Abstract
Alcohol use disorders (AUD) and chronic cigarette smoking are common among individuals with human immunodeficiency virus infection (HIV). Concurrent AUD in HIV is related to greater abnormalities in brain morphology and neurocognition than either condition alone. However, the potential influence of chronic smoking on brain morphology and neurocognition in those concurrently afflicted with AUD and HIV has not been examined. The goal of this retrospective analysis was to determine if chronic smoking affected neurocognition and brain morphology in a subsample of HIV-positive non–treatment-seeking heavy drinking participants (HD+) from our earlier work. Regional volumetric and neurocognitive comparisons were made among age-equivalent smoking HD+(n = 17), nonsmoking HD+(n = 27), and nonsmoking HIV-negative light drinking controls (n = 27) obtained from our original larger sample. Comprehensive neuropsychological assessment evaluated multiple neurocognitive domains of functioning and for potential psychiatric comorbidities. Quantitative volumetric measures of neocortical gray matter (GM), white matter (WM), subcortical structures, and sulcal and ventricular cerebral spinal fluid (CSF) were derived from high-resolution magnetic resonance images. The main findings were (1) smoking HD+ performed significantly worse than nonsmoking HD+ on measures of auditory-verbal (AV) learning, AV memory, and cognitive efficiency; (2) relative to controls, smoking HD+ demonstrated significantly lower neocortical GM volumes in all lobes except the occipital lobe, while nonsmoking HD+ showed only lower frontal GM volume compared with controls; (3) in the HD+ group, regional brain volumes and neurocognition were not influenced by viremia, highly active antiretroviral treatment, or Center for Disease Control symptom status, and no interactions were apparent with these variables or smoking status. Overall, the findings suggested that the direct and/or indirect effects of chronic cigarette smoking created an additional burden on the integrity of brain neurobiology and neurocognition in this cohort of HIV-positive heavy drinkers.
Keywords: HIV, Alcohol use disorders, Chronic cigarette smoking, Neuroimaging, Neurocognition
Introduction
Alcohol use disorders (AUD; i.e., alcohol abuse or dependence) (Cook et al., 2001; Miguez et al., 2003; Samet et al., 2004a, 2004b) and chronic cigarette smoking (Burkhalter et al., 2005; Crothers et al., 2005; Niaura et al., 2000; Turner et al., 2001) are common comorbid conditions among individuals with human immunodeficiency virus infection (HIV). Approximately 35−50% of HIV clinic attendees are reported to meet criteria for AUD (Justice et al., 2006) and 40−70% of HIV-positive individuals are chronic cigarette smokers (Crothers et al., 2005). Neuroimaging and neurocognitive studies have shown that both AUD (Oscar-Berman, 2000; Sullivan, 2000) and clinically symptomatic HIV, in the highly active antiretroviral treatment (HAART) era (Di Sclafani et al., 1997; Meyerhoff et al., 2003; Patel et al., 2003, 2002; Schifitto et al., 2001; Thompson et al., 2005), have independent adverse effects on brain neurobiology and function.
A growing body of evidence suggests chronic cigarette smoking alone is associated with abnormalities in brain morphology, blood flow, neurochemistry, and neurophysiology (Brody, 2005; Brody et al., 2004; Domino et al., 2004; Epperson et al., 2005; Gallinat et al., 2006, 2007; Hayee et al., 2003; Neuhaus et al., 2006). Furthermore, chronic smoking in adults is associated with dysfunction in multiple domains of neurocognition as well as in postural stability (Ernst et al., 2001; Fried et al., 2006; Hill et al., 2003; Iki et al., 1994; Razani et al., 2004; Richards et al., 2003; Schinka et al., 2003). Additionally, chronic cigarette smoking increases the risk for several types of bacterial and viral opportunistic infections (Arcavi and Benowitz, 2004).
With respect to common comorbidities in HIV infection, AUD have been shown to compound abnormalities in brain morphology, metabolism, neurocognition, and motor function (see Meyerhoff, 2001; Pfefferbaum et al., 2005, 2002), particularly with increasing HIV-related immunodeficiency (Meyerhoff et al., 1999, 2003; Pfefferbaum et al., 2007, 2006; Rothlind et al., 2005). Concurrent AUD in HIV seropositive persons is associated with increased probability of developing an AIDS-defining event secondary to further immunosuppression (Wang et al., 2002) and may increase susceptibility to secondary infections (Friedman et al., 2003). AUD in HIV-infected individuals is also related to increased incidence of hepatitis C, hypertension, bacterial pneumonia, and candidiasis (Justice et al., 2006). Chronic cigarette smoking is independently associated with greater risk of acquiring HIV infection (Furber et al., 2007) as well as with decreased quality of life in HIV-infected individuals (Crothers et al., 2005). Furthermore, concurrent chronic cigarette smoking in HIV-infected individuals is related to increased incidence of depression in percentage and absolute numbers of CD4+ and CD8+ cells in bronchoalveolar lavage fluid (Wewers et al., 1998) as well as with increased incidence of respiratory problems, including chronic obstructive pulmonary disease, and bacterial pneumonia, relative to their nonsmoking counterparts (Crothers et al., 2005). Lung function is relevant to brain morphology as it is reported to predict global brain volume in HIV-negative adults (Sachdev et al., 2006).
We recently reported that chronically smoking treatment-naïve HIV-negative heavy drinkers demonstrated smaller volumes than nonsmoking controls in the frontal, parietal, and temporal gray matter (GM). The chronic smokers also exhibited smaller temporal and total GM volumes than their nonsmoking heavy drinker counterparts. Notably, the nonsmoking heavy drinkers were not different from nonsmoking controls on any regional GM volume (Durazzo et al., 2007). Furthermore, in our treatment-seeking AUD cohort, chronic smokers demonstrate greater abnormalities than nonsmokers in brain morphology, metabolism, blood flow, and neurocognition (see Durazzo et al., 2007b; Durazzo et al., 2007c).
It is increasingly evident that comorbid AUD in HIV promotes increased aberrations in brain morphology, metabolism, and neurocognition. However, the effects of chronic smoking on brain morphology and neurocognition among individuals concurrently afflicted with AUD and HIV have not been considered. Thus, the primary goal of this retrospective analysis was to determine if chronic cigarette smoking modulated regional GM and white matter (WM) volumes and neurocognition in a sample of nontreatment seeking, HIV-positive heavy drinkers (HD+), a group previously described in Rothlind et al. (2005). Based on the previous findings for nonclinical chronic smokers and those from our treatment and nontreatment seeking AUD cohorts, we predicted that smoking HIV-positive heavy drinkers (sHD+) demonstrate smaller frontal, temporal, parietal, and total neocortical GM volumes than nonsmoking HIV-positive heavy drinkers (nsHD+). We also hypothesized that sHD+ exhibit inferior performance to nsHD+ on measures of auditory-verbal (AV) learning and memory, cognitive efficiency, executive skills, processing speed, working memory, and static postural stability.
Method
Participants
Participants in this report were originally recruited for a larger longitudinal study of the central nervous system (CNS) effects of concurrent HIV and AUD. From the larger HD+ sample (n = 56) described in Rothlind et al. (2005), there were 49 individuals (seven had missing data for cigarette smoking frequency) who self-reported their cigarette smoking frequency over at least the 6 months prior to study. Participants rated their smoking frequency according to the following scale: no smoking, about once a month, 2−3 times per month, 1−2 times per week, 3−4 time per week, nearly ever day, and at least once a day. The smoking participants reported consuming only cigarettes and no other form of tobacco. In the sample of 49, three individuals reported smoking 2−3 times per month and two 1−2 times per week. We included only those individuals who reported smoking nearly everyday or at least once per day over the 6 months prior to study to obtain a sample with the greatest smoking severity (n = 44). Information on number of cigarettes smoked per day and past smoking history were not obtained in the original study. All HD+ were regular, active drinkers at the time of the magnetic resonance (MR) and neurocognitive assessments.
For neurocognitive comparisons, we analyzed data from 17 nsHD+ (two females) and 27 sHD+ (three females). Nineteen sHD+ reported smoking cigarettes daily and eight smoking nearly everyday for at least 6 months prior to enrollment in the study. nsHD+ reported no consumption of any tobacco products in the 6 months prior to enrollment. Of the 44 HD+ individuals included in the neurocognitive analyses, 29 had complete regional brain volumetric MR data: there were 13 nsHD+ (one female) and 16 sHD+ (two females). In this sample, 13 (of 16) sHD+ participants reported smoking daily and three nearly everyday for at least 6 months prior to study. Neurocognitive evaluation was generally completed within 1 week of the MR study.
For both morphological and neurocognitive analyses, the HD+ groups were also compared with 27 (five females) HIV-negative, nonsmoking, light drinking controls (nsLD). nsLD were selected on the basis of completed self-report information on their smoking frequency over the 6 months prior to study and comparable age to the HD+ groups. There were not enough light drinking control participants who reported smoking nearly everyday or at least once per day to form a viable smoking, light drinking HIV-negative group. nsLD reported no consumption of any tobacco products in the 6 months prior to enrollment.
Inclusion criteria for HD+ groups required an average of at least 100 (80 for women) standard alcoholic drinks (one alcoholic drink equivalent = 12 oz of beer, 5 oz of wine, or 1.5 oz of liquor, all corresponding to approximately 13.6 g pure ethanol) per month for a minimum of 3 years prior to enrollment. All HD+ participants were actively drinking at enrollment, but were required to have zero breath alcohol levels at the time of study. Inclusion and exclusion criteria are fully detailed in Cardenas et al. (2005) and Rothlind et al. (2005). In short, all participants were free of general medical, neurologic, and neuropsychiatric conditions known or suspected to influence brain morphology. Previous history of drug dependence and unipolar mood disorders in the HIV+ groups were not exclusionary, as they are highly prevalent in HIV+ individuals (Grant et al., 1993; Haverkos, 1998). nsLD reported no history of alcohol or substance abuse. HD+ were excluded if they met DSM-IV criteria for dependence on any substance other than alcohol or nicotine in the 12 months prior to enrollment. Prior to completing any procedure, all participants gave written informed consent, which was approved by review boards of the University of California San Francisco and the San Francisco VA Medical Center.
Psychiatric/behavioral assessment and clinical labs
At the time of enrollment, participants completed the Structured Clinical Interview for DSM-IV Axis I disorders (SCID I/P v2.0; First et al., 1998), and interviews and questionnaires assessing depressive symptomatology (Beck Depression Inventory, BDI; [Beck, 1978]), lifetime alcohol consumption (Lifetime Drinking History, LDH, [Skinner and Sheu, 1982; Sobell and Sobell, 1992; Sobell et al., 1988]), and substance use (in-house questionnaire assessing substance type and quantity and frequency of use). From the LDH, we derived the average number of drinks per month in the previous year, three years, and over lifetime, and number of months of heavy drinking (i.e., total number of months over lifetime in which the participant drank in excess of 100 drinks per month). Average number of drinks per day over the week prior to testing was also calculated as this measure was related to neurocognition in Rothlind et al. (2005). Clinical laboratories obtained are described in detail in Rothlind et al. (2005).
MRI acquisition and processing
MRI data acquisition was conducted on a clinical 1.5 Tesla MR scanner (Vision, Siemens Medical Systems, Iselin, NJ) and consisted of two sequences (1) double spin-echo (TR/TE1/TE2 = 2,500/20/80 ms, 1 × 1 mm2 in-plane resolution, 3 mm slice thickness, and no slice gap), which yielded oblique-axial proton density and T2-weighted MR images and (2) Magnetization prepared rapid acquisition gradient echo (TR/TI/TE = 9.7/300/4 ms, 1 × 1 mm2 in-plane resolution, and 1.5 mm slice thickness), which yielded coronal T1-weighted (T1-w) MR images. Three-tissue intensity based segmentation was applied to T1-w images to assign a set of probabilities of WM, GM, or cerebral spinal fluid (CSF) to each voxel. The segmentation procedure and validity studies are fully described in Cardenas et al. (2005). An atlas-based deformable registration method was used to automatically identify regions of interest in the brain as detailed in Cardenas et al. (2005). In summary, a single MRI from a 36-year-old man served as a reference atlas and was manually edited to delineate regions including the major lobes of the brain (frontal, temporal, parietal, and occipital), lateral ventricles, thalamus, caudate, lenticular nuclei, brainstem, and cerebellum. A B-Spline Free Form deformation algorithm driven by normalized mutual information was used to estimate the spatial transformation from the atlas to each individual's T1-w MRI (Studholme et al., 2003, 2001a, 2001b). This transformation was then inverted and used to apply the atlas labels to demarcate participant-specific regions on each scan, which were combined with tissue segmentation results to yield regional volumes of WM, GM, and CSF. All automatically marked MRIs were carefully reviewed by a trained operator to insure accuracy of automated markings. Total cortical GM, WM, and sulcal CSF volumes represent the summed tissue or CSF volumes for the four main lobar regions.
Assessment of neurocognitive function, motor and postural stability
Measures were administered as described in Rothlind et al. (2005). General verbal intellectual functioning was assessed by the American version of the Nelson Adult Reading Task (Grober and Sliwinski, 1991) and the Information subtest of the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III; Wechsler, 1997). The domains and their constituent measures in this report were as follows:
Cognitive efficiency
A composite index, which consisted of all tests that were timed, or in which the time to complete the task influenced the score achieved was calculated by averaging the individual z-scores of those measures. Timed tests included the Luria-Nebraska Item 99 from the Luria-Nebraska Neuropsychological Battery (Golden, Hemmeke, & Purisch, 1978), Stroop word, color, and color-word tests (Golden, 1978), Symbol Digit Modalities Test (Smith, 1982), and Trails A and B (Reitan and Wolfson, 1985). Higher scores on these measures reflect better speed and accuracy on predominantly nonverbal tasks. The timed-test composite is an approximation of the concept of cognitive efficiency described by Glenn and Parsons (1992) and others (Nixon et al., 1998, 1995).
Executive skills
Short Category Test, Booklet Format, total errors (Wetzel and Boll, 1987); Trails B (Reitan and Wolfson, 1985); Computerized Wisconsin Card Sorting Test, nonperseverative errors, perseverative errors, and perseverative responses (WCST; Heaton et al., 1993).
Fine motor skills
Lafayette Grooved Pegboard Test, average of dominant and nondominant hands (Klove, 1963).
Learning and memory
AV: California Verbal Learning Test-II (CVLT-II; Delis et al., 2000), immediate recall trials 1−5 (learning), average of short and long delay free recall, and average of short and long delay cued recall (memory).
Visuospatial
Brief Visual Memory Test-Revised (BVMT-R; Benedict, 1997), total recall (learning), and delayed recall (memory).
Postural stability
Fregly Ataxia battery, stand on one leg, eyes closed, average of left and right legs (Fregly et al., 1973).
Visuospatial skills
Rey-Osterrieth Complex Figure Drawing Test, copy trial (Osterrieth and Rey, 1944) scored according to Denman (1984) criteria; item 99 from the Luria-Nebraska Neuropsychological Battery (mental spatial rotation item).
Working memory
Brown-Peterson Auditory Consonant Trigrams, total number correct (Brown, 1958), MicroCog Computerized Assessment of Cognitive Functioning, numbers reversed total score (Powell et al., 1993).
A ratio score was created for the Luria-Nebraska Item 99 by dividing the number correct (maximum possible = 8) by the time required to complete the task. Raw scores for all neurocognitive measures, except the Fregly Ataxia battery and Luria-Nebraska Item 99 ratio, were converted to standardized scores via the appropriate normative test data adjusted for age (BVMT-R, CVLT-II, Short Categories Test, Stroop Color-Word Test, and WAIS-III subtests) or age and education (Grooved Peg Board, Trails A and B, and WCST-64 variables). Standardized scores were transformed to z-scores for formation of the neurocognitive domain summary scores for the above listed domains. For the Fregly Ataxia battery and the Luria-Nebraska Item 99 ratio score, raw scores were converted to z-scores based on the performance of the 27 nsLD, as there are no suitable norms for these measures. The summary score of domains with multiple measures represents the average of the individual z-scores of the constituent measures.
Data analyzes
For comparisons of nsHD+, sHD+, and nsLD, multivariate analysis of covariance (MANCOVA) was used, with age as a covariate, to control for age effects on neocortical GM volume (Courchesne et al., 2000; Jernigan et al., 2001). Regional WM and sulcal CSF volumes were separately evaluated with multivariate analysis of variance (MANOVA). Significant MANCOVAs/MANOVAs (P ≤ .05) were followed by univariate analysis of covariance/variance (ANCOVA/ANOVA) for individual regions of interest. Significant ANCOVA/ANOVAs (P ≤ .05) were further evaluated with pairwise t-tests using the Bonferroni method to adjust for multiple comparisons. Separate ANOVAs comparing the three groups were also conducted for caudate, lenticular nuclei, thalami, brainstem, and cerebellum, and sulcal and ventricular CSF volumes. Alpha levels for each of these ANOVAs were adjusted for the number of subcortical nuclei/regions or the sulcal CSF region to control for multiplicity of regions evaluated. Significant ANOVAs were followed up with pairwise t-tests using the Bonferroni method. Comparisons among sHD+, nsHD+, and nsLD on neurocognitive domains were conducted with ANCOVA for each of the five neurocognitive domains (i.e., cognitive efficiency, executive skills, learning and memory, visuospatial skills, and working memory) and two sensorimotor domains (i.e., fine motor skills and postural stability). Education was used as a covariate for group comparisons of the neurocognitive domains due to significantly greater level in nsLD than sHD+ (see Table 1). Alpha levels (0.05) for the individual ANCOVAs were divided by the five neurocognitive domain to control for multiplicity of domains evaluated (corrected alpha = 0.01). Separate ANOVAs were conducted for the domains of fine motor skills and static postural stability, because we view these sensorimotor functions as distinct from neurocognitive processes, as evidenced by their generally weak correlations with other neurocognitive domains evaluated in this study. Significant ANCOVA/ANOVAs were followed up with pairwise t-tests using the Bonferroni method. All a priori predicted contrasts between sHD+ and nsHD+ for brain volumetrics and neurocognitive and postural stability domains used 1-year average drinks per month as a covariate due to significantly higher consumption in nsHD+ relative to sHD+ (see Table 1). Effect sizes for multivariate and univariate group comparisons are given as eta squared (η2).
Table 1.
Demographic and clinical variables for all groups
| Variable | nsLD (n = 27) a | nsHD+ (n = 17) b | sHD+ (n = 27) c | Group comparisons* |
|---|---|---|---|---|
| Age (yr) | 43.1 ± 8.8 | 44.4 ± 4.9 | 43.8 ± 5.4 | ns |
| Education | 15.2 ± 2.0 | 14.4 ± 2.2 | 13.4 ± 2.7 | a > c |
| Male (%) | 82 | 88 | 89 | NS |
| AMNART | 117 ± 8 | 113 ± 10 | 111 ± 11 | a > c |
| WAIS-III Information z-score | 1.15 ± 0.70 | −0.06 ± 1.34 | 0.27 ± 1.07 | a > b, c |
| BDI | 5.3 ± 1.4 | 13.5 ± 1.9 | 10.4 ± 1.5 | b, c > a |
| Average drinks/day past week | 1.8 ± 1.1 | 7.4 ± 4.4 | 8.0 ± 4.1 | b, c > a |
| Average drinks/month past year | 11 ± 14 | 314 ± 348 | 203 ± 116 | b, c > a; b > c |
| Average drinks/month past 3 years | 11 ± 14 | 306 ± 343 | 239 ± 157 | b, c > a |
| Average drinks/month lifetime | 12 ± 11 | 215 ± 174 | 125 ± 140 | b, c > a |
| Months heavy drinking | NA | 205 ± 99 | 190 ± 97 | NS |
| Age onset heavy drinking | NA | 25 ± 9 | 23 ± 9 | NS |
| % Previous substance dependence | NA | 41 | 48 | NS |
| % Previous substance abuse | NA | 18 | 23 | NS |
| % on HAART | NA | 82 | 41 | b > c |
| CD4+ count | NA | 348 ± 243 | 410 ± 248 | NS |
| Current CD8+ count | NA | 891 ± 365 | 1034 ± 980 | NS |
| Viral load (log10 copies/ml) | NA | 4.54 ± 4.74 | 4.58 ± 5.03 | NS |
| % Undetectable viral load | NA | 35 | 35 | NS |
| % Viremic | NA | 40 | 45 | NS |
AMNART = American National Adult Reading test; BDI = Beck Depression Inventory; NA = Not applicable; nsHD+ = nonsmoking HIV-positive heavy drinker; nsLD = nonsmoking light drinking control; sHD+ = HIV-positive heavy drinker NS = Not significant.
P < 0.05.
For sHD+, nsHD+, and the combined HD+ group (i.e., sHD+ and nsHD+), we investigated the relationships (Spearman coefficients) between regional lobar tissue volumes and average drinks per day over the week prior to evaluation, 1- and 3-year average drinks per month, lifetime average number of drinks per month, total amount of ethanol consumed over lifetime, and years of heavy drinking. For these correlations, alpha level (0.05) for each of the four lobar GM or WM region was adjusted for the six aforementioned measures of drinking severity to correct for multiplicity of correlations (corrected alpha = 0.008). Alpha levels for relationships between regional lobar GM and WM and neurocognitive/sensorimotor domains, were adjusted for the seven domains (corrected P = .007 for GM and WM). In the HD+ groups, correlations of viral load, CD4+ and CD8+ variables, aspartate aminotransferase, alanine aminotransferase, and complete blood count variables with volumetric and neurocognitive outcome measures were corrected for multiplicity of correlations in the same manner as described above.
In the HD+ group, a series of ANOVAs (no correction for multiple comparisons) were used to evaluate for potential differences in regional brain volumes and neurocognitive and sensorimotor domains for individuals taking HAART versus those who were not, for individuals with suppressed viral loads (viral load below 400 copies/ml) versus those who were viremic (viral load above 400 copies/ml) and for asymptomatic (Center for Disease Control [CDC], stage A) versus symptomatic participants (CDC stage B and C) and for smoking versus nonsmoking participants. This 2 × 2 × 2 × 2 design allowed for assessment of the main effects for the above HIV-specific group characteristics as well as their interactions.
Results
Participant characterization
In the larger sample involved in the neurocognitive analyses, 11 nsHD+ participants were Caucasian (65%), four were African-American (23%), and two were Latino (12%); in the sHD+ group, 18 were Caucasian (66%), eight were African-American (30%), and one was Latino (4%); in the nsLD group, 20 participants were Caucasian (74%), four were African-American (7%), and three were Latino (11%). There were no significant differences between nsHD+ and sHD+ in the frequency of participants with past history of substance abuse or dependence (see Table 1). The most commonly misused illicit substances were cocaine, marijuana, and methamphetamine. In the HD+ group, the average time since criteria were met for substance abuse was 134 months (min = 0, max = 360) and for substance dependence was 73 months (min = 13, max = 300). At the time of evaluation, one nsHD+ (cannabis) and two sHD+ (cocaine and methamphetamine) met current criteria for substance abuse. Similar ethnic percentages and substance abuse/dependence prevalence were apparent for the smaller cohort comprising the brain volumetric analyses. In both neurocognitive and volumetric analyses, a greater percentage of the nsHD+ participants were on HAART than sHD+ participants (P = .05) (see Table 1). Education, estimated premorbid verbal IQ, and alcohol consumption variables were not significantly different between the daily and nearly everyday smokers in the sHD+ group.
Regional brain volumetrics
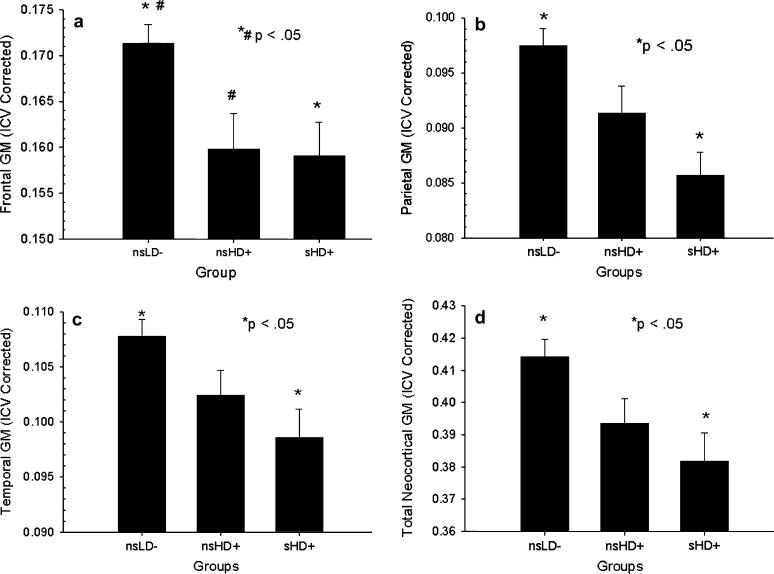
The MANCOVA comparing nsHD+, sHD+, and nsLD on regional neocortical GM was significant [F(8, 92) = 2.33, P = .025]. ANCOVAs were significant for the frontal [F(2, 48) = 6.17, P = .004, η2 = 0.22], parietal [F(2, 48) = 7.34, P = .001, η2 = 0.20], temporal GM [F(2, 48) = 4.56, P = .015, η2 = 0.25], and for total cortical GM [F(2, 48) = 6.52, P = .003, η2 = 0.21], but not for occipital GM (see Fig. 1A–D). Follow-up Bonferroni corrected pairwise t-tests indicated sHD+ had significantly lower GM volume than nsLD in the frontal (−7.7%), parietal (−12.1%), and temporal lobe (−7.1%) and for total neocortical GM volume (−8.6%)(P = .001−.020); nsHD+ had lower frontal GM volume than nsLD (−7.1%, P = .027) and showed a trend for lower total neocortical GM (−5.9%, P = .058). In planned contrasts, sHD+ demonstrated a trend for smaller parietal GM than nsHD+ (−5.1%, P = .08). No other significant differences or trends were observed between sHD+ and nsHD+. Covariation for the greater average drinks per month in nsHD+ than sHD+ did not influence findings from comparisons among nsHD+ and sHD+ for brain volumes in any region.
Fig. 1.
(a) Frontal gray matter volume for all groups. (b) Parietal gray matter volume for all groups. (c) Temporal gray matter volume for all groups. (d) Total neocortical gray matter volume for all groups. Mean ± S.E.M. ICV = Intracranial volume.
The MANOVA for regional WM was significant [F(8, 94) = 2.049, P = .049]. ANOVAs were significant only for the frontal WM [F(2, 48) = 4.56, P = .025, η2 = 0.14], where sHD+ had significantly smaller WM than nsLD (−5.9%, P = .039). The 4.0% lower frontal WM volume in nsHD+ relative to nsLD was not statistically significant (P = .14). The ANOVA for ventricular CSF was significant [F(2, 49) = 5.37, P = .008, η2 = 0.18], where sHD+ had significantly larger volume than nsLD (P = .018).
ANOVAs for caudate, lenticular, thalamus, brain stem, cerebellum volumes, or sulcal CSF volumes indicated no significant group differences on these measures. There were no within-group differences between left and right hemisphere volumes and there were no group by age interactions for any region/structure investigated. No regional volume differences were apparent between the daily-smoking and nearly-daily-smoking participants comprising the sHD+ group.
Neurocognitive, fine motor and postural stability domains
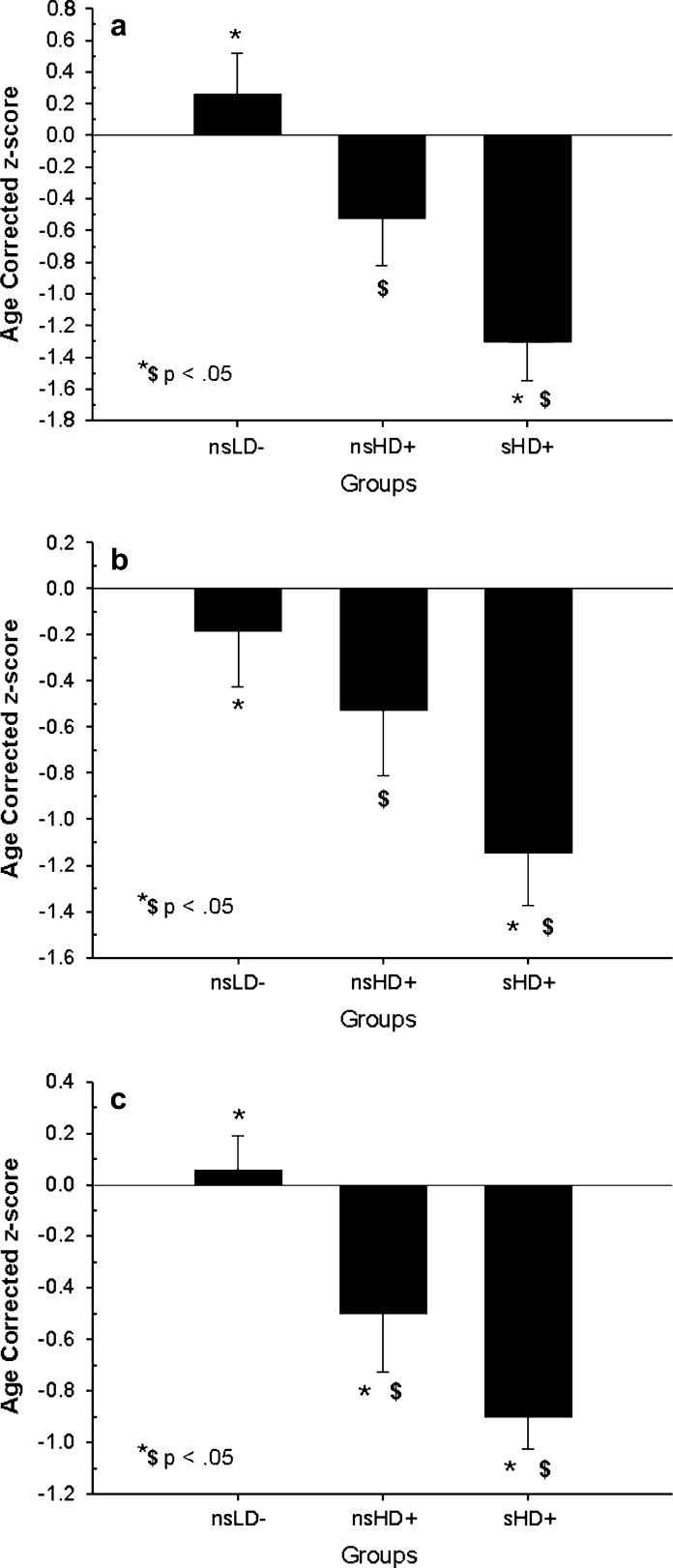
ANOVAs comparing nsHD+, sHD+, and nsLD were significant (P ≤ .01) for AV learning [F(2, 67) = 9.80, P < .001, η2 = 0.22], AV memory-free recall [F(2, 67) = 4.71, P = .01, η2 = 0.15], AV memory-cued recall [F(2, 67) = 8.80, P = .001, η2 = 0.20], cognitive efficiency [F(2, 67) = 11.43, P < .001, η2 = 0.25], executive skills, [F(2, 66) = 7.82, P = .001, η2 = 0.19], visuospatial learning [F(2, 65) = 9.80, P < .001, η2 = 0.23], visuospatial memory [F(2, 66) = 20.56, P < .001, η2 = 0.41], working memory [F(2, 66) = 7.55, P = .001, η2 = 0.18], postural stability [F(2, 65) = 13.35, P = .001, η2 = 0.29], and fine motor skills. Bonferroni corrected pairwise t-tests indicated nsLD were superior to sHD+ on all the foregoing domains (P = .003−.018), and to nsHD+ on all domains (P = .006−.049) except AV learning, AV memory-free recall, and cued AV memory (see Table 2). In planned contrasts, nsHD+ were superior to sHD+ on the domains of AV learning (P = .041), AV memory-free recall (P = .047), AV memory-cued recall (P = .049), and cognitive efficiency (P = .041), with a trend for better fine motor skills (P = .049; nonpredicted comparison; see Fig. 2A–C). The above contrasts remained significant after covarying for the greater 1-year average drinks per month in nsHD+, indicating the differences between sHD+ and nsHD+ on these domains were not mediated by differences in alcohol consumption over the year prior to enrollment. ANOVAs for visuospatial skills and postural stability were not significant. No differences were apparent on any domain between the daily-smoking and nearly-daily-smoking participants comprising the sHD+ group.
Table 2.
Group performance on neurocognitive and sensorimotor domains
| Domain | nsLD (n = 27) a | nsHD+ (n = 17) b | sHD+ (n = 27) c | Group comparisons* |
|---|---|---|---|---|
| AV learning | −0.25 ± 1.3 | −0.52 ± 1.2 | −1.30 ± 1.3 | a, b > c |
| AV memory-free recall | −0.18 ± 1.2 | −0.53 ± 1.2 | −1.15 ± 1.2 | a, b > c |
| AV memory-cued recall | 0.02 ± 1.1 | −0.24 ± 1.2 | −0.85 ± 1.4 | a, b > c |
| Cognitive efficiency | 0.06 ± 0.70 | −0.49 ± 0.93 | −0.90 ± 0.64 | a > b, c; b > c |
| Executive skills | −0.02 ± 0.58 | −0.64 ± 0.83 | −0.69 ± 0.62 | a > b, c |
| Fine motor skills | 0.46 ± 0.92 | −0.97 ± 2.1 | −1.80 ± 1.9 | a > b, c; b > c |
| Static postural stability | 0.00 ± 1.0 | −0.83 ± 1.0 | −0.87 ± 1.0 | a > b |
| Visuospatial learning | −0.31 ± 0.69 | −1.27 ± 1.7 | −1.42 ± 1.3 | a > b, c |
| Visuospatial memory | 0.97 ± 1.7 | −1.13 ± 1.3 | −1.24 ± 0.57 | a > b, c |
| Visuospatial skills | 0.06 ± 1.2 | 0.08 ± 2.5 | −0.71 ± 2.1 | NS |
| Working memory | 0.01 ± 0.72 | −0.78 ± 1.1 | −0.80 ± 0.64 | a > b, c |
Note. AV = Auditory-verbal; nsHD+ = nonsmoking HIV-positive heavy drinker; nsLD = nonsmoking light drinking control; smoking; sHD+ = HIV-positive heavy drinker; NS = Not significant.
P < 0.05.
Fig. 2.
(a) Auditory-verbal learning domain for all groups. Mean ± S.E.M. (b) Auditory-verbal memory domain (free-recall) for all groups. Mean ± S.E.M. (c) Cognitive efficiency domain for all groups. Mean ± S.E.M.
Effects of HIV disease severity on regional brain volumes and neurocognitive and sensorimotor functions
There were no main effects for HAART or CDC symptomatic status for regional brain volumes, neurocognitive/sensorimotor domains and no interactions between HAART or CDC status and smoking status. There were no main effects of viremia for regional brain volumes. Viremic individuals performed significantly worse than those with suppressed viral loads on all neurocognitive and sensorimotor domains (P = .002−.046); however, when covaried for the significantly higher average drinks per day over the week prior to evaluation in the viremic (10.6 ± 4.4 drinks/day) compared with the suppressed group (5.6 ± 2.5 drinks/day) (P = .002), all group differences on neurocognitive and sensorimotor domains were no longer significant. This indicated that alcohol consumption during the week prior to study mediated performance rather than level of viral burden.
Relationships among outcome measures in HD+
In the combined HD+ group (significant P ≤ .008), average number of drinks over past week was inversely related to fine motor skills (r = −0.56, P = .003) with a trend for executive skills (r = −0.48, P = .012). In the individual HD+ groups and the combined HD+ group (i.e., sHD+ and nsHD+), there were multiple moderately strong positive correlations (r = 0.40−0.50, P = .015−.03) between visuospatial learning and memory, visuospatial skills, and parietal, temporal, occipital WM and total lobar WM; however, these relationships did not survive our stringent correction for multiplicity (significant P ≤ .007). No significant relationships were observed among regional brain volumes and alcohol consumption variables; these correlations were generally weak and did not approach statistical significance for the individual groups or the combined HD+ sample. Furthermore, after rigorous correction for multiplicity, regional brain volumes, neurocognitive, or sensorimotor domains were not significantly related to viral load, CD4+ and CD8+ variables, aspartate aminotransferase, alanine aminotransferase, complete blood count variables (e.g., erythrocyte and leukocyte levels, hematocrit, etc), BDI, and duration of dependence on substances other than alcohol in the individual HD+ groups as well as the combined group.
Discussion
Heavy drinking HIV-infected individuals who smoke had greater abnormalities in both neurocognition and brain morphology than those who do not smoke. The specific findings in this HIV-positive nontreatment seeking heavy drinkers were (1) sHD+ performed significantly worse than nsHD+ on measures of AV learning, AV memory, and cognitive efficiency; (2) relative to nsLD, sHD+ demonstrated significantly smaller neocortical GM volumes in all but the occipital lobes as well as less total neocortical GM. nsHD+ showed only smaller frontal GM and total neocortical GM volumes; and (3) in the HD+ groups, there were no significant differences in regional brain volumes or neurocognition as a function of viremia, HAART, or CDC symptom status, and there were no interactions between these variables and smoking status. In the HD+ groups, alcohol consumption variables, viral load, CD4+ and CD8+ variables, liver function measures, complete blood count variables, depressive symptomatology, as well as past substance use disorders did not correlate with regional brain volumes or neurocognitive and sensorimotor domains.
In this study, sHD+ exhibited clinically significant levels of dysfunction in the domains of AV and visuospatial learning (mildly impaired range of functioning), AV and visuospatial memory (mildly impaired), and fine motor skills (moderately impaired; see Table 2). nsHD+ only demonstrated clinically relevant levels of dysfunction of visuospatial learning and memory, both in the mildly impaired range (see Table 2). Static postural stability for both HD+ groups was in the low average to borderline impaired range. The neurocognitive findings for the sHD+ and nsHD+ are generally consistent with previous studies reporting dysfunction in learning and memory, fine motor skills, and postural stability in those concurrently afflicted with HIV and AUD (Bornstein et al., 1993; Green et al., 2004). However, the results indicate greater overall functional impairment in sHD+, most notably in the domains of cognitive efficiency, AV and visuospatial learning and memory, and fine motor skills. This suggests the direct and/or indirect effects of chronic cigarette smoking may have added an additional insult to the CNS of this heavy drinking HIV+ cohort, which is consistent with reports indicating chronic cigarette smoking alone is associated with abnormalities in multiple domains of neurocognition (Ernst et al., 2001; Fried et al., 2006; Hill et al., 2003; Iki et al., 1994; Razani et al., 2004; Richards et al., 2003; Schinka et al., 2003).
In this report, and in our studies with HIV-negative alcoholics, chronic smokers demonstrated poorer performance than their nonsmoking counterparts on measures of AV learning and memory and cognitive efficiency (Durazzo et al., 2006b). Closer examination of group CVLT-II performances in this study revealed sHD+ did not benefit from retrieval cues (see Table 2), performed poorly on recognition testing (i.e., high false-positive rate and low discriminability), and tended to generate a higher level of intrusions than both nsHD+ and nsLD (data not shown). This profile is suggestive of deficient AV encoding/storage in sHD+ (see [Delis et al., 2000] and references therein). In adults, chronic smoking alone is associated with dysfunction in AV learning and memory (Fried et al., 2006; Hill et al., 2003; Richards et al., 2003; Schinka et al., 2003) and on tasks emphasizing fast and flexible problem solving skills (Kalmijn et al., 2002). This pattern of functioning in different populations suggests that chronic smoking may have particularly pronounced adverse effects on neural circuits involved in AV learning and memory as well as cognitive efficiency (see Durazzo et al., 2007b).
Our regional volumetric findings indicated that sHD+ demonstrated smaller neocortical GM volumes relative to nsLD in the frontal, temporal, and parietal lobes. Notably, no statistically significant volumetric differences were apparent between nsLD and nsHD+. The volume differences between sHD+ and nsHD+, however, were not statistically significant for any region. Additionally, nsHD+ tended to demonstrate lower total neocortical GM volumes than nsLD. Therefore, the overall results do not conclusively support our hypothesis that chronic cigarette smoking compounded neocortical GM volume loss or promoted other regional structural abnormalities in this HIV+ heavy drinking cohort. The significantly smaller frontal WM volume in sHD+, and the trend for nsHD+, are consistent with studies indicating both alcoholism and symptomatic HIV and their co-occurrence have particularly detrimental effects on frontal lobe WM (Meyerhoff, 2001; Pfefferbaum et al., 2007, 2006). Generally speaking, greater than normal brain atrophy is associated with increased risk for general cognitive decline and memory impairment with advancing age (Meyer et al., 1999; Visser et al., 1999). This has specific relevance for sHD+, as chronic cigarette smoking alone is associated with abnormal volume loss with increasing age (Hayee et al., 2003; Kubota et al., 1987). Correspondingly, chronic smoking is related to abnormal decline in AV memory (Richards et al., 2003), executive functions (Paul et al., 2006), psychomotor speed and cognitive flexibility (Starr et al., 2007), and general intellectual abilities (Deary et al., 2003; Ott et al., 2004) in middle aged or older adults. Therefore, in the context of HIV and continued heavy drinking, chronic smoking may have particular ramifications for the integrity of neurocognitive functioning and ability to manage the activities of daily living with advancing age of this cohort (see below for potential chronic smoking-related mechanisms contributing to the neurocognitive and brain morphological findings observed among sHD+).
There were no significant differences in neurocognitive or sensorimotor domain performance as a function of HAART, CDC symptom, or viremia (after covariation for alcohol consumption), or interactions with these variables and smoking status for any domain. Therefore, the inferior performance of sHD+ compared with nsHD+ on the domains of cognitive efficiency, AV learning and memory was not influenced by the use of antiretroviral medications or HIV disease severity in this sample. Furthermore, differences on alcohol consumption over the year prior to study (higher consumption in nsHD+ vs. sHD+) did not influence the group differences in neurocognition or the morphological results among sHD+ and nsHD+.
Limitations
Limitations of this study include the retrospective nature of the analyses and the lack of detailed information on the smoking behavior of sHD+ participants. We were unable to distinguish whether the combined effects of chronic alcohol misuse and smoking were synergistic or additive due to the lack of a group of light drinkers who smoked at levels equivalent to those in sHD+. Therefore, it is unclear if the adverse effects of chronic smoking on neurocognition observed in this HIV-positive heavy drinking cohort are apparent in chronically smoking HIV-positive light drinkers. Unfortunately, any 2 (smokers vs. nonsmokers) × 2 (heavy drinkers vs. light drinkers) analyses with our HIV+ cohort would be hopelessly confounded by the lower smoking severity in the smoking HIV+ light drinkers. Additionally, we did not have enough female participants to evaluate sex effects on our outcome measures. It is possible that the observed group differences were, at least partially, influenced by unrecorded group disparities in nutrition, exercise, overall physical health, or genetic predispositions. Both HD+ groups demonstrated a similar frequency of previous substance dependence, which primarily involved cocaine, methamphetamine, and cannabis. Chronic misuse of these substances has been reported to adversely affect human brain morphology and neurocognition in active and recently detoxified abusers (Bartzokis et al., 2000; Lawton-Craddock et al., 2003; Lundqvist, 2005; O'Neill et al., 2001; Thompson et al., 2004). However, for both HD+ groups, most participants experienced substance dependence many years prior to enrollment, and it is not clear if there are enduring effects of cocaine, methamphetamine, or cannabis on human brain morphology and neurocognition when dependence is in long-term sustained full remission.
Potential mechanisms promoting greater neurobiological and neurocognitive abnormalities in HIV-positive chronic smokers with AUD
There are several possible smoking-related mechanisms that may contribute independently, or in concert, to the greater neurobiological and neurocognitive abnormalities observed in this sample of HIV+ individuals with concurrent chronic smoking and AUD. These mechanisms may affect brain tissue in a direct and/or indirect manner. Some of the possibilities are discussed below.
Direct mechanisms
A significant number of potentially cytotoxic compounds are found in the gas and particulate phases of cigarette smoke (e.g., carbon monoxide [CO], free radicals, free radical precursors, nitrosamines, phenolic compounds, and other polynuclear aromatic compounds [Fowles et al., 2000]), which may be directly cytotoxic, promote oxidative damage, or impair the function of brain tissue (Muscat et al., 2004; Park et al., 1998). For example, CO levels are significantly higher in smokers (Deveci et al., 2004), and this elevation is associated with decreased effective hemoglobin concentrations, diminished oxygen carrying capacity of erythrocytes (Macdonald et al., 2004), as well as a diminished efficiency of the mitochondrial respiratory chain (Alonso et al., 2004). Chronic smoking has also been equated to a type of repeated acute (mild) CO poisoning (Alonso et al., 2004). Furthermore, cigarette smoke also contains high concentrations of free radical species (e.g., reactive nitrogen species and reactive oxygen species) known to promote oxidative damage or stress to cellular structures as well as macromolecules including membrane lipids, proteins, carbohydrates, and DNA (Moriarty et al., 2003). Radical species in the particulate matter are long-lived (i.e., hours to months) relative to those found in the gas phase of cigarette smoke (Ambrose and Barua, 2004) and the long-lived species can adversely affect organs other than the lungs (Panda et al., 2000; Park et al., 1998). Similarly, chronic and heavy alcohol consumption and ethanol oxidation are associated with generation of reactive oxygen species and other metabolic products that may lead to oxidative damage to various cellular molecules and structures of brain tissue, including phospholipids and DNA (Brooks, 2000; Muscat et al., 2004; Park et al., 1998).
Indirect mechanisms
Chronic exposure to cigarette smoke in rats has been shown to significantly decrease membrane-bound ATPases in brain tissue, which may alter ion homeostasis, and lead to increased intracellular levels of Ca2+ and Na+ (Anbarasi et al., 2005), and promote necrotic injury in neurons (Xiao et al. 2002). Chronic cigarette smoke exposure in rats is associated with decreased brain concentrations of enzyme-based free radical scavengers (i.e., superoxide dismutase, catalase, and glutathione reductase) and nonenzyme-based radical scavengers (i.e., glutathione and vitamins A, C, and E)(Anbarasi et al., 2006; Mendez-Alvarez et al., 1998). This may leave tissue more vulnerable to oxidative damage resulting from radical species generated by cellular metabolism or other exogenous sources. The brain in general is exceedingly susceptible to oxidative damage due to the high concentrations of unsaturated fatty acids. Additionally, chronic cigarette smoking is also associated with nocturnal hypoxia (Casasola et al., 2002), chronic obstructive pulmonary disease, and other conditions that may impair lung function (Bartal, 2001) and lead to decreased blood oxygen levels. Decreased lung function is related to poorer neurocognition and increased subcortical atrophy among community dwelling individuals 60−64 years of age (Sachdev et al., 2006). Chronic smoking is also related to a significantly increased risk for atherosclerosis (Bolego et al., 2002), as well as abnormalities in vascular endothelial function (Gerzanich et al., 2001; Hawkins et al., 2002). These processes may impact the functional integrity (e.g., vasomotor reactivity/responsivity) of the cerebrovasculature and contribute to the decreased regional cerebral blood flow (Domino et al., 2004; Rose et al., 2003; Zubieta et al., 2001) and/or WM disease (Ding et al., 2003; Stapleton et al., 2003) reported in chronic smokers. Both the neocortex and associated WM are vulnerable to the effects of diffuse ischemia ([Chalela et al., 2001] and references therein). Finally, it has been suggested that late-myelinating areas such as the frontal and temporal lobes may be particularly vulnerable to increased oxidative stress and cerebral hypoperfusion (Bartzokis, 2004a, 2004b), both of which have been reported among chronic smokers and those with AUD.
The greater regional brain volumetric and neurocognitive abnormalities observed in the sHD+ sample may be related to a combination of direct and indirect mechanisms discussed above. Specifically, sHD+ may experience chronically increased CO levels, chronic exposure to free radicals from both ethanol metabolism and cigarette smoke, decreased cerebral concentrations of free radical scavengers, and potentially compromised vascular and cardiopulmonary function.
Conclusions
The smoking and nonsmoking HD+ groups demonstrated clear deficiencies in neurocognition and sensorimotor skills relative to nsLD; however, the sHD+ participants manifested significantly greater impairment than nsHD+ on measures of AV learning and memory (marked by deficient encoding/storage), visuospatial learning and memory, cognitive efficiency, and fine motor skills. There were no significant regional brain volume differences between nsLD and nsHD+, while sHD+ demonstrated smaller neocortical GM volumes relative to nsLD in the frontal, temporal, and parietal lobes. The overall pattern of brain morphological and neurocognitive data suggests that the direct and/or indirect effects of chronic cigarette smoking impose an additional burden on brain neurobiology and neurocognitive function in HIV-positive heavy drinkers. In the context of HIV infection, the combination of continued chronic smoking and heavy drinking may convey increased risk of neurobiological injury and neurocognitive decline with advancing age. The improvements of immunological functioning associated with HAART have dramatically increased the life expectancy and quality of life for individuals with HIV. However, both AUD (Justice et al., 2006) and chronic smoking (Crothers et al., 2005) are associated with decreased quality of life in HIV-positive individuals. The co-occurrence of AUD and smoking in those living with HIV may combine to further diminish quality of life through greater compromises in neurocognition and increased incidence of health complications. The forgoing reinforces the importance of assessing for both AUD and chronic smoking in those treated for HIV (Justice et al., 2006; Niaura et al., 2000). Our results also suggest that the potential effects of cigarette smoking on neurocognition and brain morphology should be given greater consideration in research examining the neurobiological and functional effects of comorbid HIV and alcoholism as well as other conditions in which chronic smoking is prevalent (e.g., schizophrenia, mood disorders, and substance use disorders). More generally, this data, in combination with findings from HIV-negative AUD samples (Durazzo et al., 2007, 2004, 2006a, 2006b; Gazdzinski et al., 2006) and nonclinical cohorts (Brody et al., 2004 Ernst et al., 2001; Gallinat et al., 2006, 2007; Hayee et al., 2003; Razani et al., 2004; Richards et al., 2003), provide converging lines of evidence that suggest chronic cigarette smoking adversely affects both brain neurobiology and neurocognition.
Acknowledgments
This work was supported by NIH R01 AA10788 (D.J.M.), P01 AA11493 (M.W.W.), R01 MH65392 (C.S.), and Whitaker foundation award RG-01-0115 (C.S.).
References
- Alonso JR, Cardellach F, Casademont J, Miro O. Reversible inhibition of mitochondrial complex IV activity in PBMC following acute smoking. Eur. Respir. J. 2004;23:214–218. doi: 10.1183/09031936.03.00038203. [DOI] [PubMed] [Google Scholar]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J. Am. Coll. Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on membrane-bound ATPases in the brain of rats exposed to cigarette smoke. J. Biochem. Mol. Toxicol. 2005;19:59–65. doi: 10.1002/jbt.20050. [DOI] [PubMed] [Google Scholar]
- Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006;78:1378–1384. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch. Intern. Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- Bartal M. Health effects of tobacco use and exposure. Monaldi Arch. Chest Dis. 2001;56:545–554. [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging. 2004a;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49−62. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiol. Aging. 2004b;25:49–62. [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression inventory. Center for Cognitive Therapy; Pennsylvania: 1978. [Google Scholar]
- Benedict R. Brief visuospatial memory test. Revised Psychological Assessment Resources, Inc.; Odessa, FL: 1997. [Google Scholar]
- Bolego C, Poli A, Paoletti R. Smoking and gender. Cardiovasc. Res. 2002;53:568–576. doi: 10.1016/s0008-6363(01)00520-x. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Fama R, Rosenberger P, Whitacre CC, Para MF, Nasrallah HA, Fass RJ. Drug and alcohol use and neuropsychological performance in asymptomatic HIV infection. J. Neuropsychiatry Clin. Neurosci. 1993;5:254–259. doi: 10.1176/jnp.5.3.254. [DOI] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. J. Psychiatr. Res. 2005 doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol. Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Brooks PJ. Brain atrophy and neuronal loss in alcoholism: a role for DNA damage? Neurochem. Int. 2000;37:403–412. doi: 10.1016/s0197-0186(00)00051-6. [DOI] [PubMed] [Google Scholar]
- Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob. Res. 2005;7:511–522. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- Casasola GG, Alvarez-Sala JL, Marques JA, Sanchez-Alarcos JM, Tashkin DP, Espinos D. Cigarette smoking behavior and respiratory alterations during sleep in a healthy population. Sleep Breath. 2002;6:19–24. doi: 10.1007/s11325-002-0019-y. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Wolf RL, Maldjian JA, Kasner SE. MRI identification of early white matter injury in anoxic-ischemic encephalopathy. Neurology. 2001;56:481–485. doi: 10.1212/wnl.56.4.481. [DOI] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J. Gen. Intern. Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, Gibert CL, Butt AA, Justice AC. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J. Gen. Intern. Med. 2005;20:1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Pattie A, Taylor MD, Whiteman MC, Starr JM, Whalley LJ. Smoking and cognitive change from age 11 to age 80. J. Neurol. Neurosurg. Psychiatr. 2003;74:1003–1007. doi: 10.1136/jnnp.74.7.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test. 2nd ed. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Deveci SE, Deveci F, Acik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir. Med. 2004;98:551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Ding J, Nieto FJ, Beauchamp NJ, Longstreth WT, Jr., Manolio TA, Hetmanski JB, Fried LP. A prospective analysis of risk factors for white matter disease in the brain stem: the Cardiovascular Health Study. Neuroepidemiology. 2003;22:275–282. doi: 10.1159/000071190. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Mackay RD, Meyerhoff DJ, Norman D, Weiner MW, Fein G. Brain atrophy in HIV infection is more strongly associated with CDC clinical stage than with cognitive impairment. Int. J. Neuropsychol. Soc. 1997;3:276–287. [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend. 2007;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol. Clin. Exp. Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol. Clin. Exp. Res. 2006a;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. A comparison of neurocognitive function in nonsmoking and chronically smoking short-term abstinent alcoholics. Alcohol. 2006b;39:1–11. doi: 10.1016/j.alcohol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Epperson CN, O'Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal JH, Mason GF. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol. Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Fowles J, Bates M, Noiton D. The chemical constituents in cigarettes and cigarette smoke: priorities for harm reduction. Epidemiology and Toxicology Group; New Zealand: 2000. pp. 1–65. [Google Scholar]
- Fregly AR, Smith MJ, Graybiel A. Revised normative standards of performance of men on a quantitative ataxia battery. Acta Otolaryngol. 1973;75:10–16. doi: 10.3109/00016487309139631. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of cigarette smoking in young adults—a comparison with pre-drug performance. Neurotoxicol. Teratol. 2006;28:517–525. doi: 10.1016/j.ntt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin. Microbiol. Rev. 2003;16:209–219. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furber AS, Maheswaran R, Newell JN, Carroll C. Is smoking tobacco an independent risk factor for HIV infection and progression to AIDS? A systemic review. Sex. Transm. Infect. 2007;83:41–46. doi: 10.1136/sti.2005.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, von Haebler D, Seifert F, Schubert F. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J. Clin. Psychopharmacol. 2007;27:80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedfgen M. Smoking and structural brain deficits: a volumetric MR investigation. Eur. J.Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Jahng G-H, Ezekiel F, Banys P, Meyerhoff DJ. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcohol. Clin. Exp. Res. 2006;30:1–12. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca(2+) channels in cerebral arterioles. Circ. Res. 2001;88:359–365. doi: 10.1161/01.res.88.3.359. [DOI] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA. Neuropsychological efficiency measures in male and female alcoholics. J. Stud. Alcohol. 1992;53:546–552. doi: 10.15288/jsa.1992.53.546. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop color and word test. Stoelting Company; Chicago, IL: 1978. [Google Scholar]
- Golden CJ, Hammeke TA, Purisch AD. Diagnostic validity of a standardized neuropsychological battery derived from Luria's neuropsychological tests. J. Consult. Clin. Psychol. 1978;46:1258–1265. [PubMed] [Google Scholar]
- Grant I, Olshen RA, Atkinson JH, Heaton RK, Nelson J, McCutchan JA, Weinrich JD. Depressed mood does not explain neuropsychological deficits in HIV-infected persons. Neuropsychology. 1993;7:53–61. [Google Scholar]
- Green JE, Saveanu RV, Bornstein RA. The effect of previous alcohol abuse on cognitive function in HIV infection. Am. J. Psychiatry. 2004;161:249–254. doi: 10.1176/appi.ajp.161.2.249. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intellegence in the elderly. J. Clin. Exp. Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Haverkos HW. HIV/AIDS and drug abuse: epidemiology and prevention. J. Addict. Dis. 1998;17:91–103. doi: 10.1300/J069v17n04_08. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol. Sci. 2002;23:78–82. doi: 10.1016/s0165-6147(02)01893-x. [DOI] [PubMed] [Google Scholar]
- Hayee A, Haque A, Anwarullah A, Rabbani M. Smoking enhances age related brain atrophy-a quantitative study with computed tomography. Bangladesh Med. Res. Counc. Bull. 2003;29:118–124. [PubMed] [Google Scholar]
- Hill RD, Nilsson LG, Nyberg L, Backman L. Cigarette smoking and cognitive performance in healthy Swedish adults. Age Ageing. 2003;32:548–550. doi: 10.1093/ageing/afg067. [DOI] [PubMed] [Google Scholar]
- Iki M, Ishizaki H, Aalto H, Starck J, Pyykko I. Smoking habits and postural stability. Am. J. Otolaryngol. 1994;15:124–128. doi: 10.1016/0196-0709(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med. Care. 2006;44:S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am. J. Epidemiol. 2002;156:936–944. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. In: Forster FM, editor. The medical clinics of North America. Saunders; New York, NY: 1963. [PubMed] [Google Scholar]
- Kubota K, Matsuzawa T, Fujiwara T, Yamaguchi T, Ito K, Watanabe H, Ono S. Age-related brain atrophy enhanced by smoking: a quantitative study with computed tomography. J. Exp. Med. 1987;153:303–311. doi: 10.1620/tjem.153.303. [DOI] [PubMed] [Google Scholar]
- Lawton-Craddock A, Nixon SJ, Tivis R. Cognitive efficiency in stimulant abusers with and without alcohol dependence. Alcohol. Clin. Exp. Res. 2003;27:457–464. doi: 10.1097/01.ALC.0000056620.98842.E6. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol. Biochem. Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Kondor N, Yousefi V, Green A, Wong F, Aquino-Parsons C. Reduction of carboxyhaemoglobin levels in the venous blood of cigarette smokers following the administration of carbogen. Radiother. Oncol. 2004;73:367–371. doi: 10.1016/j.radonc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Mendez-Alvarez E, Soto-Otero R, Sanchez-Sellero I, Lopez-Rivadulla Lamas M. In vitro inhibition of catalase activity by cigarette smoke: relevance for oxidative stress. J. Appl. Toxicol. 1998;18:443–448. doi: 10.1002/(sici)1099-1263(199811/12)18:6<443::aid-jat530>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rauch GM, Crawford K, Rauch RA, Konno S, Akiyama H, Terayama Y, Haque A. Risk factors accelerating cerebral degenerative changes, cognitive decline and dementia. Int. J. Geriatr. Psychiatry. 1999;14:1050–1061. doi: 10.1002/(sici)1099-1166(199912)14:12<1050::aid-gps56>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ. Effects of alcohol and HIV infection on the central nervous system. Alcohol Health Res. World. 2001;25:288–298. [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Cardenas V, Studholme C, Blumenfeld R, Truran D, Ezekiel F, Lampiris H, Rothlind J, Lindgren J, Weiner MW. Evidence for brain damage in treated HIV-infected individuals. Neurology. 2003;60:A186. [Google Scholar]
- Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: impact of alcohol use. Addict. Biol. 2003;8:33–37. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]
- Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic. Biol. Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J, Richie JP., Jr. Enhanced protein glutathiolation and oxidative stress in cigarette smokers. Free Radic. Biol. Med. 2004;36:464–470. doi: 10.1016/j.freeradbiomed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Neuhaus A, Bajbouj M, Kienast T, Kalus P, von Haebler D, Winterer G, Gallinat J. Persistent dysfunctional frontal lobe activation in former smokers. Psychopharmacology (Berl.) 2006;186:191–200. doi: 10.1007/s00213-006-0366-7. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin. Infect. Dis. 2000;31:808–812. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Paul R, Phillips M. Cognitive efficiency in alcoholics and polysubstance abusers. Alcohol. Clin. Exp. Res. 1998;22:1414–1420. doi: 10.1111/j.1530-0277.1998.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Parsons OA. Behavioral dysfunction and cognitive efficiency in male and female alcoholics. Alcohol. Clin. Exp. Res. 1995;19:577–581. doi: 10.1111/j.1530-0277.1995.tb01551.x. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict. Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Review of NIAAA's neuroscience and behavioral research portfolio. NIAAA; Bethesda, MD: 2000. NIAAA research monograph No. 34: Neuropsychological vulnerabilites in chronic alcoholism. pp. 437–472. [Google Scholar]
- Osterrieth P, Rey A. Le test de copie d'une figure complex. Arch. Psychol. 1944;30:205–221. [Google Scholar]
- Ott A, Andersen K, Dewey ME, Letenneur L, Brayne C, Copeland JR, et al. Effect of smoking on global cognitive function in nondemented elderly. Neurology. 2004;62:920–924. doi: 10.1212/01.wnl.0000115110.35610.80. [DOI] [PubMed] [Google Scholar]
- Panda K, Chattopadhyay R, Chattopadhyay DJ, Chatterjee IB. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic. Biol. Med. 2000;29:115–124. doi: 10.1016/s0891-5849(00)00297-5. [DOI] [PubMed] [Google Scholar]
- Park EM, Park YM, Gwak YS. Oxidative damage in tissues of rats exposed to cigarette smoke. Free Radic. Biol. Med. 1998;25:79–86. doi: 10.1016/s0891-5849(98)00041-0. [DOI] [PubMed] [Google Scholar]
- Patel SH, Inglese M, Glosser G, Kolson DL, Grossman RI, Gonen O. Whole-brain N-acetylaspartate level and cognitive performance in HIV infection. Am. J. Neuroradiol. 2003;24:1587–1591. [PMC free article] [PubMed] [Google Scholar]
- Patel SH, Kolson DL, Glosser G, Matozzo I, Ge Y, Babb JS, Mannon LJ, Grossman RI. Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. Am. J. Neuroradiol. 2002;23:543–549. [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Cohen RA, Williams LM, Niaura R, Pogun S, Clark CR, Gunstad J, Gordon E. Cognitive status of young and older cigarette smokers: data from the international brain database. J. Clin. Neurosci. 2006;13:457–465. doi: 10.1016/j.jocn.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Cortical NAA deficits in HIV infection without dementia: influence of alcoholism comorbidity. Neuropsychopharmacology. 2005;30:1392–1399. doi: 10.1038/sj.npp.1300723. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Sullivan EV. Alcoholism and AIDS: magnetic resonance imaging approaches for detecting interactive neuropathology. Alcohol. Clin. Exp. Res. 2002;26:1031–1046. doi: 10.1097/01.ALC.0000021146.01778.55. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog assessment of cognitive functioning. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Razani J, Boone K, Lesser I, Weiss D. Effects of cigarette smoking history on cognitive functioning in healthy older adults. Am. J. Geriatr. Psychiatry. 2004;12:404–411. doi: 10.1176/appi.ajgp.12.4.404. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsycho-logical test battery: theory and interpretation. Neuropsychological Press; Tucson, AZ: 1985. [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am. J. Public Health. 2003;93:994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am. J. Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J. Int. Neuropsychol. Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Anstey KJ, Parslow RA, Wen W, Maller J, Kumar R, Christensen H, Jorm AF. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement. Geriatr. Cogn. Disord. 2006;21:300–308. doi: 10.1159/000091438. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol. Clin. Exp. Res. 2004a;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res. Hum. Retroviruses. 2004b;20:151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Kieburtz K, McDermott MP, McArthur J, Marder K, Sacktor N, et al. Clinical trials in HIV-associated cognitive impairment: cognitive and functional outcomes. Neurology. 2001;56:415–418. doi: 10.1212/wnl.56.3.415. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Belanger H, Mortimer JA, Graves AB. Effects of the use of alcohol and cigarettes on cognition in elderly African American adults. J. Int. Neuropsychol. Soc. 2003;9:690–697. doi: 10.1017/S1355617703950028. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol digit modalities test (SDMT) manual. Revised Western Psychological Services; Los Angeles, CA: 1982. [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption. The Humana Press Inc.; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J. Stud. Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [published erratum appears in J. Stud. Alcohol 1989 Jan; 50(1):92]. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003;28:765–772. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Maudsley A, Weiner M. An intensity consistent filtering approach to the analysis of deformation tensor derived maps of brain shape. Neuroimage. 2003;19:1638–1649. doi: 10.1016/s1053-8119(03)00183-6. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Schuff N, Rosen H, Miller B, Weiner M. Detecting spatially consistent structural differences in Alzheimer's and frontotemporal dementia using deformation morphometry; Paper presented at: Proceedings of Medical Image Computing and Computer Assisted Interventions (Utrecht).2001a. [Google Scholar]
- Studholme C, Cardenas V, Weiner M. Multi-scale image and multi-scale deformation of brain anatomy for building average brain atlases; Paper presented at: SPIE medical imaging conference.2001b. [Google Scholar]
- Sullivan EV. NIAAA Research Monograph No. 34: Human brain vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research portfolio. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc. Natl. Acad. Sci. USA. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use meth-amphetamine. J. Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, Page-Shafer K, Chin DP, Osmond D, Mossar M, Markstein L, Huitsing J, Barnes S, Clemente V, Chesney M. Adverse impact of cigarette smoking on dimensions of health-related quality of life in persons with HIV infection. AIDS Patient Care STDS. 2001;15:615–624. doi: 10.1089/108729101753354617. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J. Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- Wetzel L, Boll T. Short category test, booklet format. Western Psychological Services; Los Angles, CA: 1987. [Google Scholar]
- Wewers MD, Diaz PT, Wewers ME, Lowe MP, Nagaraja HN, Clanton TL. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. Am. J. Respir. Crit. Care Med. 1998;158:1543–1549. doi: 10.1164/ajrccm.158.5.9802035. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol. Psychiatry. 2001;49:906–913. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]