Abstract
1. Context
Vigilance to threat is a key feature of generalized anxiety disorder (GAD). The amygdala and ventrolateral prefrontal cortex constitute a neural circuit that is responsible for detection of threats. Disturbed interactions between these structures may underlie pediatric anxiety. To date, no study has selectively examined responses to briefly-presented threats (e.g. less than 50 msec) in GAD or in pediatric anxiety.
2. Objective
To investigate amygdala and ventrolateral prefrontal cortex activation during processing of briefly-presented threats in pediatric GAD.
3. Design
Case-control study.
4. Setting
Government clinical research institute.
5. Participants
Youth volunteers, 17 with GAD and 12 diagnosis-free.
6. Main Outcome Measures
We used functional magnetic resonance imaging to measure blood oxygenation level-dependent signal. During imaging, subjects performed an attention orienting task with rapidly presented (17 msec), masked emotional (angry or happy) and neutral faces.
7. Results
When viewing masked angry faces, GAD youth, relative to comparison subjects, showed greater right amygdala activation that positively correlated with anxiety disorder severity. Moreover, in a functional connectivity (psychophysiological interaction) analysis, right amygdala and right ventrolateral prefrontal cortex showed strong negative coupling specifically to masked angry faces. This negative coupling tended to be weaker in GAD youth than in comparisons.
8. Conclusions
GAD youth have hyper-activation of the amygdala to briefly-presented, masked threats. The presence of threat-related negative connectivity between the right ventrolateral prefrontal cortex and amygdala suggests that the prefrontal cortex modulates amygdala response to threat. In pediatric GAD, hyper-amygdala response occurs in the absence of a compensatory increase in modulation by ventrolateral prefrontal cortex.
Introduction
Vigilance for threat represents a prominent feature of generalized anxiety disorder (GAD)1–4. Neuroimaging research implicates a neural circuit encompassing the amygdala and ventrolateral prefrontal cortex in vigilance for threat4–7. Within this circuit, the amygdala is thought to support vigilance through immediate threat-processing8,9, whereas the ventrolateral prefrontal cortex facilitates later processes related to emotion regulation5,10. Disturbed amygdala-ventrolateral prefrontal cortex interactions are thought to influence anxiety10.
Developmental work in this area is important, since most adult anxiety disorders arise in adolescence. Adolescent GAD shows particularly strong ties to adult anxiety11. Studies in animal models suggest that early-life amygdala-ventral prefrontal cortex circuit dysfunction lays a foundation for persistent anxiety12,13. Translational work has begun to extend these findings to humans through brain imaging. Such studies consistently find that adults with various anxiety disorders exhibit altered activation in the amygdala and prefrontal cortex14–19, with positive correlations between amygdala activation and anxiety severity15.
Of note, these studies typically present threats under prolonged-viewing conditions where the nature of the threat can be readily discerned. Prior research implicates the amygdala and associated circuitry specifically in processing rapidly presented threats5,7. Thus, studies using brief rather than prolonged-viewing conditions of threat may better clarify the nature of amygdala-ventrolateral prefrontal cortex interactions in both adult and pediatric anxiety. Behavioral studies used spatial-orienting paradigms with briefly presented (e.g. 17 msec) threat and non-threat cues, to reveal anxiety-related attention biases27. Such paradigms might be used in the context of brain imaging research to engage regions involved in evaluating threat, under conditions that afford limited opportunities for elaborative processing.
Consistent with data implicating the amygdala in processing of briefly-presented threats, neuroimaging studies demonstrate amygdala engagement to masked threats,5,7,20 particularly among adults with elevated trait anxiety21. The only published studies using masked threatening stimuli in anxiety disorders 22,23 found heightened right amygdala activation to masked fear faces in adult post-traumatic stress disorder (PTSD). It remains unclear if these findings apply to other anxiety disorders or to youth.
We recently examined neural responses in pediatric GAD to 500 msec threat cues (angry faces)4. GAD youth exhibited greater right ventrolateral prefrontal cortex activation than healthy peers, with no between-group differences in the amygdala. Interestingly, ventrolateral prefrontal cortex activation appeared greater in GAD youth with mild relative to severe anxiety, consistent with studies implicating the ventrolateral prefrontal cortex in emotion regulation through effects on the amygdala15,24–26. However, as with most prior reports, this study involved events containing relatively prolonged presentation of threats. Events with briefly-presented, masked threats may reveal between-group differences in the amygdala and associated brain regions that are engaged by events affording limited opportunities for elaborative, strategic, or regulatory processing. No prior imaging study in healthy or anxious youth has examined neural responses to such events.
The present study uses an orienting task in pediatric GAD to monitor attention bias for rapidly presented, masked emotional (angry or happy) facial displays. Angry faces were chosen as stimuli for two reasons. First, behavioral findings in adults studied with this exact task show that anxious relative to non-anxious individuals exhibit an attention bias towards masked angry faces27. These behavioral data are consistent with other findings on related tasks that demonstrate the capacity of angry faces to disrupt attention in pediatric anxiety disorders4,28–30 and elicit attention biases in anxious adults31. Second, our previous fMRI study with GAD youth also used angry faces4. Thus, to most effectively build on this previous behavioral and imaging work, we used angry faces. Of note, as in our previous study4, we included happy faces as a comparison condition to determine whether the effects were selective to the negative emotion (anger).
The current study uses the orienting task with angry and happy faces to test two hypotheses. First, as in prior studies using rapidly-presented threats22,23, we hypothesized that youth with GAD show increased right amygdala activation relative to healthy youth in response to briefly-presented masked angry faces. Second, prior research with healthy adults shows that right ventrolateral prefrontal cortex activation inversely relates to right amygdala activation in response to masked angry faces5. Therefore, we hypothesized that the right amygdala shows negative connectivity with the right ventrolateral prefrontal cortex, particularly among healthy youth, in response to threat.
Methods
Participants
The study sample comprised 29 children and adolescents (Table 1 provides details). The NIMH Institutional Review Board approved the procedures. Parents signed the consent form and youths signed assents. All participants were evaluated with a physical examination and IQ measure. The Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) was administered to participants by trained clinicians32. Two patients and one comparison subject were left handed; all other subjects were right handed.
Table 1.
Demographics of the comparison group and patients with Generalized Anxiety Disorder.
| Variable | Comparison Group | Patient Group | Statistical Comparison |
|---|---|---|---|
| Sample Size | 12 | 17 | |
| Gender | 6 females; 6 males | 6 females; 11 males | X2(1) = 0.63 p > 0.2 |
| Age | 14.33 (1.67) | 13.12 (2.09) | t(27) = 1.67 p > 0.1 |
| IQ | 110.92 (14.24) | 105.06 (14.34) | t(27) = 1.09 p > 0.2 |
Inclusion and exclusion criteria followed our prior study4. Seventeen participants met criteria for GAD based on five requirements: 1) criteria for GAD were met based on the K-SADS; 2) GAD was the primary focus of treatment; 3) clinically significant symptoms were present (Pediatric Anxiety Rating Scale 33 score ≥ 9; Children’s Global Assessment Scale score > 60); 4) families desired treatment; 5) anxiety as measured by the Pediatric Anxiety Rating Scale persisted during a 3-week period when patients received supportive psychoeducational therapy. As in prior biological and therapeutic studies of pediatric anxiety4,34,35, provision of supportive psychoeducational therapy was provided to eliminate patients whose GAD symptoms were either transient or responsive to non-specific, supportive intervention. Stability of symptoms over the 3-week period was confirmed by the patient’s clinician immediately prior to MRI scanning.
There were 12 healthy comparison participants who were free of current and past psychiatric disorders based on the K-SADS. Controls were matched with patients on age, sex, and IQ.
Exclusion criteria followed previous work4. Specifically, we excluded for current Tourette’s syndrome, obsessive-compulsive disorder, conduct disorder, PTSD, exposure to severe trauma, suicidal ideation, lifetime history of mania, psychosis, pervasive developmental disorder, and IQ<70. We also excluded for current use of any psychoactive substance (for GAD, use of any such substance since the onset of the condition). As in our earlier study, subjects with comorbid GAD and major depressive disorder (MDD) were included, as MDD did not influence prior findings in GAD4. This decision was initially based on the fact that family-based and longitudinal investigations documented strong relationships between GAD in youth and MDD. In the current study, this decision reflected the desire to compare our current and prior findings. To evaluate the effects of MDD as well as social phobia on our results, we conducted secondary fMRI analyses between patients with and without comorbidity.
The task
The task closely followed established procedures27. Trials started with a 500-msec fixation point in the center of the screen (Figure 1). Two pictures of one actor’s face then appeared simultaneously for 17 msec. In some trials, one picture showed a neutral expression and the other an emotional expression; in other trials, both pictures showed the actor with a neutral expression. Immediately after this brief presentation, two scrambled faces (the mask) appeared for 68 msec in the same locations as the two faces. The mask was replaced by an asterisk in one hemi-field for 1100 msec. Subjects were instructed to press one button with their thumb when the asterisk appeared on the left and press another button with their index finger when the asterisk appeared on the right. The duration of the intertrial interval was 2300 msec. Previous studies using these parameters show that subjects report minimal awareness of details of the briefly presented face stimuli. Each participant was trained to perform the task before entering the MRI. These procedures are similar to our previous work4. The key difference is that the faces were presented for 17 msec and they were masked for this study, whereas in the previous study unmasked faces were presented for 500 msec. Eighty actors were each presented twice to participants, for a total of 160 trials. Forty blank trials were included to facilitate fMRI analysis.
Figure 1.
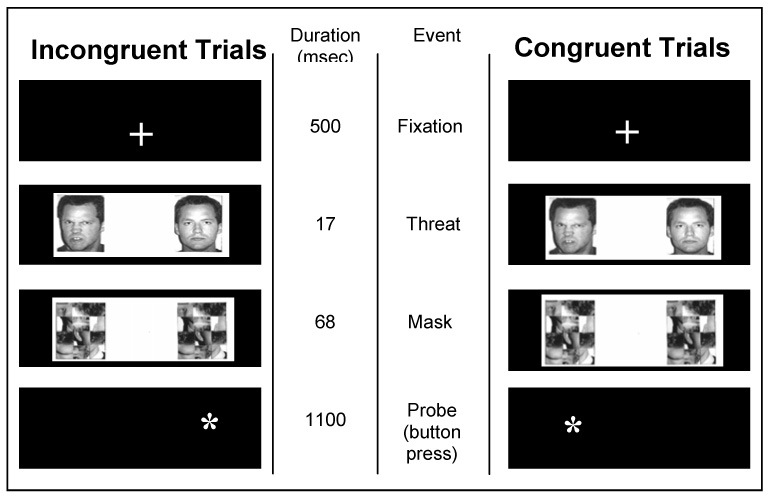
The two main trial types used to assess attention bias for masked angry faces. The columns on the far left and far right show, from top to bottom, the screens that appear in two types of trials. The same model always displays the two expressions in a given trial. The middle two columns display the duration of each event and the event name for both trial types. In the sample trial on the left, the angry face and probe are displayed on different sides of the screen (incongruent). In the sample trial on the right, the angry face and probe are on the same side (congruent). Happy/neutral and neutral/neutral trials (not shown) were also presented to subjects.
There were five trial types, including two primary conditions of interest for the behavioral measure of attention bias: congruent trials wherein a masked angry/neutral face pair was followed by an asterisk on the same side of the screen as the angry face; and incongruent trials, wherein a masked angry/neutral face pair was followed by an asterisk on the opposite side from the angry face. Also, other control conditions were included: masked happy/neutral face trials (congruent and incongruent) and masked neutral/neutral face pairs. There were 32 trials for each of the 5 conditions. For each participant, order of trial presentation was randomly determined. Emotional faces and asterisks were displayed an equal number of times on each hemi-field.
Behavioral data analysis
The same criteria for determining acceptability of trials were applied to behavioral and fMRI data. Specifically, trials with incorrect responses and responses with reaction times <200 and>1000 msec were excluded. The behavioral measure of attention bias for masked angry faces was calculated for each subject by subtracting the mean reaction time on congruent trials (asterisk in same position as the masked angry face) from the mean reaction time on incongruent trials (asterisk in different position from masked angry face). Positive values indicate an attention bias towards the spatial location of masked threat. Bias scores were similarly calculated for masked happy faces.
fMRI analysis
Images were acquired from a GE 3T scanner with 29 contiguous 3.3-mm axial slices using echo-planar, single-shot gradient echo T2 weighting (TR=2300 msec; TE=23 msec; field of view=240mm; 64×64 matrix; 3.3×3.75×3.75 mm voxel). Slices were parallel to the anterior commissure/posterior commissure line. Ramp sampling was used to correct possible distortion. For the T1-weighted volumetric scans, we used a magnetization prepared gradient echo (MP-RAGE) sequence: 180 1.0-mm axial slices; field of view = 256 mm; number of excitations=1; TR=11.4 msec; TE=4.4 msec; matrix = 256×256; TI=300 msec; bandwidth 130 Hz/pixel=33 kHz for 256 pixels; in-plane resolution=1 mm3.
We used Analysis of Functional Neuroimages (AFNI) software version 2.56b 36. Subjects were removed from the analysis if they moved more than 2.5 mm in any direction. For movement that was 2.5 mm or less, effects were reduced by registering images to one volume in each run. Participant data were smoothed with a 6-mm full width at half maximum isotropic Gaussian filter. Trials with incorrect behavioral responses or responses that were <200 msec or >1000 msec were removed from the fMRI analysis. Patients had 8.6% (SD=8.1) and comparisons had 6.0% (SD=4.8) of trials removed. Groups did not differ in the number of incorrect trials.
Using a two-level procedure, a random effects fMRI data analysis was conducted. At the subject level, we submitted each subject’s data separately to a multiple regression analysis using the 3dDeconvolve module from AFNI. Vectors were created for each of the five masked conditions (angry/neutral congruent, angry/neutral incongruent, happy/neutral congruent, happy/neutral incongruent, and neutral/neutral) with the onset time of each trial for each condition. Blank trials were modeled as an implicit baseline. An additional vector modeled nuisance trials, i.e., trials that contained incorrect responses, responses that were too fast or slow, and null responses. Vectors were transformed into waveforms using a gamma variate37 and coefficients were created for each subject and condition. Contrast values were derived from comparisons of coefficients for specific conditions.
For the second level of analysis, individual data sets were converted to Talairach space and group-level analyses were performed using AFNI’s 3dttest comparing GAD and comparison subjects. The principal effect of interest was the amygdala response to masked angry faces. The masked neutral/neutral face pairs were the comparison for examining group differences in activation to masked angry faces. Thus, the main hypothesis concerned group differences in the contrast of masked angry/neutral vs. neutral/neutral face pairs. The only difference between these trial types was the presence of a 17 msec angry face in the angry/neutral trials. We also examined responses to happy/neutral pairs relative to neutral/neutral pairs. To evaluate the fMRI data, we used AlphaSim from AFNI with 1000 Monte Carlo simulations4,38 to control for multiple comparisons within the amygdala.
Connectivity analyses
We performed two connectivity analytic procedures. First, because we were primarily interested in group differences in brain interactions in response to threat, we implemented a psychophysiological interaction analysis to examine connectivity between the right amygdala and ventrolateral prefrontal cortex during angry relative to neutral trials. To accomplish this, we adapted established procedures39,40 for use with AFNI. We deconvolved the BOLD signal with an assumed form of hemodynamic response function before the interaction term was created40. Each participant’s EPI time series was placed in Talairach space. The first eigenvariate time series from the amygdala cluster (derived from the main contrast of masked angry/neutral pair vs. neutral/neutral pair) was the “seed”. To selectively examine activation related to the conditions of interest, we entered the masked angry/neutral pair vs. neutral/neutral pair conditions as covariates. The results of this procedure show condition-related changes in the interaction of the right amygdala cluster and ventrolateral prefrontal cortex. The threshold for the VLPFC activation was set to p<.005 based on similar paradigms with rapidly presented emotional faces5.
Second, to be consistent with previous work34, we performed a standard connectivity analysis to examine interactions across all trials41,42. The first eigenvariate time series from the amygdala cluster as above was the “seed” and the time series within it was extracted. For each subject, we performed a voxel-wise correlation analysis between each individual voxel’s time series and the seed’s time series. The threshold was p<.005.
The psychophysiological interaction connectivity analysis selectively focuses on threat-related conditions and the standard connectivity analyses examines interactions across the entire task. Therefore, it is to be expected that the approaches would yield different results. The focus of this paper is on group differences on the brain response to threat and, therefore, psychophysiological interaction analysis is particularly important. Nevertheless, the connectivity analysis across all conditions is informative as it documents group differences in all trials.
Results
Behavioral Results
GAD youth showed an attentional bias of 8.0 msec (SD=24.8) and comparison subjects showed an attention bias of 11.6 msec (SD=18.6) to masked angry faces. No group difference was found for attention bias to masked angry faces, t(27)=0.42, p=0.68. All subjects considered together manifested an attention bias toward masked angry faces, t(28)=2.27, p=0.03. For GAD patients, mean reaction times were 578.7 msec (SD=74.2) for masked angry/neutral congruent trials and 586.7 msec (SD=74.6) for masked angry/neutral incongruent trials. For the comparison group, mean reaction times were 539.1 msec (SD=113.8) for masked angry/neutral congruent trials and 550.7 (SD=116.7) for masked angry/neutral incongruent trials. There was no group difference in reaction times to trials containing masked angry faces, t(27)=1.08, p=0.29. Moreover, we found no group difference in attention bias to masked happy faces, t(27)=.67, p=0.51, and the groups together did not show an attention bias toward or away from masked happy faces, t(28)=.77, p=0.45. Finally, there was no group difference in reaction time overall to trials containing masked happy faces, t(27)=.95, p=0.35.
fMRI activation results
To test our first hypothesis, we examined group differences in activation to trials containing masked angry faces vs. trials with masked neutral face pairs. Relative to comparison subjects, as hypothesized, GAD youth showed greater right amygdala activation, xyz coordinates=28 −1 −18, t(27)=2.74, p<.05, corrected using a Monte Carlo simulation for multiple comparisons within the amygdala (Figure 2). Areas of activation outside the amygdala are reported in Table 2. To evaluate the association between severity of anxiety symptoms and amygdala activation, patients’ Pediatric Anxiety Rating Scale (PARS) scores were entered in a covariate analysis using AFNI’s 3dRegAna4. This analysis showed that increased anxiety symptoms were associated with increased activation with the right amygdala, xyz coordinates= 18 −5 −10, t(15)=3.96, p=.001 (Figure 2). Anxiety severity and activation within the cluster derived from this analysis significantly correlated, Pearson r=.60, p=.01. (The cluster from this association was in an adjacent but distinct, area from that associated with a diagnosis of anxiety). For masked happy faces relative to masked neutral faces, we found no group difference in the amygdala, t(27)=2.00, p=0.15.
Figure 2.
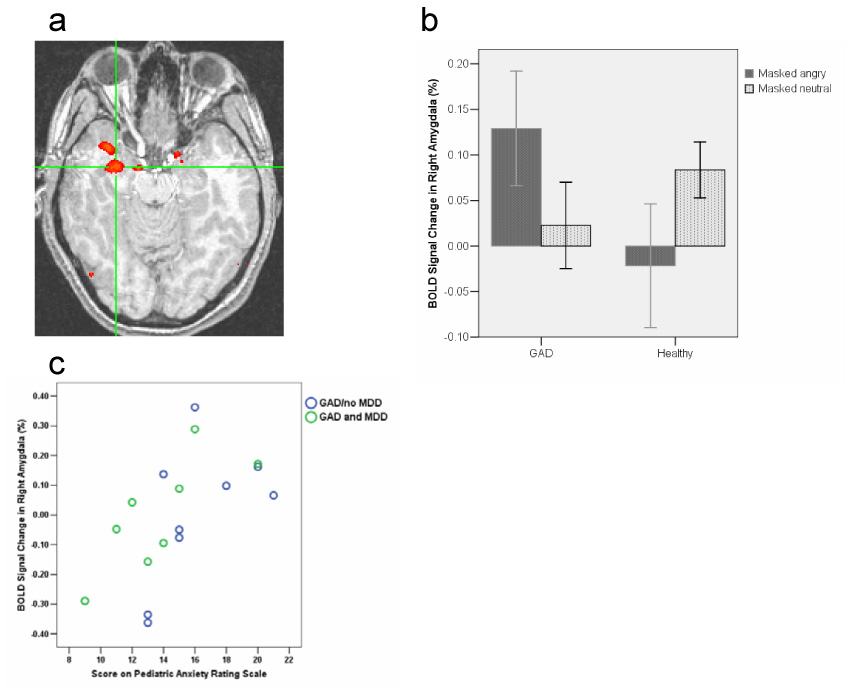
a. In the comparison of trials in which the angry face appeared relative to trials with neutral faces, youth with GAD show greater activation than controls in the right amygdala (right is left and left is right). Coordinates (xyz) for peak activation are 28 −1 −18. b. Bar graphs depicting activation to masked angry and neutral faces separately by group (error bars indicate standard errors of the mean). Bar graphs represent mean activation within the amygdala cluster. Within subject post-hoc t-tests showed that GAD participants had a significantly greater activation to masked angry faces relative to masked neutral faces, t(16) = 2.47, p = .025, and there was no significant difference in healthy comparisons between masked angry and masked neutral faces, t(11) = 1.29, p = .22. c. Relationship between patients’ BOLD response in right amygdala and severity of anxiety symptoms (PARS), Pearson r =.60, p=.01. The location of the amygdala cluster of activation (xyz coordinates 18 −5 −10) is distinct from the cluster in a. GAD patients with and without MDD are differentiated.
Table 2.
Activation areas outside of the amygdala (p < .001 uncorrected) in patients and comparison subjects in the primary contrast (angry vs. neutral faces). Negative activation in the separate groups indicates greater activation to the neutral relative to the angry faces.
| GAD vs. Comparisons (df = 27) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| −46 −62 −25 | 3.89 | Left Cerebellum | |
| Comparisons vs. GAD (df = 27) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| −58 −32 −16 | 4.06 | Left Inferior Temporal Gyrus | 20 |
| −53 −40 −12 | 3.71 | Left Middle Temporal Gyrus | 20 |
| GAD (df = 16) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| 16 36 −1 | −4.84 | Right anterior cingulate | 32 |
| Comparisons (df = 11) | |||
| Coordinates | T value | Location | Brodmann’s Area |
| 15 −65 25 | −4.79 | R Precuneus | 21 |
| 8 −2 30 | −5.05 | R Cingulate Gyrus | 24 |
| 13 −46 29 | −4.80 | R Cingulate Gyrus | 31 |
Functional connectivity analysis
For our second hypothesis, we examined connectivity between the right amygdala cluster (derived from results from the first hypothesis) and the ventrolateral prefrontal cortex during the masked angry relative to masked neutral face conditions. To be consistent with our previous work, we first report connectivity in all subjects together34. Activation in the amygdala cluster negatively coupled with activation in the right ventrolateral prefrontal cortex for all subjects (Table 3; Figure 3). A post-hoc t-test showed weaker negative connectivity in GAD relative to comparisons in the same area of activation, but this effect was modest, xyz coordinates=29 31 −10, t(29)=−2.12 p<.05. At the peak location for the group difference results (30 25 −10), GAD youth considered alone showed modest negative connectivity, t(16)=−2.21 p<.05, whereas healthy youth considered alone showed strong negative connectivity, t(11)=−4.48 p<.001.
Table 3.
Activation from the psychophysiological connectivity analysis (p < .001 uncorrected) in patients and comparison subjects in the primary contrast (angry vs. neutral faces). Positive t values indicate positive connectivity with the seed region (the amygdala cluster). Negative t values represent negative connectivity with the seed region.
| All Subjects (df = 28) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| 29 25 −10 | −4.08 | Right ventrolateral prefrontal cortex | 47 |
| 17 −85 10 | −3.70 | Right cuneus | 17 |
| 25 −57 57 | 4.04 | Right superior parietal lobule | 7 |
| GAD vs. Comparisons (df = 27) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| 42 −30 15 | 3.86 | Right superior temporal gyrus | 41 |
| −57 −9 12 | 3.91 | Left precentral gyrus | 43 |
| Comparisons vs. GAD (df = 27) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| 39 38 28 | 4.18 | Right middle frontal gyrus | 9 |
| GAD (df = 16) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| −14 −49 −1 | −4.02 | Left lingual gyrus | 19 |
| Comparisons (df = 11) | |||
| Coordinates | t value | Location | Brodmann’s Area |
| 29 25 −10 | −4.52 | Right ventrolateral prefrontal cortex | 47 |
| −37 21 −11 | −5.07 | Left ventrolateral prefrontal cortex | 47 |
| −28 25 −14 | −4.52 | Left ventrolateral prefrontal cortex | 47 |
| −43 −16 8 | −4.90 | Left insula | 13 |
| −43 34 31 | 5.31 | Left middle frontal gyrus | 9 |
| 39 −27 41 | 4.44 | Right postcentral gyrus | 2 |
Figure 3.
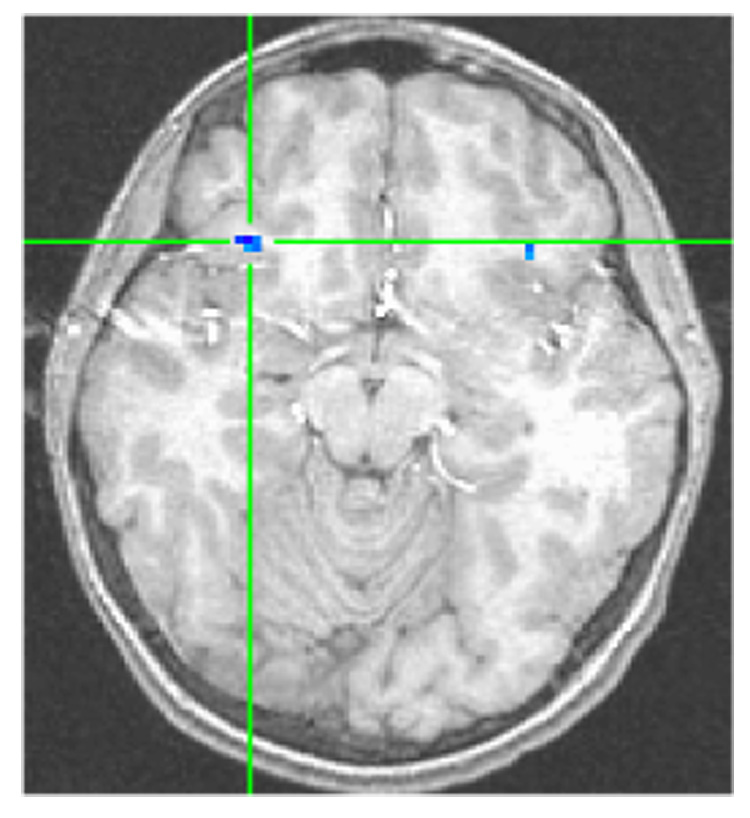
From the psychophysiological interaction analysis with the right amygdala cluster as the seed, subjects show negative coupling in the right ventrolateral prefrontal cortex, 29 25 −10, t(28) = −4.08, p < .001.
In the connectivity analysis for all conditions, there was a positive coupling between the right amygdala cluster and right ventrolateral prefrontal cortex in all subjects, xyz coordinates= 44 23 −6, t(28)=4.84, p<.001. GAD had a significantly stronger positive functional connectivity relative to comparison subjects in a slightly more anterior region, xyz coordinates=45 30 −6, t(27)=3.13, p=.004. GAD youth considered alone showed positive connectivity, xyz coordinates=44 28 −6 (t(16)=3.60 p=.002 uncorrected), whereas healthy youth considered alone showed positive connectivity in a slightly more posterior area, xyz coordinates=47 23 −6 (t(16)=5.71 p<.001 uncorrected). Both groups of subjects showed strong positive connectivity in the right ventrolateral prefrontal cortex, but GAD had greater positive connectivity in a slightly more anterior area.
Examination of comorbid conditions
Eight of the 17 subjects with GAD also had MDD. To evaluate whether MDD accounted for the amygdala findings, we conducted analyses with uncorrected t-tests within the right amygdala. For the comparison of GAD with MDD vs. GAD without MDD, there was no difference, t(15)=.04, p=.72. Relative to the comparison group, GAD/MDD showed greater amygdala activation, xyz coordinates=25 −1 −18, t(18)=2.31 p=0.04. Similarly, relative to comparison subjects, GAD without MDD showed greater amygdala activation, xyz coordinates=30 −1 −18, t(19)=2.56 p=.02.
Eight of the youth with GAD also had social phobia. To evaluate whether social phobia contributed uniquely to the amygdala findings, we followed the same procedures described above. There was no significant difference in amygdala activation between GAD without social phobia and GAD with social phobia, t(15)=1.60, p=.13. There was significantly greater amygdala activation in GAD patients with social phobia relative to healthy controls, xyz coordinates=26 −1 −18, t(18)=3.07 p=.007, with a similar trend between GAD without social phobia vs. controls, xyz coordinates=33 −1 −17, t(19)=1.97 p=.06.
Behavioral performance and fMRI associations
To evaluate associations between the attention bias to masked angry faces and amygdala activation, bias scores to masked angry faces were entered into covariate analyses using 3dRegAna separately for the two groups. GAD youth displayed a significant positive association between attention bias for masked angry faces and the strength of activation in the right amygdala (xyz coordinates 21 −6 −15) t(15)=4.96, p<.001 (Figure 4). This bias measure significantly correlated with level of amygdala activation within this cluster, Pearson r=0.74, p=.001. No significant association was found for comparison subjects. A Fisher’s Z-score transformation43 showed that there was a significant difference between the two correlations, Z=2.41, p=.02.
Figure 4.
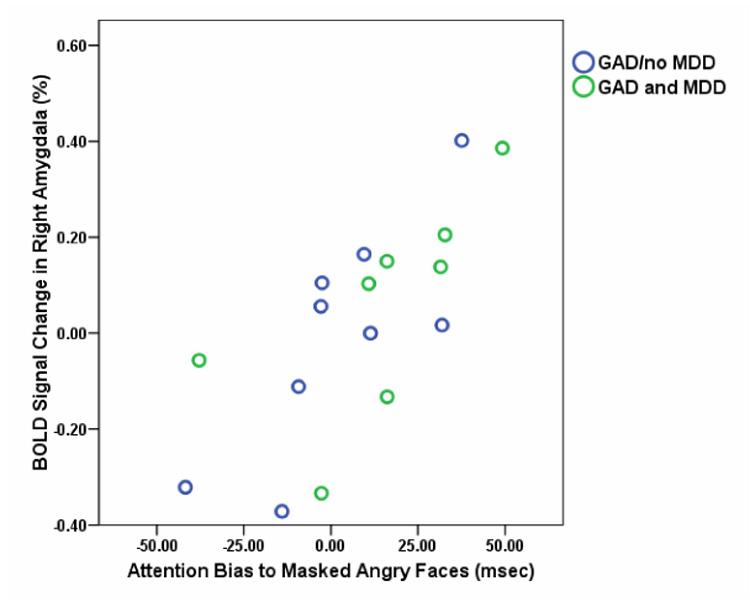
Association between right amygdala activation and attention bias to masked angry faces in youth with GAD. xyz coordinates for peak activation in this cluster was 21 −6 −15. Pearson r = 0.74, p = .001.
Anxiety severity and behavioral performance associations
There was no association between anxiety severity and attention bias to angry faces. Anxiety severity and attention bias to angry faces were each associated with increased activation in non-overlapping clusters of the amygdala.
Comment
In response to rapidly presented threats, youth with GAD show specific disturbances in neural activation. Consistent with our first hypothesis, when viewing briefly displayed, masked angry faces, GAD youth have greater amygdala activation relative to comparison subjects. Moreover, there is a positive correlation between degree of amygdala activation and anxiety-symptom severity. Results also supported our second hypothesis. As predicted, right amygdala and right ventrolateral prefrontal cortex activation did exhibit negative connectivity during threat trials specifically, which was evident in both groups. Post-hoc analysis revealed reduced negative coupling in GAD relative to healthy youth at a liberal statistical threshold. Finally, although both groups show an attention bias of similar magnitude to masked angry faces, attention bias correlates with amygdala activation in patients but not in healthy subjects.
The present findings and our previous work4 indicate that GAD youth process threat faces atypically at both behavioral and neural levels. Behaviorally, when angry faces are presented briefly as in the present study, youth with GAD and comparison subjects both show an initial attention bias toward the spatial location of threat. However, when angry faces were presented for longer periods (500 ms), GAD youth, relative to comparison subjects, showed an attention bias away from threat4. Neurally, when threat is presented briefly, GAD youth show increased amygdala activation which positively correlates with anxiety severity. In contrast, when angry faces were displayed for 500 msec, GAD youth showed no difference from healthy peers in the amygdala, but they did show greater right ventrolateral prefrontal cortex activation. Moreover, when using the 500 msec threat exposures, GAD patients with mild symptoms showed greater ventrolateral prefrontal cortex activation than GAD patients with severe symptoms, suggesting that right ventrolateral prefrontal cortex compensates for a GAD-related disturbance in functioning elsewhere, potentially in the amygdala.
Presently, little is known about the development of the amygdala-ventrolateral prefrontal cortex circuit and how it relates to the emergence of anxiety disorders. Work from animal models indicates that the developmental timing of alterations to the amygdala-prefrontal cortex circuit greatly impacts anxiety-related behavior44,45. Turning to humans, the question is how do neural disturbances relate to the onset of anxiety during development. Presently, it is not known if disturbances in this circuit precede the onset of GAD and are, therefore, risk markers, or if such disturbances arise with the disorder. Consistent with a risk-marker hypothesis, recent findings indicate that amygdala hyperactivation does relate to risk for depression and anxiety in youth46,47. Clearly, more work is needed to understand how the development of this circuit relates to the emergence of anxiety and other disorders that increase in prevalence during adolescence.
Work with a non-clinical sample of adults found that the right ventrolateral prefrontal cortex modulates amygdala responses to briefly presented, masked threat cues5. Extending these findings, our psychophysiological interaction connectivity analysis indicates that the strength of amygdala activation varies as a function of right ventrolateral prefrontal cortex activity in youth and that the negative coupling may be weaker in GAD than comparisons. Consistent with neurobiological models of emotion8,48,49, our results suggest that GAD in youth is associated with dysfunction in a threat detection system, involving a balance between sub-cortical and cortical regions, in particular, the amygdala and ventrolateral prefrontal cortex.
Some research examining the relationship between the amygdala and right ventrolateral prefrontal cortex in response to threat has emphasized the role of the right ventral prefrontal cortex in modulating amygdala responses in relation to strategic emotion regulation processes likely to be engaged over relatively long time periods10. Other work has emphasized the amygdala-ventrolateral prefrontal cortex relationship in terms of emotion regulation processes that are engaged, even when a threat stimulus is briefly presented5,48. The present findings are compatible with the latter view.
Of note, although only detected at a liberal statistical threshold, patients exhibited less negative coupling between the amygdala and ventrolateral prefrontal cortex relative to comparisons. Given that patients show greater amygdala response to threat, the reduced negative coupling in patients relative to comparisons may represent a sign of impaired amygdala modulation. Thus, from this perspective, GAD may relate more to the balance between amygdala and right ventrolateral prefrontal cortex activation, as opposed to overall increases in amygdala activation. Further research is required to clarify these relationships.
In addition, we performed a connectivity analysis across all task conditions. In contrast to the psychophysiological interaction connectivity analysis, this approach showed that both groups had a stronger positive coupling between the same regions and that GAD had greater positive connectivity. Such positive amygdala-ventrolateral prefrontal cortex connectivity has been observed previously in various populations and age groups studied with the standard approach used here34,50. Differences in the approaches of these two connectivity procedures may provide insight into the discrepant findings. The goal of the psychophysiological interaction analysis was to examine task-dependent interactions, specifically related to threat. In contrast, this connectivity analysis reveals association in activation across the entire course of the task. Thus, it is not surprising that these procedures yield different results. Further work using both connectivity approaches is necessary to confirm and understand the manner in which threat content modulates amygdala-ventrolateral prefrontal cortex connectivity in healthy and abnormal development.
In another line of research, we recently found that when stimuli are presented for a relatively long duration in specific attention conditions (e.g. participants subjectively evaluate threat-related facial expressions shown individually for several seconds), youth with GAD selectively show greater amygdala activation34. Thus, taken together with the current findings, these investigations suggest that differential amygdala response profiles are task dependent. The cognitive correlates of amygdala hyperactivation in these two studies are likely to differ. The present study may map neural correlates of threat orienting and detection related to vigilance in clinical anxiety. These correlates appear to involve the amygdala as well as disturbances in the balance between the amygdala and ventrolateral prefrontal cortex. Other tasks may map neural correlates of psychological processes distinct from threat orienting and detection, such as the subjective experience of fear. Work in this area with anxious youth demonstrates that amygdala hyperactivation, in tandem with enhanced ventral prefrontal activation and amygdala-prefrontal coupling, may be correlates of subjective fear34. Further work is necessary to understand exactly what situations lead to normal and abnormal neural activation in youth with GAD.
Finally, even though both anxious and comparison groups show an attention bias toward masked angry faces, amygdala activation only correlates with attention bias in GAD and not in comparisons. This suggests that different neural processes underlie the common behavioral result of attention bias to masked angry faces. For patients, it may be that the amygdala mediates processing rapidly presented threats and this is part of a profile of cognitive responses to threat that underlies GAD. Since the comparison subjects also show attention toward masked angry faces, the bias toward masked angry faces is not a unique feature of GAD in youth.
Limitations
The present study of GAD youth included patients with comorbid MDD and other anxiety disorders, particularly social phobia. However, follow-up analyses showed no group differences in right amygdala activation among patients with different diagnoses. Moreover, each patient subgroup showed greater activation in the same area of the amygdala relative to comparison subjects. These analyses indicate that the group differences in amygdala activation were not due to MDD or social phobia. Another limitation is the small sample. However, since small samples lead to reduced power and the hypothesized findings were confirmed, this limitation is less problematic. A final limitation is that there was a wide age range in both groups and the small sample size made it unfeasible to examine interactions between age and diagnosis. Future work on youth with GAD may wish to select specific age groups to examine how developmental changes relate to anxiety-related influences on brain function.
Acknowledgements
C.S.M. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This research was supported in part by the National Institute of Mental Health (K22 MH068017 to C.S.M), the National Institute of Mental Health Intramural Research Program (D.S.P), and the Wellcome Trust (K.M.). We thank the families who participated. We also thank Drs. K. Towbin, A. Zametkin, and J. Cameron for medical oversight, and H. Iwamoto for programming.
References
- 1.Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol. 1999;38:267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- 2.Mogg K, Bradley BP, Millar N, White J. A follow-up study of cognitive bias in generalized anxiety disorder. Behav Res Ther. 1995;33:927–935. doi: 10.1016/0005-7967(95)00031-r. [DOI] [PubMed] [Google Scholar]
- 3.Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J Abnorm Psychol. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- 4.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 5.Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, Yonekura Y. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage. 2004;21:352–363. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 7.Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–187. [Google Scholar]
- 8.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 9.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 10.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 11.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early- adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 12.Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29:1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- 13.Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- 14.Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- 15.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdale hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual Imagery and perception in postraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 17.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 18.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdale activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 19.Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 20.Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. A direct brainstem-amygdala-cortical 'alarm' system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdale to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 23.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 24.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 26.Neill DB. Frontal-striatal control of behavioral inhibition in the rat. Brain Res. 1976;105:89–103. doi: 10.1016/0006-8993(76)90925-2. [DOI] [PubMed] [Google Scholar]
- 27.Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 28.Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, Casey BJ. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. J Child Psychol Psychiatry. 2006;47:1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 29.Pine DS, Mogg K, Bradley BP, Montgomery LA, Monk CS, McClure EB, Guyer A, Ernst M, Charney DS, Kaufman J. Attention bias to threat in maltreated children: Implications for vulnerability to stress-related psychopathology. Am J Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- 30.Waters A, Mogg K, Bradley B, Pine D. Attention bias for emotional faces in children with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. doi: 10.1097/CHI.0b013e3181642992. in press. [DOI] [PubMed] [Google Scholar]
- 31.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children - present lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Walkup J, Davies M, editors. The Pediatric Anxiety Rating Scale (PARS): a reliable study. Abstract, Annual Meeting, American Academy of Child and Adolescent Psychiatry.1999. [Google Scholar]
- 34.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, Fromm S, Charney DS, Leibenluft E, Ernst E, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 35.RUPP. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. N Engl J Med. 2001;344:1279–1285. doi: 10.1056/NEJM200104263441703. [DOI] [PubMed] [Google Scholar]
- 36.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 38.Rissman J, Eliassen JC, Blumstein SE. An event-related FMRI investigation of implicit semantic priming. J Cogn Neurosci. 2003;15:1160–1175. doi: 10.1162/089892903322598120. [DOI] [PubMed] [Google Scholar]
- 39.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 40.Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 41.Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34:1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen J, Cohen P. Applied Multivariate Regression/Correlation Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- 44.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 45.Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 46.Monk CS, Klein RG, Telzer EH, Schroth EA, Blair RJR, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- 47.Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 49.LeDoux J. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- 50.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]


