Abstract
Hepatic ischemia-reperfusion injury is commonplace in liver surgery, particularly in hepatic transplantation, hepatic resection, and trauma. The signaling events contributing to local hepatocellular damage are diverse and complex, and involve the interaction between hepatocytes, sinusoidal endothelial cells, Kupffer cells, as well as infiltrating neutrophils, macrophages, and platelets. Signaling mediators include cytokines, reactive oxygen and nitrogen species, calcium, complement, and several transcription factors. The purpose of this review article is to summarize the factors that contribute to the pathophysiology of hepatic ischemia-reperfusion injury.
Keywords: Liver, ischemia-reperfusion, IR injury, nitric oxide, NO, iNOS, adenosine, cytokines. Endothelial and Kupffer cells, ROS
Introduction
Interruption of an organ’s blood flow, with its subsequent lack of oxygen and nutrient supply, is an inherent phenomenon during diverse surgical procedures. In liver surgery, there are clinical situations in which the ischemic periods can be particularly long, such as during the resection of large hepatic tumors, management of hepatic trauma of diverse origins, vascular reconstructions, and liver procurement for transplantation.1–3 Once the blood flow and oxygen supply are reestablished, reperfusion enhances the injury caused by the ischemic period, aggravating the damage caused at the cellular level.4, 5 This phenomenon, known as ischemia-reperfusion (IR) injury, impacts directly on liver viability, especially during transplantation and liver surgery.3, 6 During an ischemic period, several functional changes occur at the cellular level that promote cell injury. A decrease in oxidative phosphorylation results in ATP depletion and derangements in calcium homeostasis.7
The deleterious effects of ATP catabolism modification are further enhanced by the production of several substances, including reactive oxygen species (ROS), cytokines, adhesion molecules, and vasoactive agents (endothelin and thromboxane-A2). These alterations are accompanied by a decrease of cytoprotective substances including nitric oxide, prostacyclin and others.8 Hepatic cell death occurs due to both necrosis and apoptosis. 9
MICROCIRCULATORY FAILURE
During the ischemic period, the lack of energetic substrate interferes with active transmembrane transport, producing edema in Kupffer cells (KC) and endothelial cells (EC).10 Loss of the delicate equilibrium between nitric oxide (NO) and endothelin (ET) induces vasoconstriction and narrowing of the sinusoidal lumen, compromising leukocyte flow and bringing them in close contact with the capillary wall.11 The increase in contact between leukocytes and EC promotes leukotaxis, and although not occluding the capillary lumen completely, the trapped leukocytes interfere with the flow of blood through the sinusoidal capillaries.12–14 Platelet aggregation within the hepatic sinusoids further aggravates the turbulent flow rate through the partially occluded capillaries.15 On reperfusion of the ischemic liver, the collapse of the microcirculation maintains areas of ischemic liver parenchyma, in a phenomenon known as “no-reflow”.8, 16, 17 In addition to the microcirculatory failure, the activation of KC and neutrophils leads to the synthesis of inflammatory cytokines, further aggravating the severity of the ischemic injury. The cytokines most frequently implicated in IR injury are the tumor necrosis factor-alpha (TNF-α), interleukins 1 (IL-1) and 6 (IL-6), prostaglandins (PG), and ROS, especially superoxide (O2−) and hydrogen peroxide (H2O2).18–23 Several relevant factors and mediators such as NO are involved in the ischemic injury of the liver. NO is synthesized from L-arginine by the action of nitric oxide synthase (NOS). NO is an important mediator of immunomodulation, neurotransmission, and platelet aggregation.24 Within EC, NO triggers cGMP to reduce the vascular tone and act as a vasodilator,24,25 NO can mediate the intensity of the IR injury by modulating neutrophil adhesion, platelet aggregation, and stellate (Ito) cell relaxation.4, 15, 26 Stellate cells contract when exposed to endothelin-1 (ET-1), whereas sodium nitroprusside (NO donor) induces their relaxation.27 Therefore, one of the mechanisms involved in IR injury is loss of the equilibrium between ET and NO levels during reperfusion.27, 28 At the beginning of reperfusion, NO levels decrease and ET levels increase, favoring microcirculatory vasoconstriction.29, 30 Ischemia reduces intracellular NADPH and oxygen (factors necessary for NO synthesis) and induces the release of arginase,31 producing and important reduction in NO synthesis, with a significant increase in degradation of its precursor L-arginine.31, 32 Both endogenous and exogenous NO protect hepatocytes and EC against IR injury, apparently by vasodilation, and by inhibiting the expression of adhesion molecules (E-selectin) within the sinusoidal lumen.25, 26 In order to produce significant amounts of NO in response to a specific stimulus such as IR, the inducible nitric oxide synthase enzyme (iNOS) is synthesized de novo, a process that takes 4 to 6 hours.24, 33 Blockade of the L-arginine/NO synthase pathway has been shown to worsen hepatic apoptosis and liver transplant preservation injury.34, 35 Augmenting graft iNOS expression with adenoviral iNOS transduction has also been shown to improve liver transplant preservation injury an improve survival in severe preservation injury.35 In clinical practice, an increase in NO concentration, as well as the reduction of ET, have been shown to decrease the severity of IR injury.36
FACTORS INVOLVED
CELL TYPES
Kupffer cells
During the initial stages of reperfusion KC are activated, producing morphologic changes that cause them to protrude into the sinusoids, contributing to the reduction of blood flow within the sinusoidal lumen.18, 37 Activated KC release a large amount of both proinflammatory (TNF-α, IL-6, IL-1, and prostaglandins) and anti-inflammatory mediators (IL-10, IL-13), as well as ROS.22, 23 Some studies show that IR injury can be attenuated or aggravated by the suppression or potentiation of KC activity, respectively.37, 38 Cold liver preservation induces strong KC activation;39 modulation of KC activity can therefore attenuate the IR damage in transplanted organs and consequently improve their survival.
Neutrophils
Activated neutrophils contribute to IR damage through the release of ROS and several proteases.40 Neutrophils accumulate in the liver at the initial stages of reperfusion, and their adhesion to EC is mediated by the interaction between selectins and integrins expressed in the neutrophil membrane, and intercellular adhesion molecules (ICAM) expressed on EC.41, 42 IR increases ICAM-1 expression in hepatic EC, probably through TNF-α and IL-1 synthesis.43, 44 In fact, increased ICAM-1 expression has been associated with acute liver rejection,45, 46 and neutralization of ICAM-1 decreases the severity of IR injury.47, 48
Recent studies propose that NK-T cell and T cells also play an important role in hepatic IR injury.49 Resident lymphocytes found within the liver include conventional alphabeta TCR cells as well as unconventional NK and gammadelta T cells. These lymphocytes can alter inflammation through the secretion of soluble mediators such as cytokines and chemokines or through cognate interactions in an antigen-dependent manner. Expression of these mediators will then result in the recruitment of more lymphocytes and neutrophils.50
Platelets
Platelets adhere to the hepatic sinusoids and induce programmed EC death upon reperfusion of transplanted organs.51 Platelets synthesize and release several factors that play an important role in the liver IR and hepatic regeneration.52 These include cytokines, growth factors such as transforming growth factor-β (TGF-β), serotonin, and calpain. Platelet-derived serotonin has recently been shown to promote tissue repair after normothermic hepatic ischemia in mice. 53 In human platelets, calpain activation is dependent on fibrinogen binding to integrin and subsequent platelet aggregation, suggesting a potential role for this protease in the regulation of post-aggregation responses.54 Platelets also produce NO that leads to the production of peroxynitrite, which acts as a potent inductor of programmed cell death in EC.55, 56
MEDIATORS
Cytokines
Cytokines play a relevant role in IR injury, both by starting and maintaining the inflammatory response, as well as modulating its severity.57, 58 The substances most studied in this context are the TNF-α and interleukins IL-1 and IL-6. These cytokines have large proinflammatory activity, inducing IL-6 and IL-8 synthesis59 and lower anti-inflammatory IL-10 levels.60, 61 IL-8 is a potent neutrophil chemotactic and activating factor, and correlates with the neutrophil infiltration in an IR model.61 The expression of adhesion molecules (β2-integrins and selectins) also promote leukocyte-EC interaction.43 These factors, together with chemokines and complement factors, recruit polymorphonuclear leukocytes (PMN)8 that infiltrate the liver, perpetuating and amplifying the ischemic injury by releasing additional ROS, TNF-α, and diverse proteases.62 TNF-α by itself produces leukocyte chemotaxis and activation,19 and induces ROS production by KC.63 In turn, IL-1 induces TNF-α synthesis by KC and induces neutrophil recruitment, which in turn produce ROS.20, 64 Both TNF-α and IL-1 levels are increased during hepatic IR injury,39,45 and their neutralization decreases the intensity of IR injury.20, 64 Recent studies have confirmed the relationship between IL-1 and neutrophil recruitment within hepatic tissue after IR, but its relationship to the extent of hepatocellular injury remains unclear.65
Reactive oxygen species (ROS)
Aerobic metabolism releases ROS, which under normal circumstances are neutralized through diverse antioxidant mechanisms.66 Under stress conditions, the balance between ROS and antioxidants shifts towards the former, resulting in oxidative stress and cytotoxicity.40, 67
Some of the processes involved both directly and indirectly in IR injury by ROS synthesis include the transformation of xanthine dehydrogenase into xanthine oxidase (an oxygen-dependant process that produces uric acid, releasing the ROS superoxide and hydrogen peroxide),68 induction of NADPH oxidase by activated KC and neutrophils (ROS production is blocked when NADPH oxidase is inhibited), and NO production and its conversion to peroxynitrite (both considered reactive nitrogen species).56, 67 Within the liver, the cytotoxic effects of ROS translate into nitrosylation of iron-sulfur groups and tyrosine residues, inactivation of the heme group, and lipid peroxidation.5, 56
Because of the potential inhibition of ROS by antioxidant agents, several studies have focused in modulating the severity of IR injury with different mechanisms, including pharmacologic α-tocopherol,69 allopurinol,70, 71 N-acetylcysteine,72 and enzymatic superoxide dismutase (SOD)73 and catalase73, 74 therapies. Endogenous antioxidant levels decrease significantly during reperfusion.69, 75 Therefore, the administration of exogenous antioxidants, particularly in the early stages of reperfusion, could significantly decrease the severity of IR damage in transplanted livers.
Complement system
Activation of the complement system has been demonstrated during IR. The complement system consist of about 30 soluble and membrane bound protein can be activated by any one of three pathways, the antibody-dependent classical pathway, the alternative pathway, or mannose-binding lectin (MBL) pathway.76 Activated complement acts both directly through the formation and deposition of membrane attack complexes,77 and indirectly by stimulating the production of chemotactic agents and proinflammatory cytokines, resulting in migration and adhesion of leukocytes and neutrophil recruitment within the sinusoids.78, 79 Complement inhibitors have been shown to be effective in reducing pathology of various organ-specific I/R injuries. For example, a partial IR rat model was used to investigate the efficacy of a small molecule C5a receptor antagonist against hepatic I/R injury. This antagonist ameliorated neutrophil infiltration, liver injury, and mortality.80 However, only a few complement inhibitors such as the small molecule C5a receptor antagonist, and recombinant sCR1 or C5 antibody are currently suitable for clinical testing in humans. 81
Calcium
Calcium was one of the first factors implicated in IR, by modulating the severity of IR with Ca2+ channel blockers. During IR, Ca2+ is essential for the activation of calcium-dependent phospholipases, nucleases, and proteases, and it plays a key role in the interruption of oxidative phosphorylation by decreasing ATP levels.82 Modulation of mitochondrial calcium management has also been shown to attenuate hepatic warm ischemia-reperfusion injury. 89
Adenosine
Adenosine is an endogenous compound that is produced by the enzymatic metabolism of ATP, ADP, and AMP. At high concentrations, it confers certain protection against ischemia by inhibiting platelet aggregation,83 neutrophil activation,84 and ET and ROS production while enhancing NO production.84, 85 During liver ischemia reperfusion, adenosine and inosine are released from the liver, which in turn contributes to homeostasis by releasing glucose from the hepatic glycogen through stimulation of A3 adenosine receptors.86 With reperfusion, inosine can be washed out of the organ,87 thus eliminating completely its protective effect. Inosine, when converted to hypoxanthine and xanthine, is also involved in ROS.88
Molecular mechanisms involved in liver ischemia reperfusion
When the liver is subjected to an ischemic insult, the alterations induced by oxidative stress can exceed the compensatory capacity of the liver, producing cell death. The ischemic event can reprogram gene expression of the surviving cells, initiating cellular mechanisms that allow them to regenerate and remodel. ATP is depleted during the ischemic period, and then liver injury is further exacerbated during reperfusion. One of the important transcription factors involved in mediating hepatic IR injury is nuclear factor kappa B (NF-κB).89–91
NF-κB is normally found in the cytoplasm attached to the inhibitory protein IκB.92 During oxidative stress, IκB is degraded, allowing for the translocation of NF-κB to the nucleus.92, 93 When activated, NF-κB induces the synthesis of iNOS, cytokines (TNF-α), chemokines, and adhesion molecules (ICAM-1).27, 93 The most important mechanism for NF-κB activation is ROS production, particularly hydrogen peroxide (H2O2),94 whereas the administration of antioxidants decreases its activation. NF-κB is activated during two different stages of IR, with different actions: at an early stage (from 30 min to 3 h of reperfusion), it induces an increase in the expression of proinflammatory cytokines (IL-1β, IL-6, and TNF-α). At a later stage (9 to 12 h after reperfusion), it acts as an anti-inflammatory agent.95 Other genes that may participate in IR include those of ET-1, NOS-3, heme-oxygenase, and those of the heat stress factor proteins.96 ROS have been documented to either activate or modulate all these pathways.
Apoptosis and necrosis
During reperfusion, TNF-α and other mediators activate many of the proteins involved in apoptosis, such as the proteases caspase-3 and caspase-8, along with mitochondria cytochrome-C release to the cytoplasm.81 The cascade of events that starts with these substances leads to DNA destruction and cell death.56 With this in mind, it is reasonable to think that apoptosis is the final effector of cell death during IR. However, in spite of the fact that suppression of apoptosis improves survival after ischemia and decreases reperfusion damage,97–100 some investigators argue that the predominate cell death event during IR is massive necrosis,101 particularly in steatotic livers. In view of this controversy, Lemasters in 1999, proposed the theory of “necroapoptosis”,102 emphasizing the main mechanisms that participate in IR at the cellular level and suggesting that both cell death mechanisms, necrosis and apoptosis, occur simultaneously during ischemia, and that they even imbricate during reperfusion. The ischemic stimulus can culminate in necrosis or in apoptosis, depending on the interaction with other determining factors, such as a significant reduction of ATP levels103 or in the fat content of the liver.104
Conclusion
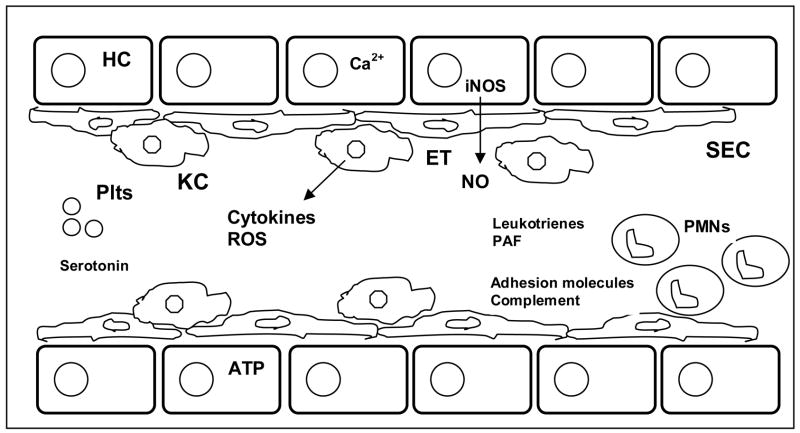
The cell signaling pathways and mediators of hepatic ischemia reperfusion are summarized in figure 1. In vitro and pre-clinical animal studies have led to an overall better understanding of liver anatomy, physiology, and the complex signaling events during IR injury. 105
Figure 1.
Mechanisms involved in hepatic ischemia-reperfusion injury. Hepatocyte (HC), Sinusoidal Endothelial cell (SEC), Kupffer cell (KC), Neutrophil (PMN), Platelets (Plts), nitric oxide (NO), Endothelin (ET), Calcium (Ca2+), Adenosine triphosphate (ATP), Platelet activating factor (PAF), Reactive oxygen species (ROS)
Application of pharmacologic, genetic, and surgical approaches to reduce hepatic IR injury have been applied and are increasingly being utilized. Therapeutic approaches include pharmacologic use of N-acetylcysteine, prostaglandins, prostacyclin, and ischemic pre-conditioning 56, 106 107, 108 109 110 Careful liver manipulation, and efforts to minimize warm ischemia time are also important principles. Strategies to improve liver outcomes and minimize I/R injury were summarized recently in a review by Clavien and colleagues111 Ultimately, the goal is application to safer clinical liver surgery during hepatic resections, liver transplantation, and the operative management of liver trauma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huguet C, Addario-Chieco P, Gavelli A, Arrigo E, Harb J, Clement RR. Technique of hepatic vascular exclusion for extensive liver resection. Am J Surg. 1992;163:602–5. doi: 10.1016/0002-9610(92)90567-b. [DOI] [PubMed] [Google Scholar]
- 2.Delva E, Camus Y, Nordlinger B, Hannoun L, Parc R, Deriaz H, Lienhart A, Huguet C. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg. 1989;209:211–8. doi: 10.1097/00000658-198902000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powner DJ. Factors during donor care that may affect liver transplantation outcome. Prog Transplant. 2004;14:241–7. 248–9. doi: 10.1177/152692480401400310. [DOI] [PubMed] [Google Scholar]
- 4.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–6. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 5.Romanque UP, Uribe MM, Videla LA. Molecular mechanisms in liver ischemic-reperfusion injury and ischemic preconditioning. Rev Med Chil. 2005;133:469–76. doi: 10.4067/s0034-98872005000400012. [DOI] [PubMed] [Google Scholar]
- 6.Henderson JM. Liver transplantation and rejection: an overview. Hepatogastroenterology. 1999;46(Suppl 2):1482–4. [PubMed] [Google Scholar]
- 7.de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39:481–4. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 9.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 10.Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Vollmar B, Menger MD, Glasz J, Leiderer R, Messmer K. Impact of leukocyte-endothelial cell interaction in hepatic ischemia-reperfusion injury. Am J Physiol. 1994;267:G786–93. doi: 10.1152/ajpgi.1994.267.5.G786. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. Faseb J. 1990;4:3355–9. [PubMed] [Google Scholar]
- 13.Yadav SS, Howell DN, Gao W, Steeber DA, Harland RC, Clavien PA. L-selectin and ICAM-1 mediate reperfusion injury and neutrophil adhesion in the warm ischemic mouse liver. Am J Physiol. 1998;275:G1341–52. doi: 10.1152/ajpgi.1998.275.6.G1341. [DOI] [PubMed] [Google Scholar]
- 14.Vollmar B, Richter S, Menger MD. Leukocyte stasis in hepatic sinusoids. Am J Physiol. 1996;270:G798–803. doi: 10.1152/ajpgi.1996.270.5.G798. [DOI] [PubMed] [Google Scholar]
- 15.Cywes R, Packham MA, Tietze L, Sanabria JR, Harvey PR, Phillips MJ, Strasberg SM. Role of platelets in hepatic allograft preservation injury in the rat. Hepatology. 1993;18:635–47. [PubMed] [Google Scholar]
- 16.Pretto EA., Jr Reperfusion injury of the liver. Transplant Proc. 1991;23:1912–4. [PubMed] [Google Scholar]
- 17.Casillas-Ramirez A, Mosbah IB, Ramalho F, Rosello-Catafau J, Peralta C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006 doi: 10.1016/j.lfs.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Lemasters JJ, Ji S, Thurman RG. Centrilobular injury following hypoxia in isolated, perfused rat liver. Science. 1981;213:661–3. doi: 10.1126/science.7256265. [DOI] [PubMed] [Google Scholar]
- 19.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology. 1996;23:506–14. doi: 10.1002/hep.510230315. [DOI] [PubMed] [Google Scholar]
- 20.Shirasugi N, Wakabayashi G, Shimazu M, Oshima A, Shito M, Kawachi S, Karahashi T, Kumamoto Y, Yoshida M, Kitajima M. Up-regulation of oxygen-derived free radicals by interleukin-1 in hepatic ischemia/reperfusion injury. Transplantation. 1997;64:1398–403. doi: 10.1097/00007890-199711270-00004. [DOI] [PubMed] [Google Scholar]
- 21.Cavalieri B, Perrelli MG, Aragno M, Ramadori P, Poli G, Cutrin JC. Ischaemic preconditioning modulates the activity of Kupffer cells during in vivo reperfusion injury of rat liver. Cell Biochem Funct. 2003;21:299–305. doi: 10.1002/cbf.1028. [DOI] [PubMed] [Google Scholar]
- 22.Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200–8. doi: 10.1016/s0891-5849(02)01017-1. [DOI] [PubMed] [Google Scholar]
- 23.Tsukamoto H. Redox regulation of cytokine expression in Kupffer cells. Antioxid Redox Signal. 2002;4:741–8. doi: 10.1089/152308602760598882. [DOI] [PubMed] [Google Scholar]
- 24.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2):249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ignarro LJ. Physiology and pathophysiology of nitric oxide. Kidney Int Suppl. 1996;55:S2–5. [PubMed] [Google Scholar]
- 26.Clemens MG. Nitric oxide in liver injury. Hepatology. 1999;30:1–5. doi: 10.1002/hep.510300148. [DOI] [PubMed] [Google Scholar]
- 27.Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213:815–23. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- 28.Clemens MG, Bauer M, Pannen BH, Bauer I, Zhang JX. Remodeling of hepatic microvascular responsiveness after ischemia/reperfusion. Shock. 1997;8:80–5. doi: 10.1097/00024382-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura E, Yamanaka N, Okamoto E, Tomoda F, Furukawa K. Response of plasma and tissue endothelin-1 to liver ischemia and its implication in ischemia-reperfusion injury. Hepatology. 1995;21:1138–43. [PubMed] [Google Scholar]
- 30.Pannen BH, Bauer M, Noldge-Schomburg GF, Zhang JX, Robotham JL, Clemens MG, Geiger KK. Regulation of hepatic blood flow during resuscitation from hemorrhagic shock: role of NO and endothelins. Am J Physiol. 1997;272:H2736–45. doi: 10.1152/ajpheart.1997.272.6.H2736. [DOI] [PubMed] [Google Scholar]
- 31.Shiraishi M, Hiroyasu S, Nagahama M, Miyaguni T, Higa T, Tomori H, Okuhama Y, Kusano T, Muto Y. Role of exogenous L-arginine in hepatic ischemia-reperfusion injury. J Surg Res. 1997;69:429–34. doi: 10.1006/jsre.1997.5094. [DOI] [PubMed] [Google Scholar]
- 32.Vodovotz Y, Kim PK, Bagci EZ, Ermentrout GB, Chow CC, Bahar I, Billiar TR. Inflammatory modulation of hepatocyte apoptosis by nitric oxide: in vivo, in vitro, and in silico studies. Curr Mol Med. 2004;4:753–62. doi: 10.2174/1566524043359944. [DOI] [PubMed] [Google Scholar]
- 33.Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, Lee JH. Hepatic ischemia/reperfusion in rats induces iNOS gene transcription by activation of NF-kappaB. Biochem Biophys Res Commun. 1999;261:917–22. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- 34.Yagnik GP, Takahashi Y, Tsoulfas G, Reid K, Murase N, Geller DA. Blockade of the L-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology. 2002;36:573–81. doi: 10.1053/jhep.2002.35058. [DOI] [PubMed] [Google Scholar]
- 35.Kaizu T, Ikeda A, Nakao A, Takahashi Y, Tsung A, Kohmoto J, Toyokawa H, Shao L, Bucher BT, Tomiyama K, Nalesnik MA, Murase N, Geller DA. Donor graft adenoviral iNOS gene transfer ameliorates rat liver transplant preservation injury and improves survival. Hepatology. 2006;43:464–73. doi: 10.1002/hep.21067. [DOI] [PubMed] [Google Scholar]
- 36.Scommotau S, Uhlmann D, Loffler BM, Breu V, Spiegel HU. Involvement of endothelin/nitric oxide balance in hepatic ischemia/reperfusion injury. Langenbecks Arch Surg. 1999;384:65–70. doi: 10.1007/s004230050176. [DOI] [PubMed] [Google Scholar]
- 37.Shiratori Y, Kiriyama H, Fukushi Y, Nagura T, Takada H, Hai K, Kamii K. Modulation of ischemia-reperfusion-induced hepatic injury by Kupffer cells. Dig Dis Sci. 1994;39:1265–72. doi: 10.1007/BF02093792. [DOI] [PubMed] [Google Scholar]
- 38.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–62. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 39.Arii S, Monden K, Adachi Y, Zhang W, Higashitsuji H, Furutani M, Mise M, Fujita S, Nakamura T, Imamura M. Pathogenic role of Kupffer cell activation in the reperfusion injury of cold-preserved liver. Transplantation. 1994;58:1072–7. [PubMed] [Google Scholar]
- 40.Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, Ward PA. Ischemia/reperfusion injury. J Surg Res. 2002;105:248–58. doi: 10.1006/jsre.2002.6385. [DOI] [PubMed] [Google Scholar]
- 41.Gopalan PK, Smith CW, Lu H, Berg EL, McIntire LV, Simon SI. Neutrophil CD18-dependent arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow can be activated through L-selectin. J Immunol. 1997;158:367–75. [PubMed] [Google Scholar]
- 42.Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–4. [PubMed] [Google Scholar]
- 43.Pober JS. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology Am J Pathol. 1988;133:426–33. [PMC free article] [PubMed] [Google Scholar]
- 44.Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368–74. [PubMed] [Google Scholar]
- 45.Kiuchi T, Oldhafer KJ, Schlitt HJ, Nashan B, Deiwick A, Wonigeit K, Ringe B, Tanaka K, Yamaoka Y, Pichlmayr R. Background and prognostic implications of perireperfusion tissue injuries in human liver transplants: a panel histochemical study. Transplantation. 1998;66:737–47. doi: 10.1097/00007890-199809270-00008. [DOI] [PubMed] [Google Scholar]
- 46.Scoazec JY, Durand F, Degott C, Delautier D, Bernuau J, Belghiti J, Benhamou JP, Feldmann G. Expression of cytokine-dependent adhesion molecules in postreperfusion biopsy specimens of liver allografts. Gastroenterology. 1994;107:1094–102. doi: 10.1016/0016-5085(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 47.Nakano H, Kuzume M, Namatame K, Yamaguchi M, Kumada K. Efficacy of intraportal injection of anti-ICAM-1 monoclonal antibody against liver cell injury following warm ischemia in the rat. Am J Surg. 1995;170:64–6. doi: 10.1016/s0002-9610(99)80255-4. [DOI] [PubMed] [Google Scholar]
- 48.Vollmar B, Glasz J, Menger MD, Messmer K. Leukocytes contribute to hepatic ischemia/reperfusion injury via intercellular adhesion molecule-1-mediated venular adherence. Surgery. 1995;117:195–200. doi: 10.1016/s0039-6060(05)80085-6. [DOI] [PubMed] [Google Scholar]
- 49.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–48. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caldwell CC, Tschoep J, Lentsch AB. Lymphocyte function during hepatic ischemia/reperfusion injury. J Leukoc Biol. 2007 doi: 10.1189/jlb.0107062. [DOI] [PubMed] [Google Scholar]
- 51.Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Platelets induce sinusoidal endothelial cell apoptosis upon reperfusion of the cold ischemic rat liver. Gastroenterology. 2000;118:183–91. doi: 10.1016/s0016-5085(00)70427-6. [DOI] [PubMed] [Google Scholar]
- 52.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 53.Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–76. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 54.Schoenwaelder SM, Yuan Y, Cooray P, Salem HH, Jackson SP. Calpain cleavage of focal adhesion proteins regulates the cytoskeletal attachment of integrin alphaIIbbeta3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin clots. J Biol Chem. 1997;272:1694–702. doi: 10.1074/jbc.272.3.1694. [DOI] [PubMed] [Google Scholar]
- 55.Gow AJ, Thom SR, Ischiropoulos H. Nitric oxide and peroxynitrite-mediated pulmonary cell death. Am J Physiol. 1998;274:L112–8. doi: 10.1152/ajplung.1998.274.1.L112. [DOI] [PubMed] [Google Scholar]
- 56.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 57.Fong Y, Moldawer LL, Shires GT, Lowry SF. The biologic characteristics of cytokines and their implication in surgical injury. Surg Gynecol Obstet. 1990;170:363–78. [PubMed] [Google Scholar]
- 58.Strieter RM, Kunkel SL, Bone RC. Role of tumor necrosis factor-alpha in disease states and inflammation. Crit Care Med. 1993;21:S447–63. doi: 10.1097/00003246-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 59.Thornton AJ, Strieter RM, Lindley I, Baggiolini M, Kunkel SL. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol. 1990;144:2609–13. [PubMed] [Google Scholar]
- 60.Morariu AM, Loef BG, Aarts LP, Rietman GW, Rakhorst G, van Oeveren W, Epema AH. Dexamethasone: benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: a study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest. 2005;128:2677–87. doi: 10.1378/chest.128.4.2677. [DOI] [PubMed] [Google Scholar]
- 61.Chen X, Kidder LS, Schmidt AH, Lew WD. Osteogenic protein-1 induces bone formation in the presence of bacterial infection in a rat intramuscular osteoinduction model. J Orthop Trauma. 2004;18:436–42. doi: 10.1097/00005131-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Lichtman SN, Lemasters JJ. Role of cytokines and cytokine-producing cells in reperfusion injury to the liver. Semin Liver Dis. 1999;19:171–87. doi: 10.1055/s-2007-1007108. [DOI] [PubMed] [Google Scholar]
- 63.Shibuya H, Ohkohchi N, Tsukamoto S, Satomi S. Tumor necrosis factor-induced, superoxide-mediated neutrophil accumulation in cold ischemic/reperfused rat liver. Hepatology. 1997;26:113–20. doi: 10.1053/jhep.1997.v26.pm0009214459. [DOI] [PubMed] [Google Scholar]
- 64.Shito M, Wakabayashi G, Ueda M, Shimazu M, Shirasugi N, Endo M, Mukai M, Kitajima M. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation. 1997;63:143–8. doi: 10.1097/00007890-199701150-00026. [DOI] [PubMed] [Google Scholar]
- 65.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toledo-Pereyra LH, Toledo AH, Walsh J, Lopez-Neblina F. Molecular signaling pathways in ischemia/reperfusion. Exp Clin Transplant. 2004;2:174–7. [PubMed] [Google Scholar]
- 67.Videla LA, Fernandez V. Biochemical aspects of cellular oxidative stress. Arch Biol Med Exp (Santiago) 1988;21:85–92. [PubMed] [Google Scholar]
- 68.McCord JM. Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc. 1987;46:2402–6. [PubMed] [Google Scholar]
- 69.Marubayashi S, Dohi K, Ochi K, Kawasaki T. Protective effects of free radical scavenger and antioxidant administration on ischemic liver cell injury. Transplant Proc. 1987;19:1327–8. [PubMed] [Google Scholar]
- 70.Nordstrom G, Seeman T, Hasselgren PO. Beneficial effect of allopurinol in liver ischemia. Surgery. 1985;97:679–84. [PubMed] [Google Scholar]
- 71.Kusumoto K, Morimoto T, Minor T, Uchino J, Isselhard W. Allopurinol effects in rat liver transplantation on recovery of energy metabolism and free radical-induced damage. Eur Surg Res. 1995;27:285–91. doi: 10.1159/000129411. [DOI] [PubMed] [Google Scholar]
- 72.Koeppel TA, Lehmann TG, Thies JC, Gehrcke R, Gebhard MM, Herfarth C, Otto G, Post S. Impact of N-acetylcysteine on the hepatic microcirculation after orthotopic liver transplantation. Transplantation. 1996;61:1397–402. doi: 10.1097/00007890-199605150-00020. [DOI] [PubMed] [Google Scholar]
- 73.Mizoe A, Kondo S, Azuma T, Fujioka H, Tanaka K, Hashida M, Kanematsu T. Preventive effects of superoxide dismutase derivatives modified with monosaccharides on reperfusion injury in rat liver transplantation. J Surg Res. 1997;73:160–5. doi: 10.1006/jsre.1997.5215. [DOI] [PubMed] [Google Scholar]
- 74.Younes M, Strubelt O. The involvement of reactive oxygen species in hypoxic injury to rat liver. Res Commun Chem Pathol Pharmacol. 1988;59:369–81. [PubMed] [Google Scholar]
- 75.Marubayashi S, Dohi K, Yamada K, Kawasaki T. Changes in the levels of endogenous coenzyme Q homologs, alpha-tocopherol, and glutathione in rat liver after hepatic ischemia and reperfusion, and the effect of pretreatment with coenzyme Q10. Biochim Biophys Acta. 1984;797:1–9. [PubMed] [Google Scholar]
- 76.Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol. 2006;3:333–40. [PubMed] [Google Scholar]
- 77.Chavez-Cartaya RE, DeSola GP, Wright L, Jamieson NV, White DJ. Regulation of the complement cascade by soluble complement receptor type 1. Protective effect in experimental liver ischemia and reperfusion. Transplantation. 1995;59:1047–52. doi: 10.1097/00007890-199504150-00023. [DOI] [PubMed] [Google Scholar]
- 78.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264:G801–9. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 79.Collard CD, Lekowski R, Jordan JE, Agah A, Stahl GL. Complement activation following oxidative stress. Mol Immunol. 1999;36:941–8. doi: 10.1016/s0161-5890(99)00116-9. [DOI] [PubMed] [Google Scholar]
- 80.Arumugam TV, Woodruff TM, Stocks SZ, Proctor LM, Pollitt S, Shiels IA, Reid RC, Fairlie DP, Taylor SM. Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J Hepatol. 2004;40:934–41. doi: 10.1016/j.jhep.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 81.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–9. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 82.Nauta RJ, Tsimoyiannis E, Uribe M, Walsh DB, Miller D, Butterfield A. The role of calcium ions and calcium channel entry blockers in experimental ischemia-reperfusion-induced liver injury. Ann Surg. 1991;213:137–42. doi: 10.1097/00000658-199102000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitakaze M, Hori M, Sato H, Takashima S, Inoue M, Kitabatake A, Kamada T. Endogenous adenosine inhibits platelet aggregation during myocardial ischemia in dogs. Circ Res. 1991;69:1402–8. doi: 10.1161/01.res.69.5.1402. [DOI] [PubMed] [Google Scholar]
- 84.Ward PA, Cunningham TW, McCulloch KK, Johnson KJ. Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab Invest. 1988;58:438–47. [PubMed] [Google Scholar]
- 85.McKie LD, Bass BL, Dunkin BJ, Harmon JW. Nitric oxide mediates the blood flow response to intravenous adenosine in the rabbit. Circ Shock. 1994;43:103–6. [PubMed] [Google Scholar]
- 86.Guinzberg R, Cortes D, Diaz-Cruz A, Riveros-Rosas H, Villalobos-Molina R, Pina E. Inosine released after hypoxia activates hepatic glucose liberation through A3 adenosine receptors. Am J Physiol Endocrinol Metab. 2006;290:E940–51. doi: 10.1152/ajpendo.00173.2005. [DOI] [PubMed] [Google Scholar]
- 87.Van Belle H, Goossens F, Wynants J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am J Physiol. 1987;252:H886–93. doi: 10.1152/ajpheart.1987.252.5.H886. [DOI] [PubMed] [Google Scholar]
- 88.Van Belle H, Wynants J, Xhonneux R, Flameng W. Changes in creatine phosphate, inorganic phosphate, and the purine pattern in dog hearts with time of coronary artery occlusion and effect thereon of mioflazine, a nucleoside transport inhibitor. Cardiovasc Res. 1986;20:658–64. doi: 10.1093/cvr/20.9.658. [DOI] [PubMed] [Google Scholar]
- 89.Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic TNF signaling and NF-{kappa}B: effects on liver homeostasis and beyond. Endocr Rev. 2007 doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- 90.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 91.Tsoulfas G, Geller DA. NF-kappaB in transplantation: friend or foe? Transpl Infect Dis. 2001;3:212–9. doi: 10.1034/j.1399-3062.2001.30405.x. [DOI] [PubMed] [Google Scholar]
- 92.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 93.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Zhang W, Mantell LL, Kazzaz JA, Fein AM, Horowitz S. Nuclear factor-kappaB is activated by hyperoxia but does not protect from cell death. J Biol Chem. 1997;272:20646–9. doi: 10.1074/jbc.272.33.20646. [DOI] [PubMed] [Google Scholar]
- 95.Takahashi Y, Ganster RW, Gambotto A, Shao L, Kaizu T, Wu T, Yagnik GP, Nakao A, Tsoulfas G, Ishikawa T, Okuda T, Geller DA, Murase N. Role of NF-kappaB on liver cold ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1175–84. doi: 10.1152/ajpgi.00515.2001. [DOI] [PubMed] [Google Scholar]
- 96.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–99. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 97.Rudiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–10. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 98.Natori S, Selzner M, Valentino KL, Fritz LC, Srinivasan A, Clavien PA, Gores GJ. Apoptosis of sinusoidal endothelial cells occurs during liver preservation injury by a caspase-dependent mechanism. Transplantation. 1999;68:89–96. doi: 10.1097/00007890-199907150-00018. [DOI] [PubMed] [Google Scholar]
- 99.Cursio R, Gugenheim J, Ricci JE, Crenesse D, Rostagno P, Maulon L, Saint-Paul MC, Ferrua B, Auberger AP. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. Faseb J. 1999;13:253–61. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- 100.Bilbao G, Contreras JL, Eckhoff DE, Mikheeva G, Krasnykh V, Douglas JT, Thomas FT, Thomas JM, Curiel DT. Reduction of ischemia-reperfusion injury of the liver by in vivo adenovirus-mediated gene transfer of the antiapoptotic Bcl-2 gene. Ann Surg. 1999;230:185–93. doi: 10.1097/00000658-199908000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 102.Lemasters JJV. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol. 1999;276:G1–6. doi: 10.1152/ajpgi.1999.276.1.G1. [DOI] [PubMed] [Google Scholar]
- 103.Cursio R, Gugenheim J, Panaia-Ferrari P, Lasfar A, Tovey M, Chastanet S, Saint-Paul MC, Ferre C, Mouiel J. Improvement of normothermic rat liver ischemia/reperfusion by muramyl dipeptide. J Surg Res. 1998;80:339–44. doi: 10.1006/jsre.1998.5445. [DOI] [PubMed] [Google Scholar]
- 104.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–8. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 105.Massip-Salcedo M, Rosello-Catafau J, Prieto J, Avila MA, Peralta C. The response of the hepatocyte to ischemia. Liver Int. 2007;27:6–16. doi: 10.1111/j.1478-3231.2006.01390.x. [DOI] [PubMed] [Google Scholar]
- 106.Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77:577–92. doi: 10.1007/s001099900029. [DOI] [PubMed] [Google Scholar]
- 107.Clavien PA, Selzner M, Rudiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–50. 851–2. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vajdova K, Heinrich S, Tian Y, Graf R, Clavien PA. Ischemic preconditioning and intermittent clamping improve murine hepatic microcirculation and Kupffer cell function after ischemic injury. Liver Transpl. 2004;10:520–8. doi: 10.1002/lt.20126. [DOI] [PubMed] [Google Scholar]
- 109.Clavien PA, Emond J, Vauthey JN, Belghiti J, Chari RS, Strasberg SM. Protection of the liver during hepatic surgery. J Gastrointest Surg. 2004;8:313–27. doi: 10.1016/j.gassur.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 110.Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921–8. 928–30. doi: 10.1097/01.sla.0000246834.07130.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]