Abstract
Hypoxia serves as a physiological cue to drive angiogenic response via HIF-dependent mechanisms. Interestingly, minor elevation of lactate levels in the tissue produces the same effect under aerobic conditions. Aerobic glycolysis contributes to lactate accumulation in the presence of oxygen especially under inflammatory conditions. We have previously postulated that aerobic lactate accumulation, already known to stimulate collagen deposition, will also stimulate angiogenesis. If substantiated, this concept would advance understanding of wound healing and aerobic angiogenesis because lactate accumulation has many aerobic sources. In this study, Matrigel® plugs containing a powdered, hydrolysable lactate polymer were implanted into the subcutaneous space of mice. Lactate monomer concentrations in the implant were consistent with wound levels for over 11 days. They induced little inflammation but considerable VEGF production and were highly angiogenic as opposed to controls. Arterial hypoxia abrogated angiogenesis. Furthermore, inhibition of lactate dehydrogenase using oxamate also prevented the angiogenic effects of lactate. Lactate monomer, at concentrations found in cutaneous wounds, stabilized HIF-1α and increased VEGF levels in aerobically cultured human endothelial cells. Accumulated lactate, therefore, appears to convey the impression of “metabolic need” for vascularization even in well-oxygenated and pH-neutral conditions. Lactate and oxygen both stimulate angiogenesis and matrix deposition.
INTRODUCTION
Hypoxia, caused by disrupted vasculature, peripheral vasculopathies or pulmonary disorders is a key factor that limits dermal wound healing (16, 18, 23). A threshold level of oxygenation is required for tissue remodeling. Hypoxia can initiate neovascularization by inducing growth factors (12, 19, 36, 39). However, sustained periods of equally severe hypoxia limit neovascularization (17), proliferation of dermal cells (28), collagen deposition (33), bacterial killing and resistance to infection (3), and more severe hypoxia causes tissue death and dysfunction (33). On the other hand, supplemental O2, in vivo accelerates wound vessel growth (12, 16, 17), collagen deposition (34, 41) and angiogenesis (12). Intermittent hyperbaric oxygen stimulates vessel growth (17), accelerates the proliferation of granulation tissue (23, 24), and facilitates epithelial healing (34). VEGF is a major long-term angiogenic stimulus at the wound site. Others and we have noted that O2 treatment induces VEGF gene expression (29) and increases VEGF protein expression in wounds (40). These apparently contradictory data need justification.
Contrary to views held for almost a century, it is now agreed that most lactate is produced under aerobic conditions, and there is little correlation between lactate and oxygen until pO2 falls below about 1 mm Hg(15). Tissue lactate can accumulate to high levels even under conditions of oxygen sufficiency particularly in wounds where, regardless of pO2, lactate rises to the range of 5 to 15 mM as opposed to the 1 to 3 mM found normally in blood and most uninjured, resting tissues (13). Furthermore, lactate is now understood to have many fundamental metabolic and signaling functions (15).
Angiogenesis may be viewed as a long-term response to unmet metabolic need. It is well demonstrated that hypoxia and/or dysoxia are master controllers of the signals that drive new vessel formation (37). Lactate, on the other hand is also a known instigator of cytokines and growth factors such as VEGF, TGF-β, and IL-1 (10, 41). Lactate stabilizes HIF-1α even in the presence of oxygen because lactate and pyruvate bind to and inhibit the HIF prolyl hydroxylases that would otherwise hydroxylate HIF-1α and mark it for rapid degradation (21, 22). Thus lactate accumulation, often associated with, but not necessarily a consequence of hypoxia, is poised to express impending hypoxia while not necessarily incurring the hazards of hypoxia itself. It may, therefore, be a more sensitive stimulus for angiogenesis than hypoxia.
In this investigation, we explored the role of lactate accumulation in two putative roles: a) as a redox-regulating angiogenic agent, and b) as a means of reducing NAD+ level and hence ADP ribosylations that regulate gene transcription and post-translational modification of many proteins. Both influence angiogenesis and connective tissue deposition (35, 44). The primary goals of this study were to test the central hypothesis that accumulated lactate ion, no matter how derived, (i) is sufficient to induce significant angiogenesis if a physiological concentration of oxygen is present, and (ii) acts via redox and polyADP-ribosylation mechanisms. If this hypothesis can be defended, the implication will be that lactate accumulation in the presence of oxygen is a relatively common circumstance and may drive new vessel formation in many aerobic circumstances.
EXPERIMENTAL APPROACH
Lactate delivery
Testing of the central hypotheses in vivo required development of an isolated site in tissue in which high, sustained levels of exogenous lactate ion can be achieved by injection without stimulating endogenous production (e.g. via inflammation (8)) and where pO2 can be independently controlled. To meet these requirements, we devised a Matrigel® implant model that shows no ability by itself to induce neovascularization but supports new vessel growth when made host to an angiogenic signal (10). A hydrolysable lactate polymer was identified that, when added to Matrigel®, would raise and sustain an increased lactate monomer level in the gel at a concentration relevant to dermal wounds. In preliminary experiments, we identified a poly-DL-lactide-co-glycolide (lactide:glycolide 50:50, mol wt 40,000–75,000; Cat#P2191; Sigma, MO) that served the purpose. Matrigel® implants supplemented with this lactate delivery agent are referred to as lactate-supplemented implants. Hypoxia, below about 1 mm Hg, is known to raise lactic acid production and lower pH (15). Hydrolysis of the said lactide polymer in water, however, released lactate ion that slightly increases pH because of it’s association with protons in water. When the lactate polymer was placed in wounds in which extracellular fluid could be collected, the level of lactate monomer rose to a concentration relevant to wounds (4–12 mM in mice, (41)), and no pH change was detected (41). To test whether surface characteristics of the lactide polymer might be inflammatory and hence might stimulate endogenous lactate production, we tested the effects of a larger polymer poly(L-lactide) (mol wt 85,000–160,000; Cat#P1566, Sigma, MO). The larger polymer did not release lactate at levels released by the above-said lactide polymer (lactide:glycolide 50:50, mol wt 40,000–75,000; Cat#P2191; Sigma, MO). In contrast, smaller polymers induced more rapid but more transient angiogenesis (not shown).
Matrigel® implantation and harvest
Six month old Swiss Webster female retired breeder mice were used in this study. Powdered lactate polymer was mixed into cold matrigel (30 mg/ml), and l ml of the mixture was injected subcutaneously in each flank (dorsally, immediately caudal to the ribcage) via a #18 needle under isofluorane anesthesia. Controls were similarly injected with control Matrigel®. In all, 245 retired breeder mice were injected with 490 implants. The animals were housed in the University of California Laboratory Animal Resource Center. Animals were killed and implants removed at the noted intervals. The institutional committee on animal use approved all procedures before experiments were performed. Implants found in (as opposed to on) muscle or the peritoneal cavity (10 of 490 implants) were discarded. On removal, sections were taken from mid implant, and were stained with: a) hematoxylin and eosin, b) purified Rat Anti-mouse CD31 (Pecam-1) monoclonal antibody (BD Biosciences), c) biotin-conjugated Rat Anti- Mouse CD34 monoclonal antibody (BD Biosciences), d) Rat anti-mouse mac-3 monoclonal antibody (BD Biosciences) to identify macrophages, and e) Mallory’s trichrome (http://stainsfile.info/StainsFile/stain/conektv/tri_mallory.htm) to identify collagen.
Experiment 1
The hypothesis that lactate induces an early invasion of endothelial cells that would be followed by vascularization was tested. The types of cells invading the implants were identified as above and quantified by averaging the number of each cell type in four high power (20×10) fields of 2 microscopic sections of each implant and then averaging the number for 4 implants. It was evident that the changes of interest were most efficiently assessed at 11 days. Sections from day 11 day implants were stained with hematoxylin and eosin, and Mallory’s trichrome stain and were graded for the extent of neovascular formation by two independent and blinded graders. Grading was stepwise as shown in Fig. 1. Note that the day 11 time point was decide based our examination of sections from days 11, 16, 21, 35 1nfd 50. Results from the time points other than day 11 are not shown.
Figure 1. Grading system used in assessing angiogenesis in Matrigel® implants.
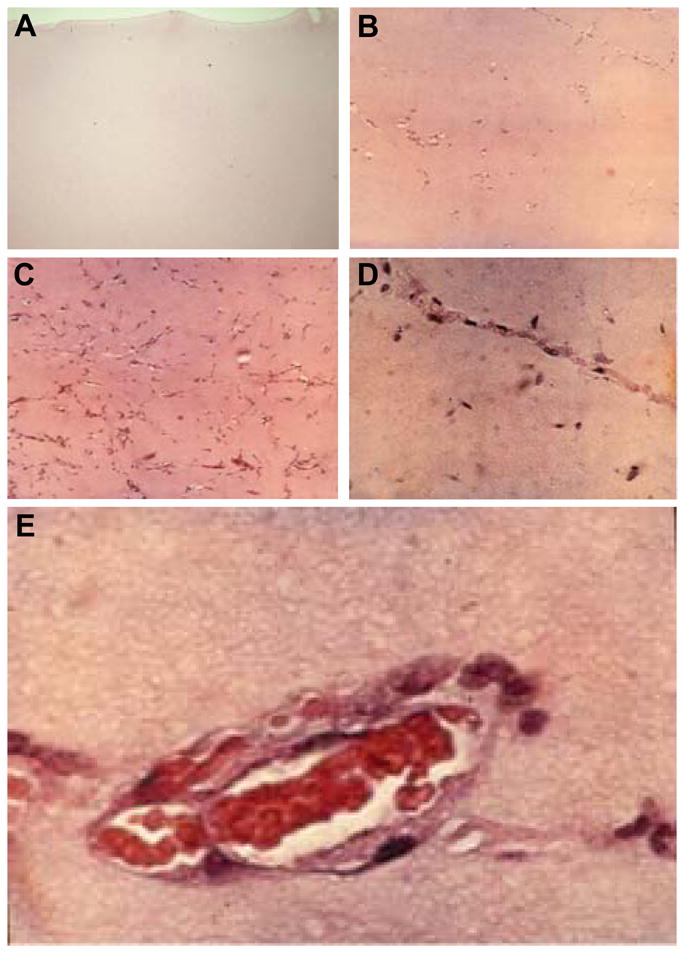
B , a score of 0 was given for acellular or rare cells; B, a score of 1 reflected scattered endothelial cells in small groups or linear arrangements but without lumens; C, a score of 2 represented endothelial cells in all quadrants of the section, prominent linear arrangements and some tube formation; D, a score of 3 was assigned for easily identified capillary tube formation, many containing red blood cells and small amounts of collagen; E, a score of 4 was reserved for larger vessels that accommodated more than 4 red cells abreast and multilayered vessels containing layers of collagen in vessel walls. Agreement between graders was >90%. In no case was the score-difference difference greater than 1 for the same observation by two different graders. The average of three repeated observations by the same graders was noted as the recordable score. Reproduced with permission from the publisher of Hopf et al. (17). For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars.
Experiment 2
The hypothesis that inhibition of lactate dehydrogenase (LDH) C4 with sodium oxamate (27) prevents angiogenesis was examined. This was aimed at expanding on our prior finding that depletion of NAD+ by excess lactate inhibits ADP- and poly(ADPRibosyl)ations and, subsequently, collagen synthesis and deposition (44). An alternate, or more likely, supplementary interpretation would be that conversion of lactate to pyruvate is required for the enhancement of HIF-1α by lactate (21, 22). Sixteen Matrigel® implants were placed in eight mice. Eight implants (in 4 mice) were lactate-supplemented and contained sodium oxamate (36 mM; Sigma, MO). Eight implants in the remaining four mice contained oxamate but no lactate polymer.
Experiment 3
The hypothesis that the concentrations of lactate and VEGF in the Matrigel® implants are quantitatively related was tested. Ten lactate-supplemented and ten untreated implants were injected into ten animals on a pair-matched basis. Implants were removed at 11 days and immediately placed in liquid nitrogen. When all implants had been collected, they were quickly thawed, weighed, placed in 0.5 ml saline and thoroughly macerated, homogenized and centrifuged. The pellet was used for DNA extraction and the liquid phase was saved for VEGF analyses with 0.4 ml of supernatant put aside for the lactate assay (Yellow Springs Instruments Co, Ohio, Model 2700 biochemical analyzer with a lactate sensitive membrane). For VEGF assays, ELISA was used (R&D Systems Minneapolis, MN).
Experiment 4
The hypothesis that both oxygen and lactate are necessary for neovascularization was examined. We adopted our previously published approach (17) with the single difference that one of the two implants in each animal was lactate-supplemented. Twelve animals were held in each of three atmospheres: 13%, 21%, and 50% oxygen for 11 days continuously, removed only for cage cleaning. The animals tolerated the various oxygen concentrations, showed no signs of discomfort, and lost no weight. Implants were removed at 11 days and stained with both H and E and Mallory’s trichrome stain for collagen.
Experiment 5
Finally, we tested the hypothesis that that exposure to lactate increases oxidant production by human endothelial cells. Nitroblue tetrazolium (NBT) test (9) was utilized for the detection of superoxide anion radical. To test whether lactate induced superoxide generation is dependent on NADPH oxidases, the flavoprotein inhibitor diphenylene iodonium (DPI) was employed.
Statistical analyses
Because the data on histologic scores are step-wise (ordinal), the Kruskal-Wallis statistic and the Mann Whitney test were used to evaluate differences between groups. Regression analysis was used to assess the correlations between lactate, DNA and VEGF. Difference between means was examined by Students t-test.
RESULTS
On explantation, microscopic sections were examined as noted in Fig. 1, and grades assigned as described. New vessels could be seen in lactate-supplemented implants with a hand-held (3x) magnifying glass (Fig. 2). Polymer granules could be seen for 30 to almost 50 days. Individual endothelial cells and macrophages in equal numbers were found in all lactate-supplemented implants through 6 days (Fig. 3). Thereafter, in lactate-supplemented implants the count of endothelial cells and macrophages increased by 3 to 4-fold and usually occupied most of each of the implants by the ninth and eleventh day. The numbers of fibroblasts remained unchanged (Fig. 3). Non-supplemented implants had too few cells to analyze. In the implants supplemented with the lactate-delivery polymer, VEGF levels were the highest in gel containing 4–5 mM lactate ion (Fig. 4). We conclude that there is a quantitative relationship between lactate and VEGF. The VEGF response at the higher (>5 mM) residual levels probably deteriorated as if at some time-point lactate concentrations may have been high enough to denature VEGF during the 11 day exposure.
Figure 2. Blood vessel growth in lactate-supplemented Matrigel®.
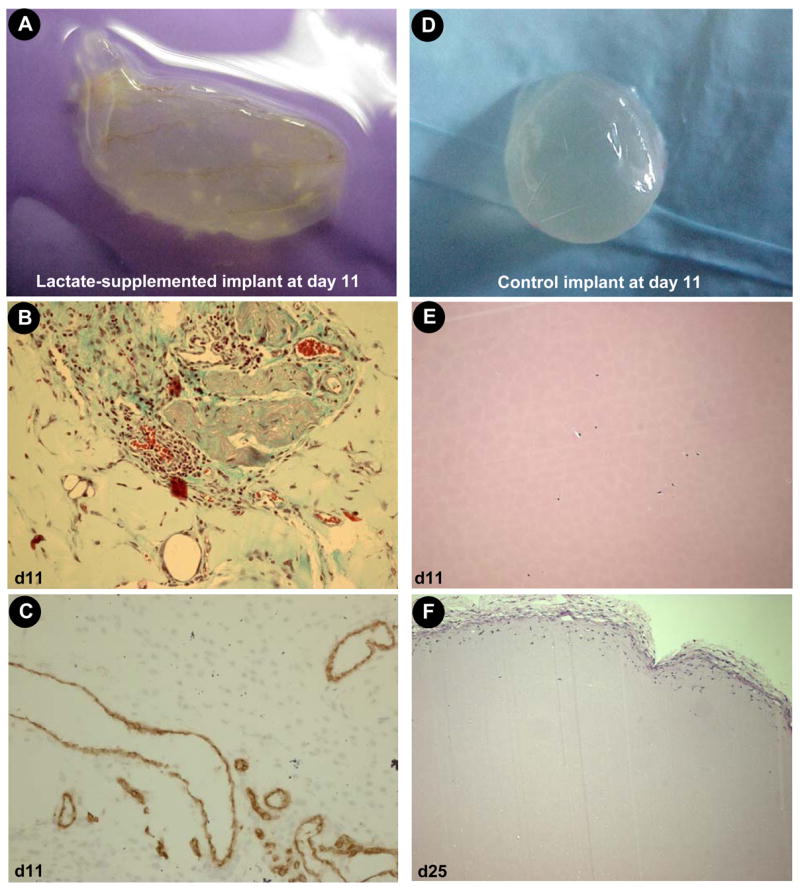
Matrigel® (n=80) containing hydrolysable poly-DL-lactide-co-glycolide (lactide:glycolide 50:50, mol wt 40,000–75,000) or no additive (control, n=100) were implanted subcutaneously, one injection in each flank just caudal to the ribcage. Four such lactate-supplemented implants were removed and examined microscopically at day 11 or 25 (control) as shown. A–C, lactate-supplemented (day 11); D–F, control (D&E, day 11; F, day 25). A&D, intact Matrigel® harvest; B&E, hematoxylin & eosin stain; C, CD31 immunostain. F, hematoxylin & eosin stain on day 25. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars.
Figure 3. Kinetics of cellular infiltration in lactate-supplemented Matrigel®.
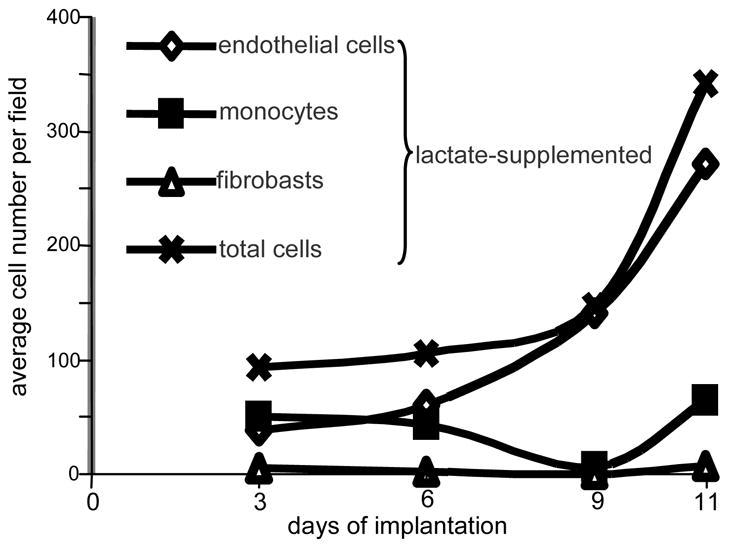
Differential cell counts were done on the basis of cellular morphology as identified by hematoxylin & eosin stain. From each implant, two sections were examined. Four fields per slide were examined at 40x magnification avoiding fields in which the periphery of the implant could be seen. Control slides, from implants either non-treated or treated with high molecular weight lactide polymer, showed too few cells to enable differential scoring. Clearly, lactate attracted primarily monocytes and endothelial cells.
Figure 4. The relationship between lactate and VEGF levels in lactate-supplemented Matrigel®.
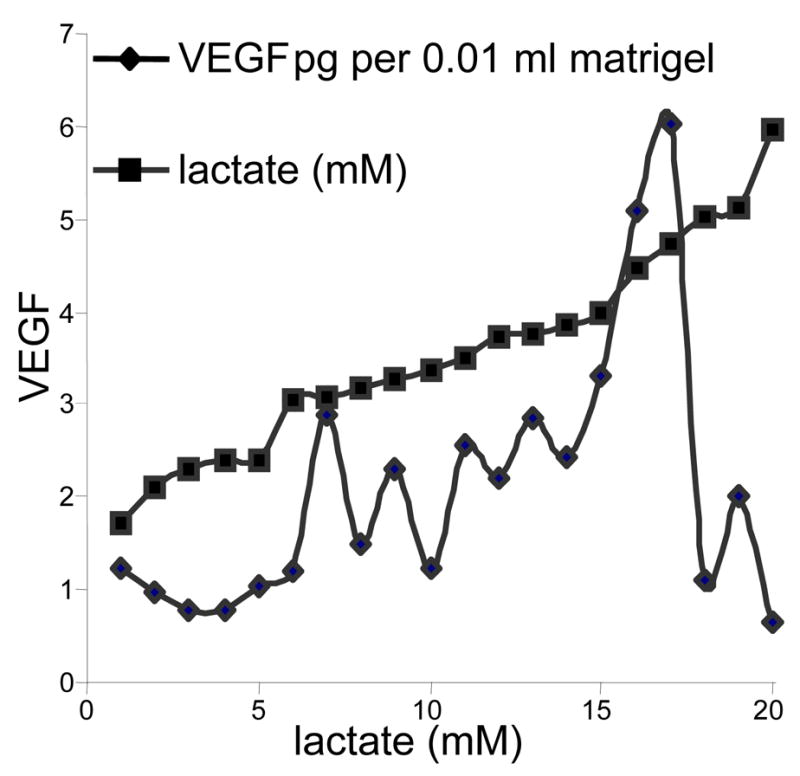
Twenty implants, including both lactate-supplemented and controls were numbered in ascending order of magnitude of lactate as shown on the horizontal axis and the squared line. They were then matched with their corresponding VEGF level. When displayed in this manner, VEGF content with respect to lactate at 11 days appears to peak at about 6 mM lactate. However, the lactate concentrations are somewhat illusory in that the hydrolysis of the lactate powder is not linear with time. Thus they can give only an impression of what the concentrations were on days 1 to 11. Nevertheless, the data supports prior experience reported by Beckert (5) who found in cultured endothelial cells that VEGF concentration increased in response to lactate to a peak at about 15 mM and cells deteriorated above that level. This figure, therefore, indicates a significant relationship to increasing lactate in vivo that reaches a peak and then apparently deteriorates. Lactate levels in controls were in the range of 0.75 to 4 mM, the same range as found by others in blood and subcutaneous tissues.
Every implant of eighty that contained hydrolysable lactate (and were not hypoxic) exhibited neovascular tube formation by 11 days of implantation with an average score of 2.8 i.e. most contained linear arrangements and tubules containing erythrocytes (Fig. 5). The lowest score in lactate-supplemented implants at day 11 was 1.5 (see Fig. 1 for scoring scale). There were relatively few clear examples of true angiogenesis, i.e. invasion of macrophage-led tubes into the implant edge demonstrating development of blood vessels from pre-existing vessels. Instead there was more evidence of isolated endothelial cells and tubes in the Matrigel® substance, consistent with vasculogenesis (32). By day 11 of implantation, many tubules contained red blood cells suggesting that hematopoetic stem cells may have differentiated to form functional vessels. Control implants of the 70 examined, whether loaded with high molecular weight polymer (n=20) or containing no polymer (n=50), displayed no appreciable tube formation, and most showed no cellularity except at the implant edge (Fig. 2). The highest score was 2, and the average was less than 1. There was, therefore no overlap between control and lactate-supplemented implants (p<0.001). Controls with high molecular weight lactate (n=25) were indistinguishable from non-treated controls except for the presence of polymer granules (not shown). A mild non-specific inflammation developed around some control implants but migrated into them no further than about 150 μm from the edge of the implant on day 3. In no such case, there was any advancement after day 6. At 50 days, most of the Matrigel® granules had disappeared but the lactate-rich sites were identified mainly by a vascularized scar. CD31 positive cells were apparent in appreciable numbers at 6 days (not shown). CD31-lined conduits indicative of blood vessel in formation was evident in day 11(Fig. 2C). Abundant CD117 (Fig. 6) and CD34 (not shown) positive cells were seen in the lactate-supplemented Matrigel® at day 9. Treatment of Matrigel® with the LDH inhibitor oxamate abolished angiogenesis that was evident in lactate-supplemented implants. The average score was 1.0 as opposed to 2.5 in the lactate-supplemented oxamate non-treated implants. There was no overlap in the scores between the two groups and the difference between the means of the two groups was statistically significant (p<0.01). Thus, LDH activity is required for lactate-induced vasculogenesis.
Figure 5. Histological characterization of Matrigel® implants in normoxic and hypoxic mice.
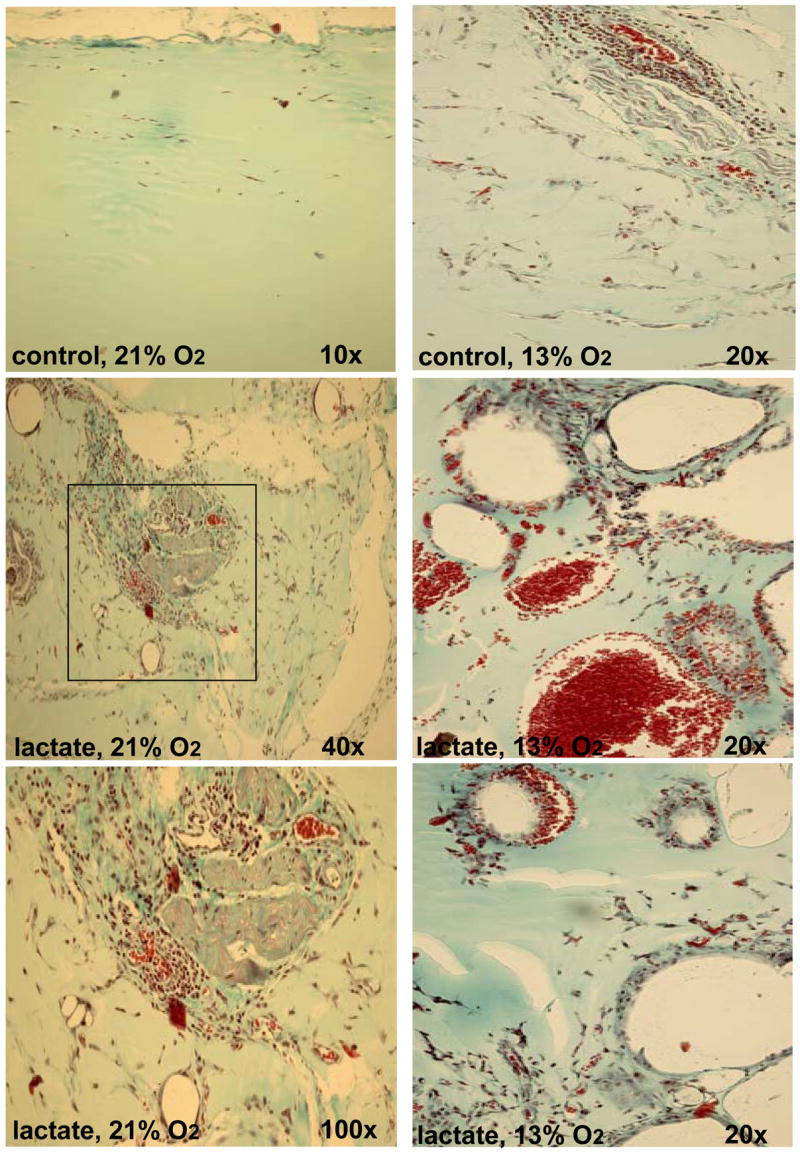
Thirty-six mice were each implanted with lactate-supplemented implant and one non-treated control. Twelve animals were held in each of three atmospheres: 13% (right column), 21% (left column), and 50% (not shown) oxygen for 11 days. A Pro-ox compact oxygen controller model 110 was used to maintain pO2 (Reming Bioinstruments, Redfield, NY). FiO2, humidity (60–80%), CO2 (<2 mm Hg) and temperature (23–25°C) were maintained within normal limits. Hypoxic (13%O2 ambience) were housed in a plexiglas chamber with a single gas inlet and outlet. Air was delivered at 1.0 L/min and nitrogen at 4.5 L/min from tanks connected to the chamber by thick-walled polyvinyl tubing. This reliably delivered 13.3 ± 1.3% oxygen to the chamber. Carbon dioxide levels were maintained at 0 mm Hg within the chamber with a CO2 absorber (Baralyme, Chemetron Medical Division, St. Louis, MO). A Datex Capnomac II [Datex Medical Instruments, Inc, Tewksbury, MA] was used to sample exhaust gas from the oxygen chambers to confirm the exposure pO2 and to ensure that CO2 did not accumulate. Compared to that in normoxic mice (left column), implants in hypoxic mice produced rare and large, thin-walled vessels. These photographs were selected to show as many vessels as possible and are not representative of their abundance. Compared to that in normoxic mice, small amounts of collagen were evident in implants in hypoxic mice. The sections were stained with Masson’s trichrome. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars.
Figure 6. Recruitment of multipotent hematopoietic stem and progenitor cells in lactate-supplemented Matrigel®.
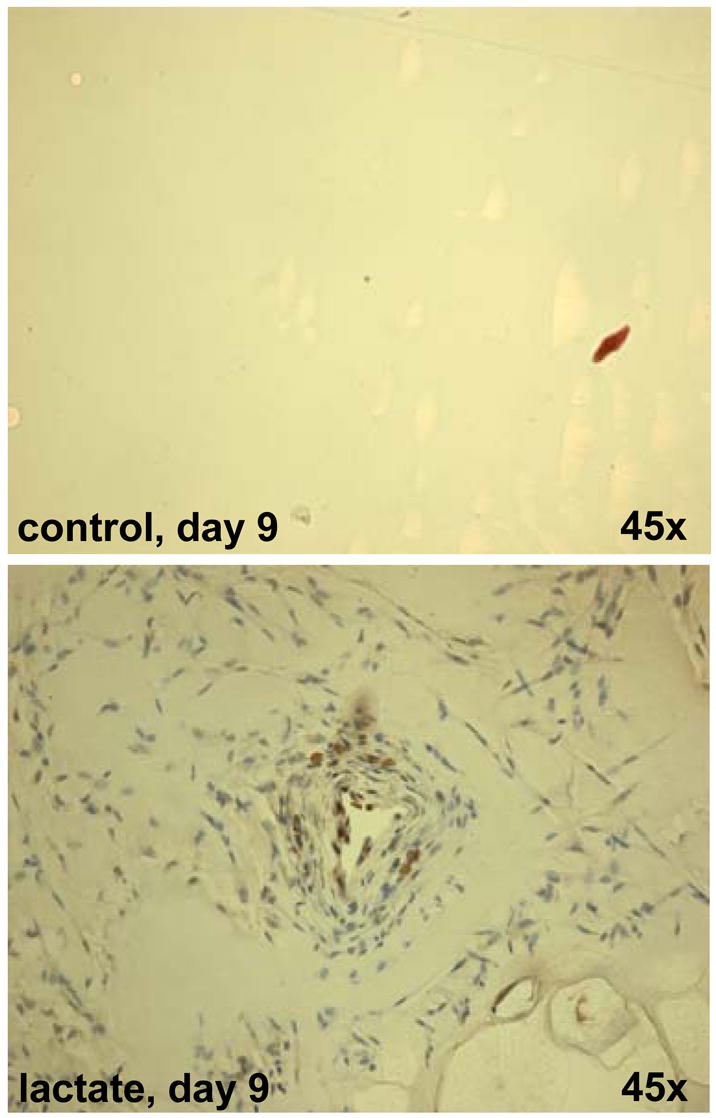
CD117 staining is shown. CD117, also known as c-kit, steel factor receptor and stem cell factor receptor, encodes a 145 kD cell surface glycoprotein belonging to the class III receptor tyrosine kinase family. It is expressed on the majority of hematopoietic progenitor cells including multipotent hematopoietic stem cells as well as committed myeloid, erythroid, and lymphoid precursor cells. In addition to the potential for the differentiation of hematopoietic cells, CD117+ stem cells from murine bone marrow were reported to be capable of differentiation into smooth muscle cells, myocytes, and endothelial cells in vivo.1,2 CD117 is also expressed on few mature hematopoietic cells, e.g. mast cells. The sections were also richly populated with CD34+ cells (not shown). For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars.
New vessels were found in all lactate-supplemented implants in air-breathing (Fig. 5, left panel) and hyperoxic (50% O2, not shown) groups. No angiogenesis score over 1.5 was found in any control implant whether normoxic, hyperoxic, or hypoxic. The “hypoxic implants” (Fig. 5, right panel shows the characteristic of one of the rare vessels found) showed few new vessels or endothelial cells. Whether lactate-supplemented or control, the few vessels that were seen in hypoxic implants were far larger in size than noted in the room-air breathing group and some contained many red cells (Fig. 5, right panel). The vascular conduits in the implants of hypoxic mice were also immature, poor in collagen, thin-walled, and apparently friable, often ruptured, and resembled cells seen in ascorbic acid depletion when collagen deposition is impaired (6).
Endothelial cells treated with NBT and lactate showed a clear increase in formazan crystals and VEGF production under standard aerobic conditions (Fig. 7). Formazan formation in response to the oxidant-donor SIN-1 demonstrated that the assay used was effective to detect reactive oxygen species. The distribution was in general perinuclear. This is consistent with a redistribution or redirection of intracellular oxygen to peroxide production at specific sites as reported (20). Non-specific inhibition of the NADPH-linked oxidase by DPI decreased lactate-induced production of superoxides (Fig. 7). VEGF level in lactate-supplemented cultures was 3.5 times higher than that in cultures not treated with the lactate polymer (p<0.01; Fig. 7). In confirmation of previous reports (20, 22), lactate treatment not only induced VEGF expression but also stabilized HIF-1α expression (Fig. 8).
Figure 7. Lactate-induced oxidant production in microvascular endothelial cells is likely to be NADPH oxidase dependent.
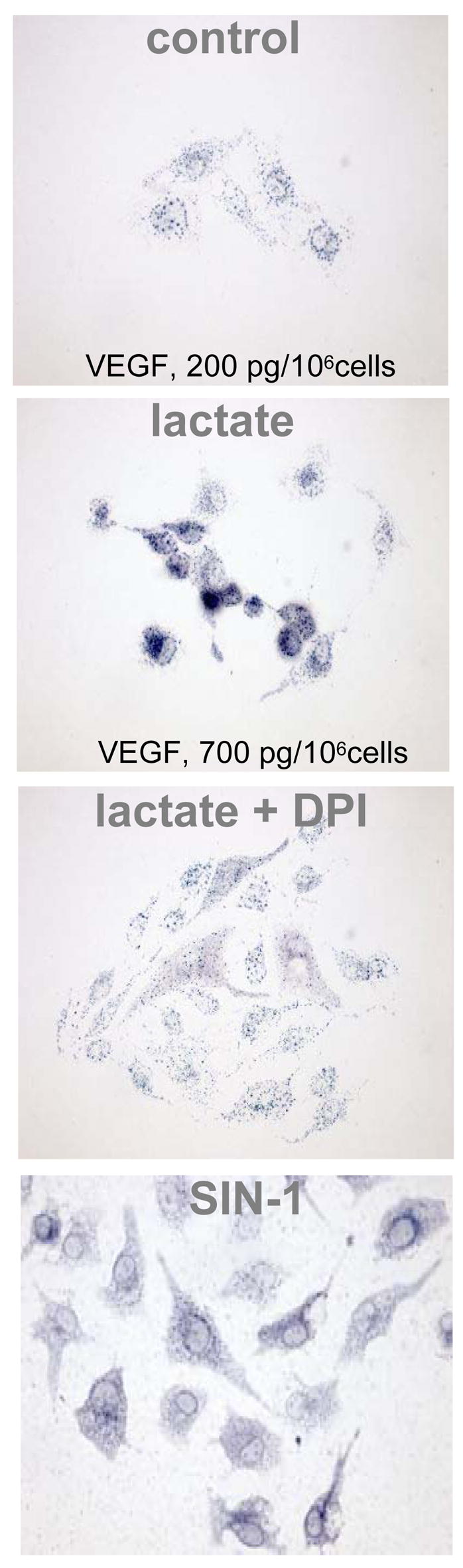
. Human micro-vascular endothelial cells (HMVEC-d) purchased from Cambrex Bio Science (Walkersville, MD) and passages 5–9 were used for experiments. Cells were grown in endothelial basal medium 2 (CC-3156) containing EGM-2, MV (SingleQuots, Cambrex, Walkersville, MD, CC-4147), 100 units/ml penicillin, 100 units/ml streptomycin and 0,2 μg/ml amphotericin B. Cultures were maintained at 37° in humidified 95% air and 5% CO2 atmosphere. Cells were growth-arrested by incubating overnight in endothelial basal medium 2. Nitroblue tetrazolium (NBT) (1 mg/ml) was added followed by lactate (15 mM). pH was adjusted to 7.4. In similar experiments, effect of the NADPH oxidase inhibitor diphenylene iodonium (DPI, 10μM) and the oxidant donor SIN-1 (1 mM; 3-morpholino-syndnonimine) was tested. Note the differences in VEGF in response to lactate-supplementation.
Figure 8. Lactate-induced stabilization of HIF-1 α in oxygenated endothelial cells.
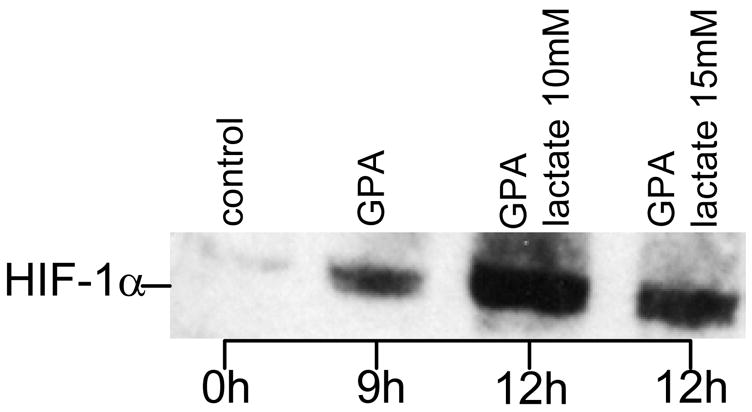
. Endothelial cells were grown in 6 well plates to sub-confluent monolayers with a cell density of approximately 106 cells/well. After overnight serum starvation, cells were treated for nine hours with the prolyl-hydroxylase inhibitor GPA1734 (8,9-dihydroxy-7-methyl-benzo[b]quinolizinium bromide; kindly provided by Dr. M. Maragoudakis, Patras, Greece) and HIF-1α protein was detected by Western blotting. For immunoblotting, cells were washed with ice-cold PBS and collected by scraping into 1 ml PBS. After centrifugation at 1000 g for 5 min, pellets were homogenized with an equal volume of lysis buffer (20 mM Tris-HCl, pH 7.5 containing 150 mM NaCl) (Cell Signaling, Beverly, MA) containing protease inhibitors and incubated for 20 min on ice. After sonication for 15 seconds samples were subjected to centrifugation at 15,000 g for 20 min at 4 ° to separate membrane and cytosolic fractions, suspended in 2X SDS sample buffer (Cell Signaling, Beverly, MA) containing 1 mM DTT, and boiled for 5 minutes. Equal quantities of protein were separated by SDS-PAGE electrophoresis under reducing conditions using a 7.5% Tris-HCl gel (Bio-Rad, Hercules, CA). After transfer onto 0.45 μm nitrocellulose membranes (Millipore, Marlborough, MA), the membranes were blocked with 5% non-fat dry milk and probed with anti HIF-1α (1:1500; Novus Biologicals, Littleton, CO). GPA 1734 was used to block the proteosomal degradation pathway of HIF-1α.
DISCUSSION
Lactate is unusual among products of glycolysis in that it has many aerobic sources including exercise, hyperglycemia, lipolysis, sympathetic nervous system activation, and rapid cell division that characterize wounds and most tumors (15, 17, 21, 22). Leukocytes in particular have limited mitochondria and when they are activated, as they are in wounds, they derive most of their energy from glycolysis thus releasing large amounts of lactate regardless of oxygen concentration (7, 26). Transient inflammation is an obligatory precursor to wound healing. One of the few axioms of wound healing is “no inflammation, no healing”. Furthermore, the recent enthusiasm for hypoxia-generated angiogenic signals seems to have obscured the fact that hypoxia impairs collagen synthesis and deposition, both of which are required for angiogenesis (7). No matter how much angiogenic stimulant is present, the functional response cannot proceed in severe hypoxia because new vessels require collagen for strength to withstand the pressures of blood flow. Collagen deposition proceeds only at half its maximal rate at 25 mm Hg pO2. Neither can wounds resist bacterial infection at that level of pO2 (3). Without collagen, angiogenesis cannot proceed beyond the stage of fragile endothelial buds (7). The problem of decreased collagen deposition in angiogenesis is illustrated by scurvy (ascorbate deficiency) that impairs hydroxylation of collagen, prevents collagen deposition, interferes with angiogenesis, and severely depresses wound healing by producing precisely the same lesion as hypoxia (30). Both hypoxia and ascorbate deficiency block the essential step of collagen deposition in which ascorbate complexes iron, molecular oxygen, and oxoglutarate and hydroxylates selected proline residues. Without this step, collagen cannot be released from the cell.
Findings of this study demonstrate that relatively minor accumulation of lactate in adequately oxygenated cells and tissues is itself sufficient to initiate the full sequence of wound healing including vasculogenesis and collagen deposition. This is, however, only one of many important features of lactate accumulation. Lactate enhances a remarkable set of actions that could be causally linked to its ability to induce the formation of reactive oxygen species. Elevated lactate induces: (i) HIF-1α stabilization, (ii) VEGF and TGFβ (41), (iii) metalloproteinases (25), (iv) endothelial cell mobility (5), (v) vascularization (current data), (vi) increased collagen synthesis, and its post translational modification and deposition (13, 41), (vii) cell proliferation (43), (viii) transcription of genes for proteoglycans, CD44, caveolin-1, Hyal-1 and –2 (11), and (ix) an environment suitable for the recruitment of progenitor cells (current data).
The current study supports that while hypoxia protects the angiogenic transcription factor HIF-1α from degradation, it inhibits angiogenesis. Impairment of angiogenesis by hypoxia in VEGF-supplemented Matrigel® has been reported (17). The hypoxia-induced compromise of angiogenesis is coincident with impaired collagen deposition. Consistently, progressive hypoxia has been noted to compromise bursting strength (a function of collagen deposition) in colon anastomoses while increasing their VEGF content (4). HIF is first seen in the wound tissue well before pO2 falls whereas lactate levels rise immediately after injury (1). On the contrary, while we observed neither benefit nor inhibitory effects of continuous hyperoxia up to 50% oxygen without added lactate (not shown), Hopf et al showed enhancement of angiogenesis in response to intermittent exposure to 100% O2 (pO2 ≅ 180 mm Hg) (17). Thus, in this context, HIF-1α level and angiogenesis appear to occur in proportion to O2 and lactate concentrations with an added factor of time of exposure.
The present data fit within a mosaic of oxidant and ADPribosylation mechanisms that are now known to support wound healing. First, it was noted that a lactate:iron chelate produces hydroxyl radical (·OH) in the presence of H2O2 by a variant of Fenton chemistry (2). Next, iron-containing structures were observed in the endoplasmic reticulum (ER) and the importance of pO2 and H2O2 that enhanced a Fenton reaction at these sites was reported. H2O2 and ·OH were generated in proportion to pO2. This, in turn, led to translocation of the HIF-1α gene from the ER into the nucleus, elevated HIF-1α and induced expression of the HIF-1 target genes plasminogen activator inhibitor 1 and heme oxygenase (20). Scavenging of the .OH attenuated compromise of prolyl hydroxylase activity. They also showed that the increased oxidant flux did not occur in mitochondria, lysosomes, or peroxisomes. It was thus concluded that generation of. OH in a Fenton reaction at the ER contributes to HIF-1α regulation by either inhibition of prolyl hydroxylase activity or interference with redox-sensitive residues. Thus, under normal physiological conditions, changes of the redox status within a cell may contribute to an efficient and fast-responding oxygen sensing system. The current data go further to suggest that lactate, which may be derived from multiple aerobic sources, increases the sensitivity of this system by binding to iron and enhancing .OH flux thus preparing for neovascularization under the threat if not the fact of hypoxia (22). In this sense it is plausible that the degree of hypoxia that the HIF mechanism responds to depends on the amount of lactate that is present in the environment. It appears that considerable, apparently contradictory, experimental evidence may be resolved by measuring or precisely controlling lactate levels in the relevant experimental systems.
HIF-1α levels in cultured and aerated cells rise in response to the addition of as little as 3 mM of the α-hydroxy acids, lactate, oxalacetate, or pyruvate (22). The authors proposed that introduction of any of these glycolytic products, would significantly amplify glycolysis, inhibit HIF-1α prolyl hydroxylase(s) and therefore extend the life of HIF-1α. The observation that HIF itself promotes glycolytic metabolism was portrayed as a novel feed-forward signaling mechanism. This concept is defended by our findings demonstrating that elevated lactate induces the production of reactive oxygen species. The reactive oxygen species, in turn, induces VEGF by a HIF-independent mechanism as well (38). H2O2, a reactive oxygen species, is now known to be required to support wound angiogenesis and healing (31).
Lactate exerts its effects by at least one other mechanism, by reducing ADPribosylation. The consequence includes many cytoplasmic and nuclear processes, few of them well understood (35, 42). This mechanism clearly regulates angiogenesis and collagen deposition via oxidant-independent mechanisms. ADPribosylations fall in response to lactate accumulation because NAD+ (not NADH) is the source of ADPribose. Excessive lactate accumulation reduces the pool of NAD+ by converting it to NADH by the action of LDH. This is significant because NAD+ is the only substrate for synthesis of ADPribose. Hence lactate diminishes ADPribosylations. Indeed, addition of NAD+ reverses the effect of lactate on collagen deposition in cell cultures. Furthermore, the LDH-inhibitor oxamate abolishes the effects of lactate of vasculaization.
Ghani proposed a role for LDH and lactate in collagen production upon noting that oxamate abrogates the effects of lactate in cultured cells (14). ADPribose (ADPR) monomer binds to and inhibits collagen prolyl hydroxylase, and increased lactate decreases ADPribosylation and enhances collagen hydroxylation and deposition. Similarly, removal of monoADPR activates VEGF in the absence of protein synthesis (44). Results of experiment 2 confirm some of these results in vivo.
Current data on CD117 positive cells indicates that lactate, with oxygen, induces wound angiogenesis in two distinctly different ways. Steep oxygen and lactate gradients in rabbit ear chambers allowed Knighton to demonstrate that new capillaries in wounds sprout from existing, functional vessels where pO2 is high and grow toward high lactate and low O2 concentrations toward the core of the wound (19). Reversal of the oxygen gradient halted angiogenesis. Beckert and colleagues demonstrated that lactate enhances cell motility (5). Thus, there is a continuous, directional advancement of new vessels from old. In the current experiments, however, new vessels in the Matrigel® appear to develop also by vasculogenesis, from individual endothelial cells that enter the implant prior to any connection with preexisting vessels. In lactate-supplemented Matrigel®, most tubes were well developed before erythrocytes entered them. Whether, however, erythrocytes enter by angiogenesis or from differentiation of hematopoetic stem cells remains unclear.
The significance of these findings is that lactate has many aerobic sources and would seem to contribute to aerobic angiogenesis and/or vasculogenesis. In all likelihood, this concept reaches beyond wound healing. The data suggests that frequent or prolonged and severe episodes of high lactate in blood or arterial tissue may become a mechanism for arteriosclerosis, perhaps retinopathy and other complications of diabetes in which lactate blood levels run high. Such events combined with “lactate-donating” inflammation from other causes may well accelerate an already established momentum of new vessel formation and matrix deposition. It is pertinent to ask whether lactate might explain, at least in part, why subintimal fibrosis is confined to the arterial system. Is it because the pO2 is high there? Is lactate accumulation in the presence of oxygen otherwise known as “pseudohypoxia”? Will minimal or intermittent episodes of lactate accumulation add to the explanation of slowly advancing arterial disease by complementing the redox consequences of episodes of hypoxia in sleep apnea?
Summary and Conclusions
Accumulated lactate, by itself, is able to initiate angiogenesis, and connective tissue synthesis provided that oxygen is present
Sustained hypoxia inhibits neovascularization
VEGF responds to variations in lactate concentration in wounds from less than 3 mM to at least 5 mM
Lactate appears to participate in a normal homeostatic mechanism that enhances sensitivity to metabolic need. However, it can’t be ignored, that while lactate accumulation is providential as sensor of metabolic need and a source of healing, it can also instigate undesirable consequences, particularly where inflammation is present and pO2 is high enough to produce pathological amounts of connective tissue.
The unique feature of lactate that places it in this powerful position is that it has both aerobic and anaerobic sources, and no matter where or what the source, lactate accumulation stimulates angiogenesis and matrix deposition.
Acknowledgments
Supported by NIH awards NIGMS GM27345, GM08258, GM069589 and GM 077185 to TKH and CKS.
Literature cited
- 1.Albina JE, Mastrofrancesco B, Vessella JA, Louis CA, Henry WL, Jr, Reichner JS. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am J Physiol Cell Physiol. 2001;281:C1971–1977. doi: 10.1152/ajpcell.2001.281.6.C1971. [DOI] [PubMed] [Google Scholar]
- 2.Ali MA, Yasui F, Matsugo S, Konishi T. The lactate-dependent enhancement of hydroxyl radical generation by the Fenton reaction. Free Radic Res. 2000;32:429–438. doi: 10.1080/10715760000300431. [DOI] [PubMed] [Google Scholar]
- 3.Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, Chang M, Le AX, Hopf HW, Hunt TK. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 4.Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48:1460–1470. doi: 10.1007/s10350-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 5.Beckert S, Farrahi F, Aslam RS, Scheuenstuhl H, Konigsrainer A, Hussain MZ, Hunt TK. Lactate stimulates endothelial cell migration. Wound Repair Regen. 2006;14:321–324. doi: 10.1111/j.1743-6109.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 6.Berthod F, Germain L, Tremblay N, Auger FA. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol. 2006;207:491–498. doi: 10.1002/jcp.20584. [DOI] [PubMed] [Google Scholar]
- 7.Biswas S, Ray M, Misra S, Dutta DP, Ray S. Is absence of pyruvate dehydrogenase complex in mitochondria a possible explanation of significant aerobic glycolysis by normal human leukocytes? FEBS Lett. 1998;425:411–414. doi: 10.1016/s0014-5793(98)00273-7. [DOI] [PubMed] [Google Scholar]
- 8.Britigan BE, Klapper D, Svendsen T, Cohen MS. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J Clin Invest. 1988;81:318–324. doi: 10.1172/JCI113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HS, Kim JW, Cha YN, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem. 2006;27:31–44. doi: 10.1080/15321810500403722. [DOI] [PubMed] [Google Scholar]
- 10.Constant JS, Feng JJ, Zabel DD, Yuan H, Suh DY, Scheuenstuhl H, Hunt TK, Hussain MZ. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair Regen. 2000;8:353–360. doi: 10.1111/j.1524-475x.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- 11.Formby B, Stern R. Lactate-sensitive response elements in genes involved in hyaluronan catabolism. Biochem Biophys Res Commun. 2003;305:203–208. doi: 10.1016/s0006-291x(03)00723-x. [DOI] [PubMed] [Google Scholar]
- 12.Fries RB, Wallace WA, Roy S, Kuppusamy P, Bergdall V, Gordillo GM, Melvin WS, Sen CK. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005;579:172–181. doi: 10.1016/j.mrfmmm.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Ghani QP, Wagner S, Becker HD, Hunt TK, Hussain MZ. Regulatory role of lactate in wound repair. Methods Enzymol. 2004;381:565–575. doi: 10.1016/S0076-6879(04)81036-X. [DOI] [PubMed] [Google Scholar]
- 14.Ghani QP, Wagner S, Hussain MZ. Role of ADP-ribosylation in wound repair. The contributions of Thomas K. Hunt, MD. Wound Repair Regen. 2003;11:439–444. doi: 10.1046/j.1524-475x.2003.11608.x. [DOI] [PubMed] [Google Scholar]
- 15.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186:259–263. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 17.Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD, Zamirul Hussain M, Hunt TK. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13:558–564. doi: 10.1111/j.1524-475X.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 18.Hunt T, Twomey P, Zederfeldt B, Dunphy J. Respiratory gas tensions and pH in healing wounds. Amer J Surg. 1967;114:302–307. doi: 10.1016/0002-9610(67)90388-1. [DOI] [PubMed] [Google Scholar]
- 19.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–270. [PubMed] [Google Scholar]
- 20.Liu Q, Berchner-Pfannschmidt U, Moller U, Brecht M, Wotzlaw C, Acker H, Jungermann K, Kietzmann T. A Fenton reaction at the endoplasmic reticulum is involved in the redox control of hypoxia-inducible gene expression. Proc Natl Acad Sci U S A. 2004;101:4302–4307. doi: 10.1073/pnas.0400265101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 22.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 23.Markus YM, Bell MJ, Evans AW. Ischemic scleroderma wounds successfully treated with hyperbaric oxygen therapy. J Rheumatol. 2006;33:1694–1696. [PubMed] [Google Scholar]
- 24.Nakada T, Saito Y, Chikenji M, Koda S, Higuchi M, Kawata K, Ishida S, Takahashi S, Kondo S, Kubota Y, Kubota I, Shimizu Y. Therapeutic outcome of hyperbaric oxygen and basic fibroblast growth factor on intractable skin ulcer in legs: preliminary report. Plast Reconstr Surg. 2006;117:646–651. doi: 10.1097/01.prs.0000197206.48963.60. discussion 652–643. [DOI] [PubMed] [Google Scholar]
- 25.Nareika A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. Sodium lactate increases LPS-stimulated MMP and cytokine expression in U937 histiocytes by enhancing AP-1 and NF-kappaB transcriptional activities. Am J Physiol Endocrinol Metab. 2005;289:E534–542. doi: 10.1152/ajpendo.00462.2004. [DOI] [PubMed] [Google Scholar]
- 26.Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- 27.O’Flaherty C, Breininger E, Beorlegui N, Beconi MT. Acrosome reaction in bovine spermatozoa: role of reactive oxygen species and lactate dehydrogenase C4. Biochim Biophys Acta. 2005;1726:96–101. doi: 10.1016/j.bbagen.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Oberringer M, Jennewein M, Motsch SE, Pohlemann T, Seekamp A. Different cell cycle responses of wound healing protagonists to transient in vitro hypoxia. Histochem Cell Biol. 2005;123:595–603. doi: 10.1007/s00418-005-0782-5. [DOI] [PubMed] [Google Scholar]
- 29.Patel V, Chivukala I, Roy S, Khanna S, He G, Ojha N, Mehrotra A, Dias LM, Hunt TK, Sen CK. Oxygen: From the benefits of inducing VEGF expression to managing the risk of hyperbaric stress. Antioxidants & Redox Signaling. 2005;7:1377–1387. doi: 10.1089/ars.2005.7.1377. [DOI] [PubMed] [Google Scholar]
- 30.Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S–1140S. doi: 10.1093/ajcn/54.6.1135s. [DOI] [PubMed] [Google Scholar]
- 31.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–220. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy S, Khanna S, Sen CK. Redox regulation of the VEGF signaling path and tissue vascularization: Hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free Radic Biol Med. 2007 doi: 10.1016/j.freeradbiomed.2007.01.025. in press. [DOI] [PubMed] [Google Scholar]
- 33.Safran M, Kaelin WGJ. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Said HK, Hijjawi J, Roy N, Mogford J, Mustoe T. Transdermal sustained-delivery oxygen improves epithelial healing in a rabbit ear wound model. Arch Surg. 2005;140:998–1004. doi: 10.1001/archsurg.140.10.998. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol. 2006;91:803–806. doi: 10.1113/expphysiol.2006.033498. [DOI] [PubMed] [Google Scholar]
- 38.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002;277:33284–33290. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- 39.Sen CK, Semenza GL. Oxygen sensing. In: Abelson JA, Simon MI, editors. Methods in Enzymology. SAn Diego: Academic Press; 2004. p. 824. [Google Scholar]
- 40.Sheikh AY, Rollins MD, Hopf HW, Hunt TK. Hyperoxia improves microvascular perfusion in a murine wound model. Wound Repair Regen. 2005;13:303–308. doi: 10.1111/j.1067-1927.2005.130313.x. [DOI] [PubMed] [Google Scholar]
- 41.Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain MZ, Rosen N, Seremetiev A, Becker HD, Hunt TK. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen. 2003;11:504–509. doi: 10.1046/j.1524-475x.2003.11621.x. [DOI] [PubMed] [Google Scholar]
- 42.Wagner S, Hussain MZ, Beckert S, Ghani QP, Weinreich J, Hunt TK, Becker HD, Konigsrainer A. Lactate down-regulates cellular poly(ADP-ribose) formation in cultured human skin fibroblasts. Eur J Clin Invest. 2007;37:134–139. doi: 10.1111/j.1365-2362.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- 43.Wagner S, Hussain MZ, Hunt TK, Bacic B, Becker HD. Stimulation of fibroblast proliferation by lactate-mediated oxidants. Wound Repair Regen. 2004;12:368–373. doi: 10.1111/j.1067-1927.2004.012315.x. [DOI] [PubMed] [Google Scholar]
- 44.Zabel DD, Feng JJ, Scheuenstuhl H, Hunt TK, Hussain MZ. Lactate stimulation of macrophage-derived angiogenic activity is associated with inhibition of Poly(ADP-ribose) synthesis. Lab Invest. 1996;74:644–649. [PubMed] [Google Scholar]


