Summary
Lentiviral vectors are useful for gene transfer to dividing and non-dividing cells. Feline immunodeficiency virus (FIV) vectors transduce most human cell types with good efficiency and may have advantages for clinical gene therapy applications. This article reviews significant progress in the development and refinement of FIV vector systems.
Keywords: gene therapy, retroviral vectors, feline immunodeficiency virus, innate immunity
Introduction
Several years after HIV-1 was described as the cause of human AIDS, a feline lentivirus was shown to cause a similar immunodeficiency in domestic cats (Pedersen et al., 1987). Like HIV-1, FIV is also T-lymphotropic, but displays broader tropism, since it infects CD8+ T cells and B cells as well as CD4+ T cells and macrophages. Many other non-domestic feline species are infected with highly related feline lentiviruses. Although some immune abnormalities have been reported (Roelke et al., 2006), non-domestic feline species rarely if ever suffer detectable illness (Troyer et al., 2005). This situation parallels the record in primates, where Old World monkeys remain healthy despite highly productive infection with lentiviruses (Beer et al., 1998; Broussard et al., 2001; Chakrabarti, 2004; Chakrabarti et al., 2000). No lentiviruses are transmitted across primate-human-ungulate barriers and FIV is no exception: the virus has not been transmitted to humans despite presumably widespread exposure via the same efficient mechanism operative in inter-feline transmission (biting) (Pedersen, 1993) and despite use of similar chemokine co-receptors (Willett et al., 1997a; Willett et al., 1997b; Poeschla and Looney, 1998). The primary receptor, analogous to CD4, is CD134 (Shimojima et al., 2004).
Lentiviral vectors were first demonstrated in 1995 (Parolin and Sodroski, 1995). Here and subsequently, the basic concept followed that of previous retroviral vectors: place the cis-acting viral nucleic acid signals needed for packaging, reverse transcription and integration into a “transfer vector” that also contains the non-viral gene or genes to be transferred. Then supply the viral particle proteins in trans to this artificial genome. The resulting integrated transfer vector will lack any viral protein encoding genes and will be replication-defective. Because these initial lentiviral vectors used the native HIV-1 envelope glycoprotein, they were restricted to targeting CD4+ T cells and the achievable titer was too low to be useful practically (Parolin and Sodroski, 1995; Parolin et al., 1996). At the same time, others were establishing that alternative envelope glycoproteins, e.g. from rhabdoviruses, could replace the native envelope protein in retroviral particles and dramatically enhance both infectivity and host range (Burns et al., 1993; Yee et al., 1994). Adapting this envelope “pseudotyping” strategy to HIV vectors, along with their design of a more optimally configured transfer vector and viral protein expression (packaging) construct, Naldini and colleagues first demonstrated effective transduction of nondividing cells in vitro and in vivo (Naldini et al., 1996a; Naldini et al., 1996b). The potential of nonprimate lentiviral vectors was at that point uncertain since the parental lentiviruses were much less studied and none can replicate in human cells. The realization that the block to FIV particle production in human cells was essentially only transcriptional and could be completely overcome with promoter (U3 element) substitution (Poeschla and Looney, 1998) enabled the first FIV vector, which was shown to transduce dividing and nondividing human, feline and murine cells (Poeschla et al., 1998). Iterative refinements have further streamlined the systems. The general design of a current three component FIV vector system is shown in Fig. 1 and the molecular events that occur during vector particle production are shown in Fig. 2. The concept of using non-primate lentivirus vectors for gene transfer to human cells was further validated using a lentivirus in the ungulate group, equine infectious anemia virus ( Olsen, 1998; Mitrophanous et al., 1999). So far, other ungulate lentiviruses have been more difficult to adapt into high-titer vector systems.
Fig. 1. Overview of a three-component FIV vector system.
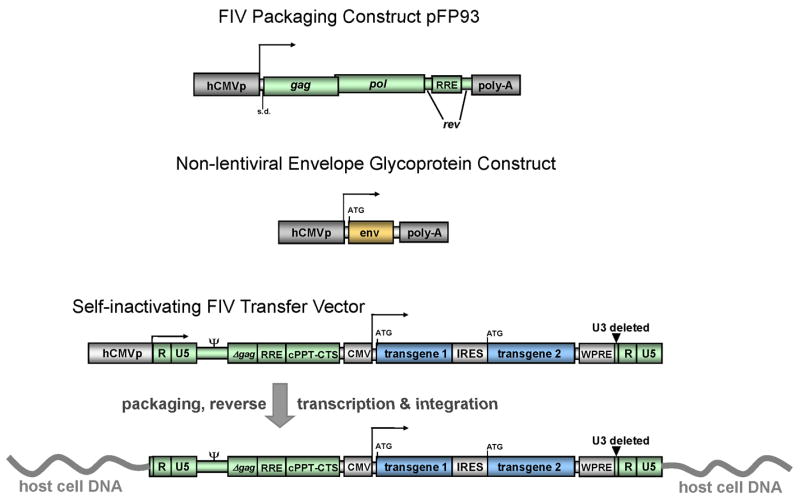
FIV-derived elements are shown in green. Normal long terminal repeats (LTRs) contain U3 (3′ unique element), R (repeat element) and U5 (5′ unique element) in that order. The transfer vector illustrated here lacks any U3 element (either 5′ or 3′), so that the viral promoter has been deleted from the system entirely. In the integrated vector, the only active promoter is the internal non-lentiviral one that drives transgene expression. The packaging construct expresses only Gag, Pol and Rev (vif, env, orf2 are abolished, as are cis-acting sequences such as both LTRs, the 5′ leader sequence, and encapsidation signal). CMV: human cytomegalovirus immediate early gene promoter. □□packaging elements. RRE: Rev response element. WPRE: woodchuck hepatitis virus posttranscriptional regulatory element. cPPT-CTS: central polypurine tract – central termination sequence (central DNA flap). Δgag: 230 proximal nt of gag, which is part of the FIv encapsidation signal.
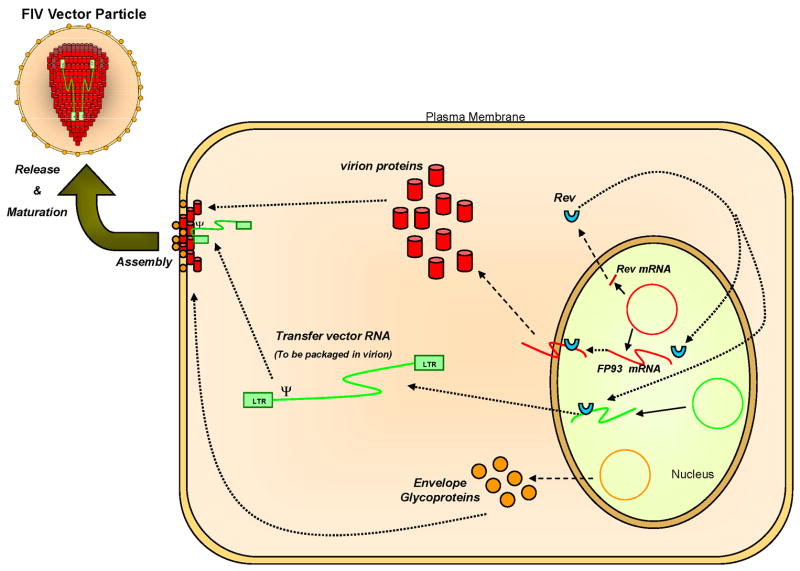
Fig. 2. Overview of events occurring during vector particle production.
The packaging construct (red circle in nucleus) is the source of Rev (blue semi-circular shape) and Gag/Pol-derived proteins (viral structural proteins and enzymes such as reverse transcriptase and integrase); the latter are shown as red cylinders. The transfer vector 513 construct (green circle in nucleus) is exported and packaged in trans. The envelope 514 glycoprotein (the expression construct is the yellow circle in the nucleus) is transported to 515 the plasma membrane and incorporated into particles.
Thus, when re-engineered into a replication-defective vector, FIV retains in human cells the core feature that has motivated lentiviral vector development – the ability to translocate its pre-integration complex across the nuclear envelope and complete integration in nondividing cells (Poeschla et al., 1998). The mechanism of this process, which has fascinated retrovirologists for decades and sharply differentiates these vectors from gammaretroviral vectors (e.g., murine leukemia virus vectors), remains poorly understood at the cell biological level. As noted above, the negligible transcriptional activity of the FIV long terminal repeat (LTR) in human cells was an initial obstacle to producing such vectors in well-characterized and highly transfectable adherent human cell lines. The problem was circumvented by replacing the 5′ U3 element with a heterologous promoter, a step that revealed that the remainder of the productive phase proceeds with high efficiency in human producer cells. Since the U3 element is regenerated at the upstream end of the integrated vector, the FIV U3 inactivity may be considered to provide a first level protection against unwanted promoter interference or activation of human cellular genes, (e.g., oncogenes). To eliminate such potential completely, U3-deleted FIV vectors have been constructed (Khare et al., 2007) following principles well-established for other retroviral vectors (Yu et al., 1986).
Although in our view there are no substantial grounds to doubt that HIV-1 can be adapted to clinical vector use safely, certain potential advantages of FIV vectors have been noted: (i) there is a substantial epidemiologic record of clinically uneventful human exposure to FIV; (ii) FIV particles do not induce HIV-cross-reactive immune responses in humans; and (iii) the possibility that a non-HIV system may result in greater patient acceptance. A fourth consideration – the built-in impediment to potential replication-competent retrovirus (RCR) propagation afforded by saturable intrinsic immunity mechanisms -- is discussed below. Initial experiments showed that FIV vectors could efficiently transduce various human cell lines, primary neurons and macrophages (Poeschla et al., 1998). These results have been confirmed with this system and with independently-derived similar systems in other laboratories (Johnston et al., 1999; Curran et al., 2000; Song et al., 2003). FIV vectors have now been applied with favorable results in the brain, eye, ear, airway, liver, muscle, pancreas and hematopoietic system (Poeschla et al., 1998; Johnston et al., 1999; Wang et al., 1999; Alisky et al., 2000; Curran et al., 2000; Loewen et al., 2001; Stein et al., 2001; Brooks et al., 2002; Curran et al., 2002; Curran and Nolan, 2002a, b; Derksen et al., 2002; Djalilian et al., 2002; Hughes et al., 2002; Kang et al., 2002; Loewen et al., 2002; Lotery et al., 2002; Price et al., 2002; Stein and Davidson, 2002; Loewen et al., 2003a; Loewen et al., 2003b; Haskell et al., 2003; Sinn et al., 2003; Loewen et al., 2004; Saenz and Poeschla, 2004;Kang et al., 2005; Harper et al., 2006; Saenz et al., 2006; Khare et al., 2007). In addition to animal models, success has been evident in explanted human organs (Wang et al., 1999; Loewen et al., 2001; Loewen et al., 2002).
For reviews and detailed protocols for FIV vector production, see (Loewen et al., 2003a; Poeschla, 2003; Saenz and Poeschla, 2004; Loewen and Poeschla, 2005; Saenz et al., 2006). These and other lentiviral vectors are generally produced by high efficiency transfection of three components: (i) a transfer vector (encodes the minimal genome RNA having the transgene, which is generally internally promoted), (ii) a packaging construct (encodes viral structural proteins and Rev in trans, and is engineered to lack encapsidation signals), and (iii) a non-lentiviral envelope glycoprotein construct for pseudotyping. This three-component system (Fig. 1) permits the production of replication-incompetent FIV viral particles containing two copies of plus-stranded RNA transfer vector. Thus, viral protein encoding sequences are not transferred to the target cell, ensuring replication-defectiveness. Existing FIV vector systems have been constructed from FIV molecular clone 34TF10 (Talbott et al., 1989).
FIV vector system overview
Advancements in basic FIV virology have continued to contribute to engineering improved vectors. The FIV vector system currently utilized in our laboratory (illustrated in Fig. 1), is described, and further details of the production components and protocols are referenced here (Loewen et al., 2003a; Saenz and Poeschla, 2004; Saenz et al., 2006;).
Transfer vector
An optimal transfer vector should exclude all native viral sequences except for the encapsidation signal and elements necessary for reverse transcription and integration (Fig. 1). Currently, standard transfer vectors incorporate these native elements and two others: the FIV rev responsive element (RRE) to facilitate efficient nuclear export of unspliced transfer vector RNA, and the central polypurine tract and central termination sequences (or cPPT-CTS) to facilitate reverse transcription and, possibly, efficient nuclear import. The encapsidation signal permits selective packaging of the transfer vector RNA. Most other elements in transfer vectors are derived from other viruses. For example, internal promoters, such as the human cytomegalovirus immediate early gene promoter (CMV) or EF1alpha promoter drive transgene expression. Other strong promoters or tissue-specific promoters may be exchanged. A picornavirus internal ribosomal entry site (IRES) is frequently used to allow simultaneous expression of two transgenes from a single internally-promoted mRNA. The woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) enhances transgene mRNA expression (Donello et al., 1998). A marker gene, such as GFP, can be used in such a vector to evaluate transduction and titrate vector stocks.
Packaging construct
The packaging construct serves as a source of FIV gag and pol genes required for necessary virion structural and enzymatic proteins. It also encodes the viral protein Rev to rescue the RRE-containing transfer vector RNA (Phillips et al., 1992). Elimination of most or all sequence homology with the transfer vector is important to reduce the risk of replication-competent retrovirus (RCR) production. Our current packaging construct, pFP93 (Fig. 1) contains sequence overlap with the transfer vector only at a short region in the 5′ end of gag (discussed below), and the RRE (Loewen et al., 2003a; Saenz and Poeschla, 2004). Compared to the initial version (Poeschla et al., 1998), vif, orf 2, additional env sequence, and the 3′ LTR have been removed. In addition, except for 11 nt containing the major splice donor, all sequences in the viral mRNA upstream of the gag ORF have been eradicated (Poeschla et al., 1998; Loewen et al., 2003a).
Pseudotyping
Pseudotyping constructs provide heterologous glycoproteins in trans to expand or re-direct cellular tropism, increase infectivity, and add stability to vector particles. Vesicular stomatitis virus glycoprotein (VSV-G) remains the most widely used glycoprotein for this purpose (Burns et al., 1993; Yee et al., 1994). Alternative glycoproteins may be used for pseudotyping lentiviral vectors including glycoproteins from rabies virus, mokola virus, gammaretroviruses, and other viruses (Cronin et al., 2005).
Progress in basic FIV virology
Encapsidation
Encapsidation is the process whereby a virion selectively packages its genomic RNA in lieu of other RNA species. The process is not completely understood, but the signals contained within the genomic RNA have been systematically mapped. Kemler and colleagues utilized RNAse protection assays to demonstrate that FIV encapsidation requires two discrete RNA segments, one in R-U5 region and one in the proximal 230 nucleotides of gag (Kemler et al., 2002; Kemler et al., 2004). The location of the latter element just downstream of the major splice donor provides a mechanism for selectively packaging full length RNA into virions. Maintenance of these encapsidation elements in the transfer vector yields wild-type levels of packaging (Kemler et al., 2004).
The feature of lentiviral vectors that most distinguishes them from conventional gammaretroviral (e.g., murine type C) vectors is their ability to transduce non-dividing cells. The pre-integration complex -- the product of reverse transcription and 3′ end processing by integrase -- is more than twice the maximum diameter of the nuclear pore complex (Cullen, 2001). Thus nuclear import cannot occur by passive diffusion. However, the mechanism of import remains controversial despite years of effort and numerous discarded hypotheses (Fouchier and Malim, 1999; Stevenson, 2000; Cullen, 2001; Vodicka, 2001). A number of peptide signals in virion proteins have been implicated as has the central DNA flap, a triple-stranded DNA structure that results at the center of the genome from a second locus of plus strand initiation (Zennou et al., 2000; Cullen, 2001). Inclusion of the HIV-1 central DNA flap elements (cPPT and CTS) augments the efficiency of HIV-1 vectors (Follenzi et al., 2000; Sirven et al., 2000; Dardalhon et al., 2001; Van Maele et al., 2003). Whitwam et al. identified and characterized the cPPT and CTS (Whitwam et al., 2001). Unlike the situation in HIV-1, the FIV cPPT and FIV 3′ PPT are not identical in sequence, which suggests the cPPT could be a partially degenerate element in FIV; alternatively, coding constraints in integrase prevent identity with the 3′ PPT. Flap formation occurs both in the context of the full length FIV and in replication-defective FIV vectors, but appears to happen less efficiently per round of replication than does flap formation in HIV-1, and a major contribution to vector efficiency has so far not been evident (Whitwam T., and Poeschla E., unpublished data).
Integration
Integration is catalyzed by the viral integrase protein (IN). Mutations at certain IN residues that participate in the catalytic center of the enzyme (e.g., D64 and D116 in HIV-1 integrase, D66 and D118 in FIV) result in “class I” phenotypes -- only integration catalysis is blocked (Engelman and Craigie, 1992; Leavitt et al., 1993; Saenz et al., 2004). Many other point mutations and virtually all wholesale deletions so far studied produce class II IN phenotypes, in which various other early and late life cycle events are defective. These complex phenotypes arise in part because integrase initially is expressed as the C-terminal portion of the Gag/Pol precursor. Class I HIV-1 and FIV mutant vectors are capable of high-level transgene expression in growth-arrested or non-dividing cells, but not dividing cells, provided an internal promoter is used (Loewen et al., 2003b; Saenz et al., 2004). These vectors illustrate the potential for transgene expression by unintegrated DNA. Indeed, this property has been utilized for retinal gene transfer with HIV-1 vectors (Yanez-Munoz et al., 2006). While such long-term durability of unintegrated lentiviral vector DNA in diverse situations was not evident in all studies so far (Naldini et al., 1996a; Loewen et al., 2003b), intensive focus on this as a distinct goal may identify more roles for non-integrating lentiviral vectors in avoiding consequences of insertional mutagenesis.
The transcriptional coactivator LEDGF/p75 was recently established to be a cellular cofactor for lentiviral integration (Llano et al., 2006; Vandekerckhove et al., 2006; Shun et al., 2007). This appears to be a lentiviral-specific role since HIV-1 and FIV, but not a gammaretrovirus, MLV, were affected by LEDGF/p75 knockdown. Lentiviruses integrate preferentially into actively transcribed genes (Schroder et al., 2002; Hacker et al., 2006; Kang et al., 2006;). Genome-wide integration site analysis showed that LEDGF/p75 also plays a significant role in determining this property (Ciuffi et al., 2005). Further understanding of the role of LEDGF/p75 could allow progress in targeting of lentiviral vector integration.
Restriction
Lentiviruses and other retroviruses display distinctive species-specific tropisms. While entry blocks (i.e., specific receptors) are one important factor, it is also clear that species-specific post-entry blocks (or restrictions) to infection occur. The so-called Ref1 and Lv1 post-entry restrictions in human and non-human primate cells act at a step at or immediately after reverse transcription in target cells, which is similar in many respects to a previously described pattern (Fv1) for gammaretroviruses (Bieniasz, 2003). Recently, the tripartite motif protein TRIM5α was determined to account for Ref1 and Lv1 restriction activities (Stremlau et al., 2004). Briefly, TRIM5α is a host factor that appears to bind to viral capsid protein trimers and disable incoming viral particles. A key caveat is that this factor is saturable, i.e., moderate to high multiplicity of infection permits efficient transduction even in a restricted cell. Saturable TRM5α mediated manner restriction has been shown for EIAV and FIV (Saenz et al., 2005; Saenz et al., 2006). For FIV, the restricting effect of TRIM5α is fairly substantial in macaque cells, but is rather mild in human cells. In some situations, e.g., very low multiplicity transduction, post-entry restriction may reduce the efficiency of FIV vectors in human targets. Such restriction may be overcome by increased dosage or simply by adding genome-less pseudotyped viral particles since cytoplasmic availability of fully mature capsids to TRIM5alpha is the determinant of saturation. However, in many if not most gene therapy situations, particularly those involving focal deposition of vector, particle concentrations are well above those needed to saturate Ref1/TRIM5α activity. In such common situations, this kind of restriction could provide a net advantage by providing an innate barrier to systemic propagation of any replication-competent retroviruses (RCR) that might theoretically arise.
Conclusion
Understanding of basic FIV molecular virology is increasing, providing a basis for efficient and safe vector design. These vectors are highly useful for genetically modifying dividing and nondividing cells ex vivo or in vivo. Further discoveries of molecular events that underlie key steps in the lentiviral life cycle are likely to foster vectors with enhanced capabilities.
None of the authors has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the paper entitled “Human Gene Therapy Vectors Derived from Feline Lentiviruses”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alisky JM, Hughes SM, Sauter SL, Jolly D, Dubensky TW, Jr, Staber PD, Chiorini JA, Davidson BL. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport. 2000;11:2669–2673. doi: 10.1097/00001756-200008210-00013. [DOI] [PubMed] [Google Scholar]
- Beer B, Denner J, Brown CR, Norley S, zur Megede J, Coulibaly C, Plesker R, Holzammer S, Baier M, Hirsch VM, Kurth R. Simian immunodeficiency virus of African green monkeys is apathogenic in the newborn natural host. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:210–220. doi: 10.1097/00042560-199807010-00003. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD. Restriction factors: a defense against retroviral infection. Trends Microbiol. 2003;11:286–291. doi: 10.1016/s0966-842x(03)00123-9. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Stein CS, Hughes SM, Heth J, McCray PM, Jr, Sauter SL, Johnston JC, Cory-Slechta DA, Federoff HJ, Davidson BL. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc Natl Acad Sci USA. 2002;99:6216–6221. doi: 10.1073/pnas.082011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti LA. The paradox of simian immunodeficiency virus infection in sooty mangabeys: active viral replication without disease progression. Front Biosci. 2004;9:521–539. doi: 10.2741/1123. [DOI] [PubMed] [Google Scholar]
- Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, Martin LN, Skulsky E, Ho DD, Cheng-Mayer C, Marx PA. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J Virol. 2000;74:1209–1223. doi: 10.1128/jvi.74.3.1209-1223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P, Ecker JR, Bushman F. A role for LEDGF/p75 in targeting HIV DNA integration. Nat Med. 2005;11:1287–1289. doi: 10.1038/nm1329. [DOI] [PubMed] [Google Scholar]
- Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. Journey to the center of the cell. Cell. 2001;105:697–700. doi: 10.1016/s0092-8674(01)00392-0. [DOI] [PubMed] [Google Scholar]
- Curran MA, Kaiser SM, Achacoso PL, Nolan GP. Efficient transduction of nondividing cells by optimized feline immunodeficiency virus vectors. Mol Ther. 2000;1:31–38. doi: 10.1006/mthe.1999.0007. [DOI] [PubMed] [Google Scholar]
- Curran MA, Nolan GP. Nonprimate lentiviral vectors. Curr Top Microbiol Immunol. 2002a;261:75–105. doi: 10.1007/978-3-642-56114-6_4. [DOI] [PubMed] [Google Scholar]
- Curran MA, Nolan GP. Recombinant feline immunodeficiency virus vectors. Preparation and use Methods Mol Med. 2002b;69:335–350. doi: 10.1385/1-59259-141-8:335. [DOI] [PubMed] [Google Scholar]
- Curran MA, Ochoa MS, Molano RD, Pileggi A, Inverardi L, Kenyon NS, Nolan GP, Ricordi C, Fenjves ES. Efficient transduction of pancreatic islets by feline immunodeficiency virus vectors. Transplantation. 2002;74:299–306. doi: 10.1097/00007890-200208150-00003. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Herpers B, Noraz N, Pflumio F, Guetard D, Leveau C, Dubart-Kupperschmitt A, Charneau P, Taylor N. Lentivirus-mediated gene transfer in primary T cells is enhanced by a central DNA flap. Gene Ther. 2001;8:190–198. doi: 10.1038/sj.gt.3301378. [DOI] [PubMed] [Google Scholar]
- Derksen TA, Sauter SL, Davidson BL. Feline immunodeficiency virus vectors. Gene transfer to mouse retina following intravitreal injection. J Gene Med. 2002;4:463–469. doi: 10.1002/jgm.267. [DOI] [PubMed] [Google Scholar]
- Djalilian HR, Tsuboi Y, Ozeki M, Tekin M, Djalilian AR, Obritch W, Lin J. Feline immunodeficiency virus-mediated gene therapy of middle ear mucosa cells. Auris Nasus Larynx. 2002;29:183–186. doi: 10.1016/s0385-8146(01)00131-6. [DOI] [PubMed] [Google Scholar]
- Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman A, Craigie R. Identification of conserved amino acid residues critical for human immunodeficiency virus type 1 integrase function in vitro. J Virol. 1992;66:6361–6369. doi: 10.1128/jvi.66.11.6361-6369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Malim MH. Nuclear import of human immunodeficiency virus type-1 preintegration complexes. Adv Virus Res. 1999;52:275–299. doi: 10.1016/s0065-3527(08)60302-4. [DOI] [PubMed] [Google Scholar]
- Hacker CV, Vink CA, Wardell TW, Lee S, Treasure P, Kingsman SM, Mitrophanous KA, Miskin JE. The integration profile of EIAV-based vectors. Mol Ther. 2006;14:536–545. doi: 10.1016/j.ymthe.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, Beck CR, Fineberg SK, Stein C, Ochoa D, Davidson BL. Optimization of feline immunodeficiency virus vectors for RNA interference. J Virol. 2006;80:9371–9380. doi: 10.1128/JVI.00958-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell RE, Hughes SM, Chiorini JA, Alisky JM, Davidson BL. Viral-mediated delivery of the late-infantile neuronal ceroid lipofuscinosis gene, TPP-I to the mouse central nervous system. Gene Ther. 2003;10:34–42. doi: 10.1038/sj.gt.3301843. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Moussavi-Harami F, Sauter SL, Davidson BL. Viral-mediated gene transfer to mouse primary neural progenitor cells. Mol Ther. 2002;5:16–24. doi: 10.1006/mthe.2001.0512. [DOI] [PubMed] [Google Scholar]
- Johnston JC, Gasmi M, Lim LE, Elder JH, Yee JK, Jolly DJ, Campbell KP, Davidson BL, Sauter SL. Minimum requirements for efficient transduction of dividing and nondividing cells by feline immunodeficiency virus vectors. J Virol. 1999;73:4991–5000. doi: 10.1128/jvi.73.6.4991-5000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Moressi CJ, Scheetz TE, Xie L, Tran DT, Casavant TL, Ak P, Benham CJ, Davidson BL, McCray PB., Jr Integration site choice of a feline immunodeficiency virus vector. J Virol. 2006;80:8820–8823. doi: 10.1128/JVI.00719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD, Ratliff KL, Shen H, Barker CK, Martins I, Sharkey CM, Sanders DA, McCray PB, Jr, Davidson BL. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River Virus glycoproteins. J Virol. 2002;76:9378–9388. doi: 10.1128/JVI.76.18.9378-9388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Xie L, Tran DT, Stein CS, Hickey M, Davidson BL, McCray PB., Jr Persistent expression of factor VIII in vivo following nonprimate lentiviral gene transfer. Blood. 2005;106:1552–1558. doi: 10.1182/blood-2004-11-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I, Azmi I, Poeschla EM. The critical role of proximal gag sequences in feline immunodeficiency virus genome encapsidation. Virology. 2004;327:111–120. doi: 10.1016/j.virol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Kemler I, Barraza R, Poeschla EM. Mapping of the encapsidation determinants of feline immunodeficiency virus. J Virol. 2002;76:11889–11903. doi: 10.1128/JVI.76.23.11889-11903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare P, Loewen N, Barraza R, Teo W, Fautsch MDHJ, Poeschla EM. Durable, safe, multi-gene lentiviral vector expression in feline trabecular meshwork. Mol Ther. 2007 doi: 10.1038/sj.mt.6300318. “in press”. [DOI] [PubMed] [Google Scholar]
- Leavitt AD, Shiue L, Varmus HE. Site-directed mutagenesis of HIV-1 integrase demonstrates differential effects on integrase functions in vitro. J Biol Chem. 1993;268:2113–2119. [PubMed] [Google Scholar]
- Llano M, Saenz DT, Meehan A, Wongthida P, Peretz M, Walker WH, Teo W, Poeschla EM. An Essential Role for LEDGF/p75 in HIV Integration. Science. 2006;314:461–464. doi: 10.1126/science.1132319. [DOI] [PubMed] [Google Scholar]
- Loewen N, Bahler C, Teo W, Whitwam T, Peretz M, Xu R, Fautsch M, Johnson DH, Poeschla EM. Preservation of aqueous outflow facility after second-generation FIV vector-mediated expression of marker genes in anterior segments of human eyes. Investig Ophthalmol Vis Sci. 2002;43:3686–3690. [PubMed] [Google Scholar]
- Loewen N, Barraza R, Whitwam T, Saenz DT, Kemler I, Poeschla E. FIV Vectors. Methods Mol Biol. 2003a;229:251–271. doi: 10.1385/1-59259-393-3:251. [DOI] [PubMed] [Google Scholar]
- Loewen N, Fautsch M, Peretz M, Bahler C, Cameron JD, Johnson DH, Poeschla EM. Genetic modification of human trabecular meshwork with lentiviral vectors. Hum Gene Ther. 2001;12:2109–2119. doi: 10.1089/10430340152677449. [DOI] [PubMed] [Google Scholar]
- Loewen N, Leske D, Chen Y, Teo W, Saenz DT, Peretz M, Holmes J, Poeschla EM. Comparison of wild-type and class I integrase mutant-FIV vectors in retina demonstrates sustained expression of integrated transgenes in retinal pigment epithelium. J Gene Med. 2003b;5:1009–1017. doi: 10.1002/jgm.447. [DOI] [PubMed] [Google Scholar]
- Loewen N, Leske DA, Cameron JD, Chen Y, Whitwam T, Simari RD, Teo WL, Fautsch MP, Poeschla EM, Holmes JM. Long-term retinal transgene expression with FIV versus adenoviral vectors. Mol Vis. 2004;10:272–280. [PubMed] [Google Scholar]
- Loewen N, Poeschla EM. Lentiviral vectors. Adv Biochem Eng Biotechnol. 2005;99:169–191. doi: 10.1007/10_007. [DOI] [PubMed] [Google Scholar]
- Lotery AJ, Derksen TA, Russell SR, Mullins RF, Sauter S, Affatigato LM, Stone EM, Davidson BL. Gene transfer to the nonhuman primate retina with recombinant feline immunodeficiency virus vectors. Hum Gene Ther. 2002;13:689–696. doi: 10.1089/104303402317322258. [DOI] [PubMed] [Google Scholar]
- Mitrophanous K, Yoon S, Rohll J, Patil D, Wilkes F, Kim V, Kingsman S, Kingsman A, Mazarakis N. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Ther. 1999;6:1808–1818. doi: 10.1038/sj.gt.3301023. [DOI] [PubMed] [Google Scholar]
- Naldini L, Bloemer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996a;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996b;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JC. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther. 1998;5:1481–1487. doi: 10.1038/sj.gt.3300768. [DOI] [PubMed] [Google Scholar]
- Parolin C, Sodroski J. A defective HIV-1 vector for gene transfer to human lymphocytes. J Mol Med. 1995;73:279–288. doi: 10.1007/BF00231614. [DOI] [PubMed] [Google Scholar]
- Parolin C, Taddeo B, Palu G, Sodroski J. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology. 1996;222:415–422. doi: 10.1006/viro.1996.0438. [DOI] [PubMed] [Google Scholar]
- Pedersen NC. The feline immunodeficiency virus. In: Levy JA, editor. The Retroviridae. Plenum Press; New York: 1993. pp. 181–228. [Google Scholar]
- Pedersen NC, Ho EW, Brown ML, Yamamoto JK. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Phillips TR, Lamont C, Konings DA, Shacklett BL, Hamson CA, Luciw PA, Elder JH. Identification of the Rev transactivation and Rev-responsive elements of feline immunodeficiency virus. J Virol. 1992;66:5464–5471. doi: 10.1128/jvi.66.9.5464-5471.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeschla E, Looney D. CXCR4 is required by a non-primate lentivirus: heterologous expression of feline immunodeficiency virus in human, rodent and feline cells. J Virol. 1998;72:6858–6866. doi: 10.1128/jvi.72.8.6858-6866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeschla E, Wong-Staal F, Looney D. Efficient transduction of nondividing cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- Poeschla EM. Non-primate lentiviral vectors. Curr Opin Mol Ther. 2003;5:529–540. [PubMed] [Google Scholar]
- Price MA, Case SS, Carbonaro DA, Yu XJ, Petersen D, Sabo KM, Curran MA, Engel BC, Margarian H, Abkowitz JL, Nolan GP, Kohn DB, Crooks GM. Expression from second-generation feline immunodeficiency virus vectors is impaired in human hematopoietic cells. Mol Ther. 2002;6:645–652. [PubMed] [Google Scholar]
- Roelke ME, Pecon-Slattery J, Taylor S, Citino S, Brown E, Packer C, Vandewoude S, O’Brien SJ. T-lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J Wildl Dis. 2006;42:234–248. doi: 10.7589/0090-3558-42.2.234. [DOI] [PubMed] [Google Scholar]
- Saenz DT, Barraza R, Loewen N, Teo W, Poeschla E. Production and Use of Feline Immunodeficiency Virus (FIV)-based lentiviral vectors. In: Rossi J, Friedman T, editors. Gene Transfer: A Cold Spring Harbor Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2006. pp. 57–74. [Google Scholar]
- Saenz DT, Loewen N, Peretz M, Whitwam T, Barraza R, Howell K, Holmes JH, Good M, Poeschla EM. Unintegrated lentiviral DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. J Virol. 2004;78:2906–2920. doi: 10.1128/JVI.78.6.2906-2920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz DT, Poeschla EM. FIV: from lentivirus to lentivector. J Gene Med. 2004;6(Suppl 1):S95–104. doi: 10.1002/jgm.500. [DOI] [PubMed] [Google Scholar]
- Saenz DT, Teo W, Olsen JC, Poeschla E. Restriction of Feline Immunodeficiency Virus by Ref1, LV1 and Primate TRIM5a Proteins. J Virol. 2005;79:15175–15188. doi: 10.1128/JVI.79.24.15175-15188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Shimojima M, Miyazawa T, Ikeda Y, McMonagle EL, Haining H, Akashi H, Takeuchi Y, Hosie MJ, Willett BJ. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–1195. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- Shun MC, Raghavendra NK, Vandegraaff N, Daigle JE, Hughes S, Kellam P, Cherepanov P, Engelman A. LEDGF/p75 functions downstream from preintegration complex formation to effect gene-specific HIV-1 integration. Genes Dev. 2007;21:1767–1778. doi: 10.1101/gad.1565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn PL, Hickey MA, Staber PD, Dylla DE, Jeffers SA, Davidson BL, Sanders DA, McCray PB., Jr Lentivirus vectors pseudotyped with filoviral envelope glycoproteins transduce airway epithelia from the apical surface independently of folate receptor alpha. J Virol. 2003;77:5902–5910. doi: 10.1128/JVI.77.10.5902-5910.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirven A, Pflumio F, Zennou V, Titeux M, Vainchenker W, Coulombel L, Dubart-Kupperschmitt A, Charneau P. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood. 2000;96:4103–4110. [PubMed] [Google Scholar]
- Song JJ, Lee B, Chang JW, Kim JH, Kwon YK, Lee H. Optimization of vesicular stomatitis virus-G pseudotyped feline immunodeficiency virus vector for minimized cytotoxicity with efficient gene transfer. Virus Res. 2003;93:25–30. doi: 10.1016/s0168-1702(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Stein CS, Davidson BL. Gene transfer to the brain using feline immunodeficiency virus-based lentivirus vectors. Methods Enzymol. 2002;346:433–454. doi: 10.1016/s0076-6879(02)46070-3. [DOI] [PubMed] [Google Scholar]
- Stein CS, Kang Y, Sauter SL, Townsend K, Staber P, Derksen TA, Martins I, Qian J, Davidson BL, McCray PB., Jr In vivo treatment of hemophilia A and mucopolysaccharidosis type VII using nonprimate lentiviral vectors. Mol Ther. 2001;3:850–856. doi: 10.1006/mthe.2001.0325. [DOI] [PubMed] [Google Scholar]
- Stevenson M. HIV nuclear import: What’s the flap? Nat Med. 2000;6:626–628. doi: 10.1038/76191. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Talbott RL, Sparger EE, Lovelace KM, Fitch WM, Pedersen NC, Luciw PA, Elder JH. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer JL, Pecon-Slattery J, Roelke ME, Johnson W, VandeWoude S, Vazquez-Salat N, Brown M, Frank L, Woodroffe R, Winterbach C, Winterbach H, Hemson G, Bush M, Alexander KA, Revilla E, O’Brien SJ. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J Virol. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maele B, De Rijck J, De Clercq E, Debyser Z. Impact of the central polypurine tract on the kinetics of human immunodeficiency virus type 1 vector transduction. J Virol. 2003;77:4685–4694. doi: 10.1128/JVI.77.8.4685-4694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove L, Christ F, Van Maele B, De Rijck J, Gijsbers R, Van den Haute C, Witvrouw M, Debyser Z. Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus. J Virol. 2006;80:1886–1896. doi: 10.1128/JVI.80.4.1886-1896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodicka MA. Determinants for lentiviral infection of non-dividing cells. Somat Cell Mol Genet. 2001;26:35–49. doi: 10.1023/a:1021022629126. [DOI] [PubMed] [Google Scholar]
- Wang G, Slepushkin V, Zabner J, Keshavjee S, Johnston JC, Sauter SL, Jolly DJ, Dubensky TW, Jr, Davidson BL, McCray PB., Jr Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect [see comments] J Clin Invest. 1999;104:R55–62. doi: 10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwam T, Peretz M, Poeschla EM. Identification of a central DNA flap in feline immunodeficiency virus. J Virol. 2001;75:9407–9414. doi: 10.1128/JVI.75.19.9407-9414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett BJ, Hosie MJ, Neil JC, Turner JD, Hoxie JA. Common mechanism of infection by lentiviruses [letter] Nature. 1997a;385:587. doi: 10.1038/385587a0. [DOI] [PubMed] [Google Scholar]
- Willett BJ, Picard L, Hosie MJ, Turner JD, Adema K, Clapham PR. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997b;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Munoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, Buch P, MacLaren RE, Anderson PN, Barker SE, Duran Y, Bartholomae C, von Kalle C, Heckenlively JR, Kinnon C, Ali RR, Thrasher AJ. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Yee JK, Miyanohara A, LaPorte P, Bouic K, Burns JC, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SF, von Ruden T, Kantoff PW, Garber C, Seiberg M, Ruther U, Anderson WF, Wagner EF, Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci USA. 1986;83:3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]