Abstract
This review summarizes the evidence for a detrimental role of nitric oxide (NO) derived from inducible NO synthase (iNOS) and/or reactive nitrogen species such as peroxynitrite in acutely-rejecting cardiac transplants. In chronic cardiac transplant rejection, iNOS may have an opposing beneficial component. The purpose of this review is primarily to address issues related to acute rejection which is a recognized risk factor for chronic rejection. The evidence for a detrimental role is based upon strategies involving non-selective NOS inhibitors, NO neutralizers, selective iNOS inhibitors and iNOS gene deletion in rodent models of cardiac rejection. The review is discussed in the context of the impact on various components including graft survival, histological rejection and cardiac function which may contribute in toto to the process of graft rejection. Possible limitations of each strategy are discussed in order to understand better the variance in published findings including issues related to the potential importance of cell localization of iNOS expression. Finally, the concept of a dual role of NO and its down-stream product, peroxynitrite, in rejection vs. immune regulation is discussed.
Introduction
The first human heart transplantation was performed in 1967 but it awaited the introduction of cyclosporine immunotherapy in the early 1980’s before success was routine leading to acceptable levels of graft survival and quality of life for the recipient. Despite these advances, acute rejection in cardiac transplant recipients continues to be a leading factor in first year mortality and morbidity following transplantation [1]. Acute rejection has been shown to account to as much as 30% of all deaths after transplantation according to the registry of the International Society for Heart and Lung Transplantation [2]. Many clinical trials to improve outcomes use the incidence of allograft rejection episodes as an endpoint in order to develop newer strategies to improve clinical outcomes.
Clinical suspicion of acute rejection is confirmed by evidence derived histologically upon endomyocardial biopsy which is the current ‘gold standard’. Rejection may be associated with functional problems such as contractile dysfunction as determined by echocardiography or other diagnostic procedures. The management of rejection varies depending on the severity of rejection, frequency of episodes, and evidence of associated compromise in hemodynamic performance. The therapeutic strategies include the concomitant use of steroids and antibody treatment such as OKT3 [1]. Amelioration of left ventricular dysfunction can also be compensated by inotropic therapy but this strategy does not resolve the underlying pathology of rejection.
Histological rejection is usually manifested by inflammatory cell infiltration along with the possibility of associated myocardial injury including parenchymal cell necrosis and apoptosis [3,4]. In the case of cardiac transplantation, the specific loss of cardiac myocytes due to apoptosis can be a significant determinant of cardiac graft function due to fact that the mature cardiac myocyte has a poor proliferative capacity to replace the loss of cells via apoptosis.
Despite the use of immunosuppression therapy, recurrent episodes of acute rejection can persist and in some cases tolerance to drugs such as cyclosporine can ensue making the recurrence of acute rejection more likely. This recurrence is of concern as there is growing evidence that there is a significant cumulative impact of acute rejection on the development of cardiac allograft vasculopathy [5]. The latter remains one of the most common complications that limits long-term graft survival.
Acute allograft rejection is a complex and incompletely understood process. The complexity arises from the interaction of several cell types as well as a variety of mediators of inflammation. This complexity makes it difficult to isolate with precision all of the factors and cell-cell interactions that regulate graft rejection. In the simplest context, antigen-presenting cells interact with macrophage and T-lymphocytes in the initial stages of organ rejection [6,7]. CD4+ lymphocytes stimulated by allo-immune activation release inflammatory cytokines such as interleukin-2 (IL-2), interferon-γ (IFN-γ), IL-4 and other mediators. The synthesis of IL-2 is a pivotal event which serves to stimulate CD8+ lymphocytes to produce IFN-γ. The cytokine, IFN-γ, in turn, activates macrophage cells to produce IL-1, IL-6, TGF-β, TNF-α and nitric oxide (NO). The latter is produced following up-regulation of inducible NO synthase (iNOS) which occurs initially predominately in macrophage cell infiltrates and later within graft parenchymal cells [8].
NO arising from iNOS, is believed to play a significant detrimental role in a variety of allo-transplanted cells or tissues including lung [9,10], kidney [11], pancreatic islets [12,13], cornea [14], and aorta [15]. There are disparate findings regarding the role of iNOS in solid organ transplant rejection, in general [see below]. Clearly, iNOS may not always play a detrimental role in rejection of all types of organ transplants. So caution is advised when extrapolating findings from one form of transplant rejection to another.
To this end, this review focuses on the role of iNOS in acute cardiac transplant rejection where much of the research has evolved. It is hoped that the reader appreciates that the issue is far more complex and that the lack of experimental data in several key areas limits a complete understanding of its role.
The importance of other NOS isoforms in acute cardiac rejection has received much less attention as has the interaction between individual NOS isoforms. Certainly, there is sufficient evidence showing coronary endothelial dysfunction in experimental heart rejection in porcine [16] and canine [17] models of rejection. This dysfunction is assumed to be via defects in endothelial NOS (eNOS). In this context, eNOS (NOS3) immunoreactivity was shown to slowly decrease with time in human heart transplantation [18]. Interestingly in humans the dysfunction in endothelium-dependent relaxations was found only in patients with high TNFα levels and with higher iNOS mRNA expression [19,20] suggesting a linking between iNOS up-regulation and defects in eNOS-dependent function.
In experimental cardiac transplantation, there was no difference in time to rejection in cardiac allografts between eNOS knockout and wild-type mice [21]. This would tend to arguer that eNOS does not play a role in acute cardiac rejection. There have been studies to examine the role of eNOS over-expression in graft rejection. In one of the first studies, coronary flow and contractility were found to be unchanged by over-expression of eNOS by adenoviral transduction [22]. This finding raises doubts about the protective role of eNOS at least in regard to hemodynamic function. However, the study did not address issues of allo-immune rejection since both donor and recipient animals were of the same inbred rat strain. A subsequent study conducted in rabbit allogeneic cardiac transplant model showed that liposome-mediated transfer of eNOS gene had a beneficial effect to increase graft survival time and to decrease by halve the amount of neutrophil and T-lymphocyte infiltration into the cardiac graft [23]. This finding would seem to conflict with findings in lung allograft rejection in which adenoviral-mediated gene transfer did not alter histological rejection [24]. So the issue of whether the eNOS isoform is protective in acute cardiac rejection remains an open question. No data exist to the authors’ knowledge on the possible role of nNOS in acute cardiac rejection.
Opposing role of iNOS in acute vs. chronic cardiac transplant rejection
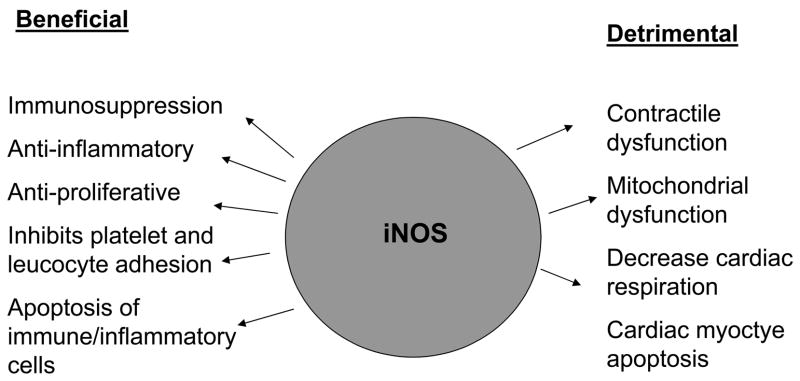
This review examines the evidence supporting the detrimental role of iNOS in acute cardiac rejection. It should be recognized; however, that iNOS appears to play an opposing beneficial role in chronic rejection (see Figure 1) as has been shown by genetic deletion of iNOS in models for aorta [25], heart [26,27] and kidney [28]. In this case, iNOS functions as an anti-inflammatory effector molecule to limit chronic transplant-induced atherosclerosis and vasculopathy. The mechanism of this protective action has been explained by the ability of NO to limit adhesion of platelets and leukocytes to vascular endothelium and to induce apoptosis of macrophages and proliferating vascular smooth muscle cells [29]. In contrast to these studies in a model of chronic airway rejection, the absence of iNOS in cyclosporine-treated mice receiving tracheal transplants has been shown to reduce luminal obliteration and rejection [30]. Thus, again caution is advised in extrapolating the role of iNOS in chronic rejection of a specific type of solid organ transplantation to another type of organ transplantation.
Figure 1.
The opposing roles of iNOS in cardiac graft rejection.
Traditionally, investigators have relied on different endpoints including graft survival, histological rejection scoring and in some cases graft function in experimental transplant rejection models. The first two endpoints are the most commonly used in evaluating experimental transplant rejection. In order to better understand the complex role of NO, it is important to understand that these endpoints may reflect different aspects of the overall process of graft rejection. Thus, one must consider that there are potentially several levels by which iNOS expression and/or elevations of NO could regulate the process of cardiac rejection.
The issue of chronic rejection is not a focus of the present review owing to other underlying etiologies and the outstanding uncertainties about the additional complication of the direct effects of immunosuppressant therapy on vasculopathy. The importance of acute rejection is that it is recognized as a major risk factor for chronic graft failure and rejection [31,32].
Role of iNOS on myocardial graft function in humans
Clinically, the role of NO arose from early studies in human cardiac transplantation in which a positive correlation was shown between iNOS expression and left ventricular contractile dysfunction measured by echocardiography and Doppler techniques [33]. An association between left ventricular dysfunction and iNOS expression was confirmed in another human study one year later [34]. Paradoxically, it was shown that iNOS expression was not related to histological rejection based on standardized International Society for Heart and Lung Transplantation (ISHLT) scoring criteria. This dissociation between functional endpoints and histological rejection is unclear but not unprecedented. However, these findings illustrate the limitations of relying on a sole endpoint parameter when evaluating the overall phenomenon of effects of NO on acute rejection/graft dysfunction in cardiac transplants.
Limitations
In the clinical setting, the intrinsic difficulty to directly determine cause-effect relationships limits precise determination of the role of iNOS in acute rejection. Overlaying this limitation are the potential problems inherent in evaluation of patients who receive changing modalities of different regimens of immunosuppressant therapy. So, the basic understanding has naturally arisen from a variety of models using experimental laboratory animals.
Evidence using broad-spectrum NOS inhibitors
Early, and more recent, studies yielded inconsistent findings utilizing non-specific, substrate-based, NOS inhibitors, including L-NG-monomethylarginine (L-NMMA) or L-nitroarginine methyl ester (L-NAME) (summarized in Table 1). Treatment of recipients with these agents either prolonged graft survival [35,36], had no effect on graft survival [37,38] or decreased graft survival [39]. None of these studies provided information on either graft function prior to graft failure or independent evaluation of histological rejection scores. The reason for the disparity in findings on graft survival among these reports likely relates to the use of various NOS inhibitors that lack specificity for iNOS. Indeed, doses of these agents used may have been in the range that inhibits the constitutive NOS (cNOS) activity; therefore, counteracting any benefits arising from inhibition of iNOS activity. In this context, the study showing decreased graft survival following treatment with L-NAME identified a hypertensive side-effect of the L-NAME regimen [39]. Antagonizing the hypertensive action of L-NAME with concomitant treatment with the angiotensin converting enzyme inhibitor, cilazapril, resulted in significantly improved graft survival times [39].
Table 1.
Summary of effects of NO modulation on acute cardiac allograft survival and rejection
| Source | Agent | Graft Survival | Histological Rejection |
|---|---|---|---|
| Non-selective NOS inhibitors | |||
| Winlaw (1995) | L-NMMA | ⬆ | ➡ |
| Menon (1998) | L-NMMA | ⬆ | ➡ |
| Bastian (1994) | L-NMMA | ➡ | n.d. |
| Paul (1996) | L-NAME | ⬇ | |
| Yamamoto (2007) | L-NMMA | ➡ | n.d. |
| NO neutralizers | |||
| Cooper (1998) | PDTC | ⬆ | ➡ |
| Pieper (2002) | DETC-Fe | ⬆ | ⬇ |
| Roza (2000) | NOX-100 | ⬆ | ➡ |
| Pieper (2001) | ⬆ | ||
| Pieper (2004) | NOX-700 | ⬆ | ⬇ |
| Pieper (2002) | AMD6221 | ⬆ | ⬇ |
| iNOS inhibitors | |||
| Worrall (1995) | Aminoguanidine | ⬆ | ⬇ |
| Takahashi (2000) | Aminoguanidine | ⬆ | ⬇ |
| Szabolcs (2002) | BBS-1 | ⬆ | ⬇ |
| BBS-2 | ⬆ | ➡ | |
| Pieper (2004) | L-NIL | ⬆ ➡ | ⬇ |
| Pterin-based NOS inhibitor | |||
| Brandacher (2001) | 4-aminobiopterin | ⬆ | ⬇ |
| Brandacher (2006) | 4-aminobiopterin | ⬆ | ⬇ |
| iNOS knockout | |||
| Koglin (1999) | INOS−/− recipients | n.d. | ⬇ |
| Mannon (1999) | iNOS−/− recipients | ➡ | ➡ |
| iNOS−/− donors | ➡ | ➡ | |
| Lee (2000) | iNOS−/− donors | ⬇ | n.d. |
| Szabolcs (2001) | iNOS−/− recipients | ➡ | ➡ |
| iNOS−/− recipients | ➡ | ➡ | |
| iNOS−/− donors and recipients | ⬆ | ⬇ | |
DETC-Fe, diethyldithiocarbamate-iron complex; L-NAME, L-nitroarginine methyl ester; L-NIL, N6-(1-iminoethyl)-L-lysine; L-NMMA; L-NG-monomethylarginine; PDTC, pyrrolidine dithiocarbamate; ⬆ increased; ⬇ decreased; ➡ unchanged; n.d., not determined
n.d. (not determined)
Limitations
These original studies hinted at a potentially negative role of iNOS in acute cardiac allograft rejection. The limitation of the use of these agents is that these compounds do not adequately distinguish individual isoforms of NOS but at the time of most of these studies more selective inhibitors of iNOS were not generally available for wide-spread experimental use. Thus, these compounds may have untoward side-effects which may counteract any benefits derived by inhibition of NO derived from iNOS.
Evidence using NO neutralizing agents
An alternative strategy to evaluate a role of NO arises from the ability of certain compounds to scavenge and neutralize detrimental effects of elevated NO levels due to up-regulation of iNOS. These agents take advantage of the avid binding characteristics of metal complexes. Among the most characterized are NO complexes with iron [40–42], cobalt [43–45] and ruthenium [46,47] derivatives. At the time that other laboratories were evaluating the effect of broad-spectrum NOS inhibitors, early work in our laboratory took into consideration the principle that dithiocarbamate derivatives bind iron and this complex serves to bind NO.
In the first experiment, pyrrolidine dithiocarbamate, an iron-chelator, prolonged grafts survival and inhibited down-stream effects on nitrosylation of myocardial heme protein [48,49]. While this agent inhibited NF-κB binding activity, it was not determined whether the decreased heme protein nitrosylation was due secondarily to NO scavenging or to decreased iNOS protein expression. The benefits on graft survival appeared to be independent on gross changes in inflammatory cell infiltration into the graft suggesting that this intervention did not significantly impact histological rejection but rather some undefined process involved in overall graft survival [48].
These studies were followed by evaluation of two related compounds, NOX-100 and NOX-700 developed by Medinox, Inc., San Diego, CA. These compounds were shown to be able to scavenge NO in vivo and to significantly improve graft survival [50–52]. In combination with low-dose cyclosporine, both agents dramatically improved graft survival beyond that achieved by either agent used alone. In addition, in a long-term combination treatment strategy, re-transplantation of a 2nd donor heart several weeks after cessation of treatment resulted in permanent graft acceptance [50]. This finding suggests latent drug-induced effects of allo-immune activation. We had assumed that the action related to a de-sensitization of lymphocytes to initiate allo-immune responses. This property was delineated in follow-up experiments using NOX-700 in which treatment results in refractoriness of lymphocytes to allo-immune activation including lymphocyte proliferation and blunted inflammatory cytokine release indicating a novel action [52].
An alternative strategy to that of iron-dithiocarbamate molecules was to evaluate metal-based NO scavengers of different chemical structures. We chose AMD6221, a ruthenium (III) polyaminocarboxylate complex [47]. This compound was shown in our studies to inhibit the increased plasma levels of NO metabolites [53]. Furthermore, scavenging of NO by the ruthenium-based compound in vivo was confirmed by HPLC analysis of the nitrosylated ruthenium complex species [53]. Treatment also prolonged cardiac allograft survival, inhibited histological rejection and inhibited down-stream effects on heme protein nitrosylation. In agreement with the previous studies using dithiocarbamate-based NO scavengers, AMD6211 also potentiated graft survival when used in combination with low-dose cyclosporine.
Limitations
A limitation of assessing a role of iNOS using iron-dithiocarbamate is that these compounds are NO scavengers which may also possess other properties. In this context, findings using the ruthenium-based compound with different chemical structure than the iron-dithiocarbamate class of compounds indirectly suggests that NO scavenging likely plays a significant role in protecting from acute rejection. However, this alternative approach still does not give information what portion of the action or iron-dithiocarbamate compounds were related to NO scavenging vs. what portion might have arisen from NO-independent properties of the compounds. Collectively, both classes of agents decrease bulk NO levels in vivo. Thus, these agents have in common that they do not differentiate the NO produced by the iNOS isoform vs. that produced by other NOS isoforms. This caveat is offset by the higher solubility of the compounds in the aqueous phase that leave sufficient residual levels of NO intact which essentially acts to preserve constitutive NO levels. While these agents are effective in protection from acute rejection, they can only be regarded as giving indirect evidence supporting a role of iNOS in acute rejection.
Evidence using semi-selective iNOS inhibitors
Aminoguanidine is a hydrazine-based NOS inhibitor that has a higher iNOS to cNOS selectivity [54]. Aminoguanidine prolonged cardiac allograft survival [55,56]. This was associated with decreased histological rejection [55,56] and specifically a decreased infiltration of CD4+ and CD8+ T-lymphocytes. Treatment with aminoguanidine was also associated with improved contractile function ex vivo based on length-tension measurements in isolated papillary muscles [55] or by improved left ventricular pressure-volume curves generated in isolated, perfused heart grafts [57]. Collectively, these studies provided more consistent evidence than that arising from data derived from broad spectrum NOS inhibitors that iNOS is implicated in acute cardiac rejection.
Limitations
A limitation of using aminoguanidine arises from the fact that this compound exhibits other properties. Under in vitro conditions aminoguanidine has been shown to inhibit diamine oxidase at nM concentrations as opposed to μM concentrations for iNOS inhibition [58]. Aminoguanidine also contains antioxidant properties and can inhibit aldose reductase [59]. As aldose reductase is up-regulated by NO [60], it is possible that this enzyme may also be induced under conditions of iNOS induction. Despite these alternative actions, there are no independent data available to determine whether aminoguanidine acts via these alternative mechanisms in models of acute cardiac rejection. Nevertheless, the importance of the latter property is relevant by the fact that sorbinil, an aldose reductase inhibitor, inhibited lipopolysaccharide (LPS)-induced expression of inflammatory cytokines and iNOS in cardiac myocytes [61]. This effect may not be peculiar to aldose reductase inhibitors but perhaps relative to the presence or absence of aldose reductase activity based on new studies using siRNA for aldose reductase. These studies show a diminished NO production and iNOS expression in LPS-treated macrophages [62].
Evidence using highly-selective, iNOS inhibitors
Other more selective, substrate-based, imidazole-like, iNOS inhibitors have been developed by Berlex Biosciences. These compounds act to limit NO production by iNOS by allosterically inhibiting iNOS subunit dimerization via a pterin- and L-arginine-independent mechanism [63]. Szabolcs et al. [64] using two pyrimidylimidazole-based iNOS dimerization inhibitors (BBS-1 and BBS-2) showed significant prolongation of graft survival. Interestingly, while both inhibitors decreased histological rejections scores and decreased infiltration of inflammatory T cells and macrophages, the effect on reduction of histological rejection was only statistically significant for BBS-2 and not BBS-1. Both inhibitors appeared to be equi-effective in decreasing apoptosis.
An interesting finding is that the expression of iNOS protein was also decreased by this treatment strategy. The significance of this finding is unclear considering the small sample size evaluated in this study. However, similar findings arise from reports in other cell types in culture that these iNOS inhibitors also decrease iNOS protein expression [65]. So, these iNOS inhibitors may have additional properties under certain conditions to also decrease iNOS expression.
Other studies conducted in our laboratory using the iNOS inhibitor, N6-(1-iminoethyl)-L-lysine (L-NIL) show that this agent improves graft survival and histological rejection scores [66]. Interestingly, graft survival per se was not prolonged at higher doses despite the finding that the same dose did improve rejection scores. This finding underscores the idea that rejection scores and graft survival times may have different etiologies. The improvement in rejection scores following treatment with L-NIL occurred without any change on iNOS expression which distinguishes it from the actions above of the iNOS inhibitors, BBS-1 and BBS-2.
Evidence using pterin-based iNOS inhibitor
Alternative to the iNOS inhibitors above, a pterin derivative, 4-aminobiopterin, which binds to the pterin domain of iNOS has been developed. This analog inhibits with equivalent efficacy the NO production by individual purified NOS isoforms but shows selectivity for inhibiting the iNOS isoform in cells [67]. Treatment of mouse cardiac allograft recipients with 4-aminobiopterin decreased histological rejection from ISHLT grade 4 in untreated allografts to scores between ISHLT grade 1B and grade 3B [68,69]. Graft survival was also significantly prolonged by treatment of recipient animals with 4-aminobiopterin. These findings were especially interesting in that the degree of prolongation of graft survival was equivalent to that achieved by a high dose of cyclosporine. This occurred despite the finding that this intervention unlike cyclosporine did not alter expression of inflammatory cytokines suggesting a novel mechanism of its protective action. Other studies show that this 4-amino derivative down-regulated T-cell activation by dendritic cells via an iNOS-independent pathway [70]. Thus it is possible that the action in vivo on cardiac graft survival might also be related, at least in part, to an iNOS-independent pathway.
Limitations
The use of selective iNOS inhibitors (substrate-based or pterin-based) provides some of the best evidence to date that iNOS plays a detrimental role in acute cardiac rejection. However, each of the class of selective iNOS inhibitors may have caveats owing to different additional mechanisms of action in vivo. As with all NOS inhibitor studies, such interventions do not discriminate the source of iNOS. The eventual development of cell-specific inhibitors of iNOS could be an advantage owing to the finding that iNOS may arise from within the graft and from infiltrating immune cells from the recipient (see next section below). This latter finding makes some findings using iNOS deletion strategies subject to other possible interpretations.
Evidence using gene deletion strategies
The role of iNOS in cardiac allograft rejection has also been evaluated by utilizing the strengths of gene deletion strategies. Similar to the pharmacological approaches, this strategy has also revealed contradictory information including either increasing graft survival, decreasing graft survival or having no effect as detailed below.
In the first study, transplantation of donor hearts into iNOS−/− and iNOS+/+ recipient mice indicated that the deletion of iNOS gene decreased histological rejection although no information on graft survival per se was evaluated [71]. This was accompanied by a reduction in apoptosis as evaluated by a variety of end-points which pointed to apoptosis as a key factor in iNOS-derived acute cardiac allograft rejection. In the same year, Mannon et al. [72] found no difference in either histological rejection or cardiac allograft survival time between iNOS−/− and wild-type mice. In contrast, another group found decreased graft survival times in iNOS−/− donor hearts vs. wild-type donor hearts in mice [21].
Theoretically, the differences in the findings above may arise from the specific design of the study specifically whether the iNOS−/− animal was used as the donor or the recipient. In the first study [71], the experimental design tested the effects of iNOS on the recipient immune response by varying gene expression in the recipient rather than in the donor heart. This is important considering that iNOS is derived from both infiltrating inflammatory cells of the recipient as well as iNOS arising from parenchymal cells of the donor heart. The significance of this distinction in cellular localization will be elaborated upon later in this review.
In the 2nd study [72], these authors also first tested the effects of iNOS on the recipient immune response and found no statistical difference in either graft rejection time or histological rejection between donor hearts transplanted into iNOS−/− and iNOS+/+ recipient mice, respectively. The only apparent difference between the studies is the choice of mice strains.
To evaluate the role of iNOS expression in the donor, the 2nd study also evaluated the actions of transplantation of iNOS−/− and iNOS+/+ donor hearts into the same allogenic recipients. Here again, there was no difference between graft survival time and rejections scores between the two groups.
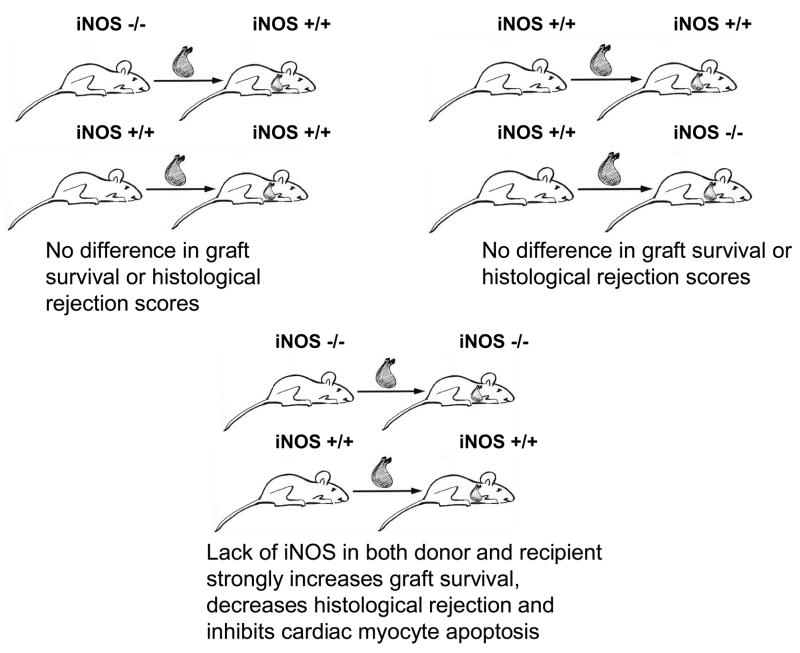
In a 3rd more recent study (illustrated in Figure 2), Szabolcs et al. [73] showed in an initial strategy that there was no difference in graft survival time or ISHLT rejection scores for donor hearts transplanted into iNOS−/− and iNOS+/+ recipient mice. Similar findings were obtained in a second strategy if iNOS−/− and iNOS+/+ donor hearts were transplanted into the same iNOS+/+ strain allograft recipients. Finally in a third strategy, elimination of iNOS in both donor and recipient mice strongly augmented graft survival, decreased ISHLT rejections scores and inhibited apoptosis in cardiac myocytes. This report was enlightening as it demonstrated that elimination of iNOS in either the donor or recipient did not prevent iNOS up-regulation in the graft owing to iNOS arising from both inflammatory cell infiltrate due to the immune response of the recipient and to iNOS in the parenchymal cells of the donor organ.
Figure 2.
Illustration showing that iNOS derived from the donor and recipient influence cardiac graft survival and acute histological rejection. Upper left illustrates the effects of iNOS deletion from donor sources. Upper right illustrates the effects of iNOS deletion from recipient sources. Lower panel illustrates the effects of iNOS deletion in both donor and recipients. Conclusions are a summary of the findings by Szabolcs et al. [2001] using various surgical strategies.
Implications of gene deletion studies
There are significant implications for the findings in this latter study. The iNOS deletion strategy in the study above illustrated nicely that both donor and recipient sources of iNOS may contribute to the process of cardiac allograft rejection and that the divergent findings to date in the gene deletion strategy must be understood in the context of the individual experimental designs. In the studies using selective iNOS inhibitors, the pharmacological strategy would not be impacted by the source of iNOS. Collectively, it is more clear from these studies that overall iNOS does, in fact, play a significant detrimental role in acute cardiac allograft rejection.
Limitations
As explained in a latter section, NO may have biphasic actions in biological systems both beneficial and detrimental which may be related, in part, to its other action as an immunosuppressant molecule. This factor should be considered in individual cases using iNOS deletion strategy since ablation eliminates the beneficial effects of NO and this factor could come into play for donor and recipient deletion strategies when assessing chronic cardiac allograft rejection.
A potential complication is that iNOS gene deletion can up-regulate inflammatory cytokine production in some models. This action could counteract any benefits to preventing the damaging activities of high NO production via iNOS. For example TNF-α gene expression is up-regulated in skin grafts of iNOS knockout vs. wild-type mice [74]. Assessment of lymphocyte proliferation revealed significant up-regulation of IFN-γ by iNOS−/− vs. wild type lymphocytes [75]. This finding could potentially explain the lack of change in skin allograft rejection as a consequence of iNOS gene deletion strategies. However, skin grafts are tissue that are most susceptible to rejection and differ from other organ grafts in that they often are least likely to respond to immunosuppression therapy.
In cardiac transplant models, the current information is incomplete. Deletion of iNOS in recipients apparently has no effect on mRNA for TNFα [71] or other cytokines [72]. However, the effects of iNOS depletion on donors or donors plus recipients on inflammatory cytokine expression in cardiac transplant rejection models are not known.
Finally, another potential limitation in the use of iNOS knockout strategies is the possibility that the findings may be masked by compensation for the loss of a given NOS isoform by increased expression of other NOS isoforms. This is phenomenon is well-established, for example, by several studies in other models showing that the deletion of eNOS results in increased expression of nNOS isoform [76–79]. It is less clear; however, whether this isoform compensation occurs in the case of iNOS deletion strategies. Unfortunately, studies using iNOS deletion strategies in the transplant research area and more specifically for heart have not addressed this issue.
NO interaction with metalloproteins in cardiac rejection
Early studies established the biological reaction of NO with metal-containing proteins, notably, iron-containing proteins [see reviews, 80–82]. Exploration of this potential interaction in acute cardiac rejection has been extremely limited. Lancaster and colleagues [83] were the first to report iron-nitrosylation of both heme- and non-heme-containing complexes coincidental with rejection using low-temperature (i.e. 77°K), electron paramagnetic resonance (EPR) spectroscopy of frozen samples in rat cardiac allografts. Signals in grafts were attributed to nitrosylation of the heme protein, myoglobin, and to non-heme dinitrosyl complexes of iron-sulfur cluster proteins. Heme nitrosylation was also seen in packed red blood cells taken from allograft recipients that were attributed to nitrosylated hemoglobin. The EPR signals in red blood cells and grafts were attributable to immune activation as they were absent in cardiac isografts and blocked by treatment of allograft recipients with FK506. Similar conclusions were obtained using cyclosporine [50].
Two other individual laboratories showed that heme- and non-heme-containing iron-nitrosylated EPR signals were eliminated by using L-NMMA [37] or aminoguanidine [55]. This suggested that the EPR signals detected in cardiac allografts arise from a NOS-dependent nitrosylation process. Subsequently, we showed that the nitrosylated heme EPR signal was significantly decreased using a selective iNOS inhibitor, L-NIL, confirming a role of enzymatically-derived NO from iNOS in this nitrosylated EPR signal [84]. However, a small fraction of nitrosylated EPR signal in cardiac allografts remained after L-NIL treatment. Studies in anoxic or ischemic myocardium have revealed that NO might also be formed via NOS-independent pathways such as a xanthine oxidase catalyzed reduction of endogenous nitrate or nitrite [85,86]. We found that a combined treatment of allograft recipients with L-NIL plus allopurinol completely inhibited the remaining L-NIL-resistant component of mononitrosyl iron complexes [84].
This suggested that only a small, but measureable, component of heme-nitrosylation occurs via a NOS-independent, xanthine oxidase-catalyzed NO formation pathway.
Limitations
A variety of agents that inhibit graft rejection are usually found to significantly decrease or prevent nitrosylation of heme protein in acute cardiac rejection. The question was proposed earlier [83] whether formation of these modified heme proteins causes some functional change that contributes to the process of rejection or whether it could be a useful marker to monitor NO-mediated graft rejection. Thus the precise functional consequences of heme nitrosylation on graft function or rejection remain unclear.
Regarding non-heme iron nitrosylation, it is believed that this represents a cytotoxic effect of NO in target cells [87]. It was found that the formation of the dinitrosyl, non-heme iron EPR signal at g =2.04 and g =2.015 was blocked by either cyclosporine or the iNOS inhibitor, L-NIL [88]. This implied that formation of these other nitrosylated EPR complexes arises from an iNOS-dependent pathway. Subsequent measurements of aconitase enzymatic activity, an important non-heme, Fe-S cluster protein in heart mitochondria revealed this as a potential iron-containing, protein target of NO [88]. Here again, the inhibition of aconitase enzymatic activity in acute cardiac allograft rejection was prevented by both cyclosporine or L-NIL treatments. This inactivation preceeded the nitration of MnSOD reported elsewhere [89]. Since most all of the aconitase activity in heart is mitochondrial in origin, it is intriguing to speculate that mitochondrial aconitase inactivation could provide, at least in part, a source of reactive oxygen for mitochondrial protein nitration.
iNOS and negative chronotropism
In laboratory animals, cardiac allograft rejection is frequently associated with severe bradycardia and impaired contractile activity. In several cases, contractile dysfunction may not directly parallel histological rejection suggesting that different mechanisms contribute to the total process of graft survival. In addition, it has not been precisely delineated what proportion of graft dysfunction is due to concurrent reversible effects of NO derived from iNOS vs. the dysfunction related to irreversible pathology via iNOS-dependent and iNOS-independent etiologies.
The issue of bradycardia also is important in the clinical condition in cardiac transplant recipients. Sinus bradycardia may occur early after cardiac transplantation and contributes to a higher risk of mortality. Clinically, pacemaker implantation or theophylline are two strategies used to manage bradycardia post-transplantation [90]. Some studies suggest that adenosine plays a role in the pathogenesis of sinus bradycardia following transplantation. The role of NO and, specifically iNOS expression, on bradycardia following transplantation is incompletely understood.
Interestingly, NOS inhibitors can induce bradycardia while addition of NO can increase heart rate. In addition, NO can positively and negatively regulate heart rate at high and low concentrations, respectively. The effects of NO in vivo are complex and even more difficult to understand owing to the effects of central actions of NO on the autonomic nervous system and the modulatory role of NO via the parasympathetic pathway [91,92].
In this context, one must consider that the cardiac graft of human and experimental laboratory transplant recipients is denervated. One study was able to show a positive chronotropic activity of nitroprusside and a negative chronotropic activity of the NOS inhibitor, L-NMMA, in denervated human cardiac transplant recipients [93]. However, the direct role of NO derived from iNOS on heart rate is unclear and more complex owing to recent findings in our laboratory that heart rate in situ was not different at post-transplant day 4 in untreated allografts vs. isograft controls but was decreased at post-transplant day 6 despite equivalent levels of iNOS gene expression at both time periods [94].
A possible explanation of this dichotomy in heart despite equivalent iNOS expression may be related to a potential uncoupling of iNOS. Indeed, in one study conducted in a non-transplanted heart model, authors could not show any changes in either basal heart rate or fractional shortening determined by echocardiography between hearts of cardiac-specific iNOS transgenic vs. wild-type mice [95]. However, in another study using a conditional transgenic expression strategy controlled by tetracycline-responsive transcriptional activator, investigators showed prolonged PR intervals at both atrial and AV sites due to iNOS transgene compared to wild-type mice. Furthermore, this finding was associated with protein nitration, heart block and increased incidence of sudden death in iNOS over-expressing but not wild-type mice [96]. While not determined, it is intriguing to speculate that knowledge of levels of peroxynitrite may be more important than actual NO levels in determining chronotropic abnormalities in disease states such as acute cardiac allograft rejection. For detailed information regarding the inotropic, chronotropic and lusitropic actions of NO on the heart, readers are referred to an excellent review on this subject [97].
Limitations
The role of iNOS in modulating heart rate in cardiac transplantation remains to be examined. Much of the current understanding about the relationship of NO and heart rate derives from studies conducted in normal, innervated hearts. Little information is available how up-regulation of iNOS influences chronotropism in the setting of the pathology of acute transplant rejection and how this response is influenced by the additional factors of sympathetic and parasympathetic denervation to the heart following transplant surgery. Most of the primary focus has been on the role of iNOS up-regulation on inotropic responses.
Effects of NO and cytokines on contraction in isolated cardiac myocytes
Using isolated cardiac myocytes in the absence of inflammatory stimuli, it was shown that NO released from NO donor compounds decreased contractile activity [98–100]. This mechanism appeared to be via NO-induced inhibition of cardiac calcium current [101]. These studies clearly showed that NO can have a direct effect on contractile properties of cardiac myocytes. However, other factors present in allo-immune rejection of cardiac transplants such as cytokines could also play a role and this effect may or may not be mediated via NO or iNOS.
In regards to the mechanism(s) of cytokine-induced contractile dysfunction, the findings are variable and attributed to constitutive NOS, iNOS or a NOS-independent pathways. In order to discern these conflicting findings, one must consider the possibility that these divergent mechanisms may relate to duration of cytokine exposure and/or whether the dysfunction is a reversible or irreversible phenomenon.
In papillary muscle and isolated neonate cardiac myocyte preparations, TNFα and other cytokines caused contractile dysfunction within 5 min. This effect was reversed by L-NMMA [102]. Similar findings were confirmed in TNFα-stimulated neonate cardiac myocytes which was reversed by addition of L-arginine but not D-arginine [103]. In both cases, the contractile dysfunction occurred in a very short period of time. Thus, up-regulation of iNOS protein expression could not be expected to be yet increased. Accordingly, activation of the constitutive NOS activity rather than iNOS up-regulation was implicated in the acute phase of contractile dysfunction stimulated by TNFα.
Another group showed that incubation of adult feline cardiac myocytes for 18 hr with TNFα caused contractile dysfunction and reduction in Ca2+ transients albeit in the absence of any increased NO levels or cGMP generation. Furthermore, the contractile abnormalities seen in this model were not restored in the present of NOS inhibitors, L-NMMA and L-nitroarginine (L-NA) [104]. These findings were supported by similar finding in two other studies following treatment with LPS in adult guinea pig cardiac myocytes and isolated perfused rat hearts [105,106].
It is unclear what can explain why NOS inhibitors alter cytokine-mediated actions in one case and not in the other. First, one possibility may be the differences in responses of neonate vs. adult cardiac myocytes. The studies suggesting a role of NO in contractile dysfunction induced by cytokines were conducted in neonate cells. In contrast, the studies showing that NOS inhibitors did not alter contractile abnormalities in response to cytokines were conducted in adult cardiac myocyte preparations. Second, it should be noted that the studies in neonate myocytes showed the negative effects on contraction acutely (i.e. in minutes) while the studies showing contractile dysfunction in adult myocytes were conducted several hours later. Finally, another consideration is that the effects may be unique to different cytokines. In line with this hypothesis, it has been reported using the same model system that L-NMMA reversed effects on cell shortening and Ca2+ that are induced by IL-6 but not by TNF-α [107].
Limitations
Collectively, it is concluded that our understanding of the etiology of contractile impairment in isolated cardiac myocytes stimulated with cytokines remains incompletely characterized. The relative contribution of eNOS, iNOS or NOS-independent mechanisms to dysfunction at various stages after cytokine stimulation still needs further evaluation.
iNOS and contractile dysfunction in allograft-derived cardiac myocytes
A limited number of studies have examined contractile behavior of isolated cardiac myocytes or cardiac tissue taken from allograft recipients. When cardiac myocytes were isolated from adult mouse allografts at a time of mild-to-moderate rejection, basal shortening was found to be either unaltered [108] or decreased [109], respectively. In the case of the mouse cardiac myocytes, the investigators found no difference in calcium current between cardiac myocytes isolated from isografts and allografts. However, β-adrenergic challenging of cardiac myocytes revealed impaired contractile activity in myocytes from rat allografts [110] but again not in mouse allografts [111]. The differences in the findings are unknown but may simply represent species differences or differential level of rejection in each species.
A role of NO in contractile dysfunction in cardiac myocytes isolated from cardiac allografts was subsequently proven by two strategies. The findings are summarized in Table 2. First, the addition of L-arginine to bathing media decreased myocyte shortening in cells derived from both rat and mouse cardiac allografts but not did not decrease myocyte shortening in cells derived from isografts [108,111]. Second, the addition of the iNOS inhibitor, aminoguanidine, reversed the impaired contractile shortening and the decreased calcium current in rat cardiac allograft myocytes [109,111]. Collectively, despite the acknowledged caveats of use of aminoguanidine, these studies appear to show that in early rejection the contractile abnormalities due to NO derived from iNOS are still reversible. However, it is recognized that the etiology of contractile dysfunction at later stages of rejection may or may not be acutely reversible by interventions to antagonize NO production from iNOS and may arise from cumulative effects of rejection involving additional etiologies including protein nitration (see below).
Table 2.
Summary showing the effects of NO modulation on function of cardiac myocytes from allograft recipients
| Source | Result |
|---|---|
| Ex vivo function in cardiac myocytes | |
| Ziolo (2001) | normal basal ICa but ICa decreased in presence of L-arginine |
| Ziolo (1998) | decreased basal shortening; improved shortening by aminoguanidine in the cell culture |
| Ritter (2001) | normal L-type Ca2+ currents, [Ca2+]i, transient amplitude and fractional shortening and normal isoproterenol-stimulated peak [Ca2+]I; addition of L-arginine unmasked decreased cell shortening |
| Ex vivo function in isolated papillary muscles | |
| Worrall (1997) | decreased β-adrenergic- and calcium-stimulated maximum tension, (+) dP/dt and (−) dP/dt; improved by aminoguanidine treatment |
| Worrall (1994) | decreased calcium-activated increase in contractile tension; reversed by aminoguanidine treatment |
| Ex vivo function in isolated perfused grafted hearts | |
| Soto (2000) | lower left ventricular filling (left-shifted, LV pressure-LV volume curves); reversed by aminoguanidine treatment |
| In situ function | |
| Koglin (1998) | decreased graded palpation scoring of contractility in iNOS−/− recipients vs. wild-type recipients |
| Menon (1998) | impaired tension-contraction response curves; partially reversed by L-NMMA |
iNOS and contractile dysfunction ex vivo in isolated allograft tissue
Several investigators using isolated right ventricular papillary muscles derived from transplanted rodent hearts [55,112] or perfusion of intact hearts from transplant recipients show contractile dysfunction before the onset of significant myocyte necrosis. Contractile dysfunction was shown after stimulation with the β-adrenoceptor agonist, isoproterenol, or increasing calcium concentrations but not at baseline. In contrast to the cell culture studies above, this dysfunction was present despite the absence of exogenous L-arginine added to buffers.
Therefore, it is not clear whether the latent contractile defects examined may or may not be related to acute cardiac depressive activities of NO put rather due to pathology arising from chronic NO up-regulation. It was concluded that iNOS played a role, at least in part, by findings that chronic treatment with aminoguanidine inhibited the level of contractile dysfunction [55,112]. Authors also reported that iNOS was expressed in macrophage infiltrating cells but not endothelium or adjacent cardiac myocytes. Therefore, authors conclude that the contractile dysfunction was primarily mediated via immune cells rather than via a paracrine effect of iNOS up-regulation within cardiac myocytes.
Limitations
A current limitation in our knowledge of the role of iNOS in contractile dysfunction in acute rejection is that in vitro measurements of quantitative contractile function of tissue derived from allografts have not yet been determined using more highly-selective iNOS inhibitors. In addition, an assumption in the findings cited above is that contractile dysfunction in the right ventricular papillary muscle is representative, in general, of left ventricular dysfunction in the context of an intact organ. Finally, an underlying limitation to date in most studies is that the ex vivo strategies have not evaluated contractile dysfunction in the milieu of other factors present under in vivo conditions.
iNOS and contractile dysfunction in cardiac allografts in vivo
In situ or in vivo measurements of cardiac dysfunction in allografts have been determined; however, there are remarkably few studies which have specifically examined the role of NO-derived from iNOS in contractile dysfunction in vivo. In mouse heart allografts using strain-gauges, increasing the resting diastolic tension resulted in impaired contractile response curves that were partially reversed by chronic treatment with a non-selective NOS inhibitor, L-NMMA [36].
In situ measurement of allograft dysfunction has been determined by echocardiography [113] and in our laboratory using sonomicrometry. Impaired function was inhibited by chronic treatment of allograft recipients with vitamin E, a superoxide dismutase (SOD) mimetic or a peroxynitrite decomposition catalyst [114–116]. While the effect of treatment with an iNOS inhibitor had not been evaluated in the context of graft function, the results with the SOD mimetic or the peroxynitrite decomposition catalyst suggest the possibility that peroxynitrite formation via interaction of superoxide and NO may play a significant role in acute cardiac allograft rejection. This conclusion would be consistent with the known cardiac depressive activity of ONOO− given to normal hearts [117] and the reversal of cardiac dysfunction in cytokine perfused rat hearts using an SOD mimetic, L-NA or a peroxynitrite decomposition catalyst [118].
Limitations
To date there are no quantitative measurements of contractile function in vivo using techniques such as sonomicrometry or the less invasive echocardiography in allografts treated with highly-selective iNOS inhibitors. Likewise, these in vivo quantitative techniques have not been used to evaluate the role of iNOS in contractile function in acute cardiac rejection in iNOS knockout vs. wild-type mice.
Peroxynitrite and Protein Nitration in Cardiac Allograft Rejection/Graft Dysfunction
The studies above hint that NO bioactivity from iNOS rather that iNOS expression per se may be more important to our understanding of its effects in acute cardiac allograft failure. One of the possibilities to be considered is that peroxynitrite and other downstream effects of iNOS up-regulation such as protein nitration rather than NO per se may contribute to acute cardiac allograft dysfunction and rejection.
Earlier studies documented the formation of nitrotyrosine in experimental cardiac allograft rejection [119]. Likewise, in human cardiac transplantation, nitrotyrosine formation was seen in biopsies of cardiac grafts with grade III rejection scores but not in biopsies of grafts with grade 0 rejection scores suggesting that nitration of cardiac protein and rejection were possibly related [120]. Besides cardiac transplantation, nitrotyrosine has been shown in other forms of organ rejection including hepatic [121], renal [11] and corneal rejection [122]. Thus, protein nitration may be a process that is not unique to cardiac rejection.
Far fewer studies have examined the nitration of specific proteins in graft rejection. Nitration of specific proteins such as the mitochondrial proteins MnSOD or cytochrome c has been demonstrated in both renal and cardiac allograft rejection [89,123]. Collectively, nitration of these proteins may be a mechanism to signal a cascade of events leading to apoptosis. The nitration of these mitochondrial proteins is believed to be especially pivotal for cardiac allografts as these organs like brain are especially rich in the number of mitochondria. In our laboratory, the loss of MnSOD protein and activity in rejecting cardiac allografts was shown to be eliminated by treatment of recipients with the selective iNOS inhibitor, L-NIL implicating an iNOS-dependent pathway.
Limitations
Despite the findings above, there are no published data in any model of transplant rejection that has documented nitration of any specific protein including MnSOD or cytochrome c arising specifically via an iNOS-dependent pathway.
Pathways of Protein Nitration in Cardiac Rejection
It is recognized that protein nitration can occur via the action of peroxynitrite from the interaction of superoxide and NO derived from NOS. It is also acknowledged that nitrotyrosine can be formed by peroxynitrite-dependent and peroxynitrite -independent pathways. An example of the latter is proposed by myeloperoxidase catalyzed nitration in the presence of nitrite and H2O2 [124]. In this context, it is known that myeloperoxidase activity is increased in experimental and human rejecting cardiac grafts [116,125,126]. Currently, there are no direct studies which have definitely ruled in or out this latter pathway of protein nitration in acute cardiac allograft rejection, specifically, nor in organ rejection, in general.
A role of NO and allo-immune activation in protein nitration in acute cardiac rejection was first suggested by studies showing blockade of increased nitration by the NO scavenger, NOX-700 or by the immunosuppressant agent, cyclosporine [52,116]. The theory here is that the former scavenges NO while the latter inhibits iNOS up-regulation. Additional studies in our laboratory using L-NIL show that this treatment blocked the increase in total protein nitration within the graft without altering iNOS expression, thereby, implicating a role of iNOS-derived protein nitration presumably [89]. This finding agreed with previous studies showing inhibition of nitration using other selective iNOS inhibitors [64,127]. Finally, the role of iNOS in nitrotyrosine formation was firmly established in mouse heart transplants using genetic ablation of iNOS [73].
To evaluate a role of peroxynitrite in protein nitration, studies were conducted using a peroxynitrite decomposition catalyst. This strategy resulted in decreased nitration in cardiac allografts [116]. The fact that the same treatment did not alter myeloperoxidase activity suggests that myeloperoxidase-derived nitration is likely not a major pathway of nitration in acute cardiac rejection. Collectively, it is concluded from the studies above that the major pathway of protein nitration in acute cardiac rejection arises from peroxynitrite formed via an iNOS-dependent pathway.
Cell localization of nitration
Clinically, immunoreactive nitrotyrosine is seen in biopsies of cardiac grafts displaying grade III rejection but not in biopsies with grade 0 histological rejection scores suggesting that protein nitration is associated with human cardiac graft rejection [120]. In experimental cardiac allograft rejection, nitrotyrosine has been detected within infiltrating inflammatory cells and in cardiac myocytes [52,64,116,119,128].
Therapeutic interventions that are shown to decrease nitrotyrosine formation in cardiac myocytes per se have, in general, also been shown to increase graft survival or inhibit histological rejection [52,129,130]. In studies using a COX-2 inhibitor, graft survival was prolonged with weak staining for nitrotyrosine which was confined to inflammatory cells [129]. Furthermore after treatment with a selective iNOS inhibitor, staining for nitrotyrosine within cardiac myocytes was negative [64]. That nitration of cardiac grafts arises specifically from iNOS was clear in studies conducted in iNOS−/− and wild-type mice. Nitrotyrosine was present when either donor or recipient was iNOS positive; however, nitrotyrosine was absent when iNOS−/− donor hearts were transplanted into iNOS−/− recipient mice [73].
Conclusions
Emerging evidence suggests that cardiac myocytes are targeted for apoptosis in acute cardiac rejection and the pathway is related, at least in part, to protein nitration. As cardiac myocytes loose the capacity to proliferate upon maturation, it is hypothesized that the loss of these cardiac muscle cells via nitrosative stress and apoptosis may critically contribute to cardiac dysfunction and graft rejection.
NO actions on inflammatory cells in acute cardiac rejection
The role of NO in acute cardiac rejection has been considered mainly in the context of effects on cardiac muscle. Another aspect worthy of consideration is the role of NO on immune cell function in acute cardiac rejection. In this context, NO is known to have significant immunosuppressive activity to down-regulate T-lymphocyte proliferation as well as down-regulate T-lymphocyte activation [131–138]. This action potentially confounds the general understanding of the totality of the role of NO derived from iNOS on acute cardiac allograft rejection.
In addition, NO is reported to preferentially act on T cell sub-types. Early studies indicated that NO inhibited the function of Th1 cells but not Th2 cells [139,140]. This action has the effect of altering the balance of Th1 vs Th2 cytokine response favoring the anti-inflammatory Th2 type response. Indeed, one study even showed that NO could up-regulate anti-inflammatory Th2 cytokines such as IL-4 and IL-10. This shift from a Th1 to Th2 response is considered as an additional possible explanation by which NO positively regulates transplant tolerance [135]. Another mechanism is the inhibition by NO of IL-12 synthesis from macrophages. This occurs at the transcriptional level [141]. IL-12 is a major inducer for the differentiation of Th1 cells. Thus, inhibition of IL-12 short-circuits Th1 cell differentiation and subsequent amplification of IFN-γsignaling and release by Th1 cells [141].
A subsequent study by the same group revealed a bimodal action of NO on Th1 cell differentiation in CD4+ subset of Th1 cells [142]. Here the NO donor, S-nitroso-acetyl-penicillamine (SNAP), was shown to increase IFN-γ production by Th1 cells at low levels of NO donor (i.e. 1 to 10 μM) whereas IFN-γ production was strongly inhibited at high levels of NO donor (i.e. 100 μM).
Other studies failed to confirm that NO-derived from SNAP selectively inhibited Th1 cells but rather equally inhibited proliferation of both Th1 and Th2 cells [133]. This effect was concentration-dependent up to 100 μM SNAP followed by increasing apoptosis with concentrations of SNAP about 300 to 1,000 μM. This biphasic effect of NO on T cell function is reminiscent of the biphasic actions of NO seen in other blood cells which indicated stimulation at low concentrations and inhibition at high concentrations of NO [141–145]. Furthermore, the finding that proliferation was inhibited when SNAP was added 24 hours after stimulation suggests that NO acts at a later stage to limit proliferation rather than at the initial induction stage [133]. This finding has implications for understanding the role of NO in allo-immune activation in vivo since up-regulation of iNOS lags behind inflammatory cell activation.
Limitations and implications
Crucial in all of these studies is the lack of understanding of the actual concentrations of NO achieved in the environment of T cells in vivo during allo-immune activation in acute cardiac allograft rejection. Then there is the issue with in vitro work using NO donors which produce kinetics of NO release that may not precisely mimic the continuous release seen by NO generated enzymatically via iNOS in the biological setting.
Nevertheless, the in vitro findings of the influence of NO on the response of immune cells have potential implications for a better understanding of many of the controversial findings reported earlier in this review regarding the effects of limitation of NO production by pharmacological means and effects on graft survival. In this context, studies showing improved cardiac allograft survival using NOS inhibitors or NO neutralizers were generally seen when the treatment strategies left residual elevated levels of NO. It is intriguing to speculate that the residual NO might exhibit a protective action to counteract rejection via the immunosuppressive action on T cell function (Figure 1).
Actions of peroxynitrite on inflammatory cells in acute cardiac rejection
There is the other issue of the NO-derived nitration in different cell types in rejection. It is well recognized that nitration can lead to apoptosis in many cell types. The fact that cardiac cells have limited capacity to regenerate makes loss of cardiac cells via nitration-induced apoptosis a significant concern for acute cardiac allograft rejection. In contrast, apoptosis in inflammatory cells could be desirable to serve to counter further immune activation during graft rejection.
A possible explanation for the inhibitory action of NO on inflammatory cell function could be via peroxynitrite formation. Indeed, peroxynitrite has been shown to inhibit T lymphocyte activation and proliferation. This action is believed mediated by blocking tyrosine phosphorylation signaling and subsequently favoring peroxynitrite-mediated apoptosis of T lymphocytes [146].
Limitations
Currently, there is incomplete understanding of the ramifications of the specific localization of nitrotyrosine within infiltrating lymphocytes and macrophage cells as opposed to parenchymal cells such as cardiac myocytes in the setting of acute cardiac rejection. However, future studies in this area may help to better understand the complex role of NO and down-stream reactive nitrogen species such as peroxynitrite in the etiology of cardiac graft rejection.
Issues of iNOS expression vs. iNOS-derived NO bioactivity
An emerging viewpoint is that NO bioactivity via iNOS rather than iNOS expression per se may be more important to understanding the complexities of NO. This notion should be a consideration for understanding a role of NO in acute allograft rejection. Traditional measurements in a plethora of studies of experimental cardiac transplant rejection have shown increased levels of plasma or serum or urinary NO metabolites, nitrate plus nitrite [35,37,50]. The fact that these NO metabolites were increased in allografts but not isografts excludes any increases due to complications of surgery. The finding that urinary NO metabolites returned to baseline after cardiac allografts are fully-rejected has been interpreted to be due to decreased release of NO into the systemic circulation since the graft is no longer functionally-perfused. While an important probe of increase NO, these peripheral measures of NO metabolites have limitations in that they did not give information that is specific to the graft.
Complimentary studies utilizing ex vivo measurements of NO production based on arginine-to-citrulline conversion assays of cardiac tissue homogenates. Studies by Worrall et al. [10,55] have shown a several fold increase in calcium-independent, L-NMMA-inhibitable, iNOS activity in cardiac allografts as compared to isograft controls [10,55]. This increase in iNOS activity was blocked by using the iNOS inhibitor, N-aminomethyl-L-lysine [147]. One study using a NO-selective electrode showed increased in situ levels of NO at 13.41 μM in allografts compared to 3.43 μM in isograft control levels [148]. The increase in NO levels was decreased to isograft levels using the iNOS inhibitor, aminoguanidine. The latter is the only study to our knowledge to measure in situ levels of NO bioactivity using this technology.
Limitations
A limitation of the arginine-to-citrulline assays for iNOS enzyme activity is that the measurements are made ex vivo using tissue homogenates incubated under saturating levels of substrate and co-factors. Such conditions may or may not exist under in vivo conditions. The in situ measurements with a NO electrode mentioned above have certain advantages in that these measurements do not suffer from this limitation.
Post-translational regulation of NO bioactivity from iNOS
A new area of research that deserves greater attention is the actual NO bioactivity as determined by post-translational factors in the setting of acute cardiac rejection. We have recently reported increases in iNOS activity ex vivo at post-transplant days 4 and 6 in rat cardiac allografts using citrulline assays. In contrast, NO content per se measured without addition of exogenous excess arginine and tetrahydrobiopterin to the assay was increased at initial early stages of acute cardiac allograft rejection (i.e. post-transplant day 4) but then return to isograft controls at later stages of rejection (i.e. post-transplant day 6) [94]. This later decreased NO content was associated with impaired GTP cyclohydrolase synthesis of tetrahydrobiopterin at post-transplant day 6 but not at early stages of rejection at post-transplant day 4. This suggests that tetrahydrobiopterin levels may be unable to support NO production via iNOS.
Interestingly, this secondary decline in NO content from its early peak in graft tissue per se does not parallel the increases in plasma NO metabolites seen previously in our laboratory which remain elevated to equivalent levels at both post-transplant days 4 and 6 [94]. This illustrates that previous peripheral measurements of NO production may not be reliable indicators of the actual NO content within cardiac grafts. Collectively, these studies indicate that iNOS enzyme activities measured under optimal conditions ex vivo may not accurately reflect the actual production of NO occurring within cardiac allografts at various stages of rejection. Our recent findings may suggest that NO bioactivity rather than iNOS expression may be more important in understanding better the etiology of effects of iNOS on graft rejection.
The concept that post-translational factors can determine NO bioactivity derived from iNOS raises questions about the possible uncoupling of NO production derived from iNOS in rejecting cardiac allografts in vivo. This consideration requires that more studies are necessary to better understand the post-translational modification of NO production from iNOS by tetrahydrobiopterin in transplant rejection. This concept is inferred from other recent findings in a renal transplant model in which supplementation with sepiapterin to increase levels of biopterin via the salvage pathway increased NO content and decreased superoxide production [149]. It was not clearly determined; however, that iNOS was the source of superoxide production in renal rejection and the improved NO production following treatment with sepiapterin occurred via iNOS or constitutive NOS.
We have hypothesized that the iNOS present in acute cardiac rejection may be uncoupled [94]. This concept would be consistent with our earlier findings that protein nitration is increased at post-transplant day 6 [89] when cardiac NO content is decreased but nitration is not increased at post-transplant day 4 when cardiac NO content is elevated in cardiac allografts.
Future Studies
It is clear that much still needs to be understood regarding the role of NO derived from iNOS in acute cardiac rejection. This includes the possibility that post-translational regulation of iNOS bioactivity must be part of the consideration. Future studies which examine iNOS bioactivity and iNOS uncoupling may provide new insights into this complex understanding.
Acknowledgments
Supported, in part, by NIH grant HL078937.
Abbreviations
- cNOS
constitutive nitric oxide synthase
- EPR
electron paramagnetic resonance
- iNOS
inducible nitric oxide synthase
- IFN-γ
interferon-γ
- IL-2
interleukin-2
- ISHLT
International Society of Heart and Lung Transplantation
- L-NIL
N6-(1-iminoethyl)-L-lysine
- L-NA
L-nitroarginine
- L-NAME
L-nitroarginine methyl ester
- L-NMMA
L-NG-monomethylarginine
- LPS
lipopolysaccharide
- SNAP
S-nitroso-acetyl-penicillamine
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gotts WG, Johnson MR. The challenge of rejection and cardiac allograft vasculopathy. Heart Failure Rev. 2001;6:227–240. doi: 10.1023/a:1011414307636. [DOI] [PubMed] [Google Scholar]
- 2.Kriett JM, Kaye MP. The registry of the International Society for Heart Transplantation: seventh office report. J Heart Lung Transplant. 1990;9:323–330. [PubMed] [Google Scholar]
- 3.Billingham ME, Cary NR, Hamond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A. A working formulatin for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:587–593. [PubMed] [Google Scholar]
- 4.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Anderson CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Stoica SC, Cafferty F, Pauriah M, Taylor CJ, Sharples LD, Wallwork J, Large SR, Parameshwar J. The cumulative effect of acute rejection on development of cardiac allograft vasulopathy. J Heart Lung Transplant. 2006;25:420–425. doi: 10.1016/j.healun.2005.11.449. [DOI] [PubMed] [Google Scholar]
- 6.Halloran PF. The immune response to an organ transplant. In: Wood K, editor. Handbook of Transplant Immunology. Stokes Pages, Bucks, UK: MedSci Publications; 1995. p. 223. [Google Scholar]
- 7.Nagano H, Nadeau KC, Takada M, Kusaka M, Tilney NL. Sequential cellular and molecular kinetics in acutely rejecting renal allografts in rats. Transplantation. 1997;63:1101–1108. doi: 10.1097/00007890-199704270-00009. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Chowdhury N, Cai B, Brett J, Marboe C, Sciacca RR, Michler ME, Cannon PJ. Induction of myocardial nitric oxide synthase by cardiac allograft rejection. J Clin Invest. 1994;94:714–721. doi: 10.1172/JCI117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiraishi T, DeMeester SR, Worrall NK, Ritter JH, Misko TP, Ferguson TB, Jr, Cooper JD, Patterson GA. Inhibition of inducible nitric oxide synthase ameliorates rat lung allograft rejection. J Thorac Cardiovasc Surg. 1995;110:1449–1460. doi: 10.1016/S0022-5223(95)70068-4. [DOI] [PubMed] [Google Scholar]
- 10.Worrall NK, Boasquevisque CH, Botney MD, Misko TP, Sullivan PM, Ritter JH, Ferguson TB, Jr, Patterson GA. Inhibition of inducible nitric oxide synthase ameliorates functional and changes of acute lung allograft rejection. Transplantation. 1997;63:1095–1101. doi: 10.1097/00007890-199704270-00008. [DOI] [PubMed] [Google Scholar]
- 11.Vos IHC, Joles JA, Schurink M, Weckbecker G, Stojanovic T, Rabelink TJ, Gröne HJ. Inhibition of inducible nitric oxide synthase improves graft function and reduces tubulointerstitial injury in renal allograft rejection. Eur J Pharmacol. 2000;391:31–38. doi: 10.1016/s0014-2999(00)00021-2. [DOI] [PubMed] [Google Scholar]
- 12.Stevens RB, Ansite JD, Mills CD, Lokeh A, Rossini RJ, Saxena M, Brown RR, Sutherland DER. Nitric oxide mediates early dysfunction of rat and mouse islets after transplantation. Transplantation. 1996;61:1740–1749. doi: 10.1097/00007890-199606270-00014. [DOI] [PubMed] [Google Scholar]
- 13.Brandhorst D, Brandhorst H, Zwolinski A, Nahidi F, Bretzel RG. Prevention of early islet graft failure by selective inducible nitric oxide synthase inhibitors after pig to nude rat intraportal islet transplantation. Transplantation. 2001;71:179–184. doi: 10.1097/00007890-200101270-00002. [DOI] [PubMed] [Google Scholar]
- 14.Støeštíkováa P, Pišková J, Filipec M, Farhali H. FK 506 and aminoguanidine suppress iNOS induction in orthotopic corneal allografts and prolong graft survival in mice. Nitric Oxide. 2003;9:111–117. doi: 10.1016/j.niox.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang J, Xu D, Zhang X, Qi S, Ma A, Jiang W, Chida N, Sudo Y, Tamura K, Daloze P, Chen H. Effect of a novel inducible nitric oxide synthase inhibitor in prevention of rat chronic aortic rejections. Transplantation. 2005;79:1386–1392. doi: 10.1097/01.tp.0000159144.08519.e2. [DOI] [PubMed] [Google Scholar]
- 16.Perrault LP, Bidouard JP, Janiak P, Villeneuve N, Bruneval P, Villaine JP, Vanhoutte PM. Time course of coronary endothelial dysfunction in acute untreated rejection after heterotopic heart transplantation. J Heart Lung Transplant. 1997;16:643–657. [PubMed] [Google Scholar]
- 17.Demers P, Elkouri S, Sirois MG, Cartier R. Coronary artery endothelial dysfunction after ischemia-reperfusion and acute untreated rejection in a canine heterotopic heart transplantation model. Transplantation. 2001;71:26–32. doi: 10.1097/00007890-200101150-00005. [DOI] [PubMed] [Google Scholar]
- 18.Vejlstrup NG, Andersen CB, Boesgaard S, Mortensen SA, Aldershvile J. Temporal changes in myocardial endothelial nitric oxide synthase experession following human heart transplantation. J Heart Lung Transplant. 2002;21:211–216. doi: 10.1016/s1053-2498(01)00359-x. [DOI] [PubMed] [Google Scholar]
- 19.Wildhirt SM, Weis M, Schulze C, Conrad N, Pehlivanli S, Rieder G, Enders G, von Scheidt W, Reichart B. Expression of endomyocardial nitric oxide synthase and coronary endothelial function in human cardiac allografts. Circulation. 2001;104(suppl I):I-336–I343. doi: 10.1161/hc37t1.094598. [DOI] [PubMed] [Google Scholar]
- 20.Wildhirt SM, Weis M, Schulze C, Conrad N, Pehlivanli S, Rieder G, Enders G, von Scheidt W, Reichart B. Coronary flow reserve and nitric oxide synthases after cardiac transplantation in humans. Eur J Cardio-Thorac Sug. 2001;19:840–847. doi: 10.1016/s1010-7940(01)00681-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee PC, Wang ZL, Mahidhara R, Qian S, Watkins SC, Griffith BP, Billiar TR, Shears LL., II Inducible nitric oxide synthase attenuates acute cardiac rejection. J Am Coll Surg. 2000;191:S98–S99. [Google Scholar]
- 22.Yap J, O’Brien T, Pellegrini C, Barber DA, Tazelaar HD, Severson SR, Miller VM, McGregor CGA. Distribution and function of recombinant endothelial nitric oxide synthase in transplanted hearts. Cardiovasc Res. 1999;42:720–727. doi: 10.1016/s0008-6363(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 23.Iwata A, Sai S, Nitta Y, Chen M, de Fries-Hallstrand R, Dalesandro J, Thomas R, Allen MD. Liposome-mediated gene transfection of endothelial nitric oxide synthase reduces endothelial activation and leukocyte infiltration in transplanted hearts. Circulation. 2001;103:2753–2759. doi: 10.1161/01.cir.103.22.2753. [DOI] [PubMed] [Google Scholar]
- 24.Jeppsson A, Pellegrini C, O’Brien T, Miller JM, Tazelaar HD, Taner CB, McGregor CGA. Gene transfer of endothelial nitric oxide synthase to pulmonary allografts: impact on acute rejection. Transplant Int. 13(suppl 1):S591–S596. doi: 10.1007/s001470050409. [DOI] [PubMed] [Google Scholar]
- 25.Shears LL, II, Kawaharada N, Tzeng E, Billiar TR, Watkins SC, Kovesdi I, Lizonova A, Pham SM. Inducible nitric oxide synthase suppresses the development of allograft arteriosclerosis. J Clin Invest. 1997;100:2035–2042. doi: 10.1172/JCI119736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koglin J, Glysing-Jensen T, Mudgett JS, Russell ME. NOS2 mediates opposing effects in models of acute and chronic cardiac rejection. Insights from NOS-2 knockout mice. Am J Pathol. 1998;153:1371–1376. doi: 10.1016/S0002-9440(10)65723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Z, Gelzer-Bell R, Yang SX, Cao W, Ohnishi T, Wasowska BA, Hruban RH, Rodriguez ER, Baldwin WM, III, Lowenstein CJ. Inducible nitric oxide synthase inhibition of Weibel-Palade body release in cardiac transplant rejection. Circulation. 2001;104:2369–2375. doi: 10.1161/hc4401.098471. [DOI] [PubMed] [Google Scholar]
- 28.Du C, Jiang J, Guan Q, Diao H, Yin Z, Wang S, Zhong R, Jevnikar AM. NOS2 (iNOS) deficiency in kidney donor accelerates allograft loss in a murine model. Am J Transplant. 2007;7:17–26. doi: 10.1111/j.1600-6143.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- 29.Weis M, Cooke JP. Cardiac allograft vasculopathy and dysregulation of the NO synthase pathway. Arterioscler Thromb Vasc Biol. 2002;23:567–575. doi: 10.1161/01.ATV.0000067060.31369.F9. [DOI] [PubMed] [Google Scholar]
- 30.Minamoto K, Pinsky DJ. Recipient iNOS but not eNOS deficiency reduces luminal narrowing in tracheal allografts. J Exp Med. 2002;196:1321–1333. doi: 10.1084/jem.20012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosenpud JD, Boyle TM, Hensler H, Sanford G, Khanna AK. The relationship between acute rejection and chronic rejection is highly dependent on specific MHC matching. A multi-strain rat heterotopic heart transplant study. Transplantation. 2000;69:2173–2178. doi: 10.1097/00007890-200005270-00037. [DOI] [PubMed] [Google Scholar]
- 32.Meier-Kriesche HU, Ojo AO, Hanson JA, Cibrik DM, Punch JD, Leichtman AB, Kaplan B. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation. 2000;70:1098–1100. doi: 10.1097/00007890-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 33.Lewis NP, Tsao PS, Rickenbacher PR, Xue C, Johns RA, Haywood GA, von der Leyen H, Trindale PT, Cooke JP, Hunt SA, Billingham ME, Valantine HA, Fowler MB. Induction of nitric oxide synthase in the human cardiac allograft is associated with contractile dysfunction of the left ventricle. Circulation. 1996;93:720–729. doi: 10.1161/01.cir.93.4.720. [DOI] [PubMed] [Google Scholar]
- 34.Paulus WJ, Kästner S, Pujadas P, Shah AM, Drexler H, Vanderheyden M. Left ventricular contractile effects of inducible nitric oxide synthase in the human allograft. Circulation. 1997;96:3436–3442. doi: 10.1161/01.cir.96.10.3436. [DOI] [PubMed] [Google Scholar]
- 35.Winlaw DS, Schyvens CG, Smythe GA, Du ZY, Rainer SP, Lord RS, Spratt PM, Macdonald PS. Selective inhibition of nitric oxide production during cardiac allograft rejection causes a small increase in graft survival. Transplantation. 1995;60:77–82. doi: 10.1097/00007890-199507150-00015. [DOI] [PubMed] [Google Scholar]
- 36.Menon SG, Zhao L, Xu S, Samlowski WE, Shelby J, McGregor J, Barry WH. Relative importance of cytotoxic T lymphocytes and nitric oxide-dependent cytotoxicity in contractile dysfunction of rejecting murine cardiac allografts. Transplantation. 1998;66:413–419. doi: 10.1097/00007890-199808270-00001. [DOI] [PubMed] [Google Scholar]
- 37.Bastian NR, Xu S, Shao XL, Shelby J, Granger DL, Hibbs JB., Jr Nω-monomethyl-L-arginine inhibits nitric oxide production in murine cardiac allografts but does not affect graft rejection. Biochim Biophys Acta. 1994;1226:225–231. doi: 10.1016/0925-4439(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto M, Maeda H, Hirose N, Radhakrishnan G, Katare RG, Hayashi Y, Rao P, Lee GH, Yamaguchi T, Sasaguri S. Bilirubin oxidation provoked by nitric oxide radicals predicts the progression of acute cardiac allograft rejection. Am J Transplant. 2007;7:1897–1906. doi: 10.1111/j.1600-6143.2007.01868.x. [DOI] [PubMed] [Google Scholar]
- 39.Paul LC, Myllärniemi M, Muzaffar S, Benediktsson H. Nitric oxide synthase inhibition is associated with decreased survival of cardiac allografts in the rat. Transplantation. 1996;62:1193–1195. doi: 10.1097/00007890-199610270-00032. [DOI] [PubMed] [Google Scholar]
- 40.Komarov A, Mattson D, Jones MM, Singh PK, Lai CS. In vivo spin trapping of nitric oxide in mice. Biochem Biophys Res Commun. 1993;195:1191–1198. doi: 10.1006/bbrc.1993.2170. [DOI] [PubMed] [Google Scholar]
- 41.Komarov AM, Lai CS. Detection of nitric oxide production in mice by spin-trapping electron paramagnetic resonance spectroscopy. Biochim Biophys Acta. 1995;1272:29–36. doi: 10.1016/0925-4439(95)00061-8. [DOI] [PubMed] [Google Scholar]
- 42.Mikoyan VD, Kubrina LN, Serezhenkov VA, Stukan RA, Vanin AF. Complexes of Fe2+ with diethyldithiocarbamate or N-methyl-D-glucamine dithiocarbamate as traps of nitric oxide in animal tissues: comparative investigations. Biochim Biophys Acta. 1997;1336:225–234. doi: 10.1016/s0304-4165(97)00032-9. [DOI] [PubMed] [Google Scholar]
- 43.Rochelle LG, Morana SJ, Kruszyna H, Russell MA, Wilcox DE, Smith RP. Interactions between hydroxocobalamine and nitric oxide (NO): evidence for a redox reaction between NO and reduced cobalamin and reversible NO binding to oxidized cobalamin. J Pharmacol Exp Thera. 1995;275:48–52. [PubMed] [Google Scholar]
- 44.Greenberg SS, Zie J, Zatarain JM, Kapusta DR, Miller MJS. Hydroxocobalamin (vitamine B12a) prevents and reverses endotoxin-induced hypotension and mortality in rodents: role of nitric oxide. J Pharmacol Exp Thera. 1995;273:257–265. [PubMed] [Google Scholar]
- 45.Brouwer M, Chamulitrat W, Ferruzzi G, Sauls DL, Weinberg JB. Nitric oxide interactions with cobalamins: biochemical and functional consequences. Blood. 1996;88:1857–1864. [PubMed] [Google Scholar]
- 46.Marmion CJ, Cameron B, Mulcahy C, Fricker SP. Ruthenium as an effective nitric oxide scavenger. Curr Top Med Chem. 2004;4:1585–1603. doi: 10.2174/1568026043387322. [DOI] [PubMed] [Google Scholar]
- 47.Mosi R, Sequin B, Cameron B, Amankwa L, Darkes MC, Fricker SP. Mechanistic studies on AMD6221: a ruthenium-based nitric oxide scavenger. Biochem Biophys Res Commun. 2002;292:519–529. doi: 10.1006/bbrc.2002.6685. [DOI] [PubMed] [Google Scholar]
- 48.Cooper M, Lindholm P, Pieper G, Seibel R, Moore G, Nakanishi A, Dembny K, Komorowski R, Johnson C, Adams M, Roza A. Myocardial nuclear factor-κB activity and nitric oxide production in rejecting cardiac allografts. Transplantation. 1998;66:838–844. doi: 10.1097/00007890-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 49.Nakanishi AL, Roza AM, Adams MB, Seibel R, Moore-Hilton G, Kalyanaraman B, Pieper GM. Electron spin resonance analysis of heme-nitrosyl and reduced iron-sulfur centered complexes in allogeneic, heterotopic cardiac transplants: effects of treatment with pyrrolidine dithiocarbamate. Free Radical Biol Med. 1998;25:201–207. doi: 10.1016/s0891-5849(98)00051-3. [DOI] [PubMed] [Google Scholar]
- 50.Roza AM, Cooper M, Pieper G, Hilton G, Dembny K, Lai CS, Lindholm P, Komorowski R, Felix C, Johnson C, Adams M. NOX 100, a nitric oxide scavenger, enhances cardiac allograft survival and promotes long-term graft acceptance. Transplantation. 2000;69:227–231. doi: 10.1097/00007890-200001270-00006. [DOI] [PubMed] [Google Scholar]
- 51.Pieper GM, Adams MB, Felix CC, Roza AM. Role of nitric oxide in cardiac transplant rejection: documentation by EPR spectroscopy and other techniques. In: Gryglewksi RJ, Minuz P, editors. Nitric Oxide: Basic Research and Clinical Applications; Proceedings of the NATO Advanced Study Institute; September 7–17, 2000; Erice, Italy. Amsterdam: IOS Press; 2001. pp. 71–79. [Google Scholar]
- 52.Pieper GM, Khanna AK, Kampalath B, Felix CC, Hilton G, Johnson CP, Adams MB, Roza AM. Inhibition of nitrosylation, nitration, lymphocyte proliferation, and gene expression in acute and delayed cardiac allograft rejection by an orally active dithiocarbamate. J Cardiovasc Pharmacol. 2004;43:522–530. doi: 10.1097/00005344-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Pieper GM, Roza AM, Adams MB, Hilton G, Johnson M, Felix CC, Kampalath B, Darkes M, Wanggui Y, Cameron B, Fricker SP. A ruthenium (III) polyaminocarboxylate complex, a novel nitric oxide scavenger, enhances graft survival and decreases nitrosylated heme protein in models of acute and delayed transplant rejection. J Cardiovasc Pharmacol. 2002;39:441–448. doi: 10.1097/00005344-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, McDaniel ML, Williamson JR, Currie MG. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 55.Worrall NK, Lazenby WD, Misko TP, Lin TS, Rodi CP, Manning PT, Ferguson TB., Jr Modulation of in vivo alloreactivity by inhibition of inducible nitric oxide synthase. J Exp Med. 1995;181:63–70. doi: 10.1084/jem.181.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi W, Suzuki J, Izawa A, Takayama K, Yamazaki S, Isobe M. Inducible nitric oxide-mediated myocardial apoptosis contributes to graft failure during acute cardiac allograft rejection in mice. Jap Heart J. 2000;41:493–506. doi: 10.1536/jhj.41.493. [DOI] [PubMed] [Google Scholar]
- 57.Soto PF, Jia CX, Rabkin DG, Hart JP, Carter YM, Sardo MJ, Hsu DT, Fisher PE, Pinsky DJ, Spotnitz HM. Improvement of rejection-induced diastolic abnormalities in rat cardiac allografts with inducible nitric oxide synthase inhibition. J Thorac Cardiovasc Surg. 2000;120:39–46. doi: 10.1067/mtc.2000.107124. [DOI] [PubMed] [Google Scholar]
- 58.Schuler W. Zur Hemming der Diamniooxylase (Histaminase) Experientia. 1952;8:230–232. doi: 10.1007/BF02170726. [DOI] [PubMed] [Google Scholar]
- 59.Kumari K, Umar S, Bansal V, Sahib MK. Inhibition of diabetes-associated complications by nucleophilic compounds. Diabetes. 1991;40:1079–1084. doi: 10.2337/diab.40.8.1079. [DOI] [PubMed] [Google Scholar]
- 60.Wani J, Carl M, Henger A, Nelson PJ, Rupprecht H. Nitric oxide modulates expression of extracellular matrix genes linked to fibrosis in kidney mesangial cells. Biol Chem. 2007;388:497–506. doi: 10.1515/BC.2007.056. [DOI] [PubMed] [Google Scholar]
- 61.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 62.Ramana KV, Reddy ABM, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-production of nitric oxide and cytoxicity in murine macrophages. Free Radical Biol Med. 2007;42:1290–1302. doi: 10.1016/j.freeradbiomed.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blasko E, Glaser CB, Devlin JJ, Xia W, Feldman RI, Polokoff MA, Phillips GB, Whitlow M, Auld DS, McMillan K, Ghosh S, Stuehr DJ, Parkinson JF. Mechanistic studies with potent and selective inducible nitric-oxide synthase dimerization inhibitors. J Biol Chem. 2002;277:295–302. doi: 10.1074/jbc.M105691200. [DOI] [PubMed] [Google Scholar]
- 64.Szabolcs MJ, Sun J, Ma N, Albala A, Sciacca RR, Philips GB, Parkinson J, Edwards N, Cannon PJ. Effects of selective inhibitors of nitric oxide synthase-2 dimerization on acute cardiac allograft rejection. Circulation. 2002;106:2392–2396. doi: 10.1161/01.cir.0000034719.08848.26. [DOI] [PubMed] [Google Scholar]
- 65.Kolodziejski PJ, Koo JS, Eissa NT. Regulation of inducible nitric oxide synthase by rapid cellular turnover and cotranslational down-regulation by dimerization inhibitors. Proc Natl Acad Sci USA. 2004;101:18141–18146. doi: 10.1073/pnas.0406711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pieper GM, Nilakantan V, Hilton G, Zhou X, Khanna AK, Halligan NLN, Felix CC, Kampalath B, Griffith OW, Hayward MA, Roza AM, Adams MB. Variable efficacy of N6-(1-iminoethyl)-L-lysine in acute cardiac transplant rejection. Am J Physiol Heart Circ Physiol. 2004;286:H525–H534. doi: 10.1152/ajpheart.00356.2003. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt K, Werner-Felmayer G, Mayer B, Werner ER. Preferential inhibition of inducible nitric oxide synthase in intact cells by the 4-amino analogue of tetrahydrobiopterin. Eur J Biochem. 1999;259:25–31. doi: 10.1046/j.1432-1327.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 68.Brandacher G, Zou Y, Obrist P, Steurer W, Werner-Felmayer G, Margreiter R, Werner ER. The 4-amino analogue of tetrahydrobiopterin efficiently prolongs murine cardiac-allograft survival. J Heart Lung Transplant. 2001;20:747–749. doi: 10.1016/s1053-2498(00)00329-6. [DOI] [PubMed] [Google Scholar]
- 69.Brandacher G, Maglione M, Schneeberger S, Obrist P, Thoeni G, Wrulich OA, Werner-Felmayer G, Margreiter R, Werner ER. Tetrahydrobiopterin compounds prolong allograft survival independently of their effect on nitric oxide synthase activity. Transplantation. 2006;81:583–589. doi: 10.1097/01.tp.0000188949.03683.fd. [DOI] [PubMed] [Google Scholar]
- 70.Thoeni G, Stoitzner P, Brandacher G, Romani N, Heufler C, Werner-Felmayer G, Werner ER. Tetrahydro-4-aminobiopterin attenuates dendritic cell-induced T cell priming independently from inducible nitric oxide synthase. J Immunol. 2005;174:7584–7591. doi: 10.4049/jimmunol.174.12.7584. [DOI] [PubMed] [Google Scholar]
- 71.Koglin J, Granville DJ, Glysing-Jensen T, Mudgett JS, Carthy CM, McManus BM, Russell ME. Attenuated acute cardiac rejection in NOS2-/-recipients correlates with reduced apoptosis. Circulation. 1999;99:836–842. doi: 10.1161/01.cir.99.6.836. [DOI] [PubMed] [Google Scholar]
- 72.Mannon RB, Roberts K, Ruiz P, Laubach V, Coffmann TM. Inducible nitric oxide synthase promotes cytokine expression in cardiac allografts but is not required for efficient rejection. J Heart Lung Transplant. 1999;18:819–827. doi: 10.1016/s1053-2498(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 73.Szabolcs MJ, Ma N, Athan E, Zhong J, Ming M, Sciacca RR, Husemann J, Albala A, Cannon PJ. Acute cardiac allograft rejection in nitric oxide synthase-2−/− and nitric oxide synthase-2+/+ mice. Effects of cellular chimeras on myocardial inflammation and cardiomyocyte damage and apoptosis. Circulation. 2001;103:2514–2520. doi: 10.1161/01.cir.103.20.2514. [DOI] [PubMed] [Google Scholar]
- 74.Most D, Efron DT, Shi HP, Tantry US, Barbul A. Differential cytokine expression in skin graft healing in inducible nitric oxide knockout mice. Plast Reconstr Surg. 2001;108:1251–1259. doi: 10.1097/00006534-200110000-00024. [DOI] [PubMed] [Google Scholar]
- 75.Casey JJ, Wei XQ, Orr DJ, Gracie JA, Huan FP, Bolton EM, Liew FY, Bradley JA. Skin allograft rejection in mice lacking inducible nitric oxide synthase. Transplantation. 1997;64:589–593. doi: 10.1097/00007890-199708270-00007. [DOI] [PubMed] [Google Scholar]
- 76.Huang A, Sun D, Shesely EG, Levee EM, Koller A, Kaley G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2002;282:H429–H436. doi: 10.1152/ajpheart.00501.2001. [DOI] [PubMed] [Google Scholar]
- 77.Al-shabrawey M, El-Remessy AB, Brooks SS, Hamed MS, Huang P, Caldwell RB. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis. 2003;9:549–558. [PubMed] [Google Scholar]
- 78.Biecker E, Neef M, Sägesser H, Shaw S, Koshy A, Reichen J. Nitric oxide synthase 1 is partly compensating for nitric oxide synthase 3 deficiency in nitric oxide synthase 3 knock-out mice and is elevated in murine and human cirrhosis. Liver Int. 2004;24:345–353. doi: 10.1111/j.1478-3231.2004.0933.x. [DOI] [PubMed] [Google Scholar]
- 79.Talukder MAH, Fujiki T, Morikawa K, Motoishi M, Kubota H, Morishita T, Tsutsui M, Takeshita A, Shimokawa H. Up-regulated neuronal nitric oxide synthase compensates coronary flow response to bradykinin in endothelial nitric oxide synthase-deficient mice. J Cardiovasc Pharmacol. 2004;44:437–445. doi: 10.1097/01.fjc.0000139450.64337.cd. [DOI] [PubMed] [Google Scholar]
- 80.Henry Y, Lepoivre M, Drapier JC, Ducrocq C, Boucher JL, Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993;7:1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- 81.Henry Y, Guissani A. Interactions of nitric oxide with hemoproteins: roles of nitric oxide in mitochondria. CMLS Cell Mol Life Sci. 1999;55:1003–1014. doi: 10.1007/s000180050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown GC. Nitric oxide inhibition of cytochrome oxidase and mitochondrial respiration: implications for inflammatory, neurodegenerative and ischaemic pathologies. Mol Cell Biochem. 1997;174:189–192. [PubMed] [Google Scholar]
- 83.Lancaster JR, Jr, Langrehr JM, Bergonia HA, Murase N, Simmons RL, Hoffman RA. EPR detection of heme and nonheme iron-containing protein nitrosylation by nitric oxide during rejection of rat heart allograft. J Biol Chem. 1992;267:10994–10998. [PubMed] [Google Scholar]
- 84.Pieper GM. Protection against allograft rejection by iron-dithiocarbamate complexes. In: Van Faassen E, Vanin AF, editors. Radicals for Life: The Various Forms of Nitric Oxide. Amsterdam: Elsevier; 2007. pp. 407–422. [Google Scholar]
- 85.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 86.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine-oxidase catalyzed nitrate reduction. Evaluation of its role in nitrite and nitric oxide generation in anoxic tissue. Biochemistry. 2003;42:1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 87.Lancaster JR, Jr, Hibbs JB., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci USA. 1990;87:1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pieper GM, Halligan NLN, Hilton G, Konorev EA, Felix CC, Roza AM, Adams MB, Griffith OW. Non-heme iron protein: a potential target of nitric oxide in acute cardiac allograft rejection. Proc Natl Acad Sci USA. 2003;100:3125–3130. doi: 10.1073/pnas.0636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nilakantan V, Halligan NLN, Nguyen TK, Hilton G, Khanna AK, Roza AM, Johnson CP, Adams MB, Griffith OW, Pieper GM. Post-translational modification of manganese superoxide dismutase in acutely rejecting cardiac transplants: role of inducible nitric oxide synthase. J Heart Lung Transplant. 2005;24:1591–1599. doi: 10.1016/j.healun.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 90.Bertolet BD, Eagle DA, Conti JB, Mills RM, Jr, Belardinelli L. Bradycardia after heart transplantation: reversal with theophylline. J Am Coll Cardiol. 1996;28:396–399. doi: 10.1016/0735-1097(96)00162-3. [DOI] [PubMed] [Google Scholar]
- 91.Chowdhary S, Ng A, Nuttall SL, Coote JH, Ross HF, Townend JN. Nitric oxide and cardiac parasympathetic control in human heart failure. Clin Sci. 2002;102:397–402. [PubMed] [Google Scholar]
- 92.Chowdhary S, Marsch AM, Coote JH, Townend JN. Nitric oxide and cardiac muscarinic control in humans. Hypertension. 2004;43:1023–1028. doi: 10.1161/01.HYP.0000126171.46645.f9. [DOI] [PubMed] [Google Scholar]
- 93.Chowdhary S, Harrington D, Bonser RS, Coote JH, Townend JN. Chronotropic effects of nitric oxide in the denervated human heart. J Physiol. 2002;541:645–651. doi: 10.1113/jphysiol.2001.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pieper GM, Nilakantan V, Halligan NLN, Khanna AK, Hilton G, Vásquez-Vivar J. Nitric oxide formation in acutely rejecting cardiac allografts correlates with GTP cyclohydrolase I activity. Biochem J. 2005;391:541–547. doi: 10.1042/BJ20050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heger J, Gödecke A, Flögel U, Merx MW, Molojavyi A, Kühn-Velten WN, Schrader J. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ Res. 2002;90:93–99. doi: 10.1161/hh0102.102757. [DOI] [PubMed] [Google Scholar]
- 96.Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ, Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–743. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function. Ten years after, and continuing. Circ Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 98.Brady AJB, Waren JB, Poole-Wilson PA, Williams TJ, Harding SE. Nitric oxide attenuates cardiac myocyte contraction. Am J Physiol. 265 doi: 10.1152/ajpheart.1993.265.1.H176. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 1993;34:H176–H182. [Google Scholar]
- 99.Joe EK, Schussheim AE, Longrois D, Mäki T, Kelly RA, Smith TW, Balligand J-L. Regulation of cardiac myocyte contractile function by inducible nitric oxide synthase (iNOS): mechanisms of contractile depression by nitric oxide. J Mol Cell Cardiol. 1998;30:303–315. doi: 10.1006/jmcc.1997.0593. [DOI] [PubMed] [Google Scholar]
- 100.Tatsumi T, Matoba S, Kawahara A, Keira N, Shiraishi J, Akashi K, Kobara M, Tanaka T, Katamura M, Nakagawa C, Ohta B, Shirayama T, Takeda K, Asayama J, Fliss H, Nakagawa M. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J Am Coll Cardiol. 2000;35:1338–1346. doi: 10.1016/s0735-1097(00)00526-x. [DOI] [PubMed] [Google Scholar]
- 101.Wahler GM, Dollinger SJ. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am J Physiol. 268 doi: 10.1152/ajpcell.1995.268.1.C45. [DOI] [PubMed] [Google Scholar]; Cell Physiol. 1995;37:C45–C54. [Google Scholar]
- 102.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 103.Kumar A, Brar R, Wang P, Dee L, Skorupa G, Khadour F, Schulz R, Parrilo JE. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol. 276 doi: 10.1152/ajpregu.1999.276.1.R265. [DOI] [PubMed] [Google Scholar]; Reg Integr Comp Physiol. 1999;45:R265–R276. [Google Scholar]
- 104.Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-α in the adult mammalian heart. J Clin Invest. 1993;92:2303–2012. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stein B, Frank P, Schmitz W, Scholz H, Thoenes M. Endotoxin and cytokines induce direct cardiodepressive effects in mammalian cardiomyocytes via induction of nitric oxide synthase. J Mol Cell Cardiol. 1996;28:1631–1639. doi: 10.1006/jmcc.1996.0153. [DOI] [PubMed] [Google Scholar]
- 106.Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, Mayer K, Bohle RM, Seeger W, Grimminger F, Sibelius U. Endotoxin-induced myocardial tumor necrosis factor-α synthesis depresses contractility of isolated rat hearts. Evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102:2758–2764. doi: 10.1161/01.cir.102.22.2758. [DOI] [PubMed] [Google Scholar]
- 107.Sugishita K, Kinugawa K, Shimizu T, Harada K, Matsui H, Takahashi T, Serizawa T, Kohmoto O. Cellular basis for the acute inhibitory effects of IL-6 and TNF-α on excitation-contraction coupling. J Mol Cell Cardiol. 1999;31:1457–1467. doi: 10.1006/jmcc.1999.0989. [DOI] [PubMed] [Google Scholar]
- 108.Ritter M, Su Z, Yao A, Zubair I, Xu S, Shelby J, Barry WH. Myocyte function and [Ca2+]I homeostasis during early allogeneic heart transplant rejection. Transplantation. 2001;72:1603–1607. doi: 10.1097/00007890-200111270-00005. [DOI] [PubMed] [Google Scholar]
- 109.Ziolo MT, Dollinger SJ, Wahler GM. Myocytes isolated from rejecting transplanted rat hearts exhibit reduced basal shortening which is reversible by aminoguanidine. J Mol Cell Cardiol. 1998;30:1009–1017. doi: 10.1006/jmcc.1998.0665. [DOI] [PubMed] [Google Scholar]
- 110.Pyo RT, Wahler GM. Ventricular myocytes isolated from rejecting cardiac allografts exhibit a reduced β-adrenergic contractile response. J Mol Cell Cardiol. 1995;27:773–776. doi: 10.1016/0022-2828(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 111.Ziolo MT, Harshbarger CH, Roycroft KE, Smith JM, Romano FD, Sondergroth KL, Wahler GM. Myocytes isolated from rejecting transplanted rat hearts exhibit a nitric oxide-mediated reduction in the calcium current. J Mol Cell Cardiol. 2001;33:1691–1699. doi: 10.1006/jmcc.2001.1420. [DOI] [PubMed] [Google Scholar]
- 112.Worrall NK, Pyo RT, Botney MD, Misko TP, Sullivan PM, Alexander DG, Lazenby WD, Ferguson TB. Inflammatory cell-derived NO modulates cardiac allograft contractile and electrophysiological function. Am J Physiol. 273 doi: 10.1152/ajpheart.1997.273.1.H28. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 1997;42:H28–H37. doi: 10.1152/ajpheart.00133.2024. [DOI] [PubMed] [Google Scholar]
- 113.Scherrer-Crosbie M, Glysing-Jensen T, Fry SJ, Vançon AC, Gadiraju S, Picard MH, Russell ME. Echocardiography improves detection of rejection after heterotopic mouse cardiac transplantation. J Am Soc Echocardiogr. 2002;15:1315–1320. doi: 10.1067/mje.2002.124644. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen TK, Nilakantan V, Felix CC, Khanna AK, Pieper GM. Beneficial effect of α-tocopheryl succinate in rat cardiac transplants. J Heart Lung Transplant. 2006;25:707–715. doi: 10.1016/j.healun.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 115.Nilakantan V, Zhou X, Hilton G, Shi Y, Baker JE, Khanna AK, Pieper GM. Antagonizing reactive oxygen by treatment with a manganese (III) metalloporphyrin-based superoxide dismutase mimetic in cardiac transplants. J Thorac Cardiovasc Surg. 2006;131:898–906. doi: 10.1016/j.jtcvs.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 116.Pieper GM, Nilakantan V, Chen M, Zhou J, Khanna AK, Henderson JD, Jr, Johnson CP, Roza AM, Szabó Protective mechanisms of a metalloporphyrinic peroxynitrite decomposition catalyst, WW85, in rat cardiac transplants. J Pharmacol Exp Thera. 2005;314:53–60. doi: 10.1124/jpet.105.083493. [DOI] [PubMed] [Google Scholar]
- 117.Schulz R, Dodge KL, Lopaschuk GD, Clanachan AS. Peroxynitrite impairs cardiac contractile function by decreasing cardiac efficiency. Am J Physiol. 272 doi: 10.1152/ajpheart.1997.272.3.H1212. [DOI] [PubMed] [Google Scholar]; Heart Circ Physiol. 1997;41:H1212–H1219. [Google Scholar]
- 118.Ferdinandy P, Danial H, Ambrus I, Rothery RA, Schulz R. Peroxynitrite is a major contributor to cytokine-induced myocardial contractile failure. Circ Res. 2000;87:241–247. doi: 10.1161/01.res.87.3.241. [DOI] [PubMed] [Google Scholar]
- 119.Sakurai M, Fukuyama N, Iguchi A, Akimoto H, Ohmi M, Yokoyama H, Nakazawa H, Tabahashi K. Quantitative analysis of cardiac 3L-nitrotyrosine during acute allograft rejection in an experimental heart transplantation. Transplantation. 1999;68:1818–1822. doi: 10.1097/00007890-199912150-00031. [DOI] [PubMed] [Google Scholar]
- 120.Szabolcs MJ, Ravalli S, Minanov O, Sciacca RR, Michler RE, Cannon PJ. Apoptosis and increased expression of inducible nitric oxide synthase in human allograft rejection. Transplantation. 1998;65:804–812. doi: 10.1097/00007890-199803270-00007. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi Y, Okabe K, Matsumura F, Akizuki E, Matsuda T, Ohshiro H, Liang J, Yamada S, Mori K, Ogawa M. Peroxynitrite formation during rat hepatic allograft rejection. Hepatology. 1999;29:777–784. doi: 10.1002/hep.510290354. [DOI] [PubMed] [Google Scholar]
- 122.Bourges JL, Valamanesh F, Torriglia A, Jeanny JC, Savoldelli M, Renard G, BenEzra D, de Kozak Y, Behar-Cohen F. Cornea graft endothelial cells undergo apoptosis by way of an alternate (caspase-independent) pathway. Transplantation. 2004;78:316–323. doi: 10.1097/01.tp.0000128614.63503.d5. [DOI] [PubMed] [Google Scholar]
- 123.MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radical Biol Med. 2001;31:1603–1608. doi: 10.1016/s0891-5849(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 124.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by meyloperoxidase in neutrophils. Nature (London) 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 125.Murata S, Miniati DN, Kown MH, Koransky ML, Lijkwan MA, Balsam LB, Robbins RC. Superoxide dismutase mimetic M40401 reduces ischemia-reperfusion injury and graft coronary artery disease in rodent cardiac allografts. Transplantation. 2004;78:1166–1171. doi: 10.1097/01.tp.0000137321.34200.fa. [DOI] [PubMed] [Google Scholar]
- 126.Healy DG, Watson RWG, O’Keane C, Egan JJ, McCarthy JF, Hurley J, Fitzpatrick J, Wood AE. Neutrophil transendothelial migration potential predicts rejection severity in human cardiac transplantation. Eur J Cardio-Thorac Surg. 2006;29:760–766. doi: 10.1016/j.ejcts.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 127.Egi K, Conrad NE, Kwan J, Schulze C, Schulz R, Wilhirt SM. Inhibition of inducible nitric oxide synthase and superoxide production reduces matrix metalloproteinase-9 activity and restores coronary vasomotor function in rat cardiac allografts. Eur J Cardio-Thorac Surg. 2004;26:262–269. doi: 10.1016/j.ejcts.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 128.Akizuki E, Akaike T, Okamoto S, Fujii S, Yamaguchi Y, Ogawa M, Maeda H. Role of nitric oxide and superoxide in acute cardiac allograft rejection in rats. Proc Soc Exp Biol Med. 2000;225:151–159. doi: 10.1046/j.1525-1373.2000.22519.x. [DOI] [PubMed] [Google Scholar]
- 129.Ma N, Szabolcs MJ, Sun J, Albala A, Sciacca RR, Zhong M, Edwards N, Cannon PJ. The effect of selective inhibition of cyclooxygenase (COX)-2 on acute cardiac allograft rejection. Transplantation. 2002;74:1528–1534. doi: 10.1097/00007890-200212150-00009. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y, Son NH, Szabolcs MJ, Ma N, Sciacca RR, Albala A, Edwards N, Cannon PJ. Effects of inhibition of poly(adenosine diphosphate-ribose) synthase on acute cardiac allograft rejection. Transplantation. 2004;78:668–674. doi: 10.1097/01.tp.0000131662.01491.2e. [DOI] [PubMed] [Google Scholar]
- 131.Hoffman RA, Langrehr JM, Wren SM, Dull KE, Ildstad ST, McCarthy SA, Simmons RL. Characterization of the immunosuppressive effects of nitric oxide in graft vs. host disease. J Immunol. 1993;151:1508–1518. [PubMed] [Google Scholar]
- 132.Allione A, Bernabei P, Bosticardo M, Ariotti S, Forni G, Novelli F. Nitric oxide suppresses human T lymphocyte proliferation through IFN-γ dependent and IFN-γ-independent induction of apoptosis. J Immunol. 1999;163:4182–4191. [PubMed] [Google Scholar]
- 133.Van der Veen RC, Dietlin TA, Pen L, Gray JD. Nitric oxide inhibits the proliferation of T-helper 1 and 2 lymphocytes without reduction in cytokine secretion. Cell Immunol. 1999;193:194–201. doi: 10.1006/cimm.1999.1471. [DOI] [PubMed] [Google Scholar]
- 134.Van der Veen RC, Dietlin TA, Pen L, Gray JD, Hofman FM. Antigen presentation to Th1 but not Th2 cells by macrophages results in nitric oxide production and inhibition of T cell proliferation: interferon-γ is essential but not sufficient. Cell Immunol. 2000;206:125–135. doi: 10.1006/cimm.2000.1741. [DOI] [PubMed] [Google Scholar]
- 135.Holán V, Krulová M, Zajícová A, Pindjáková J. Nitric oxide as a regulatory and effector molecule in the immune system. Mole Immunol. 2001;38:989–995. doi: 10.1016/s0161-5890(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 136.Hoffman RA, Ford HR. Nitric oxide regulation of lymphocyte function. In: Salvemini D, Billiar TB, Vodovotz Y, editors. Nitric Oxide and Inflammation. Basel: Birkhäuser Verlag; 2001. pp. 131–143. [Google Scholar]
- 137.Blesson S, Thiery J, Gaudin C, Stancou R, Kolb JP, Moreau JL, Theze J, Mami-Chouaib F, Chouaib S. Analysis of the mechanisms of human cytotoxic T lymphocyte response inhibition by NO. Int Immunol. 2002;14:1169–1178. doi: 10.1093/intimm/dxf081. [DOI] [PubMed] [Google Scholar]
- 138.Mahidhara RS, Hoffman RA, Huang S, Wolf-Johnson A, Vodovotz Y, Simmons RL, Billiar TB. Nitric oxide-mediated inhibition of caspase-dependent T lymphocyte proliferation. J Leukoc Biol. 2003;74:403–411. doi: 10.1189/jlb.0602293. [DOI] [PubMed] [Google Scholar]
- 139.Taylor-Robinson AW, Liew FY, Severn A, Xu D, McSorley SJ, Garside P, Padron J, Phillips RS. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994;24:980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 140.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 141.Huang FP, Niedbala W, Wei XQ, Xu D, Feng GJ, Robinson JH, Lam C, Liew FY. Nitric oxide regulates Th1 cell development through inhibition of IL-12 synthesis by macrophages. Eur J Immunol. 1998;28:4062–4070. doi: 10.1002/(SICI)1521-4141(199812)28:12<4062::AID-IMMU4062>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 142.Niedbala W, Wei XQ, Piedrafita D, Xu D, Liew FY. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur J Immunol. 1999;29:2498–2505. doi: 10.1002/(SICI)1521-4141(199908)29:08<2498::AID-IMMU2498>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 143.Kalyanaraman B, Hogg N, Pathasarathy S. The biphasic effect of sodium nitroprusside on the oxidative modification of LDL by macrophages. Free Radical Biol Med. 1993;15:496–xxx. [Google Scholar]
- 144.Pieper GM, Clarke GA, Gross GJ. Stimulatory and inhibitory action of nitric oxide donor agents vs. nitrovasodilators on reactive oxygen production by isolated polymorphonuclear leukocytes. J Pharmacol Exp Thera. 1994;269:451–456. [PubMed] [Google Scholar]
- 145.Lee C, Miura K, Liu X, Zweier JL. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:38965–38972. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- 146.Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota AM. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- 147.Yang X, Ma N, Szabolcs MJ, Zhong J, Athan E, Sciacca RR, Michler RE, Anderson GD, Wiese JF, Leahy KM, Gregory S, Cannon PJ. Upregulation of COX-2 during cardiac allograft rejection. Circulation. 2000;101:430–438. doi: 10.1161/01.cir.101.4.430. [DOI] [PubMed] [Google Scholar]
- 148.Joshi MS, Lancaster JR, Jr, Liu X, Ferguson TB., Jr In situ measurement of nitric oxide production in cardiac isografts and rejecting allografts by an electrochemical method. Nitric Oxide: Biol Chem. 2001;5:561–565. doi: 10.1006/niox.2001.0369. [DOI] [PubMed] [Google Scholar]
- 149.Huisman A, Vos I, Van Faassen EE, Joles JA, Gröne HJ, Martasek P, Van Zonneveld AJ, Vanin AF, Rabelink TJ. Anti-inflammatory effects of tetrahydrobiopterin on early rejection in renal allografts: modulation of inducible nitric oxide synthase. FASEB J. 2002;16:1135–11376. doi: 10.1096/fj.01-0890fje. [DOI] [PubMed] [Google Scholar]