Abstract
The major circulating form of vitamin D, 25-hydroxycholecalciferol (25D3), circulates bound to vitamin D-binding protein (DBP). Prior to activation to 1,25-dihydroxycholecalciferol in the kidney, the 25D3-DBP complex is internalized via receptor-mediated endocytosis, which is absolutely dependent on the membrane receptors megalin and cubilin and the adaptor protein disabled-2 (Dab2). We recently reported that mammary epithelial cells (T-47D) expressing megalin, cubilin, and Dab2 rapidly internalize DBP via endocytosis, whereas cells that do not express all 3 proteins (MCF-7) do not. The objectives of this study were to characterize megalin, cubilin, and Dab2 expression and transport of DBP in human mammary epithelial cells. Using immunoblotting and real-time PCR, we found that megalin, cubilin, and Dab2 were expressed and dose dependently induced by all-trans-retinoic acid (RA) in T-47D human breast cancer cells and that RA-treated T-47D cells exhibited enhanced DBP internalization. These are the first studies to our knowledge to demonstrate that mammary epithelial cells express megalin, cubilin, and Dab2, which are enhanced during differentiation and may explain, at least in part, our finding that receptor-mediated endocytosis of DBP is upregulated in differentiated mammary epithelial cells.
Introduction
The biologically active derivative of vitamin D, 1,25-dihydroxycholecalciferol (1,25D3)6, is a potent inhibitor of tumor growth and proliferation (1–3). Hence, there is growing interest in the development of strategies that can optimize vitamin D signaling in tissues that are sensitive to vitamin D-mediated growth inhibition. The major circulating form of cholecalciferol, 25-hydroxycholecalciferol (25D3), is delivered to the periphery tightly bound to vitamin D-binding protein (DBP). Upon delivery to target cells, uptake of the 25D3-DBP complex requires receptor-mediated endocytosis, after which the 25D3 is released and activated to 1,25D3 by cytochrome p450 (CYP27B1). Virtually all circulating 25D3 is tightly bound to DBP (>99%); therefore, endocytosis of DBP appears to be essential for the cellular uptake of 25D3 and its subsequent antitumorigenic and procalcemic effects. In the epithelial cells of the renal proximal tubule, where the majority of systemic 1,25D3 is produced, endocytosis of the 25D3–DBP complex process is absolutely dependent on the membrane proteins megalin and cubilin (4,5). In fact, 1,25D3 is virtually absent in the plasma of megalin knockout mice, animals that develop severe vitamin D deficiency and bone disease due to increased urinary loss of 25D3 (4). Additionally, pronounced urinary loss of 25D3 was observed in both humans and dogs with cubilin dysfunction, a condition that also causes a marked loss of DBP in the urine (5). Recently, it has also been demonstrated that disabled-2 (Dab2), an adaptor protein localized to the cytoplasmic tail of megalin (6,7), is essential for megalin- and cubilin-mediated endocytosis of DBP in kidney (8).
Although it has been shown that a number of extra renal tissues express megalin, cubilin, and/or Dab2 (9–15), little is known about uptake of vitamin complexes in these tissues or whether their uptake requires megalin, cubilin, and/or Dab2-mediated endocytosis. However, recent studies have shown that this megalin-mediated endocytosis occurs in numerous absorptive epithelial cell types, such as thyroid and reproductive tract, and megalin is expressed in both breast and prostate, tissues that have functional CYP27B1 (16,17) and sensitivity to 1,25D3 (18–22). Moreover, evidence suggests that differentiation plays a role in the expression and endocytic function of megalin, cubilin, and Dab2. In support of this concept, when F9 mouse embryocarcinoma cells are differentiated by all-trans-retinoic acid (RA), megalin and Dab2 expression are markedly elevated (23,24).
Despite identification of a functional CYP27B1 in a number of extra-renal sites, the mechanism by which 25D3 trafficking occurs and the regulation of these processes remain unclear in these tissues. We recently reported that mammary epithelial cells readily internalized DBP via receptor-mediated endocytosis and that this process was inhibited by a known inhibitor of megalin-and cubilin-mediated endocytosis (25). Thus, the presence of megalin, cubilin, and Dab2 may identify tissues that have the ability to transport 25D3 and locally produce antitumorigenic 1,25D3. In the present study, our first objectives were to assess megalin, cubilin, and Dab2 expression in mammary epithelial cells and determine whether levels of expression could be modulated by compounds known to induce these proteins in other cell types. Furthermore, we tested our hypothesis that stimulation of megalin, cubilin, and/or Dab2 expression in breast cancer cells would be associated with enhanced uptake of DBP.
Materials and Methods
Cell lines and culture conditions
T-47D breast cancer cells and F9 mouse embryocarcinoma cells were obtained from the American Type Culture Collection. Cells were routinely grown in a humidified incubator with 5% CO2 and a temperature of 37°C. T-47D cells were cultured in RPMI media containing 10% fetal bovine serum, penicillin (100 kU/L), and streptomycin (0.1 g/L). F9 cells were grown in DMEM media containing 10% fetal bovine serum, penicillin (100 kU/L), and streptomycin (0.1 g/L).
Assessment of differentiation
To assess the ability of known differentiating agents to induce differentiation of T-47D cells, 5 × 103 cells were plated in 6-well plates, then treated with various combinations of 1 μmol/L RA, 100 nmol/L 1,25D3, and 10 μmol/L forskolin for 7 d. Lipid accumulation (i.e. terminal differentiation) was assessed as described by Ramirez-Zacarías et al. (26). Accumulation of lipid droplets was quantitated by measuring the absorbance of solubilized Oil Red O at 510 nm following the addition of 1 mL isopropyl alcohol.
Real-time PCR
T-47D cells (2 × 105) were plated in 6-well plates and treated with various combinations of RA (0–100 μmol/L), 100 nmol/L 1,25 D3, and forskolin (0–100 μmol/L) for 2–7 d. mRNA from cells was then either immediately isolated using a commercial kit (RNeasy Mini kit, Qiagen) or cells were incubated an additional 48 h in control media prior to mRNA isolation. Total mRNA was then used for first-strand cDNA synthesis using TaqMan Reverse Transcription Reagents (N808–0234, Applied Biosystems). For each sample, reactions were performed in triplicate, generating 3 2.0-μg cDNA stocks/sample. For T-47D cells, each of the cDNA stocks were independently analyzed in duplicate (200 ng per well) for megalin, cubilin, and Dab2 mRNA expression via real-time PCR using SYBR Green Detection reagents (4309155, Applied Biosystems) with primer sets specific for human megalin (forward primer, AAATTGAGCACAGCACCTTTGA, reverse primer, TCTGCTTTCCTGACTCGAATAATG), human cubilin (forward primer, GGTTCCCTGCCAATTATCCAA, reverse primer, CCGCCATCCAAAATTTCTACA) or human Dab2 (forward primer, GGTGTAACTGTCACACTCCCTCAG, reverse primer, TGACCACCCATCATGGCTC) and were normalized against glyceraldehyde 3-phosphate dehydrogenase mRNA (forward primer, CCACCCATGGCAAATTCC, reverse primer, TGATGGGATTTCCATTGATGAC).
Western blotting
T-47D cells were plated in 100-mm dishes (5.0 × 105 cells/dish) and treated with various combinations of 1,25D3 (100 nmol/L), RA (0–100 μmol/L), and forskolin (0–100 μmol/L). After 2 d, cell monolayers were scraped into 200 μL 2× Laemmli buffer containing protease inhibitors (10 mmol/L benzamidine, 10 mmol/L sodium fluoride, 100 mmol/L sodium vanadate, 25 μg/μL aprotinin, 25 μg/μL pepstatin, 25 μg/μL leupeptin, and 1 mmol/L phenylmethylsulfonyl fluoride). Total protein was analyzed by the Micro BCA assay (Bio-Rad). For analysis of megalin and cubilin protein abundance, 60 μg protein was separated by SDS-PAGE under nonreducing conditions using 6% polyacrylamide gels. For analysis of Dab2 protein abundance, 60 mg protein was separated by SDS-PAGE under reducing conditions using 7.5% polyacrylamide gels. Proteins were transferred to nitrocellulose and Ponceau stained to confirm equal loading. Membranes were blocked at room temperature (RT) for 1 h in 5% skim milk in PBS/1% Tween 20. For detection of megalin, the membrane was incubated for 2 h at RTwith a 1:500 dilution of polyclonal rabbit anti-megalin antibody (kindly provided by Scott Argraves, University of South Carolina, Columbia, SC) in 5% bovine serum albumin followed by 1 h incubation with goat anti-rabbit IgG horseradish peroxidase (1:5000). For detection of cubilin, membranes were incubated for 3 h at RT with a 1:100 dilution of a polyclonal goat anti-cubilin antibody (Santa Cruz Biotechnology). Donkey anti-goat IgG horseradish peroxidase-conjugated secondary antibody (1:10,000) was applied for 1 h at RT. For detection of Dab2, membranes were incubated with a 1:500 dilution of a monoclonal mouse anti-Dab2 antibody (BD Biosciences Pharmingen) for 3 h at RT, followed by a 1-h incubation with a sheep anti-mouse IgG horseradish peroxidase-conjugated secondary antibody (1:7500). For all proteins, specific binding was detected by chemiluminescence and exposure to autoradiography film (Kodak Biomax).
Fluorescein conjugation and endocytosis of DBP
DBP (Calbiochem) was conjugated to Alexa-488 using a commercial kit (A10235, Molecular Probes). For DBP uptake studies, subconfluent T-47D and F9 cells were grown in 0.1 μmol/L RA for 7 d, then plated on 4-well Lab-Tek II CC2 chamber slides (Nalge Nunc International) for 48 h in RA-free media. Cells were then switched to serum-free media for 1 h, then incubated with 0.02 g/L Alexa-DBP at 37°C (the optimal temperature for endocytosis) for 30 min. Cells were rapidly fixed in ice-cold methanol, incubated with Hoechst (1 mg/L) in PBS for visualization of nuclei, and were then mounted in anti-fade on cover slips. Because we have observed uptake of Alexa-DBP by T-47D cells under control conditions (25), we viewed untreated and RA-treated T-47D cells 72 h after mounting to allow for fading of Alexa-DBP and a more accurate comparison of uptake efficiency. Because untreated F9 cells internalize virtually no appreciable DBP (M. J. Rowling and J. Welsh, unpublished data), F9 cells were viewed 2 h following mounting. Cells were viewed on an Olympus AX70 microscope equipped with a Spot RT digital camera. Fluorescent and UV images were acquired with a constant exposure time.
Statistical analysis
Data were analyzed by 1- or 2-way ANOVA as appropriate using InStat software (version 3.0 for Windows, GraphPad Software) or XLSTAT (Addinsoft). Differences between means were considered significant at P <0.05. We used Dunnett’s post-test to identify which means were significantly different from control values after ANOVA.
Results
RA treatment elevated intracellular lipid accumulation in T-47D cells
To determine whether megalin, cubilin, and/or Dab2 expression was associated with differentiation of T-47D cells, we quantitated the accumulation of lipid droplets, a marker of terminal differentiation in mammary cells. RA treatment significantly enhanced intracellular lipid accumulation (i.e. terminal differentiation) ~2-fold in T-47D cells. In contrast, 1,25D3 and forskolin did not affect lipid accumulation, but these observations do not preclude effects of 1,25D3 and forskolin on markers of earlier stages of differentiation (Fig. 1).
FIGURE 1.
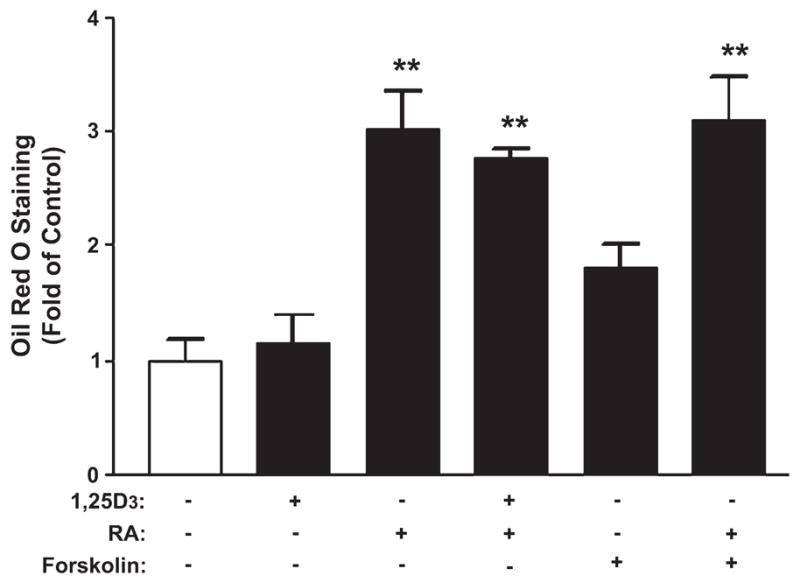
Differentiation of T-47D breast cancer cells by RA. T-47D cells were treated for 7 d with vehicle (ethanol and/or DMSO), 100 nmol/L 1,25D3, 10 μmol/L RA, or 10 μmol/L forskolin in various combinations, after which cells’ terminal differentiation was assessed by Oil Red O staining. Data are means ± SEM, n = 6, **Different from untreated control cells, P < 0.01.
Induction of megalin, cubilin, and Dab2 expression by differentiating agents in T-47D cells
Previous studies demonstrated up-regulation of megalin and Dab2 expression in rodent embryocarcinoma cells upon RA-mediated differentiation (23,24). We therefore reasoned that megalin, cubilin, and/or Dab2 expression may be similarly modulated in mammary tumor cells after treatment with compounds known to induce terminal differentiation of mammary cells. Megalin mRNA levels were elevated 1.8-fold in T-47D breast cancer cells treated with RA (Fig. 2A) and treatment with both RA and 1,25D3 produced a 4.2-fold increase in megalin mRNA levels. Whereas Western blotting identified megalin protein in T-47D cells (Fig. 2B) and revealed an increased expression after 48 h treatment with RA, 1,25D3, or in combination, only RA was effective in inducing cubilin and Dab2 protein (Fig. 3). Interestingly, RA did not increase cubilin mRNA abundance (data not shown) but elevated Dab2 mRNA expression (6.2-fold increase; Fig. 4A), which persisted for at least 2 d after RA was removed from culture media (Fig. 4B). To determine whether other differentiation-inducing agents could also impact megalin, cubilin, and Dab2 expression, we used the cAMP agonist, forskolin. After a 48-h treatment with 100 μmol/L forskolin, megalin mRNA (Fig. 5A) and protein abundance (Fig. 5B) were significantly greater than in untreated cells and this effect was potentiated by RA (Fig. 5). In contrast, forskolin did not affect cubilin (data not shown) or Dab2 expression (Fig. 4A). In the presence of RA, a significant induction of megalin mRNA occurred in forskolin treated at concentrations as low as 1 μmol/L [2-fold greater than the –RA, dimethylsulfoxide (DMSO) control], whereas maximal induction (8.6-fold increase) was achieved at 100 μmol/L. Megalin protein expression appeared to be maximal in both vehicle- and RA-treated cells treated with 0.1 μmol/L forskolin (Fig. 5B).
FIGURE 2.
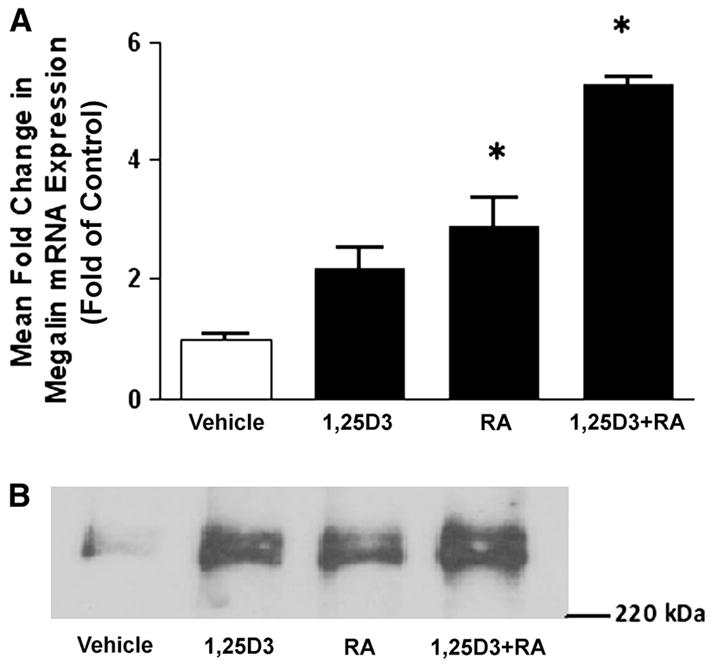
Induction of megalin by RA and 1,25D3 in T-47D breast cancer cells. Cells were treated with 10 μmol/L RA and/or 100 nmol/L 1,25D3 for 48 h. Megalin mRNA and protein abundance were analyzed as described in “Materials and Methods.” (A) Megalin mRNA abundance (determined by real-time PCR) in T-47D cells treated with RA and/or 1,25D3. Data are means ± SEM, n = 5. *Different from vehicle, P < 0.05. (B) Western blot analysis of megalin protein abundance in T-47D cells treated with RA and/or 1,25D3. Immunoblot is representative of 3 independent experiments.
FIGURE 3.
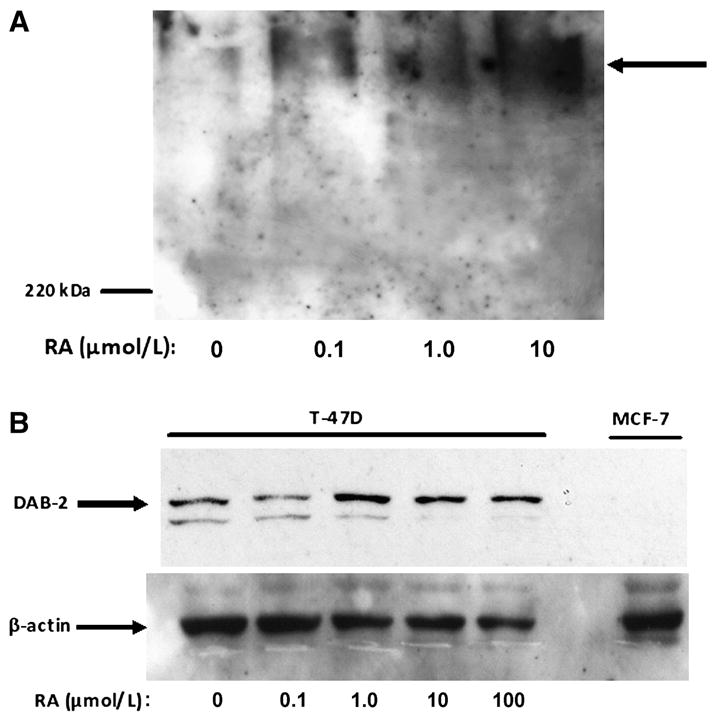
Induction of cubilin and Dab2 protein by RA in T-47D breast cancer cells. (A) T-47D cells were treated with 0, 0.1, 1.0, or 10 μmol/L RA or vehicle (DMSO) for 48 h. Lysates were separated by 6% SDS-PAGE under nonreducing conditions and immunoblotted with polyclonal rat anti-cubilin antibody. (B) T-47D cells were treated with 0–100 μmol/L RA for 48 h, whereas MCF-7 cells were treated with vehicle (DMSO). Proteins were separated using 7.5% SDS-PAGE and incubated with monoclonal mouse anti-Dab2 antibodies. Immunoblots are representative of 3 independent experiments and Dab2 bands were at the same molecular weight as Dab2 detected in kidney lysates (data not shown).
FIGURE 4.
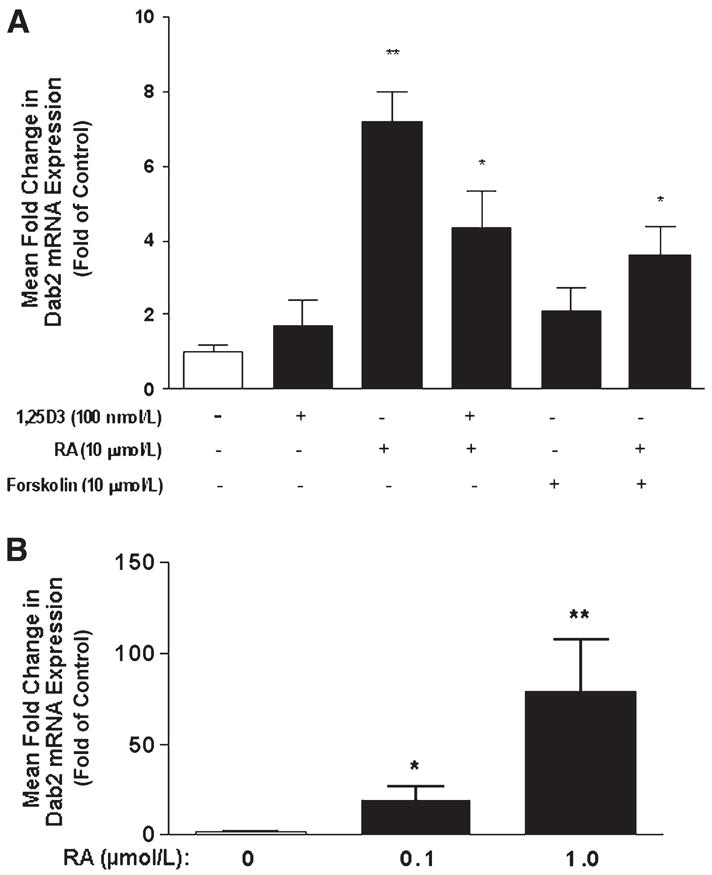
Induction of Dab2 mRNA by RA in T-47D breast cancer cells. (A) T-47D cells were treated with 10 μmol/L RA, 100 nmol/L 1,25D3, or 10 μmol/L forskolin for 48 h. (B) Induction of Dab2 mRNA by RA in T-47D cells persists after removal of RA. T-47D cells were treated with 0, 0.1, or 1.0 μmol/L RA for 7 d, after which RA was removed and cells were grown for an additional 48 h. Dab2 mRNA levels were analyzed by real-time PCR as described in “Materials and Methods.” Data are means ± SEM, n = 5. Asterisks indicate different from untreated control cells, *P < 0.05; **P < 0.01.
FIGURE 5.
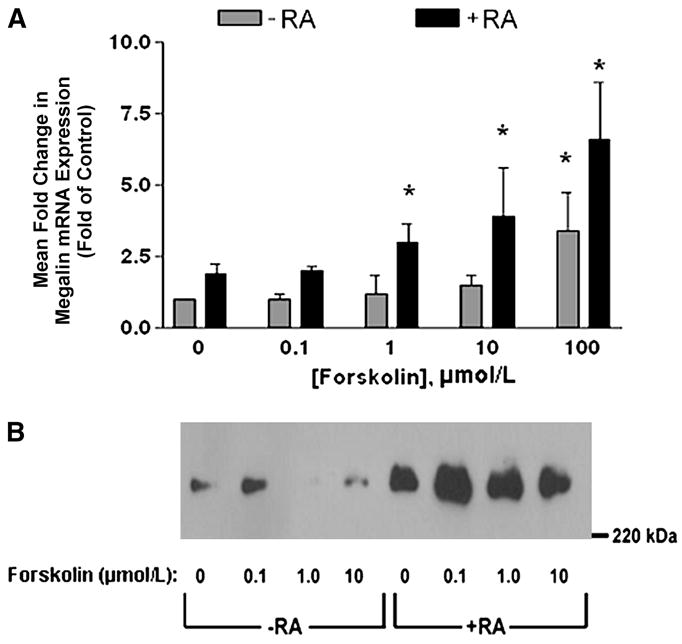
Forskolin- and RA-mediated induction of megalin mRNA and protein in T-47D breast cancer cells. (A) Megalin mRNA expression measured by real-time PCR in T-47D cells treated for 48 h with increasing concentrations of forskolin in the presence (+) or absence (−) of 10 μmol/L RA. Data are means ± SEM, n = 5. *Different from untreated control cells, P < 0.05. (B) Representative immunoblot illustrating megalin protein levels in T-47D cells treated for 48 h with increasing concentrations of forskolin in the presence (+) or absence (−) of 10 μmol/L RA.
RA treatment enhanced uptake of DBP in T-47D cells
Induction of megalin and Dab2 expression has been linked to RA-mediated differentiation of F9 embryocarcinoma cells (23,24). Consistent with this concept, we found that DBP uptake was enhanced in F9 cells that were grown in RA-supplemented media (Fig. 6A). We therefore hypothesized that increased expression of megalin, cubilin, and Dab2 would similarly correlate with uptake of DBP by T-47D cells. When T-47D cells were cultured in RA-supplemented media, like F9 cells, they internalized significantly more DBP than vehicle-treated cells (Fig. 6B).
FIGURE 6.
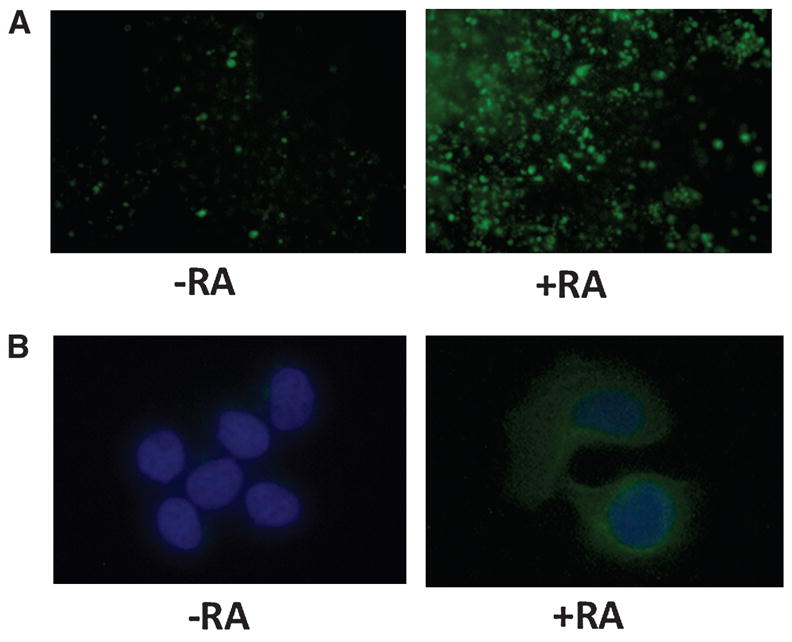
Uptake of DBP was enhanced in F9 mouse embryocarcinoma and T-47D breast cancer cells cultured in RA-supplemented media. F9 and T-47D cells were cultured for 7 d with 0.1 μmol/L RA, then incubated with 0.02 g/L DBP conjugated to Alexa-488. Cells were stained with Hoechst for visualization of nuclei. (A) F9 cells were viewed 2 h following mounting. (B) At 72 h after mounting, images of T-47D cells were captured. All images were captured on an Olympus AX70 microscope with a Spot RT digital camera.
Discussion
Megalin is a large transmembrane protein (600 kDa), which, together with its endocytic partners cubilin and Dab2, is essential for renal uptake of the major circulating form of calciferols (25D3) (4,5,8). Practically all 25D3 (>99%) circulates and is internalized as an intact 25D3-DBP complex. Once internalized by renal proximal tubule cells, the vitamin-carrier complex dissociates, allowing for metabolic activation of 25D3 into 1,25D3, the active vitamin D metabolite that binds the vitamin D receptor, which mediates antiproliferative and pro-differentiating signaling in a number of extra-renal tissues (27). Hence, considerable attention has been focused on whether tissues other than the kidney can generate 1,25D3. In support of this concept, the enzyme capable of converting 25D3 to 1,25D3 (CYP27B1) has now been conclusively identified in many additional cell types, including keratinocytes, colonocytes, and the epithelial cells of the prostate and mammary gland (16, 28,29). Although the presence of CYP27B1 in extra-renal tissues suggests that local activation of 25D3 can occur, this suggestion presumes that circulating 25D3 is accessible to the enzyme. Thus, determining the mechanisms by which the 25D3-DBP complex can be internalized by extra-renal tissues could be a critical step in determining why serum 25D3 levels are inversely correlated with incidence of numerous types of cancer.
Despite the recognition that 25D3 is delivered to sites of metabolism and storage in complex with DBP, the mechanisms for cellular uptake of 25D3–DBP have not been clearly defined in tissues other than the kidney. Previously, we demonstrated that coexpression of megalin and cubilin in mammary cells correlated with the rapid internalization of DBP via receptor-mediated endocytosis (25). Furthermore, we found that receptor-associated protein, a known inhibitor of megalin-mediated endocytosis (30), drastically reduced the ability of T-47D cells to internalize DBP. In the present study, we extended our previous work by demonstrating that treatment of T-47D cells with RA, a compound that induces terminal differentiation of T-47D cells, markedly increased expression of megalin, cubilin, and/or Dab2 and enhanced DBP uptake. These findings are consistent with our previous assessment of megalin and cubilin expression in murine mammary gland and human mammary cell lines (25) and a marked increase in mammary-specific megalin and Dab2 expression we have found in pregnant, lactating, and estrogen-/progesterone-supplemented mice (M.J. Rowling and J. Welsh, unpublished data). Moreover, these data are supported by reports of modulated megalin, cubilin, and Dab2 expression in epithelial cells from nonmammary sources (31–33). Characterizing the mechanisms of vitamin D transport in the mammary gland may therefore not only provide important information relevant to breast cancer but may identify possible targets for the prevention of other types of cancer. Additionally, future assessment of the effects of tumorigenesis on megalin, cubilin, and Dab2 expression may dramatically affect nutritional strategies for the prevention/treatment of cancer. For example, if in future studies researchers discover that mechanisms for vitamin uptake are deregulated as cells lose their differentiated phenotype, then attention to optimal supplies of vitamin D would be most critical in preventing cancer development. Conversely, if mechanisms of vitamin D transport remain functional even in transformed cells, then optimal nutritional status of vitamin D may prove important in both prevention and treatment strategies.
Although administration of 1,25D3 has been proven to be effective at inhibiting tumorigenesis, 1,25D3 produces a toxic calcemic response when administered at pharmacological doses (34). In addition, strict regulation of renal 1,25D3 synthesis ensures that under physiological conditions, serum 1,25D3 concentrations will not reach levels that can inhibit tumor growth regardless of the amount of vitamin D acquired from the diet (35). Therefore, evaluating the ability of mammary cells to locally produce 1,25D3 may be more predictive of breast cancer risk and may lead to a more feasible strategy for the use of dietary vitamin D in the context of cancer prevention. In support of this concept, epidemiological studies report that both sunlight exposure and dietary vitamin D are inversely correlated with breast cancer risk or disease progression (36–40). Rats fed diets low in vitamin D develop significantly more mammary tumors when treated with chemical carcinogens than rats with adequate vitamin D status (41). Furthermore, women with suboptimal vitamin D status exhibited increased mammographic breast density (42). Taken together, this evidence suggests that determination of uptake mechanisms and tissue stores of vitamin D in the mammary gland is a critical step toward understanding the role of dietary or sunlight-derived vitamin D in breast cancer prevention.
Footnotes
Supported by NIH grants CA103018 and CA96700 to J.W., and CA128091 and a Susan G. Komen Foundation Post-Doctoral Award (PDF0403052) to M.R.
Author disclosures: T. M. Chlon, D. A. Taffany, J. Welsh, and M. J. Rowling, no conflicts of interest.
Abbreviations used: CYP, cytochrome p450; Dab2, disabled-2; DBP, vitamin D-binding protein; 1,25D3, 1,25-dihydroxycholecalciferol; DMSO, dimethylsulfoxide; 25D3, 25-hydroxycholecalciferol; RA, all-trans-retinoic acid; RT, room temperature.
Literature Cited
- 1.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer. 2002;9:45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 2.Colston KW, Welsh JE. Vitamin D and breast cancer. 2. Oxford: Elsevier Press; 2005. [Google Scholar]
- 3.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 4.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen E, Willnow T. An endocytic pathway essential for renal uptake and activation of the steroid 25(OH)hydroxyvitamin D3. Cell. 1999;96:507–15. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 5.Nykjaer A, Fyfe J, Kozyraki R, Leheste J-R, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proc Natl Acad Sci USA. 2001;98:13895–900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher H, Oleinikov AV, Fenske C, Newman DJ. The adaptor disabled-2 binds to the third psi xNPxY sequence on the cytoplasmic tail of megalin. Biochimie. 2004;86:179–82. doi: 10.1016/j.biochi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Oleinikov AV, Zhao J, Makker SP. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem J. 2000;347:613–21. [PMC free article] [PubMed] [Google Scholar]
- 8.Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J. 2002;21:1555–64. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurer ME, Cooper JA. Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J Cell Sci. 2005;118:5345–55. doi: 10.1242/jcs.02650. [DOI] [PubMed] [Google Scholar]
- 10.Marino M, Zheng G, McCluskey RT. Megalin (gp330) is an endocytic receptor for thyroglobulin on cultured fisher rat thyroid cells. J Biol Chem. 1999;274:12898–904. doi: 10.1074/jbc.274.18.12898. [DOI] [PubMed] [Google Scholar]
- 11.Schwahn DJ, Medina D. p96, a MAPK-related protein, is consistently downregulated during mouse mammary carcinogenesis. Oncogene. 1998;17:1173–8. doi: 10.1038/sj.onc.1202038. [DOI] [PubMed] [Google Scholar]
- 12.Van Praet O, Argraves WS, Morales CR. Co-expression and interaction of cubilin and megalin in the adult male rat reproductive system. Mol Reprod Dev. 2003;64:129–35. doi: 10.1002/mrd.10245. [DOI] [PubMed] [Google Scholar]
- 13.Argraves WS, Morales CR. Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol Reprod Dev. 2004;69:419–27. doi: 10.1002/mrd.20174. [DOI] [PubMed] [Google Scholar]
- 14.Lundgren S, Carling T, Hjalm G, Juhlin C, Rastad J, Pihlgren U, Rask L, Akerstrom G, Hellman P. Tissue distribution of human gp330/megalin, a putative calcium sensing protein. J Histochem Cytochem. 1997;45:383–92. doi: 10.1177/002215549704500306. [DOI] [PubMed] [Google Scholar]
- 15.Barth JL, Argraves WS. Cubilin and megalin: partners in lipoprotein and vitamin metabolism. Trends Cardiovasc Med. 2001;11:26–31. doi: 10.1016/s1050-1738(01)00080-9. [DOI] [PubMed] [Google Scholar]
- 16.Kemmis CM, Salvador SM, Smith KM, Welsh J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr. 2006;136:887–92. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- 17.Hsu JY, Feldman D, McNeal JE, Peehl DM. Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. 2001;61:2852–6. [PubMed] [Google Scholar]
- 18.Welsh J. Induction of apoptosis in breast cancer cells in response to vitamin D and antiestrogens. Biochem Cell Biol. 1994;72:537–45. doi: 10.1139/o94-072. [DOI] [PubMed] [Google Scholar]
- 19.Welsh J, Simboli-Campbell M, Narvaez CJ, Tenniswood M. Role of apoptosis in the growth inhibitory effects of vitamin D in MCF-7 cells. Adv Exp Med Biol. 1995;375:45–52. doi: 10.1007/978-1-4899-0949-7_4. [DOI] [PubMed] [Google Scholar]
- 20.Rashid SF, Moore JS, Walker E, Driver PM, Engel J, Edwards CE, Brown G, Uskokovic MR, Campbell MJ. Synergistic growth inhibition of prostate cancer cells by 1 alpha,25 dihydroxyvitamin D(3) and its 19-nor-hexafluoride analogs in combination with either sodium butyrate or trichostatin A. Oncogene. 2001;20:1860–72. doi: 10.1038/sj.onc.1204269. [DOI] [PubMed] [Google Scholar]
- 21.Sung V, Feldman D. 1,25-Dihydroxyvitamin D3 decreases human prostate cancer cell adhesion and migration. Mol Cell Endocrinol. 2000;164:133–43. doi: 10.1016/s0303-7207(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhao XY, Peehl DM, Navone NM, Feldman D. 1alpha,25-Dihydroxyvitamin D3 inhibits prostate cancer cell growth by androgen-dependent and androgen-independent mechanisms. Endocrinology. 2000;141:2548–56. doi: 10.1210/endo.141.7.7549. [DOI] [PubMed] [Google Scholar]
- 23.Czekay RP, Orlando RA, Woodward L, Adamson ED, Farquhar MG. The expression of megalin (gp330) and LRP diverges during F9 cell differentiation. J Cell Sci. 1995;108:1433–41. doi: 10.1242/jcs.108.4.1433. [DOI] [PubMed] [Google Scholar]
- 24.Cho SY, Lee SH, Park SS. Differential expression of mouse Disabled 2 gene in retinoic acid-treated F9 embryonal carcinoma cells and early mouse embryos. Mol Cells. 1999;9:179–84. [PubMed] [Google Scholar]
- 25.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–9. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intra-cytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–7. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF. Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol. 2006;92:49–59. doi: 10.1016/j.pbiomolbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11:1961–70. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 29.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 30.Sousa MM, Saraiva MJ. Internalization of transthyretin. Evidence of a novel yet unidentified receptor-associated protein (RAP)-sensitive receptor. J Biol Chem. 2001;276:14420–5. doi: 10.1074/jbc.M010869200. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Yu WR, Carling T, Juhlin C, Rastad J, Ridefelt P, Akerstrom G, Hellman P. Regulation of gp330/megalin expression by vitamins A and D. Eur J Clin Invest. 1998;28:100–7. doi: 10.1046/j.1365-2362.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 32.Erranz B, Miquel JF, Argraves WS, Barth JL, Pimentel F, Marzolo MP. Megalin and cubilin expression in gallbladder epithelium and regulation by bile acids. J Lipid Res. 2004;45:2185–98. doi: 10.1194/jlr.M400235-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Smith ER, Capo-chichi CD, He J, Smedberg JL, Yang DH, Prowse AH, Godwin AK, Hamilton TC, Xu XX. Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J Biol Chem. 2001;276:47303–10. doi: 10.1074/jbc.M106158200. [DOI] [PubMed] [Google Scholar]
- 34.Gross C, Stamey T, Hancock S, Feldman D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol) J Urol. 1998;159:2035–9. doi: 10.1016/S0022-5347(01)63236-1. [DOI] [PubMed] [Google Scholar]
- 35.Breslau NA. Normal and abnormal regulation of 1,25-(OH)2D synthesis. Am J Med Sci. 1988;296:417–25. doi: 10.1097/00000441-198812000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Knekt P, Jarvinen R, Seppanen R, Pukkala E, Aromaa A. Intake of dairy products and risk of breast cancer. Br J Cancer. 1996;73:687–91. doi: 10.1038/bjc.1996.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I epidemiologic follow up study, 1971–1975 to 1992. Cancer Epidemiol Biomark Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 38.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–10. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 39.Grant WB. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003;164:371–7. doi: 10.1007/978-3-642-55580-0_27. [DOI] [PubMed] [Google Scholar]
- 40.Mawer EB, Walls J, Howell A, Davies M, Ratcliffe WA, Bundred NJ. Serum 1,25-dihydroxyvitamin D may be related inversely to disease activity in breast cancer patients with bone metastases. J Clin Endocrinol Metab. 1997;82:118–22. doi: 10.1210/jcem.82.1.3642. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson E, James K, Newmark HL, Carroll K. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989;49:6300–3. [PubMed] [Google Scholar]
- 42.Berube S, Diorio C, Verhoek-Oftedahl W, Brisson J. Vitamin D, calcium and mammographic breast densities. Cancer Epidemiol Biomark Prev. 2004;13:1466–72. [PubMed] [Google Scholar]


