Abstract
The vacuolar (H+)-ATPases (V-ATPases) are ATP-dependent proton pumps that operate by a rotary mechanism in which ATP hydrolysis drives rotation of a ring of proteolipid subunits relative to subunit a within the integral V0 domain. In vivo dissociation of the V-ATPase (an important regulatory mechanism) generates a V0 domain that does not passively conduct protons. EM analysis indicates that the N-terminal domain of subunit a approaches the rotary subunits in free V0, suggesting a possible mechanism of silencing passive proton transport. To test the hypothesis that the N-terminal domain inhibits passive proton flux by preventing rotation of the proteolipid ring in free V0, factor Xa cleavage sites were introduced between the N- and C-terminal domains of subunit a (the Vph1p isoform in yeast) to allow its removal in vitro after isolation of vacuolar membranes. The mutant Vph1p gave rise to a partially uncoupled V-ATPase complex. Cleavage with factor Xa led to further loss of coupling of proton transport and ATP hydrolysis. Removal of the N-terminal domain by cleavage with factor Xa and treatment with KNO3 and MgATP did not, however, lead to an increase in passive proton conductance by free V0, suggesting that removal of the N-terminal domain is not sufficient to facilitate passive proton conductance through V0. Photoactivated cross-linking using the cysteine reagent maleimido benzophenone and single cysteine mutants of subunit a demonstrated the proximity of specific sites within the N-terminal domain and subunits E and G of the peripheral stalk. These results suggest that a localized region of the N-terminal domain (residues 347–369) is important in anchoring the peripheral stator in V1V0.
The vacuolar (H+)-ATPases (V-ATPase)2 are a family of ATP-dependent proton pumps that transport protons into both intracellular compartments and across the plasma membrane (1–4). V-ATPase-dependent acidification of lysosomes and endosomes is important for protein degradation and endocytic trafficking (5), respectively, while acidification of secretory vesicles drives coupled transport of small molecules, like neurotransmitters (6). Envelope viruses (like influenza virus) and bacterial toxins (like anthrax toxin) make use of the acidic lumenal environment of endosomes to enter cells (7). Plasma membrane V-ATPases in renal intercalated cells secrete acid into the urine (3), while plasma membrane V-ATPases in epididymal clear cells aid in sperm maturation (8). Osteoclasts utilize plasma membrane V-ATPases to degrade bone (9), and certain tumor cells use plasma membrane V-ATPases to aid in invasion (10). Thus V-ATPases serve many important functions in both normal physiology and human disease.
Like the related family of F1F0 ATP synthases and the even more closely related family of archaebacterial ATPases (A-ATPases) (11), the V-ATPases are composed of a peripheral domain (V1) that carries out ATP hydrolysis and an integral domain (V0) responsible for proton translocation (1). All of these enzymes operate by a common rotary mechanism in which ATP hydrolysis is able to drive rotation of a central rotary domain relative to the remainder of the complex (referred to as the stator) (12, 13). V1 is composed of subunits A–H, and V0 is composed of subunits a, d, e, c, c′, and c″ (1). The rotary complex contains subunits D and F of V1 attached to subunit d and a hexameric ring of proteolipid subunits (c, c′, and c″) of V0 (14). The stator domain includes a hexameric head of alternating A and B subunits responsible for hydrolyzing ATP and driving rotation of the rotor domain. This A3B3 head is held fixed relative to subunit a within V0 by peripheral stalks containing subunits C, E, G, H, and the N-terminal domain of subunit a (15–19). Rotation of the proteolipid ring relative to subunit a within V0 drives vectorial transport of protons across the membrane.
Reversible dissociation of V1 and V0 represents an important mechanism of regulating V-ATPase activity in vivo (1, 2, 20). This mechanism has been demonstrated in a variety of organisms, including yeast (2), insect cells (20), mammalian dendritic cells (21), and renal tubular cells (22). In yeast and insect cells, V-ATPase dissociation occurs in response to nutrient depletion as a means of conserving cellular stores of ATP. V-ATPase dissociation in yeast is rapid and requires no new protein synthesis (23). Reassembly requires interaction with the glycolytic enzyme aldolase (24, 25) and a novel protein complex named RAVE (26–28), whereas dissociation requires an intact microtubular network (29). V-ATPase dissociation is also a sensitive function of the cellular environment in which the complex resides (30, 31). In renal tubular cells glucose-dependent assembly is under control of the PI 3-kinase pathway (22) while in cells of the salivary gland of the blowfly, protein kinase A is important in controlling assembly (32).
An important property of this regulatory mechanism is the silencing of the V1 and V0 domains following dissociation. Thus the free V1 domain is inactive as an ATPase (33), and the free V0 domain does not passively conduct protons (34). This is important to avoid release into the cytosol of an uncoupled ATPase activity or generation of an unregulated proton conductance. Subunit H has previously been shown to silence the activity of free V1 (35), and we have recently demonstrated that this is accomplished by directly bridging the stator and rotor stalks following dissociation (36). The mechanism of preventing passive proton conductance through V0, however, has remained elusive.
The a subunit of the V-ATPase is a 100-kDa protein containing an N-terminal hydrophilic domain located on the cytosolic side of the membrane and a C-terminal hydrophobic domain containing multiple transmembrane helices (37). The N-terminal domain contains information necessary to target V-ATPases to different intracellular sites whereas the C-terminal domain contains residues essential for proton translocation (38). Recent electron microscopy studies of the bovine V-ATPase suggest that the orientation of the N-terminal domain of subunit a changes from being oriented up toward the V1 domain in intact V1V0 to bending down toward the proteolipid ring in free V0 (17, 39). This has led to the hypothesis that the N-terminal domain of subunit a blocks passive proton translocation through free V0 by preventing rotation of the proteolipid ring. This report is focused on testing this hypothesis and on characterizing the interactions that occur between the N-terminal domain of subunit a and other subunits in the V-ATPase complex.
EXPERIMENTAL PROCEDURES
Materials and Strains—Zymolyase 100T was obtained from Seikagaku America, Inc. Restriction endonucleases, T4 DNA ligase, and other molecular biology reagents as well as competent cells were from New England Biolabs. Precast gels, nitrocellulose membranes, Tween 20, and horseradish peroxidase-conjugated goat anti-mouse/rabbit IgG were purchased from Bio-Rad. The chemiluminescence substrate for horseradish peroxidase was from Kirkegaard & Perry Laboratories. Protein A-Sepharose, protein G-agarose, and most other chemicals such as valinomycin and CCCP were purchased from Sigma. Materials for Escherichia coli and yeast culture and carrier DNA were purchased from BD Biosciences.
Yeast strain MM112 (MATa vph1::LEU2 stv1::LYS2 his3-D200 leu2 lys2 ura3-52) (40) was used for expressing the mutant a subunit. Yeast cells were grown in yeast extract-peptone-dextrose medium or synthetic dropout medium (41).
Antibodies—The mouse monoclonal antibody 10D7 against the yeast V-ATPase subunit a (Vph1p) was purchased from Molecular Probes. The polyclonal antibody against subunit E (Vma4p) was a gift from Dr. Daniel Klionsky (University of Michigan). The polyclonal antibody against subunit G (Vma10p) was a gift from Dr. Tom Stevens (University of Oregon).
Construction of Mutants—Site-directed mutagenesis was performed to introduce four tandem repeats of the factor Xa site, whose sequence is IEGR, after the amino acid His-374 of wild-type Vph1p (subunit a) using the Altered Sites II in vitro mutagenesis system (Promega). Factor Xa sites were introduced on the SalI-EcoRI fragment from the MM332 plasmid (pRS316-VPH1) (40) by using the mutagenic oligonucleotide 5′-AGT TCT GTG GAA GGT AGG TGG AGT CCT TCC CTC GAT CCT TCC CTC GAT CCT TCC CTC GAT CCT TCC CTC GAT GTG GTT TGT ATC CAG GAC TTG GAT-3′, and then the fragment with the mutation was substituted back into MM332. Using the same mutagenesis system, single cysteine mutants were constructed starting with the cysteineless form of Vph1p as previously described (37). The mutagenic oligonucleotides used for these constructs were as follows: 5′-GCT TGC AAA GTA CAC AAT TCG TCT CT-3′ (A347C), 5′-TCA CCA AGA CGA CAT TGC AAA GTA GC-3′ (A351C), 5′-TTG TAT CCA GGA CAC AGA TAA TGG ATG GG-3′ (Q369C). The single copy plasmid pRS316 carrying either the mutant Vph1p containing the tandem factor Xa sites or the single cysteine-containing forms of Vph1p were transformed into MM112 by the lithium acetate method (42). The transformants were selected on Ura– plates.
In Vitro Dissociation of V1 Complexes—The peripheral V1 complexes were dissociated from vacuolar membranes by KNO3 plus MgATP treatment as described previously (43). Vacuolar membrane was treated with 150 mm KNO3 and 4 mm MgATP in buffer containing 20 mm HEPES (pH 7.0), 0.2 mm EGTA, and 2 mm 2-mercaptoethanol for 1 h at 4 °C, and then sedimented by ultracentrifugation at 100,000 × g and 4 °C for 15 min.
Isolation of Vacuolar Membrane Vesicles—Vacuolar membrane vesicles were isolated using the protocol described previously (18). For the purpose of measuring passive proton flux, vacuolar membrane vesicles were incubated in the presence of assay buffer (20 mm HEPES pH 7.0, 0.2 mm EGTA, 2 mm 2-mercaptoethanol, 10% glycerol, 0.5 mg/ml BSA) containing 100 mm potassium sulfate to load them with a high potassium concentration.
Proteolysis of the Mutant a Subunit Containing Tandem Factor Xa Sites—Vacuolar membrane vesicles were prepared from the yeast strain expressing the mutant a subunit containing tandem factor Xa sites. Membrane vesicles were resuspended in 1 ml of factor Xa buffer (20 mm Tris-HCl, pH 8.0, 2 mm CaCl2) with 4 mg of factor Xa protease and incubated at 4 °C for various lengths of time followed by sedimentation at 100,000 × g.
Measurement of Passive Proton Flux in Yeast Vacuolar Membrane Vesicles—Passive proton uptake driven by a lumenal negative membrane potential was monitored by fluorescence quenching of ACMA using an excitation wavelength of 410 nm and an emission wavelength of 490 nm (34). Intact vacuolar membrane vesicles or those treated with factor Xa protease followed by dissociation of V1 with KNO3 and MgATP were loaded with 100 mm potassium sulfate prior to assay by incubation overnight at 4 °C. Vesicles were then diluted into assay buffer (150 mm NaCl, 20 mm HEPES pH 7.0, 0.2 mm EGTA, 2 mm 2-mercaptoethanol, 10% glycerol, 0.5 mg/ml BSA) with 2 μm ACMA. Fluorescence intensity was recorded using a PerkinElmer LS50B Luminescence Spectrometer LS50B with FL WinLab software. Addition of valinomycin (2 μm) led to potassium flux out of the vesicles and generated a membrane potential, which was interior negative. As a positive control, proton influx was induced by addition of 2 μm CCCP, which led to the rapid quenching of ACMA fluorescence.
Other Procedures—Photoactivated cross-linking mediated by the chemical cross-linker MBP was described previously (18). Protein concentrations were determined by the method of Lowry et al. (44). ATPase activity was measured using a coupled spectrophotometric assay in the presence or absence of 1 μm concanamycin A (45). ATP-dependent proton transport was measured using the fluorescent dye ACMA as described previously (46).
RESULTS
Introduction of Tandem Factor Xa Sites into Yeast Subunit a (Vph1p) and Effect on Growth Phenotype and V-ATPase Activity—To test the role of the N-terminal domain of subunit a in preventing passive proton translocation through the integral V0 domain, tandem factor Xa protease cleavage sites were introduced between the N- and C-terminal domains of subunit a (Vph1p) in yeast. This allowed for protease cleavage at a site between the N- and C-terminal domains of subunit a. Four tandem factor Xa sites (IEGR) were introduced following residue His-375 of Vph1p, which is 30 amino acids N-terminal to the first predicted transmembrane helix in the C-terminal domain (as shown in Fig. 1). Four tandem sites were employed as mutant constructs containing fewer sites were either not cleaved or were cleaved inefficiently. The mutant protein was expressed in a yeast strain (MM112) disrupted in both isoforms of subunit a (Vph1p and Stv1p) and the phenotype assessed by the ability of the strain to grow at pH 7.5. It has previously been shown that disruption of V-ATPase genes in yeast results in a conditional lethal phenotype in which cells are able to grow at pH 5.5 but not at pH 7.5 (2). As shown in Fig. 2, both wild-type and mutant Vph1p (subunit a) are able to support growth at pH 7.5, indicating that the factor Xa-containing form of Vph1p is able to complement the vma– phenotype and give rise to functional V-ATPase complexes.
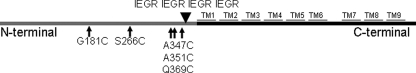
FIGURE 1.
Schematic diagram of the positions of factor Xa sites and single cysteine mutations in subunit a (Vph1p). Transmembrane helices are shown by black bars labeled TM1–TM9. Four tandem factor Xa sites (IEGR) are indicated near the border of the N- and C-terminal domains.
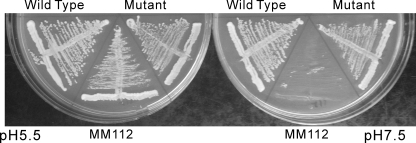
FIGURE 2.
Subunit a (Vph1p) containing four tandem factor Xa cleavage sites is able to complement the vma– phenotype of a strain disrupted in both Vph1p and Stv1p. The vph1 and stv1 double deletion strain MM112 was transformed with the pRS316 vector alone (MM112) or the vector carrying sequences encoding wild-type Vph1p (wild type), mutant Vph1p with 4 factor Xa sites inserted after H374 (mutant). The strains were streaked on plates containing YPD medium buffered to pH 5.5 or 7.5 and incubated at 30 °C for 1 day. The double deletion strain, MM112, is able to grow on acidic medium but not on neutral medium (vma– phenotype). The transformed strains containing the plasmids carrying wild-type or mutant subunit a (Vph1p) grow at both pH 5.5 and pH 7.5, indicating complementation of the vma– phenotype.
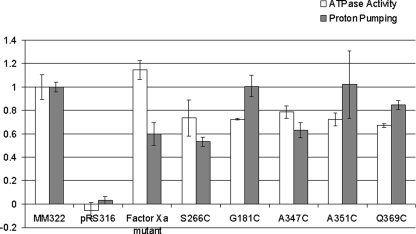
Because it has previously been shown that mutant complexes possessing as little as 25% of wild-type V-ATPase activity are still able to complement the vma– phenotype (47, 48), concanamycin-sensitive ATPase activity and proton transport (measured as the rate of quenching of ACMA fluorescence) were determined for vacuolar membranes isolated from the strain expressing the mutant protein. As can be seen in Fig. 3, the mutant form of Vph1p (subunit a) gave V-ATPase complexes possessing 115 (+10) % of ATPase activity but only 60 (+10)% of proton transport relative to the wild-type form of Vph1p. These results indicate that V-ATPase complexes containing the mutant form of subunit a are partially uncoupled with respect to proton transport.
FIGURE 3.
Concanamycin-sensitive ATPase activity and proton transport of vacuolar membrane vesicles isolated from strains expressing wild-type and mutant forms of subunit a (Vph1p). Vacuolar membrane vesicles were isolated from yeast strains expressing wild-type Vph1p, the indicated mutant forms of Vph1p or the vector alone. Concanamycin-sensitive ATPase activity (open bars) and proton pumping activity (closed bars) were measured as described under “Experimental Procedures” by measuring activity in the presence or absence of 1 μm concanamycin A. Values represent an average of at least two determinations on two independent vacuolar membrane preparations and are expressed relative to the wild type. Error bars represent S.D.
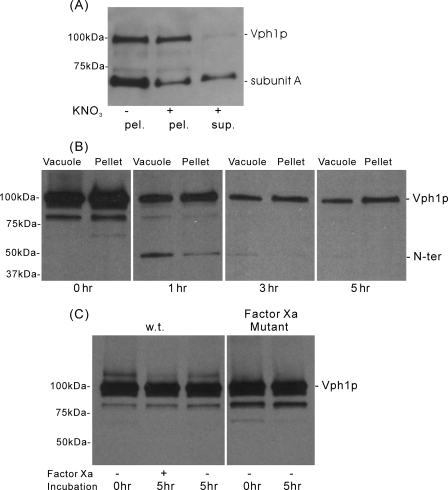
Cleavage of Factor Xa-containing Subunit a (Vph1p) by Factor Xa Protease and Release of the N-terminal Domain from Vacuolar Membranes—Vacuolar membrane vesicles were incubated with factor Xa protease at 4 °C for various lengths of time, and samples were then split into two equal aliquots. One aliquot was denatured with sample buffer and directly applied to SDS-PAGE (vacuole), while the other aliquot was incubated with 150 mm KNO3 and 1 mm MgATP followed by sedimentation to release the V1 domain and any noncovalently attached N-terminal domain (pellet). As can be seen in Fig. 4, panel A, KNO3/MgATP treatment (without prior protease treatment), is effective at removing the peripheral V1 domain (as indicated by the presence in the supernatant fraction of subunit A) while leaving behind in the pellet fraction the V0 domain (as indicated by the presence of uncleaved subunit a (Vph1p)). No detectable subunit A was released in the absence of treatment with KNO3 and MgATP (data not shown). Western blotting of the samples obtained by proteolysis (Fig. 4B) shows the transient appearance of a 45-kDa fragment corresponding to the N-terminal domain (the epitope for the anti-Vph1p antibody has been mapped to a site in the N terminus) coincident with the disappearance of the intact subunit a (Vph1p) at 100 kDa. No detectable cleavage of the wild-type Vph1p lacking the factor Xa sites was observed after 5 h of incubation with factor Xa protease, nor was mutant Vph1p cleaved in the absence of factor Xa (Fig. 4C). Quantitation of the blots indicated cleavage of ∼85% of the intact subunit a (Vph1p) after 3 h of protease treatment. In addition, KNO3/MgATP treatment resulted in release from the membrane of 75% of the N-terminal fragment observed after 1 h of factor Xa cleavage. No significant N-terminal fragment could be observed in the supernatant following sedimentation of the membranes, suggesting that this fragment, once released from the membranes, is particularly susceptible to proteolysis. Because the N-terminal domain does not contain any factor Xa cleavage sites, this proteolysis may be occurring by proteases endogenous to the vacuolar membrane preparation. The results indicate that this procedure is effective at generating vacuolar membranes lacking the N-terminal domain of subunit a.
FIGURE 4.
Cleavage of factor Xa-containing subunit a (Vph1p) by factor Xa protease and removal of V1 by treatment with KNO3 and MgATP. A, vacuolar membranes isolated from yeast expressing wild-type subunit a (Vph1p) were incubated with or without 150 mm KNO3 and 4 mm MgATP followed by sedimentation and SDS-PAGE of supernatant and pellet fractions followed by Western blot analysis using monoclonal antibodies against subunits A and Vph1p, as described under “Experimental Procedures.” Results show release of a substantial fraction of subunit A by treatment with KNO3 and MgATP without significant loss of subunit a (Vph1p) from the pellet fraction. B, vacuolar membranes from yeast expressing the mutant form of subunit a (Vph1p) containing four tandem factor Xa sites were treated for various lengths of time with factor Xa followed by treatment with 150 mm KNO3 and 4 mm MgATP and sedimentation as described under “Experimental Procedures.” Samples of vacuoles (before KNO3/MgATP treatment) or pellet (after treatment and sedimentation) were separated by SDS-PAGE and analyzed by Western blot using a monoclonal antibody against subunit a (Vph1p). Quantitation of blots revealed cleavage of ∼85% of intact Vph1p after 3 h and removal of 75% of the 45-kDa N-terminal fragment by KNO3/MgATP. C, vacuolar membranes isolated from yeast expressing wild type (wt) or mutant (factor Xa mutant) forms of Vph1p were treated in the absence or presence of factor Xa for the indicated length of time followed by separation of samples by SDS-PAGE and analysis by Western blot using anti-Vph1p antibody. Results show no cleavage of wild-type Vph1p by factor Xa or cleavage of the mutant protein in the absence of factor Xa.
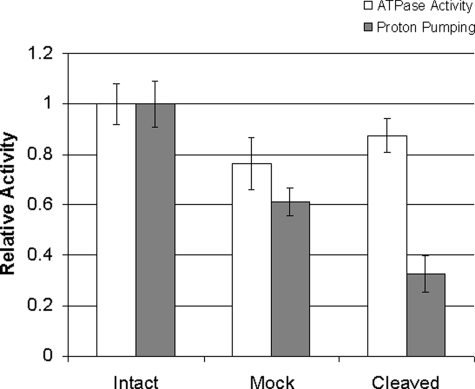
Cleavage of Factor Xa-containing Subunit a (Vph1p) Alters the Coupling Efficiency for ATP-driven Proton Transport—To address whether cleavage of mutant subunit a (Vph1p) with factor Xa protease altered the coupling efficiency of the V-ATPase, ATP-dependent proton transport and ATP hydrolysis were measured before and after cleavage with factor Xa, but without dissociation of the N-terminal domain using KNO3 and MgATP. As shown in Fig. 5, cleavage of Vph1p with factor Xa protease led to a ∼60% reduction in the coupling efficiency (defined as the ratio of relative proton transport divided by relative ATPase activity) compared with untreated vacuolar membranes. Treatment of membranes under identical conditions but in the absence of factor Xa protease led to a ∼20% reduction in coupling efficiency. Because no detectable cleavage of subunit a (Vph1p) was detected under these conditions (Fig. 4C), this reduction in the absence of factor Xa may reflect uncoupling due to cleavage of some other subunit. This result indicates that cleavage of subunit a between the N- and C-terminal domains reduces the efficiency of coupling of proton transport to ATP hydrolysis by complexes containing the mutant Vph1p even further than is observed for the uncleaved mutant (Fig. 3).
FIGURE 5.
Factor Xa cleavage of mutant subunit a (Vph1p) reduces the ratio of proton transport to ATP hydrolysis of the V-ATPase. Vacuolar membrane vesicles isolated from the yeast strain expressing the mutant form of subunit a (Vph1p) containing four tandem factor Xa cleavage sites were untreated (intact) or treated in the absence (mock) or presence (cleaved) of factor Xa for 3 h at 4 °C. Concanamycin-sensitive ATPase activity (open bars) and concanamycin-sensitive proton transport activity (closed bars) were measured as described under “Experimental Procedures.” Results are the average of three determinations from two separate experiments and are expressed relative to the untreated membranes, with the error bars corresponding to the S.D.
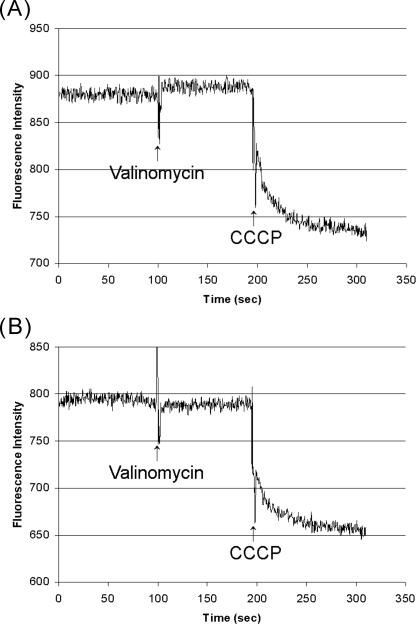
Removal of the N-terminal Domain of Subunit a (Vph1p) Does Not Increase the Passive Proton Permeability of Vacuolar Membrane Vesicles—To measure the passive proton permeability of vacuolar membrane vesicles, a negative interior membrane potential was generated across the vesicle membrane to drive proton influx, which was monitored using fluorescence quenching of ACMA (34). The interior negative membrane potential was generated by loading vacuolar membrane vesicles by incubation with 100 mm potassium sulfate and diluting them at the time of assay into potassium-free buffer containing 150 mm NaCl in the presence of valinomycin. As a positive control, the membranes were made leaky to protons by the addition of 2 μm CCCP. As shown in Fig. 6, the passive proton permeability of vacuolar membrane vesicles containing the mutant subunit a (Vph1p) which had been treated with factor Xa and KNO3/MgATP to release the N-terminal fragment of subunit a (panel B) showed the same low passive proton permeability as mutant membranes treated only with KNO3/MgATP but without factor Xa protease (panel A). As previously demonstrated (34), membranes containing wild-type V0 were also impermeable to protons (data not shown). Addition of 2 μm CCCP gave rise to a rapid and significant quenching of ACMA fluorescence. These results suggest that removal of the N-terminal domain of subunit a (Vph1p) does not significantly increase the passive proton permeability of the V0 domain.
FIGURE 6.
V0-containing vacuolar membrane vesicles with uncleaved or factor Xa cleaved subunit a (Vph1p) do not allow passive proton translocation. Vacuolar membrane vesicles isolated from yeast expressing mutant subunit a (Vph1p) containing factor Xa sites were incubated with (panel B) or without (panel A) factor Xa protease for 3 h at 4 °C as described in Fig. 4. Membranes were then treated with KNO3/MgATP to dissociate V1 and any cleaved N-terminal domain followed by sedimentation to pellet the membranes. Membranes were resuspended in assay buffer (20 mm HEPES pH 7.0, 0.2 mm EGTA, 2 mm 2-mercaptoethanol, 10% glycerol, 0.5 mg/ml BSA) containing 100 mm K2SO4 and incubated for 12 h at 4 °C to equilibrate potassium across the membrane. Aliquots were then removed and diluted into assay buffer containing 2 μm ACMA and 150 mm NaCl in place of K2SO4 (150 mm NaCl, 20 mm HEPES pH 7.0, 0.2 mm EGTA, 2 mm 2-mercaptoethanol, 10% glycerol, 0.5 mg/ml BSA) with 2 μm ACMA. Once a stable fluorescence signal was obtained, the signal was recorded for ∼90 s followed by addition of 2 μm valinomycin to induce potassium efflux and a negative interior membrane potential. The signal was recorded for an additional 100 s followed by addition of 2 μm CCCP to induce increased passive proton permeability and consequent quenching of ACMA fluorescence.
Construction and Characterization of Single Cysteine-containing Mutants of Subunit a (Vph1p)—To obtain information about the proximity of other V-ATPase subunits in the complex to specific sites within the N-terminal domain of subunit a, site-directed mutagenesis was employed to introduce unique cysteine residues into the N-terminal domain, which would then be used as the site of attachment of the photoactivatable cross-linking reagent maleimido benzophenone (MBP). We have previously employed cysteine mutagenesis and MBP-mediated cross-linking to define the structural arrangement of subunits within the V-ATPase complex (15–18). Twenty-four unique cysteine residues were introduced into the N-terminal domain of subunit a, which had previously been made Cys-less by removing the seven endogenous cysteine residues in Vph1p. We have previously shown that the Cys-less form of subunit a (Vph1p) gives rise to V-ATPase complexes possessing ∼78% of wild-type levels of bafilomycin-sensitive ATP-dependent fluorescence quenching of ACMA (37). As shown in Fig. 3, vacuolar membranes isolated from the five single cysteine mutants of Vph1p giving identifiable cross-linked products with MBP (see below), including G181C, S266C, A347C, A351C, and Q369C, all showed concanamycin-sensitive ATPase activity and proton transport at least 50% of that observed for V-ATPase complexes containing wild-type Vph1p.
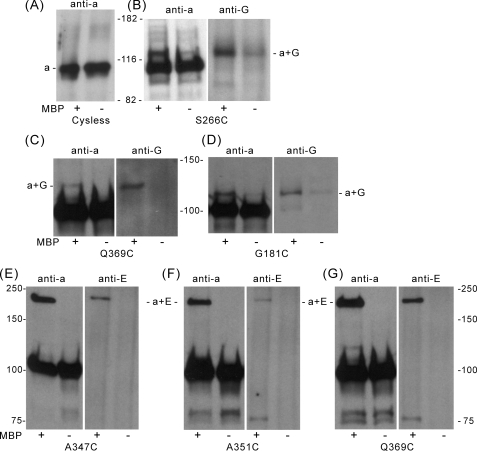
Identification of V-ATPase Subunits in Proximity to Specific Sites within the N-terminal Domain of Subunit a (Vph1p)—Photoactivated cross-linking using the sulfhydryl reagent MBP was performed on vacuolar membranes isolated from the single cysteine containing mutants as well as the Cys-less form of subunit a (Vph1p). Proteins were then solubilized with SDS, separated on SDS-PAGE, and Western blotting was performed using either the 10D7 antibody against subunit a or other subunit-specific antibodies. Higher molecular weight bands were observed in the Western blot performed using the anti-a subunit antibody for the single cysteine mutants G181C, S266C, A347C, A351C, and Q369C (whose positions are shown in Fig. 1). No higher molecular weight bands were observed for the Cys-less Vph1p control (Fig. 7A), indicating that where cross-linking was observed, these were not due to MBP reaction at cysteine residues in other subunits, which subsequently led to cross-linking to subunit a. As can be seen in Fig. 7, B–D, an MBP-dependent cross-linked band of molecular mass ∼120 kDa, which was recognized by antibodies against both subunit a and subunit G was observed for the S266C and G181C mutants. Although a fainter band, which is recognized by both anti-a and anti-G subunit antibodies is also observed for the S266C mutant in the absence of MBP, the intensity of this band increases dramatically in the presence of MBP. A band of slightly lower mobility, which was recognized by both anti-a and anti-G subunit antibodies, was also observed for the Q369C mutant. In addition (Fig. 7, E–G), an MBP-dependent cross-linked band of molecular mass of ∼200 kDa, which was recognized by antibodies against both subunits a and E, was observed for the A347C, A351C, and Q369C mutants. Because the molecular mass of this latter cross-linked product (200 kDa) is so much greater than the sum of the masses of subunits a and E (∼130 kDa), it is possible that this 200-kDa product contains an additional subunit (see “Discussion”), although no reaction of these cross-linked products with antibodies against subunits A, B, C, or H was observed. Three single cysteine mutants of subunit a (Vph1p) (S46C, T215C, and S282C) gave cross-linked products that could be identified using the anti-Vph1p subunit antibody but not with any of the other anti-V-ATPase subunit antibodies (data not shown), and these mutants were not further characterized. Sixteen single cysteine mutants of Vph1p (E3C, S26C, S74C, D89C, S95C, S115C, Q130C, S147C, T161C, A192C, A235C, N259C, S292C, S308C, S326C, and S392C), gave no cross-linked products detectable with the anti-Vph1p antibody and were also not further characterized.
FIGURE 7.
The N-terminal domain of subunit a (Vph1p) can be cross-linked to subunits E and G of V1 using the photoactivated cross-linking reagent MBP. Vacuolar membrane vesicles isolated from yeast expressing the Cys-less form of subunit a (Vph1p) or the indicated single cysteine-containing forms of Vph1p were incubated in the presence (+) or absence (–) of 1 mm MBP for 30 min at room temperature. Excess MBP was quenched by addition of 10 mm dithiothreitol and removed by washing the samples with phosphate-buffered saline-EDTA buffer by sedimentation. After resuspension, the samples were irradiated with a long wavelength ultraviolet lamp (366 nm) for 5 min at 4 °C, solubilized with 2% C12E9 and immunoprecipitated with an antibody against subunit A to precipitate intact complexes. The immunopurified proteins were separated by SDS-PAGE using a 7.5% Pre-Cast Gel and transferred to nitrocellulose membranes. Western blot analysis was then performed using an antibody against subunit a (Vph1p) or other subunit-specific antibodies, as indicated. A, vacuolar membranes from yeast expressing the Cys-less subunit a (Vph1p); staining performed with the anti-Vph1p antibody, indicating the absence of higher molecular weight cross-linked products in the membranes containing the Cys-less form of Vph1p. B–D, vacuolar membranes from the S266C, Q369C, and G181C mutants; staining performed with antibodies against subunits a and G, showing the MBP-dependent formation of a cross-linked product of molecular mass 110–130 kDa recognized by antibodies against both subunits a (Vph1p) and G. E–G, vacuolar membranes from the A347C, A351C, and Q369C mutants; staining performed using antibodies against subunits a and E, showing the MBP-dependent formation of a cross-linked product of molecular mass of ∼200 kDa recognized by antibodies against both subunits a (Vph1p) and E.
DISCUSSION
An important difference between the V-ATPases and the related family of F1F0 ATP synthases is the activity properties of the separated domains. For the ATP synthases, the dissociated F1 domain is active as an ATPase and the free F0 domain is functional as a passive proton pore (49). By contrast, the free V1 domain does not hydrolyze MgATP (33), and the free V0 domain does not passively conduct protons (34). Because the V-ATPase complex undergoes dissociation in vivo into its component V1 and V0 domains, this silencing of activities of the separate domains is essential to prevent unproductive ATP hydrolysis by V1 and dissipation of proton gradients across intracellular membranes by V0.
Binding of subunit H has previously been shown to inhibit ATP hydrolysis by V1 (35). Subunit H appears to accomplish this function by bridging the rotor and stator domains and physically preventing the rotational motion associated with catalysis (36). Two lines of evidence suggested the possibility that the N-terminal domain of subunit a might similarly inhibit passive proton translocation through the free V0 domain by physically interacting with the rotary subunits (subunit d and the proteolipid ring) and preventing rotation following dissociation of V1. First, the large N-terminal domain of subunit a is present in the a subunits of all V-ATPases (50) but is absent from the a subunit of the related F-ATPases, which as described above possess integral domains that passively conduct protons. Second, electron microscopic images of the V-ATPase from bovine clathrin-coated vesicles show an apparent movement of the N-terminal domain of subunit a from an orientation perpendicular to the membrane in intact V1V0 complexes to a “folded down” orientation in which it comes into close proximity to the remaining rotary complex (subunit d and the proteolipid ring) in the free V0 domain (17, 39).
The results presented in the current work suggest that removal of the N-terminal domain of subunit a is not sufficient to facilitate passive proton conductance of the free V0 domain. Thus cleavage of the factor Xa-containing a subunit with factor Xa protease and removal of the N-terminal domain by treatment with KNO3/MgATP gave vacuolar membrane vesicles possessing the same low passive proton permeability as native membranes. The apparent protease sensitivity of the released N-terminal domain prevented its detection in the supernatant, but ∼85% of the a subunit had been cleaved following a 3-h incubation with factor Xa protease. Moreover, the KNO3/MgATP treatment appeared effective at removing most of the residual N-terminal domain attached to the membrane. It is thus unlikely that proton conductance is being inhibited by fragments of the N-terminal domain still attached to V0.
It is still possible that the N-terminal domain of subunit a plays a role in suppressing the passive proton permeability of the C-terminal domain, but if so, it appears likely that it is not the only mechanism at work. An alternative (or additional) mechanism that may act to suppress the passive proton conductance of free V0 involves the structure of the proteolipid ring itself. Unlike the proteolipid ring of the F-ATPases, which contains a buried carboxyl group on each of the proteolipid transmembrane helices exposed to the bilayer (51), the proteolipid ring of the V-ATPases contains a buried carboxyl group on every other bilayer-exposed transmembrane helix (1). Because these buried carboxyl groups on the proteolipid subunits are thought to interact with the buried arginine residue in subunit a (52), the transmembrane helices not bearing such buried carboxyl groups may present an energy barrier for passive rotation past the a subunit arginine residue that may prevent passive proton transport. By contrast, ATP-driven rotation of the proteolipid ring may overcome this energy barrier to passive rotation. We are currently testing this hypothesis.
Comparison of the proton transport and ATPase activities of vacuolar membranes cleaved with factor Xa but not treated to remove V1 with those of untreated membranes revealed an ∼60% reduction in the coupling efficiency of proton transport to ATP hydrolysis, suggesting that a covalently intact subunit a is required to maintain tightness of coupling. This result is interesting in light of the previous observation that mild proteolysis of the reconstituted bovine V-ATPase leads to rapid cleavage of subunit a and almost complete loss of proton transport while ATP hydrolysis of the complex is reduced only 2-fold (53). In this previous study, however, trypsin cleavage was observed to occur at a site ∼20 kDa from the C terminus of the protein.3 Moreover, results obtained using chimeric a subunit constructs in yeast have demonstrated that the difference in coupling efficiency observed for V-ATPase complexes containing different isoforms of subunit a is linked to the C-terminal rather than the N-terminal domain (54). Taken together, these results indicate that changes in either the C-terminal domain or the linkage between the N- and C-terminal domains can alter coupling efficiency of the V-ATPase. While the structural basis of these changes in coupling efficiency is not clear, it is possible that, upon proteolysis of subunit a, some slippage of the A3B3 head domain relative to the integral domain of subunit a occurs. This would not be surprising if the N-terminal domain is envisioned as a sort of collar to which other parts of the peripheral stalk are attached (1) (see below). Cleavage of the collar from the anchor domain of subunit a might therefore allow the entire peripheral complex to rotate along with the A3B3 head. If this happens, the amount of rotation of the proteolipid ring relative to the integral domain of subunit a, and hence proton transport, will be reduced.
Previous studies have provided some information on how the N-terminal domain of subunit a interacts with the remainder of the complex. Co-immunoprecipitation studies have revealed that the N-terminal domain of subunit a is able to interact with both subunits A and H of V1 (19). Photoactivated cross-linking to unique cysteine residues in subunit C using the reagent MBP revealed that the foot domain of the C subunit (containing both the N and C terminus of the protein) is in close proximity to subunit a (18). Because subunits A, H, and C are all part of the stator portion of the V-ATPase complex (17), these studies suggested that the N-terminal domain of subunit a also forms part of the stator, consistent with its localization to the peripheral stalk in EM images of V1V0 (17). Coimmunoprecipitation studies have also identified an interaction between the N-terminal domain of subunit a and the glycolytic enzyme aldolase, which plays a role in controlling reversible dissociation of the V-ATPase in vivo (24). None of the previous studies, however, have identified where within the N-terminal domain these interactions take place.
In the present study we have identified sites within the N-terminal domain that are in close proximity to peripheral stalk subunits. Subunit G cross-links to three sites within this domain (G181C, S266C, and Q369C) whereas subunit E cross-links to one of these sites (Q369C) as well as two additional sites (A347C and A351C). The apparent molecular mass (200 kDa) of this a-E cross-linked product is considerably higher than that predicted from the sum of the molecular masses of subunits a and E (∼130 kDa). It is possible that this anomalous migration behavior is due to the hydrodynamic properties of the cross-linked product, as we have previously observed such aberrant migration depending upon where within the primary sequence cross-linking occurs (15–18, 36). It is also possible that another subunit (or unknown protein) is present in the cross-linked complex. We have previously observed cross-linking of subunits C and E to give a cross-linked product of molecular mass 70–80 kDa for multiple single cysteine mutants of subunit C (18). Because subunit C in the present experiments contains its endogenous cysteine residue at position 340, it is possible that subunits E and C form an MBP-dependent heterodimer that is then further cross-linked to subunit a for mutants A347C, A351C and Q369C. In fact an MBP-dependent band of ∼75 kDa is observed in the anti-E subunit antibody blot for both the A351C and Q369C mutants that could correspond to such a C-E heterodimer, although no cross-reaction of this band or that at 200 kDa is observed using the anti-C subunit antibody (data not shown). It is possible that these products are not recognized by the anti-C subunit antibody due to a blocking of the epitope recognized by this antibody as a result of cross-linking. It should also be noted that the intensity of this 75 kDa band recognized by the anti-E subunit antibody is somewhat variable for reasons that are uncertain, as it is detectable for the A347C mutant of subunit a only at longer exposure times. If the observed band does contain both subunits C and E, it would appear that the heterodimer of C and E is positioned to crosslink to subunit a in a way that neither subunit E nor C is able to alone, because no bands of intermediate molecular mass are observed for these mutants. It should also be noted that the presence of subunits A, B, or H, which have molecular masses in the range of 50–70 kDa, could also cause the observed mobility of the 200-kDa cross-linked product, although again antibodies against these subunits do not recognize this product.
In any case, the data clearly identify a region of the N-terminal domain near the border of the N- and C-terminal domain that functions in binding other subunits of the peripheral stalk. It is possible that changes in this region may help to trigger the loosening of the interaction between V1 and V0 that occurs during in vivo dissociation of the complex.
Acknowledgments
We thank Dr. Daniel Klionsky (University of Michigan) and Dr. Tom Stevens (University of Oregon) for the kind gift of polyclonal antibodies against subunits E and G, respectively. We also thank Drs. Daniel Cipriano, Ayana Hinton, Masashi Toei, and Yanru Wang as well as Sarah Bond and Kevin Jefferies for many helpful discussions. E. coli strains were provided through National Institutes of Health Grant DK34928.
This work was supported, in whole or in part, by National Institutes of Health Grant GM34478 (to M. F.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: V-ATPase, vacuolar proton-translocating adenosine 5′-triphosphatase; F-ATPase, F1F0 ATP synthase; MBP, 4-(N-maleimido)benzophenone; C12E9, polyoxyethylene 9-lauryl ether; ACMA, 9-amino-6-chloro-2-methoxyacridine; CCCP, carbonyl cyanide 3-chlorophenylhydrazone; BSA, bovine serum albumin; EM, electron microscopy.
I. Adachi and M. Forgac, unpublished data.
References
- 1.Forgac, M. (2007) Nat. Rev. Mol. Cell. Biol. 8 917–929 [DOI] [PubMed] [Google Scholar]
- 2.Kane, P. M. (2006) Microbiol. Mol. Biol. Rev. 70 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner, C. A., Finberg, K. E., Breton, S., Marshansky, V., Brown, D., and Geibel, J. P. (2004) Physiol. Rev. 84 1263–1314 [DOI] [PubMed] [Google Scholar]
- 4.Nelson, N. (2003) J. Bioenerg. Biomembr. 35 281–289 [DOI] [PubMed] [Google Scholar]
- 5.Maxfield, F. R., and McGraw, T. E. (2004) Nat. Rev. Mol. Cell. Biol. 5 121–132 [DOI] [PubMed] [Google Scholar]
- 6.Moriyama, Y., Maeda, M., and Futai, M. (1992) J. Exp. Biol. 172 171–178 [DOI] [PubMed] [Google Scholar]
- 7.Gruenberg, J., and van der Goot, F. G. (2006) Nat. Rev. Mol. Cell. Biol. 7 495–504 [DOI] [PubMed] [Google Scholar]
- 8.Pietrement, C., Sun-Wada, G. H., Silva, N. D., McKee, M., Marshansky, V., Brown, D., Futai, M., and Breton, S. (2006) Biol. Reprod. 74 185–194 [DOI] [PubMed] [Google Scholar]
- 9.Toyomura, T., Murata, Y., Yamamoto, A., Oka, T., Sun-Wada, G. H., Wada, Y., and Futai, M. (2003) J. Biol. Chem. 278 22023–22030 [DOI] [PubMed] [Google Scholar]
- 10.Sennoune, S. R., Bakunts, K., Martinez, G. M., Chua-Tuan, J. L., Kebir, Y., Attaya, M. N., and Martinez-Zaguilan, R. (2004) Am. J. Physiol. Cell. Physiol. 286 C1443–C1452 [DOI] [PubMed] [Google Scholar]
- 11.Cross, R. L., and Muller, V. (2004) FEBS Lett. 576 1–4 [DOI] [PubMed] [Google Scholar]
- 12.Imamura, H., Nakano, M., Noji, H., Muneyuki, E., Ohkuma, S., Yoshida, M., and Yokoyama, K. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2312–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirata, T., Iwamoto-Kihara, A., Sun-Wada, G. H., Okajima, T., Wada, Y., and Futai, M. (2003) J. Biol. Chem. 278 23714–23719 [DOI] [PubMed] [Google Scholar]
- 14.Iwata, M., Imamura, H., Stambouli, E., Ikeda, C., Tamakoshi, M., Nagata, K., Makyio, H., Hankamer, B., Barber, J., Yoshida, M., Yokoyama, K., and Iwata, S. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arata, Y., Baleja, J. D., and Forgac, M. (2002) Biochemistry 41 11301–11307 [DOI] [PubMed] [Google Scholar]
- 16.Arata, Y., Baleja, J. D., and Forgac, M. (2002) J. Biol. Chem. 277 3357–3363 [DOI] [PubMed] [Google Scholar]
- 17.Wilkens, S., Inoue, T., and Forgac, M. (2004) J. Biol. Chem. 279 41942–41949 [DOI] [PubMed] [Google Scholar]
- 18.Inoue, T., and Forgac, M. (2005) J. Biol. Chem. 280 27896–27903 [DOI] [PubMed] [Google Scholar]
- 19.Landolt-Marticorena, C., Williams, K. M., Correa, J., Chen, W., and Manolson, M. F. (2000) J. Biol. Chem. 275 15449–15457 [DOI] [PubMed] [Google Scholar]
- 20.Beyenbach, K. W., and Wieczorek, H. (2006) J. Exp. Biol. 209 577–589 [DOI] [PubMed] [Google Scholar]
- 21.Trombetta, E. S., Ebersold, M., Garrett, W., Pypaert, M., and Mellman, I. (2003) Science 299 1400–1403 [DOI] [PubMed] [Google Scholar]
- 22.Sautin, Y. Y., Lu, M., Gaugler, A., Zhang, L., and Gluck, S. L. (2005) Mol. Cell. Biol. 25 575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane, P. M. (1995) J. Biol. Chem. 270 17025–17032 [PubMed] [Google Scholar]
- 24.Lu, M., Sautin, Y. Y., Holliday, L. S., and Gluck, S. L. (2004) J. Biol. Chem. 279 8732–8739 [DOI] [PubMed] [Google Scholar]
- 25.Lu, M., Ammar, D., Ives, H., Albrecht, F., and Gluck, S. L. (2007) J. Biol. Chem. 282 24495–24503 [DOI] [PubMed] [Google Scholar]
- 26.Seol, J. H., Shevchenko, A., and Deshaies, R. J. (2001) Nat. Cell Biol. 3 384–391 [DOI] [PubMed] [Google Scholar]
- 27.Smardon, A. M., Tarsio, M., and Kane, P. M. (2002) J. Biol. Chem. 277 13831–13839 [DOI] [PubMed] [Google Scholar]
- 28.Smardon, A. M., and Kane, P. M. (2007) J. Biol. Chem. 282 26185–26194 [DOI] [PubMed] [Google Scholar]
- 29.Xu, T., and Forgac, M. (2001) J. Biol. Chem. 276 24855–24861 [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki-Nishi, S., Nishi, T., and Forgac, M. (2001) J. Biol. Chem. 276 17941–17948 [DOI] [PubMed] [Google Scholar]
- 31.Qi, J., and Forgac, M. (2007) J. Biol. Chem. 282 24743–24751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voss, M., Vitavska, O., Walz, B., Wieczorek, H., and Baumann, O. (2007) J. Biol. Chem. 282 33735–33742 [DOI] [PubMed] [Google Scholar]
- 33.Puopolo, K., Sczekan, M., Magner, R., and Forgac, M. (1992) J. Biol. Chem. 267 5171–5176 [PubMed] [Google Scholar]
- 34.Zhang, J., Myers, M., and Forgac, M. (1992) J. Biol. Chem. 267 9773–9778 [PubMed] [Google Scholar]
- 35.Parra, K. J., Keenan, K. L., and Kane, P. M. (2000) J. Biol. Chem. 275 21761–21767 [DOI] [PubMed] [Google Scholar]
- 36.Jefferies, K. C., and Forgac, M. (2008) J. Biol. Chem. 283 4512–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leng, X. H., Nishi, T., and Forgac, M. (1999) J. Biol. Chem. 274 14655–14661 [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki-Nishi, S., Nishi, T., and Forgac, M. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12397–12402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkens, S., and Forgac, M. (2001) J. Biol. Chem. 276 44064–44068 [DOI] [PubMed] [Google Scholar]
- 40.Manolson, M. F., Wu, B., Proteau, D., Taillon, B. E., Roberts, B. T., Hoyt, M. A., and Jones, E. W. (1994) J. Biol. Chem. 269 14064–14074 [PubMed] [Google Scholar]
- 41.Sherman, F. (2002) Methods Enzymol. 350 3–41 [DOI] [PubMed] [Google Scholar]
- 42.Gietz, D., St Jean, A., Woods, R. A., and Schiestl, R. H. (1992) Nucleic Acids Res. 20 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parra, K. J., and Kane, P. M. (1996) J. Biol. Chem. 271 19592–19598 [DOI] [PubMed] [Google Scholar]
- 44.Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J. Biol. Chem. 193 265–275 [PubMed] [Google Scholar]
- 45.Shao, E., and Forgac, M. (2004) J. Biol. Chem. 279 48663–48670 [DOI] [PubMed] [Google Scholar]
- 46.Feng, Y., and Forgac, M. (1992) J. Biol. Chem. 267 5817–5822 [PubMed] [Google Scholar]
- 47.Liu, J., and Kane, P. M. (1996) Biochemistry 35 10938–10948 [DOI] [PubMed] [Google Scholar]
- 48.MacLeod, K. J., Vasilyeva, E., Baleja, J. D., and Forgac, M. (1998) J. Biol. Chem. 273 150–156 [DOI] [PubMed] [Google Scholar]
- 49.Yoshida, M., Muneyuki, E., and Hisabori, T. (2001) Nat Rev Mol. Cell. Biol. 2 669–677 [DOI] [PubMed] [Google Scholar]
- 50.Nishi, T., and Forgac, M. (2000) J. Biol. Chem. 275 6824–6830 [DOI] [PubMed] [Google Scholar]
- 51.Fillingame, R. H., Angevine, C. M., and Dmitriev, O. Y. (2002) Biochim. Biophys. Acta 1555 29–36 [DOI] [PubMed] [Google Scholar]
- 52.Vik, S. B., Long, J. C., Wada, T., and Zhang, D. (2000) Biochim. Biophys. Acta 1458 457–466 [DOI] [PubMed] [Google Scholar]
- 53.Adachi, I., Arai, H., Pimental, R., and Forgac, M. (1990) J. Biol. Chem. 265 960–966 [PubMed] [Google Scholar]
- 54.Kawasaki-Nishi, S., Bowers, K., Nishi, T., Forgac, M., and Stevens, T. H. (2001) J. Biol. Chem. 276 47411–47420 [DOI] [PubMed] [Google Scholar]