Abstract
Skeletal muscle fibrosis is a major pathological hallmark of chronic myopathies in which myofibers are replaced by progressive deposition of collagen and other extracellular matrix proteins produced by muscle fibroblasts. Recent studies have shown that in the absence of the endogenous muscle growth regulator myostatin, regeneration of muscle is enhanced, and muscle fibrosis is correspondingly reduced. We now demonstrate that myostatin not only regulates the growth of myocytes but also directly regulates muscle fibroblasts. Our results show that myostatin stimulates the proliferation of muscle fibroblasts and the production of extracellular matrix proteins both in vitro and in vivo. Further, muscle fibroblasts express myostatin and its putative receptor activin receptor IIB. Proliferation of muscle fibroblasts, induced by myostatin, involves the activation of Smad, p38 MAPK and Akt pathways. These results expand our understanding of the function of myostatin in muscle tissue and provide a potential target for anti-fibrotic therapies.
Fibroblasts play an important role in the repair response of tissues to injury by secreting extracellular matrix proteins including collagen and growth factors. However, in a variety of disease states, continued fibrosis contributes to the pathological process. In chronic myopathies and muscular dystrophies, fibrosis is considered to be associated with decreased strength and elasticity of muscle and may inhibit the diffusion of nutrients to myofibers (1–3). Identification of factors that regulate fibrosis is an important goal not only in understanding the pathogenesis of muscular dystrophies but also in developing novel therapies to treat these disorders. Several potential future therapies for the muscular dystrophies, including those involving gene and myoblast transfer, may be hampered by significant fibrosis.
Myostatin is a highly conserved transforming growth factor-β (TGF-β)2 family member that is expressed in skeletal muscle, which is also the primary target tissue (4). Deletion of the myostatin gene (MSTN) in mice leads to muscle hypertrophy and hyperplasia with an approximate doubling of muscle mass (4). This function of myostatin, as an endogenous inhibitor of muscle growth, is also conserved in humans, as demonstrated by the identification of a hypermuscular child with a loss-of-function mutation in the myostatin gene (5). One mechanism by which myostatin regulates muscle growth in adult animals appears to be direct inhibition of the proliferation and differentiation of resident muscle precursor cells (6–8).
The potential effect of myostatin inhibition on muscle degenerative diseases has been explored in various animal models. In the absence of myostatin, muscle regenerates more quickly and completely following acute and chronic injury (8, 9). In the mdx mouse, a model of Duchenne and Becker muscular dystrophy, myostatin deletion, or postnatal inhibition increases muscle mass and strength (10–12). An early observation of mdx/mstn null mice was that the diaphragm muscle of these animals showed significantly less fibrosis compared with mdx littermates as determined by histology and hydroxyproline content, a modified amino acid of collagen (12). Reduced fibrosis has also been observed in mdx mice treated postnatally with a neutralizing antibody to myostatin (10) and with a modified myostatin propeptide delivered via adeno-associated virus (11). Decreased fibrosis of limb muscle in myostatin null animals after acute injury with notexin has been reported (9). Recently, Parsons et al. (13) studied the effect of myostatin loss or postnatal inhibition in δ-sarcoglycan (scgd–/–) mice, a mouse model of limb-girdle muscle dystrophy (LGMD2F). Fibrosis, as defined by morphometric analysis and hydroxyproline content, was significantly decreased in the scgd–/– myostatin null mouse although not in the scgd–/– treated with a neutralizing antibody to myostatin (13). These studies in models of acute and chronic injury all support the emerging concept that myostatin is a negative regulator of muscle precursors and that inhibition of myostatin improves muscle growth and regeneration. However, it is unclear from these previous studies whether the observed reduction in fibrosis is secondary to increased myofiber regeneration or whether myostatin may have a direct effect on fibrosis.
EXPERIMENTAL PROCEDURES
Growth Factors and Inhibitors—Recombinant mouse myostatin and follistatin were obtained from R & D Systems (Minneapolis, MN). Rapamycin was obtained from Cell Signaling (Danvers, MA). SB202190, SB203580, and Smad3-specific inhibitor (SIS3) were obtained from Sigma.
Antibodies—Primary antibodies used in these studies included: mouse anti-proliferating cell nuclear antigen (PCNA); goat anti-activin receptor IIB; mouse anti-cyclin D1; rabbit anti-human fibronectin; mouse anti-vimentin; and mouse anti-α-smooth muscle antigen (SMA) from Sigma. Mouse anti-HSP47 was obtained from Abcam (Cambridge, MA). Rabbit anti-phospho-Smad 2 (Ser465/467) was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY). Rabbit anti-phospho-Smad3 (Ser433/435) was obtained from EMD Chemicals Inc. (Darmstadt, Germany). Rabbit anti-Smad2; rabbit anti-Smad3; rabbit anti-phospho-Akt (Ser473); rabbit anti-Akt; rabbit anti-phospho-p38 MAPK; rabbit anti-p38 MAPK; rabbit anti-phospho-mTOR; rabbit anti-mTOR; and rabbit anti-α-tubulin antibodies were obtained from Cell Signaling. Mouse anti-MyoD was obtained from BD Biosciences (San Jose, CA). Rabbit anti-collagen I was obtained by Cell Sciences (Canto MA). Mouse anti-MF20 (developed by Donald A. Fischman), mouse anti-CD45 (by J. Thomas August and James E. K. Hildreth), and mouse anti-Pax 7 (by Atsushi Kawakami) were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). A polyclonal anti-myostatin antibody was raised in rabbits to a 15-amino acid peptide corresponding to an epitope in the C-terminal domain of myostatin (DFGLDCDEHSTESRC (Ahx)K-amide) and affinity-purified. This antibody specifically recognizes purified myostatin and a 12-kDa protein band in wild type but not myostatin null cell cultures (see supplemental Fig. S1). The secondary antibodies used in this study included: Alexa Fluor 488, 555, 594, and 647 (Invitrogen); donkey anti-mouse or anti-rabbit IgG peroxidase conjugate (Molecular Probes); and anti-goat IgG peroxidase conjugate (Sigma).
Primary Muscle Fibroblast Culture and Treatments—All of the animal experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee at The Johns Hopkins University, School of Medicine. Myostatin null mice (mstn–/–/C57BL/6) were generated by Se-Jin Lee (4). Activin receptor type IIB null mice (ActRIIB–/–) were generated by En Li (14). C57BL/6 and mdx/C57BL/6 mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Hind limb skeletal muscles and diaphragm were harvested from 4–6-week-old mice. After removing fascia, fat, and other connective tissue, the muscles were washed several times in PBS and mechanically dissociated by mincing with scissors. Minced muscles were digested with 0.2% collagenase (Sigma) diluted in Dulbecco's modified Eagle's medium (Invitrogen) at 37 °C for 30 min. After filtering through a 40-μm Nylon cell strainer (BD Falcon), the digested muscles were centrifuged at 2000 rpm for 5 min. The cell pellets were resuspended in culture medium (10% fetal bovine serum with 1% penicillin/streptomycin in Dulbecco's modified Eagle's medium). The cells were incubated at 37 °C and 5% CO2 to allow adherence to noncoated 10-cm culture dishes. As previously described, muscle fibroblasts adhere within minutes to noncoated plastic dishes, whereas myogenic cells require longer time periods (15, 16). Following 30 min of preplating, non-adherent cells were removed, and adherent cells were washed with PBS to further discard any potential remaining myogenic cells. The cultured cells were washed with PBS every day until cells became confluent. The adherent cells were shown to be exclusively fibroblasts by RT-PCR and immunohistochemistry (see supplemental Fig. S2). Before treatment with myostatin or other growth factor, the cells were grown in serum-free culture medium for 12 h. For transient transfections of primary muscle fibroblasts with pCMV-Smad3-FLAG or pCMV-empty, Lipofectamine® 2000 transfection reagent (Invitrogen) was used as per the manufacturer's instructions.
Immunocytochemistry—Primary muscle fibroblasts and C2C12 myoblasts (ATCC, Manassas, VA) were grown on microscope cover glasses (Fisherbrand) overnight. The cells or 10-μm cryosections of muscle were washed with PBS and fixed with 4% paraformadehyde for 10 min. After blocking with 5% goat serum with PBS at room temperature for 30 min, the cells were incubated with primary antibodies at 4 °C overnight and then with secondary antibodies at room temperature for 1 h. All of the primary antibodies were used at 1:200 except for anti-Pax 7, which was used at 1:5.
Cell Proliferation Assays—For [3H]thymidine and [3H]proline incorporation assays, the fibroblasts were plated at a density of 105 cells/well in 96-well plates and cultured overnight. After the cells were cultured in serum-free Dulbecco's modified Eagle's medium for 12 h, a final concentration of 12–300 ng/ml recombinant myostatin or myostatin preincubated with equimolar amounts of follistatin was added to each well for an additional 12 h. The cells were pretreated with 10 nmol rapamycin (Cell Signaling) for 1 h prior to the addition of myostatin where indicated. 4 μCi/ml [3H]thymidine or [3H]proline was added to culture medium for an additional 24 h. Incorporation of tritium into cells was counted in triplicate wells using a liquid scintillation system (Beckman LS 5801).
RT-PCR—Total cellular RNA was isolated using TRIzol reagent as per the manufacturer's directions (Invitrogen). Full-length first strand cDNA was generated from 500 ng of total RNA (Amersham Biosciences). Primers included: Pax 7 (sense, GGTCCCCAGGATGATGAGA, and antisense, TTGATGAAGACCCCACCAAG); Desmin (sense, TCGCGGCTAAGAACATCTCT, and antisense, ACATCCAAGGCCATCTTCAC); HSP47(sense, CCTGAGGTCACCAAGGATGT, and antisense, CTGCAGCTTCTCCTTCTCGT); prolyl 4-hydroxylase (sense, CCGAAGATTTTTGGAGGTGA, and antisense, CGCCCCAACCAGTACTTTTA); and myostatin (sense, CTGGTCCTGGGAAGGTTACA, and antisense, ACGCTACCACGGAAACAATC). Either glyceraldhyde-3-phosphate dehydrogenase (sense, CAACTACATGGTTTACATGTTC, and antisense, GCCAGTGGACTCCACGAC) or β-actin (sense, GCTCGTCGTCGACAACGGCTC, and antisense, CAAACATGATCTGGGTCATCTTCTC) cDNA was used as an internal standard. For studies on myostatin expression, PCR products were excised, purified, and sequenced.
p(CAGA)12 Luciferase Reporter Assay—A204 (human rhabdomyosarcoma) cells that were stably transfected with p(CAGA)12-MLP-Luc were a gift from Li-fang Liang (MetaMorphix Inc., Savage, MD) (17). Muscle fibroblasts isolated from wild-type mice and myostatin null mice were washed three times with PBS and then cultured in serum-free McCoy's 5A medium for 48 h. The conditioned culture medium was collected, centrifuged at 2000 rpm for 5 min, and heat-activated at 100 °C for 5 min. Transfected A204 cells were incubated with the conditioned medium for 24 h, and the activity of luciferase was measured using an Lmax II luminometer (Molecular Devices).
Immunoblot Analysis—SDS-PAGE and Western blot transfer were performed on cell lysates using standard techniques. The primary antibodies were used as described above. The blots were incubated with horseradish peroxidase secondary antibodies, and chemiluminescent reagent (Amersham Biosciences) was used to detect the secondary antibody on the membrane. The relative density of each band was determined by using Scio Image software (National Institutes of Health).
Injection of Myostatin Beads into Skeletal Muscle in Vivo—Heparin acrylic beads, 125–250 μm in diameter (Sigma), were soaked in 100 μg/ml myostatin, myostatin plus equimolar follistatin, or PBS for 1 h at room temperature prior to injection. Following anesthesia, tibialis muscles of 4–6-week-old C57BL/6 mice were exposed, and ∼10 beads were injected into each muscle. The muscles were removed from euthanized mice 1 week after injection. Transverse sections (10 μm thick) were obtained at 500-μm intervals throughout the injected region. The sections were stained with hematoxylin and eosin or Sirius red or used for immunohistochemistry as described above. A fibrosis index was calculated by averaging the area of fibrosis per area of beads in multiple sections per tibialis-injected muscle.
Statistical Evaluation—All of the data are presented as the means and standard deviations. Student's t test is used for comparison of the means between groups.
RESULTS
Myostatin Increases Proliferation of Muscle Fibroblasts in Vitro and in Vivo—To address the role of myostatin in the regulation of fibroblast proliferation, primary muscle fibroblasts were isolated and cultured from limb muscles of C57BL/6 mice. Primary muscle fibroblasts had a fibroblast-like morphology and expressed common antigenic markers of fibroblasts including vimentin, collagen I, HSP47, and α-SMA but did not express satellite cell or myogenic markers such as Pax 7, MyoD, desmin, or myosin heavy chain (supplemental Fig. S2, a and b). RT-PCR from mRNA isolated from cultures demonstrated expression of HSP47 and prolyl 4-hydroxylase but not Pax7 or desmin (supplemental Fig. S2, c and d), consistent with pure cultures of muscle fibroblasts devoid of myogenic cells.
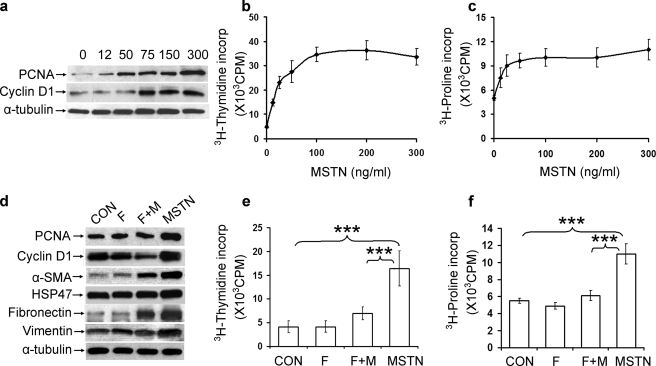
Cultured muscle fibroblasts were treated with recombinant myostatin at concentrations of 12–300 ng/ml for 24 h, and proliferation was assayed by various measures. Expression of PCNA and cyclin D1, subcellular markers of cell proliferation, increased in a dose-dependent manner (Fig. 1a). [3H]Thymidine incorporation, an assay of cellular DNA synthesis, and [3H]proline incorporation, a marker of collagen production, increased linearly with concentrations of myostatin from 12.5 to 50 ng/ml and reached a plateau at higher concentrations (Fig. 1, b and c). This proliferative effect of myostatin on muscle fibroblasts could be partially blocked with follistatin, an endogenous inhibitor of myostatin (18, 19). Myostatin-induced expression of PCNA and cyclin D1, as well as fibroblast-associated proteins α-SMA, HSP47, fibronectin, and vimentin, were blocked or reduced by co-treatment of myostatin and equimolar concentrations of follistatin (Fig. 1d). [3H]Thymidine incorporation was significantly reduced (p < 0.001) with follistatin plus myostatin compared with myostatin alone (Fig. 1e). Similarly, incorporation of [3H]proline was blocked by co-treatment of follistatin with myostatin and was statistically indistinguishable from controls (Fig. 1f).
FIGURE 1.
Myostatin induces fibroblast proliferation in vitro. a–c, primary muscle fibroblasts were treated with increasing doses of myostatin for 24 h. a, immunoblot analysis of PCNA and cyclin D1 expression in treated muscle fibroblasts. Equal amounts of protein were loaded in each lane as shown by α-tubulin immunoreactivity. b, [3H]thymidine incorporation. c, [3H]proline incorporation in treated primary muscle fibroblasts. d–f, fibroblasts were treated with either the diluent (CON), myostatin (MSTN), follistatin (F), or both myostatin and follistatin (F+M). d, immunoblot analysis of PCNA, cyclin D1, α-SMA, HSP47, fibronectin, and vimentin, markers of proliferation and fibroblast proteins. e, [3H]thymidine incorporation in treated fibroblasts. f, [3H]proline incorporation in treated fibroblasts. n = 5 for all data points; ***, p < 0.001.
Skeletal muscles, and particularly diaphragm, of the dystrophic mdx mouse model have enhanced interstitial fibrosis (20). To determine whether fibroblasts from mdx muscle are also responsive to myostatin, primary fibroblasts were isolated from mdx and wild-type mouse hindlimb and diaphragm muscles. Myostatin induced PCNA expression in fibroblasts from mdx diaphragm in a dose-dependent fashion (supplemental Fig. S3a). Cyclin D1 immunoreactivity increased 2–3-fold following the addition of myostatin to the medium of cultured fibroblasts from mdx hindlimb or diaphragm muscle similar to the increases observed in wild-type muscle (supplemental Fig. S3b).
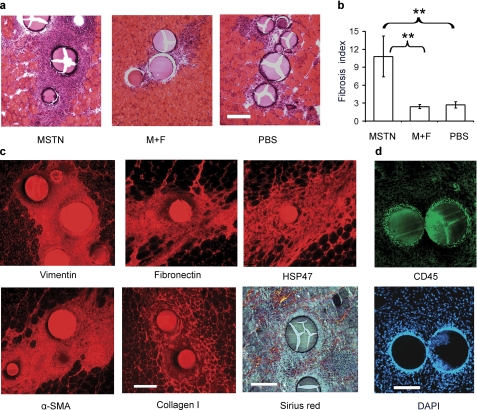
To determine whether myostatin directly induces fibroblast proliferation and fibrosis in vivo, heparin acrylic beads preincubated with recombinant myostatin were injected into tibialis muscle of adult wild-type mice. Using a similar bead injection method, previous investigators have been able to demonstrate myostatin inhibition of myogenesis in chick embryos (18). Tibialis muscle was harvested 1 week following injection with beads coated with myostatin, equimolar concentrations of myostatin and follistatin, or PBS. Histochemistry of transverse sections demonstrated endomysial cellular infiltrates and collagen deposition around myostatin-coated beads (Fig. 2a). There were slight cellular infiltrates surrounding PBS-coated beads. The average area of fibrosis per area of beads (fibrosis index) was approximately five times greater for myostatin-coated than PBS-coated beads (p < 0.005) (Fig. 2b). Preincubation of myostatin with equimolar concentrations of follistatin abolished myostatin-induced fibrosis (Fig. 2a, middle panel), and the fibrosis index of beads coated with myostatin and follistatin was statistically indistinguishable from that of beads coated with PBS (Fig. 2b). Picro-Sirius Red staining of collagen and immunohistochemistry with antibodies to vimentin, fibronectin, HSP47, α-SMA, and collagen I confirmed the identity of the cellular infiltrates as predominantly fibroblasts with extracellular matrix protein production (Fig. 2c). Conversely, there was only a thin rim of inflammatory cells surrounding the myostatin-coated beads as seen by immunohistochemistry with antibodies to CD45, a marker of T and B cells (Fig. 2d). These results confirm that myostatin induces fibroblast proliferation and extracellular matrix protein production in vivo.
FIGURE 2.
Myostatin induces fibrosis in vivo. a, hematoxylin and eosin-stained sections of tibialis muscle from 3-month-old C57BL/6 mice injected with beads coated with myostatin (MSTN), myostatin and follistatin (M+F), or PBS. Bar, 150 μm. b, fibrosis index of muscle from myostatin-coated beads is significantly increased compared with that of beads coated with PBS or myostatin plus follistatin. n = 5 for each condition; **, p < 0.005. c, immunohistochemistry of tibialis muscle injected with myostatin-coated beads showing vimentin, fibronectin, HSP47, α-SMA, and collagen. Birefringence of Picro Sirius Red staining collagen around myostatin-coated beads. d, immunohistochemistry with antibodies to CD45, a T and B cell marker, identifies only a small ring of inflammatory cells bordering the myostatin-coated beads.
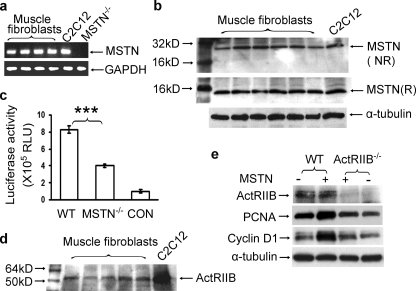
Myostatin Is Produced by Fibroblasts—Myostatin mRNA and protein are predominantly detected in total skeletal muscle tissue and have been reported to be produced by myofibers and satellite cells (6, 21). To determine whether the fibroblasts included in total muscle homogenates also express myostatin, we examined the myostatin RNA and protein from isolated muscle fibroblasts. Myostatin mRNA was detected by RT-PCR of RNA isolated from primary muscle fibroblast cultures derived from C57/BL/6 mice as well as from a positive control of C2C12 myoblasts but not from cultures of muscle fibroblasts derived from myostatin null mice (Fig. 3a). Myostatin protein was detected using a polyclonal antibody developed to the C-terminal peptide of myostatin (see “Experimental Procedures”). This antibody recognized a 25-kDa protein band from muscle fibroblasts and C2C12 myoblasts on nonreducing condition gels and a 12-kDa protein band on reducing condition gels consistent with the molecular weights of the disulfide-bridged dimer and monomer, respectively, of active myostatin (Fig. 3b). Interestingly, myostatin expression in fibroblasts is not limited to muscle. Primary fibroblasts derived from a variety of organs produced low levels of myostatin transcript as determined by RT-PCR and a subset had detectable protein product as determined by immunoblot (supplemental Fig. S4).
FIGURE 3.
Muscle fibroblasts express myostatin and ActRIIB. a, determination of myostatin mRNA expression by RT-PCR in primary muscle fibroblasts isolated from wild-type, myostatin null mice (mstn–/–) or C2C12 myoblasts. b, polyclonal antibodies to myostatin peptide recognize a 25-kDa band in nonreducing (NR) and a 12-kDa band in reducing (R) conditions from protein isolated from primary muscle fibroblasts and C2C12 cells. c, induction of p(CAGA)12 luciferase reporter construct by muscle fibroblasts. Condition medium from wild-type (WT) and mstn–/– muscle fibroblasts as well as serum-free medium (CON) was heat-activated and added to A204 cells stably transfected with the p(CAGA)12-MLP-Luc reporter construct. Each bar represents the mean ± S.D. luciferase activity (relative light units (RLU)) of A204 cells assayed after 24 h in each media. n = 6 for each data point; ***, p < 0.001. d, ActRIIB expression in primary muscle fibroblasts as shown by immunoblot. e, immunoblot of proliferation markers (PCNA and cyclin D1) in muscle fibroblasts from wild-type (WT) and ActRIIB null mice (ActRIIB–/–) treated (+) or not treated (–) with 300 ng/ml myostatin for 12 h. MSTN, myostatin.
Myostatin produced from fibroblasts is biologically active as determined by a luciferase reporter assay. The p(CAGA)12-MLP-Luc reporter construct, containing 12 copies of the consensus SBE sequence CAGA, has been widely used as a TGF-β family member and myostatin-inducible promoter system (22–24). Heat-activated conditioned medium from primary wild-type muscle fibroblasts displayed luciferase activity severalfold greater than that from myostatin null fibroblasts (Fig. 3c). Muscle fibroblasts express the putative receptor for myostatin (ActRIIB) (Fig. 3d) (19, 23). ActRIIB is a likely fibroblast receptor for myostatin because myostatin does not induce expression of markers of cell proliferation in muscle fibroblasts cultured from skeletal muscle of mice with a targeted disruption of the ActRIIB gene (Fig. 3e) (14). Taken together, these results suggest that muscle fibroblasts produce functional myostatin, which induces proliferation through the ActRIIB receptor.
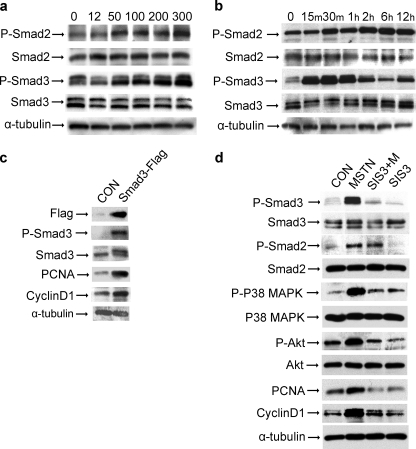
Myostatin Induces Early Activation of Smad Followed by Delayed Activation of p38 MAPK and PI3K-Akt Pathways in Muscle Fibroblasts—Myostatin inhibits myocyte proliferation and differentiation in part by stimulating phosphorylation of both Smad2 and Smad3 (19, 23). When muscle fibroblasts were treated with recombinant myostatin at concentrations from 12.5 to 300 ng/ml for 24 h, phosphorylation of both Smad2 and Smad3 increased in a dose-dependent manner, whereas the total levels of Smad2 and Smad3 were unchanged (Fig. 4a). Increased phospho-Smad2 and phospho-Smad3 could be detected at 15 min after the addition of myostatin (Fig. 4b). To determine whether the Smad signaling pathway was directly involved in myostatin-induced muscle fibroblast proliferation, we examined the effect of Smad3 on markers of proliferation. Previous reports have indicated that Smad3, but not Smad2, mediates TGF-β induced proliferation of nonmuscle fibroblasts (25–27). Muscle fibroblasts were transiently transfected with pCMV-Smad3-FLAG, resulting in a corresponding increase in phospho-Smad3, indicating that the overexpressed Smad3-FLAG was active (Fig. 4c). Smad3-FLAG-expressing fibroblasts had increased levels of proliferative markers PCNA and cyclin D1. Next, we examined the effect of inhibiting Smad3 on myostatin-treated muscle fibroblasts. Endogenous Smad3 activity was blocked with SIS3 in muscle fibroblasts. SIS3 is a potent and specific inhibitor of Smad3 that inhibits the phosphorylation of Smad3 and its interaction with Smad 4 but does not affect the phosphorylation of Smad2 (25). Immunoblot analysis showed that treatment of muscle fibroblasts with 3 μm SIS3 blocked base-line levels of Smad3 phosphorylation, but total Smad3 remained unchanged (Fig. 4d). Phospho-Smad2 and total Smad2 were unaffected by SIS3 treatment. Pretreatment of fibroblasts with SIS3 led to inhibition of myostatin-mediated increases in phospho-Smad3 and PCNA. (Fig. 4d) Together, these results suggest that myostatin activates the canonical Smad signaling pathway in fibroblasts and that Smad3 phosphorylation induces fibroblast proliferation.
FIGURE 4.
Myostatin activates the Smad signaling pathway in fibroblasts. Immunoblot analysis of cell lysate from primary muscle fibroblasts with antibodies to phospho-Smad2 (P-Smad2), total Smad2 (Smad2), phospho-Smad3 (P-Smad3), total Smad3 (Smad3), PCNA, cyclin D1, phospho-Akt (P-Akt), total Akt (Akt), phospho-p38MAPK (P-P38 MAPK), total p38 MAPK (P38 MAPK), and α-tubulin, as a loading control. a, treated with increasing concentrations of myostatin for 24 h; b, treated with 300 ng/ml of myostatin for increasing duration; c, Transfected with pCMV-empty (CON) or pCMV-Smad3-FLAG; d, untreated (CON) or treated for 6 h with 300 ng/ml of myostatin (MSTN), pretreated for 1 h with 3 μm SIS3 prior to myostatin (SIS3+M) or SIS3 alone (SIS3).
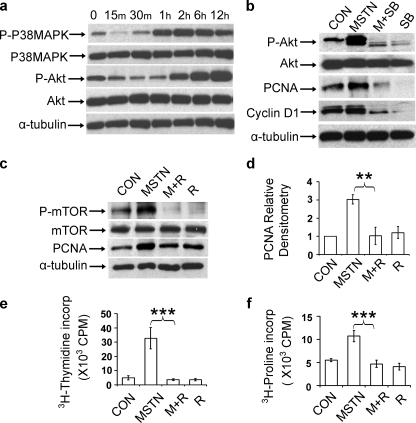
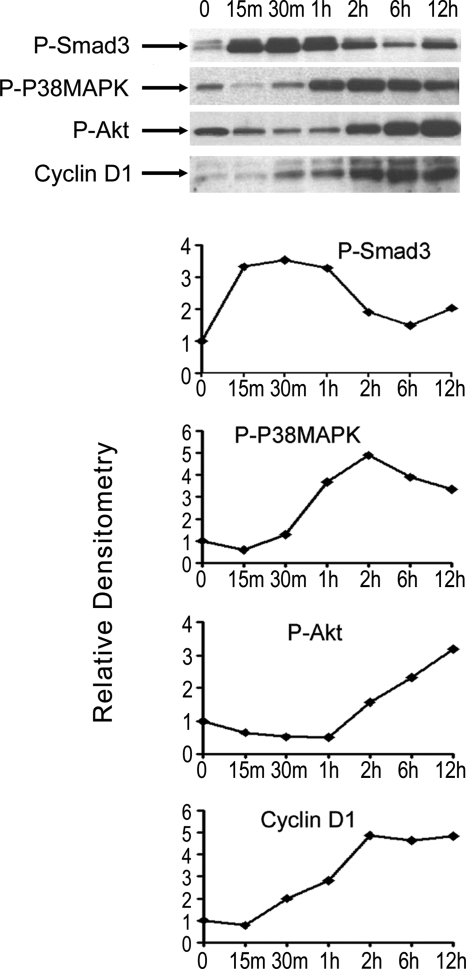
Interestingly, we found that p38 MAPK and Akt phosphorylation were induced by myostatin and reduced by inhibition of Smad3 phosphorylation with SIS3 in cultured fibroblasts (Fig. 4d). This raised the possibility of activation of p38MAPK and PI3K/Akt pathways downstream of Smad signaling of myostatin. Phosphorylation of p38-MAPK and Akt, at amino acid 473, were respectively induced at 1 and 2 h after addition of myostatin, whereas total levels of p38-MAPK and Akt were unchanged (Fig. 5a). Myostatin-induced phosphorylation of Akt and induction of PCNA and cyclin D1 expression could be blocked by pretreating cells with p38-MAPK inhibitors SB202190 (Fig. 5b) or SB203580 (data not shown). Treatment of muscle fibroblasts with myostatin increased phosphorylation of mTOR, a target of Akt. Further, inhibition of mTOR phosphorylation by pretreatment with rapamycin reduced phospho-mTOR and PCNA to control levels (p < 0.005) (Fig. 5, c and d). Inhibition of Akt/mTOR signaling with rapamycin also blocked the proliferative effects of myostatin, measured by [3H]thymidine incorporation, and the production of collagen, measured by [3H]proline incorporation (Fig. 5, e and f). These results demonstrate that myostatin induces proliferation of muscle fibroblasts via stimulation of the Smad, p38-MAPK, and PI3K/Akt signaling pathways in a sequential time course (summarized in Fig. 6).
FIGURE 5.
Delayed activation of p38 MAPK and Akt pathways regulates fibroblast growth. Immunoblot analysis of cell lysate from primary muscle fibroblasts. a, treated with 300 ng/ml of myostatin for increasing duration demonstrating expression levels of phospho-p38 MAPK (P-P38MAPK), total p38 MAPK (P38MAPK), phospho-Akt (P-AKT), total Akt (AKT), and α-tubulin, as a loading control; b, untreated (CON) or treated with 300 ng/ml of myostatin (MSTN), myostatin plus 10 μm SB202190 (M+SB), or SB202190 alone (SB). c and d, fibroblasts treated for 24 h with 300 ng/ml myostatin (MSTN), myostatin and rapamycin (M+R), or rapamycin alone (R). c, evaluated by immunoblot of PCNA, phospho-mTOR (P-mTOR), total mTOR (mTOR), and α-tubulin, as a loading control. d, evaluated by histogram of mean densities of PCNA immunoreactive band relative to control (CON). n = 3 for each data point; **, p < 0.005. e and f, [3H]thymidine incorporation (e) and [3H]proline incorporation (f) of treated fibroblasts. n = 5 for each data point; ***, p < 0.001.
FIGURE 6.
Sequential stimulation of Smad, p38 MAPK, and Akt pathways in the myostatin induction of fibroblast proliferation. Shown is a summary of immunoblot (a) and relative densitometry data (b) of primary muscle fibroblasts treated with 300 ng/ml myostatin over time.
DISCUSSION
To date, most studies of myostatin have focused on its role as an inhibitor of muscle growth that occurs via its effects on the activation, proliferation, and differentiation of myoblasts (6–8). However, in studies of acute and chronic injury, reduced fibrosis has been observed in the absence of myostatin, suggesting that myostatin may have additional roles (8, 9, 28). Recently, Zhu et al. (28) have shown that myofibroblast proliferation is induced by myostatin in cell culture. In the current study, we have provided evidence that myostatin directly stimulates muscle fibroblast proliferation and expression of extracellular matrix proteins in vivo as well as in vitro. We have shown that muscle fibroblasts express functional myostatin, which induces proliferation through the ActRIIB receptor. We have also demonstrated that in muscle fibroblasts, myostatin stimulates the canonical Smad signaling pathway common to TGF-β family members, as well as delayed activation of the p38 MAPK and the PI3K/Akt/mTOR signaling pathways and that all contribute to mediating fibroblast proliferation.
In fibroblasts, as reported for myoblasts (24, 29), we find that myostatin induces the phosphorylation and activation of canonical TGF-β signal transducers Smad2 and Smad3. We have shown that specifically, Smad3 overexpression induces muscle fibroblast proliferation and that SIS3, a specific inhibitor of Smad3, reduces myostatin-induced muscle fibroblast proliferation. These results differ from what has been described in fibroblasts isolated from tendons. In these cells, myostatin activates p38 MAPK and Smad2/3 signaling, but only p38 MAPK activation was found to be associated with proliferation (30). We also found that myostatin has a strong delayed activation of the p38 MAPK as well as the PI3K/Akt pathways in fibroblasts. Further, phospho-AKT levels are blocked by both inhibitors of Smad3 and p38 MAPK and are required for myostatin-mediated fibroblast proliferation. In myoblasts, myostatin acts to inhibit cell proliferation, and myostatin treatment of C2C12 myoblasts leads to decreased phosphorylation of Akt and downstream elements to stimulate cyclin D1 degradation (31). Conversely, we find in muscle fibroblasts that myostatin increases Akt, and mTOR phosphorylation and inhibition of Akt activity with rapamycin block the effect of myostatin on fibroblast growth and extracellular matrix protein production. This stimulatory effect of myostatin on the PI3K/Akt/mTOR pathway has not been previously described to our knowledge but is congruous to the signaling of TGF-β1 inducing fibroblast proliferation via Akt activation in other cell types (27, 32). The results presented in the current paper suggest that myostatin, similar to TGF-β1 has cell-specific signaling and effects.
TGF-β signaling is perhaps the most potent mediator of fibrogenesis in multiple organs including liver, lung, kidney, and heart (33–36). TGF-β signaling also plays a significant role in muscle fibrosis. TGF-β1 expression parallels development of fibrosis and neutralizing antibodies to TGF-β1 reduce fibrosis in mdx diaphragms (37). Similarly, TGF-β1 expression correlates with fibrosis in dystrophic patient muscle (38). TGF-β1 is a ubiquitously distributed cytokine with prominent immunosuppressive, anti-inflammatory, and tumor-suppressive roles. Therefore, it is reasonable to expect that mechanisms to sequester or block synthesis of TGF-β1 will have serious adverse effects in long term systemic treatment. Although myostatin is expressed in low levels in heart and fat (39–42) and, as shown in the current study, in fibroblasts from a variety of organs, its predominant expression and actions appear to be in skeletal muscle tissue (43). Myostatin may therefore be a more selective therapeutic target than TGF-β1 for combating muscle fibrosis.
Therapies that are currently being designed to decrease necrosis in muscle diseases may have little utility unless fibrosis is simultaneously addressed. For example, gene therapy to restore dystrophin to the sarcolemma for Duchenne muscular dystrophy may be inefficient in gene delivery or ineffective in producing increased muscle strength in the setting of significant endomysial fibrosis. Myostatin inhibitors have entered clinical trials in patients with muscle wasting and degenerative diseases. Such inhibitors have the potential of increasing muscle regeneration through the effects of myostatin on myoblasts and also of inhibiting fibrosis through its effects on fibroblasts presented in this paper.
Supplementary Material
Acknowledgments
We thank Alexandra McPherron for helpful suggestions and Reena Shetty for technical assistance. The pCMV-Smad3-FLAG construct was a gift of Jeffery L. Wrana. The p(CAGA)12-MLP-Luc transfected A204 cell line was a gift of Li-fang Liang.
This work was supported, in whole or in part, by National Institutes of Health Grant U54AR052646. This work was also supported by Muscular Dystrophy Association Grant 69566 (to K. R. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
Footnotes
The abbreviations used are: TGF, transforming growth factor; ActRIIB, activin receptor IIB; PCNA, proliferation cell nuclear antigen; MAPK, mitogen-activated protein kinase; PBS, phosphate-buffered saline; RT, reverse transcription; HSP, heat shock protein; SMA, smooth muscle actin; PI3K, phosphatidylinositol 3-kinase; SIS3, Smad3-specific inhibitor.
References
- 1.Bischoff, R. (1994) in Myology (Engel, A. G., and Franzini-Armstrong, C., eds) 2nd Ed., pp. 97–118, McGraw-Hill, New York
- 2.Huard, J., Li, Y., and Fu, F. H. (2002) J. Bone Joint Surg. Am. 84 822–832 [PubMed] [Google Scholar]
- 3.Kääriäinen, M., Järvinen, T., Järvinen, M., Rantanen, J., and Kalimo, H. (2000) Scand. J. Med. Sci. Sports 10 332–337 [DOI] [PubMed] [Google Scholar]
- 4.McPherron, A. C., Lawler, A. M., and Lee, S. J. (1997) Nature 387 83–90 [DOI] [PubMed] [Google Scholar]
- 5.Schuelke, M., Wagner, K. R., Stolz, L. E., Hubner, C., Riebel, T., Komen, W., Braun, T., Tobin, J. F., and Lee, S. J. (2004) New Engl. J. Med. 350 2682–2688 [DOI] [PubMed] [Google Scholar]
- 6.McCroskery, S., Thomas, M., Maxwell, L., Sharma, M., and Kambadur, R. (2003) J. Cell Biol. 162 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas, M., Langley, B., Berry, C., Sharma, M., Kirk, S., Bass, J., and Kambadur, R. (2000) J. Biol. Chem. 275 40235–40243 [DOI] [PubMed] [Google Scholar]
- 8.Wagner, K. R., Liu, X., Chang, X., and Allen, R. E. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 2519–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCroskery, S., Thomas, M., Platt, L., Hennebry, A., Nishimura, T., McLeay, L., Sharma, M., and Kambadur, R. (2005) J. Cell Sci. 118 3531–3541 [DOI] [PubMed] [Google Scholar]
- 10.Bogdanovich, S., Krag, T. O., Barton, E. R., Morris, L. D., Whittemore, L. A., Ahima, R. S., and Khurana, T. S. (2002) Nature 420 418–421 [DOI] [PubMed] [Google Scholar]
- 11.Qiao, C., Li, J., Jiang, J., Zhu, X., Wang, B., Li, J., and Xiao, X. (2008) Hum. Gene Ther. 19 241–254 [DOI] [PubMed] [Google Scholar]
- 12.Wagner, K. R., McPherron, A. C., Winik, N., and Lee, S. J. (2002) Ann. Neurol. 52 832–836 [DOI] [PubMed] [Google Scholar]
- 13.Parsons, S. A., Millay, D. P., Sargent, M. A., McNally, E. M., and Molkentin, J. D. (2006) Am. J. Pathol. 168 1975–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh, S. P., and Li, E. (1997) Genes Dev. 11 1812–1826 [DOI] [PubMed] [Google Scholar]
- 15.Blau, H. M., and Webster, C. (1981) Proc. Natl. Acad. Sci. U. S. A. 78 5623–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richler, C., and Yaffe, D. (1970) Dev. Biol. 23 1–22 [DOI] [PubMed] [Google Scholar]
- 17.Thies, R. S., Chen, T., Daview, M. V., Tomkinson, K. N., Pearson, A. A., Shakey, Q. A., and Wolfman, N. M. (2001) Growth Factors 18 251–259 [DOI] [PubMed] [Google Scholar]
- 18.Amthor, H., Nicholas, G., McKinnell, I., Kemp, C. F., Sharma, M., Kambadur, R., Patel, K. (2004) Dev. Biol. 270 19–30 [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. J., and McPherron, A. C. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stedman, H. H., Sweeney, H. L., Shrager, J. B., Maguire, H. C., Panettieri, R. A., Petrof, B., Narusawa, M., Leferovich, J. M., Sladky, J. T., and Kelly, A. M. (1991) Nature 352 536–539 [DOI] [PubMed] [Google Scholar]
- 21.Ríos, R., Carneiro, I., Arce, V. M., and Devesa, J. (2001) Biochem. Biophys. Res. Commun. 280 561–566 [DOI] [PubMed] [Google Scholar]
- 22.Hill, J. J., Davies, M. V., Pearson, A. A., Wang, J. H., Hewick, R. M., Wolfman, N. M., and Qiu, Y. (2002) J. Biol. Chem. 277 40735–40741 [DOI] [PubMed] [Google Scholar]
- 23.Rebbapragada, A., Benchabane, H., Wrana, J. L., Celeste, A. J., and Attisano, L. (2003) Mol. Cell. Biol. 23 7230–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu, X., Topouzis, S., Liang, L. F., and Stotish, R. L. (2004) Cytokine 26 262–272 [DOI] [PubMed] [Google Scholar]
- 25.Jinnin, M., Ihn, H., and Tamaki, K. (2006) Mol. Pharmacol. 69 597–607 [DOI] [PubMed] [Google Scholar]
- 26.Piek, E., Ju, W. J., Heyer, J., Escalante-Alcalde, D., Stewart, C. L., Weinstein, M., Deng, C., Kucherlapati, R., Bottinger, E. P., and Roberts, A. B. (2001) J. Biol. Chem. 276 19945–19953 [DOI] [PubMed] [Google Scholar]
- 27.Runyan, C. E., Schnaper, H. W., and Poncelet, A.-C. (2004) J. Biol. Chem. 279 2632–2639 [DOI] [PubMed] [Google Scholar]
- 28.Zhu, J., Li, Y., Shen, W., Qiao, C., Ambrosio, F., Lavasani, M., Nozaki, M., Branca, M., and Huard, J. (2007) J. Biol. Chem. 282 25852–25863 [DOI] [PubMed] [Google Scholar]
- 29.Langley, B., Thomas, M., Bishop, A., Sharma, M., Gilmour, S., and Kambadur, R. (2002) J. Biol. Chem. 277 49831–49840 [DOI] [PubMed] [Google Scholar]
- 30.Mendias, C. L., Bakhurin, K. I., and Faulkner, J. A. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, W., Zhang, Y., Li, Y., Wu, Z., and Zhu, D. (2007) J. Biol. Chem. 282 3799–3808 [DOI] [PubMed] [Google Scholar]
- 32.Horowitz, J. C., Rogers, D. S., Sharma, V., Vittal, R., White, E. S., Cui, Z., and Thannickal, V. J. (2007) Cell Signal. 19 761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gressner, A. M., and Weiskirchen, R. (2006) J. Cell Mol. Med. 10 76–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berk, B. C., Fujiwara, K., and Lehoux, S. (2007) J. Clin. Investig. 117 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gagliardini, E., and Benigni, A. (2007) Expert. Opin. Biol. Ther. 7 293–304 [DOI] [PubMed] [Google Scholar]
- 36.Mutsaers, S. E., Kalomenidis, I., Wilson, N. A., and Lee, Y. C. (2006) Curr. Opin. Pulm. Med. 12 251–258 [DOI] [PubMed] [Google Scholar]
- 37.Andreetta, F., Bernasconi, P., Baggi, F., Ferro, P., Oliva, L., Arnoldi, E., Cornelio, F., Mantegazza, R., and Confalonieri, P. (2006) J. Neuroimmunol. 175 77–86 [DOI] [PubMed] [Google Scholar]
- 38.Bernasconi, P., Torchiana, E., Confalonieri, P., Brugnoni, R., Barresi, R., Mora, M., Cornelio, F., Morandi, L., and Mantegazza, R. (1995) J. Clin. Investig. 96 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen, D. L., Cleary, A. S., Speaker, K. J., Lindsay, S. F., Uyenishi, J., Reed, J. M., Madden, M. C., and Mehan, R. S. (2008) Am. J. Physiol. 294 E918–E927 [DOI] [PubMed] [Google Scholar]
- 40.McPherron, A. C., and Lee, S. J. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 12457–12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morissette, M. R., Cook, S. A., Foo, S., McKoy, G., Ashida, N., Novikov, M., Scherrer-Crosbie, M., Li, L., Matsui, T., Brooks, G., and Rosenzweig, A. (2006) Circ. Res. 99 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma, M., Kambadur, R., Matthews, K. G., Somers, W. G., Devlin, G. P., Conaglen, J. V., Fowke, P. J., and Bass, J. J. (1999) J. Cell. Physiol. 180 1–9 [DOI] [PubMed] [Google Scholar]
- 43.Wagner, K. R. (2005) Curr. Opin. Rheumatol. 17 720–724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.