Abstract
Purpose
To estimate the prevalence of elevated blood pressure in adult patients with acute stroke in the United States (U.S.).
Methods
Stroke patients were classified by initial systolic blood pressure into four categories using demographic, clinical, and treatment data from the National Hospital Ambulatory Medical Care Survey, the largest study of utilization and provision of emergency department services in the U.S. We also compared the age-, sex-, and ethnicity-adjusted rates of elevated blood pressure strata comparable with stages 1 and 2 hypertension in the U.S. population.
Results
Of the 563,704 stroke patients evaluated, initial systolic blood pressure was <140 mm Hg in 173,120 patients (31%), 140–184 mm Hg in 315,207 patients (56%), 185–219 mm Hg in 74,586 patients (13%), and ≥220 mm Hg in 791 patients (0.1%). The mean time interval between presentation and evaluation was 40 ± 55, 33 ± 39, 25 ± 27, and 5 ± 1 minutes for increasing systolic blood pressure strata (p=0.009). A 3- and 8-fold higher rate of elevated blood pressure strata was observed in acute stroke than the existing rates of stages 1 and 2 hypertension in the U.S. population. Labetalol and hydralazine were used in 6,126 (1%) and 2,262 (0.4%) patients, respectively. Thrombolytics were used in 1,283 patients (0.4%), but only in those with SBP of 140–184 mm Hg.
Conclusions
In a nationally representative large dataset, elevated blood pressure was observed in over 60% of the patients presenting with stroke to the emergency department. Elevated blood pressure was associated with an earlier evaluation, however, the use of thrombolytics was restricted to ischemic stroke patients with systolic blood pressure <185 mm Hg.
Keywords: Emergency department, Blood pressure, Intracerebral hemorrhage, Ischemic stroke, Prevalence, Stroke, Systolic blood pressure
In 1981, Wallace and Levy [1] reported that blood pressure was elevated in 84% of the 334 consecutive admissions for acute stroke on the day of admission. There was spontaneous reduction of blood pressure (an average of 20 mm Hg systolic and 10 mm Hg diastolic) within ten days following the acute event without any specific antihypertensive therapy, with only one third of the cases remaining hypertensive on the tenth day of hospitalization. Subsequently, several other studies [2–8] have also described elevation of blood pressure in the acute period of stroke. In a systematic review of 18 studies [9], 52% of the patients with stroke had elevated blood pressure at the time of admission. Further studies have evaluated the prognostic significance of the initial elevated blood pressure observed in stroke patients [2–8]. Either lower or higher blood pressure after ischemic stroke and higher blood pressure after intracerebral hemorrhage were found to be associated with poor outcomes [5, 6]. Furthermore, elevated blood pressure among patients with intracerebral hemorrhage may increase the risk of hematoma expansion with subsequent neurological deterioration [10, 11].
Recently, there has been renewed interest in the treatment of elevated blood pressure in acute stroke. Among patients with ischemic stroke, use of intravenous or intra-arterial thrombolysis within 6 hours of symptom onset reduces death and disability at 3 to 6 months [12, 13]. Also, acute elevated blood pressure increases the risk of thrombolytic-related intracranial hemorrhages in ischemic stroke and may require concomitant antihypertensive treatment [14–16]. However, optimal management strategies of elevated blood pressure in acute ischemic stroke patients are unclear.
Prior to initiating further studies examining antihypertensive treatment strategies, it is necessary to define the magnitude of the problem. Most of the existing data addressing this issue are derived either from single center studies or post-hoc analysis of multicenter studies which evaluated novel neuroprotective agents [2–8]. In such studies, the magnitude of the problem could not be evaluated because of variability in patient selection, study design, referral patterns, and the definition of elevated blood pressure. We, therefore, performed the present study to determine the national prevalence of elevated blood pressure in adult patients with stroke using a nationally representative sample of the U.S. population.
METHODS
National Hospital Ambulatory Medical Care Survey (NHAMCS)
We used the data from the NHAMCS. The NHAMCS is designed to collect data on the utilization and provision of ambulatory care services in hospital emergency departments [17–19] using a national probability sample of visits in noninstitutional general and short-stay hospitals in the 50 States and the District of Columbia. A total of 663 hospitals were selected for the NHAMCS sample. Within the emergency department, 100 patient visits were systematically selected over a 4-week reporting period. The hospital staff collected data following an in-service by specially trained interviewers from the U.S. Bureau of the Census.
Data was collected on various aspects of patient visits, including patient, hospital, and visit characteristics. Among the items collected were: patient’s age, gender, race, and ethnicity; patient’s expressed reason for visit; physician’s diagnoses; diagnostic services ordered or provided; procedures provided; medications; providers seen; visit disposition; immediacy with which patient should be seen; and, expected source of payment. Items collected that are specific to the emergency department include: mode of arrival, waiting time; duration of time in the emergency department; initial vital signs; and cause of injury. All data were submitted to and coded centrally by Constella Group, Inc., and subjected to quality control procedures. The error rate was less than 2% and nonresponse rates were 5% or less for NHAMCS data items. National estimates were determined using 1) inflation by reciprocals of the sampling selection probabilities; 2) adjustment for nonresponse; and 3) a population weighting ratio adjustment [10,11].
Identification of patients with stroke in the emergency department
We selected all the emergency department visits for adult patients (aged 20 years or older) with any stroke and stroke subtypes from NHAMC data. We identified the patients with stroke and stroke subtypes using International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) primary diagnosis codes [20]. Diagnostic code fields were screened for specific codes to identify patients with stroke using ICD-9 codes 430, 431, 432, 433,434, 436, and 437 as primary diagnoses; ischemic stroke was identified by 433, 434, 436, 437.0, 437.1; intracerebral hemorrhage by 431, 432, and subarachnoid hemorrhage by 430.
Categorization of initial blood pressure in the emergency department
In the first analysis, we determined the prevalence of high systolic blood pressure (defined by value ≥140 mm Hg), high diastolic blood pressure (defined by value ≥90 mm Hg), and high mean arterial pressure (defined by value ≥107 mm Hg). The systolic and diastolic blood pressure criteria were derived from the definition of hypertension by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) [21]. In the second analysis, we determined the proportion of patients in four elevated blood pressure categories defined by systolic and diastolic blood pressures; 1). < 120/< 80 mm Hg; 2). 120-139/80-89 mm Hg; 3). 140-159/90-99 mm Hg; and, 4). ≥160/≥100 mm Hg. These categories are comparable with pre-hypertension, stage 1 hypertension, and stage 2 hypertension categories defined by JNC 7.
Statistical analyses
We calculated age-, sex-, and ethnicity-adjusted rates for elevated blood pressure categories. To calculate the adjusted rates, NHAMCS provided weights were adjusted to the U.S. census population by dividing into 42 strata (two sex types, three race types and seven age decades) using the previously described method (13): Wnew = Wij x (Ci /ΣjWij) x ΣWij, where Wnew is the weight adjusted for age, sex and race to the U.S. census population; Wij is the original NHAMCS provided weight for each individual j in stratum i; Ci is the proportion of population within each stratum i defined by sex race and age decade; ΣjWij is the summation of NHAMCS -provided weights within each stratum i defined by proportion of population ; and ΣWij is the sum of all original NHAMCS - provided weights. The above method was used to separately calculate Wnew for any strokes, ischemic strokes, and intracerebral hemorrhage.
Since high systolic blood pressure was the most prevalent, further analysis was performed using systolic blood pressure defined strata. Variables pertaining to the emergency department visit were compared across four systolic blood pressure groups: <140mm Hg, hypertension 140–184 mm Hg, severe hypertension 185–219 mm Hg, and ≥220 mm Hg. These groups were chosen to differentiate between patients with no significant elevated blood pressure (<140 mm Hg), elevated blood pressure (140–184 mm Hg), severe elevated blood pressure precluding thrombolytic therapy (185–219 mm Hg), and severe elevated blood pressure requiring emergent treatment (>220 mm Hg) (14). The variables compared were age, gender, ethnicity, mode of arrival, time interval between presentation and physician contact, type of primary provider, stroke subtype, medication used including thrombolytics and antihypertensive medication, and disposition following emergency department evaluation. The prevalence of systolic blood pressure strata was also presented for each stroke subtype.
All values are presented as frequencies with percentages [for categorical values] and means with standard deviation [for continuous values]. We used the Cochran-Mentel-Haenszel test and analysis of variance for categorical and continuous data, respectively, using SUDAAN software (Release 9.0.1, Research Triangle Institute, Research Triangle Park, NC). Bonferroni correction procedure was used to adjustment for multiple comparisons.
RESULTS
Of the 563,704 adult patients evaluated with stroke, systolic blood pressure ≥140 mm Hg was observed in 63%, diastolic blood pressure ≥90 mm Hg in 28%, and mean arterial pressure ≥107 mm Hg in 38% of the patients. The proportion of patients with systolic blood pressure ≥140 mm Hg according to stroke subtypes was as follows: ischemic stroke (67%), intracerebral hemorrhage (75%), and subarachnoid hemorrhage (100%) (see Table 1). The proportion of patients with diastolic blood pressure ≥90 mm Hg or mean arterial pressure≥107 mm Hg according to stroke subtypes is presented in Table 1. The age-, sex-, and ethnicity-adjusted rates for elevated blood pressure categories defined by both systolic and diastolic blood pressure are also presented in Table 1.
Table 1.
Prevalence of various categories of elevated blood pressure based on initial measurement among adult patients with stroke (National Hospital Ambulatory Medical Care Survey 2003).
| Elevated blood pressure strata | All Strokes (n=563704) | Ischemic stroke (n=276734) | Intracerebral hemorrhage (n=45330) | Subarachnoid hemorrhage (n=4245) |
|---|---|---|---|---|
| SBP ≥140 mmHg | 390584 (69.3%) | 211713 (76.5%) | 33992 (75.0%) | 4245 (100.0%) |
| DBP ≥90 mmHg | 172186 (30.5%) | 89315 (32.3%) | 10707 (23.6%) | 1756 (41.4%) |
| MAP ≥107 mmHg | 235843 (41.8%) | 125227 (45.3%) | 14938 (33.6%) | 4245 (100.0%) |
| SBP<120 and DBP<80 mm Hg | 43959 (7.8%) | 14313 (5.2%) | 3662 (8.1%) | 0 (0.0%) |
| SBP 120–139mm Hg/DBP 80–89 mm Hg | 107807 (19.1%) | 47988 (17.3%) | 5960 (13.1%) | 0 (0.0%) |
| SBP 140–159mm Hg/DBP 90–99 mm Hg | 183152 (32.5%) | 83559 (30.2%) | 20699 (45.7%) | 0 (0.0%) |
| SBP≥160 mm Hg/ DBP≥100 mm Hg | 228786 (40.6%) | 130874(47.3%) | 15009 (33.1%) | 4245 (100%) |
| Age-, sex-, and race/ethnicity adjusted rates† | ||||
| SBP<120 and DBP<80 mm Hg | 39766 (7.1%) | 24963 (9.0%) | 3278 (7.2%) | Not estimated* |
| SBP 120–139mm Hg/DBP 80–89 mm Hg | 147286 (26.1%) | 16207 (5.9%) | 14460 (31.9%) | Not estimated* |
| SBP 140–159mm Hg/DBP 90–99 mm Hg | 199559 (35.4%) | 104305 (37.7%) | 15243 (33.6%) | Not estimated* |
| SBP≥160 mm Hg/ DBP≥100 mm Hg | 177099 (31.4%) | 131190 (47.4%) | 12349 (27.2%) | Not estimated* |
Abbreviations used: SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Symbols used: Adjusted to United States population;
Not estimated due to small sample size.
An analysis of rates of categories defined by initial systolic blood pressure was as follows: <140 mm Hg (n=173,120, 31%), 140–184 mm Hg (n=315,207, 56%), 185–219 mm Hg (n=74,586, 13%), and ≥220 mm Hg (n=791, 0.1%). Figure 1 demonstrates the distribution of the different strata defined by systolic blood pressure according to all strokes and subtypes of stroke. There was no significant relationship between age strata and systolic blood pressure strata among patients with stroke (see Table 2). The mean time interval between presentation and evaluation was 40 ± 55, 33 ± 39, 25 ± 27, and 5 ± 1 minutes for increasing systolic blood pressure strata (p=0.009). Labetalol and hydralazine were used in 6,126 (1%) and 2,262 (0.4%) patients, respectively. None of the patients received intravenous nicardipine, nitroprusside, enalaprate, nitroglycerin, or nitrates. Among patients with ischemic stroke, thrombolytics were used in 1,283 patients (0.4%) with systolic blood pressure between 140–184 mm Hg and not used in any of the patients in the higher systolic blood pressure strata.
Figure 1.
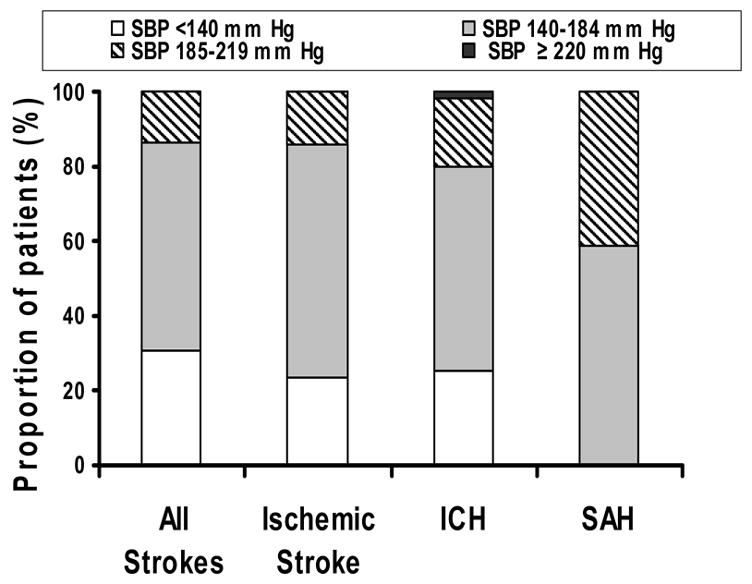
Strata of systolic blood pressure according to stroke and stroke subtypes (National Hospital Ambulatory Medical Care Survey 2003). Abbreviation used: SBP, systolic blood pressure; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage.
Table 2.
Demographic and clinical characteristics according to strata defined by initial systolic blood pressure among adult stroke patients (National Hospital Ambulatory Medical Care Survey 2003).
| < 140 mm Hg (n=173120) | 140–184 mm Hg (n=315207) | 185–219 mm Hg (n=74586) | ≥220 mm Hg | |
|---|---|---|---|---|
| Mean age (±SD) | 67.6 ± 18.2 | 69.7 ± 13.1 | 68.6 ± 10.0 | 68.9 ± 0.5 |
|
Age group
20 – 29 years 30 – 39 years 40 – 49 years 50 – 59 years 60 – 69 years 70 – 79 years ≥80 years |
4078 (2.4%) 1600 (0.9%) 29949 (17.3%) 22343 (12.9%) 23243 (13.4%) 36891 (21.3%) 55016 (31.8%) |
0 (0.0%) 4578 (1.5%) 17097 (5.4%) 55744 (17.7%) 58155 (18.4%) 113210 (35.9%) 66423 (21.1%) |
0 (0.0%) 0 (0.0%) 5237 (7.0%) 6323 (8.5%) 26048 (34.9%) 28205 (37.8%) 8773 (11.8%) |
0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 791 (100.0%) 0 (0.0%) 0 (0.0%) |
|
Sex
Men Women |
59618 (34.4%) 113502 (65.6%) |
128847 (40.9%) 186360 (59.1%) |
30814 (41.3%) 43772 (58.7%) |
26 (3.3%) 765 (96.7%) |
|
Race/ethnicity
White Blacks Other |
128018 (73.9%) 35789 (20.7%) 9313 (5.4%) |
277421 (88.0%) 30697 (9.7%) 7089 (2.2%) |
67514 (90.5%) 0 (0.0%) 7072 (9.5%) |
26 (3.3%) 765 (96.7%) 0 (0.0%) |
| Waiting time to see physician (minutes) | 40.1 ± 55.0 | 33.5 ± 38.7 | 25.0 ± 27.2 | 5.2 ± 0.9* |
|
Strata of time interval between arrival and physician evaluation:
Less than 15 minutes 15–60 minutes >1 hour or unknown/no triage |
86719 (50.1%) 64545 (37.3%) 21856 (12.6%) |
135026 (42.8%) 67502 (21.4%) 112679 (35.7%) |
34605 (46.4%) 24610 (33.0%) 15371 (20.6%) |
765 (96.7%) 26 (3.3%) 0 (0.0%) |
|
Mode of arrival
Ambulance Walk-in Unknown |
94108 (54.4%) 76493 (44.2%) 2519 (1.5%) |
178566 (56.7%) 126723 (40.2%) 9918 (3.1%) |
29976 (40.2%) 38852 (52.1%) 5758 (7.7%) |
765 (96.7%) 26 (3.3%) 0 (0.0%) |
| Length of visit (hours) | 40.3 ± 65.9 | 44.1 ± 69.3 | 50.8 ± 73.2 | 7.5 ± 29.4* |
|
Disposition
Admit to Hospital Admit to ICU Died in ED Refer to other physician/clinic for follow-up Left against medical advice Admit for 23 hour observation Transfer to other hospital |
121379 (70.1%) 7651 (4.4%) 3662 (2.1%) 34124 (19.7%) 3584 (2.1%) 0 (0.0%) 779 (0.4%) |
213205 (67.6%) 17399 (5.5%) 0 (0.0%) 44847 (14.2%) 0 (0.0%) 11052 (3.5%) 26984 (8.3%) |
54591 (73.2%) 0 (0.0%) 0 (0.0%) 13233 (17.7%) 0 (0.0%) 0 (0.0%) 326 (0.4%) |
791 (100.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
|
Treatment
Thrombolytic therapy Labetalol Hydralazine Nicaradipine/nitroprusside/enalapril/nitroglycerin/nitrates |
0 (0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) |
1283 (0.4%) 0 (0.0%) 2262 (0.7%) 0 (0.0%) |
0 (0.0%) 5361 (7.2%) 0 (0.0%) 0 (0.0%) |
0 (0.0%) 765 (96.7%) 0 (0.0%) 0 (0.0%) |
Abbreviations used: SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; ICU, intensive care unit; ED, emergency department; LPN, licensed practice nurse; and EMT, emergency medical technician.
Symbols used: significant difference derived from comparison between values from SBP (< 140 mm Hg) from SBP (140–184 mm Hg), SBP (185–219 mm Hg) and SBP (≥220 mm Hg) after adjustment for multiple comparisons.
DISCUSSION
The present study, which is one of the largest to date, demonstrates that acute elevated blood pressure was observed in over 60% of the patients presenting with stroke to the emergency department. The results are derived from settings that are representative of the nationwide admissions. Therefore, the study provides more meaningful data compared with single center studies or post-hoc analysis of randomized trials. We found that high systolic blood pressure was most prevalent in the acute period. Admission systolic blood pressure in patients with stroke has been linked to adverse outcomes, including poor clinical outcomes [2], hematoma expansion [10], and cardiovascular stress [22]. The relationship with high mean arterial pressure or diastolic blood pressure is less consistently described. We found that the age-, sex-, and ethnicity adjusted rates of elevated blood pressure categories comparable with pre-hypertension, stage 1 and stage 2 hypertension were 19%, 31%, and 30% among patients with acute stroke. These rates were several-fold higher than age-, sex-, and ethnicity adjusted rates observed for the U.S. population in 1999–2000 [prehypertension 37%, stage 1 hypertension 12%, and stage 2 hypertension 4%][23]. While a direct comparison is not possible because elevated blood pressure is not synonymous with hypertension, the indirect comparison provides some estimate of expected blood pressure ranges in general population. The recognition of elevated blood pressure led to early evaluation of the patient in the emergency department.
It has been proposed that cerebral ischemia invokes a protective response by increasing systemic blood pressure to improve cerebral perfusion [1]. Increase in systemic blood pressure has also been described in patients with increased intracranial pressure, particularly in the presence of brainstem compression [24, 25]. This pathophysiologic process has particular relevance for elevated blood pressure observed in association with intracerebral and subarachnoid hemorrhages. The mechanisms that cause elevated blood pressure in the acute period of stroke are not clearly understood. A high prevalence of chronic hypertension is observed among patients with stroke. It is, therefore, reasonable to assume that in at least a proportion of these patients, the elevated blood pressure is merely a reflection of inadequately treated or undetected chronic hypertension [26]. However, spontaneous reduction in the initial blood pressure over the next few days described previously in most patients is inconsistent with chronic hypertension [1–4]. Other mechanisms such as stress response in acute stroke leading to abnormal sympathetic activity, altered parasympathetic activity, raised levels of circulating catecholamines (18) and brain natriuretic peptide (19), have been suggested to contribute to the acute rise in blood pressure. Thus, the observed elevated blood pressure in acute stroke probably has multifactorial etiology, and therefore requires further studies.
A high prevalence of elevated blood pressure appears to be associated with all stroke subtypes (see Figure 1). However, differences in underlying pathophysiology among stroke subtypes mandates different management strategies. Ischemic stroke results from occlusion of an artery with subsequent reduction in regional cerebral blood flow (rCBF) within the affected region [27]. However, the reduction in rCBF is not homogenous but is demarcated into regions of severe reduction (core) and moderate reduction (penumbra). The penumbra remains viable for hours sustained through collateral supply, however, worsening of ischemic injury in the penumbra region with systemic blood pressure reduction is possible. In practice, neurological deterioration and worse outcome associated with blood pressure reduction has been demonstrated in a subset of patients with ischemic stroke [28]. Based on theoretical and clinical considerations, aggressive blood pressure reduction is not recommended in patients with ischemic stroke in the acute phase [29]. This may have been the possible reason for less aggressive treatment of elevated blood pressure in the current study. However, it should be noted that a lower systemic blood pressure is desirable whenever thrombolytics are considered in order to reduce the risk of post-thrombolytic intracranial hemorrhage [29].
The management of elevated blood pressure in acute intracerebral hemorrhage is based on two conflicting pathophysiologic processes. There is potential benefit of reducing rates of hematoma expansion with systemic blood pressure reduction versus the potential provocation of ischemia in the perihematoma region [24, 30, 31]. Recent data from pre-clinical and clinical studies have supported the relative safety of blood pressure reduction in acute intracerebral hemorrhage, although the benefit remains unclear [32, 33]. Patients with subarachnoid hemorrhage remain at risk of second rupture acutely following an initial rupture of intracranial aneurysm [34]. The benefit of reduction of blood pressure has been linked to reduction of hemodynamic stress on aneurysm wall and prevention of second rupture. There has been only indirect evidence available to support this concept [35].
Some issues need to be considered prior to interpretation of our results. We used the data from the NHAMCS, a large size, dataset with standardized design to provide a representative estimate of the total U.S. experience [18, 19]. Since the study is based on cross sectional survey, the data can only be used to determine the prevalence rather than incidence of diagnoses. Furthermore, the dataset provides minimal clinical details on time interval between symptom onset and evaluation, severity of neurological deficits, and diagnostic study results. Thus, there is an undefined variation in patients’ time interval between symptom onset and initial blood pressure recording. The actual prevalence of elevated blood pressure may have been higher if all blood pressure recordings were measured consistently early after symptom onset. We used primary ICD-9-CM codes to identify patients with stroke and stroke subtypes admitted to the hospital. A previous study [36] reported that if both primary and secondary ICD-9-CM codes were used, 97% of all strokes and transient ischemic attacks (TIA) could be detected but the true positive rate for stroke was 72%. Using only primary discharge diagnoses of ICD-9-CM codes, the true positive rate increased to 83%, but decreases the yield of detection of stroke and TIA to 84%. Therefore, we may have underestimated the total of number of stroke evaluations. Further underestimation may have occurred due to multiple emergency department visits by the same patient, as patients are identified by visits rather than as individuals in the NHAMCS dataset. Although the prevalence of elevated blood pressure in patients with acute stroke was determined, it was difficult to define whether or not hypertension was acute or chronic because of unavailability of supporting data. Despite these concerns, the objective of the study, however, was to determine the prevalence of elevated blood pressure associated with stroke admissions, using a methodology that has a high specificity.
In summary, we provide estimates of elevated blood pressure observed among patients with stroke evaluated in the emergency department. Elevated blood pressure is highly prevalent across different stroke subtypes. These data provide further support for planning clinical studies that address the management of elevated blood pressure and clinical outcomes in patients with acute stroke [37–39].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wallace JD, Levy LL. Blood pressure after stroke. JAMA. 1981;246(19):2177–2180. [PubMed] [Google Scholar]
- 2.Okumura K, Ohya Y, Maehara A, et al. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens. 2005;23(6):1217–1223. doi: 10.1097/01.hjh.0000170385.76826.4a. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Garcia JL, Botia E, de La Sierra A, et al. Significance of elevated blood pressure and its management on the short-term outcome of patients with acute ischemic stroke. Am J Hypertens. 2005;18(3):379–384. doi: 10.1016/j.amjhyper.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Vemmos KN, Tsivgoulis G, Spengos K, et al. Blood pressure course in acute ischaemic stroke in relation to stroke subtype. Blood Press Monit. 2004;9(3):107–114. doi: 10.1097/01.mbp.0000132424.48133.27. [DOI] [PubMed] [Google Scholar]
- 5.Vemmos KN, Tsivgoulis G, Spengos K, et al. U-shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004;255(2):257–265. doi: 10.1046/j.1365-2796.2003.01291.x. [DOI] [PubMed] [Google Scholar]
- 6.Castillo J, Leira R, Garcia MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520–526. doi: 10.1161/01.STR.0000109769.22917.B0. [DOI] [PubMed] [Google Scholar]
- 7.Aslanyan S, Fazekas F, Weir CJ, et al. Effect of blood pressure during the acute period of ischemic stroke on stroke outcome: a tertiary analysis of the GAIN International Trial. Stroke. 2003;34(10):2420–2425. doi: 10.1161/01.STR.0000091233.04524.0C. [DOI] [PubMed] [Google Scholar]
- 8.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33(5):1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 9.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43(1):18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 10.Kazui S, Minematsu K, Yamamoto H, et al. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28(12):2370–2375. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 11.Ohwaki K, Yano E, Nagashima H, et al. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004;35(6):1364–1367. doi: 10.1161/01.STR.0000128795.38283.4b. [DOI] [PubMed] [Google Scholar]
- 12.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 13.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial Prolyse in Acute Cerebral Thromboembolism. Jama. 1999;282(21):2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bowes MP, Zivin JA, Thomas GR, et al. Acute hypertension, but not thrombolysis, increases the incidence and severity of hemorrhagic transformation following experimental stroke in rabbits. Exp Neurol. 1996;141(1):40–46. doi: 10.1006/exnr.1996.0137. [DOI] [PubMed] [Google Scholar]
- 15.Huynh T, Cox JL, Massel D, et al. Predictors of intracranial hemorrhage with fibrinolytic therapy in unselected community patients: a report from the FASTRAK II project. Am Heart J. 2004;148(1):86–91. doi: 10.1016/j.ahj.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Aylward PE, Wilcox RG, Horgan JH, et al. Relation of increased arterial blood pressure to mortality and stroke in the context of contemporary thrombolytic therapy for acute myocardial infarction. A randomized trial GUSTO-I Investigators. Ann Intern Med. 1996;125(11):891–900. doi: 10.7326/0003-4819-125-11-199612010-00004. [DOI] [PubMed] [Google Scholar]
- 17.McCaig LF, Burt CW. Advance data from vital and health statistics. National Center for Health Statistics; 2004. National Hospital Ambulatory Medical Care Survey: 2002 Emergency Department Summary. [PubMed] [Google Scholar]
- 18.Selim AJ, Berlowitz DR, Fincke G, et al. Risk-adjusted mortality rates as a potential outcome indicator for outpatient quality assessments. Med Care. 2002;40(3):237–245. doi: 10.1097/00005650-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions: insights into preventable hospitalizations. Med Care. 2003;41(2):198–207. doi: 10.1097/01.MLR.0000045021.70297.9F. [DOI] [PubMed] [Google Scholar]
- 20.International Classification of Diseases. Clinical Modification. 9. Washington, DC: National Center for Health Statistics, US Public Health Service; 1980. [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 22.Hirashima Y, Takashima S, Matsumura N, et al. Right sylvian fissure subarachnoid hemorrhage has electrocardiographic consequences. Stroke. 2001;32(10):2278–2281. doi: 10.1161/hs1001.096620. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi AI, Suri MF, Kirmani JF, Divani AA. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit. 2005;11(9):CR403–409. [PubMed] [Google Scholar]
- 24.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344(19):1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi AI, Geocadin RG, Suarez JI, Ulatowski JA. Long-term outcome after medical reversal of transtentorial herniation in patients with supratentorial mass lesions. Crit Care Med. 2000;28(5):1556–1564. doi: 10.1097/00003246-200005000-00049. [DOI] [PubMed] [Google Scholar]
- 26.Arboix A, Roig H, Rossich R, et al. Differences between hypertensive and non-hypertensive ischemic stroke. Eur J Neurol. 2004;11(10):687–692. doi: 10.1111/j.1468-1331.2004.00910.x. [DOI] [PubMed] [Google Scholar]
- 27.Janardhan V, Qureshi AI. Mechanisms of ischemic brain injury. Curr Cardiol Rep. 2004;6(2):117–123. doi: 10.1007/s11886-004-0009-8. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed N, Nasman P, Wahlgren NG. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke. 2000;31(6):1250–1255. doi: 10.1161/01.str.31.6.1250. [DOI] [PubMed] [Google Scholar]
- 29.Adams HP, Jr, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34(4):1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. Pharmacologic reduction of mean arterial pressure does not adversely affect regional cerebral blood flow and intracranial pressure in experimental intracerebral hemorrhage. Crit Care Med. 1999;27(5):965–971. doi: 10.1097/00003246-199905000-00036. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi AI, Bliwise DL, Bliwise NG, et al. Rate of 24-hour blood pressure decline and mortality after spontaneous intracerebral hemorrhage: a retrospective analysis with a random effects regression model. Crit Care Med. 1999;27(3):480–485. doi: 10.1097/00003246-199903000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Powers WJ, Zazulia AR, Videen TO, et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57(1):18–24. doi: 10.1212/wnl.57.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi AI, Mohammad YM, Yahia AM, et al. A prospective multicenter study to evaluate the feasibility and safety of aggressive antihypertensive treatment in patients with acute intracrebral hemorrhage. J Intensive care Medicine. 2005;20(1):34–42. doi: 10.1177/0885066604271619. [DOI] [PubMed] [Google Scholar]
- 34.Hillman J, von Essen C, Leszniewski W, Johansson I. Significance of “ultra-early” rebleeding in subarachnoid hemorrhage. J Neurosurg. 1988;68(6):901–907. doi: 10.3171/jns.1988.68.6.0901. [DOI] [PubMed] [Google Scholar]
- 35.Wijdicks EF, Vermeulen M, Murray GD, et al. The effects of treating hypertension following aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 1990;92(2):111–117. doi: 10.1016/0303-8467(90)90085-j. [DOI] [PubMed] [Google Scholar]
- 36.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29(2):415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 37.Potter J, Robinson T, Ford G, et al. CHHIPS (Controlling Hypertension and Hypotension Immediately Post-Stroke) Pilot Trial: rationale and design. J Hypertens. 2005;23(3):649–655. doi: 10.1097/01.hjh.0000160224.94220.e7. [DOI] [PubMed] [Google Scholar]
- 38.Qureshi AI. Antihypertensive treatment of Acute Cerebral Hemorrhage; The 30th International Stroke Conference; New Orleans. 2005. [Google Scholar]
- 39.Kirmani JF. Hypertension in Acute Stroke Treatment; The 30th International Stroke Conference; New Orleans. 2005. [Google Scholar]


