Abstract
Epoxyeicosatrienoic acids (EETs) reduce infarction of the myocardium after ischemia/reperfusion injury to rodent and dog hearts mainly by opening sarcolemmal and mitochondrial potassium channels. Other mediators for the action of EET have been proposed though no definitive pathway or mechanism has yet been reported. Using cultured cells from two rodent species, immortalized myocytes from a mouse atrial lineage (HL-1) and primary myocytes derived from neonatal rat hearts, we observed that pretreatment with EETs (1 μM of 14,15-, 11,12-, or 8,9-EET) attenuates apoptosis after exposure to hypoxia/reoxygenation (HR). EETs also preserved functional beating of neonatal myocytes in culture after exposure to HR. We demonstrated that EETs increased activity of the pro-survival enzyme phosphoinositide 3 kinase (PI3K). In fact cardiomyocytes pretreated with EET and exposed to HR exhibited anti-apoptotic changes in at least five downstream effectors of PI3K: Akt, BAD (Bcl-xL/Bcl-2 associated death promoter), caspase 9 and 3 activities and expression of XIAP (X-linked inhibitor of apoptosis), when compared to vehicle-treated controls. The PI3K/Akt pathway is one of the strongest intracellular pro-survival signaling systems. Our studies show that EETs regulate multiple molecular effectors of this pathway. Understanding the targets of action of EET-mediated protection will promote development of these fatty acids as therapeutic agents against cardiac ischemia/reperfusion.
Keywords: caspase, ischemia, rat, mouse, fatty acids
Introduction
The cytochrome P450-catalysed epoxidation of arachidonic acid produces a series of epoxy fatty acids (epoxyeicosatrienoic acids, EETs) that influence a variety of cell and organ functions. Cytochrome P450-derived eicosanoids are produced in a cell- and tissue-specific manner and have many biological functions. EETs have been suggested to play diverse roles in cardiovascular homeostasis (25,44,48), regulation of vascular tone, blood pressure, ion transport, and as second messengers in numerous signaling pathways (5,8,19,52). More recently these eicosanoids have been implicated in other critical biological processes including control of cell proliferation, inflammation and homeostasis, migration and platelet aggregation (20,45,58,64). EETs have been shown to play an important role in cardioprotection in rodents and dogs subjected to ischemic-reperfusion (IR) injury (55). No clear mechanism for the action of EETs was derived in these studies. However indirect evidence shows that pharmacological inhibition of mainly ATP- and Ca+2-sensitive potassium channels (KATP, mitoKATP, KCa), (27,43,54), and in some studies p42/p44 mitogen-activated protein kinase (MAPK) (55), phosphatidylinositide-3′-OH kinase (PI3K) (54), and reactive oxygen species (27), attenuate the protection. Pharmacological inhibition often non-specifically affects signaling molecules unrelated to the focus of investigation. Furthermore some models were derived from mice in which the Ephx2 locus coding for soluble epoxide hydrolase (sEH) was disrupted (54). Though knock out of this locus increases the levels of EETs by eliminating hydrolysis to dihydroxy derivatives, there are multiple endogenous substrates for sEH, so that the exact intermediates that induce protection in these tissues may not be limited to EETs. Currently, opening of sarcolemmal and mitochondrial KATP channels is the most specific and favored mechanism by which these fatty acids are believed to mediate myocardial protection (27,43,54,55).
Regulation of cell survival is crucial to the normal physiology of multicellular organisms. The role of apoptosis in heart disease has been debated, though recent evidence suggests that it contributes to tissue injury in several cardiac disorders including myocardial infarction, atherosclerosis, transplant, myocarditis and cardiac failure. From 5-30% of cardiac myocytes undergo apoptosis in rodent and humans within 16 hours of reperfusion (2,21,47,50,60) and this trend persists for months (up to 0.25% in diseased humans, compared to controls of up to 0.002%) (28,46,60). In intact animals apoptosis occurs during myocardial infarction, heart failure and transplant injuries (13). Genetic approaches using rodents have determined that inhibition of apoptosis in cardiomyocytes reduces infarct size by 50-70% (9,50,37). Apoptotic rates as low as 0.023% are sufficient to cause lethal dilated cardiomyopathy in transgenic mice that are engineered to have cardiac restricted expression of caspase 8. This rate of apoptosis is 5-10 fold lower than that measured in cardiac tissue from patients with end stage heart failure (13,46). A recent finding in a renal proximal tubular epithelial cell line demonstrated that EETs inhibit apoptosis induced by serum withdrawal and etoposide (6,41). We have reported that EETs inhibit apoptosis induced by engagement of the death receptor Fas or by serum withdrawal in human vascular endothelial cells cultured from the heart or lung (17,36). Hence we were interested to see if EETs have the same anti-apoptotic effect in cardiomyocytes after ischemia-reperfusion (IR) since myocardial cell death caused by apoptosis is a main feature of this condition.
We investigated 4 different endpoints to report that EETs inhibit cell death/apoptosis in cultured cardiomyocytes which are subjected to hypoxia and reoxygenation (HR) to simulate conditions of IR. We describe for the first time activation of the enzyme PI3K by EETs in cultured myocytes. We also systematically followed multiple downstream anti-apoptotic signals, including phosphorylation of Akt and BAD, increased intracellular levels of X-linked inhibitor of apoptosis (XIAP) and decreased activity of caspases 9 and 3. Our results support at least 5 pro-survival effectors that may mediate EET-induced protection of cardiomyocytes via stimulation of the PI3K/Akt pathway.
Materials and Methods
Culture of Neonatal Rat Ventricular Myocytes
Hearts from one-day-old Sprague-Dawley neonatal rats were excised, and the ventricular myocardium was cut into small pieces (∼2 mm3) in ADS buffer (116 mm NaCl, 20 mm HEPES, 1 mm NaH2PO4, 5.5 mm glucose, 5.4 mm KCl, 0.8 mm MgSO4, 0.6 ml/l phenol red, pH 7.35) with 0.15 mg/ml collagenase (Worthington II, Lakewood, NJ), 0.52 mg/ml pancreatin (Life Technologies Inc., Grand Island, New York) and incubated on a shaker at 37°C for 20 min at 100 rpm. Tissue pieces were allowed to settle, and the supernatant (containing myocytes) was collected and suspended in 1 ml newborn calf serum (GIBCO, Carlsbad, CA), and centrifuged at 1,000 rpm for 6 min. The cell pellet was resuspended in 1 ml newborn calf serum and stored at 37°C. The procedure was repeated until all tissue was digested. The cells were then resuspended in Dulbecco's modified essential medium (DMEM) supplemented with 17% medium 199, 10% horse serum, 5% fetal bovine serum (FBS), 0.5% penicillin–streptomycin, and 20 mm HEPES at pH 7.2 for 2 h. This facilitates separation of ventricular myocytes from the faster-attaching non-myocytes. The ventricular myocytes in the supernatant were collected and plated on gelatin-coated dishes. They were cultured in media containing bromodeoxyuridine (5-bromo-2-deoxyuridine) at a final concentration of 0.1 mM. Cells were used for experiments after demonstrating rhythmic contractions (48–72 h).
HL-1 Cells
HL-1 cells, a cardiac muscle cell line derived from the AT-1 mouse atrial myocyte tumor lineage, were a gift from William C. Claycomb, Ph.D. (Professor of Biochemistry and Molecular Biology, Louisiana State University Health Sciences Center, New Orleans, Louisiana), and maintained according as described (11). HL-1 cells were used for experimentation after reaching approximately 70-80% confluence.
Treatment of Cells
Rat neonatal myocytes were cultured to 70% confluency and then serum starved in basal medium (DMEM +0.1% bovine serum albumin) for 24 hours for experiments in Figure 1. The cells were stimulated with one of four concentrations of 14,15-EET (0.1, 0.3, 1 or 10 μM) or vehicle (ethanol) and lysed as described under ‘Western blot’. For other experiments, separate groups of neonatal rat ventricular myocytes or mouse atrial HL-1 cells received no intervention (normoxia controls) or were exposed to hypoxia–reoxygenation (HR) after pretreatment with 2 applications of either vehicle (ethanol) or a single regioisomer of EET (1 μM of 14,15-, 11,12- or 8,9-EET). Exposure to EET was accomplished 16 hrs prior to (first application) and just before HR (second application). Hypoxia was simulated for 8 h in neonatal myocytes by exposure to 5% CO2 and 95% N2 in an airtight chamber in the presence of serum- and glucose-free DMEM containing 10 mM deoxyglucose to inhibit glycolysis. The levels of oxygen were monitored continuously and were below 2% at all times. Reoxygenation was performed for 16 h in DMEM–10% FBS except if otherwise mentioned (for PI3K activity or analyses of p-Akt). HL-1 cells were treated similarly except that deoxyglucose was not included in the serum free medium. In some experiments the PI3K inhibitor wortmannin was added 30 minutes before each application of EET. Control cells that received no intervention or HR were maintained in DMEM+10% FBS throughout the duration of the experiments.
Figure 1. EET stimulates phosphorylation of the prosurvival protein Akt.
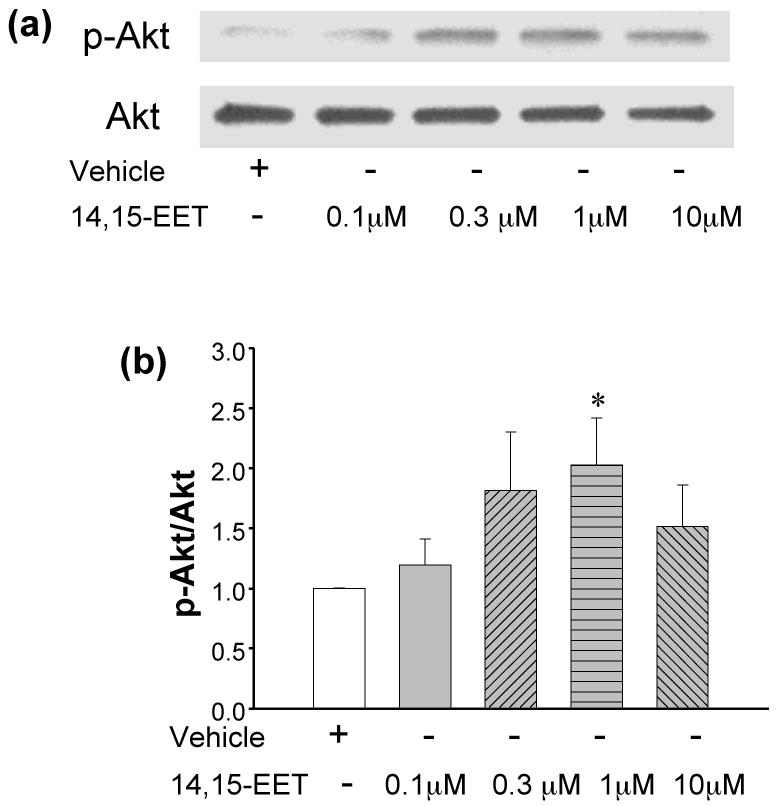
(a) neonatal myocytes were exposed to increasing concentrations of 14,15-EET for 30 minutes as indicated in the figure. Detection of pAkt and Akt were carried out by western blots. Increasing concentrations of 14,15-EET up to 1 μM enhanced phosphorylation of Akt while 10 μM was less effective (upper panel figure 1a). The lower panel depicts corresponding levels of Akt in the cells (loading controls). (b) Four independent experiments were repeated as in (a) and the densitometric intensities of the pAkt and Akt bands were measured, to calculate pAkt/Akt ratios in each sample. These were normalized against the vehicle in each experiment. * denotes p<0.01 as compared to vehicle.
MTT assay
The cells (HL-1 and neonatal myocytes) were cultured in 60 mm dishes (plated at a density approximately half million cells) to ∼80% confluency and then maintained in either complete medium or switched to basal medium after 42 hours. The samples were maintained under normoxia or treated with a single regioisomer of EET or vehicle and subjected to HR as described above. At the end of the incubation period the survival of cells was determined by the MTT assay as described in the manufacturer's protocol (Molecular Probes, Eugene, OR, V-13154). Briefly, the cells were incubated for 3 hours in phenol-red free medium containing 0.5% of the yellow mitochondrial dye, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT+). We used a slightly higher confluency (∼80%) in the MTT protocol since the assay required cell viability for an additional 3 hours of incubation with the substrate after exposure to HR. The amount of blue formazan dye generated from MTT+ was proportional to the number of live cells. The MTT+ reaction was terminated by adding DMSO to the medium followed by incubation for ten minutes at 37° C. The absorbance was read at 540 nm in a spectrophotometer. Values of the reaction were obtained after subtraction of matched blanks and the ODs of the controls were taken as 100% for comparisons with values for other samples. Readings for test cells were expressed as % of control.
Hoechst Staining
Cells were cultured in 6-well plates to 70% confluency, treated with EETs and subjected to HR as described for the MTT assay. They were then stained with 1μl of Hoechst 33342 (5 mg/ml, Molecular Probes V-13244) in 1 ml basal medium and incubated for 30 minutes. Stained cells were washed twice with PBS (Sigma, St. Louis, MO) and imaged under a fluorescent microscope (excitation 350 nm; emission 460 nm).
Annexin V binding
Annexin V binds to phosphatidyl serine (PS), which appears in the outer leaflet of the plasma membrane in early apoptotic cells. Cells (neonatal myocytes and HL-1 cells) were cultured in 60 mm dishes to 70% confluency, and subjected to normoxia or HR with or without pre-treatment with EET as described earlier. The cells were washed with PBS and treated with FITC-labeled annexin V (0.2 μg/ml) for 20 min at room temperature (17). Labeling with FITC-coupled annexin V was performed according to the manufacturer's protocol (BD Biosciences, San Diego, CA) as previously described (17). The labeled cells (10,000/sample) were analyzed by measuring fluorescence intensity using a FACScan flow cytometer (Becton Dickinson) in conjunction with CellQuest software (BD Biosciences).
Activity of caspase-3 and caspase-9
Cells (neonatal myocytes and HL-1 cells) were cultured in 60 mm dishes to 70% confluency, and subjected to HR or normoxia with or without pre-treatment with EET as described earlier. The cells were collected by centrifugation, washed twice with ice-cold PBS, resuspended in lysis buffer (5 mM MgCl2, 1 mM EGTA, 0.1% Triton X-100, and 25 mM HEPES (pH 7.5)) and stored overnight at -80°C as previously described (28). Release of free 7-amino-4-trifluoromethyl coumarin (AFC) from the synthetic substrate Ac-DEVD-AFC (50 μM) for caspase-3 and release of free AFC from the acetyl-LEHD AFC substrate for caspase-9 at 37°C was determined by fluorescence measurements in a SpectraFluor Plus plate reader after excitation at wavelength 390 nm. Emission was measured at 535 nm.
Detection of cleaved caspase-3 activity
Cells were treated under normoxia or HR with or without EET and cleaved caspase was detected according to manufacturer's protocol (Cell Signaling, Charlottesville, VA). Briefly cells were treated as described above and at the end of the treatment time cells were washed with PBS and fixed in PBS containing 3% paraformaldehyde for 20 minutes at 4°C. The cells were then washed three times with Tris-Buffered Saline-Tween-20 (TBST, 10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween-20) for 5 mins each time and incubated with 1 ml blocking buffer for 45-60 minutes. Cells were rinsed and incubated in cleaved caspase antibody (1:200 dilution) in TBST/BSA for 24 hrs at 4° C. They were washed three times and incubated with appropriate fluorescent secondary antibody and detected using a FITC filter (excitation 490 nm and emission 525 nm).
PI3K activity
PI3 kinase was estimated according to the manufacturer's protocol (Echelon, Salt lake City, Utah). Briefly cells were pretreated with EET and subjected to hypoxia for 8 hrs and reoxygenated for 60 mins. The cells were then scraped in ice cold lysis buffer (137 mM NaCl, 20 mM Tris-HCl, pH 7.4, 1mM CaCl2, 1 mM MgCl2 and 0.1mM sodium orthovanadate with 1% NP-40 and 1mM PMSF). The lysate was centrifuged and supernatant incubated with 5 μl anti-PI3K antibody for one hour. Protein A-agarose beads (60 μl of a 50% slurry) were added and the lysates were mixed gently for one hour at 4°C. The immunoprecipitated enzyme was collected by centrifugation for 5 seconds at 3000×g and washed once with the lysis buffer and again with TNE buffer (10 mM Tris-HCl, pH7.4, 150 mM NaCl, 5 mM EDTA) containing 0.1 mM sodium orthovanadate. The PI(4, 5)P2 substrate was added to the enzyme along with reaction buffer and left at room temperature for 3 hrs. The kinase reaction was stopped by addition of 2.5 μl 100 mM EDTA. The plate was then set with standards, blanks and samples, incubated with the detector protein and read using a plate reader at 450 nm.
Immunofluorescence Studies for Phospho-Akt
Neonatal rat ventricular myocytes were plated on collagen-coated coverslips, pretreated with EET and exposed to HR or normoxia for 8 hrs as described above. After reoxygenation for 60 minutes the cells were fixed in 4% paraformaldehyde, permeabilized in methanol (-20°C), and incubated for 30 min at 37°C with primary monoclonal antibody for phospho-Akt (Ser 473; Cell Signaling, Beverly, MA) at a dilution of 1:200 in PBS (0.5X). Samples were washed and incubated with anti-mouse biotinylated secondary antibodies (Santa Cruz Biotechnology; 1:200 dilution) for 30 min at 37°C. After washing with 0.5X PBS, cells were incubated for 15 min at 37°C with avidin-conjugated 1:500 fluorescein isothiocyanate followed by 1:1,000 DAPI (to stain the nuclei) for 5 min at room temperature. Samples were washed again with 0.5X PBS, and mounted on microscopic slides. Images were captured using confocal microscopy using appropriate filters for visualization of FITC (described above) and DAPI (excitation ultraviolet, emission 461 nm).
Western Blot
HL-1 cells and neonatal myocytes were cultured in 35 mm dishes to 70% confluency, washed and incubated with basal medium for 24 hours. The cells were treated under normoxia or HR with or without EETs. They were kept on ice and washed 3 times with cold PBS. Proteins were solubilized and extracted with 50 μl RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.5% SDS, 1% Nonidet P40, 0.5% sodium deoxycholate, 1 mM EDTA, 1X protease inhibitor cocktail (Pharmingen, San Diego, CA), 1X phosphatase inhibitors (Calbiochem, San Diego, CA)). The lysates were used to estimate protein content with the Biorad DC Protein Assay Reagent (Biorad, Hercules, CA). Equal amounts of protein (10-60 μg) from each sample were electrophoresed on a 10% SDS-polyacrylamide gel with running buffer and transferred to nitrocellulose as described (17,36). The membranes were treated with primary antibody for XIAP, p-BAD, BAD or p-AKT (1:1000 dilution, Cell Signaling, Charlottesville, VA) for 18 hour. They were again washed 3 times before incubating with matched secondary antibody (1:5000) for 45 minutes. The protein bands were developed with chemiluminescence reagents (ECL or ECL plus, GE Healthcare, Piscataway, NJ).
Functional evaluation of contractility of neonatal myocytes
Cultured neonatal myocytes (∼ 5 days old) were washed and incubated with basal medium for 8 h and treated with vehicle or EET and further incubate hours. The cells were treated under normoxia, or exposed to hypoxia for specific times after pretreatment with two applications of either vehicle or a regioisomer of EET (1 μM of 14,15- or 11,12-EET). In pilot experiments, vehicle-treated cells stopped beating between 30 minutes and up to 8 hours of exposure to anoxic conditions. Test plates from each batch were incubated under hypoxia and observed at half hour intervals. When ∼85% vehicle-treated cells maintained in anoxic conditions stopped beating, all test and control groups were exposed to 1 h of reoxygenation. Dishes were removed from the incubator and immediately observed under a light microscope. Islands of beating cells were counted in a blinded manner in at least 4 fixed fields per dish, from four independent batches of cells. Frequency of beating of at least 25 cells/treatment were also counted in the same samples using a stopwatch. Average number of beating cells/field and average frequency (beats/min) for each treatment was recorded, decoded and then used to calculate the mean and standard error of the mean for statistical analysis.
Statistical comparisons
All values are expressed as the means ± standard error of the means (sem) from at least 3 or more samples in each experiment. Comparisons between controls and treatments were analyzed by ANOVA followed by TUKEYs test when permitted. Values for p<0.05 were considered significant.
Results
(I) EETs induce phosphorylation of Akt in neonatal, rat, cardiac myocytes
Primary cultures of myocytes were stimulated with increasing concentrations of 14,15-EET to test the effect of the fatty acid on activating the prosurvival kinase Akt (Fig 1). After 30 minutes of treatment the cells were lysed and analyzed by Western blot to determine p-Akt levels. As seen in Fig 1a, there is an increase in p-Akt (upper panel) as compared to Akt (lower panel) in EET-treated cells. Analysis from 4 independent experiments demonstrated a significant increase in the densitometric ratio of p-Akt/Akt, versus the vehicle treated cells at the 1 μM concentration of 14,15-EET (n=4, Fig 1b). At an even higher concentration of 14,15-EET (10 μM) the ratio was lower than for 1 μM 14,15-EET as shown in Figure 1. In cells treated with 10 μM EET, this ratio was statistically indistinguishable to that of the vehicle.
(II) EETs protect mouse atrial (HL-1) and rat neonatal cardiac myocytes against HR-induced apoptosis
(i) MTT cell viability assay
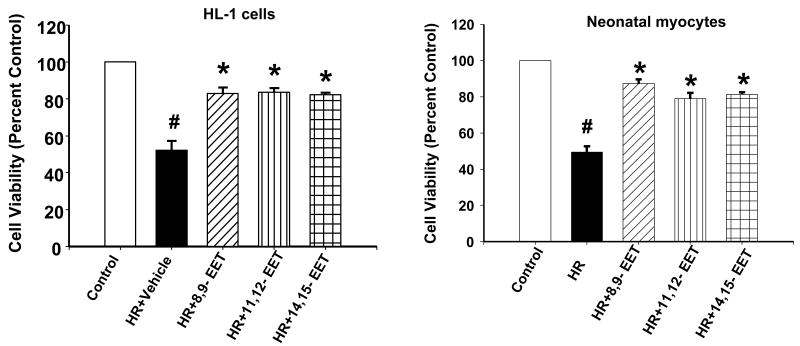
Both the cell types (HL1 and primary neonatal cardiomyocytes) showed increased survival rates when maintained under normoxia (control) or pre-treated with 8,9-, 11,12- and 14,15-EETs (1 μM of each) as compared to the cells which were subjected to HR after pretreatment with vehicle (Figure 2). The HR induced a decrease in cell viability to 50% of control as opposed to the cells pre-treated with EETs where the cell survival was ∼80% of control.
Figure 2. EETs increase cell viability after HR.
HL-1 cells (a) and neonatal myocytes (b) were subjected to HR in presence of vehicle or EET. Cell viability was determined using the MTT assay (see Methods) and normalized to ‘percent control’. Bar graph showing the mean ± sem of percent decrease of viable cells over control (100%). * denotes p<0.01 for analyses compared to HR and # denotes p<0.01 as compared to control.
(ii) Annexin V binding
We tested all 3 EET regioisomers, 8,9-, 11,12- or 14,15-EET (1μM each) and demonstrated significant protection of HL-1 cells (Figures 3a-d) and neonatal myocytes (Figures 3e-h) from increased fluorescence due to binding of annexin V after induction of apoptosis by HR. Controls in these experiments were maintained under normoxic conditions. There was a shift in the intensity of fluorescence towards control in EET pretreated cells as compared to cells pretreated with vehicle (labeled HR, Figure 3).
Figure 3. EETs decrease annexin V binding after HR.
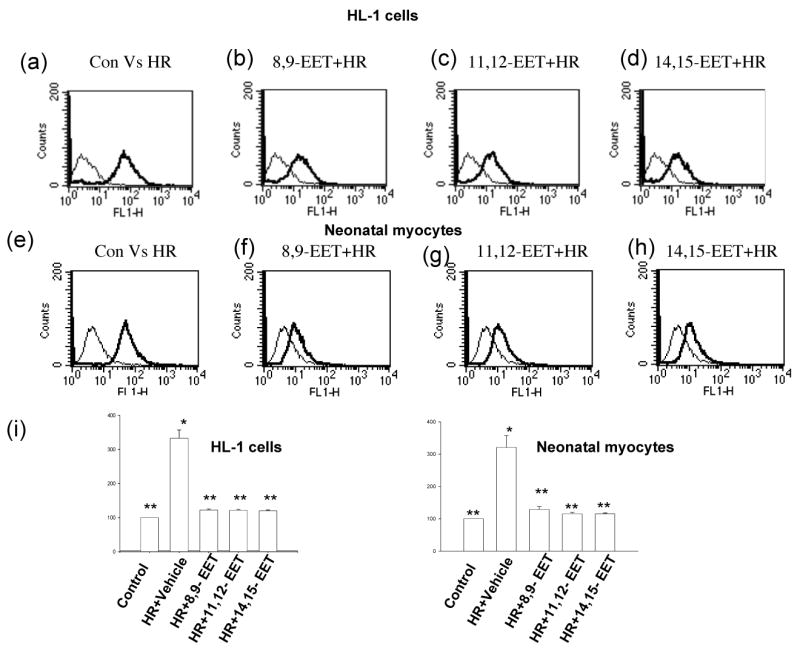
HL-1 cells (a-d) and neonatal myocytes (e-h) were subjected to HR in presence of vehicle or EET. Cells were incubated with binding buffer containing annexin V as described in Materials and Methods. Graphs (a-h) represent typical histograms comparing fluorescent intensity of control cells (exposed to normoxia) with those exposed to HR in the presence of vehicle (labeled HR) or EET (traced in bold lines). Note an increase in fluorescence after HR which shifts towards control in samples pretreated with EET. (I) Bar graph showing the mean ± sem of percent increase of annexin binding over control (100%). * denotes p<0.05 compared to control; ** denotes p<0.05 compared to HR-treated cells.
(iii) Nuclear fragmentation
Typical nuclei stained with Hoechst 33342 are shown in Figure 4. HR considerably increased DNA condensation in HL-1 cells and myocytes. We observed pyknotic nuclei after HR in HL-1 cells (Fig 4a) and neonatal myocytes (Fig 4b). 14,15-EET protected DNA fragmentation, which is confirmed by the appearance of normal nuclei in EET pretreated cells.
Figure 4. EET blocks fragmentation of nuclei by HR.
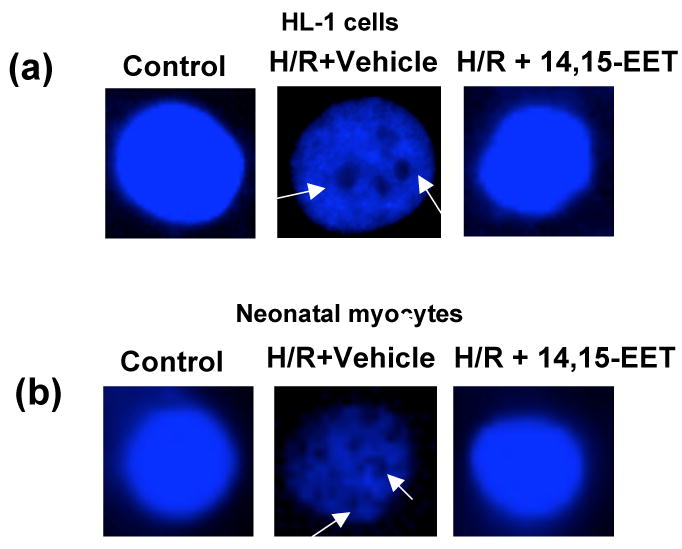
HL-1 cells (a) and neonatal myocytes (b) were subjected to HR in presence of vehicle or 14-15-EET (1 μM). Nuclei were stained with Hoechst reagent and imaged. There is visible condensation of chromatin (marked with arrows) in serum deprived cells that is absent in control cells and reduced in cells pretreated with EET after HR.
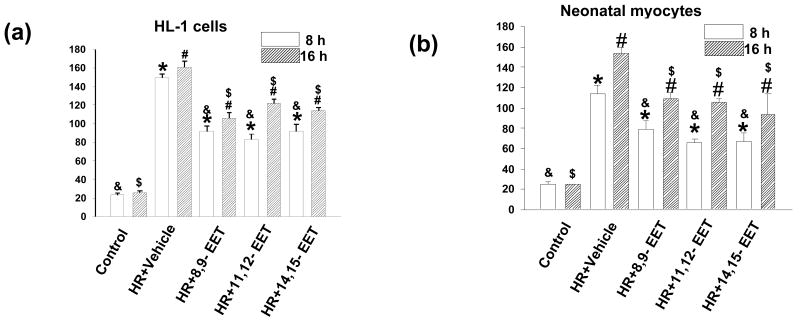
(iv) Caspase 3 activity
Caspase-3 activity was increased in HL-1 cells and myocytes (Figures 5a&b) after treatment with HR (reoxygenation was carried out for 2 time points, 8 h and 16 h). There was however a significant decrease in caspase-3 activity in both cell types that were pre-treated with one of the 3 EET regioisomers (1 μM). We also performed immunofluorescence studies by incubating the treated cells with specific caspase antibody which only recognizes the activated or cleaved form of the enzyme. The results were similar to the activity assay: HR exposed cells showed an increase in fluorescence compared to the HR group pretreated with EET, both in HL-1 cells (Figure 6a) and neonatal myocytes (Figure 6b).
Figure 5. EETs decrease caspase-3 activity.
HL-1 cells (a) and neonatal myocytes (b) were subjected to HR after pretreatment with vehicle (marked HR) or one of the 3 regioisomers of EET as labeled. Caspase-3 activity was measured as described in the Materials and Methods. * indicates a significant difference (p<0.05) compared to control (8h); # indicates a significant difference (p<0.05) compared to control (16h); & indicates a significant difference (p<0.05) compared to HR (8h); $ indicates a significant difference (p<0.05) compared to HR (16h). Controls were treated under normoxic conditions. Both cell types showed increased caspase-3 activity after HR which was attenuated by 1 μM of 8,9-, 11,12-, or 14,15-EET.
Figure 6. EET decreases intracellular cleaved caspase 3.
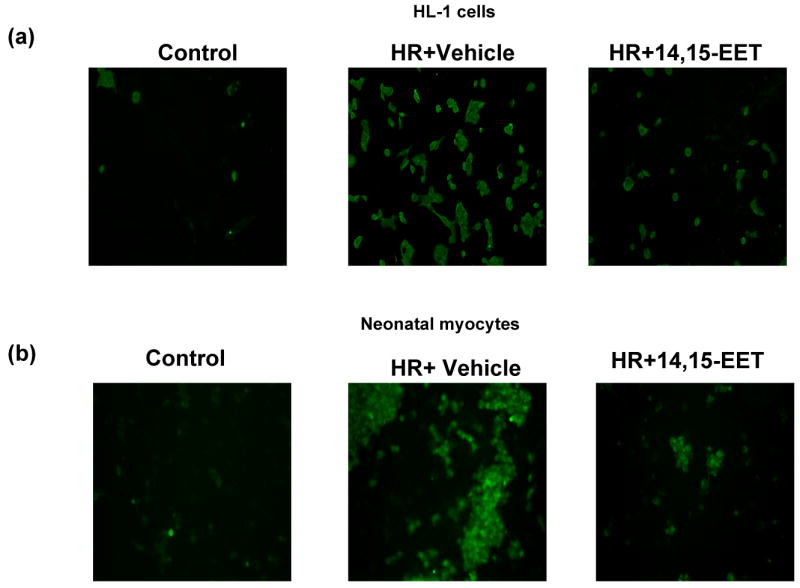
HL-1 cells (a) and neonatal myocytes (b) were subjected to HR after pretreatment with vehicle or 14,15-EET (1 μM). After treatment cells were processed as described in ‘Materials and Methods’ and treated with a caspase-3 antibody which recognizes only the cleaved caspase and not the pro-caspase. The cells were then stained with corresponding FITC-tagged secondary antibody and analyzed by fluorescent microscopy. Note increased caspase 3 activity in HR as compared to HR+14,15-EET or control (normoxia).
(III) EETs activate multiple targets of the PI3K/Akt survival pathway
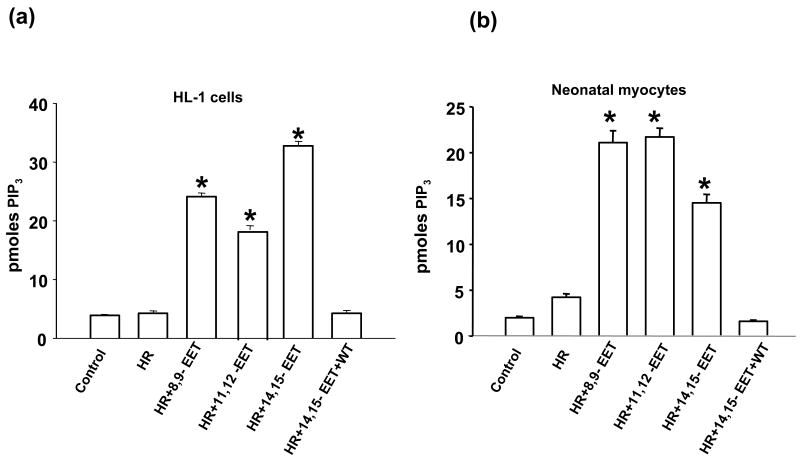
(i) EETs activate PI3K
To elucidate a mechanistic pathway of protection by EETs we evaluated PI3K activity in HL-1 cells (Fig 7a) and neonatal myocytes (Fig 7b). There was an increase in PI3K activity in EETs pretreated cells subjected to HR as compared to vehicle treated cells. The assay was carried out using immunoprecipitated enzyme to measure generation of PIP3 which is the product of PI3K from the substrate PIP2, and measured colorimetrically. The PI3K inhibitor wortmannin (WT) blocked the increase in PI3K activity induced by EET.
Figure 7. EETs increase PI3K activity.
HL-1 cells (a) and neonatal myocytes (b) were subjected to HR after pretreatment with vehicle or EET in the absence or presence of wortmannin (WT, 0.2-1 μM). Reoxygenation was performed for 1 hour. PI3K assays were conducted as mentioned in ‘Materials and Methods’. The values were determined by plotting the OD on the standard curve obtained from the known values (standards). * p< 0.001 as compared to control or HR-treated group.
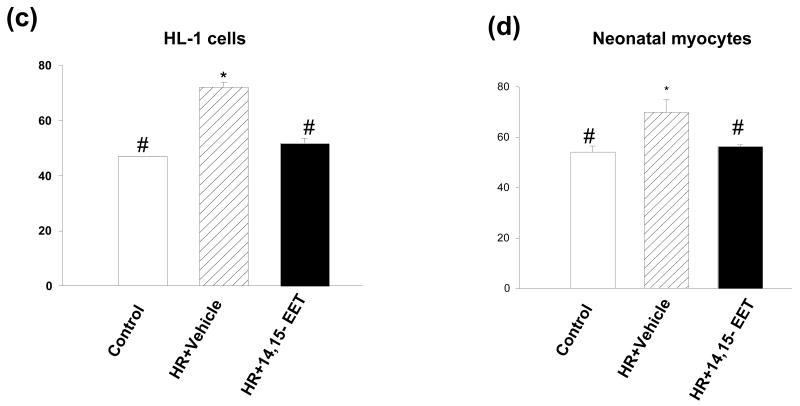
(ii) EETs induce phosphorylation of Akt
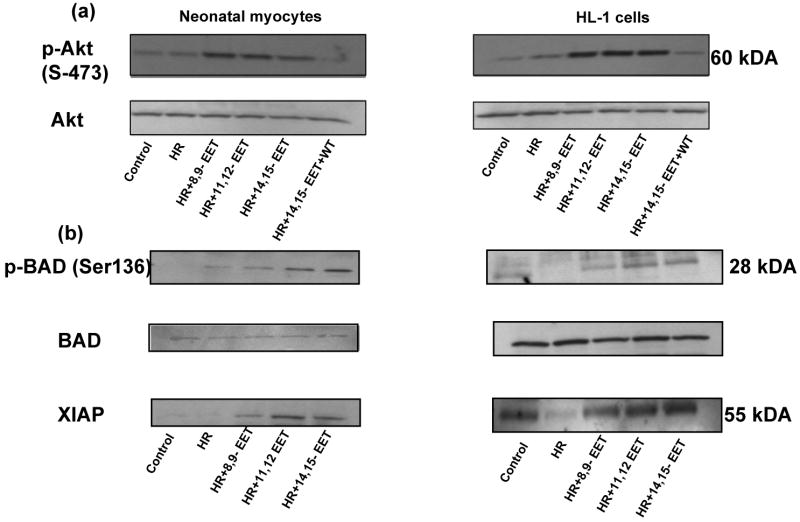
Figure 8 depicts the phosphorylation of Akt in HR induced HL-1 cells (Fig 8a) and myocytes (Fig 8b). The cells treated with 14,15-EET (1 μM) showed increase in p-AKT (green) as compared to the HR treated cells or normoxic controls (graphically represented in Figs 8c&d). Nuclei were counterstained with DAPI (blue). The Akt phosphorylation by each EET-regioisomer was further confirmed by western blots of proteins (labeled in each panel) from myocytes and HL-1 cells (Figure 9). Pretreatment with EET increased levels of p-Akt versus Akt (loading controls) as compared to vehicle pretreated controls (Figure 9). This increase was blocked by WT.
Figure 8. EET increases intracellular p-Akt.
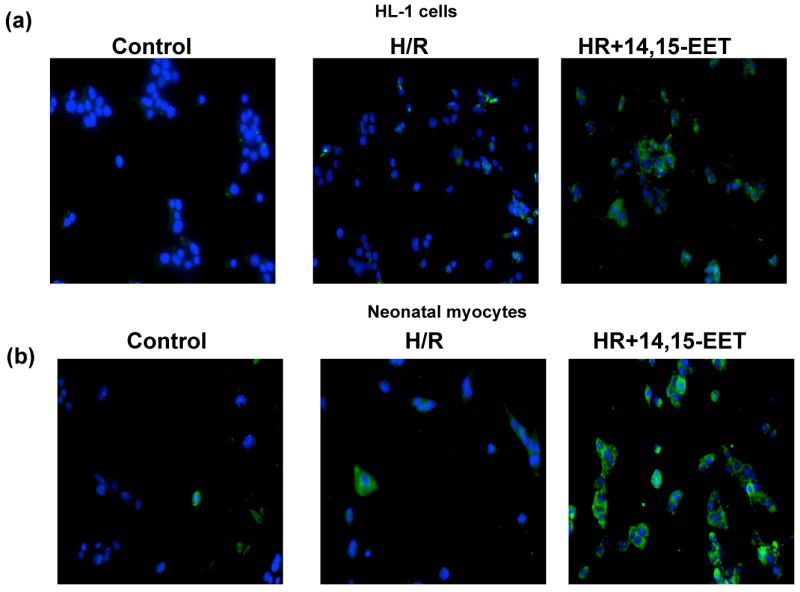
HL-1 cells (a) and neonatal myocytes (b) were subjected to HR after pretreatment with vehicle or 14,15 EET. Cells were treated as described in ‘Materials and Methods’ and stained with p-AKT antibody. Nuclei were counterstained with DAPI and the cells were viewed under fluorescent microscopy. Images were captured and overlaid as shown. Figures (c) and (d) represent the quantitation of green fluorescence, which indicates the increase in p-Akt levels. * p< 0.05 as compared to control and # p<0.05 as compared to HR.
Figure 9. Western analysis demonstrating effect of EETs on Akt, BAD and XIAP.
HL-1 cells and neonatal myocytes were grown and subjected to HR with or without pretreatment with EETs. The cells were lysed in the presence of phosphatase inhibitors and the lysates were developed by western analysis with antibody to (a) p–Akt and Akt; samples labeled WT were pretreated with wortmannin (0.2-1 μM) for 30 min (b) p-BAD, BAD and XIAP in neonatal myocytes (left panel) and HL-1 cells (right panel). Samples treated with EET showed increase in phosphorylation of Akt (p-Akt) and BAD (p-BAD) and higher levels of XIAP in both cell types, while Akt and BAD remain unaltered and demonstrate equal protein loading in all lanes in (a) and (b) respectively.
(iii) EETs initiate phosphorylation of BAD
To further study the involvement of downstream factors of PI3K/Akt, we performed western blots for p-BAD (Fig 9) since it is a target for p-AKT. We found that there was an increase in phosphorylation of BAD in EET-pretreated HL-1 cells (right panel) and neonatal myocytes (left panel) as compared to cells exposed to HR after pretreatment with vehicle. Expression of BAD (loading control) in the corresponding lysates did not appear altered.
(iv) EETs increase intracellular levels of XIAP
XIAP is an anti-apoptotic protein that is a downstream effector of the PI3K/Akt pro-survival pathway. We found that there was an increase in XIAP expression in EET-pretreated HL-1 cells (Fig 9 right panel) and neonatal myocytes (left panel). These samples were derived from the same protein lysates as the BAD panel (above them), which demonstrate equal protein loading in each lane.
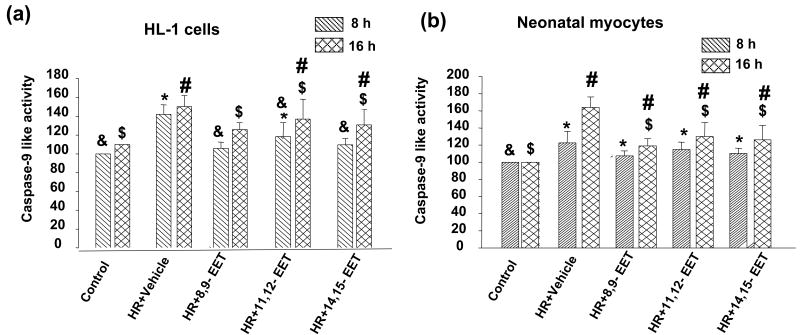
(v) EETs decrease activity of caspase 9
Caspase 9 is another downstream target of the Akt pathway. Since we found an increase in caspase-3 activity after HR and its subsequent decrease in EET pretreated cells we also investigated caspase-9 activity in HL-1 cells and neonatal myocytes (Figure 10a&b). We saw a similar trend in caspase-9 activity in these cells with a significant increase in HR treated cells and a subsequent decrease when the cells were pretreated with EET.
Figure 10. EETs decrease caspase-9 activity.
HL-1 cells (a) and neonatal myocytes (b) were subjected to HR after pretreatment with vehicle or the 3 regioisomers of EET. Caspase-9 activity was measured as described in the ‘Materials and Methods’. * indicates a significant difference (p<0.05) compared to control (8h); # indicates a significant difference (p<0.05) compared to control (16h); & indicates a significant difference (p<0.05) compared to HR (8h); $ indicates a significant difference (p<0.05) compared to HR (16h). Controls were treated under normoxic conditions. Both cell types showed increased caspase-9 activity after HR, which was attenuated by 8,9-, 11,12-, or 14,15-EET by 16 h.
(IV) EETs protect function of rat neonatal cardiac myocytes against HR-induced injury
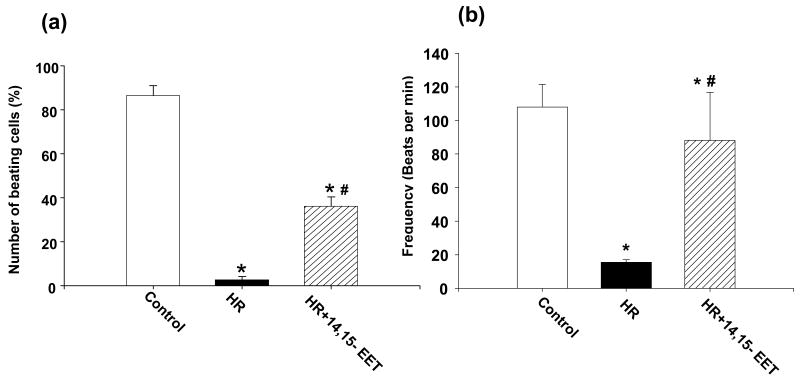
EETs maintain rhythmic beating of rat neonatal myocytes during HR
Neonatal rat myocytes beat spontaneously in culture. Exposure to hypoxia retards rhythmic contractions of these cells (Fig 11). Beating frequency was preserved by pre-incubation with 14,15- or 11,12-EET as compared to vehicle. We observed only a few beating cells in each field of myocytes after HR (the hypoxia was followed by 1 h reperfusion) as compared to those pre-incubated with 14,15-EETs (1 μM) 16 h prior to and just before hypoxia (Fig 11a). The average frequency of these contractions was 108/min under normoxia, which was reduced to 15 beats/minute in the few cells still beating after HR. Cells pretreated with EET were beating at an average frequency of 88 beats/min (Fig 11b).
Figure 11. EET protects contractility of primary neonatal myocytes in culture.
Neonatal myocytes were subject to HR after pretreatment with vehicle or 14,15-EET (1 μM). The number of beating cells in a microscope field (a) and the frequency (beats/min) (b) were counted as described in Materials and Methods (a total of 3-5 batches of cells were examined for each treatment). Pretreatment with EET increased both total number and frequency of beating cells as compared to myocytes that were exposed to HR in the presence of vehicle.
Discussion
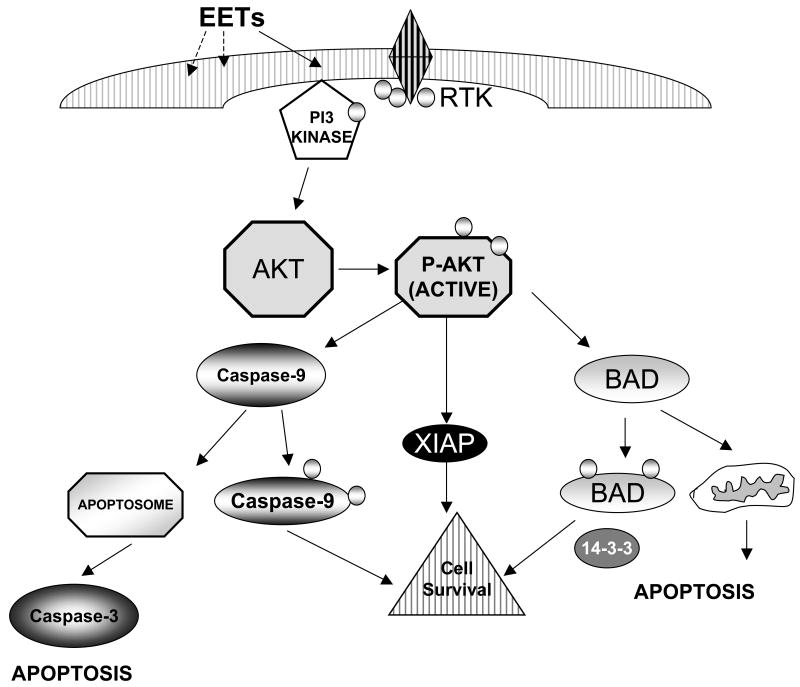
Our results demonstrate by four independent assays (viability using MTT, nuclear fragmentation by Hoechst staining, increase in the early apoptosis marker annexin V, and activation of the late apoptotic protease, caspase-3) that EETs attenuate HR-induced cell death/apoptosis of cardiomyocytes cultured from the hearts of 2 rodent species, rats and mice. Importantly, incubation with EETs not only enhanced cell survival but also maintained contractile function of neonatal myocytes that was attenuated by HR. Currently there is no accepted mechanism for protection of cardiomyocytes by EETs. Our results strongly support the hypothesis that EETs maintain cellular structure and function by stimulation of the PI3K-Akt cell survival pathway. We identified 5 targets that mediate the action of EETs, viz. PI3K, Akt, BAD, XIAP and caspase 9 (see schematic figure 12). Each one of these proteins was affected by EETs in an anti-apoptotic manner, defining a mechanism for the observed protection of cardiomyocytes after injury by HR. EETs may stimulate other pathways in the cell that also have a pro-survival role (eg. opening of KATP channels). Future studies are needed to address if activation of PI3K regulates channel function or if multiple pro-survival pathways must be functional at the same time to protect cardiomyocytes. Understanding clearly how EETs are protective will be a first step towards developing these fatty acids into therapeutic agents for cardiovascular disease.
Figure 12. Schematic representation of a pathway for protection of cardiomyocytes by EET via the PI3K/Akt pro-survival pathway.
We have reported that pretreatment with EET increases (i) PI3K activity (ii) phosphorylation of Akt (iii) phosphorylation of BAD (iv) intracellular XIAP and (v) inhibition of activity of caspases-9. Each of these events decreases activity of caspase 3, a late effector, to protect the cardiomyocyte from apoptosis by HR. These targets are known to align in a signaling cascade as illustrated in the schematic. It should be noted that the results do not implicate absence of other pro-survival or mitotic pathways that may also be targeted by EETs.
The PI3K/Akt pathway is one of the most potent intracellular mechanisms to promote cell survival. For example, in a study of four downstream effectors of growth factor receptors, PI3K, Ras, Raf and Src, PI3K was the only one to inhibit apoptosis after serum withdrawal (30). Our results indicate that PI3K activity is enhanced by EET within 30 minutes of application, and also hours later during the first hour of reoxygenation. It is not clear if EETs directly stimulate PI3K or act indirectly. The best known activators of PI3K include G-protein coupled receptors (GPCR) (10,35,40,51,69), receptor tyrosine kinases (23,33), and glycoprotein 130 (12,32,56). Heterotrimeric G-proteins and receptor tyrosine kinases mediate the action of EET. Evidence of cholera toxin sensitive, high affinity binding of EETs to mononuclear cells (61,62) or activation of K+ channels via the Gsα subunit of heterotrimeric G proteins, raises the possibility that EETs may stimulate PI3K via GPCR. The sulfonamide derivative of 14,15-EET (14,15-EET-SI, 20 μM) induced association between the EGF receptor and Src-like kinases within 1 minute of application at a concentration of 20 μM in renal epithelial cells (7). This high concentration of 14,15-EET-SI stimulated phosphorylation of the 85 kD regulatory subunit of PI3K (7). Cross-talk with the epidermal growth factor receptor, EGFR, regulates the angiogenic action of EET in the chick chorioallantoic vessels (38). In addition EETs can transcriptionally upregulate proteins such as tissue plasminogen activator (t-PA) via Gs (45), implying secondary or late signaling may occur after synthesis of new proteins or factors that are induced by EET. These observations may explain why a single exposure to free fatty acids which are rapidly metabolized in cells may trigger events appearing many hour later.
One downstream target of PI3K is the kinase Akt (67) which is reported to be activated during intracellular signal transduction of many receptors and survival factors. Members of this family include Akt1, Akt2 and Akt3 (42). Akt, also known as protein kinase B (PKB) or RAC-PK, is a serine-threonine kinase. PI3K phosphorylates inositol lipids that recruit and modify several targets containing Pleckstrin homology (PH) domains including Akt and 3-phosphoinositide–dependent kinase 1 (PDK-1) (4,22,31). Akt1 is activated by phosphorylation first on Thr308 by PDK-1 and subsequently on Ser473 (53). PDK-1 has a Pleckstrin homology domain and is therefore also recruited to the membrane after activation of PI3K. A number of downstream targets that regulate apoptosis are modulated by p-Akt including members of the Bcl-2/CED9 family Bax, Bak and BAD (14,26,63,68), XIAP, caspase 9, GSK3β (glycogen synthase kinase 3β), transcription factors of the forkhead (FKH) and NFkB families and eNOS (18,34,49,59). Bad and caspase 9 are inactivated after phosphorylation by Akt (1) while XIAP is stabilized by it. Therefore, Akt promotes cell survival by ultimately modifying the core death machinery.
Akt primarily triggers phosphorylation of BAD at Ser-136 which is sufficient to promote survival. The phosphorylation of BAD leads to the prevention of cell death via a mechanism that involves the selective association of p-BAD with isoforms of the scaffold protein 14-3-3. This interaction induces release of anti-apoptotic binding partners of BAD such as Bcl-XL or Bcl-2 (14,24,65). 14-3-3 isoforms associate with a number of cellular signaling molecules including KSR, cdc25, Raf-1 and PI3K (3,39). Growth factor deprivation or apoptotic signals cause removal of the phosphates which allow BAD to bind to anti-apoptotic BCl-2 ultimately resulting in displacement of voltage-dependent anion channel 2 (VDAC2) of the mitochondria, increasing mitochondrial outer membrane permeabilization and inducing apoptosis. BAD is therefore a pro-apoptotic molecule when it is not phosphorylated.
XIAP is, as its name (X-linked inhibitor of apoptosis) suggests, an inhibitor of apoptosis which belongs to the IAP family of proteins with an evolutionarily conserved role in regulating programmed cell death (15,16). It binds to and blocks caspases 9, 3 and 7 (16). The protein contains BIR (baculovirus inhibitor of apoptosis repeats) that limit substrate access to catalytic sites of activated caspases. In addition, this protein prevents dimerization of procaspase 9 (57) and protects the cells from accidental activation of caspases. XIAP is an important anti-apoptotic protein since it attenuates the major inhibitor of the extrinsic pathway, caspase 9, as well as the common mediator of both the intrinsic as well as extrinsic pathways, caspase 3 (13). Our results (Fig 9) demonstrate reduced levels of this protein after HR in both types of cultured myocytes. Pretreatment with EETs impressively reduces this fall in the level of XIAP induced by HR.
Though this is the first report describing anti-apoptotic actions of EETs on cardiomyocytes, enhanced survival of other cultured cells by these fatty acids and also by epoxygenase overexpression, have been reported. Examples of cell types so affected include LLCPKc14 (porcine kidney proximal tubule-like epithelial cell line (6), primary human coronary and pulmonary microvascular endothelial cells (17), bovine aortic endothelial cells (66), and the human carcinoma cell line Tca-8113 (29). The PI3K/Akt pathway has been implicated in all these studies, though much higher concentrations of 14-15-EET (10-20 μM) were used in the kidney cells (6), where Akt kinase activity was increased 10 minutes after addition of 14,15-EET to the LLCPKc14s. Levels of pAkt were higher after at least 12 hours of treatment of the carcinoma cells with 8,9-, 11,12- or 14,15-EET (100 nM) followed by induction of apoptosis by tumor necrosis factor α for 12 hours, (29). Interestingly expression of the 110 kD PI3K subunit was increased after overexpression of epoxygenase enzymes that catalyze formation of EET, in bovine aortic endothelial cells (66).
Hence we demonstrate that EETs prevent apoptosis in rat neonatal cardiac myocytes and a mouse atrial cardiomyocyte cell line (HL-1), subjected to HR injury. We report for the first time that treatment with EET enhances PI3K activity. This treatment also attenuates both the increase in activity of caspase 9 and the fall in the levels of intracellular XIAP induced by HR. We have confirmed that EETs increase phosphorylation of Akt that is upstream of these effectors. Together these observations support mechanistic evidence for the protective effect of EETs in cardiomyocytes to prevent HR-induced cell death. These data make it essential to investigate if activation of potassium channels (especially KATP channels), the mechanism favored by other investigators for protection of myocardium by EETs, is independent of PI3K or occurs by cross-talk in parallel to this pro-survival pathway.
Acknowledgments
Technical help from Laurel Dunn and Ying Gao are gratefully acknowledged.
Grants: Financial support was provided by NIH Grants HL069996 (M Medhora), HL49294 (ER Jacobs), HL68627 (ER Jacobs) and the Robert A. Welch Foundation (JR Falck) GM 31278 (JR Falck).
References
- 1.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bialik S, Geenen DL, Sasson IE, Cheng R, Horner JW, Evans SM, Lord EM, Koch CJ, Kitsis RN. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest. 1997;100:1363–1372. doi: 10.1172/JCI119656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burbelo PD, Hall A. 14-3-3 proteins. Hot numbers in signal transduction. Curr Biol. 1995;5:95–96. doi: 10.1016/s0960-9822(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 4.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 5.Campbell WB, Harder DR. Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res. 1999;84:484–488. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- 6.Chen JK, Capdevila J, Harris RC. Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol. 2001;21:6322–6331. doi: 10.1128/MCB.21.18.6322-6331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J Biol Chem. 1998;273:29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 8.Chen YJ, Jiang H, Quiley J. The nitric oxide and prostaglandin-independent component of the renal vasodilator effect of thimerosal is mediated by epoxyeicosatrienoic acids. J Pharamacol Exp Ther. 2003;304:1292–1298. doi: 10.1124/jpet.102.042671. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 10.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 11.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig R, Wagner M, McCardle T, Craig AG, Glembotski CC. The cytoprotective effects of the glycoprotein 130 receptor-coupled cytokine, cardiotrophin-1, require activation of NF-kappa B. J Biol Chem. 2001;276:37621–37629. doi: 10.1074/jbc.M103276200. [DOI] [PubMed] [Google Scholar]
- 13.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 14.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 15.Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–23. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 17.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Guttermann DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;91:H517–31. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 18.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Flemming I. Cytochrome P450 and Vascular Homeostasis. Circ Res. 2001;89:753–762. doi: 10.1161/hh2101.099268. [DOI] [PubMed] [Google Scholar]
- 20.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 21.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 22.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichilis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF--activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 23.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajewski TF, Thompson CB. Apoptosis meets signal transduction: elimination of a BAD influence. Cell. 1996;87:89–592. doi: 10.1016/s0092-8674(00)81377-x. [DOI] [PubMed] [Google Scholar]
- 25.Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J Vasc Res. 1998;35:274–284. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- 26.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross GJ, Hsu A, Falck JR, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol. 2007;42:687–691. doi: 10.1016/j.yjmcc.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–866. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- 29.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 31.Klarlund JK, Guilherme A, Holik JJ, Virbasius JV, Chawla A, Czech MP. Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 32.Kuwahara K, Saito Y, Kishimoto I, Miyamoto Y, Harada M, Ogawa E, Hamanaka I, Kajiyama N, Takahashi N, Izumi T, Kawakami R, Nakao K. Cardiotrophin-1 phosphorylates akt and BAD, and prolongs cell survival via a PI3K-dependent pathway in cardiac myocytes. J Mol Cell Cardiol. 2000;32:1385–1394. doi: 10.1006/jmcc.2000.1177. [DOI] [PubMed] [Google Scholar]
- 33.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 34.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 36.Medhora M, Dhanasekaran A, Gruenloh SK, Dunn LK, Gabrilovich M, Falck JR, Harder DR, Jacobs ER, Pratt PF. Emerging mechanisms for growth and protection of the vasculature by cytochrome P450-derived products of arachidonic acid and other eicosanoids. Prostaglandins Other Lipid Mediat. 2007;82:19–29. doi: 10.1016/j.prostaglandins.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Miao W, Luo Z, Kitsis RN, Walsh K. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J Mol Cell Cardiol. 2000;32:2397–2402. doi: 10.1006/jmcc.2000.1283. [DOI] [PubMed] [Google Scholar]
- 38.Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR) FASEB J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- 39.Morrison D. 14-3-3: modulators of signaling proteins? Science. 1994;266:56–57. doi: 10.1126/science.7939645. [DOI] [PubMed] [Google Scholar]
- 40.Naga Prasad SV, Esposito G, Mao L, Koch WJ, Rockman HA. G betagamma-dependent phosphoinositide 3-kinase activation in hearts with in vivo pressure overload hypertrophy. J Biol Chem. 2000;275:4693–4698. doi: 10.1074/jbc.275.7.4693. [DOI] [PubMed] [Google Scholar]
- 41.Natarajan R, Reddy MA. HETEs/EETs in renal glomerular and epithelial cell functions. Current Opin Pharmacol. 2003;3:198–203. doi: 10.1016/s1471-4892(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson KM, Anderson NG. The protein kinase B/Akt signaling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 43.Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids (EETs) in cardioprotection: Ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H537–542. doi: 10.1152/ajpheart.00071.2006. [DOI] [PubMed] [Google Scholar]
- 44.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by eicosatrienoic acids. J Biol Chem. 2001;276:15983–15989. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 46.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 47.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 48.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–939. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 49.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Palojoki E, Saraste A, Eriksson A, Pulkki K, Kallajoki M, Voipio-Pulkki LM, Tikanen I. Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2001;280:H2726–H2731. doi: 10.1152/ajpheart.2001.280.6.H2726. [DOI] [PubMed] [Google Scholar]
- 51.Robert P, Tsui P, Laville MP, Livi GP, Sarau HM, Bril A, Berrebi-Bertrand I. EDG1 receptor stimulation leads to cardiac hypertrophy in rat neonatal myocytes. J Mol Cell Cardiol. 2001;33:1589–1606. doi: 10.1006/jmcc.2001.1433. [DOI] [PubMed] [Google Scholar]
- 52.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 53.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 54.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheng Z, Knowlton K, Chen J, Hoshijima M, Brown JH, Chien KR. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem. 1997;272:5783–5791. doi: 10.1074/jbc.272.9.5783. [DOI] [PubMed] [Google Scholar]
- 57.Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 58.Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–7. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- 59.Wang H, Lin L, Jiang J, Wang Y, Lu ZY, Bradbury JA, Lih FB, Wang DW, Zeldin DC. Up-regulation of endothelial nitric-oxide synthase by endothelium-derived hyperpolarizing factor involves mitogen-activated protein kinase and protein kinase C signaling pathways. J Pharmacol Exp Ther. 2003;307:753–764. doi: 10.1124/jpet.103.052787. [DOI] [PubMed] [Google Scholar]
- 60.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong PY, Lai PS, Shen SY, Belosludtsev YY, Falck JR. Post-receptor signal transduction and regulation of 14(R),15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J Lipid Mediat Cell Signal. 1997;16:155–69. doi: 10.1016/s0929-7855(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 62.Wong PY, Lin KT, Yan YT, Ahern D, Iles J, Shen SY, Bhatt RK, Falck JR. 14(R),15(S)-epoxyeicosatrienoic acid (14(R),15(S)-EET) receptor in guinea pig mononuclear cell membranes. J Lipid Mediat. 1993;6:199–208. [PubMed] [Google Scholar]
- 63.Yamaguchi H, Wang HG. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene. 2001;20:7779–7786. doi: 10.1038/sj.onc.1204984. [DOI] [PubMed] [Google Scholar]
- 64.Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, Zeldin DC. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–20. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- 65.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;27:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 66.Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, Lu ZY, Wang DW, Zeldin DC. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol. 2007;293:H142–51. doi: 10.1152/ajpheart.00783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1998;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 68.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 69.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]