Abstract
In this study, we compare four different adjuvants, LT(R192G), CpG ODN, MPL®TDM and alum, for their ability to affect the magnitude, distribution, and duration of antibody responses against F1-V, the lead-candidate antigen for the next generation vaccine against plague, in a murine model. In addition, three different routes of immunization – intranasal (IN), transcutaneous (TC), and subcutaneous (SC), were compared with each adjuvant. Since aerosol exposure to biological warfare agents is of primary concern, both serum and bronchioalveolar lavage (BAL) were analyzed for antigen-specific antibody responses. The most significant findings of the study reported here are that 1) the adjuvant influences the Type 1/Type 2 balance of the antibody response in both the serum and BAL, 2) mucosal immunization is not necessary to obtain F1-V-specific BAL responses, 3) non-traditional adjuvants such as LT(R192G) work when delivered SC, 4) the route of immunization affects the magnitude of the immune response, and 5) F1-V is highly immunogenic by some routes even in the absence of an exogenously applied adjuvant. These studies provide important insights into the influence of different classes of adjuvants on the immune outcome in biodefense vaccines and for development of new generation vaccines against other pathogens as well.
Keywords: adjuvants, vaccines, plague, Y. pestis F1-V
1. Introduction
Recently, a great deal of effort has been directed towards replacement of existing whole cell or formalin inactivated vaccines with subunit vaccines that may be safer and more effective than existing vaccines. Still other efforts are directed at developing alternatives to traditional vaccine delivery, including non-parenteral (i.e., mucosal or transcutaneous) immunization. Mucosally or transcutaneously delivered vaccines offer a number of possible advantages over traditional vaccines including 1) the potential to confer mucosal as well as systemic immunity, 2) increased stability, 3) increased shelf-life, and 4) elimination of needles and the need for specially trained healthcare specialists to administer vaccines. A major limiting factor for the development of mucosal or transcutaneous vaccines is the availability of safe, effective adjuvants that function non-parenterally and that can initiate and support the transition from innate to adaptive immunity. While a number of substances of bacterial origin have been tested as mucosal or transcutaneous adjuvants, the three bacterial products with the greatest potential to function as non-parenteral adjuvants are the ADP-ribosylating enterotoxins (cholera toxin (CT), produced by various strains of Vibrio cholerae, and the heat-labile enterotoxin (LT) produced by some enterotoxigenic strains of Escherichia coli [1-5]), synthetic oligodeoxynucleotides containing unmethylated CpG dinucleotides (CpG ODN) [6], and monophosphoryl lipid A (MPL) [7-9].
The mechanism of adjuvanticity of the ADP-ribosylating enterotoxins is the subject of considerable debate. Our own view is that the adjuvanticity of these molecules is an outcome and not an event. It is likely that these molecules exert their adjuvant function by interacting with a variety of cell types, including epithelial cells, dendritic cells (DCs), macrophages, and possibly B- and T-lymphocytes. This complex and dynamic interaction changes the context in which antigen is processed and presented during the initiation phase of the immune response. LT and CT elevate intracellular cAMP in a variety of cell types and their adjuvanticity is at least, in part, related to that function. CpG and MPL are both TLR-agonists and their adjuvant activities are due to several different effects they have on innate and adaptive immune responses, acting through MyD88-dependent and MyD88–independent pathways.
In a recent study, we evaluated different prime - boost regimens, including parenteral, mucosal, and transcutaneous delivery, in order to explore the effect of changing the route of prime and boost on the ability of the recombinant Yersinia pestis-derived fusion protein (F1-V) to promote the development of long-lasting, high titer antibodies [10]. F1-V has been shown to provide protection against flea-borne, subcutaneous and aerosol challenge and has the potential to provide protective immunity against pneumonic as well as bubonic plague due to either wild type F1+ Y. pestis or to naturally occurring F1- variants [11, 12]. The most significant finding of that study is that boosting by a different (heterologous) route than the priming dose can be as or more effective than homologous boosting for induction of either serum or bronchioalveolar anti-F1-V IgG1 responses. In a follow-on aerosol challenge study [13], we demonstrated that the route of immunization and choice of adjuvant influence the magnitude of the antibody response as well as the IgG1/IgG2a ratio, and that inclusion of an appropriate adjuvant in the vaccine formulation is critical for protection against aerosol challenge following non-parenteral immunization.
In the current report, we compare four different adjuvants, a mutant of LT designated LT(R192G), CpG ODN, MPL®TDM, and alum, for their ability to alter the magnitude, distribution, and duration of systemic and bronchioalveolar lavage (BAL) antigen-specific anti-F1-V responses following intranasal (IN), transcutaneous (TC), or parenteral (SC) immunization of mice.
2. Materials and methods
2.1. Antigens and Adjuvants
LT(R192G) was prepared in our laboratory by galactose-affinity chromatography as previously described [14]. CpG ODN 1826 was obtained from Coley Pharmaceuticals. CpG ODN 1826 is mouse specific and is made with a nuclease-resistant phosphorothioate backbone with the sequence (1826-T C C A T G A C G T T C C T G A C G T T). MPL®TDM is an oil in water emulsion that contains detoxified monophosphoryl lipid A derived from Salmonella minnesota R595 and synthetic trehalose dicorynomycolate (TDM), a component of Mycobacterium tuberculosis, in 2% squalene and Tween-80, and was obtained from Sigma (M6536, Lot 103K1610). Alhydrogel® 2.0% (Alum) was obtained from Brenntag Biosector, Germany.
F1-V was a non-his-tagged version of the fusion protein, expressed by E. coli BLR(DE3)/pPW731 [15] and isolated to 99% purity by gel filtration chromatography. Briefly, following overnight growth, protein in inclusion bodies from lysed cells was denatured with 6 M urea on ice. F1-V was then purified by size exclusion chromatography on a Superdex™ 75 gel filtration column (Amersham). Purified F1-V was analyzed on SDS-PAGE gel using the Novex gel system (Invitrogen) and stained with Coomassie dye to detect the presence of the recombinant protein. Western blots were also performed using sera from goats immunized with purified F1 and V to confirm the presence and quantification of the recombinant protein.
2.2. Animal immunizations
Groups of 6-8 weeks-old female Swiss Webster mice were obtained from Harlan-Sprague and were immunized twice, at day 0 and 28, with recombinant F1-V alone or in combination with one of the four adjuvants. Immunizations were administered IN, TC or SC and animals were boosted by the same route as the priming dose. For IN immunization, mice were lightly anesthetized with Isoflurane (IsoFlo, USP, Abbott Labs). The 10-15 μl of the immunizing inoculum per mouse consisted of 10 μg of F1-V alone or admixed with 5 μg of LT(R192G), CpG ODN, or MPL®TDM and was delivered to the external nares of the animals' nostrils with a pipette tip. For TC immunization, mice were shaved on the abdomen and anesthetized intraperitoneally with ketamine/xylazine. The shaved skin was hydrated with a wet gauze before applying 100 μl of the immunizing solution containing 50 μg F1-V alone or admixed with 50 μg of LT(R192G), CpG ODN, or MPL®TDM. For SC immunization, mice were injected with 100 μl saline containing 10 μg of F1-V alone or admixed with 5 μg of LT(R192G), CpG ODN, or MPL®TDM. Since alum is commonly used in parenteral vaccines, an additional group of 30 mice immunized SC with 10 μg of F1-V adsorbed to Alhydrogel® 2.0% in a final volume of 100 μl saline was included as a parenteral vaccine control.
2.3. Sample collection
Ten animals from each group were sacrificed by CO2 inhalation on days 15, 30 and 60 post final immunization (PFI). Blood was collected from each animal by cardiac puncture. BAL was obtained by exposing the trachea and making a small incision into which an 18-gauge needle was inserted and secured. Through this cannula, the lungs were repeatedly lavaged by slowly injecting and withdrawing 1 ml of phosphate buffered saline supplemented with a protease inhibitor cocktail (Roche Laboratories).
2.4. Measurement of serum and BAL antibody
Individual serum and BAL samples were examined for anti-F1-V total IgG, IgG1, and IgG2a antibodies using a quantitative ELISA as described previously [10]. For quantitative analysis, concentrations of IgG, IgG1, and IgG2a were determined by non-linear regression from a standard curve of mouse myeloma IgG1 or IgG2a (Sigma) serially diluted as a standard on each ELISA plate. The results are expressed as the mean concentration±S.E.M.
2.5. Statistical analysis
Statistical analysis was performed using a one-way analysis of variance followed by a Bonferroni Multiple Comparisons post-test. P values of ≤ 0.05 were considered significant. Statistical analysis was performed using Prism 4 software (GraphPad Inc.).
3. Results
3.1. Serum and BAL anti-F1-V responses following SC immunization
The purpose of this group of experiments was to compare the serum and BAL anti-F1-V responses when F1-V was administered SC alone or in combination with one of the adjuvants being evaluated. Mice were immunized SC twice, at day 0 and day 28, with F1-V alone (F) or admixed with LT(R192G) (R), CpG ODN (C), or MPL®TDM (M), or adsorbed to alum (A). Groups of ten animals from each immunization regimen were sacrificed on days 15, 30, and 60 PFI. Antigen-specific antibody responses were determined by ELISA and a comparison was made between the mean responses of the different groups at each time point.
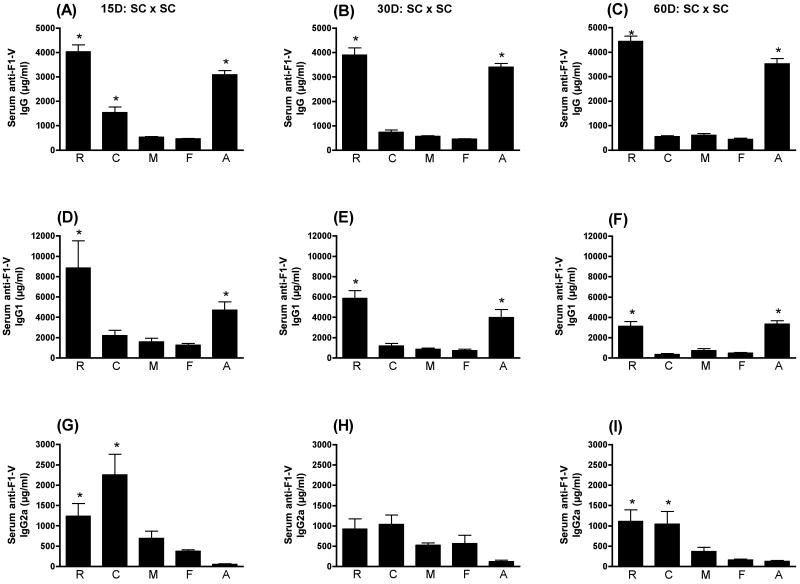
As seen in Fig. 1A, LT(R192G) (p≤0.001), CpG ODN (p≤0.001), and alum (p≤0.001) were each able to significantly enhance the serum anti-F1-V IgG response compared to animals receiving F1-V alone at day 15 PFI. There was no significant increase in the serum anti-F1-V IgG response with MPL®TDM included as an adjuvant at this time point. By day 30 PFI (Fig. 1B), only LT(R192G) (p≤0.001) and alum (p≤0.001) significantly enhanced the serum anti-F1-V IgG response when compared to animals receiving F1-V alone. Both LT(R192G) and alum continued to enhance the serum anti-F1-V IgG response through day 60 PFI (Fig. 1C). Similarly, only LT(R192G) (p≤0.001) and alum (p≤0.01) significantly enhanced the serum anti-F1-V IgG1 response when compared to animals receiving F1-V alone at all time points in this study (Figs. 1D, 1E, 1F). By contrast, both LT(R192G) (p≤0.01) and CpG ODN (p≤0.001) significantly enhanced the serum anti-F1-V IgG2a response at day 15 PFI and day 60 PFI compared to animals immunized SC with F1-V alone. There was no significant enhancement of serum anti-F1-V IgG2a by MPL®TDM or alum at day 15 PFI or thereafter. When comparing the different responses, it is interesting to note that alum enhanced the levels of serum anti-F1-V IgG1 (Figs. 1D, 1E, 1F) while CpG ODN enhanced the levels of serum anti-F1-V IgG2a (Figs. 1G, 1I) compared to antigen alone, consistent with the reported induction of Type 2 and Type 1 responses by the two adjuvants, respectively. When LT(R192G) was administered as an adjuvant by this route, antigen-specific serum IgG1 and IgG2a were both enhanced, indicating a more balanced Type 1/Type 2 response.
Figure 1.
Serum anti-F1-V responses following SC immunization. Swiss Webster mice were immunized at day 0 and 28 subcutaneously with F1-V alone (F) or in conjunction with LT(R192G) (R), CpG ODN (C), MPL®TDM (M), or alum (A). Ten animals from each group were sacrificed on days 15, 30 and 60 post final immunization. Serum was collected by cardiac puncture. Concentrations of IgG (panels A, B and C), IgG1 (panels D, E and F) and IgG2a (panels G, H and I) were determined by ELISA by non-linear regression against standard curves. Error bars represent ± SEM. Adjuvant enhanced responses that are significantly different (p≤0.05) than antigen alone are indicated with an asterisk (*).
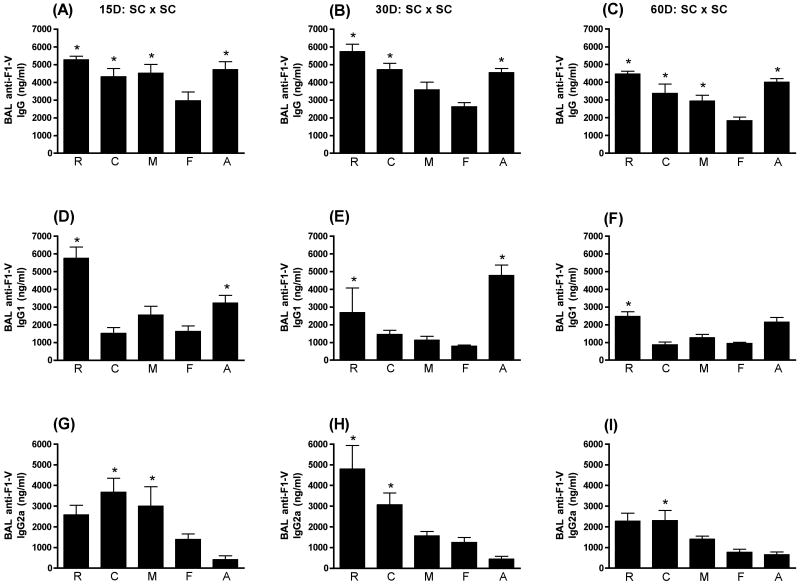
As seen in Fig. 2, SC immunization with each of the four adjuvants, LT(R192G) (p≤0.001), CpG ODN (p≤0.01), MPL®TDM (p≤0.01), and alum (p≤0.001), was able to significantly enhance the BAL anti-F1-V IgG response when compared to animals receiving F1-V alone beginning at day 15 PFI (Fig. 2A) and through day 60 PFI (Fig. 2C). That was not the case when the BAL anti-F1-V IgG1 and IgG2a responses were examined. As seen in Fig. 2D, at day 15 PFI, only LT(R192G) (p≤0.001) and alum (p≤0.05) significantly enhanced the BAL anti-F1-V IgG1 response when compared to animals receiving F1-V alone. This same pattern continued through day 30 PFI with both adjuvants (Fig. 2E) and day 60 PFI with LT(R192G) only (Fig. 2F). The difference between the BAL anti-F1-V IgG1 response induced when alum was included as an adjuvant was not significantly different than the response observed with F1-V alone at day 60 PFI. By contrast only CpG ODN (p≤0.01) significantly enhanced the BAL anti-F1-V IgG2a response through day 60 PFI, while MPL®TDM (p≤0.05) and LT(R192G) (p≤0.001) significantly enhanced the BAL anti-F1-V IgG2a response only at day 15 and 30 PFI, respectively (Fig. 2G, 2H, 2I). As seen for the serum anti-F1-V responses, alum enhanced the levels of BAL anti-F1-V IgG1 while CpG ODN enhanced the levels of BAL anti-F1-V IgG2a compared to antigen alone.
Figure 2.
BAL anti-F1-V responses following SC immunization. Swiss Webster mice were immunized at day 0 and 28 subcutaneously with F1-V alone (F) or in conjunction with LT(R192G) (R), CpG ODN (C), MPL®TDM (M), or alum (A). Ten animals from each group were sacrificed on days 15, 30 and 60 post final immunization. Lung washes were collected by bronchioalveolar lavage (BAL). Concentrations of IgG (panels A, B and C), IgG1 (panels D, E and F) and IgG2a (panels G, H and I) were determined by ELISA by non-linear regression against standard curves. Error bars represent ± SEM. Adjuvant enhanced responses that are significantly different (p≤0.05) than antigen alone are indicated with an asterisk (*).
3.2. Serum and BAL anti-F1-V responses following IN immunization
The purpose of this group of experiments was to compare the serum and BAL anti-F1-V responses when F1-V was administered IN alone or in combination with one of the adjuvants being compared. Mice were immunized IN twice, at day 0 and day 28, with F1-V alone (F) or admixed with LT(R192G) (R), CpG ODN (C), or MPL®TDM (M). Alum was not included in the IN immunization studies. Groups of ten animals from each immunization regimen were sacrificed on days 15, 30, and 60 PFI. Antigen-specific antibody responses were determined by ELISA and a comparison was made between the mean responses of the different groups at each time point.
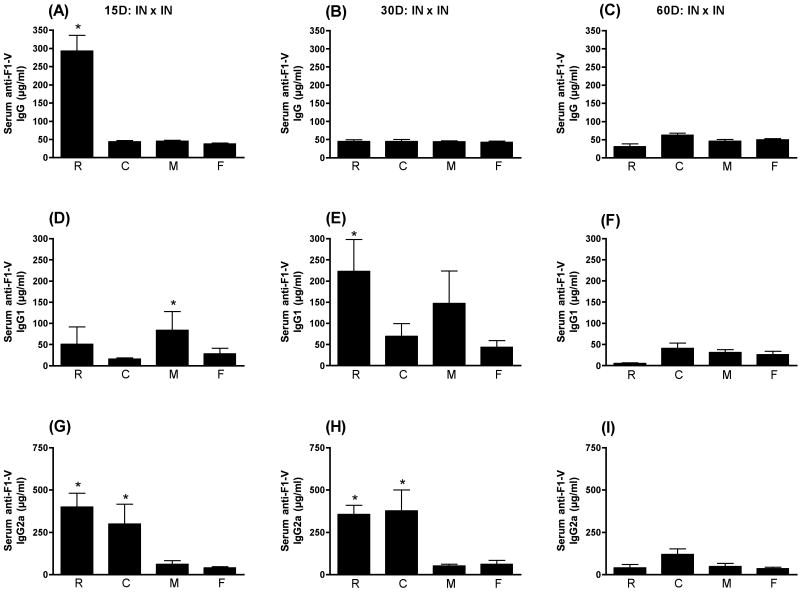
Mucosal immunization, including IN, has a great deal of potential for immunization strategies designed to elicit protection on mucosal surfaces and it is well known that mucosal immunization can elicit systemic responses as well. For most antigens, an adjuvant is essential for mucosal immunization to be effective. It was therefore of interest to compare adjuvants that are reported to function mucosally for the ability to elicit different isotypes of antigen-specific antibodies. As seen in Fig. 3, following IN immunization, only LT(R192G) significantly enhanced the serum anti-F1-V IgG response at day 15 PFI (p≤0.001). By day 30 PFI, none of the adjuvants induced a serum anti-F1-V IgG response greater than that obtained with F1-V alone (p>0.05). It is important to note that even in the absence of an adjuvant, F1-V elicited a reasonable serum IgG response when administered IN compared to non-vaccinated controls. When the serum anti-F1-V IgG1 responses were compared (Fig. 3D, 3E, 3F), only MPL®TDM at day 15 PFI (p≤0.05) and LT(R192G) at day 30 PFI (p≤0.01) elicited responses that were significantly greater than the responses observed with F1-V alone. As seen in Fig. 3G, both LT(R192G) (p≤0.001) and CpG ODN (p≤0.01) were able to significantly enhance the serum anti-F1-V IgG2a response when compared to animals receiving F1-V alone. This effect continued through day 30 PFI (Fig. 3H) but not day 60 PFI (Fig. 3I).
Figure 3.
Serum anti-F1-V responses following IN immunization. Swiss Webster mice were immunized at day 0 and 28 intranasally with F1-V alone (F) or in conjunction with LT(R192G) (R), CpG ODN (C), or MPL®TDM (M). Ten animals from each group were sacrificed on days 15, 30 and 60 post final immunization. Serum was collected by cardiac puncture. Concentrations of IgG (panels A, B and C), IgG1 (panels D, E and F) and IgG2a (panels G, H and I) were determined by ELISA by non-linear regression against standard curves. Error bars represent ± SEM. Adjuvant enhanced responses that are significantly different (p≤0.05) than antigen alone are indicated with an asterisk (*).
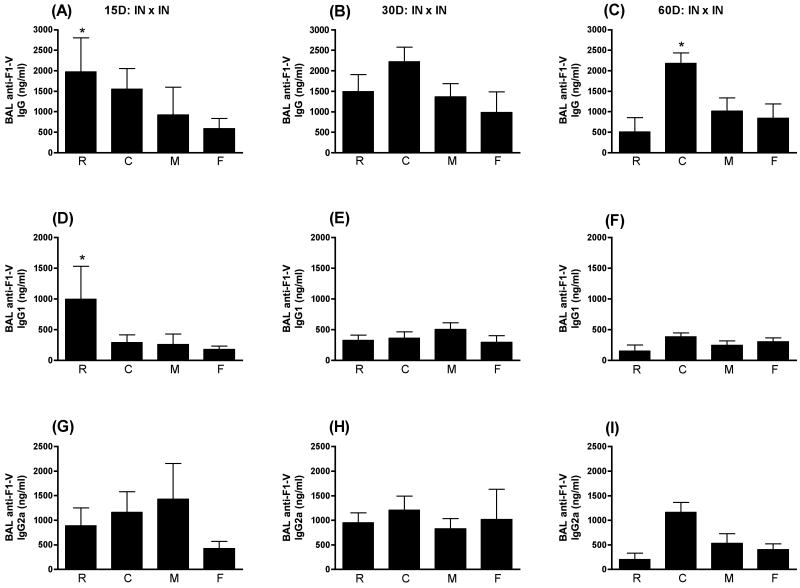
There was a great deal of variability in the BAL anti-F1-V responses following IN immunization and a significant response was elicited by F1-V without an adjuvant. Consequently, as seen in Fig. 4, the only significant enhancement of BAL anti-F1-V IgG was observed with LT(R192G) at day 15 PFI (p≤0.05) and CpG ODN at day 60 PFI (p≤0.05). Only LT(R192G) (p≤0.01) significantly enhanced the BAL anti-F1-V IgG1 response and only at day 15 PFI (Fig. 4D). None of the adjuvants significantly enhanced the BAL anti-F1-V IgG2a response when compared to F1-V alone. In addition to the greater variability following IN immunization, the levels of serum and BAL anti-F1-V antibodies were uniformly higher with all of the adjuvants tested following SC immunization (Figs. 1 and 2) when compared to IN immunization (Figs. 3 and 4) with the same amount of antigen and adjuvant.
Figure 4.
BAL anti-F1-V responses following IN immunization. Swiss Webster mice were immunized at day 0 and 28 intranasally with F1-V alone (F) or in conjunction with LT(R192G) (R), CpG ODN (C), or MPL®TDM (M). Ten animals from each group were sacrificed on days 15, 30 and 60 post final immunization. Lung washes were collected by bronchioalveolar lavage (BAL). Concentrations of IgG (panels A, B and C), IgG1 (panels D, E and F) and IgG2a (panels G, H and I) were determined by ELISA by non-linear regression against standard curves. Error bars represent ± SEM. Adjuvant enhanced responses that are significantly different (p≤0.05) than antigen alone are indicated with an asterisk (*).
3.3. Serum and BAL anti-F1-V responses following TC immunization
The purpose of this group of experiments was to compare the serum and BAL anti-F1-V responses when F1-V was administered TC alone or in combination with one of the adjuvants being compared. Mice were immunized TC twice, at day 0 and day 28, with F1-V alone (F) or admixed with LT(R192G) (R), CpG ODN (C), or MPL®TDM (M). Alum was not included in the TC immunization studies. Groups of ten animals from each immunization regimen were sacrificed on days 15, 30, and 60 PFI. Antigen-specific antibody responses were determined by ELISA and a comparison was made between the mean responses of the different groups at each time point.
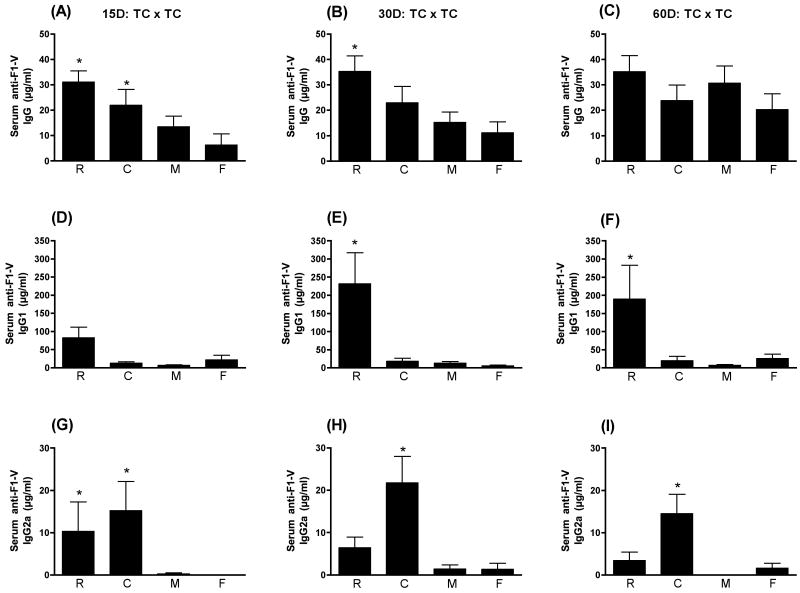
TC delivery of vaccines has been shown to be effective at inducing both humoral and cellular antigen-specific immune responses in both the systemic and mucosal compartments of immunized animals and humans. As seen in Fig. 5A, at day 15 PFI both LT(R192G) (p≤0.01) and CpG ODN (p≤0.05) were able to significantly enhance the levels of serum anti-F1-V IgG following TC immunization when compared to animals receiving F1-V alone. By day 30 PFI, only LT(R192G) (p≤0.01) significantly enhanced serum anti-F1-V IgG response. By day 60 PFI, the serum anti-F1-V response in animals receiving antigen alone has increased to the point that the differences between groups were no longer significant. As seen in Figs. 5D, 5E, and 5F, only LT(R192G) was able to significantly enhance the serum anti-F1-V IgG1 response in animals immunized TC and that enhanced response was sustained through day 60 PFI. By contrast, both LT(R192G) (p≤0.05) and CpG ODN (p≤0.01)) enhanced the serum anti-F1-V IgG2a response at day 15 PFI and CpG was the only one of the three adjuvants that significantly enhanced the serum anti-F1-V IgG2a response at day 30 PFI (p≤0.001) and day 60 PFI (p≤0.05). As with the other routes of immunization, CpG ODN elicited a more prominent antigen-specific serum Type 1 response when administered TC.
Figure 5.
Serum anti-F1-V responses following TC immunization. Swiss Webster mice were immunized at day 0 and 28 transcutaneously with F1-V alone (F) or in conjunction with LT(R192G) (R), CpG ODN (C), or MPL®TDM (M). Ten animals from each group were sacrificed on days 15, 30 and 60 post final immunization. Serum was collected by cardiac puncture. Concentrations of IgG (panels A, B and C), IgG1 (panels D, E and F) and IgG2a (panels G, H and I) were determined by ELISA by non-linear regression against standard curves. Error bars represent ± SEM. Adjuvant enhanced responses that are significantly different (p≤0.05) than antigen alone are indicated with an asterisk (*).
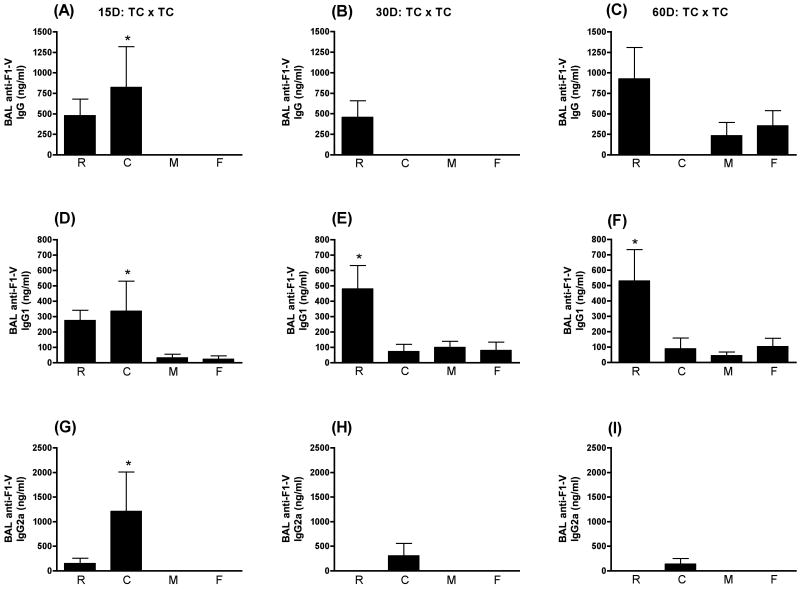
As with IN immunization, there was a great deal of variability in the antigen-specific BAL response when F1-V was administered TC alone or in combination with any of the adjuvants (Fig. 6). The only significant enhancement of BAL anti-F1-V IgG was observed with CpG at day 15 PFI (p≤0.01). With respect to BAL anti-F1-V IgG1, only CpG ODN (p≤0.05) at day 15 PFI and LT(R192G) at day 30 PFI (p≤0.01) and day 60 PFI (p≤0.01) significantly enhanced the BAL anti-F1-V IgG1 response when compared to animals receiving F1-V alone. Only CpG ODN (p≤0.001) significantly enhanced the BAL anti-F1-V IgG2a response when compared to F1-V alone and that was only at day 15 PFI. The levels of serum and BAL anti-F1-V antibodies were uniformly lower with all of the adjuvants tested following TC immunization (Figs. 5 and 6) when compared to either of the other two routes (Figs. 1, 2, 3, and 4) even with 5 times the amount of antigen (10 μg SC, IN vs. 50 μg TC) and 10 times the amount of LT(R192G), CpG, or MPL®TDM (5 μg SC, IN vs. 50 μg TC).
Figure 6.
BAL anti-F1-V responses following TC immunization. Swiss Webster mice were immunized at day 0 and 28 transcutaneously with F1-V alone (F) or in conjunction with LT(R192G) (R), CpG ODN (C), or MPL®TDM (M). Ten animals from each group were sacrificed on days 15, 30 and 60 post final immunization. Lung washes were collected by bronchioalveolar lavage (BAL). Concentrations of IgG (panels A, B and C), IgG1 (panels D, E and F) and IgG2a (panels G, H and I) were determined by ELISA by non-linear regression against standard curves. Error bars represent ± SEM. Adjuvant enhanced responses that are significantly different (p≤0.05) than antigen alone are indicated with an asterisk (*).
Interestingly, there was no detectable anti-F1-V IgA in BAL following immunization by any route or with any of the adjuvants examined, and very low levels of serum anti-F1-V IgA following SC or IN immunization. None of the serum anti-F1-V IgA responses observed with any of the four adjuvants under study were significantly different than those obtained with F1-V alone (data not shown).
4. Discussion
The objective of this study was to compare four different adjuvants, LT(R192G), CpG ODN, MPL®TDM and alum, for their ability to affect the magnitude, distribution, and duration of anti-F1-V antibody responses following IN, TC, or SC immunization. Since aerosol exposure to biological warfare agents is of primary concern, both serum and BAL were analyzed for antigen-specific antibody responses.
The adjuvants chosen for this study exert their adjuvant effects through different mechanisms. Alum adsorbs and concentrates antigen for delivery to antigen presenting cells (APCs). Alum also causes non-specific damage at the injection site, resulting in recruitment of additional APCs to the site, and enhances APC functions by inducing increased surface expression of costimulatory and adhesion molecules, including CD40, CD83, CD86, MCH II and ICAM leading to enhanced antigen uptake, activation and antigen-specific humoral responses [16, 17]. Alum is a parenteral adjuvant and induces primarily a Type 2 immune response to coadministered antigens. The ADP-ribosylating adjuvants, like LT(R192G), have been shown to induce more balanced Type 1/Type 2 responses against coadministered antigens and function when administered parenterally, mucosally, or transcutaneously. The adjuvant activity of these molecules is related to their ability to increase cAMP in epithelial cells and to activate APCs. Increasing cAMP induces a TRAF-6 independent activation of NF-kB in epithelial cells, coincident with an adjuvant-induced up-regulated expression of costimulatory molecules on, and maturation of, dendritic cells [18, 19]. The secretion of pro-inflammatory chemokines and cytokines by epithelial cells at the same time that antigen is being processed and presented by activated APCs changes the environment of the immune–synapse in which T-cell programming and B-cell activation occur. Because the receptor for LT (a family of ganglioside receptors) is fairly ubiquitous, a number of cell types are activated, including dendritic cells, macrophages, B-cells, T-cells, and epithelial cells. The outcome of this altered environment is the induction of both Type 1 and Type 2 immune responses.
CpG ODN and MPL®TDM are both TLR-agonists and signal through MyD88-dependent and MyD88-independent pathways, resulting in activation of NF-κB, p38, and JNK 1/2 in cells that express the specific TLR receptor (TLR9 for CpG and TLR2/TLR4 for MPL®TDM). It is interesting that these two TLR-agonists are associated with different immune outcomes. CpG ODN is principally associated with Type 1 immune responses while MPL®TDM is associated with Type 2 immune responses. It is possible that different cell types are activated because of different receptor distributions or that there are subtle differences in intracellular signaling pathways activated by these two molecules which results in their different adjuvant effects. More likely, since MPL®TDM contains constituents other than MPL, including synthetic trehalose dicorynomycolate and squalene, it has non-TLR-associated effects (i.e., activation of non-TLR immune pathogen recognition receptors). Specifically, squalene in the emulsion concentrates antigens and functions as a depot for both the MPL and the antigen at the injection site. As a result, MPL®TDM causes tissue damage at the injection site resulting in non-specific inflammation (similar to alum), attraction of APCs and induction of cytokine cascades [20]. Furthermore, the particulate nature of the oil droplets allows the antigen to be readily trapped in the lymph nodes and taken up by macrophages and dendritic cells leading to enhanced antigen presentation. Interestingly, in this study MPL®TDM was only marginally effective at eliciting a significant anti-F1-V antibody response, regardless of route. It is also possible that a different outcome would be obtained with MPL in the absence of TDM.
A major finding of this study is the observation that the adjuvant influences the balance of the antibody response in both the serum and BAL. This was clearly demonstrated when comparing the anti-F1-V responses following SC immunization induced by alum, MPL®TDM, CpG ODN and LT(R192G). Alum was effective at enhancing both serum and BAL anti-F1-V IgG1 (Type 2) responses but not IgG2a (Type 1) responses following SC immunization. By contrast, CpG ODN was effective at enhancing both serum and BAL anti-F1-V IgG2a (Type 1) responses but not IgG1 (Type 2) responses following SC immunization. LT(R192G) enhanced both the serum and BAL anti-F1-V IgG1 (Type 2) and IgG2a (Type 1) responses following SC immunization. These responses were consistent with the reported activation of Type 1 and Type 2 immune responses by CpG ODN and alum, respectively, as well as the ability of LT(R192G) to elicit a balanced response.
A second major finding of this study is that mucosal immunization is not necessary to obtain F1-V-specific BAL responses. Indeed, the highest anti-F1-V IgG BAL responses were obtained following SC immunization. This has significance for development of vaccines against a variety of respiratory pathogens, including potential biothreat agents for which the pulmonary surface would be the first likely productive point of contact following aerosol release. Antigen-specific serum IgG generated in response to vaccination may transudate across the lung epithelia and thus provide antigen-specific antibody at the mucosal surfaces.
Another major finding of this study is that non-traditional adjuvants also work when administered SC. ADP-ribosylating adjuvants are generally considered to be effective for mucosal and, more recently, TC delivery. Our findings suggest that appropriately attenuated mutants of the ADP-ribosylating toxin/adjuvants may be practical alternatives to alum for parenteral immunization.
Another noteworthy but anticipated finding of this study is that the route of immunization affects the magnitude of the immune response. Thus SC immunization elicited the highest levels of anti-F1-V IgG, IgG1, and IgG2a in both the serum and BAL when compared to IN or TC immunization with the same (IN) or 5-10 fold greater (TC) amount of antigen and adjuvant. Few direct comparisons of the magnitude of the immune response following these different routes have been conducted and the efficiency of TC immunization is always an issue. It is possible that more effective delivery with enhanced patch technology, permeation enhancers, or nanoparticle delivery will improve these responses.
Finally, we also observed that there is an antibody response to F1-V in the BAL following SC and IN immunization in the absence of an adjuvant. This response could be increased by including specific adjuvants in some cases but not in others. However, adjuvants do more than just increase the magnitude of the immune response and the qualitative nature of the response is also important. We recently demonstrated that SC immunization with F1-V in the absence of an adjuvant can confer 70% protection against aerosol challenge with Y. pestis [13]. However, IN immunization in the absence of adjuvant resulted in 100% mortality while the inclusion of adjuvant in the formulation provided 90% protection, demonstrating that an adjuvant is necessary for protection following IN immunization. The current studies provide important insights into the influence of different classes of adjuvants on the immune outcome in biodefense vaccines and for development of new generation vaccines against other pathogens as well.
Acknowledgments
This study was supported by Public Health Service grant AI056452 from the National Institute for Allergy and Infectious Diseases (J.D.C.). We are grateful to Bradford S. Powell at the United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, Maryland for providing the F1-V expressing construct.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clements JD, Hartzog NM, Lyon FL. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6(3):269–77. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 2.Elson CO. Cholera toxin and its subunits as potential oral adjuvants. Curr Top Microbiol Immunol. 1989;146:29–33. doi: 10.1007/978-3-642-74529-4_3. [DOI] [PubMed] [Google Scholar]
- 3.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22(9):2277–81. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 4.Xu-Amano J, Kiyono H, Jackson RJ, Staats HF, Fujihashi K, Burrows PD, et al. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178(4):1309–20. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto M, Vancott JL, Okahashi N, Marinaro M, Kiyono H, Fujihashi K, et al. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]
- 6.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 7.Carozzi S, Salit M, Cantaluppi A, Nasini MG, Barocci S, Cantarella S, et al. Effect of monophosphoryl lipid A on the in vitro function of peritoneal leukocytes from uremic patients on continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1989;27(8):1748–53. doi: 10.1128/jcm.27.8.1748-1753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henricson BE, Manthey CL, Perera PY, Hamilton TA, Vogel SN. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect Immun. 1993;61(6):2325–33. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers KR, Beining P, Betts M, Snippe H, Inman J, Golding B. Monophosphoryl lipid A behaves as a T-cell-independent type 1 carrier for hapten-specific antibody responses in mice. Infect Immun. 1995;63(1):168–74. doi: 10.1128/iai.63.1.168-174.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynn A, Freytag LC, Clements JD. Effect of homologous and heterologous prime-boost on the immune response to recombinant plague antigens. Vaccine. 2005;23(16):1957–65. doi: 10.1016/j.vaccine.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Heath DG, Anderson GW, Jr, Mauro JM, Welkos SL, Andrews GP, Adamovicz J, et al. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine. 1998;16(1112):1131–7. doi: 10.1016/s0264-410x(98)80110-2. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett CO, Sebbane F, Adamovicz JJ, Andrews GP, Hinnebusch BJ. Flea-borne transmission model to evaluate vaccine efficacy against naturally acquired bubonic plague. Infect Immun. 2004;72(4):2052–6. doi: 10.1128/IAI.72.4.2052-2056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynn A, Roy CJ, Powell BS, Adamovicz JJ, Freytag LC, Clements JD. Protection against aerosolized Yersinia pestis challenge following homologous and heterologous prime-boost with recombinant plague antigens. Infect Immun. 2005;73(8):5256–61. doi: 10.1128/IAI.73.8.5256-5261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng E, Cardenas-Freytag L, Clements JD. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT) Vaccine. 1999;18(12):38–49. doi: 10.1016/s0264-410x(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 15.Goodin JL, Nellis DF, Powell BS, Vyas VV, Enama JT, Wang LC, et al. Purification and protective efficacy of monomeric and modified Yersinia pestis capsular F1-V antigen fusion proteins for vaccination against plague. Protein Expr Purif. 2007;53(1):63–79. doi: 10.1016/j.pep.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewer JM. (How) do aluminium adjuvants work? Immunol Lett. 2006;102(1):10–5. doi: 10.1016/j.imlet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA. The Common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69(2):1151–9. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23(15):1804–13. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Martin M, Sharpe A, Clements JD, Michalek SM. Role of B7 costimulatory molecules in the adjuvant activity of the heat-labile enterotoxin of Escherichia coli. J Immunol. 2002;169(4):1744–52. doi: 10.4049/jimmunol.169.4.1744. [DOI] [PubMed] [Google Scholar]
- 20.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19(1):103–7. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]