or while the spinal deformity is being treated. Common associated conditions of CS are cardiac abnormalities, urological abnormalities and spinal cord abnormalities. CS is associated with intraspinal anomalies in approximately 20% of patients and should be examined using magnetic resonance imaging [6, 11, 26]. Bradford et al. [3] reported that 38% of the 42 patients in their study had an intraspinal anomaly using magnetic resonance imaging. Using intravenous urography, MacEwen et al. [18] identified genitourinary abnormalities in 20% of patients with CS. The precise incidence of congenital heart disease associated with CS has not been reported. Basu et al. [2] found congenital heart disease in 26% of patients with a congenital spinal deformity.
Congenital scoliosis might be associated with a low mental status. Mental retardation is often observed in patients of CS associated with syndrome [16]. Although a possible association between CS and a low mental status has been recognized, we are not aware of any report describing the mental status or cerebral metabolism in patients with CS in detail. Therefore, we investigated the mental status and cerebral glucose metabolism in patients with CS and compared the results with those from patients with adolescent idiopathic scoliosis (AIS).
Materials and methods
Subjects
We studied 12 CS and 14 AIS patients who had been admitted to our department for the surgical correction of scoliosis. The Cobb angles of the curves were measured on plain radiographs and the Risser signs were determined. All patients aged between 11 and 16 years presenting to the scoliosis were asked to voluntarily examine a mini-mental status examination [13] and an analysis of their cerebral glucose metabolism. Fourteen AIS patients were used as the control group because the cerebral glucose metabolism was reported to be similar to normal healthy controls [21]. All subjects were right-handed and had at least 5 years of education.
All subjects were also examined clinically to rule out any hidden metabolic disease, chromosomal abnormality, or psychiatric disease that could affect the cerebral glucose metabolism before entering the study. In addition, brain MR imaging studies were performed in all subjects before entering the study in order to rule out any organic brain lesion. Subjects with a history of neuromuscular disease, endocrine disease, skeletal dysplasia, connective tissue abnormalities, organic brain disease, psychiatric disease or other systemic congenital abnormality were excluded from the study. All subjects and their parents provided informed consent before the examination and measurements. The study was approved by the Clinical Research Ethics Committee of the university and the hospital.
F-18 FDG brain PET
Brain positron emission tomography (PET) scans of a single frame of 15 min were acquired starting 60 min after the intravenous injection of 370 MBq (10 mCi) F-18 fluorodeoxyglucose (FDG) per 1.7 m2 of body surface area using a Gemini PET/CT scanner (Philips, Milpitas, CA, USA). It was performed with the subjects under resting conditions with their eyes closed and ears unplugged, comfortably lying in a darkened and quiet room. Subjects were fasted for at least 8 h before PET imaging. The PET images were reconstructed using 3D RAMLA (2 repetition, 0.006 relaxation parameter) and displayed in a 128 × 128 matrix (pixel size = 2 × 2 mm, with a slice thickness of 2 mm). Attenuation correction was performed with a uniform attenuation coefficient (μ = 0.096 cm−1). In-plane and axial resolution of the scanner were 4.2 and 5.6 mm full width at half maximum (FWHM), respectively.
SPM analysis of regional cerebral glucose metabolism
Spatial preprocessing and statistical analysis were performed using the SPM2 implemented in Matlab 5.3 (The MathWorks, Inc., Natick, MA, USA). All the reconstructed F-18-FDG brain PET images were spatially normalized into Montreal Neurological Institute (MNI, McGill University, Montreal, Que., Canada) standard templates by an affine transformation (12 parameters for rigid transformations) and a non-linear transformation, then smoothed with a FWHM 8-mm Gaussian kernel to increase the signal-to-noise ratio and to account for subtle variations in anatomic structures. To remove the effects of the difference in the overall counts, the voxel counts were normalized to the mean voxel count of the gray matter in each PET image using proportional scaling. Images of the patients with CS were compared with those with AIS in a voxel-wise manner using SPM2 for between-group analysis (P < 0.001, uncorrected; extent threshold, k = 50). The clusters that passed this threshold were considered significant at P < 0.05 corrected for multiple comparisons. The Talairach brain coordinates were estimated from a non-linear transformation from MNI space to Talairach space (Talairach Daemon Client, Version 1.1, Research Imaging Center, University of Texas Health Science Center at San Antonio). The differences between the CS and AIS patients were examined using an extent threshold level of 50 voxels with a P value < 0.001 to illustrate the group differences in statistical voxel-based analysis, as well as for illustrating the result of the registration between CS and AIS. For the visualization of the t-score statistics (SPM{t} map), significant voxels were projected onto the three-dimensional rendered brain or a standard high-resolution MR image template provided by SPM2, thereby allowing anatomic identification. In addition, linear correlations of the regional brain glucose metabolism with the MMSE scores were investigated using ‘single subject: covariates only’ analysis and simple regression analysis, which are statistical models in SPM2 based on the general linear model. We looked for all voxels where the regional cerebral glucose metabolism was significantly and positively correlated with the MMSE scores. After group analysis, each F-18 FDG brain PET images of the CS patient was compared with those of the AIS patients to investigate individual brain glucose metabolism abnormalities using t statistics in SPM2.
Statistical analysis
Statistical analysis was performed with SPSS 11.5 software for Windows (SPSS, Chicago, IL, USA). Data were expressed by mean ± standard deviation. Groups were compared by using the Mann–Whitney U test. A P ≤ 0.05 was regarded as statistically significant.
Results
Patients characteristics
Table 1 shows the details of the subjects. The CS group consisted of 12 patients (6 girls and 6 boys) with a mean age of 13.0 ± 1.4 years. The mean Cobb’s angle of the major curve in the CS group was 50.2 ± 12.7 and the mean MMSE score was 22.4 ± 5.7. The AIS group consisted of 14 patients (10 girls and 4 boys) with a mean age of 13.2 ± 0.8 years. The mean MMSE score in the AIS group was 29.1 ± 1.1. The mean MMSE score was significantly lower in the CS group than in the AIS group (P < 0.01).
Table 1.
Details of the subjects
| Case | Age | Gender | Risser stage | Cobb’s anglea | MMSE score | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 14.3 | M | 3 | 41/LTL | 25 | Congenital scoliosis (T12 hemivertebra) |
| 2 | 12.1 | M | 3 | 46/RTL | 28 | Congenital scoliosis (T12, L1 hemivertebra) |
| 3 | 10.8 | F | 2 | 64/LL | 11 | Congenital scoliosis (L1, L2 hemivertebra) |
| 4 | 11.8 | M | 2 | 39/RTL | 24 | Congenital scoliosis (T11 hemivertebra) |
| 5 | 12.0 | F | 2 | 50/LTL | 17 | Congenital scoliosis (L1 hemivertebra) |
| 6 | 15.2 | M | 3 | 55/LTL | 13 | Congenital scoliosis (multiple hemivertebra) |
| 7 | 14.2 | F | 3 | 53/LTL | 24 | Congenital scoliosis (unilateral bar) |
| 8 | 12.2 | F | 2 | 42/LL | 25 | Congenital scoliosis (L3 hemivertebra) |
| 9 | 12.0 | M | 2 | 83/LT | 22 | Congenital scoliosis (unilateral bar) |
| 10 | 14.6 | M | 3 | 46/LTL | 26 | Congenital scoliosis (T12 hemivertebra) |
| 11 | 14.2 | F | 3 | 40/RT | 27 | Congenital scoliosis (multiple hemivertebra) |
| 12 | 12.6 | F | 2 | 43/LL | 27 | Congenital scoliosis (L2 hemivertebra) |
| 13 | 14.1 | M | 3 | 59/44/RT/LTL | 30 | Adolescent idiopathic scoliosis |
| 14 | 13.2 | F | 3 | 55/41/RT/LTL | 27 | Adolescent idiopathic scoliosis |
| 15 | 11.6 | F | 2 | 46/RT | 29 | Adolescent idiopathic scoliosis |
| 16 | 12.5 | F | 2 | 57/39/RT/LTL | 30 | Adolescent idiopathic scoliosis |
| 17 | 13.8 | M | 3 | 48/LTL | 29 | Adolescent idiopathic scoliosis |
| 18 | 13.3 | M | 3 | 69/RT | 30 | Adolescent idiopathic scoliosis |
| 19 | 12.5 | F | 2 | 38/59/RT/LL | 30 | Adolescent idiopathic scoliosis |
| 20 | 12.0 | F | 2 | 62/RT | 29 | Adolescent idiopathic scoliosis |
| 21 | 14.2 | F | 3 | 51/38/RT/LTL | 30 | Adolescent idiopathic scoliosis |
| 22 | 13.5 | F | 2 | 60/43/RT/LTL | 27 | Adolescent idiopathic scoliosis |
| 23 | 13.1 | M | 3 | 59/48/RT/LTL | 30 | Adolescent idiopathic scoliosis |
| 24 | 13.6 | F | 4 | 48/62/RT/LL | 29 | Adolescent idiopathic scoliosis |
| 25 | 12.7 | F | 2 | 48/49/RT/LTL | 28 | Adolescent idiopathic scoliosis |
| 26 | 14.2 | F | 4 | 53/68/RT/LL | 30 | Adolescent idiopathic scoliosis |
a LTL left thoracolumbar, RTL right thoracolumbar, LL left lumbar, LT left thoracic, RT right thoracic
Group analysis of regional cerebral metabolism abnormalities by SPM analysis
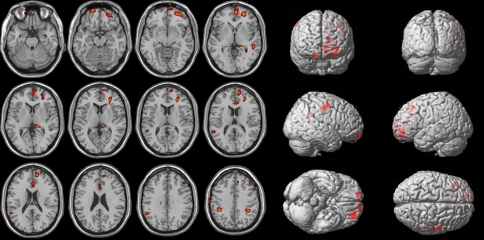
As shown in Fig. 1 and Table 2, several voxel clusters of significantly decreased cerebral metabolism were observed in the CS patients using t-statistics. The largest cluster was an area of the left prefrontal cortex (Brodmann area 10) (voxel number 585, peak Z value = 3.30, uncorrected P < 0.001). The second largest cluster area was the right orbitofrontal cortex (Brodmann area 11) (voxel number 141, peak Z value = 3.11, uncorrected P = 0.001). The third largest cluster area was the left dorsolateral prefrontal cortex (Brodmann area 9) (voxel number 119, peak Z value = 3.36, uncorrected P = 0.003). The other clusters were the left anterior cingulate gyrus (Brodmann area 24) (voxel number 105, peak Z value = 2.98, 2.54 uncorrected P = 0.001) and pulvinar of the left thalamus (voxel number 72, peak Z value = 2.70, uncorrected P = 0.001).
Fig. 1.
Hypometabolic regions in the CS patients vs. AIS subjects. These regions are displayed on the axial (left) and surface rendered (right) images. Brain regions of the abnormal glucose metabolism are displayed using a height threshold of uncorrected P = 0.001 and an extent threshold of 50 voxels
Table 2.
Group analysis results of brain areas of significantly decreased glucose metabolism in patients with CS compared to AIS
| Cluster size | Hemisphere | Talairach coordinates | Peak Z | Structure | BA | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| 585 | Left | −26 | 54 | −2 | 3.83 | Prefrontal cortex | 10 |
| 141 | Right | 16 | 62 | −18 | 3.11 | Orbitofrontal cortex | 11 |
| 119 | Left | −46 | 32 | 38 | 2.77 | Dorsolateral prefrontal cortex | 9 |
| 105 | Left | −6 | 32 | 18 | 2.98 | Anterior cingulate | 24 |
| Left | −2 | 22 | 26 | 2.54 | Anterior cingulate | 24 | |
| 72 | Left | −18 | −34 | 4 | 2.70 | Thalamus, pulvinar | |
BA Brodmann area
Correlation of MMSE and brain metabolism
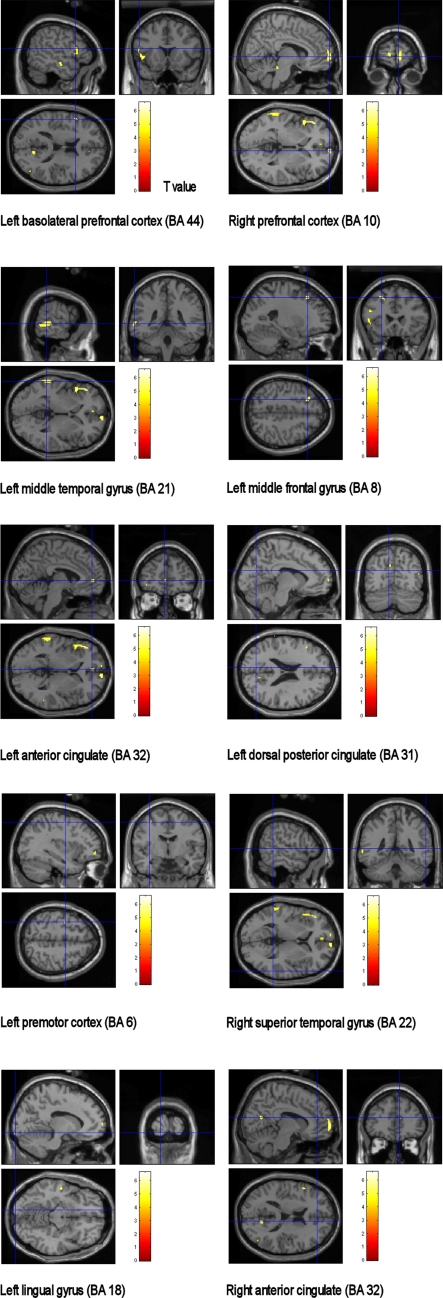
Positive correlations between the MMSE score and cerebral glucose metabolism were observed in several areas (Fig. 2). These areas were left basolateral prefrontal cortex (BA 44, peak Z = 3.65, MNI coordinate; −52, 16, 16, P = 0.001, R2 = 0.8247), right prefrontal cortex (BA 10, peak Z = 3.86, MNI coordinate; 8, 66, 0, P = 0.0013, R2 = 0.8222), left middle temporal gyrus (BA 21, peak Z = 3.76, MNI coordinate; −62, −40, 0, P = 0.0012, R2 = 0.8216), left middle frontal gyrus (BA 8, peak Z = 3.57, MNI coordinate; −24, 24, 48, P ≤ 0.001, R2 = 0.8204), left anterior cingulate (BA 32, peak Z = 3.31, MNI coordinate; −4, 48, 2, P ≤ 0.001, R2 = 0.7869), left dorsal posterior cingulate (BA 31, peak Z = 3.16, MNI coordinate; −8, −72, 22, P ≤ 0.001, R2 = 0.8256), left premotor cortex (BA 6, peak Z = 3.14, MNI coordinate; −34, −6, 54, P ≤ 0.001, R2 = 0.6448), right superior temporal gyrus (BA 22, peak Z = 3.12, MNI coordinate; 58, −44, 4, P ≤ 0.001, R2 = 0.7786), left lingual gyrus (BA 18, peak Z = 3.11, MNI coordinate; −12, −100, −12, P ≤ 0.001, R2 = 0.6364), and right anterior cingulate (BA 32, peak Z = 3.10, MNI coordinate; 10, 42, 16, P ≤ 0.001, R2 = 0.7045).
Fig. 2.
Positive correlated brain areas in the patients with CS between MMSE scores and glucose metabolism
Individual analysis of regional cerebral metabolism abnormalities by SPM analysis
Table 3 demonstrates the SPM2 results of cerebral glucose metabolism in the individual CS patients compared to AIS.
Table 3.
Individual analysis results of brain areas of significantly decreased metabolism in patients with CS compared to AIS
| Case | Hemisphere | Talairach coordinates | Peak Z | P value | Structure | BA | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 1 | Right | 38 | 62 | 4 | 3.07 | 0.033 | Prefrontal cortex | 10 |
| Left | −36 | 30 | −8 | 2.92 | 0.04 | Basolateral prefrontal cortex | 47 | |
| Right | 58 | −70 | 18 | 2.84 | 0.04 | Middle temporal gyrus | 39 | |
| 2 | Right | 6 | −20 | 10 | 3.27 | 0.035 | Thalamus, medial dorsal nucleus | |
| Left | −8 | −28 | 8 | 3.25 | 0.031 | Thalamus, pulvinar | ||
| Right | 4 | 6 | 26 | 2.70 | 0.045 | Corpus callosum | ||
| Left | −2 | 18 | 20 | 2.70 | 0.045 | Anterior cingulate | ||
| 3 | Left | 0 | −24 | 30 | 5.00 | 0.01 | Cingulate gyrus | |
| Left | −12 | 12 | 8 | 4.39 | 0.014 | Caudate nucleus | ||
| Left | −14 | −26 | 10 | 4.19 | 0.016 | Thalamus, pulvinar | ||
| Left | −12 | −38 | −2 | 4.04 | 0.018 | Parahippocampal gyrus | 27 | |
| Left | −52 | −32 | 2 | 3.66 | 0.026 | Superior temporal gyrus | 22 | |
| Left | −40 | 10 | 60 | 3.09 | 0.033 | Middle frontal gyrus | 6 | |
| Left | −30 | −14 | −18 | 3.06 | 0.035 | Hippocampus | ||
| Left | −16 | 56 | 40 | 3.01 | 0.038 | Dorsolateral prefrontal cortex | 9 | |
| Left | −66 | −36 | −12 | 2.56 | 0.049 | Middle temporal gyrus | 21 | |
| 4 | Left | −2 | 56 | −2 | 4.09 | 0.018 | Prefrontal cortex | 10 |
| Left | −16 | 38 | 18 | 2.68 | 0.045 | Anterior cingulate | ||
| Right | 54 | −42 | 6 | 2.85 | 0.04 | Superior temporal gyrus | 22 | |
| Right | 4 | 22 | 40 | 3.00 | 0.038 | Cigulate gyrus | 32 | |
| Right | 64 | 0 | 0 | 2.95 | 0.04 | Superior temporal gyrus | 22 | |
| 5 | Right | 2 | −22 | 28 | 4.08 | 0.018 | Cingulate gyrus | |
| Right | 60 | 12 | −4 | 2.78 | 0.04 | Superior temporal gyrus | 22 | |
| Right | 58 | −6 | 50 | 3.22 | 0.03 | Precentral gyrus | 6 | |
| Right | 24 | −66 | 6 | 3.16 | 0.033 | Posterior cingulate | 30 | |
| Left | −40 | 6 | 62 | 2.98 | 0.04 | Middle frontal gyrus | 6 | |
| 6 | Right | 2 | −24 | 28 | 3.67 | 0.026 | Cingulate gyrus | |
| Left | −20 | −68 | 4 | 3.45 | 0.028 | Posterior cingulate | 30 | |
| Right | 52 | −32 | 4 | 3.30 | 0.031 | Superior temporal gyrus | 22 | |
| Right | 16 | −62 | 10 | 3.05 | 0.038 | Posterior cingulate | ||
| Left | −14 | −26 | 10 | 2.97 | 0.04 | Thalamus, pulvimar | ||
| 7 | Left | −18 | −34 | −8 | 5.13 | 0.01 | Parahippocampal gyrus | |
| Right | 16 | −62 | 10 | 4.51 | 0.01 | Posterior cingulate | ||
| Left | −64 | −34 | 28 | 4.44 | 0.01 | Inferior parietal lobule | 40 | |
| Left | −64 | −44 | −2 | 3.84 | 0.02 | Middle temporal gyrus | 21 | |
| Left | −30 | −10 | 52 | 3.81 | 0.02 | Precentral gyrus | 6 | |
| Left | −12 | 68 | −6 | 3.46 | 0.028 | Prefrontal cortex | 10 | |
| Right | 12 | 68 | 2 | 3.41 | 0.028 | Prefrontal cortex | 10 | |
| Right | 2 | −22 | 28 | 3.39 | 0.03 | Cingulate gyrus | ||
| Right | 10 | −4 | 30 | 2.41 | 0.049 | Cingulate gyrus | ||
| Left | −10 | 6 | 6 | 2.82 | 0.04 | Caudate nucleus | ||
| 8 | Left | −20 | 56 | 26 | 3.73 | 0.023 | Prefrontal cortex | 10 |
| Left | −54 | 2 | −34 | 2.85 | 0.04 | Middle temporal gyrus | 21 | |
| Left | −52 | 8 | −22 | 2.82 | 0.04 | Middle temporal gyrus | 21 | |
| Left | −32 | 24 | 42 | 3.10 | 0.033 | Dorsolateral prefrontal cortex | 9 | |
| 9 | Right | 48 | −30 | 46 | 3.70 | 0.023 | Inferior parietal lobule | 40 |
| Right | 40 | 20 | 6 | 3.96 | 0.02 | Insula | ||
| 10 | Left | −42 | −42 | 40 | 3.53 | 0.026 | Inferior parietal lobule | 40 |
| Left | −4 | −36 | 32 | 3.44 | 0.028 | Cingulate gyrus | ||
| Right | 8 | −36 | 34 | 2.63 | 0.047 | Cingulate gyrus | ||
| Left | 12 | −60 | 8 | 3.43 | 0.028 | Posterior cingulate | ||
| Left | −52 | −40 | −20 | 2.97 | 0.04 | Inferior temporal gyrus | 37 | |
| Left | −50 | 4 | −36 | 2.94 | 0.04 | Middle temporal gyrus | 21 | |
| 11 | Right | 24 | −14 | 70 | 3.81 | 0.02 | Premotor cortex | 6 |
| Right | 12 | −12 | 72 | 3.27 | 0.031 | Premotor cortex | 6 | |
| Left | −16 | −12 | 72 | 3.67 | 0.026 | Premotor cortex | 6 | |
| Left | −14 | 26 | 36 | 3.35 | 0.03 | Dorsolateral prefrontal cortex | 9 | |
| Left | −16 | 28 | 28 | 3.09 | 0.033 | Anterior cingulate | ||
| Left | −10 | 30 | 20 | 2.74 | 0.04 | Anterior cingulate | ||
| Left | −50 | −30 | −2 | 2.62 | 0.047 | Superior temporal gyrus | 22 | |
| 12 | Left | −42 | −4 | 46 | 4.11 | 0.016 | Premotor cortex | 6 |
| Right | 52 | −52 | 52 | 3.82 | 0.02 | Inferior parietal lobule | 40 | |
| Left | −48 | 14 | 38 | 3.88 | 0.02 | Dorsolateral prefrontal cortex | 9 | |
| Right | 36 | 40 | 40 | 3.73 | 0.023 | Dorsolateral prefrontal cortex | 9 | |
| Right | 36 | 62 | 2 | 3.32 | 0.032 | Prefrontal cortex | 10 | |
BA Brodmann area
Discussion
There are a number of reports for associated conditions of CS. These include cardiac abnormalities, urological abnormalities and spinal cord abnormalities. In addition, mental retardation is often observed in patients of CS associated with syndrome [16]. Despite a possible association between CS and a low mental status, reports about the mental status or cerebral metabolism in patients with CS are lacking. Therefore, we investigated the mental status and cerebral glucose metabolism in patients with CS. To our knowledge, this is the first report on the cerebral metabolism using F-18 FDG brain PET in patients with CS.
In this study, we determined a regional pattern of cerebral glucose metabolism in patients with CS using objective SPM analysis method. Because most of the patient group was adolescent, the ideal control group for SPM analysis would be age and gender matched group of normal adolescent. However, ethnical problems prohibit this. The rationale for using AIS as control group is based on our previous results showing similar cerebral glucose metabolism in AIS patients and normal young adults [21]. In this study, SPM was adopted for objective evaluation of brain perfusion SPECT. The process of spatial normalization enables a voxel-based statistical comparison of brain images with different morphologies. SPM could allow the identification of abnormalities on an individual case-by-case basis, as well as group comparisons for different categories of brain diseases such as cognitive disorders, epilepsy and cerebrovascular diseases.
The group analysis found that various brain areas of the patients with CS showed glucose hypometabolisms in the left prefrontal cortex (Brodmann area 10), right orbitofrontal cortex (Brodmann area 11), left dorsolateral prefrontal cortex (Brodmann area 9), left anterior cingulate gyrus (Brodmann area 24) and pulvinar of the left thalamus. Those regions were correlated with cognitive and intellectual function. The overall decline in the cognitive function is closely correlated with the degree of cortical metabolic dysfunction [10]. The prefrontal cortical abnormalities newly emerged as patients showed further cognitive deterioration [8]. Eidelberg et al [9] reported glucose hypometabolism in the orbitofrontal cortex in cognitively impaired patients. This study demonstrates decreased cerebral glucose metabolism of prefrontal cortex by group analysis. Compared with AIS, the dorsolateral prefrontal cortex and prefrontal cortex were hypometabolic state.
The cingulate gyrus is the principal component of the limbic system, and its anterior and posterior parts possess different thalamic and cortical connections with different cytoarchitectures and subserve distinctive functions [12, 23]. The anterior cingulate gyrus can be divided into discrete anatomical and behavioral subdivisions: the affective division and the cognitive division [4]. The affective division includes the areas of Brodmann 25 and 33 and the rostal area of Brodmann 24, and plays a role in emotion and motivation. The cognitive division includes caudal areas of Brodmann 24 and 32 and plays a role in complex cognitive and attentional processing [4, 7, 24]. Previous study suggested that the major regions of age-related decline remain prominent in the prefrontal cortex as well as in the anterior cingulate gyrus [25].
The thalamus is also a principal component of the limbic system and the ascending reticular activating system. In addition, it is an input modulator where external sensory stimuli enter the cortex of the brain. Therefore, the thalamus has distinct connections with the cingulate gyrus, the reticular formation and the cerebral cortex. Decreased glucose metabolism in the thalamus is often observed in other studies regarding developmental disorders [17, 15]. From previous reports, it can be presumed that the thalamus plays an important role in brain development, especially in the early period of human life [5, 20].
From this study, CS patients might have some problems at the beginning period of brain development such as a congenital factor or a functional deficit in the subcortical areas including the thalamus, which can widely influence brain development and resulting in various developmental disorders.
The correlations between the cognitive function and cerebral glucose metabolism revealed that various brain areas had positive correlations between the MMSE scores and the cerebral glucose metabolism in this study, including the prefrontal cortex, anterior and posterior cingulate gyri, and premotor cortex. The posterior cingulate gyrus plays a role in orientation and interpretation of the environment [22, 23], and has connections and behavioral attributes distinct from those of the anterior cingulate gyrus. Therefore, the functions of these divisions are probably coordinated [12]. The posterior cingulate gyrus also has dense connections with the medial temporal memory system. These communications might play a role in the posterior cingulate gyrus in orientation [7].
Anatomically, the posterior cingulate gyrus and its adjacent medial parietal cortex connect to the anterior cingulate, prefrontal, lateral parietal, and temporal cortices, as demonstrated in a monkey model [19]; such anatomical findings indicate that the posterior cingulate area plays a role in orchestrating the multimodal associative functions. In a clinical setting, cerebral blood flow in the posterior cingulate has been shown to be correlated with the clinical severity of dementia [1], and long-term changes in the posterior cingulate cerebral blood flow could be predictive of an onset of clinical symptoms of Alzheimer disease later in life [14]. In line with these findings, the posterior cingulate glucose metabolism in our CS patients showed a positive correlation with the scores on the MMSE, which is a test of mixed cognitive-memory performance.
This study suggests the possible role of voxel-based analysis of brain FDG PET abnormalities in patients with CS. From this study, the metabolic abnormalities of the brain in patients with CS could be identified clearly using group and individual analyses of SPM2. However, the numbers of subjects in the subgroups examined was too small to allow a valid result with external variables such as age, gender or the neuropsychological test results. Therefore, more study on a larger number of patients will be needed to allow a more objective SPM analysis of patients with CS.
Acknowledgments
W. W. Park and K. T. Suh contributed equally to this study. This study was supported for two years by Pusan National University Research Grant.
References
- 1.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93–100. doi: 10.1002/1531-8249(200001)47:1<93::AID-ANA15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Basu PS, Elsebaie H, Noordeen MH. Congenital spinal deformity: a comprehensive assessment at presentation. Spine. 2002;27:2255–2259. doi: 10.1097/00007632-200210150-00014. [DOI] [PubMed] [Google Scholar]
- 3.Bradford DS, Heithoff KB, Cohen M. Intraspinal abnormalities and congenital spine deformities: a radiographic and MRI study. J Pediatr Orthop. 1991;11:36–41. doi: 10.1097/01241398-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 5.Chiron C, Raynaud C, Maziere B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33:696–703. [PubMed] [Google Scholar]
- 6.Conner AN, Young DG, Hide TA. Occult intraspinal anomalies and congenital scoliosis. J Bone Joint Surg. 1984;66:1319. [PubMed] [Google Scholar]
- 7.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 8.Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 9.Eidelberg D, Moeller JR, Dhawan V, Sidtis JJ, Ginos JZ, Strother SC, Cedarbaum J, Greene P, Fahn S, Rottenberg DA. The metabolic anatomy of Parkinson’s disease: complementary [18F]fluorodeoxyglucose and [18F]fluorodopa positron emission tomographic studies. Mov Disord. 1990;5:203–213. doi: 10.1002/mds.870050304. [DOI] [PubMed] [Google Scholar]
- 10.Foster NL, Chase TN, Mansi L, Brooks R, Fedio P, Patronas NJ, Di Chiro G. Cortical abnormalities in Alzheimer’s disease. Ann Neurol. 1984;16:649–654. doi: 10.1002/ana.410160605. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie R, Faithfull DK, Roth A, Hall JE. Intraspinal anomalies in congenital scoliosis. Clin Orthop Relat Res. 1973;93:103–109. doi: 10.1097/00003086-197306000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hirono N, Mori E, Ishii K, Ikejiri Y, Imamura T, Shimomura T, Hashimoto M, yamashita H, Sasaki M. Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64:552–554. doi: 10.1136/jnnp.64.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong SK, Cho KH, Kim JM. The usefulness of the Korean version of modified mini-mental state examination (K-mMMSE) for dementia screening in community dwelling elderly people. BMC Public Health. 2004;4:31. doi: 10.1186/1471-2458-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 15.Lee JD, Kim DI, Ryu YH, Whang GJ, Park CI, Kim DG. Technetium-99m-ECD brain SPECT in cerebral palsy: comparison with MRI. J Nucl Med. 1998;39:619–623. [PubMed] [Google Scholar]
- 16.Lee CK, Chang BS, Hong YM, Yang SW, Lee CS, Seo JB. Spinal deformities in Noonan syndrome: a clinical review of sixty cases. J Bone Joint Surg. 2001;83-A:1495–1502. [PubMed] [Google Scholar]
- 17.Lee BF, Yang P, Jong YJ, Hsu HY, Chen CC. Single photon emission computerized tomography in children with developmental language disorder-a preliminary report. Kaohsiung J Med Sci. 2002;18:373–378. [PubMed] [Google Scholar]
- 18.MacEwen GD, Winter RB, Hardy JH. Evaluation of kidney anomalies in congenital scoliosis. J Bone Joint Surg. 1972;54-A:1451–1454. [PubMed] [Google Scholar]
- 19.Pandya DN, Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstein M, Denays R, Ham HR, Piepsz A, VanPachterbeke T, Haumont D, Noel P. Functional imaging of brain maturation in humans using iodine-123 iodoamphetamine and SPECT. J Nucl Med. 1989;30:1982–1985. [PubMed] [Google Scholar]
- 21.Suh KT, Lee SS, Kim SJ, Kim YK, Lee JS. Pineal gland metabolism in patients with adolescent idiopathic scoliosis. J Bone Joint Surg. 2007;89-B:66–71. doi: 10.1302/0301-620X.89B1.18058. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci. 1988;8:1863–1872. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 24.Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–1228. doi: 10.1016/S0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 25.Willis MW, Ketter TA, Kimbrell TA, George MS, Herscovitch P, Danielson AL, Benson BE, Post RM. Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res. 2002;114:23–37. doi: 10.1016/S0925-4927(01)00126-3. [DOI] [PubMed] [Google Scholar]
- 26.Winter RB, Haven JJ, Moe JH, Lagaard SM. Diastematomyelia and congenital spine deformities. J Bone Joint Surg. 1974;56-A:27–39. [PubMed] [Google Scholar]