Abstract
BACKGROUND
The current study was performed to analyze the feasibility, safety, imaging appearance, and short-term efficacy of image-guided percutaneous radiofrequency ablation (RFA) of primary and metastatic adrenal neoplasms including adrenocortical carcinoma.
METHODS
The procedure was performed using 36 treatment spheres on 15 adrenocortical carcinoma primary or metastatic tumors in eight patients over 27 months. Tumors ranged from 15 to 90 mm in greatest dimension with a mean of 43 mm. All patients had unresectable tumors or were poor candidates for surgery. Mean follow-up was 10.3 months.
RESULTS
All patients were discharged or were free of procedure-related medical care 6−48 hours after the procedures without major complications. All treatments resulted in presumptive coagulation necrosis by imaging criteria, which manifested as loss of previous contrast enhancement in ablated tissue. Eight of 15 (53%) posttreatment thermal lesions lost enhancement and stopped growing on latest follow-up computed tomographic scan. Three of 15 (20%) demonstrated interval growth and four did not change in size. Of these four lesions, two showed contrast enhancement. For smaller tumors with a mean greatest dimension less than or equal to 5 cm, 8 of 12 (67%) tumors were completely ablated, as defined by decreasing size and complete loss of contrast enhancement. Three of 15 (20 %) tumors and related thermal lesions were found to have disappeared nearly completely on imaging.
CONCLUSIONS
Percutaneous, image-guided RFA is a safe and well tolerated procedure for the treatment of unresectable primary or metastatic adrenocortical carcinoma. The procedure is effective for the short-term local control of small adrenal tumors, and is most effective for tumors less than 5 cm. The survival rate for patients with adrenocortical carcinoma improves when radical excision is performed in selected patients. Aggressive local disease control may potentially influence survival as well. However, further study is required to evaluate survival impact, document long-term efficacy, and to determine if RFA can obviate repeated surgical intervention in specific clinical scenarios.
Keywords: adrenal metastases, radiofrequency ablation, adrenocortical carcinoma
Patients with primary and metastatic adrenocortical carcinoma (ACC) have limited treatment options. Chemotherapy with mitotane has shown limited efficacy and radiation therapy has had little significant effect on the natural course of this disease.1–5 Repeated surgical resection is used in selected patients and is associated with long-term survival.
Image-guided local tumor ablation with radiofrequency current may offer a minimally invasive and safe treatment option for patients who have had multiple recurrent surgeries, who are not ideal surgical candidates, or for whom the proven benefits of surgery do not outweigh the risks. Recent advancements in radiofrequency thermal ablation (RFA) make local tissue destruction possible in a rapid, predictable, and inexpensive manner with minimal morbidity and a short recovery time. Radiofrequency ablation has been applied safely and effectively to benign bone tumors, malignant primary and meta-static liver tumors, and renal cell carcinoma.6–11 If aggressive surgical resection improves survival in certain clinical settings, it is possible that local tissue destruction also might benefit specific patients. Radiofrequency ablation may improve survival for patients with solitary or small liver tumors compared with no treatment or medical management alone, although the long-term data are not yet available. This procedure may also provide a treatment option for patients with inoperable liver tumors.8–11
MATERIALS AND METHODS
Radiofrequency ablation was performed in 8 patients with 15 ACC recurrences or metastases. Patients were followed for more than 27 months (from February 1999 to May 2001) with mean and median follow-ups of 10.3 and 8 months, respectively. Treatments were comprisedof 36 thermal spheres applied to overlap and envelop the tumors. Tumors ranged from 15−90 mm in greatest dimension with a mean dimension of 43 mm. Mean tumor dimension was calculated from the longest dimension and its perpendicular dimension. Volumes were calculated based on the formula for the volume of a sphere (4/3 πr3). All patients underwent percutaneous tumor ablation with radiofrequency using ultrasound and/or computed tomography (CT) scan for image guidance.
Five tumors involved the adrenal bed, five tumors involved the liver, two tumors abutted or invaded the kidney, two tumors were paraspinal in location, one was retroperitoneal, and one was located in the ribs/ lung. Some tumors involved more than one organ or location. Tumors adjacent to vital organs were treated more conservatively to decrease the risk of collateral damage. All patients had unresectable tumors or were poor candidates for surgery as determined by an experienced adrenal surgeon. Disease progression was documented by imaging in all patients before they were enrolled in the RFA study. No patient had hyper-cortisolism.
The procedures were performed in the interventional radiology or CT scanning suite after extensive consultation with the patients. All patients provided informed consent. Routine protocols for conscious sedation were followed. Patients received intravenous midazolam, intravenous fentanyl, and subcutaneous bupivacaine or lidocaine.
Investigational review board consultation was not necessary because the ablation system was Food and Drug Administration 510-k cleared for soft tissue ablation. One patient required deep sedation after conscious sedation failed to control excessive intraprocedural pain. Two patients received general anesthesia because they had excessively painful tumors or concurrent comorbid disease. Patients were kept overnight because most traveled long distances to our facility. However, RFA has been performed safely as an outpatient procedure.6
Radiofrequency ablation was performed with ultrasound and/or CT scan guidance. Seven tumors were treated with ultrasound guidance, seven with both CT scan and ultrasound, and one with CT scan alone. The RFA system used in all cases was a 200-Wt, 480-kHz alternating current radiofrequency generator (Radionics, Burlington, MA). This system used chilled saline to perfuse and cool the needles so as to limit overcooking of tissue. Tissue impedance and post-RFA tissue temperature were monitored to optimize energy deposition in the target tissue. The RFA system was comprised of 1 or 3 parallel 17.5-gauge needles placed into the tumor with imaging guidance. Needles were selected according to the lesion size, location, and proximity of adjacent anatomy. Six tumors were treated with single needles and nine tumors were treated with triple parallel needles. Using monopolar RFA, two to four grounding pads were placed on the thighs to make the patient into an electrical circuit. Radiofrequency current deposition at the needle tip led to ionic agitation and frictional heat. Above 50 °C, tissue desiccated and proteins denatured, causing coagulation necrosis.9 The needle track was cauterized upon needle removal to decrease the theoretic risk of needle track seeding and to prevent track bleeding.
After the procedure, patients returned to the nursing units for monitoring and pain control. Computed tomography scans were performed fewer than 6 hours after the procedure to evaluate short-term effects and to document that patients were free of complications.
To evaluate response, contrast-enhanced CT scans were obtained 4−12 weeks after the procedure. Repeat CT scans were typically performed at 6 months and 12 months, then according to clinical need. Tumor necrosis was considered complete when enhancement was neither seen in the tumor nor at the periphery on follow-up CT scans. A change of greater than 10 CT numbers (Hounsfield units) indicated enhancement, signifying incomplete treatment or tumor recurrence. Post-RFA thermal lesion growth also indicated incomplete treatment or tumor recurrence. The two were not differentiated.
RESULTS
All patients tolerated the procedure well without periprocedural complications. One patient developed a delayed multimicrobial abscess in a 90-mm lesion 11 weeks after his third RFA treatment session, which was treated successfully with a long course of levofloxacin and prolonged catheter drainage. No hematoma, skin breakdown, skin burn, fistula, urinoma, pneumothorax, or nerve deficits were noted in any patients.
Four to 6 weeks after RFA, CT scans showed that all lesions had lost some contrast enhancement, which was consistent with coagulation necrosis (Fig. 1). On most recent follow-up, 3 of 15 (20%) thermal lesions showed continued growth after RFA, 4 of 15 (27%) had no change in size, and the remaining 8 of 15 (53%) decreased in size and lost enhancement. Three of 15 (20%) tumors and related thermal lesions almost completely disappeared on cross-sectional imaging. Residua of nonenhancing tissue were barely perceptible and probably represented scar tissue (Fig. 1). Local recurrence and incomplete treatment are managed identically so no attempt was made to differentiate the two.
FIGURE 1.
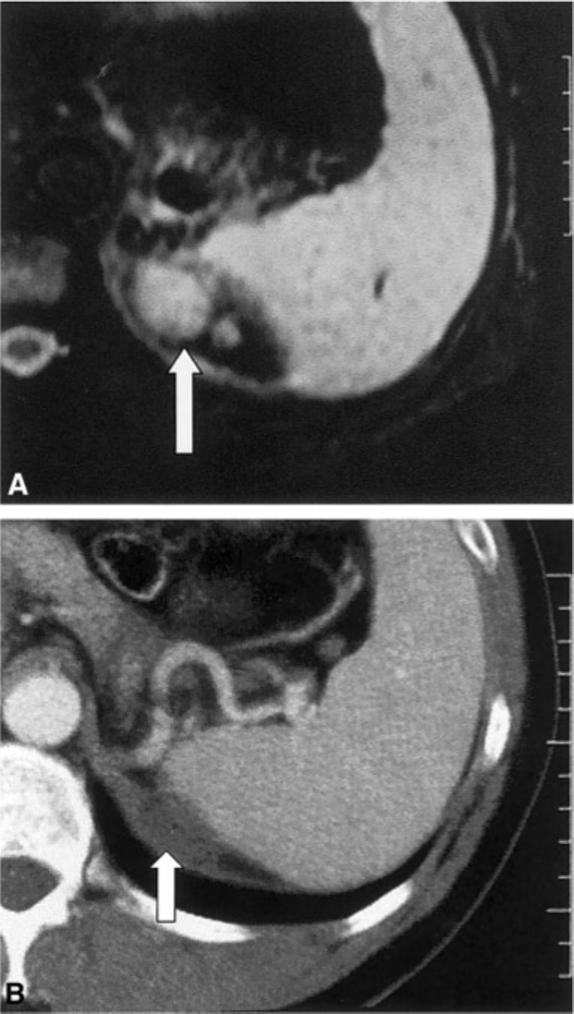
(A) T2-weighted magnetic resonance image shows bilobed tumor (arrow) in the adrenal bed adjacent to the spleen before radiofrequency ablation (RFA) was performed. (B) Enhanced computed tomograpy scan 20 months after RFA demonstrates near-complete involution of treated tumor with shrinking, unenhancing residual thermal lesion (arrow), presumably scar tissue.
Better results were achieved with smaller tumors. Eight of 12 (67%) tumors with a mean dimension less than or equal to 5 cm were completely ablated, as defined by loss of enhancement and a decrease in size. Three of 15 tumors had a mean dimension greater than 5 cm and two showed growth or enhancement. One of these stayed the same size but lost enhancement.
Computed tomographic scan and ultrasound were both required for optimal probe positioning in seven patients. In these patients, probe positioning was judged to be difficult or sub-optimal with either modality alone. In other patients, one guidance modality was used.
The mean pretreatment tumor volume was 58,077 mm3. Immediately post-RFA, mean volume increased to 60,709 mm3. Most recent follow-up mean volume decreased to 49,342 mm3, with a mean follow-up of 10.3 months and a median follow up of 8 months (Table 1). Thermal lesions had a greater volume than pre-RFA tumors and treated areas shrunk over time (Figs. 1, 2, 4; Table 1).
TABLE 1.
Lesion Volumes
| Tumor no. | Pre-RFA volume (mm3) | Post-RFA volume (mm3) | Most recent volume (mm3) | Change of post vs. pre (%) | Change of most recent vs. post (%) | Most recent follow-up (mos) |
|---|---|---|---|---|---|---|
| 1 | 31,044 | 99,491 | 38,773 | 22 | −61 | 8 |
| 2 | 65,417 | 168,209 | 94,390 | 157 | −44 | 4 |
| 3 | 40,174 | 143,720 | 19,014 | 258 | −88 | 18 |
| 4 | 33,493 | 27,598 | 113,040 | −18 | 310 | 19 |
| 5 | 33,493 | 33,493 | 10,884 | 0 | −68 | 17 |
| 6 | 17,965 | 8678 | 65 | −52 | −99 | 20 |
| 7 | 1766 | 1150 | 0 | −35 | −100 | 20 |
| 8 | 26,508 | 17,965 | 65 | −32 | −99 | 15 |
| 9 | 56,087 | 56,087 | 56,087 | 0 | 0 | 2 |
| 10 | 47,689 | 73,585 | 65,417 | 54 | −11 | 4 |
| 11 | 127,767 | 127,767 | 127,767 | 0 | 0 | 2 |
| 12 | 4187 | 14,130 | 18,807 | 237 | 33 | 5 |
| 13 | 10,884 | 10,884 | 65,417 | 0 | 501 | 18 |
| 14 | 153,902 | 96,918 | 96,918 | −37 | 0 | 2 |
| 15 | 220,781 | 33,493 | 33,493 | −57 | 0 | 1 |
| Mean | 58,077 | 60,709 | 49,342 |
RFA: radiofrequency ablation.
FIGURE 2.
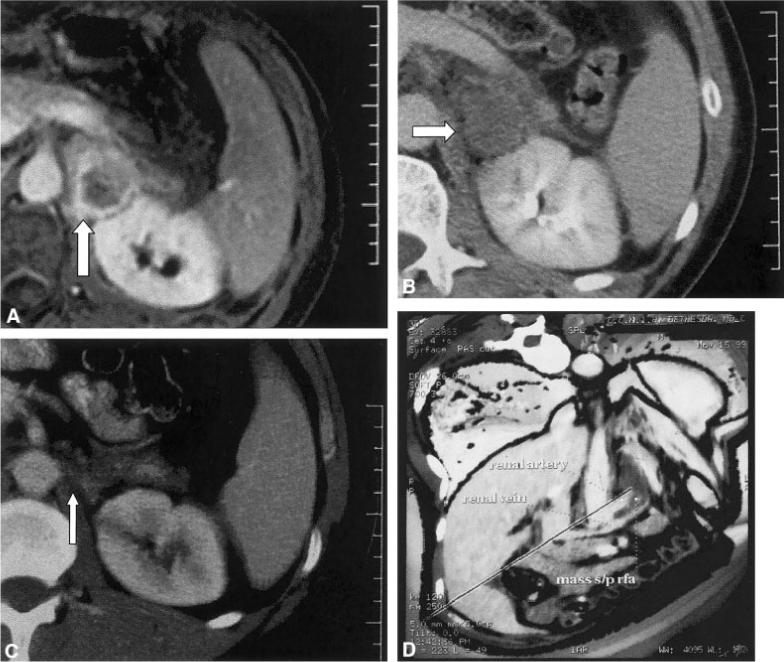
(A) Enhanced T1-weighted magnetic resonance image before radiofrequency ablation (RFA) shows the enhancing rim of a recurrent adrenocortical carcinoma (ACC) postsurgery (arrow). The ACC tumor is located in the suprarenal bed, between the aorta and kidney. (B) Enhanced computed tomography (CT) scan immediately after RFA shows devascularized tumor with loss of enhancement (arrow). (C) Enhanced CT scan 14 months after RFA shows interval shrinkage of thermal lesion or tumor with a small residual (arrow). (D) Three-dimensional shaded surface display from contrast-enhanced CT immediately after RFA. The planes are cut away in the treated region to display the renal artery, renal vein, and the intervening treated thermal lesion between these two vessels. This demonstrates the predictability of RFA near vessels.
FIGURE 4.

Lesion volumes over time.
DISCUSSION
Adrenocortical carcinoma is an uncommon, but diffi-cult to manage tumor. Its estimated annual incidence in the United States is 1−2 per million.12 Forty to 70% of patients have metastases at the time of diagnosis.1
Patients with metastatic ACC have limited treatment options. Mitotane has been used for decades as a single agent or in combination with other drugs.2,13 However, this therapy has a 20−25% response rate, at best.3,14 Some studies have failed to demonstrate significant activity for conventional chemotherapy.3–5 The value of radiation therapy is questionable.1,2 Repeated surgery remains the only therapy associated with prolonged survival in patients with metastatic ACC.1,15–17 However, surgical morbidity and cost are high.16 Therefore, there is a need for a safer and easier local therapy.
Specifically, ACC has a 20−25% 5-year survival rate.2,18 Recurrence in the adrenal bed occurs in 50% of patients. Metastases occur in 70% of patients and involve the lungs (45%), liver (42%), lymph nodes (24%), and bone (15%).12 Mitotane, the only systemic treatment, has a response rate of 20−25%, nearly all of which are partial responses.1–3,14 Most reports of cytotoxic therapy for ACC are anecdotal or include a small number of patients.3,13,14 Phase II studies of mitotane multidrug combination chemotherapies for ACC have reported response rates between 18% and 30% and a median survival period of 9.3−11.8 months.5,19
Given the lack of other treatment options, repeat surgical procedures in patients with recurrent ACC have been investigated extensively. Numerous studies have shown that surgery may prolong the disease-free survival period of certain patients with metastatic ACC.15–17 Although these studies indicate that sequential resections are beneficial in a subset of patients, this approach must be balanced against the related morbidity.
Recent technical developments in RFA allow safe, effective, and predictable ablation of large tissue volumes (Fig. 3).8–11 Radiofrequency ablation is equally effective as surgical resection for osteoid osteoma.6,7 It is a safe and effective means of local control of unresectable primary and metastatic hepatic neoplasms, with a reported complication rate of less than 3%.8–11 The adjacent vasculature is protected by the relatively cool body temperature of blood. However, this also makes tumor recurrence more likely adjacent to vessels (Fig. 3). The RFA procedure has preliminary survival curves that are similar to those for surgery for small, solitary liver tumors.
FIGURE 3.
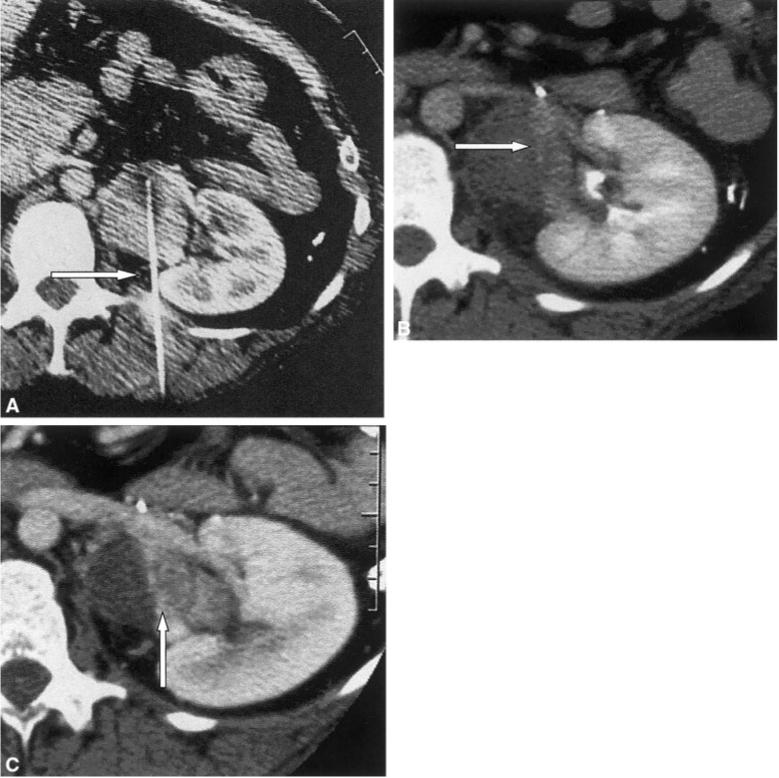
(A) Contrast-enhanced computed tomography (CT) scan shows radiofrequency ablation (RFA) probe tip (arrow) within enhancing renal hilum adrenocortical carcinoma drop metastasis. (B) Enhanced CT scan immediately after RFA shows a patent renal artery and subtle residual enhancing tissue at the lateral margin of the tumor (arrow). (C) Contrast-enhanced CT 6 weeks after RFA shows regrowth of incompletely treated tumor in a region with previous suspicious enhancement (arrow).
Our observations corroborate previous studies that showed that RFA can be performed safely in patients with metastatic disease to solid abdominal organs.9,10 In our study, one delayed abscess occurred despite prophylactic antibiotic coverage with 1 g of intravenous Cephazolin during the procedure. The timing of the abscess 11 weeks later makes superinfection of the thermal lesion a possibility, given that thermally damaged tissue may be a fertile milieu for bacterial growth. The combination of CT and ultrasound guidance and monitoring often provided better probe positioning and repositioning than either modality alone, although this is subjective and operator dependent.
Radiofrequency ablation was used as an effective alternative to repeated surgical resection in patients with ACC tumors. It was utilized as a treatment option in these patients due to its predictability, ease, safety profile, and similar results compared with local surgical resection. Perhaps, most importantly, ACC recurs in or metastasizes to regions or organs where RFA may be applied easily, including the retroperitoneum, adrenal bed, kidney, liver, bone, lymph nodes, and lung. The imaging outcome criteria of size and enhancement have validated somewhat the use of RFA in the liver and kidney, but not in other locations of adrenal tumors. The post-RFA thermal lesion volume should be much greater than the pre-RFA tumor volume because it should include a margin of normal adjacent tissue. Thereafter, the natural history of the thermal lesion should decrease in size as fibrosis occurs (i.e., if there are no tumors growing within; Fig. 2, Table 1). The post-RFA volumes were obtained at varying times after treatment, which may account for some of the variability in the post-RFA volumes. It remains unclear whether short-term shrinkage or lack of enhancement (Fig. 4) suggests long-term local control or enhanced survival.
The RFA procedure may provide a safe and effective treatment approach to patients with small adrenal tumors, including metastatic ACC.20 Our group has successfully ablated a pheochromocytoma and has treated metastases to the adrenal gland21 (Wood BJ, Ramkaransingh JR. Unpublished data, 2003). However, it is controversial whether adrenalectomy for solitary metastases to the adrenal gland from other primary tumors prolongs survival. A few small series suggest that surgery in this setting can benefit specific patients, particularly those with favorable tumor biology, long disease-free intervals, symptomatic disease or specific histology such as adenocarcinoma, lung carcinoma, renal cell carcinoma, colorectal carcinoma, or melanoma.22–24 The role of RFA in this setting is speculative.
Our study showed that RFA may be more effective for adrenal tumors smaller than 5 cm than for larger ones. These early results are preliminary and the follow-up period was short. However, as a local method of tissue destruction, RFA could impact survival in patients with advanced ACC. This is based on extrapolation from the effects of local control of disease by repeated surgical resection. Radiofrequency ablation is easy to repeat, given its low morbidity and cost, and may be safely applied to the anatomic areas to which ACC tumors commonly metastasize. Because surgical resection is the only proven effective therapy for ACC patients, the utility and impact of RFA for ACC patients should be evaluated further. Likewise, the efficacy and potential clinical impact of RFA to treat other adrenal neoplasms, including metastases to the adrenal gland, deserves more study. It may be a safe and potentially effective treatment option for patients with adrenal tumors and it may provide other useful options.
Footnotes
This article is a U.S. Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- 1.Demeure MJ, Somberg LB. Functioning and nonfunctioning adrenocortical carcinoma. Surg Oncol Clin North Am. 1998;7:791–805. [PubMed] [Google Scholar]
- 2.Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors and the effect of mitotane therapy. N Engl J Med. 1990;332:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 3.Vassilopoulou-Sellin R, Guinee VF, Klein MJ, et al. Impact of adjuvant mitotane on the clinical course of patients with adrenocortical cancer. Cancer. 1993;71:3119–3123. doi: 10.1002/1097-0142(19930515)71:10<3119::aid-cncr2820711037>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Decker RA, Elson P, Hogan TF, et al. Eastern Cooperative Oncology Group study 1879: mitotane and Adriamycin in patients with advanced adrenocrtical carcinoma. Surgery. 1991;110:1006–1013. [PubMed] [Google Scholar]
- 5.Bukowski RM, Wolfe M, Levine HS, et al. Phase II trial of mitotane and cisplatin in patients with adrenal carcinoma: a Southwest Oncology Group Study. J Clin Oncol. 1993;11:161–165. doi: 10.1200/JCO.1993.11.1.161. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal DI, Hornicek FJ, Wolfe MW, et al. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg [A] 1998;80:815–821. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Lindner NJ, Scarborough M, Ciccarelli JM, Enneking WF. CT-guided radiofrequency ablation for treatment of osteoid osteoma in comparison to traditional techniques. Z Orthop. 1997;135:522–527. doi: 10.1055/s-2008-1039739. [DOI] [PubMed] [Google Scholar]
- 8.Rossi S, Fornari F, Buscarini L. Percutaneous ultrasound guided radiofrequency electrocautery for the treatment of small hepatocelluar carcinoma. J Interven Radiol. 1993;8:97–103. [Google Scholar]
- 9.Solbiati L, Ierace T, Goldberg NS, et al. Percutaneous US-guided radiofrequency tissue ablation of liver metastases: treatment and follow up in 16 patients. Radiology. 1997;202:195–203. doi: 10.1148/radiology.202.1.8988211. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio Frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371–379. doi: 10.1148/radiology.209.2.9807561. [DOI] [PubMed] [Google Scholar]
- 11.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan MF. Adrenocortical carcinoma. CA Cancer J Clin. 1987;37:349–365. doi: 10.3322/canjclin.37.6.348. [DOI] [PubMed] [Google Scholar]
- 13.Kasperlik-Zaluska AA, Migdalska BM, Zgliczynski S, Makowska AM. Adrenocortical carcinoma. Cancer. 1995;75:2587–2591. doi: 10.1002/1097-0142(19950515)75:10<2587::aid-cncr2820751028>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Wooten MD, King DK. Adrenocortical carcinoma. Cancer. 1993;72:3145–3153. doi: 10.1002/1097-0142(19931201)72:11<3145::aid-cncr2820721105>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 15.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–971. [PubMed] [Google Scholar]
- 16.Jensen CJ, Pass HI, Sindelar WF, Norton JA. Recurrent or metastatic disease in select patients with adrenocortical carcinoma. Arch Surg. 1991;126:457–461. doi: 10.1001/archsurg.1991.01410280059008. [DOI] [PubMed] [Google Scholar]
- 17.Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian national registry for adrenal cortical carcinoma. Surgery. 1997;122:1212–1218. doi: 10.1016/s0039-6060(97)90229-4. [DOI] [PubMed] [Google Scholar]
- 18.Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. Adrenocortical carcinoma. Cancer. 1989;64:765–769. doi: 10.1002/1097-0142(19890801)64:3<765::aid-cncr2820640333>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Abraham J, Bakke S, Rutt A, et al. A phase II study of combination chemotherapy and surgical resection in the treatment of adrenocortical carcinoma: continuous infusion of doxorubicin, vincristine and etoposide with daily mitotane before and after surgical resection [abstract]. Proc Am Soc Clin Oncol. 1999;18:191a. doi: 10.1002/cncr.10487. [DOI] [PubMed] [Google Scholar]
- 20.Abraham J, Fojo T, Wood BJ. Radiofrequency ablation of metastatic lesions in adrenocortical cancer. Ann Intern Med. 2000;133:312–313. doi: 10.7326/0003-4819-133-4-200008150-00028. [DOI] [PubMed] [Google Scholar]
- 21.Pacek K, Fojo T, Goldstein DS, et al. Radiofrequency ablation (RFA): a novel approach for the treatment of metastatic pheochromocytoma. J Natl Cancer Inst. 2001;93:648–649. doi: 10.1093/jnci/93.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul CA, Virgo KS, Wade TP, Audisio RA, Johnson FE. Adrenalectomy for isolated adrenal metastases from non-adrenal cancer. Int J Oncol. 2000;17:181–187. [PubMed] [Google Scholar]
- 23.Lo CY, Van Heerden JA, Soreide JA, et al. Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg. 1996;83:528–531. doi: 10.1002/bjs.1800830432. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998;82:389–394. [PubMed] [Google Scholar]


