Abstract
Biopsies of lesions in the spine are often challenging procedures with significant risk of complications. CT-guided needle biopsies could lower these risks but uncertainties still exist about the diagnostic accuracy. Aim of this retrospective study was to evaluate the diagnostic accuracy of CT-guided needle biopsies for bone lesions of the spine. We retrieved the results of 430 core needle biopsies carried out over the past fifteen years at the authors’ institute and examined the results obtained. Of the 430 biopsies performed, in 401 cases the right diagnosis was made with the first CT-guided needle biopsy (93.3% accuracy rate). Highest accuracy rates were obtained in primary and secondary malignant lesions. Most false negative results were found in cervical lesions and in benign, pseudotumoral, inflammatory, and systemic pathologies. There were only 9 complications (5 transient paresis, 4 haematomas that resolved spontaneously) that had no influence on the treatment strategy, nor on the patient’s outcome. In conclusion we can assert that this technique is reliable and safe and should be considered the gold standard in biopsies of the spine.
Keywords: Biopsy, Spine, Computed tomography, Bone lesion
Introduction
An accurate biopsy is essential in the diagnostic work-up of any neoplastic lesion, and even if modern radiological imaging techniques have a good predictive value in differential diagnosis, an histological examination is usually necessary to plan further treatment. Traditionally, open biopsy has been the gold standard for musculoskeletal lesions, providing adequate material for histological and immunohistochemical studies in most cases, resulting in a highly diagnostic procedure. However, in vertebral neoplasm, performing an open biopsy can be a difficult procedure with significant risk of complications [13, 14].
Alternatively to open biopsy, percutaneous needle biopsy has gained popularity showing a good accuracy with a less invasive procedure. It is applicable to outpatients or day hospital patients, since general anesthesia is rarely required. Cost and time are inferior compared to open biopsy and there is less risk of tumor spread, infection and/or wound problems. In case of deep lesions, for example in the pelvis or spine, needle biopsies are challenging procedures [16]. In these cases, the aid of computed tomography (CT)-guidance has further increased accuracy, reduced complications and therefore has now become the procedure of choice [5, 8, 9, 11, 17–19].
Purpose of this paper is to assess the diagnostic accuracy and clinical usefulness of percutaneous CT-guided core needle biopsy of vertebral lesions. Therefore, we reviewed 430 spinal needle biopsies performed at our institution over the last 15 years.
Materials and methods
From July 1990 to December 2005, 1068 CT-guided core needle biopsies of the appendicular and axial skeleton were performed at our institution and almost half of the cases (430) were performed on the spine. Demographic patient data and spinal locations of the biopsies are reported in Table 1. Except for 7 children, all patients included in this study were adults. The size of the lesion (antero-posterior, transverse and cranio-caudal diameters) were between 0.5 and 2.5 cm in 31 lesions, between 2.5 and 3.5 cm in 363, and 3.5 cm or more in the remaining 36 cases.
Table 1.
Demographic and detailed spinal location of the 430 CT-guided biopsies (male/female 248/182, median age 45.5 (range 5–86), 255 in-patients and 175 out-patients)
| Spine location | |||
|---|---|---|---|
| Cervical tract (10) | Thoracic tract (124) | Lumbar tract (178) | Sacrum/tailbone (118) |
| C1 (1) | T1–T4 (23) | L1 (30) | S1 (78) |
| C2 (3) | T5–T8 (48) | L2 (30) | S2 (18) |
| C3 (2) | T9–T12 (53) | L3 (31) | S3 (12) |
| C7 (4) | L4 (50) | SI-joint (7) | |
| L5 (37) | Tailbone (3) | ||
January 1990–May 1997: CT Sytec 3000 (General Electric, Milwaukee, USA)
June 1997–December 2005: High-Speed Cti (General Electric, Milwaukee, USA)
A complete disease staging, usually with plain radiography, CT, MRI, and bone scan was carried out. In case of multiple lesions we always took the biopsy at the most easily approachable site and always tried to get at least two samples.
All biopsies were carried out with three different core needle biopsy sets: the Trap System Set (HS-Hospital Services S.p.A., Roma, Italy), the Craig-Kogler Set (Chiruma S.r.l. S.Giovanni in Persiceto, Bologna, Italy), and the small caliber Bonopty Insertion Set (Radi MS, Uppsala, Sweden). We used the small caliber set only when the site, the size or the approach did not allow for use of a larger needle. Table 2 sums up the types and features of the different needles, and the numbers of biopsies performed with each set.
Table 2.
Biopsy set used for core needle biopsies (CNB)
| Trap system | Craig-Kogler | Bonopty | |
|---|---|---|---|
| Trocar calibera | 8G/4–5 mm with 10/15 cm sharp stylet | 8G/4–5 mm with 19 cm sharp guide | 15G/1.8 mm–14G/2.1 mm with 12.2/16 cm drills |
| Trocar length | 10–15 cm | 10 cm | 9.5 cm |
| Extractor | 10/15 cm flexible steel (9G) | 21 cm coaxial (12 G) | 16 cm cannula (18G) |
| Multiple coaxial | No | Yes | Yes |
| Action systemb | |||
| No. of biopsies | 372 | 53 | 5 |
aInner and outer caliber
bWith this system the trocar remains positioned inside the lesion while multiple biopsies can be taken through the needle
After informed consent obtained, all but seven biopsies were performed in local anesthesia. Seven children, between 5 and 10 years of age, underwent a general anesthesia. Local anesthesia was performed by infiltration of mepivacaine cloridate at 2% (5–15 cc).
The patient’s position (supine, prone, lateral right or left) varied according to the segment of the spine affected and the site of the lesion in the vertebra. Whenever possible, the needle approach to the lesion was chosen according to a potential future surgical procedure, keeping the biopsy tract easily exercisable (Table 3).
Table 3.
Approaches of the 430 CT-guided biopsies
| Spine site | Patient position | Biopsy approach | |
|---|---|---|---|
| Cervical tract | Supine | Anterolateral | (1) |
| Prone | Transpeduncular | (6) | |
| Prone | Midposterior | (3) | |
| Dorsal tract | Prone | Midposterior | (2) |
| Prone, lateral left/right | Transpeduncolar | (84) | |
| Lateral left/right | Transcostovertebral | (3) | |
| Lateral lefta | Posterolateral | (35) | |
| Lumbar tract | Prone | Midposterior | (10) |
| Prone, lateral right/left | Transpeduncolar | (101) | |
| Lateral rightb | Posterolateral | (67) | |
| Sacral tract | Prone | Midposterior | (118) |
a,bLateral left/right in order to avoid aorta/vena cava
A scout-view radiogram was taken focused on the affected area. The setting of the thickness and interval of the slice was related to the site and the size of the lesion: usually 1 mm thickness and interval for the cervical spine, 3 mm thickness and 2 mm interval for the thoracic spine, 5 mm thickness and 3 mm interval for the lumbar and sacral spine, and 3 mm thickness and 2 mm interval for the coccygeus bone. Under CT-guidance the best insertion point, along the surgical line for the anatomical region, was selected and marked on the skin. Then the trocar is introduced, either over a K-wire, or parallel to a spinal needle, and the needle is inserted. In case of a coaxial technique, the trocar is left inside the lesion, in the other cases the trocar is withdrawn together with the needle. The needle positioning is done under sequential scans (Figs. 1, 2, 3). A lower rate of milli-Amperes (mAs) was used for monitoring needle approach to the lesion, in order to reduce the patient’s absorbed dose.
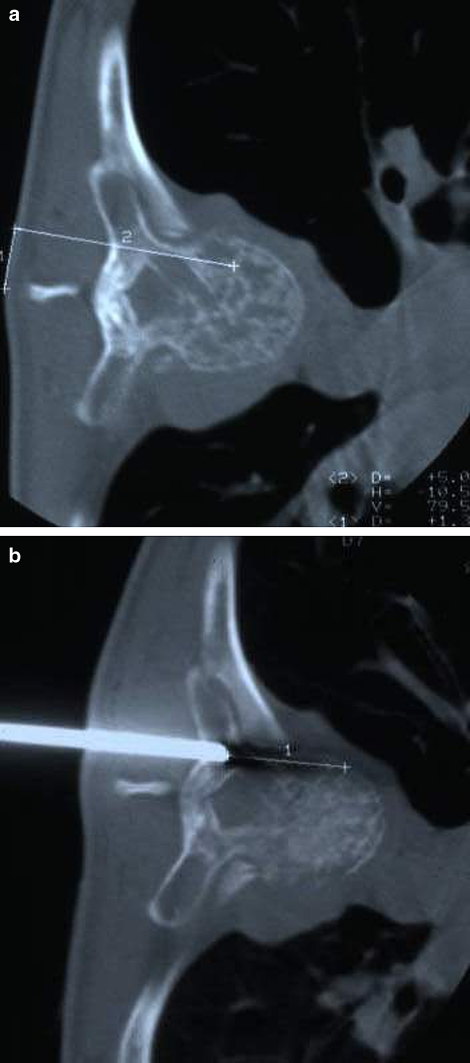
Fig. 1.
Thoracic spine (T7). Planning of the needle tract: transpeduncular approach. a Line 1 measures the distance between the spinous process and the skin insertion point. Line 2 measures the distance between the skin and the distal portion of the lesion. b CT control of the tip of biopsy needle (trap system needle). Line 1 shows the distance from the tip of the needle to the distal part of the lesion. The needle should be advanced to the most distal portion of the lesion in order to take as much diagnostic tissue as possible. Histological diagnosis: angioma
Fig. 2.
Cervical spine (C1) Lytic lesion of the right atlas process. Prone position: mid-posterior approach. a Planning of the needle tract with a lead marker attached to the skin. b Insertion of biopsy needle (bonopty set) inside the lesion. Histological diagnosis: benign inactive lesion, not further defined
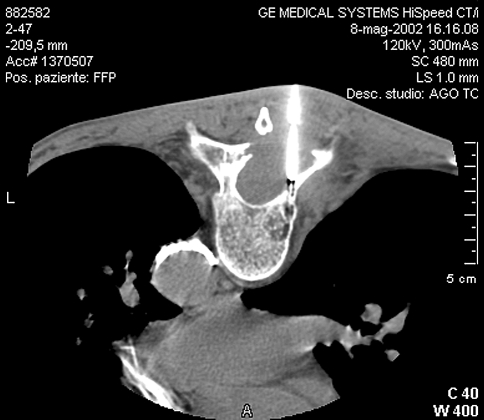
Fig. 3.
Prone position: transpeduncular approach of T9. The centimetrated trocar of Craig-Kogler set is placed on the cortex of the rigth pedicle. Introduction through the trocar of the biopsy needle approaching the moth-eaten lytic lesion of the posterior right part of the vertebra. Histological diagnosis: metastasis of adenocarcinoma
Samples were harvested from the lesion and sent to the pathologist in saline solution. In 31 cases, where an infection was suspected, a swab sample was collected for microbiology. The time required for the complete biopsy procedure, including the pre-biopsy CT, varied from 25 to 60 min (median 30).
The diagnosis was always based on a combination of histopathological findings associated with clinical and radiological information. If the diagnosis was not consistent with the clinical and radiological findings, the case was reviewed in a multidisciplinary way by orthopaedic surgeons, pathologists and radiologists, and in case of a doubtful diagnosis the biopsy was repeated.
Results
The CT-guided needle biopsy resulted in a histological diagnosis of a tumorous or pseudotumorous lesion in 385 of 430 cases (89.5%). In 291 of these, the biopsy was followed by a surgical treatment in which the needle biopsy diagnosis was confirmed: 211 primary malignant, 56 benign tumors, 24 others (aneurysmal bone syst, simple bone cyst, histiocytosis X, Paget’s disease of bone, synovitis). The other 94 biopsies (32 systemic malignant: Hodgkin lymphoma, Non-Hodgkin lymphoma, myeloma; 45 metastatic carcinoma; and 17 inflammatory lesions) were not followed by a surgical treatment. For these 94 cases the clinical course and response to the non-surgical treatment confirmed the accuracy of the biopsy results.
In 45 cases the procedure was not diagnostic. In 20 of these, (5%) the amount of sample tissue was insufficient. In 25 cases (6%) the obtained tissue was atypical (sclerotic bone, intramedullary fibrosis/sclerosis, blood cloths), but did not lead to a specific pathology diagnosis. All 45 cases underwent a second diagnostic procedure: an open biopsy in 5 cases (3 cervical spine, 2 thoracic spine) and a repeated percutaneous CT-guided core needle biopsy in 40 (1 cervical, 13 thoracic, 17 lumbar, 9 sacral lesions). In each of these cases, the second procedure was performed within 30 days from the first one.
The diagnoses of the 5 open biopsies were: 1 giant cell tumor, 1 aneurysmal bone cyst, 2 lymphoma and 1 metastasis. Therefore, the first results were all false negatives. The 40 second CT-guided needle biopsies were performed aiming at a different part of the lesion. These biopsies resulted in 16 true negatives (1 cervical, 5 thoracic, 5 lumbar, 5 sacral lesions) and 24 false negatives (8 thoracic, 12 lumbar, 4 sacral).
In the 16 true negative cases the first diagnosis was confirmed but the sample further defined: stress fracture, osteoporotic fragments with bone reabsorbement, edema in irradiated bone, bone sclerosis or necrosis, subcondral cystic lesion in osteoarthritis. The true negative cases were followed-up by clinical and radiological controls for a period variable from 0.8 to 3.0 years and no diagnostic adjustments were made. The 16 true negative cases incremented the accuracy of the initial percutaneous needle biopsy from 89.5 to 93.3%.
Table 4 shows the accuracy of CT-guided biopsies according to the different spinal segments. There were more false negative results in the cervical spine than in the thoracic, lumbar and sacral segments.
Table 4.
Results of the 430 CT-guided biopsies according to the different spinal segments
| Spine site | |||
|---|---|---|---|
| Cervical tract | 10 | Diagnostic | 7 (70%) |
| Non diagnostic | 3 (30%) | ||
| Thoracic tract | 124 | Diagnostic | 114 (92%) |
| Non diagnostic | 10 ( 8%) | ||
| Lumbar tract | 178 | Diagnostic | 166 (93%) |
| Non diagnostic | 12 ( 7%) | ||
| Sacral tract | 118 | Diagnostic | 114 (97%) |
| Tail bone | Non diagnostic | 4 (3%) | |
| Total | 430 | Diagnostic | 401 (93.3%) |
| Non diagnostic | 29 (6.7%) | ||
| Overall accuracy | 93.3% | ||
Table 5 shows the accuracy rates based on the histological diagnosis. False negative diagnosis was more frequent in benign tumors, systemic malignant forms, inflammatory lesions, and pseudotumorous lesions, while in primary and secondary malignant lesions accuracy was relatively high.
Table 5.
Accuracy rates based on the histological diagnosis
| Diagnosis | ||
|---|---|---|
| Primitive benign | Diagnostic | 55 (89%) |
| Non diagnostic | 7 (11%) | |
| Pseudotumoral | Diagnostic | 24 (83%) |
| Non diagnostic | 5 (17%) | |
| Primitive malignant | Diagnostic | 211 (99.5%) |
| Non diagnostic | 1 (0.5%) | |
| Systemic malignant | Diagnostic | 34 (77%) |
| Non diagnostic | 10 (23%) | |
| Flogosis | Diagnostic | 17 (77%) |
| Non diagnostic | 5 (23%) | |
| Secondary malignant | Diagnostic | 44 (98%) |
| Non diagnostic | 1 (2%) | |
| Othera | Diagnostic | 16 (100%) |
| Non diagnostic | 0 (0%) | |
aNonspecific non-pathologic diagnoses as stress fractures, osteoporotic fragments with bone reabsorbement, edema in irradiated bone, bone sclerosis or necrosis, and subcondral cystic lesion in osteoarthritis
Of the 31 cases in which swab samples were collected, 18 (58%) showed bacterial growth (14 bacterium of Koch, 3 Brucellosis and 1 Escherichia coli). In 13 cases (42%) no bacterial growth was detected.
Biopsy related complications were reported in 9 cases (2.1%): 5 transient paresis of lower limbs, 3 psoas muscle haematomas, 1 retroperitoneal haematoma due to accidental puncture of a lumbar vein that resolved spontaneously in one day. The nine complications neither affected the treatment strategy nor the overall patients outcome.
Discussion
The radiological study of spinal lesions, either infective, tumoral or of different nature, has been significantly improved by the contribution of CT and MR imaging. This improvement is essentially morphological and offers an excellent imaging of the anatomical extension as well as identification of the active parts of the lesion after constrast medium injection. The low specificity of these studies, however, hardly allows an etiologic diagnosis based on radiographical studies alone. For most spinal tumors and pseudotumorous lesions a histological study is therefore required in order to make a definitive diagnosis [3].
Percutaneous biopsy of musculoskeletal lesions can be performed either by fine needle aspiration (FNA) or core needle biopsy (CNB). FNA needles have a diameter of less than 1 mm, whereas CNB needles have a diameter of more than 1.5 cm. At our institution we do not perform FNA biopsies. By this method of biopsy the architecture of the obtained tissue is not preserved and only cytological study is possible. This might make a histological diagnosis more difficult and less accurate. Furthermore, sampling errors may occur due to the small amount of material obtained [1, 4, 10]. In a recent review of FNA and CNB biopsies in 359 patients with muscoloskeletal lesions the accuracy of fine needle percutaneous aspiration biopsy is reported more than 60% but significantly inferior compared to the accuracy of core needle biopsy (more than 70%) [6]. In a more recent paper (only 50 cases and no biopsies performed in spine), FNA achieved a diagnostic accuracy rate of 88% for nature of the lesion, 64% for specific diagnosis, 78% for histologic grading, and 74% for histologic typing. CNB achieved an accuracy rate of 93% for nature of lesions, 83% for specific diagnosis, 83% for histologic grading, and 90% for histologic typing. Both biopsy methods have a higher diagnostic accuracy rate for high-grade tumors than for low-grade or benign lesions in determining the nature, specific diagnosis, and histologic grading [19].
This study describes the diagnostic results of the 430 percutaneous CT-guided core needle biopsies that were performed on spinal lesions at our institution over the last 15 years. It is one of the largest studies on core needle biopsies of spinal lesions, and some interesting findings can be drawn from this series.
In the past, lower accuracy rates have been reported for CT-guided needle biopsies at the thoracic spine [9]. In our study however, accuracy rates were higher in the thoracic, lumbar and sacral tracts, and whereas the cervical tract has the lowest. The current series actually shows a decreased accuracy in the cervical tract and an increased accuracy in the thoracic and lumbar-sacral tract, when compared to a previous study performed at our institution in 1994 [15]. We think that the lower accuracy rate in the cervical tract can be correlated to the relatively smaller size of the lesions and difficulties in obtaining more than one core with a large single coaxial action needle. We underline that the results for the cervical tract in this study were based on a relatively small group of cases.
Also the histotype of the lesion seems to influence the accuracy of the percutaneous CT-guided needle biopsy. Other authors have previously described a tendency towards higher accuracy in metastatic lesions compared to primary bone tumors [8]. In our series most false negative results were found in biopsies of benign tumors, systemic malignant lesions, inflammatory lesions, and pseudotumerous lesions. The accuracy in malignant, primary and secondary, lesions was very high. Fibrotic, collagenous, deeply vascularized and sometimes colliquative are usually features more typical of benign, inflammatory, or systemic lesions, like colliquative tbc, aneurysmal bone cyst, hemangioma, whose thin membrane, endothelium or scattered nodules we probably missed in the first biopsy. Malignant lesions, on the other hand ususally contain more cohesive cells and stroma. Careful evaluation of the pre-biopsy imaging studies is necessary in order to get tissue from the viable part of the lesion: at the periphery, if the radiologic imaging suggests a sarcoma with central necrosis, in depth if the lesion is infiltrative malignant, in both bone and soft tissues if it has the aspects of an inflammatory process [2]. The goal is to obtain the sample as homogeneous as possible. In infectious lesions (osteomyelitis, spondylitis) the diagnosis can be difficult due to nonspecific histological features and frequent absence of bacterial growth in microbiology cultures, especially when the infection is chronic or when patients have been treated extensively with antibiotics. In systemic malignant disease (Hodgkin, Non-Hodgkin lymphoma and myeloma) the histological diagnosis can be difficult and is often based on immunohistochemical stainings. In some of our cases immunohistochemistry was negative in our first needle biopsy but positive in the second one. This might be due to a technical error during the needle biopsy, resulting in insufficient amount of sample tissue or non-diagnostic tissue. Also a prolonged decalcification during the histologic preparation of the obtained material could have resulted in negative immunohistochemistry. This could partially explain the false negative results in systemic malignant lesions.
Overall, our results show that percutaneous CT-guided core needle biopsy has a high accuracy rate. A correct diagnosis was obtained with the first procedure in 401 of 430 cases (93.3%). Another 24 lesions were correctly diagnosed after a second needle biopsy. In 5 cases, after the first negative percutaneous needle biopsy, an incisional biopsy was preferred over a second attempt with CT-guided needle biopsy because it was considered a safer procedure with more chance of a diagnostic sample. This was the case in 3 cervical and 2 thoracic lesions that were difficult to approach with a needle because of the site and the small size (Table 5).
Our results are consistent with the data in the international literature. Diagnostic accuracy varies from 70 to 93% [6, 7, 12, 15, 17, 20]. For high grade malignant bone tumors and metastasis, CT-guided needle biopsy has an accuracy of more than 90%, for low grade malignant and benign tumors around 80% [20]. Infections have a low accuracy rate (50%), especially when they are more cronical nonspecific than acute [6]. This study shows that the overall accuracy of percutaneous CT-guided biopsy can be considered similar to that of open biopsy [12].
According to our experience, the accurace rate is influenced by the nature of the lesion, the site, and the radiologist’s experience. If the diagnosis after a percutanous CT-guided needle biopsy is negative or the result is not consistent with the clinical suspect and/or radiological imaging, it is mandatory to repeat the needle biopsy or to carry out an open biopsy. Accuracy is further increased by a strict collaboration and communication among orthopaedic surgeon, radiologist and pathologist as well.
CT-guided percutaneous core needle biopsy is a fast, economic, and safe procedure. The diagnostic accuracy varies according to the site biopsied, type of disease, and operator’s experience. The success rate is higher in malignant lesions, primary and secondary, and lower in inflammatory lesions, especially chronic ones. In cervical spine lesions, accuracy rates are lower compared to the rest of the spine. Overall, the false negative rates are low and can be further reduced by careful pre-biopsy studying of the radiological images.
Therefore in our institution the percutaneous CT-guided biopsy is considered nowadays the procedure of choice in the musculoskeletal lesions of the spine. However, if the histological diagnosis after the percutaneous biopsy is still doubtful or the result is not consistent with the clinical suspect and/or radiological imaging, the percutaneous needle biopsy should be repeated. Only in very few cases an incisional biopsy could be necessary.
References
- 1.Ayala AG, Ro JY, Fanning CV, Flores JP, Yasko AW. Core needle biopsy and fine needle aspiration in the diagnosis of bone and soft tissue lesions. Hematol Oncol Clin North Am. 1995;9:633–651. [PubMed] [Google Scholar]
- 2.Bickels J, Jelinek J, Shmookler B, Neff RS, Malawer MM. Biopsy of muscoluloskeletal tumors. Current concepts. Cinical Orthop Relat Res. 2001;368:212–219. [PubMed] [Google Scholar]
- 3.Campanacci M, Mercuri M, Gamberini G. Biopsy. Chir Organi Mov. 1995;80(2):113–123. [PubMed] [Google Scholar]
- 4.Costa MJ, Campman SC, Davis RL, Howell LP. Fine needle aspiration cytology of sarcoma: retrospective review of diagnostic utility and specificity. Diagn Cytopathol. 1996;15:23–32. doi: 10.1002/(SICI)1097-0339(199607)15:1<23::AID-DC6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Dondelinger RF, Vanderschelden P, Capasso P, Trotteur G. Spiral tomodensitometry applied to interventional procedures. J Belge Radiol. 1995;78:118–125. [PubMed] [Google Scholar]
- 6.Hau MA, Kim JI, Kattapuram S, Hornicek FJ, Rosenberg AE, Gebhardt MC, Mankin HJ. Accuracy of CT-guided biopsies in 359 patient with musculoskeletal lesions. Skeletal Radiol. 2002;31(6):349–353. doi: 10.1007/s00256-002-0474-3. [DOI] [PubMed] [Google Scholar]
- 7.Issakov J, Flusser G, Kollender Y, Merimsky O, Lifschitz-Mercer B, Meller I. Computed tomography-guided core needle biopsy for bone and sof tissue tumors. Isr Med Assoc J. 2003;5:28–30. [PubMed] [Google Scholar]
- 8.Kattapuram SV, Rosenthal DI. Percutaneous needle biopsy of the spine. In: Sundarsen N, Schidek HH, Schliller AL, Rosenthal DI, editors. Tumor of the spine: diagnosis and clinical management. Philadelphia: WB Saunders; 1990. pp. 45–61. [Google Scholar]
- 9.Kornblum MB, Wesolowski DP, Fischgrund, Herkowitz HN (1998) Computed tomography-guided biopsy of the spine. A review of 103 patients. Spine 23(1):81–85 [DOI] [PubMed]
- 10.Layfield LF, Anders KH, Glasgow BJ, Mirra JM. Fine needle aspiration of primary soft tissue lesion. Arch Pathol Lab Med. 1986;110:420–424. [PubMed] [Google Scholar]
- 11.Lis E, Bilsky MH, Pisinski L, Boland P, Healey JH, O’Malley B, Krol G. Percutaneous CT-guided biopsy of osseous lesion of the spine in patients with known or suspected malignancy. Am J Neuroradiol. 2004;25(9):1583–1588. [PMC free article] [PubMed] [Google Scholar]
- 12.Madhavan VP, Smile SR, Chandra SS, Ratnakar C. Value of core needle biopsy in the diagnosis of soft tissue tumours. Indian J Pathol Microbiol. 2002;45:165–168. [PubMed] [Google Scholar]
- 13.Mankin HJ, Lange TE, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg. 1982;64A(8):1121–1127. [PubMed] [Google Scholar]
- 14.Mankin HJ, Mankin CJ, Simon MA. The hazards of biopsy, revisited. J Bone Joint Surg Am. 1996;78(5):656–663. doi: 10.2106/00004623-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Monti C, Rimondi E, Rollo G, Bettini N, Picci P, Marchi M. Percutaneous computed tomography-guided biopsy in spinal diseases. Radiol Med. 1994;87(3):299–304. [PubMed] [Google Scholar]
- 16.Ottolenghi CE. Aspiration biopsy of the spine. Technique for the thoracic spine and results of twenty-eight biopsies in this region and over-all results of 1050 biopsies of other spinal segments. J Bone Joint Surg Am. 1969;51(8):1531–1544. [PubMed] [Google Scholar]
- 17.Puri A, Shingade V, Agarwal M, Anchan C, Juvekar S, Desai S, Jambhekar NA. CT-guided percutaneous core needle biopsy in deep seated musculoskeletal lesions: a prospective study of 128 cases. Skeletal Radiol. 2006;35(3):138–143. doi: 10.1007/s00256-005-0038-4. [DOI] [PubMed] [Google Scholar]
- 18.Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge savings of outpatient core needle biopsy compared with open biopsy of muscoloskeletal tumors. J Bone Joint Surg Am. 1996;78(5):644–649. doi: 10.2106/00004623-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Welker JA, Henshaw RM, Jelinek J, Shmooker BM, Malawer MM. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses: outcomes analysis of 155 patients at a sarcoma referred Center. Cancer. 2000;89:2677–2686. doi: 10.1002/1097-0142(20001215)89:12<2677::AID-CNCR22>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Yang YJ, Damron TA. Comparison of needle core biopsy and fine needle aspiration for diagnostic accuracy in musculoskeletal lesions. Arch of Pathol Lab Med. 2004;128(7):759–764. doi: 10.5858/2004-128-759-CONCBA. [DOI] [PubMed] [Google Scholar]