Abstract
The aim of this study is to assess the prevalence low bone mass among girls with adolescent idiopathic scoliosis (AIS) and their siblings. The subjects of this study were Saudi Arabian girls with AIS. Patients had their weight and height measured to calculate their body mass index (BMI). Clinical examination and investigations were done to rule out any other cause of scoliosis. All had bone mineral density (BMD) measurement of hip area and the spine using DEXA scan, Hologic Inc. Patients with a BMD of < −2.6 was taken as osteoporotic and those between < −1 and −2.5 was taken as osteopenic for analysis. As control subjects, siblings of the patients with normal spine had their BMI calculated and BMD measurement done. We were able to analyze the data of 32 girls with an average age of 18.42 ± 5.71 (14–26) years with mean BMI of 17.7 ± 0.69 (16.5–18.5) kg/M2. Analysis of the scans of the hip revealed that 62.5% of the patients were osteoporotic with BMD of 0.837 (0.697–0.936) ± 0.04, T-score −3.8 ± 0.56 (−2.6 to −3.9) and Z-score. Nine (28.1%) were osteopenic with BMD of 0.768 ± 0.15 (0.638–0.878), mean T-score of −1.6 (−1.1 to 2.5) and Z-score −3.5 ± 0.63 (−2.9 to −3.9). Analysis of BMD of the spine showed similar results. In comparison to the scoliotics, girls with normal spine had higher BMI and BMD which was statistically significant at P < 0.001. T- and Z-score was also lower in scoliotic girls in comparison with girls with normal spine significant at P < 0.001 (CI 95%). Our study indicates that the scoliosis causes osteopenia and osteoporosis among girls while their siblings with normal spine remain with normal bone mass.
Keywords: Adoloscent idiopathic scoliosis, Osteopenia, Osteoporosis, Low bone mass, Bone mineral density
Introduction
Scoliosis is a deformity in which there is lateral curvature of the either thoracic or lumbar spine or together with more than 10° on a standard erect radiograph. About 10 percent of the curves progress which require surgical intervention [14]. Two to four percent of children between the age 10 and 16 years, girls more than boys are affected [16]. Reports indicate that 27–38% of the girls with scoliosis are osteopenic [3, 5, 16], and recently it was suggested that the presence of osteopenia may be taken as a prognostic factor in the progression of the curve [10].
Skeletal deformities which include scoliosis was reported to be 1.16% among Saudi Arabian school going male children [7] and the prevalence of scoliosis among school going children is not know even though screening for scoliosis was advocated [17]. It have been shown that osteopenia and osteoporosis to be common among postmenopausal women [8, 18–20], but studies indicated that there was lower incidence of osteopenia among healthy boys and girls between 25 and 30 age groups compared to other countries [1, 21]. Reports in the literature indicate high prevalence of low bone mass among girls with AIS, but it is yet to be proved that scoliosis causes low bone mass. To date we did not find any study which has compared the BMD of patients with AIS and their siblings, which could give an indication whether scoliosis inherently cause osteopenia and osteoporosis. This study is done to assess the prevalence of low bone mass among Saudi Arabian girls with scoliosis and to compare them with their siblings.
Patients and methods
Saudi Arabian girls with adolescent idiopathic scoliosis (AIS) were subjects of this study. A verbal consent was taken from the parents to include in the study. Data was gathered on a preset proforma which included age, sex, weight, height, number of siblings, the deformity among other siblings and parents. Total body mass index (BMI) was calculated. Clinical examination and investigations were done to rule out any other cause of scoliosis. AIS was confirmed by clinical examination and erect and bending radiographs. Non-AIS siblings were recruited as controls. All AIS and non-AIS had base line blood hematology and biochemistry tests like, hemoglobin concentration, sickle cell test, electrolytes, serum calcium, phosphorus, alkaline phosphatase levels, areal bone mineral density (BMD) measurement of proximal femur and lumbar spine using DEXA scan, Hologic Inc. The T-score compares the BMD at peak bone mass of 30 years of age group, whereas Z-score compares the BMD of same, age and sex. As per WHO criteria, osteoporosis is defined as T-score of <−2.6 and osteopenia between <−1 and −2.5 was taken as osteopenic for analysis. As the study group and the control had not reached peak bone mass (30 years), Z-score was taken for analysis of osteoporosis and osteopenia. The data was entered in the database and analyzed using Statistical Package for the Social Sciences (SPSS, Chicago, Illinois). Means were compared using student’s t test and Chi-square as needed and statistical significance of P value of <0.05 with confidence Interval of 95%. The study was approved by the Ethical and Research committee of the College of Medicine, King Faisal University Dammam and King Fahd Hospital of the University, Al Khobar.
Results
We were able to analyze the data of 32 girls with AIS and 27 girls in the control group. The average age of AIS group was 18.42 ± 5.71 (14–26) and non-AIS group was 17.65 ± 4.5 (14–25) years. All the children in the non-AIS group were female siblings. The mean BMI of the AIS group was 17.7 ± 0.69 (16.5–18.5) compared to the control group which was 19.84 ± 0.6 (18.9–20.5) P ≤ 0.01.
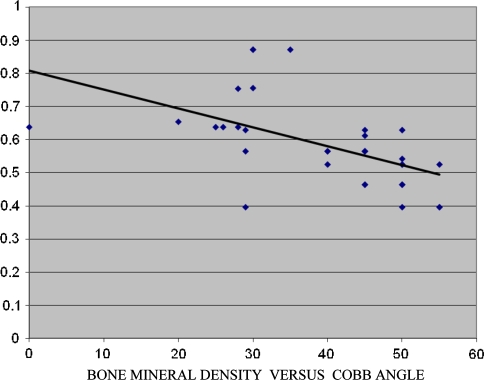
Analysis of the scans of the hip revealed that 62.5% of the patients were osteoporotic with BMD of 0.837 (0.697–0.936) ± 0.04, T-score −3.8 ± 0.56 (−2.6 to −3.9) and Z-score −3.5 ± 0.63 (−2.9 to −3.9) (Table 1). Nine (28.1%) were osteopenic with BMD of 0.768 ± 0.15 (0.638–0.878), mean T-score of −1.6 (−1.1 to 2.5) and Z-Score −3.5 ± 0.63 (−2.9 to −3.9). Analysis of BMD of the spine showed similar results (Table 2); 29/32 scoliotic girls had low bone mass (Osteopenia and osteoporosis). Nineteen of the 29 girls had a Cobb angle >30º (range 35–55) and 10/29 had a Cobb angle of <29º (range 20–29). Patients with Cobb angle >30º had low bone mass in 65% when compared to 35% in girls with Cobb angle <30º. Figure 1 gives the regression analysis between BMD and Cobb angle. In comparison to the scoliotics, girls with normal spine had a normal BMI and BMD which was statistically significant at P < 0.001, Table 3. Standardization of the BMD to BMI showed that BMD was significantly lower in the scoliotic patients than the controls P ≤ 0.023 (Table 3). T- and Z-Score was also lower in scoliotic girls in comparison with girls with normal spine significant at P < 0.001 (CI 95%). Among the non-AIS group 3/27 were osteopenic and none of the girls were osteoporotic. There was no significant differences between the other parameters.
Table 1.
Age and DEXA results of the upper femur
| Normal | Osteopenic | Osteoporotic | |
|---|---|---|---|
| Number | 3 | 9 | 20 |
| Age (years) | 18.33 ± 6.65 (14–22) | 18.42 ± 4.5 (14–25) | 17.8 ± 5.5 (14–26) |
| BMC g/cm | 22.28 ± 2.17 (19.28–23.15) | 20 ± 2.94 (19.8–22.3) | 17.32 ± 1.12 (18.5–19.02) |
| BMD g/cm2 | 0.984 ± 0.07 (0.964–1.02) | 0.837 ± 0.04 (0.697–0.936) | 0.768 ± 0.15 (0.638–0.878) |
| T-score | −0.60 ± 0.07 (−0.6 to −0.7) | −1.9 ± 0.56 (−1.1 to −2.4) | −3.8 ± 0.56 (−2.6 to −3.9) |
| Z-score | −0.26 ± 0.28 (−0.1 to −0.6) | −1.7 ± 0.51 (−1.1 to −2.4) | −3.5 ± 0.63 (−2.9 to −3.9) |
Table 2.
Age and DEXA results of the spine (L1–L4)
| Normal | Osteopenic | Osteoporotic | |
|---|---|---|---|
| Number | 3 | 9 | 20 |
| Age (Years) | 18.33 ± 6.65 (14–22) | 18.42 ± 4.5 (14–25) | 17.8 ± 5.5 (14–26) |
| BMC g/cm | 43.12 ± 0.62 (42.76–43.85) | 39.20 ± 1.94 (37.8–42.3) | 37.32 ± 1.2 (35.5–39.02) |
| BMD g/cm2 | 1.025 ± 0.04 (0.994–1.083) | 0.702 ± 0.15 (0.638–0.818) | 0.536 ± 0.08 (0.417–0.635) |
| T-score | 0.43 ± 0.21 (0.3–0.6) | −2.19 ± 0.56 (−1.9 to −2.4) | −4.1 ± 0.67 (−3.7 to −4.8) |
| Z-score | 0.6 ± 0.2 (−0.1 to 0.4) | −1.9 ± 0.61 (−1.8 to −2.7) | −3.6 ± 0.53 (−3.1 to −3.9) |
Fig. 1.
Regression analysis for BMD and Cobb angle
Table 3.
Comparison between scoliotic and nonscoliotic girls
| Scoliotic | Nonscoliotic | P value | |
|---|---|---|---|
| Number | 32 | 27 | |
| Age | 18.42 ± 5.71 (13–26) | 17.65 ± 4.5 (14–25) | 0.5 |
| BMI M/kg2 | 17.7 ± 0.69 (16.5–18.5) | 19.84 ± 0.6 (18.9–20.5) | <0.01 |
| BMC g/cm | 19.02 ± 4.42 (12.37–24.91) | 23.1 ± 5.94 (19.8–25.3) | <0.001 |
| BMD g/cm2 | 0.584 ± 0.15 (0.396–0.872) | 0.968 ± 0.19 (0.886–1.06) | <0.0001 |
| Standardized BMD/BMI% | 4.29 ± 0.0.73 | 4.68 ± 0.52 | <0.023 |
| Osteoporotic | 20 | 0 | <0.0001 |
| Osteopenia | 9 | 3 | <0.01 |
| T-score | −2.34 ± 1.08 (−0.6 to −3.7) | −0.9 ± 0.50 (−0.1 to −1.4) | <0.001 |
| Z-score | −2.25 ± 1.05 (−0.6 to −3.7) | −0.9 ± 0.51 (−0.5 to −1.7) | <0.001 |
Discussion
Recent understanding of the three-dimensional deformity of the scoliosis has added more insight in the development of the newer and better implants and revised techniques of early fixation of the curves with fusion. Animal studies have shown that there is delayed healing in osteoporotic bone [13] and human studies indicate in osteoporotic bone there is altered fracture healing [9]. Whether osteoporosis in young scoliotics cause failure of spinal fusion postoperatively is to be proved. Burner et al. [2] first raised the concern of osteopenia in AIS children using plain radiographs, but Cook et al. [6] using DEXA showed that low bone mass was common in children of AIS. Thomas et al. [22] found 50% of the children they followed up with scoliosis had osteoporosis, whereas Cheng and Guo [5] reported the prevalence of osteopenia to be 58.7% and osteoporosis of 18.7%. Firstly, we found in our study that 68% were osteoporotic, which is higher than reported in the literature and secondly patients with higher curves of >30° were significantly had low bone mass compared to those with curves < 30º (65 vs. 35%).
Why patients with AIS should suffer from low bone mass has still not passed the speculation stage. Which comes first the deformity or low bone mass? Lee [11] reported that patients with AIS had low calcium intake and this was a contributing factor in the development of scoliosis. Cheung et al. [4] suggested that the low bone mass could be due to abnormally faster growth at adolescence with low calcium intake cause abnormal mineralization. Apart from the above mentioned factors low BMI could be a contributing factor in low BMD in scoliotic patients.
The cause or causes of progression of curves in scoliosis is still evolving. Multiple factors have been suggested to be prognostic in the progression of the curve. Lonstein and Carlson [12], initially proposed a three-factor prognostic index (initial Cobb angle, Risser grade and chronological age) and later Peterson and Nachemson [15] proposed a four-factor regression equation. The report of Hung et al. [10] sheds light on this important aspect of progression of curves. In their study they found that osteopenia was an important risk factor in the progression of the curves in patients with AIS. They believe that the Z-score of bone mineral density should be added to the other predictive factors of the progression of curves.
This study has few limitations. The numbers in the study and control group were small and secondly we needed to check the Vitamin D levels to know whether osteomalacia played any role in the attainment of low bone mass. In conclusion our study suggests that scoliosis does induce low bone mass while siblings remain with normal bone mineral density. Moreover, the severity of osteopenia and osteoporosis depends on the degree of Cobb angle in children with AIS.
References
- 1.Ardawi MSM, Maimany AA, Bahksh TM, Nasrat HAN, Milaat WA, Al-Raddadi RM. Bone mineral density of the spine and femur in healthy Saudis. Osteop Int. 2005;16:43–55. doi: 10.1007/s00198-004-1639-9. [DOI] [PubMed] [Google Scholar]
- 2.Burner WL, Badger VM, Sherman FC. Osteoporosis and acquired back deformities. J Pediatr Orthop. 1982;2:383–385. doi: 10.1097/01241398-198210000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Cheng JC, Qin L, Cheung CS, Sher AH, Lee KM, Ng SW, Guo X. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15:1587–1595. doi: 10.1359/jbmr.2000.15.8.1587. [DOI] [PubMed] [Google Scholar]
- 4.Cheung CSK, Lee WTK, Tse YK, Lee KM, Guo X, Qin L, et al. Generalized osteopenia in adolescent idiopathic scoliosis-association with abnormal pubertal growth, bone turnover, and calcium intake. Spine. 2006;31:330–338. doi: 10.1097/01.brs.0000197410.92525.10. [DOI] [PubMed] [Google Scholar]
- 5.Chung JCY, Guo X. Osteopenia in adolescent idiopathic scoliosis—a primary problem or secondary to the spinal deformity? Spine. 1997;22:1716–1721. doi: 10.1097/00007632-199708010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cook SD, Harding AF, Morgan EL, Nicholson RJ, Thomas KA, Whitecloud TS, Ratner ES. Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop. 1987;7:168–174. doi: 10.1097/01241398-198703000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Corea JR, Maqbool G, Al-Arfaj AL, Sankaran-Kutty M (1987) Screening for musculoskeletal deformitiesin school children. KASCT Project Final Report; p 17
- 8.El-Desouki M. Osteoporosis in postmenopausal Saudi women using dual X-ray bone densitometry. Saudi Med. 2003;J24:953–956. [PubMed] [Google Scholar]
- 9.Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury. 2007;38(Suppl 1):S90–S99. doi: 10.1016/j.injury.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Hung VWY, Cheung CSK, Lam TP, Ng BKW, Tse YK, Guo X et al. (2005) Osteopenia: a new prognostic factor of curve progression in Adolescent Idiopathic Scoliosis. J Bone Joint Surg 87-A(12):2709–2716 [DOI] [PubMed]
- 11.Lee WTK. Generalized low bone of girls with adolescent idiopathic scoliosis is related to inadequate calcium intake and weight bearing physical activity in prepubertal period. Osteoporosis Int. 2005;16:1024–1035. doi: 10.1007/s00198-004-1792-1. [DOI] [PubMed] [Google Scholar]
- 12.Lonstein JE, Carlson JM (1984) The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg 66-A:1061–1071 [PubMed]
- 13.McCann RM, Colleary G, Geddis C, Clarke SA, Jordan GR, Dickson GR, Marsh D (2007) Effect of osteoporosis on bone mineral density and fracture repair in a rat femoral fracture model. J Orthop Res (Epub ahead of print) [DOI] [PubMed]
- 14.Miller NH. Cause and natural history of adolescent idiopathic scoliosis Ortho Clin N Am. 1999;30:343–352. doi: 10.1016/s0030-5898(05)70091-2. [DOI] [PubMed] [Google Scholar]
- 15.Peterson LE, Nachemson AL. Prediction of progression of the curve in girls who have adolescent idiopathic scoliosis of moderate. Logistic regression analysis based on data from the Brace Study of the Scoliosis Research Society. J Bone and Joint Surg. 1995;77:823–827. doi: 10.2106/00004623-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Roach JW. Adolscent idiopathic scoliosis. Ortho Clin N Am. 1999;30:353–365. doi: 10.1016/S0030-5898(05)70092-4. [DOI] [PubMed] [Google Scholar]
- 17.Sadat-Ali M. School screening for scoliosis. Have we done enough. Saudi Med J. 1998;19(2):210–211. [PubMed] [Google Scholar]
- 18.Sadat-Ali M, Al-Habdan I, Fatma Al-Mulhim, Yousef A. Bone mineral density among postmenopausal Saudi Arabian women. Saudi Med J. 2004;25(11):1623–1625. [PubMed] [Google Scholar]
- 19.Sadat-Ali M, Al-Habdan I, Al-Mulhim AA, Yousef A. Effect of parity on bonemineral density in postmenopausal Saudi women. Saudi Med J. 2005;26(10):1588–1590. [PubMed] [Google Scholar]
- 20.Sadat-Ali M, El-Hassan AY, Ezzeldin IM, Al-Frehi H, Al-Mohanna F. Postmenopausal osteoporosis in Saudi women: a pilot screening. Ann Saudi Med. 1993;13:272–274. doi: 10.5144/0256-4947.1993.272. [DOI] [PubMed] [Google Scholar]
- 21.Sadat-Ali M, Al-Habdan I, Marwah S. Bone mineral density measurements of distal radius in Saudi Arabian females. Ann Saudi Med. 1996;16(4):414–416. doi: 10.5144/0256-4947.1996.414. [DOI] [PubMed] [Google Scholar]
- 22.Thomas KA, Cook SD, Skalley TC. Lumbar spine and femoral mineral density in idiopathic scoliosis. A follow up study. J Pediatr Orthop. 1992;12:235–240. doi: 10.1097/01241398-199203000-00016. [DOI] [PubMed] [Google Scholar]