Abstract
Chinese soft-shelled turtles (Trionyx sinens) in culture farms using an artificial warming system in Zhejiang, China, often show typical signs of white-spot disease such as white spots on their bodies, skin lesions, anorexia and eventually death. The sick turtles were mostly 5~80 g in weight. A suspected fungal pathogen was isolated from the sick turtles and verified as Paecilomyces lilacinus by sequence analysis of the internal transcribed spacer (ITS) of its ribosomal DNA (rDNA). Detailed morphological examinations were also conducted to confirm the white-spot disease.
Keywords: White-spot disease, Chinese soft-shelled turtles, Paecilomyces lilacinus, Zhejiang
INTRODUCTION
Chinese soft-shelled turtles (Trionyx sinens) belong to reptile, living in fresh water and known as a nutrient-rich food in Asian countries including China, Japan, Korea, etc. (Feng et al., 1996; Yin et al., 2005). Large-scale farming of Chinese soft-shelled turtles in Zhejiang, Jiangsu and Fujian provinces, China, has been growing rapidly. Recently, annual consumption of turtles has reached about 2~3 hundred millions in China (Li et al., 2006). A so-called white-spot disease has been found in turtles weighing 5~80 g in some cultured farms in Zhejiang since 1996, leading to a severe economic loss to the industry. Infected turtles showed typical signs of white spots on whole body surfaces, skin lesions, anorexia and eventually death. Fungal infection was suspected. Fungal diseases of various animals including reptiles, amphibians and marine fishes have been described by many investigators (Bowater et al., 2003; Paré, 2003; Schumacher, 2003; Yanong, 2003); however, there are few studies examining the causal pathogens of diseases in Chinese soft-shelled turtles in culture farms.
In this study, we reported for the first time in China that the white-spot disease of Chinese soft-shelled turtles in a culture farm was caused by Paecilomyces lilacinus according to its morphological characters of the isolate, challenge studies and sequence analysis of the internal transcribed spacer (ITS) of the ribosomal DNA (rDNA).
MATERIALS AND METHODS
Fungal pathogen isolation
The diseased turtles from a commercial aquaculture farm in Hangzhou, Zhejiang, China were used to isolate the suspected fungal pathogen. The infected turtles were around 20 g in weight. Mycelia from white spots were resuspended in sterile distill water. Single mycelium was picked up from the Petri dish under microscope and inoculated onto the Czapek agar (CA) (NaNO3 3.0 g, K2HPO4 1.0 g, KCl 0.5 g, MgSO4·7H2O 0.5 g, FeSO4·7H2O 0.01 g, sucrose 30 g, agar 15 g, distilled water 1 L) containing 2 mg of ampicillin and streptomycin to prevent bacterial growth for subsequent incubation at (24±1) °C. Subcultures were transferred to new CA plates and incubated for 7 d for colony morphology examination. Purified isolates were stored at the Fungal Laboratory, Zhejiang University, China.
Identification of fungi
Microscopic observations were based on preparations mounted in lactic acid. Genomic DNA was extracted from fresh mycelia with a commercial kit (DNeasy Plant Mini Kits, Qiagen, Germany). A fragment of the rDNA containing the ITS regions 1 and 2 and the 5.8S ribosomal RNA (rRNA) gene was amplified by polymerase chain reaction (PCR) using the primer combinations ITS4 and ITS5 (White et al., 1990) in an automated thermocycler (Minicycler PTC-150, MJ Research, USA). The PCR products were used directly after purification (QIAquick PCR Purification Kit, Qiagen) for DNA sequencing on an Applied Biosystems 3730 DNA Analyzer (PE Applied Biosystems, Foster City, California, USA). BLAST (basic local alignment search tool) was used to perform similarity search of the sequence from the isolate obtained in this study with those from GenBank.
Challenge experiments
Conidia were harvested from the 7-day-old culture by washing with sterilized water. The suspension was adjusted to 5×106 conidia/ml based on counts under light microscope using a Neubauer haemocytometer, and used for challenge experiments. Forty clinically healthy turtles [weight (25±5) g] from a culture farm were immersed in water containing 10×10−6 KMnO4 for 15 min, and then randomly divided into four groups. Ten turtles each in Groups I and II were artificially incised on their back like “×” in 2-cm length. Turtles in Groups III and IV (10 each) were used for challenge experiment without incision. Turtles of each group were kept in a separate plastic container containing 4.5 L of sterilized water (pH 5.5) and fed with sterilized commercial diets. The experimental space and containers were exposed to ultraviolet radiation for 24 h before use. The water temperature for challenge experiments was controlled at (22±1) °C. The conidia suspension (500 ml) was poured into the containers for turtles of Groups II and IV, while sterilized distill water in the same volume was used for turtles of Groups I and III as controls. Lesions on the body surface were observed daily until Day 21. Fungal cells recovered from the artificially infected turtles in the first challenge experiment were also tested in the second challenge study. All procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University.
RESULTS
Isolation and identification
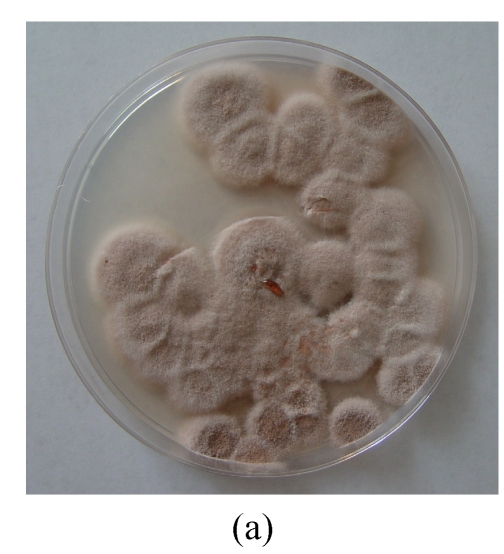
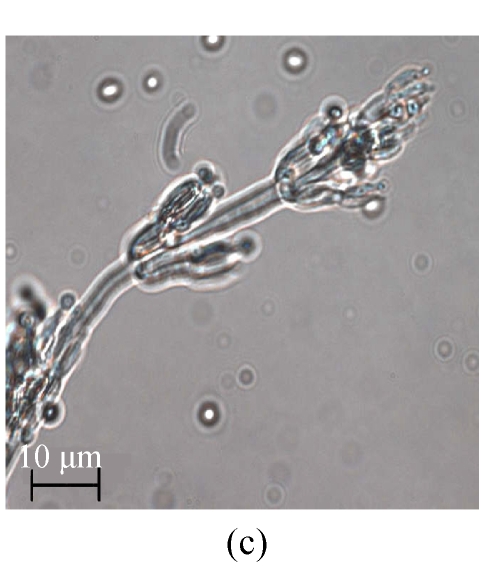
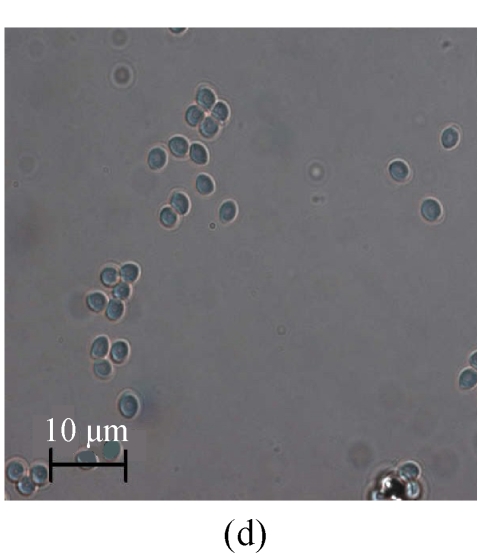
The same fungal pathogen was isolated from the diseased turtles. Growth of the fungal isolate on CA (Fig.1a) and MEA (malt extract agar) produced purplish colonies without yellow pigment after 7-day incubation at (24±1) °C under ambient daylight. Complex fruiting heads with verticillate conidiophores and divergent phialides on the culture of CA (Figs.1b and 1c) and MEA were observed. Smooth-walled and elliptical conidia were seen in long chains and measured approximately 2.5 μm×3.0 µm. The isolate was identified as P. lilacinus according to these microscopic characteristics and the lilac pigment, and verified by the 100% identity of the ITS sequence (GenBank accession No. DQ452735) of its rDNA to the sequence data from GenBank of the nearest neighbor P. lilacinus strains UWFP 674 (AY213667), UWFP 699 (AY213666), ATCC 10114 (AY213665) and NKCM1001 (AB24 4777).
Fig. 1.
Macroscopic and microscopic features of the Paecilomyces lilacinus isolate from a diseased turtle after 7 d of incubation at 25 °C on Czapek agar (CA). (a) Visual colony morphology; (b) Colony morphology viewed under stereo microscope; (c) Conidiophore from the culture; (d) Conidia from the culture
Challenge experiments
Fig.2 shows the differences of skin lesions of naturally infected, clinically healthy and artificially infected turtles. All turtles challenged with the P. lilacinus isolate in this study (Group II) exhibited the white mold on the incisions 21 d post-infection (Fig.2c). However, only two turtles in Group IV (not incised but challenged) were infected during the experiment period. There was no infection on the turtles in control Groups I (incised but not challenged) and III (neither incised nor challenged). In the second challenge experiment, the turtles inoculated with the isolate from the first challenge showed similar results.
Fig. 2.
Visualization of the turtle shell infections by Paecilomyces lilacinus. (a) Naturally infected turtle; (b) Healthy turtle; (c) Artificially infected turtle with surgical cross incisions on the back (shown in arrow); (d) Turtle with surgical incisions but without artificial challenge as control (shown as absence of the cross lines in the middle of the shell)
DISCUSSION
Paecilomyces is a ubiquitous saprophytic mold. The two major pathogenic species are P. lilacinus and P. variotii with the former causing most reported infections. Cutaneous infection with P. lilacinus is rare in reptiles, but could occur in immunocompromised human hosts (Orós et al., 1996; Saberhagen et al., 1997; Hall et al., 2004). We found that P. lilacinus was the main cause of the white-spot disease in turtles. So far, there has been no report of P. lilacinus infection in Chinese soft-shelled turtles.
The disease often occurs in turtles weighing 5~80 g in culture farms with high population density particularly under fluctuating temperature conditions. It is likely that the turtle’s immune functions were compromised due to temperature fluctuations and high density. In practice, it is difficult for the farmers to keep the water temperature constant via artificial heating systems. The farmers are also not willing to reduce the density of turtle populations for their pursuit of economic benefits.
Challenge experiments indicated that the number of infected turtles with surgical excisions was significantly higher than that of the noninfected ones. The infection style of this fungal pathogen in the turtles was similar to that in patients with soft tissue infection at prepatellar bursitis due to puncture wounds in the skin (Westenfeld et al., 1996). Biting among the populated turtles is apparently a predisposing factor to the fungal infection.
In summary, P. lilacinus is the primary cause of the white-spot disease in Chinese soft-shelled turtles at their early age. Wounds on the body surface predispose the young turtles to this opportunistic pathogen.
Acknowledgments
The authors would like to thank Mr. G.Q. Xiong, Director of the Hongzhou Zhongde Aquatic Culture Co., Ltd., China, for submitting the diseased and healthy turtles for challenge experiments.
Footnotes
Project supported by the Science and Technology Department of Zhejiang Province, China (No. 2004C26026) and the Science and Technology Department of Hangzhou City, China (No. 20051322B33)
References
- 1.Bowater RO, Thomas A, Shivas RG, Humphrey JD. Deuteromycotic fungi infecting barramundi cod, Cromileptes altivelis (Valenciennes), from Australia. Journal of Fish Diseases. 2003;26(11-12):681–686. doi: 10.1046/j.1365-2761.2003.00503.x. [DOI] [PubMed] [Google Scholar]
- 2.Feng H, Yamazaki M, Matsuki N, Saito H. Anti-tumor effects of orally administered soft-shelled turtle powder in mice. Biological & Pharmaceutical Bulletin. 1996;19(3):367–368. doi: 10.1248/bpb.19.367. [DOI] [PubMed] [Google Scholar]
- 3.Hall VC, Goyal S, Davis MD, Walsh JS. Cutaneous hyalohyphomycosis caused by Paecilomyces lilacinus: report of three cases and review of the literature. International Journal of Dermatology. 2004;43(9):648–653. doi: 10.1111/j.1365-4632.2004.02175.x. [DOI] [PubMed] [Google Scholar]
- 4.Li XL, Shuai JB, Fang WH. Protection of Carassius auratus Gibelio against infection by Aeromonas hydrophila using specific immunoglobulins from hen egg yolk. Journal of Zhejiang University SCIENCE B. 2006;7(11):922–928. doi: 10.1631/jzus.2006.B0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orós J, Ramírez AS, Poveda JB, Rodríguez JL, Fernández A. Systemic mycosis caused by Penicillium griseofulvum in a Seychelles giant tortoise (Megalochelys gigantea) The Veterinary Record. 1996;139(12):295–296. doi: 10.1136/vr.139.12.295. [DOI] [PubMed] [Google Scholar]
- 6.Paré JA. Fungal diseases of amphibians: an overview. The Veterinary Clinics of North America Exotic Animal Practice. 2003;6(2):315–326. doi: 10.1016/S1094-9194(03)00006-9. [DOI] [PubMed] [Google Scholar]
- 7.Saberhagen C, Klotz SA, Bartholomew W, Drews D, Dixon A. Infection due to Paecilomyces lilacinus: a challenging clinical identification. Clinical Infectious Diseases. 1997;25(6):1411–1413. doi: 10.1086/516136. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher J. Fungal diseases of reptiles. The Veterinary Clinics of North America Exotic Animal Practice. 2003;6(2):327–335. doi: 10.1016/S1094-9194(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 9.Westenfeld F, Alston WK, Winn WC. Complicated soft tissue infection with prepatellar bursitis caused by Paecilomyces lilacinus in an immunocompetent host: case report and review. Journal of Clinical Microbiology. 1996;34(6):1559–1562. doi: 10.1128/jcm.34.6.1559-1562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White TJ, Bruns T, Lee S, et al. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al., editors. PCR Protocols: A Guide to Methods and Applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 11.Yanong RP. Fungal diseases of fish. The Veterinary Clinics of North America Exotic Animal Practice. 2003;6(2):377–400. doi: 10.1016/S1094-9194(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 12.Yin J, Tezuka Y, Subehan , Shi L, Ueda JY, Matsushige K, Kadota S. A combination of soft-shell turtle powder and essential oil of a unicellular chorophyte prevents bone loss and decreased bone strength in ovariectomized rats. Biological & Pharmaceutical Bulletin. 2005;28(2):275–279. doi: 10.1248/bpb.28.275. [DOI] [PubMed] [Google Scholar]