Abstract
In this study the MTP1 gene, encoding a type III integral transmembrane protein, was isolated from the rice blast fungus Magnaporthe oryzae. The Mtp1 protein is 520 amino acids long and is comparable to the Ytp1 protein of Saccharomyces cerevisiae with 46% sequence similarity. Prediction programs and MTP1-GFP (green fluorescent protein) fusion expression results indicate that Mtp1 is a protein located at several membranes in the cytoplasm. The functions of the MTP1 gene in the growth and development of the fungus were studied using an MTP1 gene knockout mutant. The MTP1 gene was primarily expressed at the hyphal and conidial stages and is necessary for conidiation and conidial germination, but is not required for pathogenicity. The Δmtp1 mutant grew more efficiently than the wild type strain on non-fermentable carbon sources, implying that the MTP1 gene has a unique role in respiratory growth and carbon source use.
Keywords: Magnaporthe oryzae, Magnaporthe grisea, MTP1, cDNA, Gene knockout, Conidial germination
INTRODUCTION
Magnaporthe oryzae, distinguished from Magnaporthe grisea (Couch and Kohn, 2002), is a major fungal pathogen of rice and other cereals, causing devastating rice blast disease throughout the world (Ou, 1985). In China more than 3.8×106 ha of rice has been exposed to this disease every year since 1990. Much has been learned of the molecular mechanisms regulating pre- and post-penetration events in the development and pathogenicity of M. oryzae, such as those involved in appressorium formation and invasion growth (Lee and Dean, 1993; Xu and Hamer, 1996; Choi and Dean, 1997; DeZwaan et al., 1999; Clergeot et al., 2001; Kim et al., 2005; Zhao et al., 2005; Veneault-Fourrey et al., 2006a; Odenbach et al., 2007). Among those known important genes, Pth11 is a plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues (DeZwaan et al., 1999). Pls1 is another transmembrane protein localized in plasma membranes and vacuoles of appressoria, required for the penetration of the rice leaf by the fungal pathogen (Clergeot et al., 2001; Veneault-Fourrey et al., 2006b). However, the functions of most transmembrane proteins in this fungal pathogen have not been fully characterized. The plasma membrane is an interface interacting with plant cells when the pathogen penetrates into or grows in the plant. The signals outside of the cell are imported through the cell membrane to activate fungal developmental events, which may result in cell differentiation or growth (DeZwaan et al., 1999; Gronover et al., 2001; Dean et al., 2005). The vacuole membrane is involved in autophagy, which is required for the pathogenicity of the rice blast fungus (Veneault-Fourrey et al., 2006a; Liu et al., 2007). The proteins integrated into the mitochondrial membrane are also necessary for the function of mitochondrion. The mitochondrion is an important organelle and is the site at which fatty acid β-oxidation occurs, which is necessary to synthesize glycerol in the appressorium from storage products (Dean et al., 2005). Glycerol is necessary in order to generate turgor pressure in an appressorium, which is required for plant penetration and pathogenicity (de Jong et al., 1997).
MTP1 gene, encoding a type III integral transmembrane protein, is one of hundreds of genes isolated from the M. oryzae appressorium cDNA library in our previous study (Lu et al., 2005a). Little is known about the functional and pathological significance of the Mtp1 protein in the rice blast fungus, or its homologues in other filamentous fungi. In this study we report the molecular structure of the MTP1 gene which encodes a transmembrane protein with 8 membrane helices, its expression profile during growth and development and its function which was elucidated through gene knockout experiments in M. oryzae.
MATERIALS AND METHODS
Fungal strain, DNA/RNA isolation and manipulation
The M. oryzae wild type strain, Guy-11, and mutants (transformants and an MTP1-knockout mutant generated from Guy-11) were cultured on complete medium (CM) plates (Talbot et al., 1993) at 25 °C with a 14-h light and 10-h dark cycle using fluorescent lights. Studies involving a cross with strain 2539 were conducted on oat meal agar (OMA) medium (30 g oatmeal in 1 L distilled water). DNA and total RNA extraction, polymerase chain reaction (PCR), restriction digest reaction, gel electrophoresis and ligation reaction were carried out following standard procedures (Sambrook et al., 2002).
Isolation and sequence analysis of MTP1
The cDNA fragment containing the full coding sequence of the MTP1 gene was cloned from the cDNA library (Lu et al., 2005b) by PCR using the primers s148RNAp1 (5′-CCACCACGGCTTCAATCCCGCGAC-3′) and s148RNAp2 (5′-GATGCACATCGGTGCAGAGCTGTC-3′), cloned into the T-vector, pUCm-T (Sangon, Shanghai, China), and sequenced on an ABI377 DNA sequencer (Invitrogen, USA). The DNA fragment containing the MTP1 gene was amplified from Guy-11 genomic DNA using the primers s148p1 (5′-GTTTGGTTAATTGTCTTCCCGGCTGTTTTG-3′) and s148p2 (5′-CACCCCCTTCCCGAGTCTTGATTTGTTGT-3′), cloned into the pCR-XL®-TOPO® vector (Clontech, USA), and sequenced.
The putative protein Mtp1 was predicted for homologies by using the Blastp (Altschul et al., 1997) and ClustalW (http://www.ebi.ac.uk/clustalw/) (Thompson et al., 1994), for subcellular localization by TargetP 1.1 (http://www.cbs.dtu.dk/services/TargetP-1.1/) (Emanuelsson et al., 2000) and WoLFPSORT (http://wolfpsort.seq.cbrc.jp) (Horton et al., 2006), for the presence and location of signal peptide cleavage sites by SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP/) (Dyrløv Bendtsen et al., 2004), and for protein structure by Predictprotein (http://www.predictprotein.org/) (Rost et al., 2004).
Construction of the MTP1 knockout vector
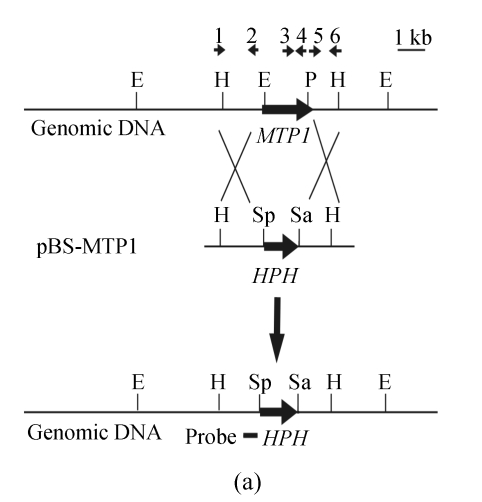
The knockout vector, pBS-MTP1, was constructed by inserting two flanking sequences of MTP1 gene into the pBS-HPH2 vector. pBS-HPH2, an intermediate vector, was constructed by inserting a 1344-bp SalI-BamHI PCR fragment containing a hygromycin phosphotransferase (HPH) gene expression cassette into the SalI-BamHI sites of the pBS(+) vector (Stratagene, USA). The HPH gene expression cassette fragment was prepared by PCR amplification from the plasmid pCB1003 (Carroll et al., 1994) by using the primers 5′-CCggatccTGGAGGTCAACACATCAAT-3′ and 5′-CCgtcgacCTACTCTATTCCTTTGCCCTCG-3′. A 1240-bp KpnI-SalI downstream flanking sequence fragment of MTP1, amplified from genomic DNA using the primers s148dnp1 (5′-AAggtaccTGCTGCTGTTACCGCGAAGGATT-3′) and s148dnp2 (5′-AAgtcgacAAGAGGCGGCGAAGAGGAAGTTAC-3′), was firstly inserted into the KpnI-SalI sites of pBS-HPH2 and then another 1231-bp SpeI-NotI upstream flanking sequence fragment obtained by PCR with the primers s148upp1 (5′-AAggtaccTGCTGCTGTTACCGCGAAGGATT-3′) and s148upp2 (5′-AAgtcgacAAGAGGCGGCGAAGAGGAAGTTAC-3′) was inserted into the SpeI-NotI sites of pBS-HPH2 to generate the knockout vector pBS-MTP1. The 3.5-kb HindIII digested pBS-MTP1 vector was used to transform protoplasts of Guy-11.
Fungal transformation and identification of MTP1 knockout mutants
The transformation procedure, including protoplast generation, was conducted as described previously (Lu et al., 2007a). Transformants were recovered and selected in CM with 20% (w/v) sucrose and 200 μg/ml hygromycin B (Roche Diagnostics GmbH, Mannheim, Germany). Transformants were further confirmed by passing through a second selection in the hygromycin B media.
Initial identification of the knockout mutants was performed by PCR using primers internal to MTP1 (s148ckp1: 5′-CGGCGTCGTCCCTCGTGTTGG-3′ and s148ckp2: 5′-TCGCGGCGCTGGGATTCTGC-3′), resulting in amplification of an 832-bp fragment of the MTP1 gene. Putative knockout transformants identified by PCR screens were purified by single conidium isolation, and confirmed further by Southern hybridization analysis. Genomic DNAs from transformants were prepared, and cut with the EcoRV restriction enzyme for Southern blot analysis. A 547-bp DNA fragment located in the upstream flanking sequence of MTP1 gene was obtained by PCR from genomic DNA with the primers s148sb2p1 (5′-CGAAACGGCGAAGACGAA-3′) and s148sb2p2 (5′-GCCGCCGCCCGCCAACAT-3′), and used as a probe for Southern blot. Southern blot hybridizations were conducted using the DIG high prime DNA labeling and detection starter kit I (Roche Diagnostics GmbH, Mannheim, Germany).
Construction and expression of the MTP1-GFP fusion protein
A 1.4-kb HPH gene fragment was amplified from the plasmid pCB1003 with the primers p1 (5′-ACgcggccgcGGAGGTCAACACATCAATG-3′) and p2 (5′-CCaggcctGGTCGGCATCTACTCTATTC-3′) and cloned into the NotI-StuI sites of pEGFP (Clontech, USA), resulting in generation of the plasmid pEGFP-HPH. A 1534-bp upstream sequence of MTP1 was amplified from Guy-11 genomic DNA with the PCR primers s148pro-p1 (5′-CTGAggatccAGCAGACGACGCGGAAGAAGAGATGAGAC-3′) and s148pro-p2 (5′-GTGTggatccCGCGGTTTAGATGCTGGTAGTATTTTG-3′) and cloned into the BamHI site of pEGFP-HPH to generate pEGFP-MTP1. In pEGFP-MTP1, the green fluorescent protein (GFP) construct was under the control of the native MTP1 promoter.
The promoter sequence of the NAR gene (Lu et al., 2007b) was amplified from Guy-11 genomic DNA using the PCR primers 5′-CaggatccGGGAAGCGATTGCGTT-3′ and 5′-GGTGccatggTGTCGGTTGTGGTG-3′, and inserted into the BamHI-NcoI sites of pEGFP to produce the pEGFP-NAR vector. The GFP construct without a stop codon (TAA) under the control of the NAR promoter was amplified from pEGFP-NAR with the primers 5′-CTgtcgacGGGAAGCGATTGCGTTT-3′ and 5′-TTcccgggCTTGTACAGCTCGTCCATGC-3′, and cloned into the SalI-SmaI sites of the pBS(+) vector to generate the vector pBS-NE. Subsequently the coding domain of the MTP1 fragment without the initiation codon (ATG) was amplified using the primers 5′-TTcccgggGCGTGGTCGAGAATTGGGAT-3′ and 5′-TTtctagaTTTCACGCAACCCGATCGCTAT-3′ from MTP1 cDNA and inserted into the SmaI-XbaI sites of pBS-NE to generate pBS-NEM. Then a 0.9-kb BAR gene fragment was obtained from pBARKS1 (Pall and Brunelli, 1993) via PCR using the primers 5′-AatctagAGAAGATGATATTGAAGGA-3′ and 5′-AatctagaCTAAATCTCGGTGACGGGC-3′, and cloned into the XbaI site of pBS-NEM to generate pEGFP-MTP2. In the pEGFP-MTP2 plasmid, the resulting MTP1-GFP fusion construct was under the control of the NAR promoter.
The resulting vector, pEGFP-MTP1, was transformed into protoplasts of Guy-11 and selected with 200 μg/ml hygromycin B. Another resulting vector, pEGFP-MTP2, linearized with KpnI, was transformed into protoplasts of the Δmtp1 mutant and selected with 150 μg/ml glufosinate-ammonium (Yongnong Ltd., Zhejiang, China). Hygromycin-resistant transformants or glufosinate-ammonium-resistant transformants were individually confirmed by DNA gel blot analysis. The expression of GFP was examined using an Olympus-BX51 fluorescence microscope with a cooled CCD camera DP50 (Olympus, Japan) and Zeiss LSM-510 laser scanning microscope (Carl Zeiss, Germany).
Growth characteristics of the Δmtp1 mutant
Vegetative growth of both the M. oryzae Δmtp1 mutant and Guy-11 strains was examined following a previously described procedure (Lu et al., 2007a) in CM medium, CM-N medium (CM medium without the nitrogen source), CM-C medium (CM medium without the carbon source), CM-hyperosmotic medium (CM medium with 1 mol/L NaCl) (Talbot et al., 1993) and YPEG medium (1% (w/v) yeast extract, 2% (w/v) bactopeptone, 2% (v/v) ethanol, 3% (v/v) glycerol, 2% (w/v) agar). With the same procedure, the influences of Cu2+ (CuSO4, 1.0×10−4 mol/L) and Fe3+ (FeCl3, 1.0×10−4 mol/L) in 1/4 YG medium (0.13% (w/v) yeast extract, 0.5% (w/v) glucose, 1.5% (w/v) agar) on vegetative growth of the mutant were evaluated. Conidiation, conidial germination and appressorium development of the mutant and Guy-11 were also counted according to a previously described procedure (Lu et al., 2007a). Appressorium turgor of the mutant was estimated using incipient cytorrhysis (cell collapse) assay (Howard et al., 1991).
Pathogenicity assays
The inoculation method was carried out as previously reported (Lu et al., 2007a). Two-week-old seedlings of rice (Oryza sativa cv. CO-39) or 8-day-old barley (Hordeum vulgare cv. ZJ-8) were spray inoculated with conidial suspension (1×105 conidia/ml) and then placed in a controlled environment chamber with a 14-h light and 10-h dark cycle using fluorescent lights for 7 d (rice) or 4 d (barley). Disease severity was recorded according to the method proposed by Bonman et al.(1986).
RESULTS
Isolation of MTP1
An EST (expressed sequence tag) (GenBank_Accn: CK828223), which encodes an M. oryzae transmembrane protein 1 (Mtp1) and is expressed in hyphae, conidium and appressorium, was identified from ESTs of a suppression subtractive hybridization cDNA library of the M. oryzae strain Guy-11 (Lu et al., 2005a). After searching the M. oryzae database (http://www.broad.mit.edu/), this EST was found to correspond to a hypothetical protein, MG05336.5, in strain 70-15. Then the cDNA fragment containing the full coding sequence was cloned from the Guy-11 appressorium cDNA library (Lu et al., 2005b) using high fidelity PCR and subsequently sequenced (GenBank_Accn: DQ085779). The cDNA obtained was 1982-bp long and included 1560 bp of the coding sequence, 195 bp of the 5′ untranslated region and 227 bp of the 3′ untranslated region. The 6073-bp MTP1 genomic DNA fragment was also cloned from genomic DNA of Guy-11 by long distance PCR and sequenced (GenBank_Accn: EF525179). The nucleotide sequence of the genomic MTP1 gene contains a putative 1853-bp open reading frame with three introns that are 140-bp, 89-bp and 61-bp long. The coding sequence of MTP1 encodes a predicted mature polypeptide of 520 amino acids. This polypeptide sequence shows 46% identity in sequence to the Ytp1 protein of Saccharomyces cerevisiae (West et al., 1996), a type III transmembrane protein, as predicted by Blastp and ClustalW (Thompson et al., 1994; Altschul et al., 1997) during sequence similarity searches. The Mtp1 protein also shows high similarity to many proteins in filamentous fungi, such as predicted protein (GenBank_Accn: CAP73000) in Podospora anserine (63% in identity), predicted protein (GenBank_Accn: EAA28954) in Neurospora crassa (62%) and predicted protein (GenBank_Accn: XP_001225052) in Chaetomium globosum (62%). However, these homologues in filamentous fungi have not been studied until now.
Predictprotein (PHDhtm) (Rost et al., 1996) predicted that the Mtp1 protein is likely to be a type III (polytopic) transmembrane protein with 8 membrane helices. WoLFPSORT (Horton et al., 2006) predicted that the Mtp1 protein is a transmembrane protein, probably located at the plasma membrane. However, TargetP 1.1 (Emanuelsson et al., 2000) predicted that Mtp1 protein is most likely to be located in the mitochondrion. SignalP 3.0 (Dyrløv Bendtsen et al., 2004) predicted that the Mtp1 protein is not a secretory protein (by neural network). These predicted results suggested that the Mtp1 protein is a transmembrane protein, possibly located at the mitochondrial membrane or the plasma membrane.
Analysis of GFP expression under the control of the native MTP1 promoter
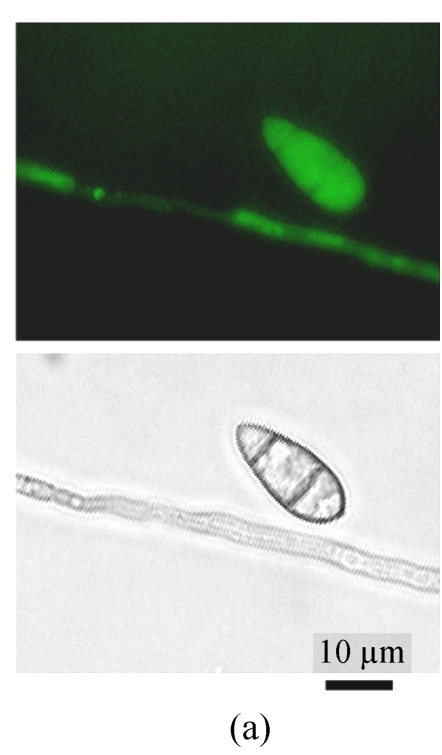
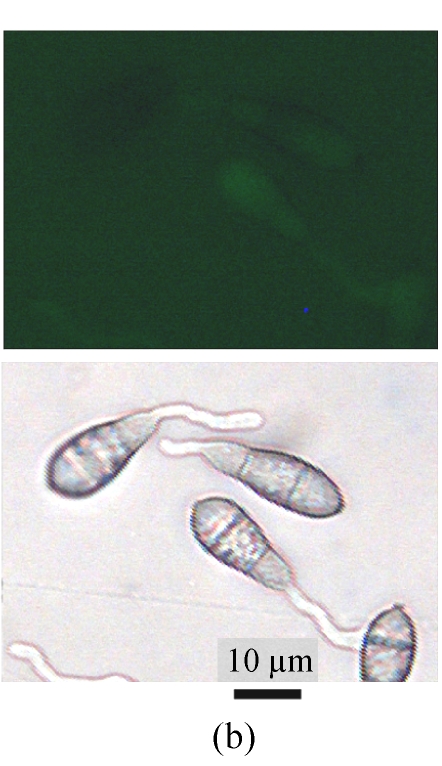
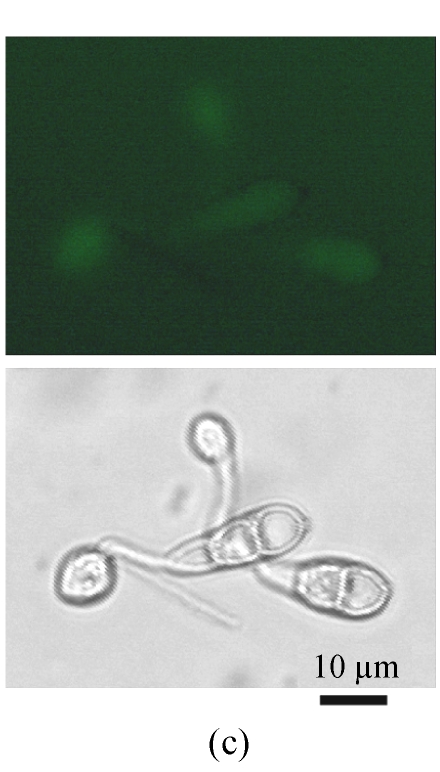
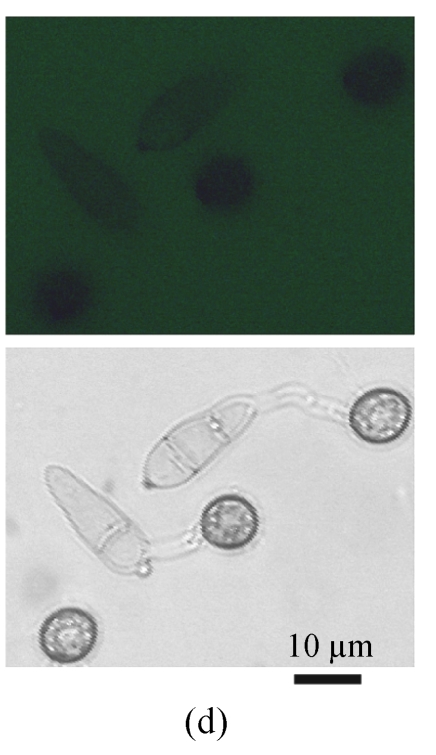
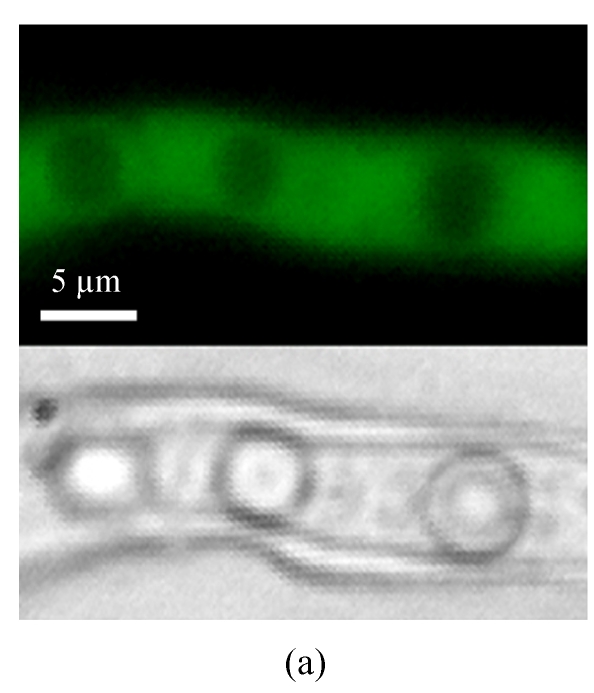
A GFP expression vector (pEGFP-MTP1), under the control of the native MTP1 promoter, was constructed and transformed into the wild type M. oryzae strain. Then the differential expression pattern of the MTP1 gene at various developmental stages was analyzed using seven transformants. Observation of GFP fluorescence in transformants under an Olympus-BX51 epifluorescence microscope showed that GFP was expressed in hyphae and spores. However, it was faintly expressed in germinating spores and appressoria of M. oryzae (Fig.1, see Page 516). This implied that the MTP1 gene is more highly expressed and active in the hyphal and conidial stages than in the appressorial stage.
Fig. 1.
Differential expression pattern of MTP1. The green fluorescence emitted by GFP protein, which was expressed under the control of the MTP1 promoter, was bright in hyphae and conidia (a), faint in germinating conidia at 2 hours post-inoculation (hpi) (b) and forming appressoria at 4 hpi (c) and invisible in mature appressoria at 24 hpi (d) of M. oryzae. The mycelia, conidia and appressoria were observed by fluorescence microscopy (top) and by light microscopy (bottom) in each panel
Knockout of MTP1
A gene knockout strategy was employed to determine the function of MTP1. A knockout vector, pBS-MTP1 (Fig.2a), containing the HPH gene cassette flanked by 5′ and 3′ ends of the MTP1 gene was constructed. One MTP1 knockout mutant (F7) out of nine hygromycin resistant transformants was determined initially by PCR using primers internal to MTP1, and then by DNA gel blot analysis, to have undergone gene deletion. In DNA gel blot analysis a 9.0-kb fragment was detected in the transformant F7 in contrast with a 4.9-kb fragment found in the wild type strain (Fig.2b). The band shift from 4.9-kb to 9-kb showed that the MTP1 gene had been deleted in the transformant F7 (namely Δmtp1 mutant) and that homologous recombination had occurred at a single site.
Fig. 2.
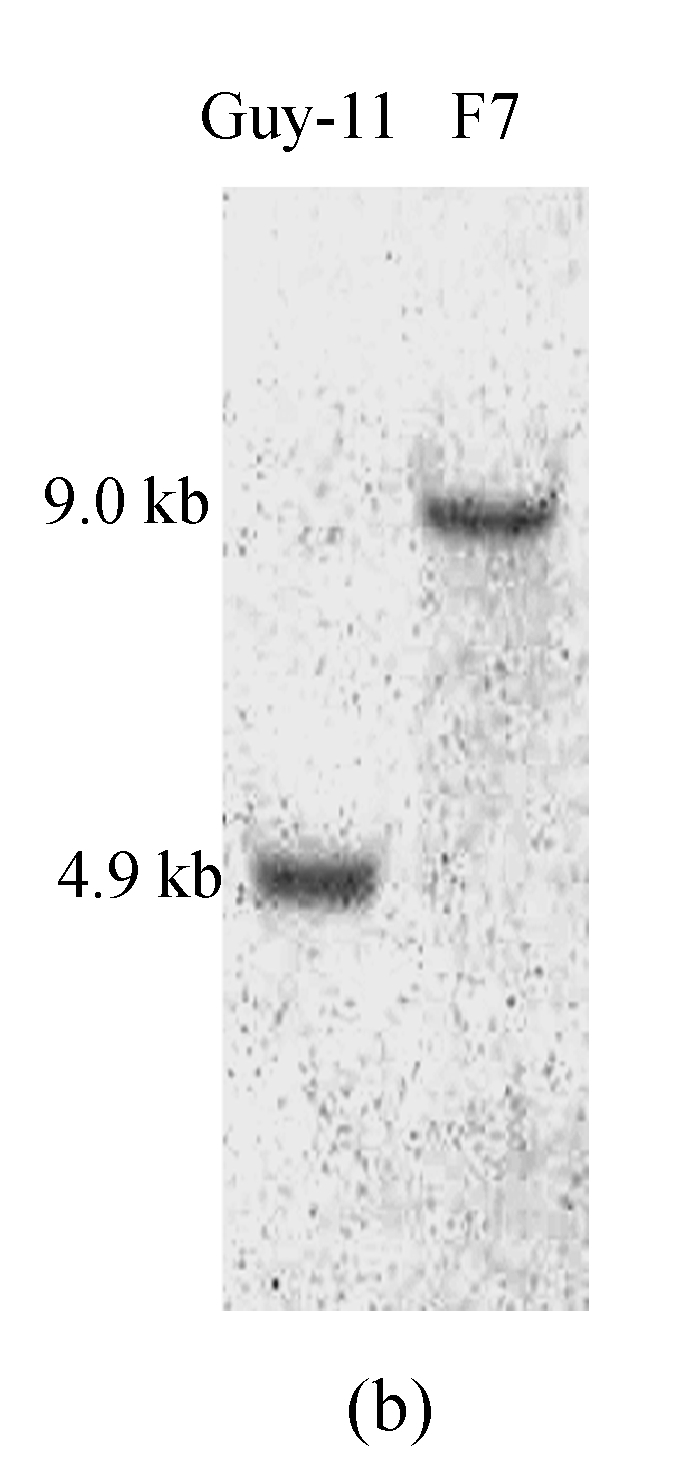
Deletion of the MTP1 gene in M. oryzae. (a) MTP1 locus and knockout vector (pBS-MTP1). Large arrows indicate orientations of the MTP1 and HPH genes. The positions and orientations of primers s148upp1, s148upp2, s148ckp1, s148ckp2, s148dnp1 and s148dnp2 are labeled with small arrows 1, 2, 3, 4, 5 and 6. The HindIII digested fragment containing the gene deletion construct was excised, purified by gel electrophoresis and used to transform M. oryzae Guy-11 protoplasts. E=EcoRV, H=HindIII, P=PvuI, Sa=SalI, Sp=SpeI; (b) DNA gel blot analysis of Δmtp1 mutant. All genomic DNA samples were digested with EcoRV, fractionated and probed with a 547-bp fragment located in the upstream flanking sequence of MTP1 shown in Fig.2a. As expected, a 9-kb band was detected in Δmtp1 mutant F7 in contrast with a 4.9-kb band in the wild type strain Guy-11
Analyses of MTP1-GFP fusion expression
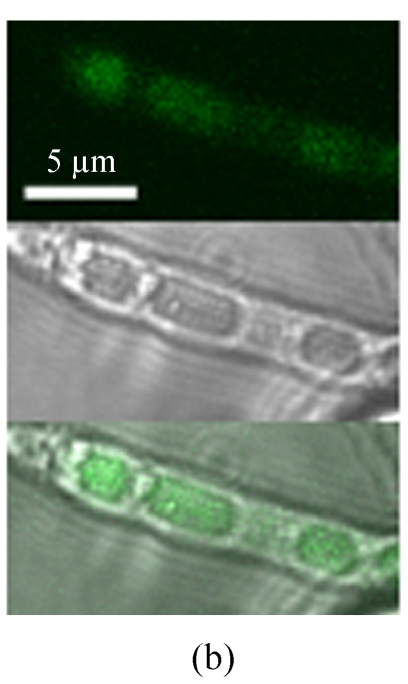
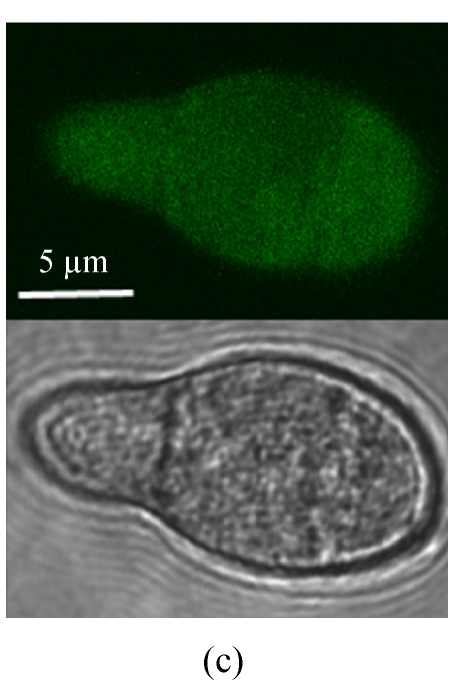
In order to analyze Mtp1-GFP localization in a cell, a MTP1-GFP fusion expression vector (pEGFP-MTP2), under the control of the NAR promoter, was constructed and transformed into the Δmtp1 mutant. When observed under a normal fluorescence microscope, the GFP fluorescence was observed strongly in the hyphal cytoplasm and weakly in the vacuoles of transformants (Fig.3a). However, under a confocal laser scanning microscope the GFP fluorescence was observed also in the hyphal vacuoles (Fig.3b) and in conidial cytoplasm with unidentified minute dot-like structures (Fig.3c). These observations of GFP fluorescence in transformants showed that GFP was located in the cytoplasm associated with the membrane of vacuoles or other membranes in the cytoplasm (Fig.3). Considering the above-mentioned program-predicted results, the MTP1-GFP fusion expression results implied that the possible locations of the Mtp1 protein are membranes on several organella in the cytoplasm.
Fig. 3.
Cellular localization of Mtp1. (a) The mycelia were observed under Olympus-BX51 fluorescence microscopy (top, eGFP; bottom, light); (b) The mycelia were observed under a laser scanning microscope (top, eGFP; middle, light; bottom, merge); (c) The conidia were observed under a laser scanning microscope (top, eGFP; bottom, light). GFP-MTP1 fusion protein appeared in the cytoplasm of the M. oryzae cell, associated with the membrane of vacuoles or other organella in the cytoplasm
Characterization of the Δmtp1 mutant
The growth in colony diameters of the Δmtp1 mutant and the wild type strain was comparable in the CM medium, the CM-N medium, the CM-C medium and the CM-hyperosmotic medium (P≤0.05). The colony growth of the Δmtp1 mutant on 1/4 YG medium was not slowed by adding Cu2+ (CuSO4, 1.0×10−4 mol/L) and Fe3+ (FeCl3, 1.0×10−4 mol/L) (P≤0.05), in contrast to that of the wild type strain. The colony color of the mutant and wild type strains became darker on 1/4 YG medium following the addition of CuSO4. Interestingly the Δmtp1 mutant [(4.44±0.09) cm in colony diameter at 12 days post-inoculation (dpi)] grew faster than the wild type strain [(3.55±0.05) cm at 12 dpi] or MTP1 rescued strain MTG-3, a Δmtp1 mutant in which the MTP1 gene was re-introduced via an MTP1-GFP expression vector (pEGFP-MTP2) [(3.63±0.07) cm at 12 dpi] on the YPEG medium (complete medium containing ethanol and glycerol as a sole non-fermentable carbon source) (P≤0.05). Also, the colony color of the mutant was darker than that of the wild type strain in the YPEG medium. Therefore the Δmtp1 mutant was devoid of colony growth defects in these solid media, and the MTP1 gene is not essential for the mitochondrial respiratory function. In mating experiments, the Δmtp1 mutant formed normal perithecia with viable ascospores after 4 weeks of incubation with the opposite mating-type strain 2539. However, conidiogenesis in the Δmtp1 mutant was reduced significantly. The mutant produced (0.70±0.05)×104 conidia/mm2 on the CM medium plate, approximately close to half of that produced by the wild type strain [(1.30±0.08)×104 conidia/mm2] or MTP1 rescued strain MTG-3 [(1.20±0.07)×104 conidia/mm2] (P≤0.05). In the germination assay, conidia of the Δmtp1 mutant were slower to germinate than those of the wild type strain (Table 1). Two hours after being placed onto plastic covers, only 21.1% of the conidia of the Δmtp1 mutant germinated compared to 90.2% of those of wild type strain. Four hours later the rate of germ tube emergence from the Δmtp1 mutant was still lower than that of the wild type strain. The rate of later appressorium formation in the Δmtp1 mutant was also delayed (Table 1). Six hours post-inoculation (hpi), the rate of appressorium formation in the mutant was 66.3%, while in the wild type strain it was 86.6%. However, 24 h later the rate of appressorium formation was similar in both. In MTP1 rescued strain MTG-3, the defects of the Δmtp1 mutant in conidium germination and appressorium formation were recovered (Table 1). To evaluate the role of MTP1 in appressorium turgor, we measured the turgor of the mature appressorium using the incipient cytorrhysis test described by Howard et al.(1991). The rate of collapsed appressoria in a series of glycerol solutions of varying strengths did not differ significantly between the Δmtp1 mutant and the wild type strain (P≤0.05).
Table 1.
Analysis of conidial germination and appressorium formation
| Strain | Conidial germination (%)1 |
Appressorium formation (%)2 |
||
| 2 hpi | 4 hpi | 6 hpi | 24 hpi | |
| Guy-11 | 90.2±1.9 a | 98.5±0.2 a | 86.6±5.2 a | 99.3±0.4 a |
| F7 | 21.1±0.6 b | 91.0±1.0 b | 66.3±4.0 b | 96.2±1.1 a |
| MTG-3 | 89.3±2.2 a | 96.5±1.8 a | 87.5±5.8 a | 97.6±0.8 a |
The conidia suspension of the wild type strain Guy-11, the Δmtp1 mutant F7 and the MTP1-rescued strain MTG-3 (1×105 conidia/ml, 20 μl) was droplet inoculated on plastic coverslips at 25 °C. The experiments were repeated three times with more than 300 conidia counted each time. The same lowercase letters in each column denote that the difference estimated by the Duncan’s test (P≤0.05) is not significant
The percentage of conidia that had elaborated a germ tube was counted at 2 hpi or 4 hpi;
The percentage of conidia forming appressoria was counted at 6 hpi or 24 hpi.
Effect of MTP1 gene deletion on pathogenicity
Infection assays were performed to assess the ability of the Δmtp1 mutant to cause disease in barley and susceptible rice. In conidial sprays no significant difference in disease symptoms could be observed on plants inoculated with either the Δmtp1 mutant or the wild type strain (Fig.4, see Page 516).
Fig. 4.
Lesions caused by M. oryzae on the leaves of rice seedlings. These leaves were photographed 7 d after the rice seedlings (cv. CO-39) were individually spray-inoculated with conidia (1×105 conidia/ml) of the wild type strain Guy-11, Δmtp1 mutant or sprayed with 0.2% (w/v) gelatin (control)
DISCUSSION
In this study we have cloned and sequenced the MTP1 gene, a YTP1 homologue in M. oryzae. The Mtp1 protein shares 46% amino acid identity with a previously identified S. cerevisiae Ytp1 protein (West et al., 1996). The YTP1 gene encodes a type III transmembrane protein, which is not essential for the growth and development of yeast (West et al., 1996). The computer programs predicted that the Mtp1 protein is a type III transmembrane protein possessing 8 membrane helices. MTP1-GFP fusion expression experiments showed that the Mtp1 protein might be located on several membranes in the cytoplasm.
The MTP1 gene is not essential for the colony growth of M. oryzae. The Δmtp1 mutant does not confer sensitivity to starvation, Cu2+ or Fe3+, and exhibits no detectable osmoregulation defect. Moreover, the Δmtp1 mutant does not lose its mating ability to cross with the opposite mating-type strain. However, in contrast to those in Δytp1 mutant of yeast, which lacked a detectable growth phenotype (West et al., 1996), the phenotypic effects displayed through deletion of the MTP1 gene in M. oryzae were marked by a reduction in conidiation and a delay in conidium germination and appressorium formation. The delay in appressorium formation may be due to a delay in conidium germination. The conidial formation and conidium germination in the rice blast fungus are a complicated process, affected by many known genes, such as a blue light receptor gene SMO1 (Hamer and Givan, 1990), adenylate cyclase gene MAC1 (Adachi and Hamer, 1998), two PAK kinase genes CHM1 and MST20 (Li et al., 2004), MgWC-1 (Lee et al., 2006), and autophagy related genes MgATG1 and MgATG8 (Veneault-Fourrey et al., 2006a; Liu et al., 2007). In Aspergillus oryzae or M. oryzae, conidium formation and conidial germination rely on endogenous sources for nutrient supply through autophagy (Kikuma et al., 2006; Liu et al., 2007). The mitochondrion is an organelle that functions at the turnover of endogenous nutrient sources and supplies adenosine triphosphate (ATP) through the tricarboxylic acid (TCA) cycle, β-oxidation and oxidative phosphorylation. Recently it was found that Mtp1 might interact with Pls1 in the appressorium of M. oryzae (personal communication, Marc-Henri Lebrun). Pls1 is a transmembrane protein (tetraspanins), only localized in plasma membranes and vacuoles of appressoria and required for the formation of the penetration peg at the base of the appressorium, probably through re-establishing cell polarity (Clergeot et al., 2001; Veneault-Fourrey et al., 2006b). And Mtp1 protein possibly plays its role in the function of mitochondrion or vacuole in the turnover of nutrient sources or the supply of energy sources, which could be used during the conidial formation stage and the early stage of conidial germination, or play its role through interacting with Pls1 protein at the stage of appressorium.
As the Δmtp1 mutant could use ethanol and glycerol as its sole carbon sources and produce similar appressorium turgor as the wild type strain, the Δmtp1 mutant exhibits no defects in the mitochondrial respiratory function and fatty acid β-oxidation. Interestingly the Δmtp1 mutant seems to use ethanol and glycerol more readily than the wild type strain, as it grows faster than the wild type strain in non-fermentable carbon sources. In yeast Δtpk2 mutants also grew better than the wild type strain in non-fermentable carbon sources, and the protein kinase A (PKA) catalytic subunit Tpk2 protein, which represses transcription of genes involved in high-affinity iron uptake, showed a unique role in respiratory growth and carbon source use (Robertson et al., 2000). The Mtp1 protein may have a similar function to the Tpk2 protein; however, more direct experimental analyses are required to confirm this.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30671351 and 30470064) and the Natural Science Foundation of Zhejiang Province, China (No. Y304211)
References
- 1.Adachi K, Hamer JE. Divergent camp signaling pathways regulate growth and pathogenesis in the rice blast fungus. Magnaporthe grisea Plant Cell. 1998;10(8):1361–1373. doi: 10.2307/3870646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul SF, Madden TL, Schafferi AA, Zhang J, Zhang Z, Miller W, Lipman DJ. BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonman JM, Vergel DDT, Khin MM. Physiologic specialization of Pyricularia oryzae in the Philippines. Plant Dis. 1986;70(8):767–769. doi: 10.1094/PD-70-767. [DOI] [Google Scholar]
- 4.Carroll AM, Sweigard JA, Valent B. Improved vectors for selecting resistance to hygromycin. Fungal Genet Newslett. 1994;41:22. [Google Scholar]
- 5.Choi W, Dean RA. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell. 1997;9(11):1973–1983. doi: 10.1105/tpc.9.11.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clergeot PH, Gourgues M, Cots J, Laurans F, Latorse MP, Pepin R, Tharreau D, Notteghem JL, Lebrun MH. PLS1, a gene encoding a tetraspanin-like protein, is required for penetration of rice leaf by the fungal pathogen Magnaporthe grisea . PNAS. 2001;98(12):6963–6968. doi: 10.1073/pnas.111132998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couch BC, Kohn LM. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea . Mycologia. 2002;94(4):683–693. doi: 10.2307/3761719. [DOI] [PubMed] [Google Scholar]
- 8.de Jong JC, McCormack BJ, Smirnoff N, Talbot NJ. Glycerol generates turgor in rice blast. Nature. 1997;389(6648):244–245. doi: 10.1038/38418. [DOI] [Google Scholar]
- 9.Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, et al. The genome sequence of the rice blast fungus Magnaporthe grisea . Nature. 2005;434(7036):980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- 10.DeZwaan TM, Carroll AM, Valent B, Sweigard JA. Magnaporthe grisea Pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell. 1999;11(10):2013–2030. doi: 10.1105/tpc.11.10.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyrløv Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340(4):783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Emanuelsson O, Nielsen H, Brunak S, Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300(4):1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 13.Gronover CS, Kasulke D, Tudzynski P, Tudzynski B. The role of G protein alpha subunits in the infection process of the gray mold fungus Botrytis cinerea . Mol Plant Microbe Interact. 2001;14(11):1293–1302. doi: 10.1094/MPMI.2001.14.11.1293. [DOI] [PubMed] [Google Scholar]
- 14.Hamer JE, Givan S. Genetic mapping with dispersed repeated sequences in the rice blast fungus: mapping the SMO locus. Mol Gen Genet. 1990;223(3):487–495. doi: 10.1007/BF00264458. [DOI] [PubMed] [Google Scholar]
- 15.Horton P, Park KJ, Obayashi T, Nakai K. Proceedings of the 4th Annual Asia Pacific Bioinformatics Conference APBC06. Taipei, Taiwan: 2006. Protein Subcellular Localization Prediction with Wolf Psort; pp. 39–48. [DOI] [Google Scholar]
- 16.Howard RJ, Ferrari MA, Roach DH, Money NP. Penetration of hard substrates by a fungus employing enormous turgor pressures. PNAS. 1991;88(24):11281–11284. doi: 10.1073/pnas.88.24.11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikuma T, Ohneda M, Arioka M, Kitamoto K. Functional analysis of the ATG8 homologue Aoatg8 and role of autophagy in differentiation and germination in Aspergillus oryzae . Eukaryotic Cell. 2006;5(8):1328–1336. doi: 10.1128/EC.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Ahn IP, Rho HS, Lee YH. MHP1, a Magnaporthe grisea hydrophobin gene, is required for fungal development and plant colonization. Mol Microbiol. 2005;57(5):1224–1237. doi: 10.1111/j.1365-2958.2005.04750.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Singh P, Chung WC, Ash J, Kim TS, Hang L, Park S. Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae . Fungal Genet Biol. 2006;43(10):694–706. doi: 10.1016/j.fgb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Lee YH, Dean RA. cAMP regulates infection structure formation in the plant pathogenic fungus Magnaporthe grisea . Plant Cell. 1993;5(6):693–700. doi: 10.1105/tpc.5.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Xue C, Bruno K, Nishimura M, Xu JR. Two PAK kinase genes, CHM1 and MST20, have distinct functions in Magnaporthe grisea . Mol Plant Microbe Interact. 2004;17(5):547–556. doi: 10.1094/MPMI.2004.17.5.547. [DOI] [PubMed] [Google Scholar]
- 22.Liu XH, Lu JP, Zhang L, Dong B, Min H, Lin FC. Involvement of a Magnaporthe grisea serine/threonine kinase gene, MgATG1, in appressorium turgor and pathogenesis. Eukaryotic Cell. 2007;6(6):997–1005. doi: 10.1128/EC.00011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu JP, Liu TB, Lin FC. Identification of mature appressorium-enriched transcripts in Magnaporthe grisea, the rice blast fungus, using suppression subtractive hybridization. FEMS Microbiol Lett. 2005;245(1):131–137. doi: 10.1016/j.femsle.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 24.Lu JP, Liu TB, Liu XY, Lin FC. Representative appressorium stage cDNA library of Magnaporthe grisea . J Zhejiang Univ Sci B. 2005;6(2):132–136. doi: 10.1631/jzus.2005.B0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu JP, Feng XX, Liu XH, Lu Q, Wang HK, Lin FC. Mnh6, a nonhistone protein, is required for fungal development and pathogenicity of Magnaporthe grisea . Fungal Genet Biol. 2007;44(9):819–829. doi: 10.1016/j.fgb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Lu JP, Duan ZB, Liu TB, Lin FC. Cloning, sequencing and expression analysis of the NAR promoter activated during hyphal stage of Magnaporthe grisea . J Zhejiang Univ Sci B. 2007;8(9):661–665. doi: 10.1631/jzus.2007.B0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odenbach D, Breth B, Thines E, Weber RW, Anke H, Foster AJ. The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea . Mol Microbiol. 2007;64(2):293–307. doi: 10.1111/j.1365-2958.2007.05643.x. [DOI] [PubMed] [Google Scholar]
- 28.Ou SH. Rice Diseases. 2nd Ed. Kew, UK: Commonwealth Mycological Institute; 1985. [Google Scholar]
- 29.Pall MC, Brunelli P. A series of six compact fungal transformation vectors containing polylinkers with multiple unique restriction sites. Fungal Genet Newslett. 1993;40:59–62. [Google Scholar]
- 30.Robertson LS, Causton HC, Young RA, Fink GR. The yeast A kinases differentially regulate iron uptake and respiratory function. PNAS. 2000;97(11):5984–5988. doi: 10.1073/pnas.100113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rost B, Fariselli P, Casadio R. Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Science. 1996;5(8):1704–1718. doi: 10.1002/pro.5560050824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Research. 2004;32(Web Server issue):W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 3rd Ed. New York, NY: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- 34.Talbot NJ, Ebbole DJ, Hamer JE. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea . Plant Cell. 1993;5(11):1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veneault-Fourrey C, Barooah MK, Egan MJ, Talbot NJ. Autophagic fungal cell death is necessary for infection by the rice blast fungus. Science. 2006;312(5773):580–583. doi: 10.1126/science.1124550. [DOI] [PubMed] [Google Scholar]
- 37.Veneault-Fourrey C, Lambou K, Lebrun MH. Fungal Pls1 tetraspanins as key factors of penetration into host plants: a role in re-establishing polarized growth in the appressorium? FEMS Microbiol Lett. 2006;256(2):179–184. doi: 10.1111/j.1574-6968.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 38.West RWJr, Crivellone MD, Ma J, Thomas S. Sequence of the Saccharomyces cerevisiae YTP1 gene encoding a deduced novel type-III transmembrane protein with domains of sequence similarity to mitochondrial electron-transport enzymes. Gene. 1996;169(1):119–124. doi: 10.1016/0378-1119(95)00774-1. [DOI] [PubMed] [Google Scholar]
- 39.Xu JR, Hamer JE. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea . Genes Dev. 1996;10(21):2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Kim Y, Park G, Xu JR. A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea . Plant Cell. 2005;17(4):1317–1329. doi: 10.1105/tpc.104.029116. [DOI] [PMC free article] [PubMed] [Google Scholar]