Abstract
The words “No” and “Yes” are involved in conditioning to prohibit or encourage behavior, respectively. We therefore hypothesized that these words would be attributed endogenous valence, activating neuronal circuits involved with valence and emotional control. Functional Magnetic Resonance Imaging (fMRI) at 4 Tesla was used to record regional brain activity while participants were exposed to emphatic vocalizations of the words. Results showed that No and Yes were associated with opposite brain-behavior responses; while No was negatively valenced, produced slower response times, and evoked a negative signal in the right lateral orbitofrontal cortex (OFC), Yes was positively valenced, produced faster response times and evoked a positive signal in a contiguous region of the OFC. Attribution of negative valence to No and trait anger control were associated with increased responsivity of the OFC to No. Inasmuch as sensitivity to the prohibitive command No develops during childhood through interaction with primary caregivers as the first social objects, our findings may implicate the lateral OFC in the neurobiology of emotion regulation and subsequent social development.
Keywords: fMRI, inferior frontal gyrus, OFC, valence, emotional control, anger, “yes”, “no”
During human development, the meaning of certain words acquires emotional valence and motivational significance via their repeated context-dependent association with rewarding or punishing events. The elementary commanding word used to prohibit or cease behavior is the word No while Yes is the word used to encourage or to continue it. The word No, considered the earliest and most potent relational word in language development, is both expressed and received while interacting with the social environment. For example, No is expressed as internal or external feedback to refuse commands, encode failure, or to negate propositions (Gopnik & Meltzoff, 1985; Gopnik & Melzoff, 1997; Peirce, 1869/1984). No is expressed from within and from the social environment, often as a command to stop ongoing or attempted behavior, and it may thus be experienced as unpleasant and be perceived as negatively valenced. Conversely, an emphatically expressed Yes could be encouraging and perceived as positively valenced. The neural mechanisms underlying the perception of the regulatory words No and Yes, and the relationship of this neural response to affective valence have not been studied thus far.
Evidence from non-human primate research demonstrates sensitivity of the orbitofrontal cortex (OFC) to gradations of intrinsic value, guiding approach/avoidance behavior preferences (Tremblay & Schultz, 1999). In humans, the OFC, which encompasses lateral and medial inferior prefrontal regions, is a paralimbic structure that receives inputs from each of the sensory association areas as well as from midbrain dopaminergic structures. As such it is well positioned to conjoin multimodal valence information as well as memory for previous punishment and reward associations (Zald & Rauch, 2006). Converging functional neuroimaging research implicates the OFC in the assignment of affective value (or valence) of a stimulus. For example, the OFC represents the reinforcement properties of feedback stimuli (Elliott, Frith, & Dolan, 1997); signaling boundaries for accepting or rejecting a choice of action (Elliott, Newman, Longe, & Deakin, 2003). Interestingly, during social conditioning No and Yes are universally used as feedback words. Saying No to oneself as internal feedback is of particular relevance to activity of the OFC since it is a common form of inner cognitive feedback affecting self control, which presumably is modulated by OFC function (Bechara, Damasio, & Damasio, 2000).
Underlying social conditioning, the perception of Yes and No probably relate to approach/avoidance and behavioral activation (BAS)/inhibition (BIS) systems (Hewig et al., 2004). Behavioral preferences triggering BIS in particular may have a role in one’s sensitivity to reinforcement contingencies (Gray & McNaughton, 1996). Hearing No, for example, could trigger a negative emotional response to the threat of losing one’s ongoing goal or motivation (as in hearing No when reaching for the cookie jar); withdrawing one’s hand from the “cookie jar” will require the enforcement of inhibitory resources which could be associated with BIS and right prefrontal regions (Sutton & Davidson, 2000).
The evidence indicates that lesions of the OFC are associated with acquired impairment in the processing of inhibitory emotional signals (Bechara et al., 2000). Alternatively, there are multiple independent reports attesting to the impairment of OFC function and structure in populations with poor behavioral control (for review see (Zald & Rauch, 2006)). The trait expression of anger is related to poor inhibitory control (R. J. Davidson, K. M. Putnam, & C. L. Larson, 2000) and not surprisingly, increased tonic OFC functioning is associated with the regulation of anger (R. J. Davidson et al., 2000) (Goldstein et al., 2005). However, healthy variability in the successful control of anger have only recently drawn attention (Hewig, Hagemann, Seifert, Naumann, & Bartussek, 2004).
This functional Magnetic Resonance Imaging (fMRI) study aims to investigate individual differences in the brain activation patterns in response to No and to Yes. We therefore recorded the behavioral and regional blood-oxygenation-level-dependent (BOLD) responses to emphatically spoken No and Yes compared to carefully matched control words in 23 non-smoking healthy male participants.
Drawing on the theoretical evidence, our first hypothesis was that an emphatically spoken No would be perceived as negatively valenced while Yes would be perceived as positively valenced. Based on the sensitivity of the OFC to emotional value attribution (O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001), we hypothesized that OFC response to these words would be associated with their respective valence attributions. Possibly, those who attend to No as a valenced signal are also more inclined to control their anger allowing them to perform the necessary behavioral change (as in hearing No and withdrawing from the cookie jar); ignoring No as a prohibitive signal would prevent its expression from reaching the threshold required to change behavior.
To test these hypotheses, we developed a simple fMRI task where participants listened to emphatically vocalized single words and also saw the words printed on the screen. No and its matched control word Up were expressed in an emphatically prohibitive tone; Yes and its matched control word Ten were expressed in an emphatically encouraging tone. Participants were required to press a button as soon as they detected the word stimuli and reaction times (RT) were recorded. Immediately following fMRI, the participants rated the words for valence, intensity and interest and reported their intrinsic (“free”) associations to the words. Self-report of anger control were obtained with the State-Trait-Anger Expression Index (STAXI) (Speilberger, 1988) prior to fMRI scanning.
Method
Participants
Twenty three healthy, non-smoking (by self-report and breath CO test), right-handed (as measured by self-report and a modified Edinburgh inventory, mean ± SD, 0.89 ± 0.36; values closer to 1 indicate right handedness) (Oldfield, 1971) male participants took part in this study. Only right-handed male participants were selected due to potential differences in hemispheric specificity of emotion (Heller & Levy, 1981) and language (Schirmer, Zysset, Kotz, & Yves von Cramon, 2004). These were young participants (10 Caucasians, 7 African Americans, 6 Hispanics and 1 Asian) ages 22 to 42 years old (29.7 ± 5.3 years), with 14.9 ± 2.5 years of education and English as first language. Participants were fully informed of the nature of the research and provided written consent in accordance with the Brookhaven National Laboratory Institutional Review Board. Initial phone screening and subsequent on site evaluation by a neurologist ensured that participants were able to understand and give informed consent and that they were 20–45 years of age and that exclusion criteria were met. These were a) cigarette smoking and/or past or present history of alcohol or drug abuse and positive pre-scan urine toxicology tests; b) contraindication to MRI (e.g., having implanted ferromagnetic parts or devices); c) history of neurological or psychiatric diseases; d) head trauma with loss of consciousness; e) history of cardiovascular or endocrinological disease; and f) current medical illness.
Task and Stimuli
A block-design task was used to present emphatic vocalizations with simultaneous visual display of four word stimuli: No, Up, Yes and Ten. No was matched to Up and Yes to Ten on number of letters, frequency of use in the English language (Kucera, 1982), and on emphatic vocalization that was accomplished as follows: eight separate utterances of each of the words were recorded from four males who were trained to sound emphatically prohibitive (for No, Up) or emphatically encouraging (for Yes, Ten). While uttering the words No and Up they were instructed to imagine that their child was running into the street and they had to stop the child. Then they were instructed to emphatically say “No Up” and “Up No” (counterbalanced pairs) to stop the child. While uttering Yes and Ten, the men were instructed to imagine that their child is winning at a game and they had to emphatically encourage the child with “Yes Ten” or “Ten Yes” (counterbalanced pairs), to cheer the child on). These recorded utterances were pseudo-randomized within and across each task run, such that there were no repeat utterances from the same male within a task ran.
These pair recordings were then visually matched for peak amplitude. A t-test analysis of the peak amplitudes (in Media wizard 11.0, CDH Productions, www.CDHNOW.com) with the No vs. Up and Yes vs. Ten stimuli was further conducted. These analyses showed no significant differences within these two pairs (p = 0.13 and 0.57, respectively). There was also no difference in peak amplitudes between No and Yes (p = 0.17). These recorded utterances were pseudo-randomized within and across each task run, such that there were no repeat utterances from the same male within a task run.
The visual stimulation (words in black Arial font size 72 on white screen) was presented via MRI-compatible goggles for visual display of the given utterance spelled out. For example, while the word No was uttered the subject could also read the word No on the screen. This visual display was time-locked with the utterances that were presented using modified headphones (Commander XG MRI Audio System, Resonance Technology INC.) that also reduced external acoustic noise by 28 dB. The combined auditory and visual presentation of the words was designed to facilitate accurate perception of the words by recruiting both auditory and visual linguistic processing.
Within each word block, there were 4 word stimuli each lasting 2000 milliseconds (ms) (1200 ms of auditory and visual and 800 ms visual only) and alternating with a 2000 ms fixation cross. There were four task runs. Each run was comprised of 8 pseudo-randomized 18-second word blocks (2 blocks of each word), alternating with 18-second fixation baseline without auditory or visual stimulation (see Figure 1 for a depiction of one run). All participants reported hearing the words. In order to acquaint the participant with task requirements (including visual and auditory stimulation and scanner noise), the task was preceded by 4 training blocks identical to the actual task (including emphatic utterances), except for the use of different words.
Figure 1.
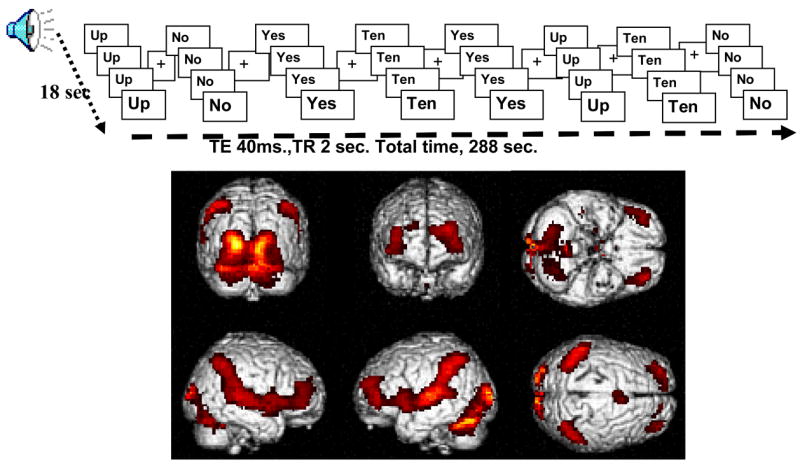
(top) A graphic scheme of one of the task runs with 8 word blocks alternating with fixation baseline of the same length. The complete task included 4 of these runs each with a different pseudorandomized order of word conditions. (bottom) BOLD images of the general task activations.
To confirm and maintain auditory and visual detection, participants were instructed to “respond by pressing the button as soon as you see and hear a word” and to“subvocally express the word.” Note that, albeit, sub-vocalizing was required across all the word stimuli in this paradigm, the ubiquitous use of No as internal feedback is an intrinsic feature of No as compared to the other words in this paradigm.
Functional Magnetic Resonance Imaging
MRI acquisition was performed on a 4-Tesla Varian/Siemens scanner, equipped with a whole-body SONATA gradient set. The BOLD responses were measured as a function of time using a T2*-weighted single-shot gradient-echo planar imaging (EPI) sequence (TE/TR = 20/2000 ms, 4 mm slice thickness, 1 mm gap, typically 33 coronal slices, 20 cm FOV, 64 × 64 matrix size, 90°-flip angle, 200 kHz bandwidth with ramp sampling, 4 dummy scans, 92 dB of sound pressure level). The coronal acquisition and the short echo time were implemented here to facilitate imaging of OFC regions susceptible to artifacts (Kringelbach & Rolls, 2004). Padding was used to minimize motion. Task performance and participant motion were determined immediately after each fMRI task run to ensure the button press response was maintained, and that motion was within the accepted threshold of 1 mm maximum displacement and 1° rotation (Caparelli, Tomasi, Arnold, Chang, & Ernst, 2003). The typical scan time was 40 minutes. A T1-weighted 3D-MDEFT sequence (Lee et al., 1995) (TE/TR = 7/15ms, 0.94 × 0.94 × 3 mm spatial resolution, axial orientation, 256 readout and 192 × 48 phase-encoding steps, 8 minutes scan time) was used to collect structural images that were inspected by the neurologist (F. Telang) to rule out gross morphological abnormalities.
Behavioral Measures
Reaction times and performance accuracy were recorded throughout the fMRI task. Immediately following the fMRI scanning, participants were instructed to rate their reactions to each of the four words (and not to the single utterances) that were presented during the scan, on “how negatively or positively you felt about the word” using a visual analogue scale (−10, extremely negative to +10, extremely positive) and “how mild or intense you felt the word to be” to rate the subjective intensity of the spoken words (−10, extremely mild to +10 extremely intense) and the same was performed for measures of interest in the words. Finally, participants were instructed to “write a sentence or a phrase on whatever comes to your mind regarding each of the words as was presented to you in the MRI scanner” (i.e., their associations to each of the words).
We chose the assessment of trait emotional control to be the control of anger (R. J. Davidson, K. M. Putnam, & C. Larson, L., 2000). The State-Trait Anger Expression Inventory-2 (STAXI-2) is a self-report questionnaire that makes an important distinction between emotional state and trait, and between anger expression and control of anger at both the state and trait levels (Speilberger, 1988). We used the subscale of Anger Control-Out (AC-O, ranging from 8, less control, to 32, more control) that assesses trait emotional control of angry feelings by inhibiting the expression of anger toward other persons or objects in the environment. In contrast, individuals with low AC-O do not successfully monitor or prevent the outward expression of their anger. The parallel state subscale was used to assess the intensity of current anger and its expression (ranging from 15, no anger, to 60, intense anger ready to be expressed currently) (Speilberger, 1988). Based on a sample of 609 healthy male adults, alpha coefficients of internal consistency were 0.84 for the AC-O and 0.94 for the state anger subscales; concurrent validity was also established and produced correlations of up to 0.71 with other known anger scales (Speilberger, 1988). All participants completed the STAXI-2 several hours (n = 18) or 2–5 days before the fMRI (n = 5).
Analysis of Behavioral Measures
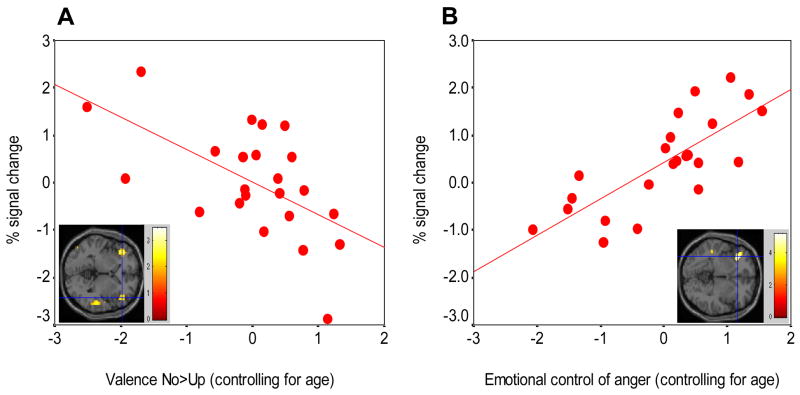
Reaction times and performance accuracy were averaged within each word condition and across all 4 runs; repeated measures ANOVA with Bonferroni correction was then used in SPSS (Stevens, 1992) for these comparisons between word conditions. For figure 2 B, RT to the differential contrasts No-Up and Yes-Ten were displayed and subjected to paired sample t-test. Distributions of the subjective behavioral scales, valence, interest and intensity were not normally distributed and were therefore analyzed with the non-parametric Wilcoxon Signed Ranks Test for pairwise comparisons (Stevens, 1992). Figures with means and standard error for the subjective behavioral scales as well as for the task performance are available in Supplementary Online Results. Age correlated slightly with valence assignment to No but not Up and with AC-O in a direction consistent with previous studies showing a relationship between young age and poorer behavioral control (Manuck, Flory, Muldoon, & Ferrell, 2002). We therefore accounted for age by saving and then using the standardized residuals from simple regressions with valence or AC-O as the dependent variables and age as the independent variable. These were used for the correlations in Figure 3 A and B.
Figure 2.
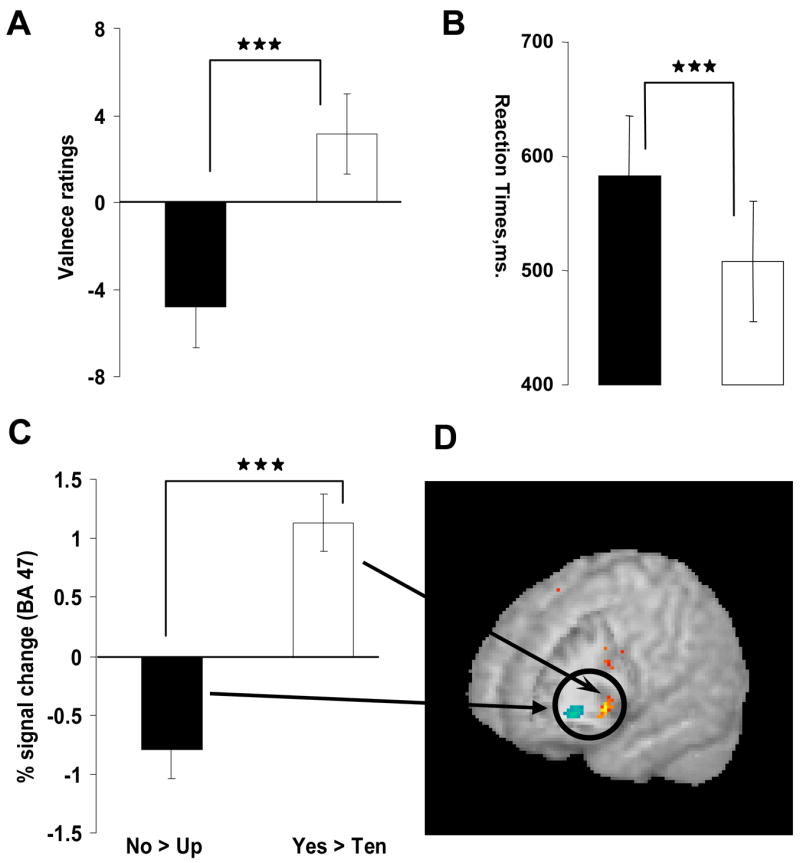
Differential brain and behavioral responses to No and Yes (N = 23). (a) Post-scan ratings of valence (−10 most negative to +10 most positive) to the contrasts No-Up (black bar) and Yes-Ten (white) (non-parametric Wilcoxon, Z = −4.6, p < 0.0001). (b) Reaction-times to the contrasts No-Up (black bar) and Yes-Ten (white) (t = −5.5, p < 0.0001). Note, the y axis starts with 400 ms. (c) percent signal change in the right lateral OFC, BA 47, for -No-Up (Talairach coordinates: 30, 33, −3) and Yes-Ten (coordinates: 33, 30, −9), t = −5.9, p < 0.0001. Error bars at a–c represent ± standard error. (d) Corresponding image.
Figure 3.
Correlations between behaviors, corrected for age, and BOLD changes (z-scores) in the lateral OFC in response to No. (a) Scatter plot shows association between the BOLD signal change for -No-Up in the right OFC (x = 42, y = 36, z = 9) and valence No-Up with a linear regression line (r = −0.59, p < 0.001) and with an imbedded corresponding image. The left OFC activation was not significant (b) Correlation between control of anger and BOLD signal change for No-baseline in the right OFC (x = 36, y = 30, z = −3) and a linear regression line (r = 0.77, p < 0.001) with an imbedded corresponding image (Left = Right).
MRI Processing and Data Analyses
Primary reconstruction of EPI scans and analysis of fMRI data sets was performed in IDL language and package (Research Systems, Boulder, CO) using phase correction to deghost the EPI time series (Buonocore & Gao, 1997). The SPM2 package (Wellcome Department of Cognitive Neurology, London UK) was used for subsequent analyses. A six-parameter rigid body transformation (3 rotations, 3 translations) was used for image realignment to correct for head motion. The realigned datasets were normalized to the Talairach frame (Talairach J, 1988) with a 12 parameters affine transformation (Ashburner, Neelin, Collins, Evans, & Friston, 1997), using a voxel size of 3x3x3 mm3. An 8-mm full-width-half-maximum Gaussian kernel was used to smooth the data. A general linear model (Friston et al., 1995) and a castle design with four conditions (No, Up, Yes and Ten) convolved with a canonical hemodynamic response function were used to calculate the activation maps. The time series were band pass filtered with the hemodynamic response function as low pass filter and 1/560 sec cut-off frequency as high-pass filter.
Statistical analysis of fMRI data
To identify significantly activated brain areas, a voxel based statistical analysis was performed with SPM2 with the contrasts (No, Up, Yes, Ten - baseline and No-Up, Yes-Ten) applied to the parameter estimates of each participant for each run separately. Examination of the differential contrasts -No-Up and Yes-Ten at each of the 4 runs separately (thus including only 8 stimuli at each run) resulted in the observation that the OFC t-values dropped with time from 3.94 in the first run to 1.59 in the 4th run. This drop in signal which was also observed for No-baseline and Yes-baseline probably reflects habituation effects (Garavan, Kelley, Rosen, Rao, & Stein, 2000) compromising statistical power. Therefore, the contrast maps for each condition and participant were averaged across all 4 runs resulting in the maximal number of events per condition (32 per word).
A voxel-based one-way repeated measures ANOVA model with the four averaged word conditions, No, Up, Yes, Ten - baseline was used to create group activation results; activation was thresholded at p < 0.05 using a Family Wise Error (FWE) correction and a minimum cluster size of 15 contiguous voxels (270 mm3). Using this repeated measures ANOVA, we also report the differential activations No-Up, Yes-Ten which were thresholded at p < 0.005 uncorrected, with a minimum cluster size of 5 contiguous voxels (135 mm3). Small volume correction with a 12 mm sphere was applied to the OFC (BA 46, 47), our a priori region of interest.
Voxel-based correlations in SPM2 using simple linear regression analyses were conducted with the SPM contrasts (No-baseline, No-Up, etc.) as dependent variables and the behavioral measures as covariates. A threshold of p < 0.005 uncorrected was applied with a minimum cluster size of 5 contiguous voxels and small volume correction as described above.
Analysis with regions-of-interest
Functional ROIs with a volume of 27 voxels (0.729 cc, isotropic) were defined at each of the brain regions that were derived from voxel-based SPM2 analyses and listed in rightmost columns of Tables 1 and 2. This was done to calculate the average BOLD responses (% signal change) in these regions and to then use these ROI measurements in SPSS with repeated measures ANOVAs to validate the voxel-based findings and to conduct post-hoc comparisons (Tomasi, Ernst, Caparelli, & Chang, 2006). For example, the bars in figure 2 C represent the difference in the means of these ROIs of OFC response for -No-Up or Yes-Ten. These ROI measurements were also used in two-tailed Pearson correlations with the selected behavioral measures valence ratings and anger control listed in tables 1 and 2 and depicted in figures 3 A and B (represented as Z scores).
Table 1.
Functional MRI: Brain Regions for the word contrasts and Correlations of the Mean Voxel Value from these Regions with Valence and Emotional Control
| BA | S | Size | T | p | x | y | z | a ROI correlations (r) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Valence | AC-O | |||||||||
| No-Up | ||||||||||
| Inferior frontal gyrus | 47 | R | 77 | 4.22 | 0.010 | −30 | 33 | −3 | −0.47* | 0.52** |
| Yes-Ten | ||||||||||
| Inferior frontal gyrus | 47 | R | 73 | 4.87 | 0.019 | −33 | 30 | −9 | 0.42* | 0.30 |
| Inferior frontal gyrus | 47 | R | 4.33 | −42 | 24 | −3 | 0.22 | 0.03 | ||
| Superior temporal gyrus | 22 | R | 215 | 4.14 | 0.020 | −54 | −42 | 9 | −0.10 | 0.14 |
| Superior temporal gyrus | 22 | R | 4.12 | −57 | −51 | 9 | −0.01 | 0.23 | ||
| Superior temporal gyrus | 29 | R | 4.18 | −51 | −33 | 15 | −0.11 | 0.15 | ||
Note. BA: Brodman Area, S: hemisphere, Size: number of voxels in cluster, P: corrected.
AC-O: Anger Control-Overt subscale of the State-Trait Anger Expression Inventory-2 (STAXI-2) ranging in scores from 8 (less control) to 32 (more control).
The two columns to the right labeled ROI correlations report the extracted ROI (as detailed in Method) from the respective voxel-based SPM2 analysis reported on the left-hand columns; these ROIs were subjected to correlational analysis with valence and AC-O.
p < 0.05,
p < 0.005
Table 2.
Voxel based correlations with BOLD to reveal the Neural Correlates of Valence and Emotional Control
| BA | S | Size | T | pb | x | y | z | a ROI correlations (r) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Valence | AC-O | |||||||||
| Valence ratings with the contrasts: | ||||||||||
| No-Up | ||||||||||
| Inferior frontal gyrus | 46 | R | 19 | 3.47 | 0.053 | −42 | 36 | 9 | −0.59† | 0.44* |
| Inferior frontal gyrus | 45 | L | 204 | 2.78 | 0.083 | 45 | 33 | 0 | −0.48* | 0.06 |
| Yes-Ten | ||||||||||
| Inferior frontal gyrus | 47 | R | 157 | 3.06 | 0.067 | −30 | 15 | −9 | 0.54† | 0.03 |
| Control of Anger (AC-O) | ||||||||||
| Inferior frontal gyrus | 47 | R | 85 | 5.87 | 0.001 | −36 | 30 | −3 | −0.51** | 0.77† |
| Inferior frontal gyrus | 45 | R | 3.55 | −45 | 33 | 3 | −0.50** | 0.62† | ||
Note.
The two columns to the right labeled ROI correlations report the extracted ROI from the respective voxel-based correlational analysis, reported on the left-hand columns.
small volume correction.
p < 0.05,
p < 0.005,
p < 0.001
Results
Behavioral Results
Behavioral results showed that No was the most negatively valenced while Yes was the most positively valenced word (Wilcoxon Signed Ranks Test, all Z = −3.60 to −4.63, p < 0.0001; No-Up-Ten-Yes) (Figure 2 A). Ratings of subjective intensity of the word stimuli did not differ for No-Up and No-Yes (Wilcoxon Signed Ranks Test, Z = −0.193 to −0.50, p > 0.61) but Yes tended to be more subjectively intense than Ten (Z = −2.20, p = 0.027). Similarly, ratings of interest did not differ between the words (Z = −1.0 to −0.48, p = 1.00 to 0.67), indicating that self-report measures of valence to No and Yes were not influenced by interest albeit somewhat by intensity (see also Supplementary Online Results). A qualitative evaluation of the participants’ associative responses was consistent with the valence ratings; associations to No were negative with a prohibitive theme (e.g., you can’t do this, dad disciplines when I was a kid, punishing a dog) while associations to Yes were positive with a theme of encouraging behavior (cheering someone on, you can do it!, women I have sex with). Associations to Up (arrow going up, getting up, ascending the escalator) and Ten (giving an answer in math class, getting the right number, ten minutes left) were more neutral.
Reaction time measures, to an extent, paralleled this subjective valence contrast between the words (Repeated measure ANOVA, F = 25.06, p < 0.0001). Participants required a significantly longer time to respond to No than to Yes (mean difference ± standard error, 78.7 ± 11.1 ms. p < 0.0001, 99% confidence interval, Bonferroni corrected) (Figure 2 B) with a trend for No-Up (25.1 ± 9.4 ms., p = 0.071). However, a significant RT difference between Up and Ten (40.5 ± 7.0 ms., p = 0.01) was also observed, indicating that response time differences are at least partly related to the emphatic vocalization difference between the word pairs No, Up and Yes, Ten. Response omissions were minimal (range 0 to 6) in this simple detection task, showing no difference across subjects or between word conditions (Wilcoxon Signed Ranks Test, all Z < −0.07, all p > 0.35) (Supplementary Online Results) and also showing that all subjects were able to hear and see the word stimuli.
Scores on the AC-O in this sample ranged from 17 to 32 (mean ± SD, 26.1 ± 4.5) which is comparable to the normative male population (24.6 ± 4.9) (Speilberger, 1988). As noted above, though not significant, younger subjects tended to report less anger control (r = −.26, p = 0.101) and to rate No (r = −.36, p = 0.095) but not Up (r = .10, p = 1.2) as less negative. Accounting for age, AC-O correlated with valence ratings to No (r = −0.45, p = 0.05) and showed a trend with Yes (r = 0.36, p = 0.09) but not with Up (r = −0.14, p = 0.43) or Ten (r = 0.05, p = 0.75). Thus the more the control of anger, the more was the tendency to assign negative valence to No and a trend toward assigning positive valence to Yes, possibly indicative of an overall more sensitivity to feedback signals. State anger levels were at minimum in this sample (15.6 ± 1.1) and they did not correlate with valence ratings to any of the words (p > 0.30).
Brain Activity
All study participants showed task related activations compared to baseline (No, Up, Yes, Ten - baseline; Figure 1, bottom) bilaterally in middle and superior frontal gyrus (Brodmann areas 9, 6, and 10), superior temporal gyrus (Wernicke, BA 22, and Heschl’s gyrus in the primary auditory cortex, BA 41), inferior parietal gyri (BA 40) and precuneus (BA 7), the primary visual areas (BA 17), and in the insula (BA 13), thalamus, caudate and putamen, and cerebellum. This activation pattern is consistent with activations for speech perception (Burton, Locasto, Krebs-Noble, & Gullapalli, 2005), visual processing and emotional prosody (Maddock, Garrett, & Buonocore, 2003) showing that the subjects were able to hear and see the word stimuli.
Table 1 lists areas of activations and the volume of the activation clusters corresponding to the a priori differential contrasts (No-Up, Yes-Ten; Figure 2 C), which by design controlled for BOLD changes related to language and prosody (See supplementary online results, Figure 3). Compared to the matched control word, fMRI response to No revealed a negative BOLD signal in the right lateral and posterior aspect of the OFC (BA 47, Figure 2C and 2D). Inspection of this region’s (at x = 30, y = 33, z = −3 mm) response to No-baseline indeed showed mean signal decreases to the word (mean = −0.21%) although responses were quite variable across subjects (SD = 1.01%, SEM = 0.21%). This negative signal uniquely to No, indicates that during rest periods (fixation baseline), subjects activated the OFC more than during the word condition, or alternatively that the No condition led to deactivation of the OFC.
In contrast, compared to the matched control word, a positive right OFC BOLD signal was observed in response to Yes. Inspection of this region’s response (at x = 33, y = 30, z = −9, BA 47) to Yes-baseline indeed showed mean signal increases (mean = 0.35), again, with pronounced variability across subjects (SD = 1.01, SEM = 0.22). Note that Yes-Ten activations were also observed in the superior temporal gyrus (BA 22, 29), possibly attributable to the intensity differences between these words; other studies have shown response in this area to subjective intensity dimensions of auditory stimulation (Belin et al., 1998; Grandjean et al., 2005).
Brain and Behavior Correlations
As evidenced by the ROIs (Table 1 right) corresponding to the OFC findings, assignment of more negative valence specifically to No or more positive valence specifically to Yes was related to an increased right lateral OFC respective response. Note that the superior temporal gyrus was not related to the corresponding valence ratings (follow-up voxel-based correlations supported these null findings). Table 2 lists voxel-based correlations that were performed to validate the above ROI correlations. As Figure 3 A shows, the more bilateral OFC signal change to No the more it was rated negatively (Right, r = −0.59, p < 0.001; Left r = −0.48, p < 0.05). Note, however, that the left side was not significant using voxel-based analysis. More positive valence ratings to Yes-Ten also correlated with increased right lateral OFC (Left, r = 0.54, p < 0.001); however this activation was only significant at a lower voxel-based threshold of p < 0.01, uncorrected.
Better trait control of anger, as measured with the AC-O variable, was associated with stronger response of the right lateral OFC to No-Up (r = 0.52, p < 0.005). The voxel-based correlation with the differential contrast No-Up produced the same OFC region but it was not significant at p < 0.005, possibly due to the restricted range of values produced by this contrast. A stronger correlation was observed between No-baseline and AC-O (r = 0.77, p < 0.001; Figure 3 B). Thus, in contrast to the main effect of deactivation to No, right OFC response actually increased over baseline in subjects who reported having more anger control and more negative valence attributions to No. Note that there were no significant correlations with state anger (data not shown).
Discussion
This study documented unique brain-behavior responses to the emphatic behavior-modifying words No and Yes. We reported opposite valence, RT, and right lateral OFC responses to No versus Yes. The results also revealed associations between greater OFC response to No and negative valence attribution and better control of anger.
Behavioral Responses to No and to Yes
Participants rated No as producing negative valence. This was predicted given No’s involvement starting at childhood in stopping behavior and registering failure (Gopnik & Melzoff, 1997). The word Yes was rated as positively valenced. Thus, it appears that No and Yes are associated with contrasting emotional assignment, at least at the self-report level (Figure 2 A). Further research on psychophysiological correlates to Yes and No utterances will provide additional validation of the emotional involvement by these regulatory words.
The results also show that RT to No was significantly slower than to Yes. This finding was surprising since we did not create conditions in this blocked detection task to evoke inhibitory control. Thus, it is possible that this finding could be due to chance and it should be regarded with caution. Another possibility, however, is that the tone in which each of the word pairs (No, Up and Yes, Ten) was expressed has produced the RT differences, as indicated by the significant RT difference between Up and Ten. Although Up and Ten are neutral in terms of their semantic meaning, these words were expressed in emotional tones - Up as forbidding and Ten as encouraging. Although we observed a greater valence contrast between No and Yes as compared to Up and Ten, it is important to note that the two emotion-related properties, semantic meaning and tone, could not be dissociated in this paradigm. Such dissociation awaits future studies.
It is also possible that the prosody and the conditioned meaning of No are not dissociable during early learning so that the synergistic relationship between the word meaning and the tone in which it is expressed is paramount to appropriate conditioning during childhood (Thompson, 1994). Nevertheless, if future studies will replicate the slowed RT to No with more appropriate controls, we suggest an interpretation reminiscent of a Stroop-like effect (Carter et al., 2000). More specifically, participants may have had to overcome interference due to the incongruence between the task demand to press a button and the conditioned tendency to stop behavior when emphatically ordered to do so (No!). This interpretation is partly supported by the finding that RT to Yes (press a button when encouraged by Yes!) was the fastest of all the words, suggestive of a facilitation effect (Figure 2 B).
Although to continue a desirable behavior is perhaps as important as to cease an undesirable behavior, it is noteworthy and interesting that Yes is not typically used or investigated in behavioral conditioning in early development. In this sense, it is not entirely accurate to suppose that No and Yes are diametrically opposite. Rather, No and Yes are related as negative and positive emotions are related, where there is a phylogenetically modulated bias toward negative information (Smith, Cacioppo, Larsen, & Chartrand, 2003).
In this study, the control of anger in daily situations related to subjective ratings of valence attribution to No. That is, participants who reported more anger control at the trait level showed emotional reactivity by assigning more affective value to No. This finding is analogous to recent empirically supported theories postulating that young children’s sensitivity to rules relates to their assignment of value to externally expressed or internalized disapproval of the parent (Kochanska & Aksan, 2006). Those who perceived No as strongly negative (which was a minority in this sample) may be particularly sensitive to negative feedback and punishment.
Involvement of the OFC in No and Yes
We documented contrasting response in the lateral OFC to No and Yes both at the resting baseline comparison and after controlling for general language related activations (e.g., No-Up). Participants uniquely produced a negative BOLD signal in the OFC to No (Figure 2 C). There are several contrasting interpretations of the negative BOLD signal in fMRI. The hypothesis that the negative BOLD signal stems from an active suppression of neural activity employed by the brain to control the distraction of task-irrelevant neural processes is of particular interest (Raichle et al., 2001; Tomasi et al., 2006). This model posits that deactivation reflects the transition from a constrained neural state (during task periods) to a less constrained state (during rest periods). Alternative models of the negative BOLD signal posit that what others call ‘deactivation’ may simply represent a hemodynamic vascular response to cerebral blood flow in adjacent regions as a compensation for the blood flow needs in another region (Hoge et al., 1999). In the context of this study, we interpret the main effect of deactivation to No as an effortful attempt to control the task-irrelevant distraction of hearing No while doing the incongruent action of pressing a button upon the word’s detection.
The positive OFC signal uniquely to Yes (Figure 2 C) is expected due to its association with positive emotions though its right lateral (versus left or medial) location raises further questions about what Yes might mean particularly as it is presented in this paradigm.
Asymmetry of Emotion and Content Specificity
Here we discuss these right lateralized OFC findings drawing from theoretical models of hemispheric asymmetry of emotion (Davidson, 1992), BIS (Ellison-Wright et al., 2004) and reward vs. punishment parsing of the OFC (Elliott, Dolan, & Frith, 2000). It has been accepted that the right prefrontal cortex is generally associated with negatively valenced material and inhibition and the left prefrontal cortex is associated with positive valence and approach motivation (Harmon-Jones, 2004).
Therefore, it would be predicted that No, being negatively valenced and inhibitive, will trigger BIS and produce right OFC response. Our findings showing right asymmetry to No are consistent with the valence model that postulates that the right hemisphere is associated with negative emotions and with behavioral inhibition (BIS) in response to punishment (i.e., the word No) (Wheeler, 1993). Similarly, according to the motivational direction model, hearing No would likely trigger motivation to withdraw, which too has been associated with right PFC/OFC activation (Davidson, 1998). Moreover, the word No is used as a punishment in operant conditioning to inhibit behavior, and behavioral inhibition (BIS) has been associated with right brain activation (Gray et al., 1982).
Following this reasoning Yes would be expected to produce left lateralized response, as it was rated positively, and Yes could also be perceived as producing BAS and motivation to initiate approach behavior. However, our findings showing right lateralized response to Yes, do not follow valence and BIS/BAS predictions. Although the OFC has been reliably represented in studies of emotional valence, there is less consistency in the interpretation of asymmetry across studies that used different imaging modalities and different targets of stimulation (visual, auditory, etc.).
Lateral versus medial parsing of the OFC has prompted great interest and investigation. A large meta-analysis by Kringelbach and Rolls (2004) reviewed and synthesized evidence that pointed to the lateral OFC being reliably represented in studies of reinforcement associations and modification of one’s own behavior in response to changed contingencies in the environment. The authors’ meta-analysis supported findings whereby medial parts of the OFC were represented in studies involving pleasant stimuli and lateral aspects of the OFC were active in response to perceived negative and punishing stimuli. With regard to reward and punishment representations in the OFC, it is conceivable to suppose that No is associated with punishing contingencies and Yes with rewarding contingencies, especially in the emphatic tone these words are expressed in the current study. However, the lateral OFC was represented in both word stimuli in our study.
There are several possible explanations to the lack of a medial versus lateral dissociation of the OFC response to Yes versus No, respectively. Here we suggest that the content specific representation of language which is robustly and consistently represented in the lateral OFC may be responsible for the exclusively lateral findings. Even language with emotional weight and arguably symbolic representation of reward and punishment is mostly represented in the lateral aspects of the OFC (Murphy, Nimmo-Smith, & Lawrence, 2003) possibly due to the use of a language task versus a task with facial emotions or with somatosensory stimuli, for example. Since, as noted earlier the anatomical connections between the OFC and visual and auditory inputs are located in the lateral and not the medial OFC, this information supports the lateral OFC representation in this fMRI task which involves auditory and visual language stimulation. The studies reviewed above are relevant to the current investigation inasmuch as the words No and Yes are hypothesized to play a role in social conditioning by modulation of OFC response to their emotional valence and its reputed role in the mediation of reinforcement learning.
Valence Attribution and Emotional Control
The distribution of OFC responses was quite variable across participants. Extensive individual differences in BOLD response specifically to emotional stimuli are not uncommon, especially in prefrontal regions which may be developmentally sensitive to environmental impacts and stressors (Liston et al., 2006). This variability is a common feature of neuroimaging studies but it may also reflect the considerable structural variability between individuals found particularly in lateral aspects of the OFC (Kringelbach & Rolls, 2004). A study investigating gray matter maturation from childhood to age 21, has shown considerable variability in gray matter density and overall late maturation of the OFC compared to other ventral brain regions (Gogtay et al., 2004). This finding aligns with studies showing individual variability in responsivity to reinforcement contingencies and the developmental trajectory of the capacity to execute goal directed behavior (Berlin, Rolls, & Kischka, 2004). This variability could be partially explained by individual differences in relevant personality traits and task-related ratings (Canli, 2004).
Here we reported that the subjects’ valence assignment to hearing and seeing No, was related to the degree of response in their bilateral OFC while hearing and seeing No (Figure 3 A). In this context we can observe right OFC activity due to its correlation specifically with negative valence attribution (Davidson, 2004). The results are consistent with the extensively documented role of the OFC in the assignment of valence (for review see (Rolls, 2004)) and in its involvement in socially or externally assigned reward and punishment contingencies (O’Doherty et al., 2001).
Next, we found that the self-reported control of anger was related to increased right OFC response to hearing and seeing No. Our findings are consistent with earlier studies that found anger control to be associated with right brain activation, withdrawal motivation and behavioral inhibition (Hewig et al., 2004). In a recent EEG study, Hewig et al. (2004) investigated the relationship between valence, motivational direction, BIS/BAS and anger control. They found that anger control was correlated with activation on the right side and concluded that these findings are best explained by the motivation direction model, in which right brain activation is associated with the motivation to withdraw.
As mentioned earlier, participants who reported better control of anger also assigned more negative valence specifically to No and activated the OFC to No (Figure 3 B). In contrast to the deactivation main effect, the correlation analyses revealed that it is this region’s activation to No that is associated with the more adaptive trait behavioral response. Therefore, these findings suggest a relationship between sensitivity to inhibitory cues, anger control, behavioral inhibition, and right OFC activation. Hence, individuals with greater sensitivity to inhibitory cues may be more adept at controlling their anger and inhibiting behavior that could elicit punishment. Note that these correlations were specific to OFC response to hearing No emphatically expressed, and did not extend to the other words.
In contrast, individuals who showed a deactivation of OFC signal to No are the ones who show reduced sensitivity to the valence of this prohibitive signal (rating it as less negative) and report less control of their own anger. From the distributions of OFC response, it could be construed that deactivation is the adaptive response, especially if deactivation connotes a state of effortful constraint. Since our sample consists of healthy control participants and the variation in anger control is well within a normal range, it would be difficult to extrapolate which direction the BOLD signal to No may take in populations exhibiting a pathological lack of anger control.
Nevertheless, the generalizability of these findings to disordered populations is of interest as it has some face validity to propose that antisocial individuals will respond differently to an emphatic No as would individuals who can control their anger. Studies with antisocial individuals and those with OFC damage reported that poor behavioral control is associated with decreased sensitivity to cues that convey emotional significance (Bechara et al., 2000; Raine, Lencz, Bihrle, LaCasse, & Colleti, 2000). Through its connections with structures subserving memory functions, the OFC has a major role in the evaluation of incoming inputs through comparison with previous experiences (London, Ernst, Grant, Bonson, & Weinstein, 2000). The decreased sensitivity associated with OFC impairment may relate to limited access to previous emotional experience. Additional fMRI studies investigating brain response to regulatory words with adults with impulse control disorders may further elucidate the role of the OFC in emotion regulation and sensitivity to prohibitive commands.
Conclusions drawn from the current study point to several broad suggestions for future studies: The specificity of the OFC response to these regulatory words and the relationship to subjective valence and control of emotion extend these novel findings for potential use in evaluating linguistic markers of inhibitory control. We suggest that use of the word No and similarly prohibitive words in paradigms of inhibitory control will shed light on the utility of prohibitive language in emotion regulation and will add to the growing number of cognitive-emotional fMRI paradigms used to advance understanding of complex brain-behavior relationships (Strauss et al., 2005).
Further, we speculate that the OFC’s unique role in processing valence information also aids in the regulation of responses by emotionally facilitated choices regarding desirable or undesirable outcomes. Our results with healthy adults specifically suggest that it is this region’s responsivity to an abstract inhibitive cue that contributes to some of the normal variability in the ability to attribute valence and to control emotion. We suggest that those who heed No, are perhaps more sensitive to reinforcement contingencies (such as punishment in the case of No) and in their daily lives, are more skilled at controlling their outward expression of anger in order to attain long-term goals and to avoid punishment.
Finally, since language acquisition and the emotional meaning of words parallels frontal brain development (Sowell et al., 2003), we further speculate that this development impacts the OFC and its interaction with internally and externally imposed inhibitory demands (Levesque et al., 2004). Thus, inasmuch as sensitivity to the prohibitive command No develops during childhood through interaction with the primary caretakers as the first social objects, our findings may implicate the lateral OFC in the neurobiological substrates of early emotion regulation and subsequent social and prefrontal brain development.
Supplementary Material
Acknowledgments
We thank the reviewers for their helpful review. We thank Alex Reben for helping us with voice recordings. This study was supported by grants from U.S. Department of Energy Office of Biological and Environmental Research (under contract DE-AC02-98CH10886), NARSAD Young Investigator Award, NIH/T32 DA07316-01A1 (to Alia-Klein) and the General Clinical Research Center (5-MO1-RR-10710).
References
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into image registration. Neuroimage. 1997;6(4):344–352. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- Bechara AB, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Belin P, Zilbovicius M, Crozier S, Thivard L, Fontaine A, Masure MC, et al. Lateralization of speech and auditory temporal processing. J Cogn Neurosci. 1998;10(4):536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Brain. 2004. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. [DOI] [PubMed] [Google Scholar]
- Buonocore MH, Gao L. Ghost artifact reduction for echo planar imaging using image phase correction. Magn Reson Med. 1997;38(1):89–100. doi: 10.1002/mrm.1910380114. [DOI] [PubMed] [Google Scholar]
- Burton MW, Locasto PC, Krebs-Noble D, Gullapalli RP. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage. 2005;26(3):647–661. doi: 10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. J Pers. 2004;72(6):1105–1132. doi: 10.1111/j.1467-6494.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage. 2003;20(2):1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20(1):125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation-A possible prelude to violence. Science. 2000;289:591–572. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Elliott R, Frith CD, Dolan RJ. Differential neural response to positive and negative feedback in planning and guessing tasks. Neuropsychologia. 1997;35(10):1395–1404. doi: 10.1016/s0028-3932(97)00055-9. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright Z, Heyman I, Frampton I, Rubia K, Chitnis X, Ellison-Wright I, et al. Heterozygous PAX6 mutation, adult brain structure and fronto-striato-thalamic function in a human family. Eur J Neurosci. 2004;19(6):1505–1512. doi: 10.1111/j.1460-9568.2004.03236.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA. Practice-related functional activation changes in a working memory task. Microsc Res Tech. 2000;51(1):54–63. doi: 10.1002/1097-0029(20001001)51:1<54::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Leskovjan AC, Fowler JS, Wang GJ, Gur RC, et al. Anger and depression in cocaine addiction: association with the orbitofrontal cortex. Psychiatry Res. 2005;138(1):13–22. doi: 10.1016/j.pscychresns.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Gopnik A, Meltzoff A. From people, to plans, to objects: changes in the meaning of early words and their relation to cognitive development. Journal of Pragmatics. 1985;9:495–512. [Google Scholar]
- Gopnik A, Melzoff AN. Words, thoughts and theories. Cambridge, Mass: Bradford, MIT Press; 1997. [Google Scholar]
- Grandjean D, Sander D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, et al. The voices of wrath: brain responses to angry prosody in meaningless speech. Nat Neurosci. 2005;8(2):145–146. doi: 10.1038/nn1392. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: Reprise. In: Hope DA, editor. Perspectives on anxiety, panic and fear. Vol. 43. Lincoln: University of Nebraska Press; 1996. pp. 61–134. [PubMed] [Google Scholar]
- Harmon-Jones E. Contributions from research on anger and cognitive dissonance to understanding the motivational functions of asymmetrical frontal brain activity. Biol Psychol. 2004;67(1–2):51–76. doi: 10.1016/j.biopsycho.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Heller W, Levy J. Perception and expression of emotion in right-handers and left-handers. Neuropsychologia. 1981;19(2):263–272. doi: 10.1016/0028-3932(81)90110-x. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Naumann E, Bartussek D. On the selective relation of frontal cortical asymmetry and anger-out versus anger-control. J Pers Soc Psychol. 2004;87(6):926–939. doi: 10.1037/0022-3514.87.6.926. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med. 1999;42(5):849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Aksan N. Children’s Conscience and Self-Regulation. Journal of Personality. 2006;74(6):1587–1618. doi: 10.1111/j.1467-6494.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kucera F. Frequency Analysis of English Usage. Boston: Houghton Mifflin; 1982. [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34(3):308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129(2):361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-Induced Alterations in Prefrontal Cortical Dendritic Morphology Predict Selective Impairments in Perceptual Attentional Set-Shifting. J Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184–06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10(3):334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Muldoon MF, Ferrell RE. Central nervous system serotonergic responsivity and aggressive disposition in men. Physiol Behav. 2002;77(4–5):705–709. doi: 10.1016/s0031-9384(02)00922-8. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Handedness Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peirce C. Grounds of validity of the laws of logic: further consequences of four incapacities. In: Fisch MH, Kloesel CJ, editors. Writings of Charles S. Peirce: A Chronological Edition. Vol. 2. Bloomington, Indiana: Indiana University Press; 18691984. pp. 211–241. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colleti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55(1):11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Zysset S, Kotz SA, Yves von Cramon D. Gender differences in the activation of inferior frontal cortex during emotional speech perception. Neuroimage. 2004;21(3):1114–1123. doi: 10.1016/j.neuroimage.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41(2):171–183. doi: 10.1016/s0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Speilberger C. Manual for the State-Trait Anger Expression Inventory. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. 2. Lawrence Erlbaum, Associates; NewJersey: 1992. [Google Scholar]
- Strauss MM, Makris N, Aharon I, Vangel MG, Goodman J, Kennedy DN, et al. fMRI of sensitization to angry faces. Neuroimage. 2005;26(2):389–413. doi: 10.1016/j.neuroimage.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia. 2000;38(13):1723–1733. doi: 10.1016/s0028-3932(00)00076-2. [DOI] [PubMed] [Google Scholar]
- Talairach JTP. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thompson RA. Emotion regulation: a theme in search of definition. Monogr Soc Res Child Dev. 1994;59(2–3):25–52. [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: An intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Zald D, Rauch S, editors. The Orbitofrontal Cortex. USA: Oxford University Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.