Abstract
Objective
To study the toxicity of inhaled PGE1 (IPGE1) in healthy ventilated piglets.
Methods
Mechanically ventilated anesthetized piglets received either high dose IPGE1 (IPGE1 group) or nebulized saline (control group) continuously for 24 hours. Cardio-respiratory parameters, complete blood counts and serum electrolytes were monitored. Lung histology was evaluated by a masked pathologist for the severity (minimal, moderate, and severe) and extent (focal, multifocal, and diffuse) of histologic injury.
Results
Ten neonatal pigs were instrumented. Four received nebulized saline and six received high dose IPGE1. There was no evidence of adverse cardio-respiratory effects, bronchial irritation or hypernatremia related to IPGE1. Diffuse/multifocal alveolar edema and focal polymorphonuclear infiltration was observed in both the control and IPGE1 groups suggesting that alveolar alterations may be secondary to effects of mechanical ventilation. The most distinct histomorphological abnormalities observed in the IPGE1 animals were focal ulceration, flattening of the bronchial epithelium and loss of cilia of moderate to severe degree in the trachea and bronchi.
Conclusion
In healthy piglets, inhalation of high dose IPGE1 was not associated with adverse cardiorespiratory effects, bronchial irritation, or hypernatremia and produced minimal signs of pulmonary toxicity even after 24 hours. Prolonged inhalation of high dose PGE1 therefore appears safe in newborn piglets.
Keywords: Pulmonary toxicity, inhaled, PGE1/Alprostadil, neonatal, piglet/animal, histomorphology, nebulizer, aerosol
1. Introduction
Neonatal hypoxemic respiratory failure (NHRF), often referred to as persistent pulmonary hypertension of the newborn (PPHN) because of failure of the elevated pulmonary vascular resistance to decrease postnatally, is associated with a variety of neonatal diseases. Inhaled nitric oxide (INO), the only selective pulmonary vasodilator approved for the treatment of NHRF, is ineffective in 30–46% of infants and requires specialized delivery systems making the treatment expensive and limiting availability (1). Intravenous PGE1 (ivPGE1) and PGI2, potent vasodilators used empirically in the treatment of NHRF, are associated with systemic hypotension and worsening of oxygenation due to increased venous admixture (2–6). This has led investigators to explore the delivery of PGE1 and PGI2 directly to the lungs as an inhalation, thus minimizing systemic effects and achieving selective pulmonary vasodilation (7–21). Compared to PGI2, PGE1 has a shorter half-life, lower pH (6.3 versus 10.5), bronchodilator action, anti-proliferative and anti-inflammatory effects on the alveolar, interstitial and vascular spaces of the lung (8, 22–25). We have described the emitted dose, stability and aerosol particle size distribution of inhaled PGE1 in a neonatal ventilator circuit and demonstrated effective pulmonary delivery using magnetic resonance imaging in a ventilated piglet model (26, 27). We have also reported the feasibility, safety and effective delivery of inhaled PGE1 (IPGE1) when administered for a maximum duration of three hours in a phase I–II study in term/near-term neonates with NHRF (28, 29). Treatment of PPHN associated with NHRF with IPGE1 would likely require inhalation for a longer period of time. Although significant data exist regarding the safety of long term ivPGE1 in neonates with heart disease, the possible toxic effects of IPGE1, especially local pulmonary toxicity, following prolonged inhalation have not been investigated. In addition, there are concerns that IPGE1 may be a bronchial irritant and that continuous aerosolization of medications dissolved in normal saline for prolonged periods may result in hypernatremia in the neonatal subject. The objective of this study is to evaluate the safety of 24-hour inhalation of PGE1 in a neonatal animal model undergoing assisted ventilation with high fractional inspired O2 concentration (FiO2) that closely mimics the intended clinical use.
2. Materials and Methods
Subjects
The study was performed on ten healthy domestic piglets (1–9 days old) from a specific pathogen free (SPF) litter after approval by the Institutional Animal Care and Use Committee. All animals received care in compliance with the NIH guidelines.
Animal Preparation
Animals were fasted 1–3 hours prior on the day of the experiment. They were intubated oro-tracheally after intramuscular pre-medication with midazolam (1 mg/kg) and ketamine (33 mg/kg). A tracheotomy was performed if oro-tracheal intubation was unsuccessful. The cephalic vein was catheterized percutaneously for vascular access. The carotid artery was cannulated through a neck incision for continuous blood pressure monitoring and arterial blood sampling. The external jugular vein was also cannulated if percutaneous intravenous access was unsuccessful. Anesthesia was maintained by intravenous infusion of ketamine (20 mg/kg/hour), midazolam (2 mg/kg/hr) and fentanyl (30 μg/kg/hr) titrated to the level of anesthesia as assessed by monitoring heart rate, blood pressure, and response to painful stimuli. Maintenance fluids consisting of dextrose-saline were administered at a rate of 5–10 ml/kg/hour and the rate modified based on the animals’ hemodynamic status. Body temperature was maintained at 38–39° C. Blood pressure was monitored continuously and recorded every 2 hours and as needed. Additional saline was infused, when necessary, to maintain baseline systemic blood pressure. Prophylactic antibiotics were given at 12 hour intervals.
Mechanical Ventilation
Time cycled, pressure limited assisted ventilation (Sechrist) was initiated at a rate of 15 bpm, FiO2 of 1.0, peak inspiratory pressure of 18 cm H2O, and positive end expiratory pressure of 4 cm H2O. During the study, ventilator parameters were adjusted to maintain normocapnia. Oxygen saturations were monitored continuously and arterial blood gas analyses performed every 6 hours and as needed. FiO2 was maintained at 1.0 throughout the experiment.
Drug Preparation
Animals were randomly assigned to receive IPGE1 (IPGE1 group) or nebulized saline (control group) continuously for 24 hours. PGE1, supplied as 500 μg of Alprostadil in 1 ml of ethanol (Gensia Sicor Pharmaceuticals, Irvine, California), was diluted in normal saline to yield a dose of 1200 ng/kg/min when infused into the nebulizer chamber at a rate of 4 ml/hr.
Administration of Continuous Aerosol
The low flow MiniHeart jet nebulizer (Westmed Inc., Lakewood, Colorado) was used to generate continuous aerosols as described previously (28). The nebulizer was placed in the inspiratory limb of the ventilator circuit ~20 inches from the andotracheal tube (ETT). The oxygen flow through the jet nebulizer was set at 2 LPM. During nebulization, the ventilator flow was adapted according to the additional flow of the nebulizer to maintain alveolar ventilation. At the start of aerosol therapy, the nebulizer chamber was primed with 2 ml of the study medication followed by continuous delivery into the nebulizer chamber at a rate of 4 ml/hour.
Blood Sampling
Blood for arterial blood gas analyses was obtained 20 minutes after initiation of mechanical ventilation and every 6 hours thereafter (ABL 5, radiometer, Copenhagen, Denmark). Additional blood gases were obtained if indicated. Blood samples were obtained at baseline and at the end of the experiment for evaluation of complete blood counts (CBC), and serum electrolytes.
Histomorphological Analysis of the Lungs
At the end of the experimental protocol, the piglets were euthanized by intravenous overdose of pentobarbital (90 mg/kg). At autopsy, the lungs and heart were removed en bloc and put in formalin. Representative sections were obtained from all pulmonary lobes (12 sections per animal) and trachea (five sections per animal), processed routinely for paraffin embedding, and stained with hematoxylin/eosin. Lung histomorphology was assessed by a single pathologist masked to group assignment using a scoring system adapted from those described in literature (30–33). Lung injury was characterized on the basis of both severity of injury and extent of injury. Severity of injury was graded as no, minimal, moderate or severe. Extent of injury was classified based on the percentage of sections per animal with abnormal histological features as none (0%), focal (<25%), multifocal (25–50%), and diffuse (>50%). Trachea, bronchi and alveoli were evaluated separately for evidence of toxicity. The trachea and bronchi were evaluated for epithelial flattening or desquamation, loss of cilia, ulceration, hemorrhage and polymorphonuclear infiltration. The alveoli were scored for the presence of hyaline membranes, edema, alveolar wall thickness, and polymorphonuclear infiltration.
Statistical Analyses
Descriptive statistics were used to summarize sample characteristics. Group differences for continuous variables were assessed using the independent samples t-test. Paired samples t-test was used to compare subjects at baseline and at the end of the experimental protocol. Heart rates and systolic blood pressures were plotted against time to evaluate trends during the experiment. The General Linear Model (GLM) was used to assess the differences in heart rate and systolic blood pressure between the PGE1 and control groups, considering effects from the repeated measurement of these variables (34). Cross-tabulation was used to evaluate severity (minimal or moderate to severe) and extent (focal, multifocal and diffuse) of lung injury. Two-tailed significance level was set at 0.05. Statistical analyses were performed using the SPSS® statistical package, version 15.0.1 (SPSS Inc., Chicago, IL, USA) and SAS/STAT® software, Version [9.1.3] (SAS Institute Inc., Cary, NC, USA).
3. Results
Ten piglets underwent the experimental protocol. Of these, six were randomized to the PGE1 group and four to the control group.
Baseline Characteristics
The mean birth weight of the piglets was 2.0 kg (range 1.4 to 2.5 kg) and the mean age was 4 days (range 1 to 9 days). Majority of the piglets were male (90%).
Hemodynamic Parameters
The mean heart rate and systolic blood pressure were comparable in animals in the IPGE1 and control groups (Figs 1 and 2). Although the heart rate was higher and the systolic blood pressure lower at the start of the experiment (probably as a result of the recent instrumentation); no significant time trends in heart rate or blood pressure were observed during the aerosol administration in either group. None of the piglets in either group required vasopressors.
Figure 1. Changes in Heart Rate during Aerosol Administration by Study Group.
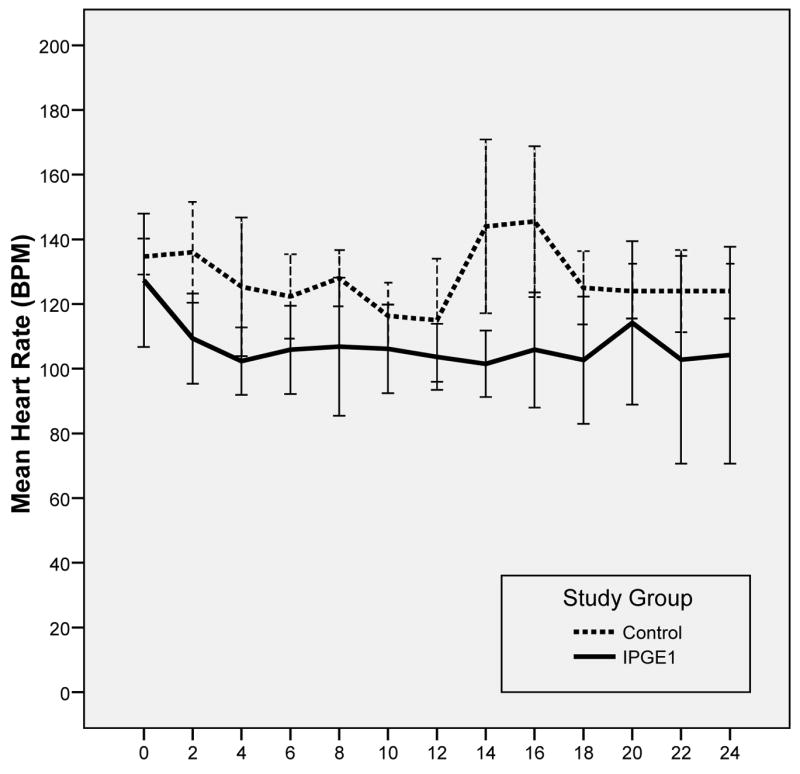
Error bars represent ± 1 SD
Figure 2. Changes in Systolic Blood Pressure During Aerosol Administration by Study Group.
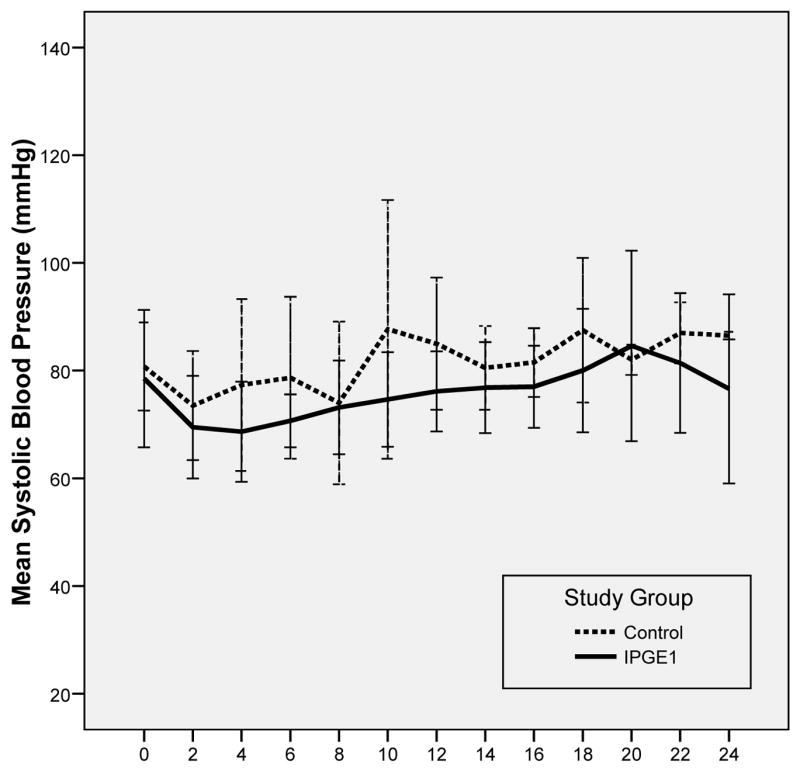
Error bars represent ± 1 SD
Respiratory Support and Arterial Blood Gases
The baseline ventilator support was comparable in the two groups (Table 1). In both groups of animals, PaO2 decreased over time (p<0.01). However, there was no difference between the two groups over the course of the study in the need for ventilator support or blood gas parameters. There was no evidence of airway irritation as manifested by coughing or wheezing associated with significant changes in breathing pattern, heart rate, blood pressure, anesthetic requirement, ventilator peak pressures, ventilator rate or PaCO2 during PGE1 or saline inhalation in the anesthetized mechanically ventilated subjects of this study.
Table 1. Respiratory parameters at baseline and at end of study.
| Baseline | 24 Hours | |||
|---|---|---|---|---|
| Control (n=4) | PGE1 (n=6) | Control (n=4) | PGE1 (n=6) | |
| Ventilator settings | ||||
| Rate (bpm) | 15±1 | 16±1 | 19±2 | 21±5 |
| Peak pressure (cm H2O) | 18±1 | 19±2 | 17±1 | 20±3 |
| Blood gas analyses | ||||
| pH | 7.37±0.14 | 7.33±0.14 | 7.29±0.1 | 7.31±0.1 |
| PaCO2 (mmHg) | 34±15 | 36±13 | 37±5 | 36±9 |
| PaO2 (mmHg) | 427±46 | 451±66 | 238±82 | 248±136 |
Values represent mean±SD
Hematological and Biochemical indices
Paired blood samples at baseline and end of study were available for total leukocyte count, hematocrit, platelet count, and serum electrolytes (Table 2). Hematological and biochemical indices were comparable between the two groups at baseline and at the end of the study. Hypernatremia was not documented in any animal in either group.
Table 2. Hematological and Biochemical indices at baseline and at end of study.
| Baseline | End of Study | |||
|---|---|---|---|---|
| Control (n=4) | PGE1 (n=6) | Control (n=4) | PGE1 (n=6) | |
| TLC (1000/mm3) | 8.2±4.4 | 13.0±6.8 | 12.9±3.5 | 16.0±5.5 |
| Hematocrit (%) | 29.6±5.2 | 34.6±7.7 | 32.4±5.1 | 31.0±5.5 |
| Platelets (1000/mm3) | 217±28 | 406±152 | 215±64 | 400±163 |
| Sodium (mMol/L) | 133.4±0.5 | 138.5±6 | 131.0±7.0 | 132.0±6.9 |
| Potassium (mMol/L) | 4.0±0.2 | 3.8±0.6 | 5.2±0.8 | 4.3±1.3 |
Values represent mean±SD
Light Microscopy
Direct pulmonary toxicity of the inhaled medication was evaluated by histologic examination of five tracheal sections and 12 parenchymal sections from each animal.
There was no evidence of severe toxic injury in the form of hyaline membranes, diffuse/severe neutrophilic infiltration or ulceration in the lungs and trachea in either the control or IPGE1 groups. Majority of the animals in the control and IPGE1 groups had normal appearing lungs (Figure 3) or showed the presence of focal injury of minimal severity (Figure 4). Two animals in the PGE1 group had evidence of moderate-severe focal tracheal or bronchial injury in the form of flattening of epithelium, loss of cilia, ulceration and polymorphonuclear infiltration. Moderate to severe multifocal/diffuse alveolar edema and focal polymorphonuclear infiltration was observed in one animal in the control group and two animals in the IPGE1 group.
Figure 3.
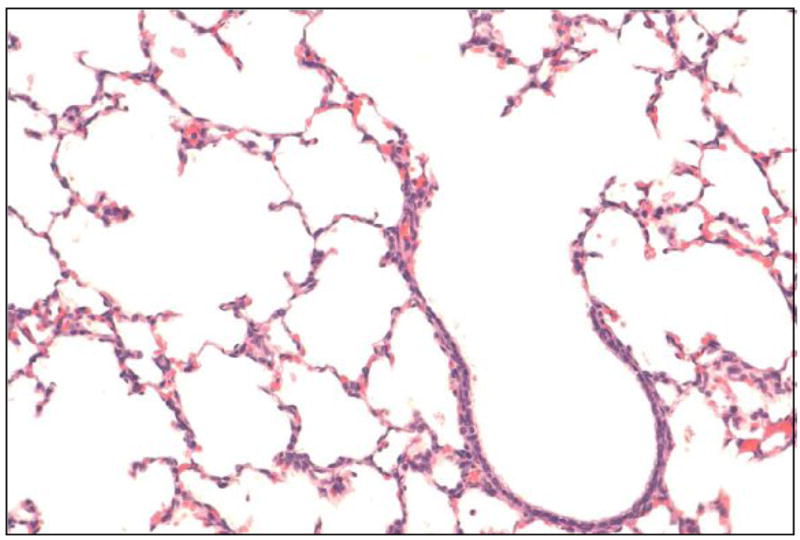
Normal bronchus and adjacent alveoli in a piglet who received IPGE1 for 24 hours
Figure 4.
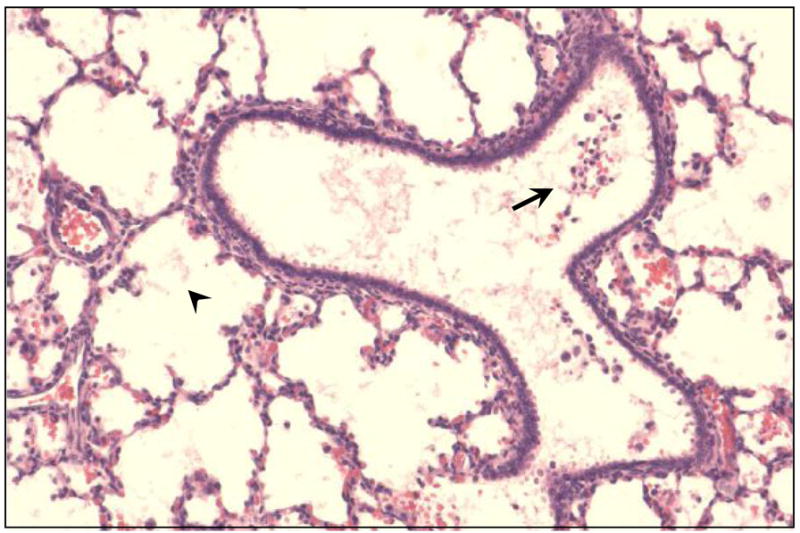
Few neutrophils and red blood cells in the bronchus (arrow) and minimal alveolar edema (arrowhead) in a piglet who received IPGE1 for 24 hours
In addition to the tracheal, bronchial and alveolar changes, atelectasis was evident in 2 piglets (one each in the control and IPGE1 groups). Evidence of bile aspiration was present in three animals in the PGE1 group and two animals in the control group.
4. Discussion
We have described for the first time a 24 hour model of anesthetized ventilated neonatal piglets to study the safety of continuous aerosol therapy. We used healthy piglets to ensure that changes observed during long-term inhalation were not confounded by underlying disease or by the experimental model of pulmonary hypertension.. Although the subjects of this study were healthy piglets, FiO2 of 1.0 was used to simulate the treatment of neonates with NHRF who typically are ventilated with 100% oxygen. This would allow the evaluation of pulmonary toxicity related to IPGE1 in the presence of high FiO2 while undergoing positive pressure ventilation.
The neonatal pig was a satisfactory model for this study as the lung of the neonatal piglet demonstrates several similarities to the lung of the human newborn, and the functional changes occurring in the pulmonary circulation during the first two weeks of life follow a similar time course to those in the human neonate (35).
Existing animal studies for testing drug safety in the treatment of respiratory failure are limited by the fact that most often spontaneously breathing, relatively mature animals have been used, and if undergoing mechanical ventilation, the duration of exposure to the inhaled agent has been relatively short (8 hours) (31). Histomorphological changes in tracheal, bronchial and alveolar epithelial tissues after 8-h inhalation of PGI2 or normal saline in 14 healthy ventilated lambs have been previously reported (28.5 to 48.5 kg in weight). There was no difference in light microscopy findings following inhalation of PGI2 or normal saline. Histological abnormalities were seen in 57% of tracheal sections and included focal flattening of the epithelium, loss of cilia, slight inflammatory cell infiltration. Alveolar changes were seen in 12% of sections and included thickening of alveolar septal space and focal inflammatory cell infiltration. Similarly, mild acute sterile tracheitis was reported following inhalation of PGI2 in four intubated large white landrace piglets (11 to 21 kg) (22). This was attributed to the alkaline glycine diluent used to prepare the PGI2 solution (pH 10.5). However, PGE1 solution prepared in normal saline has a lower pH (6.5) and therefore is less likely to be associated with pulmonary toxicity. Pulmonary toxicity following inhalation of PGE1 has not previously been described.
We have previously reported the effective delivery of IPGE1 at doses ranging from 50 to 300 ng/kg/min in a phase I–II study in term/near-term neonates with NHRF as assessed by plasma PGE1 levels (29). In an in vitro study, we have demonstrated that the emitted dose of IPGE1 following jet nebulization in a neonatal ventilator circuit was 32–40% (26). In the present study, we used a high dose of IPGE1, 1200 ng/kg/min, corresponding to a total dose of ~3,500 μg of PGE1 administered over 24 h. Compared to doses known to reduce pulmonary hypertension in patients (8–300 ng/kg/min), this represents a high dose (7–9, 20, 28, 36). A dose of 1200 ng/kg/min was chosen as this represents a dose four times the maximal dose that has been reported in humans and when given over a 24-hour period represents the cumulative dose that would be delivered over several days in the clinically used doses. Moreover, this dose is more likely to reveal evidence of toxicity especially when given continuously over 24 hours as the extent of adverse effects on the hemodynamic parameters and lung histomorphology are directly related to the dose delivered.
We chose to administer high dose IPGE1 continuously for 24 hours in the anesthetized ventilated piglets as this would allow sufficient time for the manifestation of adverse effects on hemodynamic parameters and pulmonary histomorphology. Hubbard et al reported that initial histomorphological changes in the respiratory tract appeared as early as 15 to 60 min after exposure depending on the severity of toxic exposure (37). Changes included necrosis and sloughing of respiratory epithelium, loss of cilia, and surface erosions. Inflammatory response, manifested by the formation of pseudomembranes, was observed two hours after the onset of toxic inhalation and was associated with simultaneous appearance of neutrophils in the lamina propria, epithelium, and lumen of trachea and bronchi. The acute inflammatory cell response was maximal by 24 hours. Increased mucus production and metaplastic changes were evident by 12 hours. The extent of injury was related to dose of toxic inhalation. Proceeding from these observations, appearance or worsening of pathological changes after 24-h inhalation of high doses of PGE1 is unlikely.
There was no evidence of adverse effects of high dose IPGE1 when administered continuously for 24 hours on cardiorespiratory, hematological and biochemical parameters. The absence of significant changes in arterial pressure indicates that significant systemic vasodilation did not occur during prolonged high dose IPGE1. This is in accordance with the first pass pulmonary metabolism and lack of significant active metabolites of PGE1. Although, PGE1 is known to have significant bronchodilator action, there is a concern that transient airway irritation may result in coughing, wheezing and occasionally bronchoconstriction (23, 38–40). In the current report, no evidence of coughing or wheezing associated with significant changes in breathing pattern, heart rate, blood pressure, anesthetic requirement, ventilator peak pressures, ventilator rate or PaCO2 were recorded during PGE1 inhalation, indicating that there was no significant bronchospasm as a result of airway irritation in the anesthetized subjects of this report. There is a concern that continuous administration of IPGE1 dissolved in normal saline at a rate of 4 ml/hr may result in hypernatremia in newborn infants. In the current report, normal saline was the vehicle for the administration of aerosolized PGE1 or placebo for both groups of animals. Hypernatremia was not observed in any animal either in the control or the IPGE1 groups even after 24 hours of continuous aerosol delivery.
The decline in oxygenation during the 24 hour period in animals in both groups probably reflects oxygen toxicity. Prolonged exposure to hyperoxia in newborns has been reported to be toxic resulting in destruction of the alveolar-capillary barrier leading to pulmonary edema, impaired gas exchange, pulmonary hypertension and eventually death (41–43). During the first 24 to 72 hr after exposure to 100% oxygen, most animal species do not demonstrate significant light microscopic changes although biochemical changes including increased oxygen consumption of cells and increased production of oxygen free radicals has been described. The earliest morphologic changes seen in the lung in response to hyperoxic stress involve subtle changes in endothelial cell ultra-structure seen 48 hours after hyperoxic exposure. Structural remodeling of pulmonary arteries is seen after 7 days of exposure to hyperoxia.
Pathological changes on lung histology were minimal or focal even after 24 hours of high dose IPGE1 in conjunction with positive pressure ventilation with FiO2 of 1.0. There was no evidence of severe toxic injury in the form of hyaline membranes, diffuse/severe neutrophilic infiltration or ulceration in the lungs and trachea in either the control or IPGE1 groups. Moderate to severe diffuse/mutifocal alveolar edema was observed in both the control and IPGE1 groups suggesting that the alveolar alterations may be secondary to effects of mechanical ventilation with high FiO2 (30, 44–46). The commonest abnormality observed in the IPGE1 group was the presence of moderate-severe focal tracheal or bronchial epithelial flattening, loss of cilia and ulceration in two animals. The most significant lesions after toxic inhalation have been reported to affect the trachea with the most severe changes being observed in the tissue adjacent to the tip of the ETT (31, 37). This is because, following aerosol delivery, a large fraction of the aerosol accumulates in the conducting airways, particularly in the trachea and ETT (47). Bile aspiration, evident in five animals undergoing the experimental protocol, could also be responsible for some of the histological abnormalities observed. These findings suggest that high dose IPGE1 is associated with minimal signs of pulmonary toxicity even after 24 hours of therapy. The possibility of severe pulmonary toxicity occurring after 24 hours is unlikely.
The most significant strength of our study is that we have successfully established a neonatal animal model undergoing assisted ventilation and anesthesia to study safety of continuous IPGE1 therapy over 24 hours thus closely simulating intended clinical use. Although significant data exist regarding the safety of long term ivPGE1 in neonates with heart disease, the possible toxic effects of IPGE1, especially local pulmonary toxicity, following prolonged inhalation have not been previously investigated. We have demonstrated that the continuous delivery of high dose IPGE1 over 24-hours in a ventilated newborn animal model is relatively safe without significant adverse effects on hemodynamic, respiratory, hematological, and biochemical parameters and pulmonary histology.
Despite the important findings described in this study, there are potential deficiencies. We have evaluated the direct pulmonary toxicity of IPGE1 following continuous administration for only 24 hours in mechanically ventilated anesthetized neonatal pigs. It is likely that clinical use may require aerosol administration for a longer period of time. We have tried to compensate for this by administering a high dose of IPGE1 (1200 ng/kg/min, four times the maximal dose reported in humans) representing the cumulative dose that would be delivered over several days in the clinically used doses. This dose is more likely to reveal evidence of toxicity as the extent of adverse effects on the hemodynamic parameters and lung histomorphology are directly related to the dose delivered. Additionally, based on pulmonary toxicology studies following toxic inhalation, appearance or worsening of pathological changes after 24-h inhalation of high doses of PGE1 is unlikely (37).
5. Conclusion
In summary, in healthy piglets, 24-h inhalation of high dose PGE1 was not associated with adverse cardiorespiratory effects, bronchial irritation, or hypernatremia and produced minimal signs of pulmonary toxicity. Prolonged inhalation of high dose PGE1 therefore appears safe in newborn piglets and might be applied without serious harm to human patients. Randomized controlled clinical trials are needed to document efficacy and safety in humans and to establish the role of IPGE1 as an additional selective pulmonary vasodilator in the treatment of PPHN associated with NHRF.
Acknowledgments
This research was funded in part by Grant K23 HD41423-01 from the National Institute of Child Health and Human Development and the Children’s Research Center of Michigan.
ABBREVIATIONS
- ETT
endotracheal tube
- NHRF
neonatal hypoxemic respiratory failure
- PPHN
persistent pulmonary hypertension of the newborn
- PGE1
prostaglandin E1
- IPGE1
inhaled PGE1
- ivPGE1
intravenous PGE1
- INO
inhaled nitric oxide
- FiO2
fractional inspired oxygen concentration
- NICU
neonatal intensive care unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NINOS. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. The Neonatal Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 2.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile LA, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Drummond WH, Gregory GA, Heymann MA, Phibbs RA. The independent effects of hyperventilation, tolazoline, and dopamine on infants with persistent pulmonary hypertension. J Pediatr. 1981;98:603–611. doi: 10.1016/s0022-3476(81)80775-5. [DOI] [PubMed] [Google Scholar]
- 4.Graves ED, 3rd, Redmond CR, Arensman RM. Persistent pulmonary hypertension in the neonate. Chest. 1988;93:638–641. doi: 10.1378/chest.93.3.638. [DOI] [PubMed] [Google Scholar]
- 5.Radermacher P, Santak B, Becker H, Falke KJ. Prostaglandin E1 and nitroglycerin reduce pulmonary capillary pressure but worsen ventilation-perfusion distributions in patients with adult respiratory distress syndrome. Anesthesiology. 1989;70:601–606. doi: 10.1097/00000542-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Awad JA, Soteriou MC, Drougas JG, Stokes KA, Roberts LJ, 2nd, Pinson CW. Plasma prostaglandin E1 concentrations and hemodynamics during intravenous infusions of prostaglandin E1 in humans and swine. Transplantation. 1996;61:1624–1629. doi: 10.1097/00007890-199606150-00013. [DOI] [PubMed] [Google Scholar]
- 7.Walmrath D, Schermuly R, Pilch J, Grimminger F, Seeger W. Effects of inhaled versus intravenous vasodilators in experimental pulmonary hypertension. Eur Respir J. 1997;10:1084–1092. doi: 10.1183/09031936.97.10051084. [DOI] [PubMed] [Google Scholar]
- 8.Meyer J, Theilmeier G, Van Aken H, Bone HG, Busse H, Waurick R, Hinder F, Booke M. Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg. 1998;86:753–758. doi: 10.1097/00000539-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Putensen C, Hormann C, Kleinsasser A, Putensen-Himmer G. Cardiopulmonary effects of aerosolized prostaglandin E1 and nitric oxide inhalation in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157:1743–1747. doi: 10.1164/ajrccm.157.6.9609017. [DOI] [PubMed] [Google Scholar]
- 10.Welte M, Zwissler B, Habazettl H, Messmer K. PGI2 aerosol versus nitric oxide for selective pulmonary vasodilation in hypoxic pulmonary vasoconstriction. Eur Surg Res. 1993;25:329–340. doi: 10.1159/000129297. [DOI] [PubMed] [Google Scholar]
- 11.Zobel G, Dacar D, Rodl S, Friehs I. Inhaled nitric oxide versus inhaled prostacyclin and intravenous versus inhaled prostacyclin in acute respiratory failure with pulmonary hypertension in piglets. Pediatr Res. 1995;38:198–204. doi: 10.1203/00006450-199508000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Zwissler B, Welte M, Messmer K. Effects of inhaled prostacyclin as compared with inhaled nitric oxide on right ventricular performance in hypoxic pulmonary vasoconstriction. J Cardiothorac Vasc Anesth. 1995;9:283–289. doi: 10.1016/s1053-0770(05)80322-2. [DOI] [PubMed] [Google Scholar]
- 13.Booke M, Bradford DW, Hinder F, Harper D, Brauchle RW, Traber LD, Traber DL. Effects of inhaled nitric oxide and nebulized prostacyclin on hypoxic pulmonary vasoconstriction in anesthetized sheep. Critical care medicine. 1996;24:1841–1848. doi: 10.1097/00003246-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Olschewski H, Walmrath D, Schermuly R, Ghofrani A, Grimminger F, Seeger W. Aerosolized prostacyclin and iloprost in severe pulmonary hypertension. Ann Intern Med. 1996;124:820–824. doi: 10.7326/0003-4819-124-9-199605010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153:991–996. doi: 10.1164/ajrccm.153.3.8630585. [DOI] [PubMed] [Google Scholar]
- 16.Mikhail G, Gibbs J, Richardson M, Wright G, Khaghani A, Banner N, Yacoub M. An evaluation of nebulized prostacyclin in patients with primary and secondary pulmonary hypertension. Eur Heart J. 1997;18:1499–1504. doi: 10.1093/oxfordjournals.eurheartj.a015478. [DOI] [PubMed] [Google Scholar]
- 17.Webb SA, Stott S, van Heerden PV. The use of inhaled aerosolized prostacyclin (IAP) in the treatment of pulmonary hypertension secondary to pulmonary embolism. Intensive Care Med. 1996;22:353–355. doi: 10.1007/BF01700458. [DOI] [PubMed] [Google Scholar]
- 18.Haraldsson A, Kieler-Jensen N, Nathorst-Westfelt U, Bergh CH, Ricksten SE. Comparison of inhaled nitric oxide and inhaled aerosolized prostacyclin in the evaluation of heart transplant candidates with elevated pulmonary vascular resistance. Chest. 1998;114:780–786. doi: 10.1378/chest.114.3.780. [DOI] [PubMed] [Google Scholar]
- 19.Max M, Kuhlen R, Dembinski R, Rossaint R. Effect of aerosolized prostacyclin and inhaled nitric oxide on experimental hypoxic pulmonary hypertension. Intensive Care Med. 1999;25:1147–1154. doi: 10.1007/s001340051027. [DOI] [PubMed] [Google Scholar]
- 20.Krieg P, Wahlers T, Giess W, Rohde R, Hartrumpf M, Bund M, Haverich A. Inhaled nitric oxide and inhaled prostaglandin E1: effect on left ventricular contractility when used for treatment of experimental pulmonary hypertension. Eur J Cardiothorac Surg. 1998;14:494–502. doi: 10.1016/s1010-7940(98)00210-3. [DOI] [PubMed] [Google Scholar]
- 21.Lockinger A, Schutte H, Walmrath D, Seeger W, Grimminger F. Protection against gas exchange abnormalities by pre-aerosolized PGE1, iloprost and nitroprusside in lung ischemia-reperfusion. Transplantation. 2001;71:185–193. doi: 10.1097/00007890-200101270-00003. [DOI] [PubMed] [Google Scholar]
- 22.van Heerden PV, Caterina P, Filion P, Spagnolo DV, Gibbs NM. Pulmonary toxicity of inhaled aerosolized prostacyclin therapy--an observational study. Anaesthesia and intensive care. 2000;28:161–166. doi: 10.1177/0310057X0002800206. [DOI] [PubMed] [Google Scholar]
- 23.Wasserman MA, Griffin RL, Marsalisi FB. Inhibition of bronchoconstriction by aerosols of prostaglandins E1 and E2. The Journal of pharmacology and experimental therapeutics. 1980;214:68–73. [PubMed] [Google Scholar]
- 24.Borok Z, Gillissen A, Buhl R, Hoyt RF, Hubbard RC, Ozaki T, Rennard SI, Crystal RG. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis. 1991;144:1080–1084. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- 25.Kato S, Sugimura H, Kishiro I, Machida M, Suzuki H, Kaneko N. Suppressive effect of pulmonary hypertension and leukocyte activation by inhaled prostaglandin E1 in rats with monocrotaline-induced pulmonary hypertension. Exp Lung Res. 2002;28:265–273. doi: 10.1080/01902140252964357. [DOI] [PubMed] [Google Scholar]
- 26.Sood BG, Peterson J, Malian M, Galli R, Geisor-Walter M, McKinnon J, Sharp J, Maddipati KR. Jet nebulization of prostaglandin E1 during neonatal mechanical ventilation: Stability, emitted dose and aerosol particle size. Pharmacological Research. 2007;56:531–541. doi: 10.1016/j.phrs.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sood BG, Shen Y, Latif Z, Sharp J, Joshi A, Slovis T, Haacke EM. MR Evaluation of Aerosol Delivery in the Ventilated Newborn Pig. E-PAS 61511. 2007 doi: 10.1203/PDR.0b013e3181761841. [DOI] [PubMed] [Google Scholar]
- 28.Sood BG, Delaney-Black V, Aranda JV, Shankaran S. Aerosolized PGE1: A Selective Pulmonary Vasodilator in Neonatal Hypoxemic Respiratory Failure Results of a Phase I/II Open Label Clinical Trial. Pediatr Res. 2004;56:579–585. doi: 10.1203/01.PDR.0000139927.86617.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sood BG, Glibetic M, Aranda JV, Delaney-Black V, Chen X, Shankaran S. Systemic levels following PGE1 inhalation in neonatal hypoxemic respiratory failure. Acta Paediatr. 2006;95:1093–1098. doi: 10.1080/08035250600580511. [DOI] [PubMed] [Google Scholar]
- 30.Keszler M, Klappenbach RS, Reardon E. Lung pathology after high frequency jet ventilation combined with low rate intermittent mandatory ventilation in a canine model of meconium aspiration. Pediatr Pulmonol. 1988;4:144–149. doi: 10.1002/ppul.1950040305. [DOI] [PubMed] [Google Scholar]
- 31.Habler O, Kleen M, Takenaka S, Leiderer R, Pusch R, Welte M, Zwissler B, Messmer K. Eight hours’ inhalation of prostacyclin (PGI2) in healthy lambs: effects on tracheal, bronchial, and alveolar morphology. Intensive Care Med. 1996;22:1232–1238. doi: 10.1007/BF01709341. [DOI] [PubMed] [Google Scholar]
- 32.Zhou ZH, Sun B, Lin K, Zhu LW. Prevention of rabbit acute lung injury by surfactant, inhaled nitric oxide, and pressure support ventilation. Am J Respir Crit Care Med. 2000;161:581–588. doi: 10.1164/ajrccm.161.2.9901048. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Cao L, Lam LK, Zhu L, Guo C, Sun B. Mitigation of Meconium-Induced Lung Injury by Surfactant and Inhaled Nitric Oxide Is Associated with Suppression of Nuclear Transcription Factor Kappa B. Biol Neonate. 2005;87:73–81. doi: 10.1159/000081266. [DOI] [PubMed] [Google Scholar]
- 34.Walker GA. Repeated Measures Analysis. In: Walker GA, editor. Common Statistical Methods for Clinical Research with SAS Examples. SAS Institute Inc; Cary, NC: 2002. pp. 111–156. [Google Scholar]
- 35.Haworthand SG, Hislop AA. Adaptation of the pulmonary circulation to extra-uterine life in the pig and its relevance to the human infant. Cardiovasc Res. 1981;15:108–119. doi: 10.1093/cvr/15.2.108. [DOI] [PubMed] [Google Scholar]
- 36.Booke M, Bradford DW, Hinder F. Inhaled nitric oxide versus nebulized PGE1 and nebulized prostacyclin. Appl Cardiopulm Pathophysiol. 1997;6:233–239. [Google Scholar]
- 37.Hubbard GB, Langlinais PC, Shimazu T, Okerberg CV, Mason AD, Jr, Pruitt BA., Jr The morphology of smoke inhalation injury in sheep. J Trauma. 1991;31:1477–1486. doi: 10.1097/00005373-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Smith AP, Cuthbert MF, Dunlop LS. Effects of inhaled prostaglandins E1, E2, and F2alpha on the airway resistance of healthy and asthmatic man. Clinical science and molecular medicine. 1975;48:421–430. doi: 10.1042/cs0480421. [DOI] [PubMed] [Google Scholar]
- 39.Szczeklik A, Mastalerz L, Nizankowska E, Cmiel A. Protective and bronchodilator effects of prostaglandin E and salbutamol in aspirin-induced asthma. Am J Respir Crit Care Med. 1996;153:567–571. doi: 10.1164/ajrccm.153.2.8564099. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto Y, Hirota K, Ohtomo N, Sato T, Ishihara H, Matsuki A. Prostaglandin E1 produces spasmolytic effects on histamine-induced bronchoconstriction in dogs. Critical care medicine. 1999;27:2755–2759. doi: 10.1097/00003246-199912000-00025. [DOI] [PubMed] [Google Scholar]
- 41.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annual review of physiology. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 42.Jones R, Zapol WM, Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. The American journal of pathology. 1984;117:273–285. [PMC free article] [PubMed] [Google Scholar]
- 43.Mantell LL, Horowitz S, Davis JM, Kazzaz JA. Hyperoxia-induced cell death in the lung--the correlation of apoptosis, necrosis, and inflammation. Annals of the New York Academy of Sciences. 1999;887:171–180. doi: 10.1111/j.1749-6632.1999.tb07931.x. [DOI] [PubMed] [Google Scholar]
- 44.John E, McDevitt M, Wilborn W, Cassady G. Ultrastructure of the lung after ventilation. British journal of experimental pathology. 1982;63:401–407. [PMC free article] [PubMed] [Google Scholar]
- 45.Degraeuwe PL, Thunnissen FB, Vos GD, Blanco CE. High-frequency oscillatory ventilation, partial liquid ventilation, or conventional mechanical ventilation in newborn piglets with saline lavage-induced acute lung injury. A comparison of gas-exchange efficacy and lung histomorphology. Biology of the neonate. 1999;75:118–129. doi: 10.1159/000014087. [DOI] [PubMed] [Google Scholar]
- 46.Ehlert CA, Truog WE, Thibeault DW, Garg U, Norberg M, Rezaiekhaligh M, Mabry S, Ekekezie II. Hyperoxia and tidal volume: Independent and combined effects on neonatal pulmonary inflammation. Biology of the neonate. 2006;90:89–97. doi: 10.1159/000092005. [DOI] [PubMed] [Google Scholar]
- 47.Fuller HD, Dolovich MB, Posmituck G, Pack WW, Newhouse MT. Pressurized aerosol versus jet aerosol delivery to mechanically ventilated patients. Comparison of dose to the lungs. Am Rev Respir Dis. 1990;141:440–444. doi: 10.1164/ajrccm/141.2.440. [DOI] [PubMed] [Google Scholar]


