Summary
Giardia lamblia is a ubiquitous parasite that causes diarrhea. Effective control of Giardia infections in mice has been shown to involve IgA, T cells, mast cells, and IL-6. We now show that TNFα also plays an important role in the early control of giardiasis. Mice treated with neutralizing anti-TNFα antibodies or genetically deficient in TNFα were infected with the G. lamblia clone GS/(M)-H7. In both cases, mice lacking TNFα had much higher parasite numbers than controls during the first two weeks of infections. However, anti-parasite IgA levels, mast cell responses and IL-4 and IL-6 mRNA levels do not appear significantly altered in the absence of TNFα. In addition, we show that mice infected with G. lamblia exhibit increased intestinal permeability, similar to human Giardia infection, and that this increase occurs in both wild-type and TNFα deficient mice. We conclude that TNFα is essential for host resistance to Giardia lamblia infection, and that it does not exert its effects through mechanisms previously implicated in control of this parasite.
Keywords: Giardia lamblia, TNF, Trans-epithelial electrical resistance
Introduction
Giardia lamblia (syn. G. intestinalis, G. duodenalis) is the most commonly diagnosed parasitic cause of diarrhea in the U.S. and infects ∼200 million people world-wide. There is significant variability in the outcome of these infections (reviewed in (1-4). Many infections are asymptomatic, while others result in severe diarrhea, cramps and nausea. Similarly, most infections are controlled by host immune responses, whereas others become chronic. G. lamblia infections in humans induce immune response characterized by production of large amounts of anti-parasite IgA (reviewed in (1, 3). IgA is required for prevention of chronic G. muris, a rodent specific species of Giardia, infections in mice (5-8). In contrast, in the adult mouse model of G. lamblia infection, antibodies are not required to eliminate the parasite and a cellular immune response is able to control the infection in the absence of antibodies (8-11). It remains to be determined if and how cellular immune responses might contribute to control of G. lamblia infections in humans.
The precise nature of the protective cellular response against G. lamblia in mice is unclear, but we and others have found that CD4+ T cells, mast cells and IL-6 are all required (10-13). Mice treated with depleting antibodies against CD4 or c-kit fail to eliminate this parasite (10, 13). Similarly, mice with mutations in c-kit or targeted deletion of IL-6 or the T cell receptor β gene also fail to control G. lamblia infections (10-12). However, unlike many other mouse models of parasitic infection, mice deficient in either IL-4 or IFN-γ had no defects in parasite control (10). Recently, while analyzing the role of nitric oxide during G. lamblia infections in mice, we noted that TNFα mRNA levels were lower in IL-6 deficient mice following Giardia infection than in wild-type mice, (14). We therefore decided to directly address the role of TNFα in the mouse model of G. lamblia infection.
Materials and Methods
Mice, parasites and infections
C57BL/6J, homozygous TNFα deficient mice on a B6X129 mixed background (B6;129S2-Tnftm1Gkl/J) and wild-type B6X129 F2/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). G. lamblia strain GS/(M)-H7 was cultured to confluence and used for infections as previously described (10). Female mice aged between 5 and 8 weeks were used for all experiments. Briefly, mice were infected by gavage with 1 × 106 parasites each in 0.2 ml phosphate-buffered saline (PBS) on day 0 and killed on different days after infection. Parasite numbers were determined from 10 cm of the distal duodenum and proximal jejunum just below the ligament of Trietz. Tissue was minced in 4 ml ice-cold PBS and chilled for 15 minutes on ice and parasites were counted on a hemocytometer as previously described (13). The limit of sensitivity for this assay is 10,000 parasites / 10 cm. All animals were handled in accordance with protocols approved by the Georgetown University Animal Care and Use Committee.
Anti-TNFα treatment
TNFα was neutralized in vivo as previously described (15). C57BL/6J mice were injected i.p. with 1 mg of anti-TNFα (clone HT-11−22) or isotype control (clone GL113) on the same day as infection.
IgA responses
Anti-parasite IgA in intestinal fluid was detected as previously described (11). Intestinal fluid was collected from mice by flushing the distal jejunum with 3 ml cold PBS. In vitro cultures of Giardia were allowed to attach to glass slides and fixed with cold methanol-acetone (1:1) for 5’. Fixed parasites were blocked by incubation in 5% normal goat serum in PBS and serial dilutions of intestinal fluid was applied for 60’. After washing, anti-parasite IgA was detected using FITC conjugated goat anti-mouse IgA (Southern Biotechnology, Birmingham, AL). IgA anti-Giardia monoclonal antibodies (16) were used as positive controls. Slides were mounted with Vectashield + propidium iodide (Vector Laboratories, Burlingame, CA) and viewed with a Zeiss Axiophot microscope. Images were collected with a CoolSnap fx camera (Roper Scientific, Trenton, NJ) using OpenLab software (Improvision, Cambridge, MA). Images were processed with AdobePhotoshop (Adobe Systems, San Jose, CA).
Mast cell responses
Mast cells in the small intestine were detected using chloroacetate esterase activity as previously described (13). Distal jejunum was fixed overnight in 4% formaldehyde in PBS, embedded in paraffin and sectioned (5 μ) onto glass slides. Sections were stained as described and viewed as above using brightfield imaging and a CRI color filter (Cambridge Research, Cambridge, MA).
Semi-Quantitative RT-PCR
Cytokine mRNA levels were determined as previously described (11). Total RNA was prepared from a 1−2 cm segment of the small intestine corresponding to the jejunal region immediately distal to that used for determining parasite counts. RNA was purified using RNA-STAT-60 (Tel-test, Inc., Midland, Tex.). RNA was quantified using a Nanodrop spectrophotometer and quality was assessed by MOPS-formaldehyde gel electrophoresis prior to cDNA synthesis. Five μg of total RNA from each mouse was used to generate double stranded cDNA using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). IL-4, IL-6 and HPRT cDNAs were amplified as described and analyzed on 1.5% agarose gels. PCR products were quantitated by densitometric analysis of agarose gels using AlphaEase Software and an AlphaImager (Alpha Innotech, San Leandro, CA).
Epithelial Permeability
We used a modified snap-well assay system described by Fasano and colleagues to measure electrical resistance of the intestinal mucosa (17). Briefly, segments of the jejunum were removed and the muscle layers carefully stripped away from the mucosa. For each mouse, three ∼1 cm2 pieces of mucosa were then mounted in snap-well chambers with a precisely machined aperture (Costar, Cambridge, MA) and cultured in DMEM without serum. Epithelial resistance was measured every 30 minutes using an EndOhm-24 and EVOM voltmeter (World Precision Instruments, Sarasota, FL). Resistance of the mucosae in these chambers typically increases immediately after tissues are mounted in the chambers, then stabilizes and eventually decreases over time. Two or three pieces of mucosa per mouse were analyzed in parallel.
Statistical Analysis
We applied a Student's t-test to test the hypothesis that the mean expression values for the control and infected groups were equal. Statistical probability of P < 0.05 was considered significant.
Results
The role of TNFα was initially assessed by infecting groups of wild-type C57BL/6 female mice (Jackson Laboratories, Bar Harbor, ME) with G. lamblia GS/(M)-H7 and determining parasite numbers following infection as described (10). Mice received either neutralizing anti-TNFα or control IgG on the day of infection (15). Parasite numbers in the small intestines of mice receiving anti-TNFα were more than ten-fold greater five days following infection than in mice receiving control IgG (Fig. 1A). Moreover, while control-treated mice had eliminated parasites by day 12, anti-TNFα treated mice still had parasite numbers greater than the limit of detection of our assay (∼10,000 parasites / mouse, dashed lines in Fig. 1). The reduced numbers of parasites on day 12 could reflect decreasing concentrations of anti-TNFα in these mice over time. We therefore infected genetically TNFα deficient mice on a B6X129 F2 genetic background and wild-type mice (Jackson Laboratories) and analyzed parasite numbers on days 5, 12 and 28 post-infection (Fig. 1B). Again, TNFα deficient mice had substantially more parasites than wild-type controls on days 5 and 12. However, parasite numbers on day 12 had significantly decreased in TNFα deficient mice, similar to what was seen in anti-TNFα treated mice, while control mice had no detectable parasites. By day 28, parasites were no longer detectable in TNFα deficient mice or wild-type controls. Thus, TNFα plays a critical role during the establishment and/or early control of this infection.
Figure 1.
TNFα is required for efficient control of Giardia infection. A. C57BL/6 mice were infected with G. lamblia and treated with anti-TNFα (hatched bars) or control rat IgG (open bars) on day 0. Parasite numbers were determined on different days post-infection. B. TNFα deficient mice (hatched bars) and B6X129 F2 controls (open bars) were infected on day 0 and parasite numbers were determined on different days post-infection. N = 4 / group. Data are presented as means with S.D. * p<0.05 by Mann-Whiney test. The limit of detection in this assay is 104 trophozoites / mouse and is marked by the dashed horizontal line. Data are representative of three experiments each with anti-TNFα treatment and TNFα deficient mice.
Previous studies using IL-6 deficient mice also demonstrated a significant defect in early control of G. lamblia infections (11, 12). Indeed, the parasite numbers seen in both IL-6 and TNFα deficient mice at days 5 and 12 post-infection are quite similar (Fig. 1 and ref. 13). Interestingly, while IL-6 deficient mice required 60 days to reduce parasite numbers to undetectable levels, the TNFα deficient mice are able to eliminate infections by day 28. This suggests that the defect in IL-6 deficient mice is more severe than just the lack of TNFα production.
To determine if the absence of TNFα led to changes in other immune responses that might explain the high parasite loads early in infections, anti-parasite IgA, mast cell responses, and mRNA levels for IL4 and IL-6 were examined. While IgA is not required to control the acute phase of a G. lamblia infection in mice, IgA could still contribute to parasite elimination. Intestinal fluid was therefore collected from infected mice and analyzed for the presence of parasite-specific IgA. No IgA was detected in uninfected mice or mice infected for 5 days, regardless of genotype (Fig. 2). Detectable IgA responses were seen in both wild-type and TNFα deficient mice on both day 12 and day 28 post-infection (Fig. 2). Thus, major differences in IgA responses do not account for the different parasite loads seen in wild-type and IL-6 deficient mice,
Figure 2.
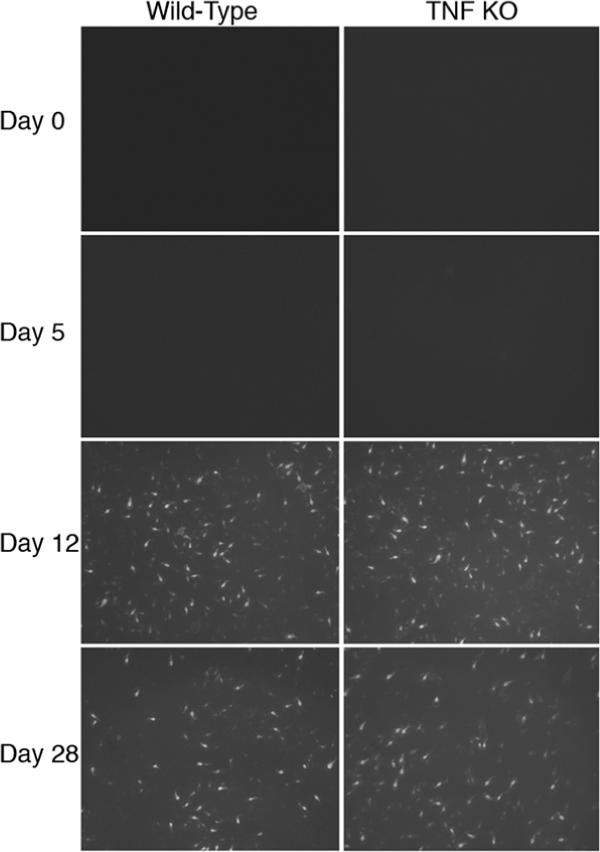
IgA responses in TNFα deficient mice. Wild-type and TNFα deficient B6X129 F2 mice were infected with G. lamblia and euthanized on days 5, 12 and 28 as indicated. Uninfected mice (Day 0) were included as controls. Intestinal fluid was used to stain in vitro cultures of G. lamblia and IgA was detected by indirect immunofluorescence. Original magnification 200X.
Mast cells have been shown to be important for control of both G. lamblia and G. muris infections in mice (13, 18). Moreover, TNFα has recently been shown to be important for mast cell development in vitro and in vivo (19). Mast cells began were first detected in the mucosa of both wild-type and TNFα deficient mice 12 days post-infection (Fig. 3), indicating that mast cell accumulation begins to occur between days 5 and 12 post-infection. No differences in mast cell numbers were seen at any time post-infection. However, at day 28 post-infection, mast cells in TNFα deficient mice appeared to be localized more in the villus regions and less in the crypt regions of the jejunum compared to mast cells in wild-type mice (Fig. 3). Given that parasites had already been eliminated, it is unclear if this difference is important for resolution of the infection. Thus, the lack of TNFα did not cause a profound reduction in two pathways known to be involved in control of Giardia infection.
Figure 3.
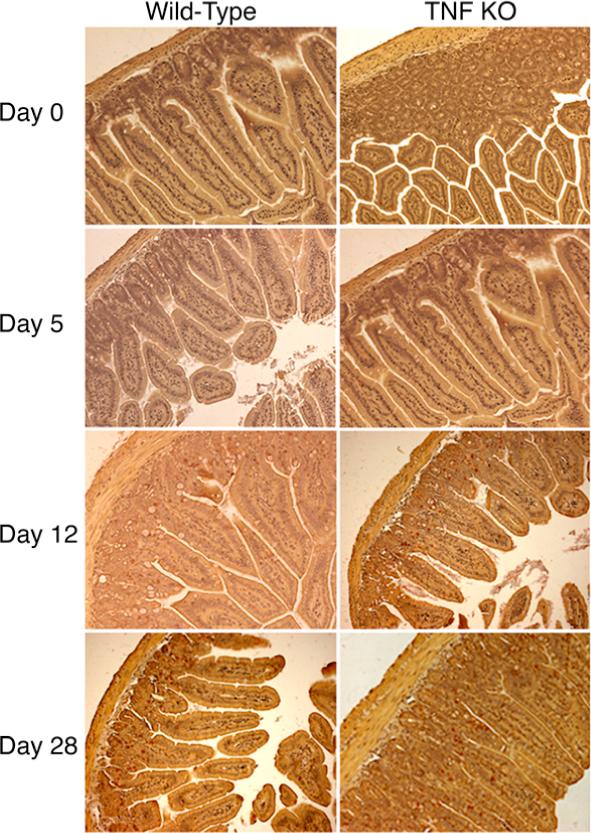
Mast cell responses in TNFα deficient mice. Wild-type and TNFα deficient B6X129 F2 mice were infected with G. lamblia and euthanized as in Fig. 2. Jejunal specimens were stained for chloroacetate esterase activity to detect mast cells (red cells). Original magnification 200X.
Like TNFα deficient mice, IL-6 deficient mice have much greater parasite burdens than wild-type mice early during infections (11, 12). A major difference in immune response between wild-type and IL-6 deficient mice is the larger increase in IL-4 mRNA levels seen in IL-6 deficient mice (12). We therefore used semi-quantitative RT-PCR to examine induction of both IL-4 and IL-6 mRNA levels in the TNFα deficient mice. IL-4 mRNA levels were elevated in both wild-type and TNFα deficient mice 5 and 12 days post-infection, compared to uninfected mice (Fig. 4 A and B). Unlike in IL-6 deficient mice, however, the IL-4 mRNA does not appear to be significantly more abundant than in wild-type mice. Furthermore, IL-4 mRNA levels were decreased in both wild-type and TNFα deficient mice on day 28. Thus, while the strong induction of IL-4 responses might contribute to the defect in parasite control observed in IL-6 deficient mice, it is unlikely to explain the high parasite numbers seen in TNFα deficient mice.
Figure 4.
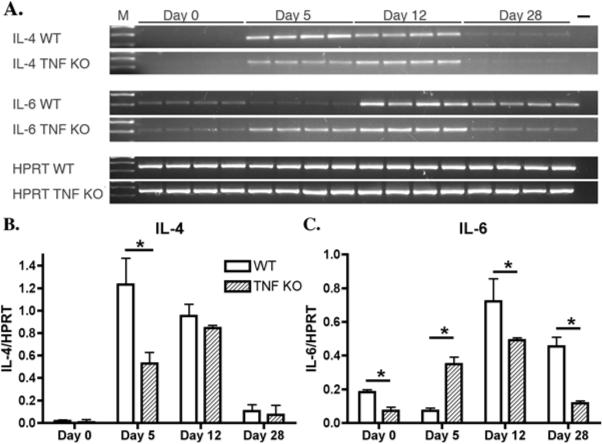
Intestinal gene expression in G. lamblia infected mice. cDNA was prepared from intestinal RNA of wild-type and TNFα deficient B6X129 F2 mice infected with G. lamblia. A. IL-4, IL-6 and HPRT were amplified and analyzed on 1.5% agarose gels (A). M- 100 bp ladder. No cDNA PCR control is shown in the far right lane (−). B and C. Densitometric analysis of the images in A. Data are presented as the ratio of pixel intensities for IL-4 vs HPRT and IL-6 vs HPRT. * p<0.05 by student t-test.
More significant differences in IL-6 mRNA levels were seen when comparing wild-type and TNFα deficient mice (Fig. 4 A and C). Five days post-infection the TNFα deficient mice had intestinal IL-6 mRNA levels that were higher than those seen in wild-type controls. The higher parasite numbers in TNFα deficient mice at this time cannot, therefore, be attributed to a failure to induce IL-6. In contrast, IL-6 mRNA levels in wild-type mice were greater than those in TNF deficient mice 12 days and 28 days post-infection. While these differences were statistically significant, the biological relevance of this is unclear, particularly because IL-6 mRNA levels in uninfected wild-type mice were also greater than those in uninfected TNF deficient mice.
Finally, giardiasis in humans causes nutrient malabsorption and one possible contributing mechanism has been reported to be decreased epithelial barrier function and increased permeability (20-22). Since TNFα is well known to increase epithelial permeability in several in vitro and in vivo systems (reviewed in (23, 24) we decided to examine permeability in wild-type and TNFα deficient mice infected with Giardia. We used a recently developed method for rapid determination of trans-epithelial electrical resistance to examine intestinal permeability in the mucosa of infected and uninfected mice (17). Mucosae from infected mice exhibited significantly lower resistance than mucosae from uninfected mice (Fig. 4A). Moreover, this decrease occurred whether or not the mice had an intact TNFα gene (Fig. 4B). These data also demonstrate that Giardia lamblia infected mice exhibit a pathologic response to infection that is characteristic of human infections (21, 22).
Discussion
G. lamblia infections in humans are marked by considerable variation in their outcomes, both in the symptoms of disease and the duration of infection. We now show that TNFα is an important cytokine for determining the parasite burden and duration of G. lamblia infection in mice. We also show that mice infected with G. lamblia develop increased epithelial permeability, a characteristic of human giardiasis. Furthermore, we show that TNFα does not contribute to control of infections through any previously described mechanism, i.e. IgA, mast cells, IL-4 or IL-6, suggesting that other effector responses downstream of this cytokine are important. Finally, we show that TNFα is not required for the increase in epithelial permeability observed in this system.
It is becoming clear that mechanisms used by the immune system to control G. lamblia infections in mice are not identical to those used to control G. muris. While antibodies, particularly IgA are crucial for control of G. muris infections in mice, cellular responses are able to control the human parasite, G. lamblia (5-8, 10). This is consistent with clinical observations in humans, where chronic Giardia infections have been associated with two particular hypogammaglobulinemia syndromes, common variable immunodeficiency (CVID) and Bruton's X-linked agammaglobulinemia (XLA), but not with selective IgA deficiency (reviewed in (1-3). It has become clear that the molecular defects in both XLA and CVID affect cells in addition to B cells (25-27), and it may be associated cellular responses that are responsible for the failure to control Giardia in these patients. For example, mutations in the btk kinase in XLA lead to reduced TNFα production by peripheral blood mononuclear cells in response to TLR signaling (25). Studies of the cellular mechanisms responsible for control of G. lamblia using mouse models are therefore highly valuable.
The two cytokines shown to have a major impact on Giardia infections in mice are IL-6 and TNFα. The defect in IL-6 deficient mice is more severe in terms of the persistence of infection. TNFα deficient mice clear their infections by day 28 whereas IL-6 deficient mice needed 60 days (11). It is possible that some of the differences observed between IL-6 and TNF deficient mice might also reflect the different genetic backgrounds used in these studies. The TNF deficient mice used in this study were on a mixed C57BL/6 X 129 genetic background, while the IL-6 deficient mice used previously (11) were inbred C57BL/6 mice. Inbred C57BL/6 mice were also used in the current study for anti-TNFα treatment. Several lines of evidence suggest that IL-6 appears to act upstream of TNFα in this Giardia model system. For example, in IL-6 deficient mice, IL-4 mRNA levels are increased following infection while TNFα mRNA levels are reduced (12, 14). Thus, IL-6 appears necessary for normal TNFα responses. Furthermore, in the absence of TNFα, mRNA levels for IL-6 and IL-4 were only mildly affected. Indeed, at day 5 post-infection, IL-6 levels were higher in TNFα deficient mice than in wild-type controls. Thus, TNFα is not needed for IL-6 responses during this infection. One possible explanation is that the greater IL-6 expression at day 5 in TNFα deficient mice reflects the greater parasite load present at this time. As recently noted, intestinal epithelial cells appear to be a significant source of IL-6 during mouse infections (28). Higher parasite burdens would therefore be expected to increase epithelial cell responses. It is likely that IL-6 and TNFα are produced by multiple cell types during G. lamblia infections in mice and experiments using tissue specific gene targeting and/or chimeric mice will be required to identify sources of these cytokines necessary for parasite elimination.
Interestingly, both IL-6 and TNFα are considered pro-inflammatory cytokines, helping to recruit macrophages and neutrophils to sites of inflammation. However, G. lamblia infections in humans and mice are typically not marked by significant infiltration of neutrophils or macrophages (29, 30). This suggests that regulatory responses in the intestinal tract may be active during Giardia infections in order to reduce immune-mediated pathology. Indeed, infections in the gastrointestinal tract are well known for inducing regulatory responses (31). Many immune effector mechanisms, e.g. acute phase responses and macrophage activation, are regulated by IL-6 and TNFα and it remains to be determined which are responsible for elimination of Giardia in this model.
A key finding in this study is the fact that mice with G. lamblia infections exhibit increased intestinal permeability. Increased permeability of intestinal epithelium has been described in vitro and in human infections (20, 22, 32, 33). Changes in intestinal permeability, however, have not been described previously in mouse infections. The modified “Snap-well” assay described here is a simple, fast and reproducible way to measure changes in trans-epithelial electrical resistance (TEER) that does not require sophisticated equipment like Ussing chambers. Our results with this system indicate that while Giardia infection causes a decrease in TEER, TNFα is not responsible. This is consistent with reduced TEER seen in vitro using only parasites and epithelial cell lines (20, 32, 33). Interestingly, the magnitude of the TEER decrease reported here was similar in wild-type and TNFα knockout mice 5 days post-infection, despite the presence of roughly 10-fold more parasites being present in the TNFα deficient mice. We and others have also recently reported that G. lamblia infected mice have altered intestinal motility and that these changes are absent in SCID mice (14, 34). Together with the altered intestinal permeability reported here, this indicates that although these mice do not have diarrhea, they do develop pathology representative of human disease.
TNFα is important for protective immune responses to many infections and also for pathological responses in inflammatory diseases like Crohn's disease and rheumatoid arthritis. These are the first data reporting a role for TNFα in protection against G. lamblia infections. Furthermore, the defect in TNFα deficient mice does not appear to be related to mechanisms previously shown to control Giardia infections, including IgA production, mast cell responses, IL-6 or IL-4 expression. Together with the observation that G. lamblia infected mice have increased intestinal permaeability, these results confirm the utility of studying G. lamblia infections in adult mice as a model of human giardiasis for understanding both pathogenesis and protective immunity against this infection.
Figure 5.
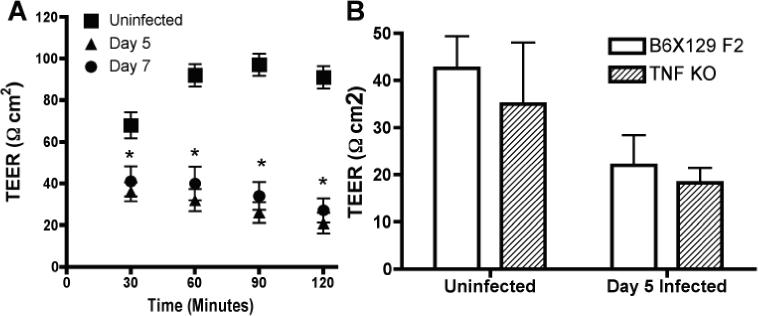
Epithelial permeability is increased in both wild-type and TNFα deficient mice. A. Mucosae from jejunum of C57BL/6 mice were mounted in modified snap-well chambers and electrical resistance measured every 30 minutes. Two pieces of tissue were analyzed / mouse and 5 mice / group were analyzed. Data are presented as means and S.D. * p<0.05 by student t-test. B. Electrical resistance was measured as in A for infected and uninfected wild-type and TNFα deficient B6X129 F2 mice. Three pieces of tissue were analyzed / mouse and 4 mice / group were analyzed. Data shown are for the 90 minute measurement. Mice in B were 6 weeks of age and those in A were 12 weeks, likely explaining the lower TEER values seen in uninfected mice in B.
Acknowledgements
The authors thank Alessio Fasano for assistance establishing the snap-well assay technique and Bob Seder (NIH) for anti-TNFα antibodies. This work was supported by NIH grant AI-049565 to S.M.S. and AI/DK-049316 to T.S.D.
References
- 1.Farthing MJ. The molecular pathogenesis of giardiasis. J Pediatr Gastroenterol Nutr. 1997;24:79–88. doi: 10.1097/00005176-199701000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Faubert G. Immune response to Giardia duodenalis. Clin Microbiol Rev. 2000;13:35–54. doi: 10.1128/cmr.13.1.35-54.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckmann L. Mucosal defences against Giardia. Parasite Immunol. 2003;25:259–270. doi: 10.1046/j.1365-3024.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 4.Muller N, von Allmen N. Recent insights into the mucosal reactions associated with Giardia lamblia infections. Int J Parasitol. 2005;35:1339–1347. doi: 10.1016/j.ijpara.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Snider DP, Gordon J, McDermott MR, Underdown BJ. Chronic Giardia muris infection in anti-IgM-treated mice. I. Analysis of immunoglobulin and parasite-specific antibody in normal and immunoglobulin-deficient animals. J Immunol. 1985;134:4153–4162. [PubMed] [Google Scholar]
- 6.Snider DP, Skea D, Underdown BJ. Chronic giardiasis in B-cell-deficient mice expressing the xid gene. Infect Immun. 1988;56:2838–2842. doi: 10.1128/iai.56.11.2838-2842.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langford TD, Housley MP, Boes M, et al. Central importance of immunoglobulin A in host defense against Giardia spp. Infect Immun. 2002;70:11–18. doi: 10.1128/IAI.70.1.11-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davids BJ, Palm JE, Housley MP, et al. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J Immunol. 2006;177:6281–6290. doi: 10.4049/jimmunol.177.9.6281. [DOI] [PubMed] [Google Scholar]
- 9.Byrd LG, Conrad JT, Nash TE. Giardia lamblia infections in adult mice. Infect Immun. 1994;62:3583–3585. doi: 10.1128/iai.62.8.3583-3585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer SM, Nash TE. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect Immun. 2000;68:170–175. doi: 10.1128/iai.68.1.170-175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou P, Li E, Zhu N, Robertson J, Nash T, Singer SM. Role of interleukin-6 in the control of acute and chronic Giardia lamblia infections in mice. Infect Immun. 2003;71:1566–1568. doi: 10.1128/IAI.71.3.1566-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bienz M, Dai WJ, Welle M, Gottstein B, Muller N. Interleukin-6-deficient mice are highly susceptible to Giardia lamblia infection but exhibit normal intestinal immunoglobulin A responses against the parasite. Infect Immun. 2003;71:1569–1573. doi: 10.1128/IAI.71.3.1569-1573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li E, Zhou P, Petrin Z, Singer SM. Mast Cell-Dependent Control of Giardia lamblia Infections in Mice. Infect Immun. 2004;72:6642–6649. doi: 10.1128/IAI.72.11.6642-6649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li E, Zhou P, Singer SM. Neuronal Nitric Oxide Synthase Is Necessary for Elimination of Giardia lamblia Infections in Mice. J Immunol. 2006;176:516–521. doi: 10.4049/jimmunol.176.1.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou P, Miller G, Seder RA. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-alpha plays a critical role in maintaining secondary immunity in the absence of IFN-gamma. J Immunol. 1998;160:1359–1368. [PubMed] [Google Scholar]
- 16.Singer SM, Nash TE. The Role of Normal Flora in Giardia lamblia Infections in Mice. J Infect Dis. 2000;181:1510–1512. doi: 10.1086/315409. [DOI] [PubMed] [Google Scholar]
- 17.El Asmar R, Panigrahi P, Bamford P, et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 18.Roberts-Thomson IC. Genetic studies of human and murine giardiasis. Clin Infect Dis. 1993;16(Suppl 2):S98–104. doi: 10.1093/clinids/16.supplement_2.s98. [DOI] [PubMed] [Google Scholar]
- 19.Wright HV, Bailey D, Kashyap M, et al. IL-3-mediated TNF production is necessary for mast cell development. J Immunol. 2006;176:2114–2121. doi: 10.4049/jimmunol.176.4.2114. [DOI] [PubMed] [Google Scholar]
- 20.Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology. 2002;123:1179–1190. doi: 10.1053/gast.2002.36002. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39:153–157. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Troeger H, Epple HJ, Schneider T, et al. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut. 2006 doi: 10.1136/gut.2006.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson PR. Increased gut permeability in Crohn's disease: is TNF the link? Gut. 2004;53:1724–1725. doi: 10.1136/gut.2004.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 25.Horwood NJ, Page TH, McDaid JP, et al. Bruton's tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J Immunol. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 26.Salzer U, Chapel HM, Webster AD, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 27.Castigli E, Wilson SA, Garibyan L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 28.von Allmen N, Christen S, Forster U, Gottstein B, Welle M, Muller N. Acute trichinellosis increases susceptibility to Giardia lamblia infection in the mouse model. Parasitology. 2006;133:139–149. doi: 10.1017/S0031182006000230. [DOI] [PubMed] [Google Scholar]
- 29.Oberhuber G, Kastner N, Stolte M. Giardiasis: a histologic analysis of 567 cases. Scand J Gastroenterol. 1997;32:48–51. doi: 10.3109/00365529709025062. [DOI] [PubMed] [Google Scholar]
- 30.Roxstrom-Lindquist K, Palm D, Reiner D, Ringqvist E, Svard SG. Giardia immunity-an update. Trends Parasitol. 2006;22:26–31. doi: 10.1016/j.pt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005;206:132–148. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 32.Teoh DA, Kamieniecki D, Pang G, Buret AG. Giardia lamblia rearranges F-actin and alpha-actinin in human colonic and duodenal monolayers and reduces transepithelial electrical resistance. J Parasitol. 2000;86:800–806. doi: 10.1645/0022-3395(2000)086[0800:GLRFAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Chin AC, Teoh DA, Scott KG, Meddings JB, Macnaughton WK, Buret AG. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect Immun. 2002;70:3673–3680. doi: 10.1128/IAI.70.7.3673-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen YS, Gillin FD, Eckmann L. Adaptive immunity-dependent intestinal hypermotility contributes to host defense against Giardia spp. Infect Immun. 2006;74:2473–2476. doi: 10.1128/IAI.74.4.2473-2476.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]



