Abstract
Somatostatin mediates inhibitory functions through five G protein–coupled somatostatin receptors (sst1–5). We used immunohistochemistry, immunofluorescence, and RT-PCR to determine the presence of somatostatin receptors sst1, sst2A, sst2B, sst3, sst4, and sst5 in normal and IgA nephropathy human kidney. All somatostatin receptors were detected in the thin tubules (distal convoluted tubules and loops of Henle) and thick tubules (proximal convoluted tubules) in the tissue sections from nephrectomy and biopsy samples. Immunopositive sst1 and sst4 staining was more condensed in the cytoplasm of tubular epithelial cells. In normal kidney tissue sections, podocytes and mesangial cells in the glomeruli stained for sst1, sst2B, sst4 and sst5, and stained weakly for sst3. In IgA kidney tissue, the expression of somatostatin receptors was significantly increased with particular immmunopositive staining for sst1, sst2B, sst4, and sst5 within glomeruli. In the epithelial cells, the staining for sst2B and sst4 in proximal tubules and sst1, sst2B, and sst5 in distal tubules was increased. The mRNA expression of sst1–5 was also detected by RT-PCR. Somatostatin and all five receptor subtypes were ubiquitously distributed in normal kidney and IgA nephropathy. The increased expression of somatostatin receptors in IgA nephropathy kidney might be the potential pathogenesis of inflammatory renal disease. (J Histochem Cytochem 56:733–743, 2008)
Keywords: human kidney, IgA nephropathy, somatostatin, somatostatin receptors, tubule, podocytes, mesangial cells
Somatostatin, also known as somatotropin-release inhibiting factor (SRIF), was first characterized in 1973 (Brazeau et al. 1973). It is a cyclic polypeptide with two biologically active isoforms: SRIF-14 and SRIF-28. This cyclic polypeptide has broad inhibitory effects on the secretion of hormones such as growth hormone in the hypothalamus and insulin and glucagon in the pancreas. Somatostatin is distributed widely throughout both the central and peripheral nervous system and in other organs including the pancreas, gut, adrenal glands, thyroid, and in particular, the kidney (Benali et al. 2000; Moller et al. 2003; Bates et al. 2004a; Pinter et al. 2006).
The biological effects of somatostatin are exerted through binding to the G protein–coupled somatostatin receptors (sst) in the plasma membrane (Schonbrunn and Tashjian 1978; Krantic 2000; Weckbecker et al. 2003). Five isoforms of sst including the spliced variants (sst2A and sst2B) have been identified (Benali et al. 2000; Moller et al. 2003). They belong to the heptahelical family of G protein–coupled receptors and have high affinity binding with endogenous somatostatin. A series of synthetic somatostatin analogs with different affinity for the subtypes have been developed for research and clinical use (Moller et al. 2003; Weckbecker et al. 2003), though the internalization of ssts evoked by agonists may abrogate their effects (Kreienkamp et al. 1998; Cescato et al. 2006; Roosterman et al. 2007). The signaling pathways for the different ssts are thought to vary and are likely to explain why the activation of different ssts displays disparate effects, such as anti-proliferation and anti-angiogenesis effects (Bruns et al. 1994; Danesi et al. 1997), whereas others, such as sst3, are involved in apoptosis induction (Sharma et al. 1996; Moller et al. 2003). These processes of cell proliferation, apoptosis, and angiogenesis are important in renal hemodynamics and function.
In the kidney, somatostatin is reported to reduce glomerular filtration rate and decrease renal blood flow through direct renal vasoconstriction (Schmidt et al. 2002). At a cellular level, somatostatin exerts an anti-diuretic affect by depressing free water clearance and by an inhibition of vasopressin-induced water permeability in the distal tubules, thus reducing urine volume (Ray et al. 1993). It has been shown that somatostatin suppresses the release of aldosterone and renin (Jones et al. 1984; Sieber et al. 1988; Mazzocchi et al. 1992; Reubi et al. 1993). In addition, somatostatin may inhibit mesangial cell proliferation and contraction (Garcia-Escribano et al. 1993; Garcia-Escribano et al. 1994), and epidermal growth factor induced human proximal tubular epithelial cell proliferation (Hatzoglou et al. 1996; Turman and Apple 1998). Somatostatin and its synthetic analogs have been shown to inhibit experimental angiogenesis in in vitro and in vivo models (Danesi et al. 1997; Adams et al. 2004). Some analogs have been used for somatostatin receptor scintigraphy for kidney cancer imaging and radiotherapy (McCaffrey et al. 2000; Vegt et al. 2006).
There are several reports on the expression of somatostatin or ssts in mouse (Bates et al. 2004b), rat (Kurokawa et al. 1983; Bruno et al. 1993; Kimura et al. 2001), and human kidney (Yamada et al. 1992; Balster et al. 2001) using northern blotting, RT-PCR, and immunostaining; however, the immunostaining for all sst subtypes and the spliced isoforms in kidney are still unknown, and no data are available for the diseased human kidney. In this study, we examined the distribution of somatostatin and somatostatin receptor subtypes (sst1–5) in normal human kidney using immunohistochemistry, immunofluorescence, and real-time RT-PCR and the expression patterns of ssts in IgA nephropathy.
Materials and Methods
Tissue Retrieval
Paraffin blocks were obtained from nephrectomy and renal biopsy samples at Hull and East Yorkshire NHS Trust (Hull, UK) with approval of the local ethics committee (Reference Elsy no. CHH 341). Twenty-five normal and four IgA nephropathy human kidney samples from patients undergoing nephrectomy or diagnostic renal biopsy were used in this study. The criteria for normal kidney tissue samples are based on pathological diagnosis rather than from healthy volunteer subjects. Four patients presenting classical IgA nephropathy underwent renal biopsy, and the diagnosis was confirmed based on pathological findings that included expansion of the mesangial matrix, proliferation of mesangial cells (hypercellularity), and immunofluorescent staining for IgA and C3 and occasional IgG in the mesangium and to a lesser extent the glomerular capillary wall.
Immunohistochemistry
Paraffin-embedded kidney tissue sections (3 μm thickness) were immunostained for sstl, sst2A, sst2B, sst3, sst4, and sst5 using polyclonal rabbit antisera (Gramsch Laboratories; Schwabbausen, Germany). Somatostatin was stained by rabbit polyclonal anti-human somatostatin antibody (AHP533; AbD Serotec, Kidlington, UK). Two staining methods were used to characterize sst expression. sst1, sst2B, sst3, and sst5 underwent tyramide signal amplification, and somatostatin, sst2A, and sst4 underwent Dako-catalyzed signal amplification. All sections underwent deparaffinization, endogenous peroxidase blocking, heat-induced epitope retrieval by microwave heating in citrate buffer, pH 6, for 20 min, and avidin/biotin blocking (Vector Laboratories; Peterborough, UK), along with blocking in nonspecific serum to ensure that staining was specific. sst1, sst2B, sst3, and sst5 primary antibodies were incubated at 1:5000 overnight at 4C, followed by biotinylated anti-mouse/rabbit immunoglobulins (Dako; Cambridgeshire, UK) at 1:1000 for 18 min. For somatostatin, the anti-somatostatin antibody as a dilution of 1:100 was used (Hall et al. 2002). sst2B and stt4 underwent incubation at a dilution of 1:10,000 overnight at 4C, using Dako Catalyzed Signal Amplification Peroxidase System (Dako), with a biotinylated secondary link antibody and streptavidin-biotin complex. All staining was visualized using 3,3′-diaminobenzidine and was counterstained with hematoxylin. Immunostaining in the various parts of the kidney sections (in relation to its intensity or positivity) was characterized and was scored as 0 (negative), 1 (weak staining), 2 (stained), or 3 (strong staining). Negative controls included the omission of the primary antibody or incubation with normal goat serum diluted 1:20 with TBS or normal rabbit serum diluted 1:2500 for the same period of time as the primary antibody.
Immunofluorescence
Frozen kidney tissue sections (20 μm thickness) were fixed with 4% paraformaldehyde and permeabilized by incubation in −20C methanol for 1 min and 0.1% Triton X-100 in PBS for 3 hr at room temperature. Sections were incubated in 1% BSA to block nonspecific binding and incubated in the appropriate sst primary antibody at 1:500 dilution in PBS with 1% BSA at 4C overnight (Gramsch Laboratories). Tissue sections were washed three times with PBS and incubated in the affinity purified sheep anti-rabbit IgG conjugated with FITC (Sigma; Poole, UK) at a dilution of 1:160 for 3 hr at room temperature. Sections were double stained by incubation for 4 hr in the Cy3-conjugated monoclonal anti-α-smooth muscle actin (SMA; Sigma) at a dilution of 1:200. After three washes with PBS, sections were coverslipped with Vectashield mounting medium with 4′-6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Staining was photographed using Bio-Rad Radiance 2100 confocal microscope and acquisition software (Bio-Rad; Hercules, CA).
Quantitative RT-PCR
Total RNA was extracted from the snap-frozen normal kidney samples in liquid nitrogen using Trizol (Invitrogen; Paisley, UK) and treated with DNase I (Invitrogen) to remove genomic DNA contamination before reverse transcription in the presence of RNasin RNase inhibitor (Promega; Southampton, UK). The RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase using random primers (Invitrogen). Quantitative RT-PCR was preformed using GeneAmp 5700 sequence detection system (Applied Biosystems; Foster City, CA). The forward and reverse primers and probes for sst1–5 were synthesized by MWG-Biotech (Ebersberg, Germany) (Table 1). The internal probes were labeled at the 5′ ends with the reporter fluorochrome 6-carboxyfluorescein (FAM) and at the 3′ ends with the quencher fluorochrome 6-carboxytetramethylrhodamine (TAMRA). Each reaction volume was 25 μl and contained 1× TaqMan Universal Master Mix (Applied Biosystems), 5 μl cDNA, 0.75 μl 300 nM forward primer, 0.75 μl 300 nM reverse primer, and 0.38 μl 150 nM internal probe. The human β-glucuronidase housekeeping gene (GUSB; Applied Biosystems) was used as an internal standard. Water was used as a non-template control. Non–reverse-transcribed samples were run in parallel to confirm that positive results were not caused by amplification of genomic DNA. Human genomic DNA or plasmid somatostatin receptor cDNAs were used as positive controls. The PCR cycle consisted of an initial cycle of 50C for 2 min followed by 95C for 10 min, 50 repeated cycles of 95C for 15 sec (denaturation), and 50C for 1 min (primer annealing and extension).
Table 1.
Primers and probes used for quantitative RT-PCR
| Subtype
|
Primer/probe sequence
|
|
| sst1 | Forward | 5′-GCTCGGAGCGCAAGATCA-3′ |
| Reverse | 5′-CGTCGTCCTGCTCAGCAAA-3′ | |
| Probe | 5′-CTTAATGGTGATGATGGTGGTGATGGTGTTT-3′ | |
| sst2 | Forward | 5′-TGGTCCACTGGCCCTTTG-3′ |
| Reverse | 5′-TTGATGCCATCCACAGTCATG-3′ | |
| Probe | 5′-CAAGGCCATTTGCCGGGTGG-3′ | |
| sst2A | Forward | ACAGCTGTGCCAACCCTATC |
| Reverse (1) | TGGACTCATGCTGAGCAATC | |
| Reverse (2) | TAAAGATCATATCCAGGCATGATCC | |
| sst2B | Forward | ACAGCTGTGCCAACCCTATC |
| Reverse | GAATTGTCTACCTTGACCAAGCA | |
| sst3 | Forward | 5′-TGGGCCTGCTGGACTC-3′ |
| Reverse | 5′-GTTGAGGATGTAGACGTTGGTGACT-3′ | |
| Probe | 5′-CCGTGTGCCGCAGGACCACA-3′ | |
| sst4 | Forward | 5′-GCGCTCGGAGAAGAAAATCA-3′ |
| Reverse | 5′-GGCTGGTCACGACGAGGTT-3′ | |
| Probe | 5′-CGTCTTTGTGCTCTGCTGGATGCCTT-3′ | |
| sst5 | Forward | 5′-TCATCCTCTCCTACGCCAACA-3′ |
| Reverse | 5′-TGGAAGCTCTGGCGGAAGT-3′ | |
| Probe | 5′-CCGTCCTCTCAGGCTTCCTCTCGGA-3′ | |
sst, somatostatin receptor subtype.
Results
Distribution of Somatostatin
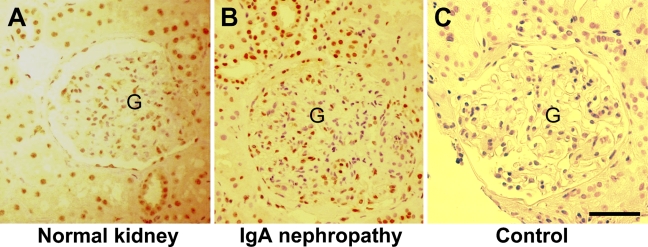
The anti-somatostatin can recognize both SRIF 14 and SRIF-28 (Hall et al. 2002). Staining was positive in human tubular epithelial cells and glomerular cells, particularly for nuclei that were more intensively stained. Epithelial cells in both the proximal convoluted tubule (PCT) and distal convoluted tubule (DCT) were clearly stained (Figure 1A). Epithelial cells in the urine pole were also positive. However, we found about one half the cells in the glomeruli were stained, and some were negative. The positively stained cells could be glomerular epithelial cells (podocytes) and vascular endothelial cells, because the staining pattern was different from that for mesangial cells (Balster et al. 2001; Bates et al. 2004a).
Figure 1.
Immunostaining for somatostatin in human kidney. Paraffin-embedded normal human kidney (A) and IgA nephropathy kidney (B) sections were stained with anti-somatostatin antibody using the horseradish peroxidase (HRP)-StrepABC kit. (C) Control staining with normal rabbit serum. Positive staining (brown) in the epithelial cells of tubules and glomeruli (G). Bar = 100 μm.
The immunostaining on the sections of IgA nephropathy showed a staining pattern that was similar to that of normal kidney (Figure 1B).
Somatostatin Receptors in Normal Kidney
We examined the protein distribution of sst1–5 in paraffin-embedded sections of normal human kidney using samples from nephrectomy or renal biopsy specimens. The rabbit polyclonal anti-somatostatin receptor antibodies used in this study have been extensively characterized in previous studies on native tissues (Schulz et al. 1998a,b,2000; Green et al. 2002; Hall et al. 2002; Stafford et al. 2003,2004; Notas et al. 2004; Taniyama et al. 2005; Vezzosi et al. 2005; Druckenthaner et al. 2007), the cell lines overexpressing somatostatin genes (Schulz et al. 1998b,2000), and controls with antibodies absorbed with antigenic peptide (Schulz et al. 1998a,b,2000; Notas et al. 2004; Druckenthaner et al. 2007). These antibodies targeting to the C terminus of the receptors had no cross-reactivity among the subtypes (Schulz et al. 1998a,b,2000). The specificity of these antibodies was examined by positive staining using human anterior pituitary, pancreas, endometrium, acoustic neuroma, and endothelial cells as previously reported (Green et al. 2002; Stafford et al. 2003,2004; Adams et al. 2005) and negative staining using normal rabbit or goat sera.
Tubular Staining
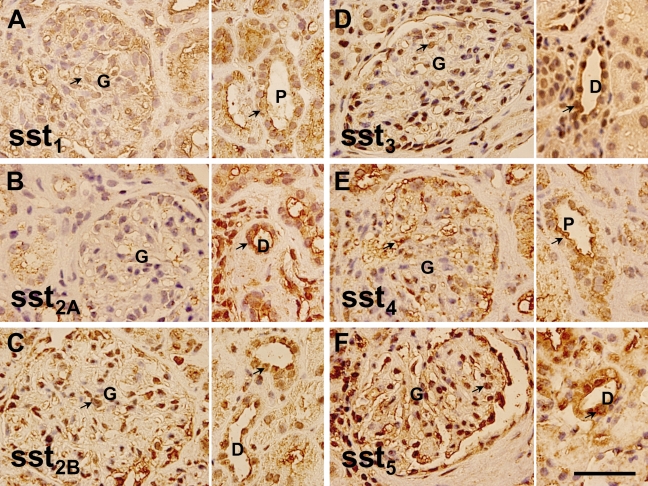
The PCTs, DCTs, and loop of Henle (LH) were identified by their histological characteristics on the tissue sections. Positive immunostaining for all isoforms of somatostatin receptors (sst1, sst2A, sst2B, sst3, sst4, and sst5) was found in the PCTs, DCTs, and LH (Figure 2). The staining pattern for nuclear areas was more condensed for all ssts, but cytoplasmic staining was also observed for sst1, sst2B, and sst5.
Figure 2.
Distribution of somatostatin receptors in normal human kidney. Examples of immunostaining for somatostatin receptor (sst)1, sst2A, sst2B, sst3, sst4, and sst5 are shown in A–F. Left panel shows the glomerular (G) staining and right panel shows the staining of proximal (P) or distal (D) tubules (n=25). Staining is indicated by arrows. Bar = 100 μm.
Glomerular Staining
The glomerular cells were positively stained by anti-sst1, anti-sst2B, anti-sst3, and anti-sst5 antibodies and weakly stained by anti-sst2A and anti-sst4. Staining for sst1 and sst4 was predominantly cytoplasmic, whereas staining for sst2B, sst3, and sst5 was observed in both nuclear and cytoplasmic areas. Positive cell types in the glomeruli were not ascertained in the paraffin sections, but proximal tubular epithelia starting from glomerular urinary poles were clearly labeled by anti-sst1, anti-sst2B, anti-sst3, and anti-sst5 (Figure 2).
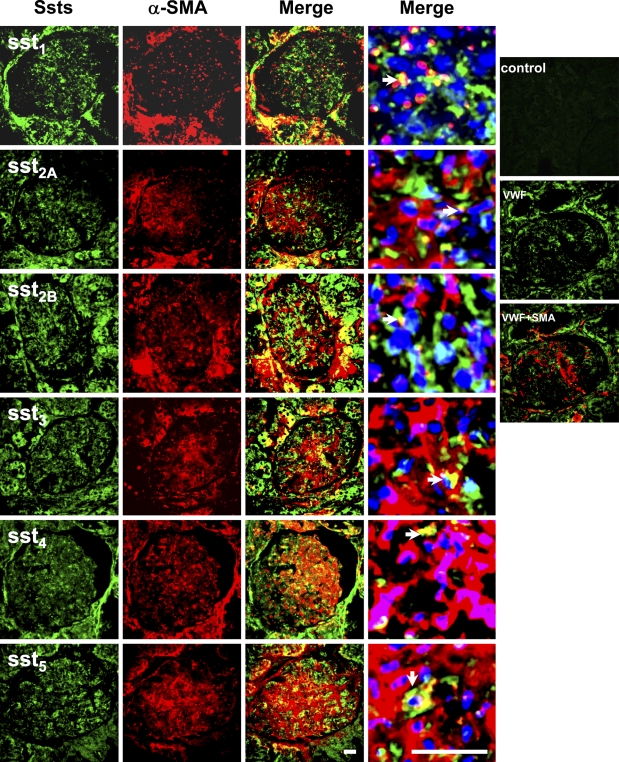
Dual Fluorescent Labeling With Anti-α-SMA
To confirm the cell types and determine the location of the somatostatin receptors in a cell, fluorescent immunostaining was performed using frozen tissue sections of normal human kidney. α-SMA was used as a cell marker of mesangial cells (Johnson et al. 1991), and von Willebrand factor (VWF) was used as a marker of vascular endothelial cell (Pusztaszeri et al. 2006). We found the anti-α-SMA antibody strongly stained vascular smooth muscle cells (data not shown) and only weakly stained mesangial cells in normal kidney tissue sections. Overlapping staining of sst1, sst2B, sst4, and sst5 with α-SMA was observed (Figure 3), suggesting that glomerular mesangial cells express sst1, sst2B, sst4, and sst5. Some glomerular cells (possibly capillaries) were labeled with anti-sst2A, but the pattern was different from mesangial cells that were labeled with anti-α-SMA, suggesting sst2A may not be present in mesangial cells. The staining for sst1, sst4, and sst5 was predominantly cytoplasmic (Figures 2 and 3). Some cells in the glomeruli were stained with anti-VWF but not colocalized with anti-α-SMA (Figure 3), suggesting they were more likely endothelial cells.
Figure 3.
Dual immunofluorescent staining for somatostatin receptors and smooth muscle α-actin (SMA) in glomeruli. Staining patterns of somatostatin receptors in the glomeruli (green, FITC) and double stained by anti-smooth muscle α-actin (red, Cy3). Merged pictures with nuclear staining (blue, 4′-6-diamidino-2-phenylindole) are the glomeruli with 6× zoom for left photo images. No staining for normal rabbit serum control (Control). There was no overlapping staining for von Willebrand factor (VWF) and SMA (VWF + SMA). Overlapping staining (yellow) is indicated by arrowheads in merged images. Bar = 50 μm.
Somatostatin Receptors in IgA Nephropathy Kidney
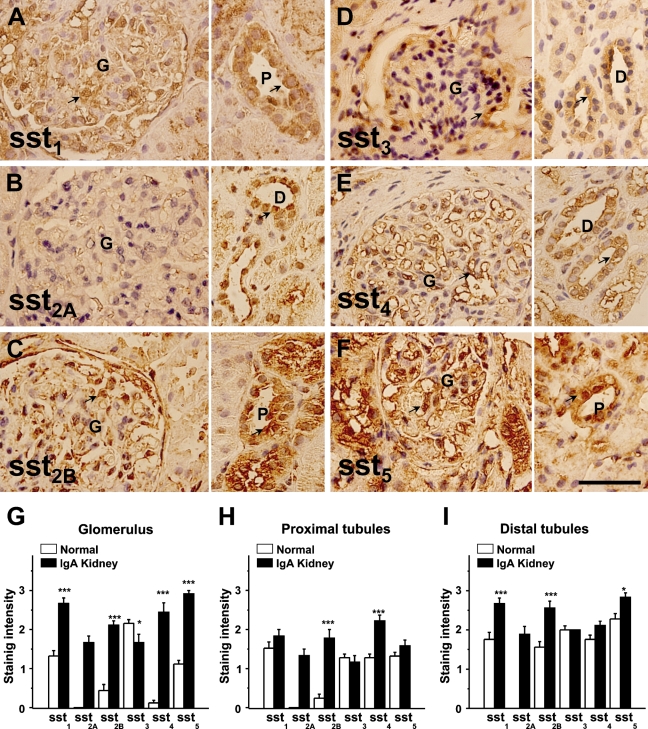
Paraffin-embedded biopsy samples from four patients diagnosed with IgA nephropathy were stained with anti-somatostatin antibodies, and normal kidney tissue sections were stained in parallel as a control. Like the staining in normal kidney, we found all somatostatin receptors (sst1–5) were positive in sections of IgA nephropathy kidney, but staining for sst1, sst2B, sst4, and sst5 was much stronger than that seen in normal kidney sections. sst3 staining was similar or perhaps less intense in the glomeruli in comparison to controls (Figure 4). This increased staining was not limited to the glomeruli but was also found in the proximal and distal tubules. In the IgA nephropathy kidney samples, more staining was observed in the cytoplasm for sst1, sst2B, sst4, and sst5. The mean data for intensity of staining scores are shown in Figures 4G–4I.
Figure 4.
Immunohistochemical staining of IgA nephropathy kidney sections. Examples of immunostaining for sst1, sst2A, sst2B, sst3, sst4, and sst5 are shown in A–F. Left panel shows the glomerular (G) staining and right panel shows the staining of proximal (P) and distal (D) tubules. Staining is indicated by arrows. Kidney samples were from four IgA nephropathy patients. (G–I) Quantitative comparison using the scorings of immunostaining in normal and IgA nephropathy kidney sections. Bar = 100 μm.
Detection of Somatostatin Receptor mRNAs in Human Kidney
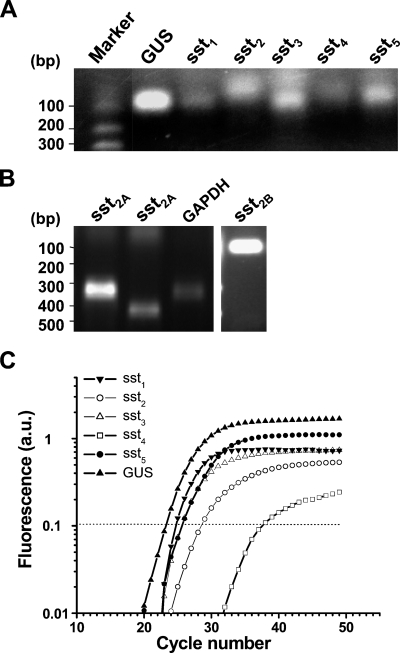
The mRNA of sst1–5 in the normal kidney was detected by RT-PCR (Figure 5A). The spliced isoform of sst2A and sst2B were also detected by an isoform-specific primer set (Figure 5B). Because sst4 has no introns, we used real-time RT-PCR to quantitatively compare mRNA after genomic DNA digestion. The mRNA expression of sst1–5 was confirmed. sst1, sst2, and sst5 were expressed consistently at high levels in four separate kidney samples, but very low levels of sst3 and sst4 were observed. The internal fluorescent probes used for real-time PCR were specific for the individual amplicon, because no fluorescent signal was observed if a nonspecific internal probe was used as a control (Figure 5C).
Figure 5.
Detection of somatostatin receptor mRNA in kidney by RT-PCR. (A) PCR products were shown in the 2% agarose gel stained with ethidium bromide. The expected size of PCR amplicons is 68 bp for sst1, 61 bp for sst2, 86 bp for sst3, 68 bp for sst4, and 79 bp for sst5. (B) The spliced variants of sst2A [Lane 1, 332 bp with reverse primer (1); Lane 2, 475 bp with reverse primer (2)] and sst2B (95 bp) were detected by RT-PCR. The housekeeping gene, GAPDH (326 bp), was used as control. The sst2B product was confirmed by direct sequencing. (C) An example of real-time PCR detection of somatostatin mRNA in kidney. No product was detected if the nonspecific probes for somatostatin were used, such as sst1 probe used for detection of sst2 (data not shown).
Discussion
In this study, we showed that somatostatin and all five somatostatin receptor subtypes including spliced variants are widely expressed in normal kidney. These receptors are predominantly distributed in the tubular epithelium and glomerular mesangial cells. In addition, we showed for the first time that somatostatin receptors are expressed more highly in IgA nephropathy human kidney and found that several isoforms (sst1, sst2B, sst4, and sst5) are upregulated in this inflammatory kidney disease. These data provide new evidence in the understanding of the role of somatostatin analogs in the normal and diseased kidney.
There are several reports on the expression of somatostatin and somatostatin receptors in animal and human kidney tissues detected by northern blotting or RT-PCR at the mRNA level or the tissue distribution by immunostaining (Table 2). Northern blotting is an insensitive method for mRNA detection that has been widely used in previous studies, and it has been replaced by RT-PCR or real-time RT-PCR. Using real-time PCR, we found all five subtypes of somatostatin receptors exist in kidney tissue. To avoid genomic DNA contamination, we treated the RNA samples with DNase and also set negative and positive (genomic DNA or plasmid somatostatin receptor cDNA) controls in parallel for RT-PCR. Because some isoforms of ssts, such as sst4, have no introns, we used real-time RT-PCR to quantitatively compare the groups with and without reverse transcriptase treatment. We found a clear shift of the real-time PCR curve between the groups, which confirms the presence of somatostatin receptor mRNAs.
Table 2.
Distribution of somatostatin and somatostatin receptor subtypes in animal and human kidney
| ss | sst1 | sst2A | sst2B | sst3 | sst4 | sst5 | References | |
|---|---|---|---|---|---|---|---|---|
| Mouse | ||||||||
| Distal tubules | + | + | Bates et al. 2003,2004a,b | |||||
| Proximal tubules | + | + | + | + | + | |||
| Collecting ducts | + | |||||||
| Glomeruli | + | + | + | + | ||||
| mRNA | + | + | + | + | + | + | ||
| Rat | ||||||||
| Distal tubules | Kurokawa et al. 1983; Bruno et al. 1993; Kimura et al. 2001 | |||||||
| Proximal tubules | ||||||||
| Collecting ducts | + | |||||||
| Glomeruli | + | + | ||||||
| mRNA | + | + | + | + | ±, + | ± | ||
| Human | ||||||||
| Distal tubules | + | + | + | + | + | + | + | Yamada et al. 1992; Rohrer et al. 1993; Turman et al. 1997; Turman and Apple 1998; Balster et al. 2001; Taniyama et al. 2005a |
| Proximal tubules | + | + | + | + | + | + | + | |
| Collecting ducts | + | + | + | + | + | + | + | |
| Glomeruli | + | + | + | + | + | + | + | |
| mRNA | + | + | + | + | ±,+ | + | + | |
Results from this study.
ss, somatostatin; sst, somatostatin receptor subtypes; +, positive staining; ±, disputable.
Immunostaining with specific antibodies is usually important for protein localization. sst1 has been reported exclusively in tubules in an earlier study (Balster et al. 2001), but we found that sst1 clearly stained in the glomerular cells (mesangial cells), as shown in both paraffin-embedded and frozen sections. This may be because of the antibody used, which targeted the 57 amino acid N terminus (Balster et al. 2001). However, we used the peptide-specific anti-sst1 antibody targeting the eight amino acids (CTSRITTL) in the C terminus. The specificity of the sst1 antibody used in this study has been extensively examined in many studies (Schulz et al. 1998a,b,2000; Green et al. 2002; Stafford et al. 2003,2004; Adams et al. 2005). Therefore, our results suggest that sst1 is widely expressed in the tubules and glomeruli. We noted that staining was possibly both cytosolic and nuclear. Because of the method used with histochemical staining of paraffin sections, we cannot clearly delineate the exact intracellular location of proteins, and hence it is difficult to be more specific. We know that several somatostatin receptors have the property of internalization, suggesting the localization in cytosol and around nuclear area is possible. In addition, the laser confocal pictures (Figure 3) also suggest that the staining may not be in the nuclei (blue staining with DAPI).
Both sst2A and sst2B isoforms are predominantly located in the tubules. Staining for sst2B was much stronger than that for sst2A in the human kidney, suggesting that the expression of the sst2B isoform could be higher. This result is in accordance with reports of sst2A being expressed in kidney tubules (Kimura et al. 2001; Taniyama et al. 2005). In addition, the autoradiography data with a high affinity sst2 analog [177Lu-DOTA] octreotate also showed the binding in proximal tubules and glomeruli (Melis et al. 2005). The renal radiation injury caused by the sst2-specific radioactive octreotide further supports the presence of sst2 in human kidney (Rolleman et al. 2007).
The finding of somatostatin and somatostatin receptor subtypes (sst1, sst2B, sst3, sst4, and sst5) within the mesangial cells suggests a role for their regulation in renal function (Ray et al. 1993; Schmidt et al. 2002). The upregulation of somatostatin receptors in IgA nephropathy suggests the involvement of these receptors in inflammatory kidney disease, especially for glomerular damage caused by abnormal proliferation of mesangial cells or glomerular podocytes. Others have shown that somatostatin can inhibit the synthesis of insulin-like growth factor, which is an important regulatory protein, leading to mesangial cell proliferation and hyperfiltration as seen in IgA and diabetic nephropathy (Serri et al. 1992). Somatostatin analogs, such as octreotide that mainly binds to sst2 and sst5, have been used for the treatment of some tumors and could also have potential effects for renal disorders through the suppression of mesangial cell proliferation (Uemasu et al. 1990; Uemasu et al. 1992; Dasgupta 2004).
In conclusion, we present the tissue distribution of somatostatin and all five somatostatin receptor subtypes in histologically normal and IgA nephropathy human kidney, with increased somatostatin receptor expression in IgA nephropathy kidney. The ubiquitous distribution of these receptors in glomeruli and tubules is consistent with the observation that somatostatin or its analogs may affect function. The findings of this study could also provide a histological basis for the treatment of autosomal-dominant polycystic kidney with somatostatin analogs (Ruggenenti et al. 2005; Grantham 2006) or the related kidney side effects using somatostatin peptide-based radiation therapy (Rolleman et al. 2007).
References
- Adams RL, Adams IP, Lindow SW, Atkin SL (2004) Inhibition of endothelial proliferation by the somatostatin analogue SOM230. Clin Endocrinol (Oxf) 61:431–436 [DOI] [PubMed] [Google Scholar]
- Adams RL, Adams IP, Lindow SW, Zhong W, Atkin SL (2005) Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Br J Cancer 92:1493–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster DA, O'Dorisio MS, Summers MA, Turman MA (2001) Segmental expression of somatostatin receptor subtypes sst(1) and sst(2) in tubules and glomeruli of human kidney. Am J Physiol Renal Physiol 280:F457–465 [DOI] [PubMed] [Google Scholar]
- Bates CM, Kegg H, Grady S (2004a) Expression of somatostatin in the adult and developing mouse kidney. Kidney Int 66:1785–1793 [DOI] [PubMed] [Google Scholar]
- Bates CM, Kegg H, Grady S (2004b) Expression of somatostatin receptors 1 and 2 in the adult mouse kidney. Regul Pept 119:11–20 [DOI] [PubMed] [Google Scholar]
- Bates CM, Kegg H, Petrevski C, Grady S (2003) Expression of somatostatin receptors 3, 4, and 5 in mouse kidney proximal tubules. Kidney Int 63:53–63 [DOI] [PubMed] [Google Scholar]
- Benali N, Ferjoux G, Puente E, Buscail L, Susini C (2000) Somatostatin receptors. Digestion 62(suppl 1):27–32 [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R (1973) Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179:77–79 [DOI] [PubMed] [Google Scholar]
- Bruno JF, Xu Y, Song J, Berelowitz M (1993) Tissue distribution of somatostatin receptor subtype messenger ribonucleic acid in the rat. Endocrinology 133:2561–2567 [DOI] [PubMed] [Google Scholar]
- Bruns C, Weckbecker G, Raulf F, Kaupmann K, Schoeffter P, Hoyer D, Lubbert H (1994) Molecular pharmacology of somatostatin-receptor subtypes. Ann NY Acad Sci 733:138–146 [DOI] [PubMed] [Google Scholar]
- Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, Culler M, et al. (2006) Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med 47:502–511 [PubMed] [Google Scholar]
- Danesi R, Agen C, Benelli U, Paolo AD, Nardini D, Bocci G, Basolo F, et al. (1997) Inhibition of experimental angiogenesis by the somatostatin analogue octreotide acetate (SMS 201–995). Clin Cancer Res 3:265–272 [PubMed] [Google Scholar]
- Dasgupta P (2004) Somatostatin analogues: multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol Ther 102:61–85 [DOI] [PubMed] [Google Scholar]
- Druckenthaner M, Schwarzer C, Ensinger C, Gabriel M, Prommegger R, Riccabona G, Decristoforo C (2007) Evidence for somatostatin receptor 2 in thyroid tissue. Regul Pept 138:32–39 [DOI] [PubMed] [Google Scholar]
- Garcia-Escribano C, Diez-Marques ML, Gonzalez-Rubio M, Rodriguez-Puyol M, Rodriguez-Puyol D (1993) Somatostatin antagonizes angiotensin II effects on mesangial cell contraction and glomerular filtration. Kidney Int 43:324–333 [DOI] [PubMed] [Google Scholar]
- Garcia-Escribano C, Diez-Marques ML, Medina-Alonso J, Rodriguez-Puyol M, Rodriguez-Puyol D (1994) Somatostatin activates particulate guanylate cyclase in cultured rat mesangial cells. Kidney Int 46:1611–1615 [DOI] [PubMed] [Google Scholar]
- Grantham JJ (2006) Does extended-release somatostatin slow the growth of renal cysts in autosomal-dominant polycystic kidney disease? Nat Clin Pract Nephrol 2:66–67 [DOI] [PubMed] [Google Scholar]
- Green VL, Richmond I, Maguiness S, Robinson J, Helboe L, Adams IP, Drummond NS, et al. (2002) Somatostatin receptor 2 expression in the human endometrium through the menstrual cycle. Clin Endocrinol (Oxf) 56:609–614 [DOI] [PubMed] [Google Scholar]
- Hall GH, Turnbull LW, Richmond I, Helboe L, Atkin SL (2002) Localisation of somatostatin and somatostatin receptors in benign and malignant ovarian tumours. Br J Cancer 87:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzoglou A, Bakogeorgou E, Papakonstanti E, Stournaras C, Emmanouel DS, Castanas E (1996) Identification and characterization of opioid and somatostatin binding sites in the opossum kidney (OK) cell line and their effect on growth. J Cell Biochem 63:410–421 [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, et al. (1991) Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 87:847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Millar JA, Lawrie C, Sumner DJ, Reid JL (1984) Specific inhibition of aldosterone responses to endogenous and exogenous angiotensin II by somatostatin. Clin Endocrinol (Oxf) 21:279–284 [DOI] [PubMed] [Google Scholar]
- Kimura N, Schindler M, Kasai N, Kimura I (2001) Immunohistochemical localization of somatostatin receptor type 2A in rat and human tissues. Endocr J 48:95–102 [DOI] [PubMed] [Google Scholar]
- Krantic S (2000) Peptides as regulators of the immune system: emphasis on somatostatin. Peptides 21:1941–1964 [DOI] [PubMed] [Google Scholar]
- Kreienkamp HJ, Roth A, Richter D (1998) Rat somatostatin receptor subtype 4 can be made sensitive to agonist-induced internalization by mutation of a single threonine (residue 331). DNA Cell Biol 17:869–878 [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Aponte GW, Fujibayashi S, Yamada T (1983) Somatostatin-like immunoreactivity in the glomerulus of rat kidney. Kidney Int 24:754–757 [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Malendowicz LK, Meneghelli V, Nussdorfer GG (1992) Evidence that endogenous somatostatin (SRIF) exerts a tonic inhibitory effect on the rat renin–angiotensin–aldosterone system. In Vivo 6:9–12 [PubMed] [Google Scholar]
- McCaffrey JA, Reuter VV, Herr HW, Macapinlac HA, Russo P, Motzer RJ (2000) Carcinoid tumor of the kidney. The use of somatostatin receptor scintigraphy in diagnosis and management. Urol Oncol 5:108–111 [DOI] [PubMed] [Google Scholar]
- Melis M, Krenning EP, Bernard BF, Barone R, Visser TJ, de Jong M (2005) Localisation and mechanism of renal retention of radiolabelled somatostatin analogues. Eur J Nucl Med Mol Imaging 32:1136–1143 [DOI] [PubMed] [Google Scholar]
- Moller LN, Stidsen CE, Hartmann B, Holst JJ (2003) Somatostatin receptors. Biochim Biophys Acta 1616:1–84 [DOI] [PubMed] [Google Scholar]
- Notas G, Kolios G, Mastrodimou N, Kampa M, Vasilaki A, Xidakis C, Castanas E, et al. (2004) Cortistatin production by HepG2 human hepatocellular carcinoma cell line and distribution of somatostatin receptors. J Hepatol 40:792–798 [DOI] [PubMed] [Google Scholar]
- Pinter E, Helyes Z, Szolcsanyi J (2006) Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther 112:440–456 [DOI] [PubMed] [Google Scholar]
- Pusztaszeri MP, Seelentag W, Bosman FT (2006) Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54:385–395 [DOI] [PubMed] [Google Scholar]
- Ray C, Carney S, Morgan T, Gillies A (1993) Somatostatin as a modulator of distal nephron water permeability. Clin Sci (Lond) 84:455–460 [DOI] [PubMed] [Google Scholar]
- Reubi JC, Horisberger U, Studer UE, Waser B, Laissue JA (1993) Human kidney as target for somatostatin: high affinity receptors in tubules and vasa recta. J Clin Endocrinol Metab 77:1323–1328 [DOI] [PubMed] [Google Scholar]
- Rohrer L, Raulf F, Bruns C, Buettner R, Hofstaedter F, Schule R (1993) Cloning and characterization of a fourth human somatostatin receptor. Proc Natl Acad Sci USA 90:4196–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleman EJ, Krenning EP, Bernard BF, de Visser M, Bijster M, Visser TJ, Vermeij M, et al. (2007) Long-term toxicity of [(177)Lu-DOTA (0),Tyr (3)]octreotide in rats. Eur J Nucl Med Mol Imaging 34:219–227 [DOI] [PubMed] [Google Scholar]
- Roosterman D, Kreuzer OJ, Brune N, Cottrell GS, Bunnett NW, Meyerhof W, Steinhoff M (2007) Agonist-induced endocytosis of rat somatostatin receptor 1. Endocrinology 148:1050–1058 [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, Remuzzi G, et al. (2005) Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 68:206–216 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Pleiner J, Schaller G, Roden M, Dallinger S, Mayer G, Schmetterer L, et al. (2002) Renal hemodynamic effects of somatostatin are not related to inhibition of endogenous insulin release. Kidney Int 61:1788–1793 [DOI] [PubMed] [Google Scholar]
- Schonbrunn A, Tashjian H Jr (1978) Characterization of functional receptors for somatostatin in rat pituitary cells in culture. J Biol Chem 253:6473–6483 [PubMed] [Google Scholar]
- Schulz S, Pauli SU, Handel M, Dietzmann K, Firsching R, Hollt V (2000) Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A. Clin Cancer Res 6:1865–1874 [PubMed] [Google Scholar]
- Schulz S, Schmitt J, Wiborny D, Schmidt H, Olbricht S, Weise W, Roessner A, et al. (1998a) Immunocytochemical detection of somatostatin receptors sst1, sst2A, sst2B, and sst3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clin Cancer Res 4:2047–2052 [PubMed] [Google Scholar]
- Schulz S, Schreff M, Schmidt H, Handel M, Przewlocki R, Hollt V (1998b) Immunocytochemical localization of somatostatin receptor sst2A in the rat spinal cord and dorsal root ganglia. Eur J Neurosci 10:3700–3708 [DOI] [PubMed] [Google Scholar]
- Serri O, Brazeau P, Kachra Z, Posner B (1992) Octreotide inhibits insulin-like growth factor-I hepatic gene expression in the hypophysectomized rat: evidence for a direct and indirect mechanism of action. Endocrinology 130:1816–1821 [DOI] [PubMed] [Google Scholar]
- Sharma K, Patel YC, Srikant CB (1996) Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol 10:1688–1696 [DOI] [PubMed] [Google Scholar]
- Sieber C, Gnadinger M, Del Pozo E, Shaw S, Weidmann P (1988) Effect of a new somatostatin analogue SMS 201–995 (Sandostatin) on the renin-aldosterone axis. Clin Endocrinol (Oxf) 28:25–32 [DOI] [PubMed] [Google Scholar]
- Stafford ND, Condon LT, Rogers MJ, Helboe L, Crooks DA, Atkin SL (2004) The immunohistochemical localisation of somatostatin receptors 1, 2, 3, and 5 in acoustic neuromas. J Clin Pathol 57:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford ND, Condon LT, Rogers MJ, MacDonald AW, Atkin SL (2003) The expression of somatostatin receptors 1 and 2 in benign, pre-malignant and malignant laryngeal lesions. Clin Otolaryngol Allied Sci 28:314–319 [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H (2005) Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J 52:605–611 [DOI] [PubMed] [Google Scholar]
- Turman MA, Apple CA (1998) Human proximal tubular epithelial cells express somatostatin: regulation by growth factors and cAMP. Am J Physiol 274:F1095–1101 [DOI] [PubMed] [Google Scholar]
- Turman MA, O'Dorisio MS, O'Dorisio TM, Apple CA, Albers AR (1997) Somatostatin expression in human renal cortex and mesangial cells. Regul Pept 68:15–21 [DOI] [PubMed] [Google Scholar]
- Uemasu J, Godai K, Tokumoto A, Kawasaki H (1992) Reduced glomerular hypertrophy by somatostatin analog, SMS 201–995, in the subtotal nephrectomized rats fed high-protein meals. J Pharmacol Exp Ther 260:505–508 [PubMed] [Google Scholar]
- Uemasu J, Tokumoto A, Godai K, Kawasaki H, Hirayama C (1990) Effects of chronic administration of somatostatin analogue SMS 201–995 on the progression of chronic renal failure in subtotal nephrectomized rats. Exp Clin Endocrinol 96:97–104 [DOI] [PubMed] [Google Scholar]
- Vegt E, Wetzels JF, Russel FG, Masereeuw R, Boerman OC, van Eerd JE, Corstens FH, et al. (2006) Renal uptake of radiolabeled octreotide in human subjects is efficiently inhibited by succinylated gelatin. J Nucl Med 47:432–436 [PubMed] [Google Scholar]
- Vezzosi D, Bennet A, Rochaix P, Courbon F, Selves J, Pradere B, Buscail L, et al. (2005) Octreotide in insulinoma patients: efficacy on hypoglycemia, relationships with Octreoscan scintigraphy and immunostaining with anti-sst2A and anti-sst5 antibodies. Eur J Endocrinol 152:757–767 [DOI] [PubMed] [Google Scholar]
- Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C (2003) Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2:999–1017 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S (1992) Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci USA 89:251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]