Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ) and retinoic acid X-receptor (RXR) heterodimer, which regulates cell growth and differentiation, represses the TGFβ1 gene that encodes for the protein involved in cancer biology. This review will introduce the novel mechanism associated with the inhibition of the TGFβ1 gene by PPARγ activation, which regulates the dephosphorylation of Zf9 transcription factor. Pharmacological manipulation of TGFβ1 by PPARγ activators can be applied for treating TGFβ1-induced pathophysiologic disorders such as cancer metastasis and fibrosis. In this article, we will discuss the opposing effects of TGFβ on tumor growth and metastasis, and address the signaling pathways regulated by PPARγ for tumor progression and suppression.
1. INTRODUCTION
Peroxisome proliferator-activated receptor-γ (PPARγ) as a ligand-activated transcription factor belongs to the members of nuclear hormone receptor superfamily. PPARγ is implicated in a wide variety of cellular functions, regulating the expression of gene networks required for cell proliferation, differentiation, morphogenesis, and metabolic homeostasis. The transforming growth factor isoforms (TGFβ1, β2, and β3) as the members of the TGFβ superfamily are ubiquitously expressed cytokines [1, 2]. TGFβ exerts multiple functions with differential expression pattern in organs: each form of TGFβ has similar biological activities [3]. Among the TGFβ forms, it is recognized that TGFβ1 plays a major role in the regulation of cell proliferation and differentiation. In this review paper, we will discuss the role of PPARγ on TGFβ gene expression.
Accumulating evidences suggest that the interplay of PPARγ and TGFβ contributes to the regulation of cell proliferation, differentiation, and their associated cellular functions. For instance, the interaction of PPARγ signaling with the proteins affected by the activation of TGFβ receptor determines the outcome of the breast tumor progression [4]. Many studies have shown that agonist-induced activation of PPARγ interferes with TGFβ/Smad-dependent or Smad-independent signaling in different cell types [5–12]. The crosstalk between PPARγ and TGFβ can be achieved not only by PPARγ-dependent modulation of the propagation of TGFβ/TGFβ receptor-mediated signaling pathways, but also by the regulation of TGFβ1 expression itself and TGFβ1-inducible target genes. Hence, suppression of TGFβ signaling by PPARγ could be counteracted by the inhibitory action of TGFβ on the PPARγ-mediated signaling [13–15].
The TGFβ1 expression is regulated at multiple levels. Diverse transcription factors are involved in the transcriptional regulation of TGFβ gene expression and post-translational modification makes precursors bound with TGFβ1 binding proteins mature to TGFβ molecule [16, 17]. The role of PPARγ activation in TGFβ1 gene repression has been examined by the experiments using thiazolidinedione PPARγ agonists [18, 19]. These studies on the regulation of the TGFβ1 gene and the molecular interaction of ligand-activated nuclear receptors for the activation of responsible transcription factor(s) brought insights into the transcriptional control mechanism. The research results showed that PPARγ activation might transrepress the TGFβ gene, interfering with TGFβ signaling and thereby altering the expression of TGFβ-inducible target genes [18], substantiating the fact that ligand activation of PPARγ modulates TGFβ receptor-mediated gene regulation.
2. TGFβ AND CANCER CELL BIOLOGY
TGFβ1 exerts its diverse biological effects by acting on distinct combinations of type I and type II receptors and recruiting downstream signal transducers including Smads, consequently regulating a group of target gene expression responsible for a specific biological activity. Smad proteins are classified into R-Smads (receptor-regulated Smads: Smads 1, 2, 3, 5, and 8), Co-Smads (common mediator Smad: Smad 4), and I-Smads (inhibitory Smads: Smad 6 and 7), and these play roles as the transcriptional regulators for the superfamily of TGFβ1-inducible target genes [1, 2, 20–22]. Smad 2 and Smad 3 are the specific mediators of TGFβ1, whereas Smad 1, Smad 5, and MADH6/Smad 9 are crucial for bone morphogenic protein signaling [22]. In particular, Smad 3 is involved in the TGFβ1 gene regulation, which is crucial for the autocrine function of TGFβ1 [23].
Following the activation of the TGFβ1 receptor by TGFβ1, TGFβ1-induced receptor kinase activation rapidly phosphorylates Smads proteins and initiates formation of functional oligomeric complexes. The resultant oligomeric complex translocates to the nucleus to regulate target gene expression. Briefly, the type I TGFβ1 receptor kinase phosphorylates serine residues at the C-terminal SSXS motif in the MH2 domain of Smad 3 (or Smad 2) [24]. Phosphorylated Smad 3 (or Smad 2) forms an oligomeric complex with Smad 4, which is crucial for the maximal transcription of diverse TGFβ1-inducible target genes [25, 26]. The oligomeric complexes of Smad 3 (or Smad 2) and Smad 4 recognize DNA binding element tetranucleotide (CAGA) or GC-rich sequences, and several copies of which are present in the promoter regions of many TGFβ1-responsive genes such as plasminogen activator inhibitor-1 (PAI-1), α2(I) procollagen, and type VII collagen [25, 27]. It is well known that the protein products encoded from these genes promote the accumulation of extracellular matrix and that abnormal accumulation of the proteins may lead to fibrosis, which represents a form of the epithelial to mesenchymal transition (EMT).
Moreover, TGFβ1-activated kinase-1, a member of MAPK kinase kinase family, activates its MAP kinase pathways [28, 29]. It is accepted that TGFβ1-activated ERK pathway synergistically enhances Smad signaling of the TGFβ1 receptor due to the positive cross talk between the ERK and Smad pathways [22, 30]. Serine phosphorylation of Smad 3/2, but not phosphorylation of the C-terminal motif, was decreased by MEK-ERK inhibitors [31]. Smad 3/2 are differentially activated by TGFβ1 in hepatic stellate cells as a result of the differential phosphorylations of the Smads. Smad 3 plays a key role in TGFβ signaling, which is strengthened by the observation that the loss of Smad 3 interfered with TGFβ1-mediated induction of target genes [32, 33]. In addition, activation of CCAAT/enhancer binding protein (C/EBP) β is also involved in the inhibition of TGFβ1 expression [34].
During the process of carcinogenesis, TGFβ action can be either tumor suppressive or tumor promoting, depending on the stage of tumor development [35–37]. In an experimental cell model, TGFβ could induce cell growth arrest and promote apoptosis of carcinoma cells [1]. The antiproliferative action of TGFβ in epithelial cells, for example, is essentially attributed to the cell cycle arrest and the apoptosis concomitantly induced. It is well known that cell cycle arrest induced by TGFβ occurs at G1 phase through enhancing transcription of cyclin-dependent kinase inhibitors, p21Cip1/WAF and p15Ink4b, while suppressing the induction of c-Myc, a progrowth transcription factor, and of Id1–3, the inhibitors of differentiation [38–43]. In a model of gastric adenocarinoma, TGFβ-mediated apoptosis contributed to tumor suppression, which resulted from TGFβ-induced caspase-8 activation [44]. Moreover, it has been shown that TGFβ reduced the expression of antiapoptotic Bcl-2 family members in prostate cancer cells [45].
By contrast, TGFβ may also lead to tumor cell proliferation as a consequence of EMT process [46–48], which is a cellular phenomenon characterized by a loss of polarized epithelial phenotype with transition to a mesenchymal or more migratory phenotype. Studies have shown that diverse signaling pathways are involved in the TGFβ-dependent EMT process. Initiation of EMT by TGFβ receptor activation is mediated by either Smad-dependent or Smad-independent pathway [1, 49, 50]. Downstream of the TGFβ receptor activation, the Smads activated by the TGFβ receptor kinase promote transcription of the genes, which eventually play crucial roles in the process of EMT. The responsible transcription factors primarily include Snail, Slug, and LEF-1 [1]. In addition, TGFβ also activates the non-Smad pathways, which include Ras, phosphatidylinositol 3-kinase (PI3K), and Par 6. These molecules regulate the expression of Snail and the activities of glycogen synthase kinase 3β (GSK3β) and RhoA, respectively [51, 52], thereby enhancing the process of EMT. It is now accepted that the EMT phenomenon of primary cancer cells promoted by the action of TGFβ may increase cancer metastasis.
TGFβ acts on tumor cells directly, playing a role in cancer cell migration and invasion. Diverse TGFβ-mediated signaling pathways are responsible for this process. In glioblastoma cells, siRNA knockdowns of TGFβ1 and TGFβ2 resulted in the inhibition of cell motility or invasiveness [53]. As a same token, TGFβ released from tumor tissues might facilitate glioma cell migration and invasion via an autocrine signaling [54]. Several lines of evidence also support the concept that TGFβ-induced Smad signaling is responsible for the invasiveness of cancer cells [55–58]. This is explained in part by the TGFβ-dependent induction of matrix metalloproteases, which are known to be responsible for cell migration and invasion [55, 59–62]. Activation of ERK and JNK by TGFβ and their association with focal complexes may also contribute to cell migration, as shown in the case of breast carcinoma [63]. Moreover, it has been shown that the activation of p38 MAPK pathway by TGFβ facilitated invasion of head and neck squamous epithelial cells [61].
In addition to the double-edged effects of TGFβ on cancer cells, TGFβ may alter cancer growth by suppressing the growth of multiple immune cells, which compromises the overall immune functions. Studies have shown that the proliferation and activity of T cells are suppressed by the TGFβ blockade of IL-2 production and expression of T cell effector molecules [64–68]. Also, TGFβ attenuates the activity of natural killer (NK) cells by inhibiting NK production of interferon-γ (IFN-γ) [69, 70]. Another study showed that TGFβ inhibited the antigen presentation function of dendritic cells through suppressing the expression of MHC class II and costimulatory molecules [71]. All of these results support the alterations by TGFβ in immune functions, which would impair immune surveillance or attack against cancer cells.
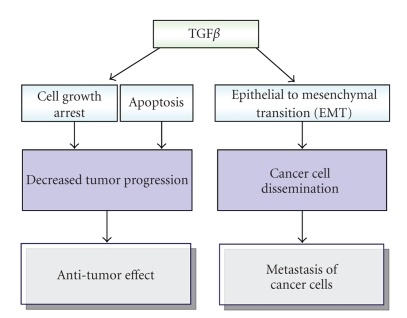
In summary, action of TGFβ1 on cancer cells switches from tumor suppression to tumor promotion, depending on the stage of tumor progression. For instance, during the early phase of breast tumorigenesis, the TGFβ signal inhibits primary tumor growth via cell growth arresting and promoting apoptosis. However, at later stage, cancer cells acquire a capacity to escape from the tumor suppressive effects of TGFβ1 via induction of EMT. Interestingly, the aforementioned conflicting functions of TGFβ might go through the same TGFβ receptor complex and the associated signaling pathways involving Smad transcription factors [1]. Probably, there should be certain stage-dependent modifications in cellular signaling system including changes in receptor function and downstream Smad signaling cascades. Taken together, it is concluded that TGFβ may not only induce growth arrest of cancer cells, but also increase cancer dissemination [1], supporting the concept that the cytokine serves a dual function in tumor development and progression (Figure 1).
Figure 1.
A scheme showing the opposing effects of TGFβ on tumor growth and metastasis.
3. PPARγ AND CANCER BIOLOGY
PPARγ has been extensively studied as an anticancer target in preclinical and clinical settings [72]. The anticancer effects appeared to be cancer cell-specific. A knock-out or loss of function mutation in PPARγ can be an important risk factor for the incidence of cancer [73–75]. In this sense, PPARγ has been considered as a novel target for designing new anticancer drugs for chemotherapy. This is further supported by the finding that PPARγ activators exert a potent tumor-suppressing activity against various human cancer cells [76–78]. As a matter of fact, PPARγ activators such as troglitazone and ciglitazone exert antiproliferative activities in epithelial cancer cell lines or animal models, which presumably results from the activation of PPARγ receptor and the PPARγ receptor-dependent pathways [76, 79–83]. Nevertheless, other anticancer pathways have also been recognized in association with PPARγ, which might be PPARγ receptor-independent [84, 85]. Multiple PPARγ-independent anticancer targets of PPARγ agonists have been suggested in several cancer cell types. The mechanisms may comprise a variety of pathways such as the blockade of G1-S phase transition by inhibiting translation initiation [86], activation of JNK-dependent cell death pathway [87], induction of the early growth response-1 (Egr-1) gene [88], inhibition of Bcl-xL and Bcl-2 function [85], counteracting TGFβ release by tumor cells [54], and induction of cyclin-dependent kinase inhibitor p21WAF1/CIP1 [89]. However, the precise antiproliferative mechanisms of the PPARγ agonists remain to be further studied. On the contrary, there are also other reports available on the opposite effects showing that PPARγ signaling promoted carcinogenesis [90, 91].
It should be noted that the antitumor effects of PPARγ may be explained at least in two different ways. One mechanism involves cell growth regulation [4], which should be further clarified, whereas the other mechanism includes cancer chemopreventive effects mediated by the induction of antioxidant enzymes [92]. It is well recognized that PPARγ affects cell survival, growth, and differentiation by acting on the peroxisomal proliferator-response element (PPRE), thereby modulating an expression of a group of genes controlling cell growth and differentiation pathways [93, 94]. The PPARγ homodimer and PPARγ-retinoic acid X receptor (RXR) α heterodimer have the specificities of DNA-binding with preferential binding of the latter to DR1, which is a PPRE DNA binding site. SRC-1 is a coactivator of PPARγ [95]. Binding of the ligand-activated PPARγ-RXRα heterodimer to its DNA binding sites stimulates the interaction between PPARγ-RXRα and p160/SRC-1 [95].
A number of studies support the concept that cancer chemoprevention is accomplished by the induction of antioxidant enzymes. The results from our laboratories indicated that oltipraz and flavonoids as potential cancer chemopreventive agents activate C/EBPβ in the antioxidant genes such as glutathione S-transferase (GST) A2 [96, 97]. In addition, treatments of cells with PPARγ activators induced the nuclear translocation of NF-E2-related factor 2 (Nrf2) and C/EBPβ, and activating Nrf2 and C/EBPβ bindings to the antioxidant response element (ARE) and C/EBP response elements, respectively [92]. Moreover, the Nrf2 and C/EBPβ genes contain PPRE sites, which account for the induction of the target antioxidant proteins by PPARγ activators. Both the ARE and the C/EBP binding site have crucial roles in transactivating the GSTA2 gene by PPARγ and RXR ligands [92]. Therefore, Nrf2 and/or C/EBPβ inductions(s) via the PPARγ and RXRα heterodimer binding to the PPREs in the promoter regions of the target genes contribute(s) to the antioxidant capacity of cells (e.g., GSTA2).
A result of our previous study indicated that specific mutations of these nuclear binding sites in the GSTA2 promoter, which are present as a three-PPRE cluster, caused the complete loss of its responsiveness to PPARγ activators [92]. All of the putative PPRE sites comprising DR1 were functionally active. Therefore, the binding of the activating PPARγ-RXR heterodimer to all of the PPRE sites appeared to be crucial for the inducible gene activation, showing that the PPAR binding site cluster is the functionally active PPRE-responsive enhancer module (PPREM) [92]. This study on the regulation of gene expression by the PPARγ-RXR heterodimer at the promoter containing multiple DR1 elements brought additional insight into the transcriptional control mechanism of the antioxidant enzymes. The identified molecular mechanism would shed light on the contribution of cell viability and cancer chemoprevention as a consequence of the induction of antioxidant targets genes by PPARγ activators.
4. TGFβ REGULATION BY PPARγ-RXR AND CELL SIGNALING
Activation of the PPARγ-RXR heterodimer represses the TGFβ1 gene through dephosphorylation of a transcription factor called zinc finger transcription factor-9 (Zf9), which has been shown to be induced by phosphatase and tensin homolog deleted on chromosome (PTEN)-mediated p70 ribosomal S6 kinase-1 (S6K1) inhibition [18]. Because RXRs are modular proteins with a highly conserved central DNA binding domain and a less conserved ligand binding domain [98], activation of the PPARγ and RXR heterodimer contributes to the gene regulation. The role of PPARγ in repression of the TGFβ1 gene was further evidenced by the effects of thiazolidinediones, and also by the reversal of TGFβ1 repression by the dominant negative mutants, supporting to the novel aspect that PPARγ activation contributes to TGFβ1 gene repression and that RXRα is necessary for the full responsiveness in the gene repression. In fact, the inhibition of TGFβ1 gene by the PPARγ and RXR heterodimer might account for either tumor suppression or tumor promotion [18]. Also, as an effort to identify the molecular basis of TGFβ1 repression by PPARγ activators, the effects of PPARγ and RXR activation on the TGFβ1 gene transactivation, that is regulated by the proximal DNA response elements, have been examined [18]. The potential regulatory sites responsible for the TGFβ1 gene expression have been explored by using the luciferase reporter gene assays, which identified the putative PPREs located at the multiple sites upstream from −453 bp of the promoter region [18]. Promoter deletion analyses indicate that neither the putative PPREs nor the activator protein-1 (AP-1) binding sites are directly regulated by PPARγ activators forthe gene repression.
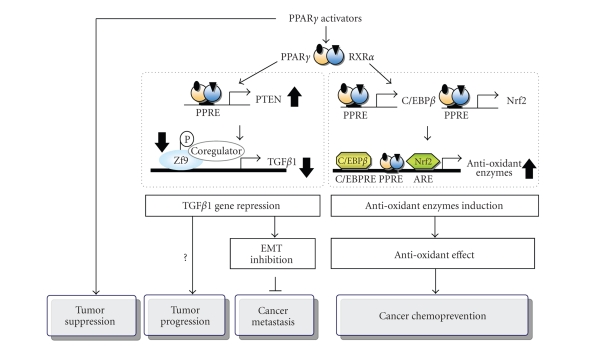
S6K1, a ubiquitous serine/threonine kinase, controls the translational efficiency by phosphorylating ribosomal S6 protein [99]. S6K1 functions as a multifunctional kinase for the phosphorylation of ribosomal S6 protein [99], CREM [100], BAD [101], and the eukaryotic elongation factor 2 kinase [102]. Rapamycin, a well-known mammalian target of rapamycin (mTOR) inhibitor, inhibited liver fibrosis and TGFβ1 expression in rats bile duct-ligated or challenged with toxicants [103, 104], with a concomitant decrease in S6K1 activity. It is well recognized that rapamycin inhibits S6K1 activity via mTOR inhibition [105]. Yet, other pharmacological agents that modulate S6K1 activity have not been reported. The mechanism of PPARγ-RXR heterodimer-mediated repression of the TGFβ1 gene has been elucidated in terms of the modulation of S6K1 activity (Figure 2).
Figure 2.
A schematic presentation of the multiple pathways regulated by PPARγ for tumor suppression, progression, inhibition of metastasis, and cancer chemoprevention.
The PI3K-mTOR pathway regulates S6K1 for the regulation of transcription factors involved in the TGFβ1 gene transactivation. A study identified the inhibition of S6K1 activity by the PPARγ-RXR, which contributes to TGFβ1 gene repression [18]. Another signaling molecule, PTEN, antagonizes the PI3-kinase-mTOR-S6K1-mediated signaling cascade [106, 107]. Thus, it has been elucidated that PPARγ activators upregulate PTEN, which leads to the S6K1 inhibition, consequently causing TGFβ1 repression [18].
5. TRANSCRIPTION FACTORS RESPONSIBLE FOR TGFβ REPRESSION BY PPARγ-RXR
In the promoter region of the TGFβ1 gene (Figure 3), the putative binding sites for PPARγ-RXR seemed to be neither active nor responsible for the gene repression by the activated PPARγ and RXR heterodimer. It has been claimed that the effects of PPARγ or retinoid ligands on TGFβ1 gene expression might be mediated in part by AP-1 inhibition [108, 109]. Nevertheless, such a result that deletion of the DNA region containing both AP-1 sites still had the capability to repress the gene by PPARγ activator suggests that the AP-1 binding sites might not be a major regulatory target in the TGFβ1 gene repression. Rather, the target molecule altered by PPARγ-RXRα-activated cell signal may be involved in the interaction with the protein recruited on the AP-1 DNA complex. It appeared that the TGFβ1 gene repression may have not resulted from the direct inhibition of AP-1, but other mechanistic basis [18].
Figure 3.
The human TGFβ1 promoter region.
Another study showed that the mechanism associated with the inhibition of TGFβ1 by PPARγ activators involves the regulation of c-Fos [108]. In the study, thiazolidinediones inhibit high-glucose-induced TGFβ1 promoter activity. A suggested mechanism was raised based on the observation that treatments of thiazolidinediones reduced high-glucose-induced, activated PKC and c-Fos-mediated TGFβ1 gene expression in mesangial cells [108].
Zf9 as an immediate early gene reduces cell proliferation with the induction of p21cip1 and the enhancement of c-Jun degradation [110, 111], thus functioning as a potential tumor suppressor gene. The transcription factors that interact with the known DNA binding sites on the region downstream within the −323 bp of the TGFβ1 gene include Zf9, NF1, and SP1. It is noteworthy that Zf9 activation induces TGFβ1 during the activation of hepatic stellate cells [112]. Also, Zf9 regulates TGFβ receptors and collagen α1(I), promoting accumulation of extracellular matrix [113]. Studies have shown that Zf9 phosphorylation enhances its nuclear localization and transcriptional activity [111]. Zf9 as a transcription factor plays a crucial role for the induction of TGFβ1 [113]. Thus, phosphorylation status of Zf9 may contribute to the promotion of its target gene expression [114]. Identification of the partners of Zf9 or phosphorylated Zf9 for the TGFβ1 gene regulation and their molecular interactions would be interesting to pursue. The constitutive Zf9 phosphorylation by S6K1 strengthened the important role of S6K1 as a multifunctional kinase for the transcription factor regulation of target genes [100–102].
The TGFβ1 gene contains the DNA response element interacting with Zf9 [16] that regulates multiple genes involved in tissue differentiation. Activation of Zf9 includes its phosphorylation at serine (or tyrosine) residues [114]. Thus, phosphorylation of Zf9 leads to transcription of its target genes [111, 114]. Although the kinase catalyzing Zf9 phosphorylation has not been completely identified, the inhibition of Zf9 phosphorylation by rapamycin that inhibits S6K1 activity via mTOR inhibition supports the role of S6K1 in Zf9 phosphorylation [18]. More importantly, the role of S6K1 in regulating TGFβ1 gene and the associated molecular mechanistic basis have been clarified in terms of Zf9 dephosphorylation [18]. In view of the previous observations that Zf9 is crucial as a transcription factor for TGFβ1 induction in hepatic stellate cells [113] and that a phosphorylated form of Zf9 plays a role in the transactivation of the target gene promoter [114], the potential ability of PPARγ activators to inhibit serine phosphorylation of the transcription factor has also been investigated. Thus, it has been demonstrated that the inhibition of the TGFβ1 gene by the activation of PPARγ-RXR includes Zf9 dephosphorylation [18]. Therefore, TGFβ1 gene repression by PPARγ activators appears to be related with dephosphorylation of Zf9, supporting the conclusion that the PPARγ-RXR heterodimer causes TGFβ1 repression via S6K1 inhibition, and that the inhibition of S6K1 activity provides a central mechanism, by which PPARγ-RXR regulates Zf9-dependent TGFβ1 gene expression (Figure 2).
Moreover, it has been shown that PPARγ activation induces PTEN, which serves as a PI(3,4,5)P3 lipid phosphatase and antagonizes PI3-kinase-mediated cell signaling [106]. Functional PPREs located in the PTEN promoter have been recognized [115]. The induction of PTEN by PPARγ activators may result in TGFβ1 gene repression following S6K1 inhibition. Furthermore, PPARγ activators inhibited phosphorylations of Akt, ERK1/2, p90 ribosomal S6 kinase-1 (RSK1), and mTOR, downstream of PTEN, indicating that PTEN induction by PPARγ activators leads to S6K1 inhibition via the pathways of ERK1/2-RSK1 as well as Akt-mTOR. In conclusion, the result showing that PPARγ activation upregulates PTEN, which has also been implicated in tumor-inhibitory or anti-inflammatory actions of PPARγ [106, 115], gives credence to the concept that PPARγ activators induce PTEN during S6K1 inhibition, and consequently causes TGFβ1 repression. Therefore, the inhibition of tumor proliferation by PPARγ activators may be explained in part by PPARγ-dependent TGFβ1 repression (Figure 2), supporting the concept that the PPARγ activators may be applied for controlling TGFβ1-induced cancer metastasis and fibrosis.
ACKNOWLEDGMENT
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Ministry of Science and Technology (MOST), South Korean government (no.R11-2007-107-01001-0).
References
- 1.Rahimi RA, Leof EB. TGF-β signaling: a tale of two responses. Journal of Cellular Biochemistry. 2007;102(3):593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 2.Piek E, Heldin C-H, Dijke PT. Specificity, diversity, and regulation in TGF-β superfamily signaling. The FASEB Journal. 1999;13(15):2105–2124. [PubMed] [Google Scholar]
- 3.Roberts AB, Sporn MB. Differential expression of the TGF-β isoforms in embryogenesis suggests specific roles in developing and adult tissues. Molecular Reproduction and Development. 1992;32(2):91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- 4.Jarrar MH, Baranova A. PPARγ activation by thiazolidinediones (TZDs) may modulate breast carcinoma outcome: the importance of interplay with TGFβ signalling. Journal of Cellular and Molecular Medicine. 2007;11(1):71–87. doi: 10.1111/j.1582-4934.2007.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu M, Zhang J, Zhu X, et al. Peroxisome proliferator-activated receptor γ inhibits transforming growth factor β-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. Journal of Biological Chemistry. 2001;276(49):45888–45894. doi: 10.1074/jbc.M105490200. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Liu F, Chen N. Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists attenuate the profibrotic response induced by TGF-β1 in renal interstitial fibroblasts. Mediators of Inflammation. 2007;2007 doi: 10.1155/2007/62641. Article ID 62641, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARγ agonists prevent TGFβ1/Smad3-signaling in human hepatic stellate cells. Biochemical and Biophysical Research Communications. 2006;350(2):385–391. doi: 10.1016/j.bbrc.2006.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Wen X, Spataro BC, Hu K, Dai C, Liu Y. Hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-γ agonists. Journal of the American Society of Nephrology. 2006;17(1):54–65. doi: 10.1681/ASN.2005030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-β/Smad3 signaling. Journal of Biological Chemistry. 2005;280(24):22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang X, Kanjanabuch T, Mao S-L, et al. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. American Journal of Physiology. 2006;290(1):E103–E113. doi: 10.1152/ajpendo.00605.2004. [DOI] [PubMed] [Google Scholar]
- 11.Redondo S, Ruiz E, Santos-Gallego CG, Padilla E, Tejerina T. Pioglitazone induces vascular smooth muscle cell apoptosis through a peroxisome proliferator-activated receptor-γ, transforming growth factor-β1, and a Smad2-dependent mechanism. Diabetes. 2005;54(3):811–817. doi: 10.2337/diabetes.54.3.811. [DOI] [PubMed] [Google Scholar]
- 12.Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-γ ligands inhibit TGF-β1-induced fibronectin expression in glomerular mesangial cells. Diabetes. 2004;53(1):200–208. doi: 10.2337/diabetes.53.1.200. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Jr, Nicholson AC. Transforming growth factor-β1 (TGF-β1) and TGF-β2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-γ . Journal of Biological Chemistry. 2000;275(2):1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- 14.Ahdjoudj S, Kaabeche K, Holy X, et al. Transforming growth factor-β inhibits CCAAT/enhancer-binding protein expression and PPARγ activity in unloaded bone marrow stromal cells. Experimental Cell Research. 2005;303(1):138–147. doi: 10.1016/j.yexcr.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Zheng S, Chen A. Disruption of transforming growth factor-β signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-γ in rat hepatic stellate cells. American Journal of Physiology. 2007;292(1):G113–G123. doi: 10.1152/ajpgi.00200.2006. [DOI] [PubMed] [Google Scholar]
- 16.Kim S-J, Glick A, Sporn MB, Roberts AB. Characterization of the promoter region of the human transforming growth factor-β1 gene. Journal of Biological Chemistry. 1989;264(1):402–408. [PubMed] [Google Scholar]
- 17.Öklü R, Hesketh R. The latent transforming growth factor β binding protein (LTBP) family. Biochemical Journal. 2000;352, part 3:601–610. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ, Yang EK, Kim SG. Peroxisome proliferator-activated receptor-γ and retinoic acid X receptor α represses the TGFβ1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: role for Zf9 dephosphorylation. Molecular Pharmacology. 2006;70(1):415–425. doi: 10.1124/mol.106.022954. [DOI] [PubMed] [Google Scholar]
- 19.Weigert C, Brodbeck K, Bierhaus A, Häring HU, Schleicher ED. c-Fos-driven transcriptional activation of transforming growth factor β-1: inhibition of high glucose-induced promoter activity by thiazolidinediones. Biochemical and Biophysical Research Communications. 2003;304(2):301–307. doi: 10.1016/s0006-291x(03)00599-0. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki Y, Nemoto T, Nakao A, et al. Interaction between GC Box binding factors and Smad proteins modulates cell lineage-specific α2(I) collagen gene transcription. Journal of Biological Chemistry. 2001;276(19):16573–16579. doi: 10.1074/jbc.M010485200. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki Y, Mamura M, Kanamaru Y, et al. Constitutive phosphorylation and nuclear localization of Smad3 are correlated with increased collagen gene transcription in activated hepatic stellate cells. Journal of Cellular Physiology. 2001;187(1):117–123. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1059>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Massagué J, Chen Y-G. Controlling TGF-β signaling. Genes and Development. 2000;14(6):627–644. [PubMed] [Google Scholar]
- 23.Piek E, Ju WJ, Heyer J, et al. Functional characterization of transforming growth factor β signaling in Smad2- and Smad3-deficient fibroblasts. Journal of Biological Chemistry. 2001;276(23):19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- 24.Wrana JL. Regulation of Smad activity. Cell. 2000;100(2):189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 25.Vindevoghel L, Lechleider RJ, Kon A, et al. SMAD3/4-dependent transcriptional activation of the human type VII collagen gene (COL7A1) promoter by transforming growth factor β . Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):14769–14774. doi: 10.1073/pnas.95.25.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng X-H, Lin X, Derynck R. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15Ink4B transcription in response to TGF-β . The EMBO Journal. 2000;19(19):5178–5193. doi: 10.1093/emboj/19.19.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier J-M. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. The EMBO Journal. 1998;17(11):3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey RS, Mulder KM. Involvement of extracellular signal-regulated kinase 2 and stress-activated protein kinase/Jun N-terminal kinase activation by transforming growth factor β in the negative growth control of breast cancer cells. Cancer Research. 1997;57(4):628–633. [PubMed] [Google Scholar]
- 29.Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. Journal of Biological Chemistry. 2001;276(17):14466–14473. doi: 10.1074/jbc.M005724200. [DOI] [PubMed] [Google Scholar]
- 30.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. The FASEB Journal. 2003;17(11):1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa F, Matsuzaki K, Mori S, et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38(4):879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- 32.Uemura M, Swenson ES, Gaça MDA, Giordano FJ, Reiss M, Wells RG. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and α-smooth muscle actin organization. Molecular Biology of the Cell. 2005;16(9):4214–4224. doi: 10.1091/mbc.E05-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Wang W, Hu H, et al. Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-β1 in cultured rat hepatic stellate cells. Pharmaceutical Research. 2006;23(1):82–89. doi: 10.1007/s11095-005-9043-5. [DOI] [PubMed] [Google Scholar]
- 34.Kang KW, Kim YG, Cho MK, et al. Oltipraz regenerates cirrhotic liver through CCAAT/enhancer binding protein-mediated stellate cell inactivation. The FASEB Journal. 2002;16(14):1988–1990. doi: 10.1096/fj.02-0406fje. [DOI] [PubMed] [Google Scholar]
- 35.Muraoka-Cook RS, Dumont N, Arteaga CL. Dual role of transforming growth factor β in mammary tumorigenesis and metastatic progression. Clinical Cancer Research. 2005;11(2):937s–943s. [PubMed] [Google Scholar]
- 36.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nature Genetics. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 37.Truty MJ, Urrutia R. Basics of TGF-β and pancreatic cancer. Pancreatology. 2007;7(5-6):423–435. doi: 10.1159/000108959. [DOI] [PubMed] [Google Scholar]
- 38.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-β-induced cell cycle arrest. Nature. 1994;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 39.Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang X-F. Transforming growth factor β induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pietenpol JA, Holt JT, Stein RW, Moses HL. Transforming growth factor β1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(10):3758–3762. doi: 10.1073/pnas.87.10.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexandrow MG, Kawabata M, Aakre M, Moses HL. Overexpression of the c-myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of transforming growth factor β1. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(8):3239–3243. doi: 10.1073/pnas.92.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang Y, Chen C-R, Massagué J. A self-enabling TGFβ response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Molecular Cell. 2003;11(4):915–926. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 43.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nature Reviews Cancer. 2003;3(11):807–820. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 44.Kim SG, Jong H-S, Kim T-Y, et al. Transforming growth factor-β1 induces apoptosis through Fas ligand-independent activation of the Fas death pathway in human gastric SNU-620 carcinoma cells. Molecular Biology of the Cell. 2004;15(2):420–434. doi: 10.1091/mbc.E03-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chipuk JE, Bhat M, Hsing AY, Ma J, Danielpour D. Bcl-xL blocks transforming growth factor-β1-induced apoptosis by inhibiting cytochrome c release and not by directly antagonizing Apaf-1-dependent caspase activation in prostate epithelial cells. Journal of Biological Chemistry. 2001;276(28):26614–26621. doi: 10.1074/jbc.M100913200. [DOI] [PubMed] [Google Scholar]
- 46.Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105(4):425–431. doi: 10.1016/s0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 47.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature Reviews Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 48.Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. Journal of Cellular Physiology. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 49.Gal A, Sjöblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained TGFβ exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008;27(9):1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- 50.Pardali K, Moustakas A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochimica et Biophysica Acta. 2007;1775(1):21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-β1-induced epithelial-mesenchymal transition. Molecular Biology of the Cell. 2006;17(4):1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes and Development. 2002;16(9):1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- 53.Friese MA, Wischhusen J, Wick W, et al. RNA interference targeting transforming growth factor-β enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Research. 2004;64(20):7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 54.Coras R, Hölsken A, Seufert S, et al. The peroxisome proliferator-activated receptor-γ agonist troglitazone inhibits transforming growth factor-β-mediated glioma cell migration and brain invasion. Molecular Cancer Therapeutics. 2007;6(6):1745–1754. doi: 10.1158/1535-7163.MCT-06-0763. [DOI] [PubMed] [Google Scholar]
- 55.Leivonen S-K, Ala-aho R, Koli K, Grénman R, Peltonen J, Kähäri V-M. Activation of Smad signaling enhances collagenase-3 (MMP-13) expression and invasion of head and neck squamous carcinoma cells. Oncogene. 2006;25(18):2588–2600. doi: 10.1038/sj.onc.1209291. [DOI] [PubMed] [Google Scholar]
- 56.Leivonen S-K, Kähäri V-M. Transforming growth factor-β signaling in cancer invasion and metastasis. International Journal of Cancer. 2007;121(10):2119–2124. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- 57.Deckers M, van Dinther M, Buijs J, et al. The tumor suppressor Smad4 is required for transforming growth factor β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Research. 2006;66(4):2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 58.Kang Y, He W, Tulley S, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-β . Journal of Neuro-Oncology. 2001;53(2):177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 60.Rooprai HK, Rucklidge GJ, Panou C, Pilkington GJ. The effects of exogenous growth factors on matrix metalloproteinase secretion by human brain tumour cells. British Journal of Cancer. 2000;82(1):52–55. doi: 10.1054/bjoc.1999.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johansson N, Ala-aho R, Uitto V, et al. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. Journal of Cell Science. 2000;113(2):227–235. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 62.Lin S-W, Lee M-T, Ke F-C, et al. TGF β1 stimulates the secretion of matrix metalloproteinase 2 (MMP2) and the invasive behavior in human ovarian cancer cells, which is suppressed by MMP inhibitor BB3103. Clinical & Experimental Metastasis. 2000;18(6):493–499. doi: 10.1023/a:1011888126865. [DOI] [PubMed] [Google Scholar]
- 63.Giehl K, Imamichi Y, Menke A. Smad4-independent TGF-β signaling in tumor cell migration. Cells Tissues Organs. 2007;185(1–3):123–130. doi: 10.1159/000101313. [DOI] [PubMed] [Google Scholar]
- 64.Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA. Transforming growth factor-β regulation of immune responses. Annual Review of Immunology. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 65.Kehrl JH, Wakefield LM, Roberts AB, et al. Production of transforming growth factor β by human T lymphocytes and its potential role in the regulation of T cell growth. Journal of Experimental Medicine. 1986;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annual Review of Immunology. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 67.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-β and the immune response: implications for anticancer therapy. Clinical Cancer Research. 2007;13(18):5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 68.Ahmadzadeh M, Rosenberg SA. TGF-β1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. Journal of Immunology. 2005;174(9):5215–5223. doi: 10.4049/jimmunol.174.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rook AH, Kehrl JH, Wakefield LM, et al. Effects of transforming growth factor β on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. Journal of Immunology. 1986;136(10):3916–3920. [PubMed] [Google Scholar]
- 70.Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U. Regulation of NK cell functions by TGF-β1. Journal of Immunology. 1995;155(3):1066–1073. [PubMed] [Google Scholar]
- 71.Geissmann F, Revy P, Regnault A, et al. TGF-β1 prevents the noncognate maturation of human dendritic Langerhans cells. Journal of Immunology. 1999;162(8):4567–4575. [PubMed] [Google Scholar]
- 72.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. Lancet Oncology. 2004;5(7):419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 73.Girnun GD, Smith WM, Drori S, et al. APC-dependent suppression of colon carcinogenesis by PPARγ . Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13771–13776. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikezoe T, Miller CW, Kawano S, et al. Mutational analysis of the peroxisome proliferator-activated receptor γ in human malignancies. Cancer Research. 2001;61(13):5307–5310. [PubMed] [Google Scholar]
- 75.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in PPARγ associated with human colon cancer. Molecular Cell. 1999;3(6):799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 76.Kubota T, Koshizuka K, Williamson EA, et al. Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Research. 1998;58(15):3344–3352. [PubMed] [Google Scholar]
- 77.Chen GG, Lee JF, Wang SH, Chan UPF, Ip PC, Lau WY. Apoptosis induced by activation of peroxisome-proliferator activated receptor-gamma is associated with Bcl-2 and Nf-κB in human colon cancer. Life Sciences. 2002;70(22):2631–2646. doi: 10.1016/s0024-3205(02)01510-2. [DOI] [PubMed] [Google Scholar]
- 78.Brockman JA, Gupta RA, Dubois RN. Activation of PPARγ leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115(5):1049–1055. doi: 10.1016/s0016-5085(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 79.Kato M, Kusumi T, Tsuchida S, Tanaka M, Sasaki M, Kudo H. Induction of differentiation and peroxisome proliferator-activated receptor γ expression in colon cancer cell lines by troglitazone. Journal of Cancer Research and Clinical Oncology. 2004;130(2):73–79. doi: 10.1007/s00432-003-0510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T. Ligands for peroxisome proliferator-activated receptor γ inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. Journal of Clinical Endocrinology and Metabolism. 2001;86(5):2170–2177. doi: 10.1210/jcem.86.5.7493. [DOI] [PubMed] [Google Scholar]
- 81.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ . Nature Medicine. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 82.Yu J, Qiao L, Zimmermann L, et al. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006;43(1):134–143. doi: 10.1002/hep.20994. [DOI] [PubMed] [Google Scholar]
- 83.Heaney AP, Fernando M, Melmed S. PPAR-γ receptor ligands: novel therapy for pituitary adenomas. Journal of Clinical Investigation. 2003;111(9):1381–1388. doi: 10.1172/JCI16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Day C. Thiazolidinediones: a new class of antidiabetic drugs. Diabetic Medicine. 1999;16(3):179–192. doi: 10.1046/j.1464-5491.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 85.Weng J-R, Chen C-Y, Pinzone JJ, Ringel MD, Chen C-S. Beyond peroxisome proliferator-activated receptor γ signaling: the multi-facets of the antitumor effect of thiazolidinediones. Endocrine-Related Cancer. 2006;13(2):401–413. doi: 10.1677/erc.1.01182. [DOI] [PubMed] [Google Scholar]
- 86.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Research. 2001;61(16):6213–6218. [PubMed] [Google Scholar]
- 87.Bae M-A, Song BJ. Critical role of c-Jun N-terminal protein kinase activation in troglitazone-induced apoptosis of human HepG2 hepatoma cells. Molecular Pharmacology. 2003;63(2):401–408. doi: 10.1124/mol.63.2.401. [DOI] [PubMed] [Google Scholar]
- 88.Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) ligand, selectively induces the early growth response-1 gene independently of PPARγ: a novel mechanism for its anti-tumorigenic activity. Journal of Biological Chemistry. 2003;278(8):5845–5853. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- 89.Sugimura A, Kiriyama Y, Nochi H, et al. Troglitazone suppresses cell growth of myeloid leukemia cell lines by induction of p21WAF1/CIP1 cyclin-dependent kinase inhibitor. Biochemical and Biophysical Research Communications. 1999;261(3):833–837. doi: 10.1006/bbrc.1999.1049. [DOI] [PubMed] [Google Scholar]
- 90.Lefebvre A-M, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 91.Saez E, Rosenfeld J, Livolsi A, et al. PPARγ signaling exacerbates mammary gland tumor development. Genes and Development. 2004;18(5):528–540. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park EY, Cho IJ, Kim SG. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-γ and retinoid X receptor heterodimer. Cancer Research. 2004;64(10):3701–3713. doi: 10.1158/0008-5472.CAN-03-3924. [DOI] [PubMed] [Google Scholar]
- 93.Chou F-S, Wang P-S, Kulp S, Pinzone JJ. Effects of thiazolidinediones on differentiation, proliferation, and apoptosis. Molecular Cancer Research. 2007;5(6):523–530. doi: 10.1158/1541-7786.MCR-06-0278. [DOI] [PubMed] [Google Scholar]
- 94.Thompson EA. PPARγ physiology and pathology in gastrointestinal epithelial cells. Molecules and Cells. 2007;24(2):167–176. [PubMed] [Google Scholar]
- 95.Kodera Y, Takeyama K, Murayama A, Suzawa M, Masuhiro Y, Kato S. Ligand type-specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. Journal of Biological Chemistry. 2000;275(43):33201–33204. doi: 10.1074/jbc.C000517200. [DOI] [PubMed] [Google Scholar]
- 96.Kang KW, Cho IJ, Lee CH, Kim SG. Essential role of phosphatidylinositol 3-kinase-dependent CCAAT/enhancer binding protein β activation in the induction of glutathione S-transferase by oltipraz. Journal of the National Cancer Institute. 2003;95(1):53–66. doi: 10.1093/jnci/95.1.53. [DOI] [PubMed] [Google Scholar]
- 97.Kang KW, Park EY, Kim SG. Activation of CCAAT/enhancer-binding protein β by 2′-amino-3′-methoxyflavone (PD98059) leads to the induction of glutathione S-transferase A2. Carcinogenesis. 2003;24(3):475–482. doi: 10.1093/carcin/24.3.475. [DOI] [PubMed] [Google Scholar]
- 98.Holmbeck SMA, Dyson HJ, Wright PE. DNA-induced conformational changes are the basis for cooperative dimerization by the DNA binding domain of the retinoid X receptor. Journal of Molecular Biology. 1998;284(3):533–539. doi: 10.1006/jmbi.1998.2207. [DOI] [PubMed] [Google Scholar]
- 99.Jenö P, Ballou LM, Novak-Hofer I, Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(2):406–410. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Groot RP, Ballou LM, Sassone-Corsi P. Positive regulation of the cAMP-responsive activator CREM by the p70 S6 kinase: an alternative route to mitogen-induced gene expression. Cell. 1994;79(1):81–91. doi: 10.1016/0092-8674(94)90402-2. [DOI] [PubMed] [Google Scholar]
- 101.Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(17):9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90RSK1 and p70 S6 kinase. The EMBO Journal. 2001;20(16):4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu J, Wu J, Frizell E, et al. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999;117(5):1198–1204. doi: 10.1016/s0016-5085(99)70406-3. [DOI] [PubMed] [Google Scholar]
- 104.Biecker E, De Gottardi A, Neef M, et al. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. Journal of Pharmacology and Experimental Therapeutics. 2005;313(3):952–961. doi: 10.1124/jpet.104.079616. [DOI] [PubMed] [Google Scholar]
- 105.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 106.Lee KS, Park SJ, Hwang PH, et al. PPAR-gamma modulates allergic inflammation through up-regulation of PTEN. The FASEB Journal. 2005;19(8):1033–1035. doi: 10.1096/fj.04-3309fje. [DOI] [PubMed] [Google Scholar]
- 107.Liu J-L, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WKA. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Molecular and Cellular Biology. 2005;25(14):6211–6224. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weigert C, Brodbeck K, Bierhaus A, Häring HU, Schleicher ED. c-Fos-driven transcriptional activation of transforming growth factor β-1: inhibition of high glucose-induced promoter activity by thiazolidinediones. Biochemical and Biophysical Research Communications. 2003;304(2):301–307. doi: 10.1016/s0006-291x(03)00599-0. [DOI] [PubMed] [Google Scholar]
- 109.Salbert G, Fanjul A, Piedrafita FJ, et al. Retinoic acid receptors and retinoid X receptor-α down-regulate the transforming growth factor-β1 promoter by antagonizing AP-1 activity. Molecular Endocrinology. 1993;7(10):1347–1356. doi: 10.1210/mend.7.10.8264664. [DOI] [PubMed] [Google Scholar]
- 110.Narla G, Heath KE, Reeves HL, et al. KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science. 2001;294(5551):2563–2566. doi: 10.1126/science.1066326. [DOI] [PubMed] [Google Scholar]
- 111.Slavin DA, Koritschoner NP, Prieto CC, López-Díaz FJ, Chatton B, Bocco JL. A new role for the Krüppel-like transcription factor KLF6 as an inhibitor of c-Jun proto-oncoprotein function. Oncogene. 2004;23(50):8196–8205. doi: 10.1038/sj.onc.1208020. [DOI] [PubMed] [Google Scholar]
- 112.Ratziu V, Lalazar A, Wong L, et al. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9500–9505. doi: 10.1073/pnas.95.16.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim Y, Ratziu V, Choi S-G, et al. Transcriptional activation of transforming growth factor β1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1: potential mechanisms for autocrine fibrogenesis in response to injury. Journal of Biological Chemistry. 1998;273(50):33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- 114.Warke VG, Nambiar MP, Krishnan S, et al. Transcriptional activation of the human inducible nitric-oxide synthase promoter by Krüppel-like factor 6. Journal of Biological Chemistry. 2003;278(17):14812–14819. doi: 10.1074/jbc.M300787200. [DOI] [PubMed] [Google Scholar]
- 115.Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Current Biology. 2001;11(10):764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]