Abstract
We previously reported [Cardenas, V.A., Studholme, C., Meyerhoff, D.J., Song, E., Weiner, M.W., 2005. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 138, 115−130] that non-treatment-seeking, active heavy drinkers (HD) demonstrated smaller regional neocortical gray matter volumes compared to light drinking controls; however, the potential effects of chronic cigarette smoking on regional brain volumes were not addressed. The goal of this retrospective analysis was to determine if chronic smoking affected brain structure in the non-treatment-seeking heavy drinking sample from our earlier report (i.e., Cardenas et al., 2005). Regional volumetric comparisons were made among age-matched smoking HD (n = 17), non-smoking HD (n = 16), and non-smoking light drinkers (nsLD; n = 20) from our original sample. Quantitative volumetric measures of neocortical gray matter (GM), white matter (WM), subcortical structures, and cerebral spinal fluid (CSF) were derived from high-resolution magnetic resonance imaging. Smoking HD demonstrated smaller volumes than nsLD in the frontal, parietal, temporal GM, and for total neocortical GM. Smoking HD also demonstrated smaller temporal and total GM volumes than non-smoking HD. Non-smoking HD and nsLD did not differ significantly on GM volumes. Further, the three groups did not differ on lobar WM, subcortical structures or regional CSF volumes. These retrospective analyses indicate neocortical GM volume reductions in non-treatment-seeking smoking HD, but not in non-smoking HD, which are consistent with our studies in recently detoxified treatment-seeking alcohol-dependent samples.
Keywords: Alcoholism, Chronic cigarette smoking, MRI, Brain volume, Neuroimaging
1. Introduction
The prevalence of chronic cigarette smoking among individuals afflicted with alcohol use disorders (i.e., alcohol abuse or dependence) is approximately 50−80% (Durazzo et al., in press; Hurt et al., 1994; Pomerleau et al., 1997; Romberger and Grant, 2004). In 1-week-abstinent, treatment-seeking alcoholics, we observed that chronic cigarette smokers exhibited smaller regional gray matter volumes relative to non-smokers, and alcoholics had smaller regional gray matter volumes compared to light drinking controls, irrespective of smoking status (Gazdzinski et al., 2005). Treatment-seeking individuals constitute only a small fraction of persons afflicted with alcohol use disorders (AUD) in the United States, yet most of what is known about the effects of AUD on human brain morphology stems from magnetic resonance imaging (MRI) studies with individuals recruited from inpatient and outpatient treatment programs. Treatment-naïve alcoholics represent the vast majority of individuals with AUD. Compared to treatment-seeking alcoholics, treatment-naïve alcoholics have been reported to demonstrate a different drinking trajectory and less severe levels of lifetime alcohol consumption (Fein and Landman, 2005), as well as lower magnitudes of alcohol-induced cerebral morphological abnormalities (Fein et al., 2002). Therefore, morphological findings obtained for treatment-seeking alcoholics may not necessarily generalize to treatment-naïve individuals. To further address this issue, we studied treatment-naïve, community-dwelling active heavy drinkers with high-resolution quantitative magnetic resonance imaging and observed that chronic and excessive alcohol consumption in these individuals was associated with cerebral morphological abnormalities (Cardenas et al., 2005). Specifically, treatment-naïve, active heavy drinkers demonstrated smaller regional cortical gray matter (GM) volumes compared to light drinking controls. However, the potential effects of chronic cigarette smoking on brain structure were not addressed in our previous MRI studies with treatment-naïve, active heavy drinkers.
Our quantitative volumetric studies with 1-week-abstinent, treatment-seeking alcoholics suggested that chronic cigarette smoking modulated neocortical GM volumes (Gazdzinski et al., 2005). In population-based samples, the prevalence of chronic smoking is significantly higher in persons with AUD than light social drinkers (Dawson, 2000; John et al., 2003). Therefore, it is possible that chronic smoking in our cohort of active heavy social drinkers is also associated with a similar pattern of regional tissue dysmorphology evidenced by our treatment-seeking alcoholics. Thus, the primary goal of this retrospective analysis was to determine the potential influence of chronic cigarette smoking on regional GM and WM volumes in the non-treatment-seeking, heavy drinking community-based sample described in Cardenas et al. (2005).
2. Methods
2.1. Participants
Participants in this report and Cardenas et al. (2005) were originally recruited for a larger longitudinal study of the central nervous system effects of HIV and AUD. From the 49 actively drinking HD participants in Cardenas et al. (2005), we identified 17 smoking HD (sHD; 3 females) who reported smoking daily (n = 10) or nearly everyday (n = 7) for at least 6 months prior to enrolment in the study. We focused on individuals with the greatest smoking frequency and did not include sporadic smokers. Information on number of cigarettes smoked per day and past smoking history were not obtained in the original study. The “daily” sHD were 8 years younger (p = .04) than the “nearly everyday” sHD, but they were equivalent on education, estimated premorbid verbal IQ and alcohol consumption variables. The 16 non-smoking HD (nsHD; 3 females) and 20 non-smoking light drinkers (nsLD; 3 females) were selected from the larger cohort by age matching to the sHD sample. nsHD reported no consumption of any tobacco products in the 6 months prior to enrolment. The original LD sample did not have a sufficient number of daily or nearly everyday smokers to form a viable group. nsLD participants consumed a lifetime average of less than or equal to 45 (35 for women) standard alcoholic drinks per month (one alcoholic drink equivalent = 12 oz of beer, 5 oz of wine, or 1.5 oz of liquor, all corresponding to approximately 13.6 g pure alcohol) with no history of current or past alcohol or substance abuse and no history of drinking more than 100 drinks per month. Classification as a HD required average consumption of at least 100 (80 for women) standard alcoholic drinks per month for a minimum of 3 years prior to enrolment and active alcohol consumption at time of study. Exclusion criteria are fully detailed in Cardenas et al. (2005). In short, all participants were free of general medical, neurologic and neuropsychiatric conditions known or suspected to influence brain morphology. Subjects were excluded if they met DSM-IV criteria for dependence on any other substance than alcohol or nicotine in the 6 months prior to enrolment. Prior to completing any procedure, all participants gave written informed consent, which was approved by review boards of the University of California San Francisco and the San Francisco VA Medical Center.
2.2. Psychiatric/behavioral assessment
At the time of enrolment, participants completed the Structured Clinical Interview for DSM-IV Axis I disorders (American Psychological Association, 1994), and interviews and questionnaires assessing depressive symptomatology (Beck Depression Inventory, BDI; Beck, 1978), lifetime alcohol consumption (Lifetime Drinking History, LDH; Skinner and Sheu, 1982; Sobell and Sobell, 1992; Sobell et al., 1988), and substance use (in-house questionnaire assessing substance type, and quantity and frequency of use). From the LDH, we derived the number of drinks in the week prior to enrolment, and average number of drinks per month in the previous year and over lifetime, number of months of heavy drinking (i.e., total number of months over lifetime in which the participant drank in excess of 100 drinks per month), and cumulative ethanol consumption over lifetime in kilograms (total number of drinks over lifetime × 0.0136 kg ethanol/drink). General verbal intellectual functioning was predicted by the American version of the Nelson Adult Reading Task (AMNART; Grober and Sliwinski, 1991). The neurocognitive implications of chronic smoking in this HD cohort will be reported elsewhere.
2.3. MRI acquisition and processing
MRI data acquisition was conducted on a clinical 1.5 T MR scanner (Vision, Siemens Medical Systems, Iselin, NJ) and consisted of two sequences: (1) double spin-echo (TR/TE1/TE2 = 2500 ms/20 ms/80 ms, 1 mm × 1 mm in-plane resolution, 3 mm slice thickness, no slice gap, oriented at the orbital-metal angle +5° as seen in the midsagittal scout) yielded proton density and T2-weighted MR images and (2) Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE; TR/TI/TE = 9.7 ms/300 ms/4 ms, 1 mm × 1 mm in-plane resolution, 1.5 mm slabs; oriented orthogonal to the long axis of the hippocampus) yielded coronal T1-weighted (T1-w) MR images. Three-tissue intensity based segmentation was applied to T1-w images to assign a set of probabilities of WM, GM, or CSF to each voxel. The procedure details and validity studies are fully described in Cardenas et al. (2005). An atlas-based deformable registration method was used to automatically identify regions of interest (ROIs) in the brain as described in Cardenas et al. (2005). In summary, a single MRI from a 36-year-old man served as a reference atlas and was manually edited to delineate ROIs including the major lobes of the brain (frontal, temporal, parietal, and occipital), lateral ventricles, thalamus, caudate, lenticular nuclei, brainstem, and cerebellum. Temporal GM volume included some tissue from the amygdala and the hippocampal complex, and volumes for the thalamus, basal ganglia nuclei, brainstem and cerebellum represent the sum of GM and WM within these structures. A B-Spline Free Form deformation algorithm driven by normalized mutual information (Studholme et al., 2001a,2001b, 2003) was employed to estimate the spatial transformation from the atlas to each individual's T1-w MRI. This transformation was then inverted and used to apply the atlas labels to demarcate participant-specific ROIs on each scan, which, after combination with tissue segmentation results, yielded lobar volumes of WM, GM, and CSF. All automatically marked MRIs were carefully reviewed by a trained operator to insure accuracy of automated markings.
2.4. Statistical analyses
Studies with healthy adult controls generally reveal a linear decline of neocortical GM volume with increasing age (e.g., Courchesne et al., 2000; Jernigan et al., 2001). We used multivariate analysis of covariance (MANCOVA) with age as a covariate to control for age–volume relationships in all group comparisons. Similar to analyses described in Cardenas et al. (2005), regional cortical GM, WM, and sulcal CSF were separately evaluated with MANCOVA for the HD group irrespective of smoking status (i.e., sHD and nsHD combined) compared to the nsLD group. Significant MANCOVAs (p ≤ .05) were followed by univariate comparisons. We then separately evaluated regional and total cortical GM, WM and sulcal and ventricular CSF for sHD, nsHD, and nsLD with MANCOVA. Total cortical GM, WM and sulcal CSF volumes for sHD, nsHD, and nsLD represent the summed tissue volume the four main lobar regions. In addition, separate MANCOVAs comparing the three groups were also conducted for basal ganglia and thalami, brainstem and cerebellum, and sulcal and ventricular CSF volumes. Significant MANCOVAs (p ≤ .05) were followed up with univariate tests. Significant univariate tests were further evaluated for group differences among sHD, nsHD, and nsLD with Bonferroni corrected pairwise t-tests. In the HD groups, we also separately investigated the relationships (Spearman coefficients) between regional lobar tissue volumes and lifetime average number of drinks per month, total amount of ethanol consumed over lifetime, and years of heavy drinking. For these correlations, alpha level (.05) was adjusted for the three aforementioned measures of drinking severity and four lobar regions separately for each tissue type (i.e., GM or WM) to correct for multiplicity of correlations. Therefore, for correlations between measures of drinking severity and lobar GM or WM volumes adjusted alpha = .004. Statistical analyses were performed with SPSS v12.0 and S-plus v6.1.
3. Results
3.1. Participant characterization
Groups were equivalent in age and on BDI scores. nsLD and nsHD were equivalent on education, but nsHD had 2 more years of education than sHD (p = .04; see Table 1). Fourteen of the nsLD participants were Caucasian and two each were African American, Latino, and Asian. Ten nsLD were Caucasian, five African American, and two Latino. Nine sHD were Caucasian and eight were African American. Fourteen of 17 sHD, and 12 of 16 nsHD met criteria for alcohol dependence, and the rest of the HD met criteria for alcohol abuse. In the nsHD group, criteria for past dependence were met by three participants for cocaine (average number of months since last dependent = 132; min = 12, max = 300), three for methamphetamine (average number of months since last dependent = 176; min = 72, max = 373), and three for cannabis (average number of months since last dependent = 36; min = 12, max = 72). In the sHD group, criteria for past dependence were met by three participants for cocaine (average number of months since last use = 57; min = 15, max = 120), three for methamphetamine (average number of months since last dependent use = 240; min = 120, max = 312), five for cannabis (average number of months since last dependent = 242; min = 36, max = 372) and one for heroin (number of months since last dependent = 120). No HD participant concurrently met criteria for current or past substance abuse on compounds other than those listed above (except alcohol and nicotine). sHD consumed significantly more kilograms of ethanol over lifetime (p = .05) and tended to have a higher average number of drinks per month over lifetime and greater number of years of heavy drinking than nsHD (both p = .06).
Table 1.
Demographics and alcohol use histories all groups (mean ± S.D.)
| Measure | nsLD (n = 20) | nsHD (n = 16) | sHD (n = 17) |
|---|---|---|---|
| Age (years) | 43 ± 10 | 43 ± 11 | 45 ± 8 |
| Education (years) | 14 ± 2 | 15 ± 2 | 13 ± 2 |
| %Caucasian | 70 | 63 | 53 |
| AMNART | 117 ± 7 | 116 ± 7 | 112 ± 11 |
| BDI | 6 ± 6 | 10 ± 9 | 10 ± 9 |
| Average drinks/day over last week | 2 ± 1 | 6 ± 3 | 8 ± 5 |
| 1-yr average drinks/month | 12 ± 16 | 204 ± 101 | 231 ± 126 |
| 3-yr average drinks/month | 12 ± 16 | 200 ± 99 | 236 ± 115 |
| Lifetime average drinks/month | 10 ± 8 | 134 ± 77 | 193 ± 96 |
| Months heavy drinking | na | 144 ± 108 | 204 ± 84 |
| Total ethanol consumption (kg) | 44 ± 45 | 367 ± 233 | 597 ± 400 |
| Years regular drinking | 22 ± 10 | 26 ± 11 | 27 ± 8 |
AMNART, American National Adult Reading Test; 1-yr average, average number of drinks per month over 1-year prior to study; 3-yr average, average number of drinks per month over 3-years prior to study; lifetime average drinks/month, average number of drinks per month over lifetime; total ethanol consumption, amount of ethanol consumed over lifetime; months heavy drinking, number of months drinking at greater than 100 drinks per month; years regular drinking, number of years of consumption of at least one alcoholic beverage per month; BDI, Beck Depression Inventory.
3.2. Volumetric comparisons
3.2.1. HD and nsLD
MANCOVA (Wilks' Lambda) comparing the combined HD group (i.e., nsHD + sHD) to the nsLD group on regional neocortical GM was significant [F(4, 47) = 2.77, p = .05)]. ANCOVAs were significant for the frontal, parietal, temporal GM, and total cortical GM (p = .004−.03), with a trend for occipital GM (p = .06). The HD sample demonstrated smaller volumes in these regions than the nsLD group. MANCOVAs for lobar WM and sulcal CSF indicated no significant differences between HD and nsLD, and there was no group by age interaction. These findings are consistent with findings for the larger cohort in examined Cardenas et al. (2005).
3.2.2. sHD, nsHD, and nsLD
MANCOVA (Wilks' Lambda) for regional neocortical GM was significant [F(2, 92) = 2.19, p = .04)]. ANCOVAs were significant for the frontal, parietal, temporal GM, and total cortical GM (p = .001−.03), with a trend (p = .09) for occipital GM. There was no group by age interaction. Bonferroni corrected pairwise comparisons indicated significantly smaller volumes in sHD compared to nsLD in the frontal, parietal, and temporal GM and for total cortical GM (p = .001−.02) (see Figs. 1–4). Notably, sHD demonstrated smaller temporal GM and total GM volumes than nsHD (both p ≤ .02), with a trend (p = .07) for smaller parietal GM in sHD (see Figs. 2 and 4). Given the greater total ethanol consumption and number of drinks per month over lifetime in sHD compared to nsHD, we used these variable as well as months of heavy drinking as covariates in pairwise comparisons of sHD and nsHD. The neocortical GM differences between sHD and nsHD remained significant after covarying for these variables. This suggests that differences in cumulative ethanol dose or months of heavy drinking did not account for the smaller temporal GM and total GM volumes observed in sHD compared to nsHD. No significant differences were observed between nsLD and nsHD for regional lobar GM or total GM volumes.
Fig. 1.
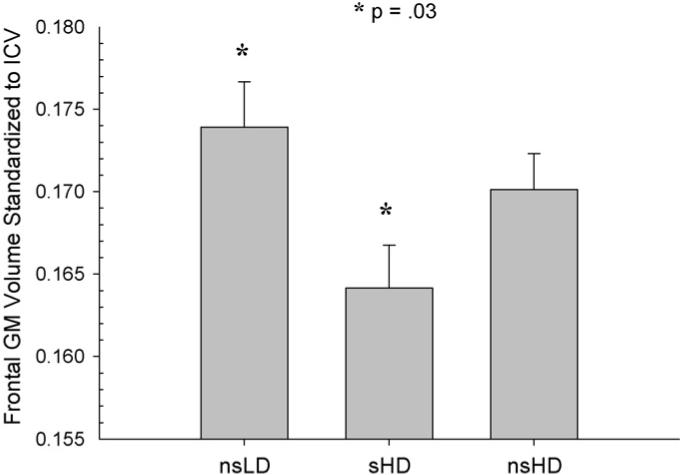
Frontal GM volume standardized to intracranial volume for nsLD, sHD, and nsHD; ICV, intracranial volume (mean and standard error).
Fig. 4.
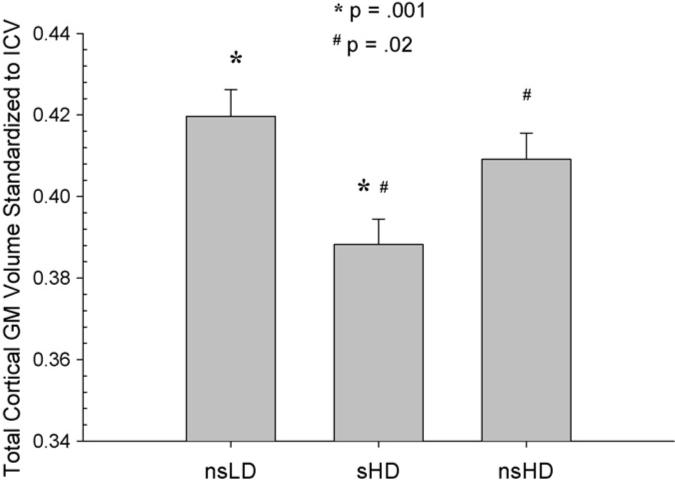
Total cortical GM volume standardized to intracranial volume for nsLD, sHD, and nsHD; ICV, intracranial volume (mean and standard error).
Fig. 2.
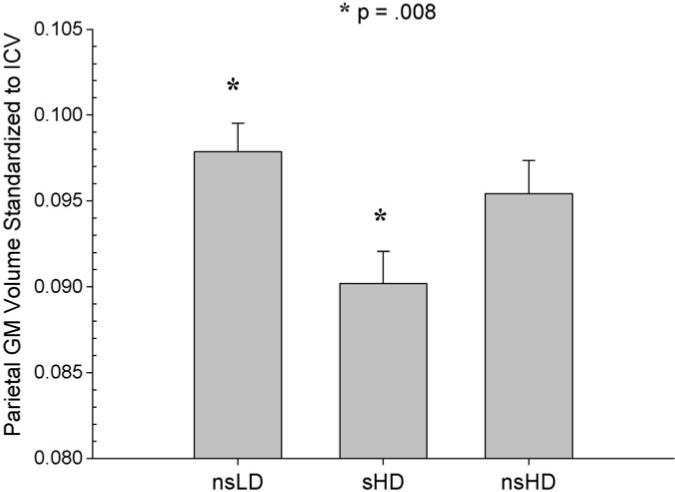
Parietal GM volume standardized to intracranial volume for nsLD, sHD, and nsHD; ICV, intracranial volume (mean and standard error).
MANCOVA for lobar WM, basal ganglia and thalamus, and brain stem and cerebellum volumes indicated no significant differences among the three groups. MANCOVAs for sulcal, ventricular, and total CSF volumes were not significant, although sHD tended to have more ventricular CSF than both nsHD and nsLD (both p = .07). There were no group by age interactions in the above analyses. Additionally, there were no volumetric disparities in any region or structure between the daily-smoking and near-daily-smoking participants comprising the sHD group. Finally, after our conservative correction for multiple correlations (see Section 2), no measure of alcohol consumption was significantly related to regional GM or WM volumes for sHD, nsHD, or the combined group (i.e., sHD + nsHD).
4. Discussion
The primary objective of this retrospective analysis was to examine the potential effects of chronic cigarette smoking on MRI-derived regional brain volumes in a subset of non-treatment-seeking heavy drinkers from our previous study (Cardenas et al., 2005). Similar to the larger predominantly male cohort in Cardenas et al. (2005), this HD subset had significantly less regional cortical GM than the subset of nsLD. Most importantly, analyses contrasting sHD, nsHD, and nsLD groups indicated that the GM volume differences observed between the nsLDs and the entire HD group in this study were primarily driven by smaller volumes in sHD relative to nsLD. In addition, sHD demonstrated smaller temporal GM and total GM volumes than nsHD, which was not attributable to group differences in drinking severity.
The absence of significant cortical GM volume differences between the nsHD and nsLD groups in this study are consistent with our volumetric findings in a somewhat older sample of treatment-seeking alcohol-dependent individuals with approximately 1 week of abstinence (Gazdzinski et al., 2005). In Gazdzinski et al. (2005), which employed the same MR acquisition and processing methods as this report, the non-smoking, treatment-seeking alcoholics did not show significant reductions in regional cortical GM compared to non-smoking controls. As in this report, the non-smoking, treatment-seeking alcoholics also consumed considerably less alcohol over lifetime than their smoking counterparts. The lower cumulative alcohol exposure in the non-smoking alcoholics in both studies may partially explain why they did not show significant atrophy relative to non-smoking controls. However, the non-smoking alcoholics in Gazdzinski et al. (2005) consumed nearly three times as much ethanol (in kilograms) over lifetime than the nsHD and nearly twice as much as sHD in this report, yet they still did not demonstrate significant neocortical GM volume reductions in any lobe relative to their non-smoking control participants. Overall, the findings from Gazdzinski et al. (2005) and the present study suggest that chronic excessive alcohol consumption per se was not associated with significant abnormalities in neocortical GM morphology in these alcoholic cohorts, but the combination of chronic alcohol misuse and cigarette smoking resulted in significant neocortical GM volume loss relative to controls.
Recent brain neuroimaging studies reported structural abnormalities in non-alcoholic chronic smokers. Specifically, MRI indicated smaller neocortical GM volumes and lower densities in the prefrontal cortex, smaller left anterior cingulate volume, and lower GM densities in the right cerebellum of chronic smokers compared to non-smokers (Brody et al., 2004), and computed tomography showed increased generalized brain atrophy with advancing age in chronic smokers (Akiyama et al., 1997; Hayee et al., 2003; Kubota et al., 1987). Volume reductions in these regions are also commonly reported in alcoholism (see Sullivan, 2000, for review). The brain structural findings emerging from the smoking literature, and from our own studies, suggest that the combination of chronic alcohol misuse and cigarette smoking explain the regional dysmorphology observed in the cerebrum of individuals with AUD.
A growing body of evidence suggests that chronic smoking, independent of substance abuse disorders, is associated with adverse effects on several domains of neurocognition, including executive skills, learning and memory, processing speed, and working memory (e.g., Ernst et al., 2001; Heffernan et al., 2005; Kalmijn et al., 2002; Paul et al., 2006; Razani et al., 2004; Richards et al., 2003). In a large cohort of community-recruited actively drinking and abstinent alcoholics, Glass et al. (2006) found that both alcoholism and smoking severity were inversely related to neurocognitive function, and smoking severity (i.e., pack years) was a unique predictor of general intelligence and cognitive proficiency (i.e., an index of both speed and accuracy). Correspondingly, Friend et al. (2005) reported that both chronicity of alcohol misuse and cigarette smoking were inversely related to measures of general intellectual functioning, set-shifting and processing speed in a large community-based group of actively drinking and abstinent alcoholics. Also, non-smoking alcoholics were superior to smoking alcoholics on measures of processing speed and set-shifting. In 1-month-abstinent-alcoholics in treatment, we observed chronic smokers performed significantly worse than non-smokers on measures of learning and memory, processing speed, cognitive efficiency, and postural stability (Durazzo et al., in press). Therefore, in addition to greater brain morphological abnormalities, chronic cigarette smoking among individuals with AUD appears to modulate neurocognition. In general, brain shrinkage is a risk factor for cognitive decline and memory impairment in the elderly (e.g., Meyer et al., 1999; Visser et al., 1999), and, if occurring in middle age, may increase the risk for earlier and more rapid cognitive decline with advancing age.
4.1. Potential mechanisms for neocortical GM dysmorphology in concurrent alcoholism and cigarette smoking
Chronic cigarette smoking is associated with significantly increased risk for development of atherosclerosis (Bolego et al., 2002), and nicotine has been shown to induce alterations of vascular endothelial function (Hawkins et al., 2002). These processes may impact the functional integrity of the cerebrovasculature and contribute to the decreased regional cerebral blood flow (Domino et al., 2004; Rose et al., 2003; Zubieta et al., 2001) and/or white matter disease (Ding et al., 2003; Fukuda and Kitani, 1996) reported in chronic smokers. The neocortical GM is particularly vulnerable to the effects of diffuse ischemia (see Chalela et al., 2001, and references therein). The particulate and gas phases of cigarette smoke contain many toxic compounds (e.g., carbon monoxide, free radicals, nitrosamines, polynuclear aromatic compounds; Fowles et al., 2000) that may directly or indirectly compromise brain tissue. For example, carbon monoxide (CO) levels are significantly higher in smokers (Deveci et al., 2004) which is associated with lower effective hemoglobin concentrations and, consequently, diminished oxygen carrying capacity of the blood (Macdonald et al., 2004), as well as decreased efficiency of the mitochondrial respiratory chain (Alonso et al., 2004). Chronic smoking has also been equated to a type of repeated acute (mild) CO poisoning (Alonso et al., 2004), and is linked to nocturnal hypoxia (Casasola et al., 2002) as well as to respiratory risks such as chronic obstructive pulmonary disease and other conditions that may diminish or compromise lung function (Bartal, 2001). Decreased lung function was associated with poorer neurocognition and increased subcortical atrophy in community-dwelling adults aged 60−64 years (Sachdev et al., 2006). Cigarette smoke also contains high concentrations of free radical species (e.g., reactive oxygen species, ROS) known to promote oxidative damage or stress to cellular structures and macromolecules including proteins, membrane lipids, carbohydrates, and DNA (Moriarty et al., 2003). Similarly, chronic and heavy alcohol consumption and ethanol catabolism are both associated with generation of ROS and other metabolic products that may also promote oxidative damage to various cellular molecules and structures, including phospholipids and DNA (Brooks, 2000). Therefore, a combination of chronically increased CO levels, chronic exposure to ROS from both ethanol metabolism and cigarette smoke, and potentially compromised vascular and pulmonary function may contribute to the significantly lower GM volumes observed in this sHD cohort. Additionally, it is possible that the brain regions (e.g., neocortical GM) adversely affected by chronic and excessive ethanol consumption are rendered more vulnerable to the effects of the potentially noxious compounds found in cigarette smoke (or vice versa).
Limitations of this study include the modest numbers of participants, the retrospective nature of the analyses, and the lack of detailed information on the smoking behavior of the sHD group. Additionally, the designation of smoking status was based on self-report rather than confirmation with biological measures (e.g., breath carbon monoxide level, positive plasma nicotine). We were unable to distinguish whether the combined effects of chronic alcohol misuse and smoking were synergistic or additive due to the lack of a group of light drinkers who smoked at levels equivalent to those in sHD. Additionally, we did not have enough female participants to evaluate sex effects on outcome measures. It is also possible that the observed group differences are premorbid in nature or that potential unrecorded group differences in nutrition, exercise, overall physical health, or other genetic predispositions not examined contributed to the findings. Furthermore, both HD groups demonstrated a similar frequency of previous substance dependence, which primarily involved cocaine, methamphetamine, and cannabis. Chronic misuse of these substances has been reported to adversely affect human brain morphology in active and recently detoxified abusers (e.g., Bartzokis et al., 2000; Matochik et al., 2005; O'Neill et al., 2001; Thompson et al., 2004). The long-term, enduring effects of cocaine, methamphetamine, or cannabis on human brain morphology are largely unknown, especially when dependence is in long-term sustained full remission. In both of our HD groups, participants meeting criteria for substance dependence, in most cases, had not used illicit compounds for many years prior to enrolment; therefore, it is unlikely that past substance dependence on illicit drugs significantly affected our structural findings.
In conclusion, results from these predominately Caucasian male cohorts indicate that consideration of smoking status in the evaluation of brain morphology of non-treatment-seeking individuals with AUD is warranted. Results from this report, together with the findings from Gazdzinski et al. (2005), suggest the combination of chronic heavy alcohol consumption and cigarette smoking has particularly deleterious effects on cortical GM in both treatment-seeking and non-treatment-seeking individuals with AUD. Considering our findings that chronic smoking appears to compound abnormalities in brain morphology, metabolites and perfusion in treatment-seeking alcoholics (Durazzo et al., 2004, 2006; Gazdzinski et al., 2005, 2006), larger prospective studies that better characterize the smoking behavior of both men and women in non-treatment-seeking alcoholics and its effects on both brain neurobiology and neurocognition are indicated. Finally, the potential effects of cigarette smoking on human brain morphology and function also should be given greater consideration in other conditions where it is prevalent (e.g., schizophrenia, mood disorders, and non-alcohol substance abuse).
Fig. 3.
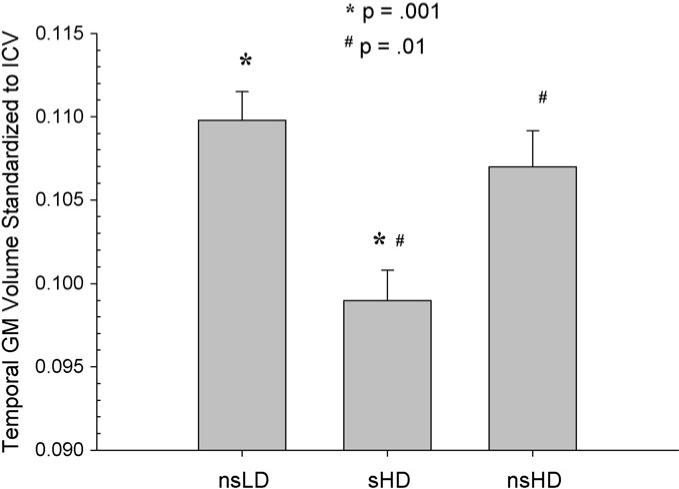
Temporal GM volume standardized to intracranial volume for nsLD, sHD, and nsHD; ICV, intracranial volume (mean and standard error).
Acknowledgements
This work was supported by NIH P01 AA11493 (M.W.W.), R01 AA10788 (D.J.M.), R01 MH65392 (C.S.), and Whitaker Foundation Award RG-01-0115 (C.S.).
References
- Akiyama H, Meyer JS, Mortel KF, Terayama Y, Thornby J, Konno S. Normal human aging: factors contributing to cerebral atrophy. J. Neurol. Sci. 1997;152:39–49. doi: 10.1016/s0022-510x(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Alonso JR, Cardellach F, Casademont J, Miro O. Reversible inhibition of mitochondrial complex IV activity in PBMC following acute smoking. Eur. Respir. J. 2004;23:214–218. doi: 10.1183/09031936.03.00038203. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Bartal M. Health effects of tobacco use and exposure. Monaldi Arch. Chest Dis. 2001;56:545–554. [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry. Res. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- Bolego C, Poli A, Paoletti R. Smoking and gender. Cardiovasc. Res. 2002;53:568–576. doi: 10.1016/s0008-6363(01)00520-x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol. Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Brooks PJ. Brain atrophy and neuronal loss in alcoholism: a role for DNA damage? Neurochem. Int. 2000;37:403–412. doi: 10.1016/s0197-0186(00)00051-6. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Casasola GG, Alvarez-Sala JL, Marques JA, Sanchez-Alarcos JM, Tashkin DP, Espinos D. Cigarette smoking behavior and respiratory alterations during sleep in a healthy population. Sleep Breath. 2002;6:19–24. doi: 10.1007/s11325-002-0019-y. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Wolf RL, Maldjian JA, Kasner SE. MRI identification of early white matter injury in anoxic–ischemic encephalopathy. Neurology. 2001;56:481–485. doi: 10.1212/wnl.56.4.481. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dawson D. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;78:263–273. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Deveci S, Deveci F, Acik Y, Ozan A. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir. Med. 2004;98:551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Ding J, Nieto FJ, Beauchamp NJ, Longstreth WT, Jr., Manolio TA, Hetmanski JB, Fried LP. A prospective analysis of risk factors for white matter disease in the brain stem: the Cardiovascular Health Study. Neuroepidemiology. 2003;22:275–282. doi: 10.1159/000071190. [DOI] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin. Exp. Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind J, Gazdzinski S, Banys P, Meyerhoff DJ. A comparison of neurocognitive function in non-smoking and chronically smoking short-term abstinent alcoholics. Alcohol: An Inter. Biomed. J. doi: 10.1016/j.alcohol.2006.06.006. in press. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Rothlind J, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin. Exp. Res. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naïve alcohol dependent individuals. Alcohol Clin. Exp. Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naïve alcoholics come from different populations. Alcohol. 2005;35:19–26. doi: 10.1016/j.alcohol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Friend KB, Malloy PF, Sindelar HA. The effects of chronic nicotine and alcohol use on neurocognitive function. Addict. Behav. 2005;30:193–202. doi: 10.1016/j.addbeh.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Fowles J, Bates M, Noiton D. The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction. Epidemiology and Toxicology Group; New Zealand: 2000. [6/2/2006]. pp. 1–65. http://www.ndp.govt.nz/tobacco/documents/tobaccochem.pdf. [Google Scholar]
- Fukuda H, Kitani M. Cigarette smoking is correlated with the periventricular hyperintensity grade of brain magnetic resonance imaging. Stroke. 1996;27:645–649. doi: 10.1161/01.str.27.4.645. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: Preliminary evidence for the effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin. Exp. Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Jahng GH, Ezekiel F, Meyerhoff DJ. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion—a preliminary magnetic resonance study. Alcohol Clin. Exp. Res. 2006;30:947–958. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JM, Adams KM, Nigg JT, Wong MM, Puttler LI, Buu A, Jester JM, Fitzgerald HE, Zucker RA. Smoking is associated with neurocognitive deficits in alcoholism. Drug Alcohol Depend. 2006;20:119–126. doi: 10.1016/j.drugalcdep.2005.08.013. Epub September 15, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J. Clin. Exp. Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol. Sci. 2002;23:78–82. doi: 10.1016/s0165-6147(02)01893-x. [DOI] [PubMed] [Google Scholar]
- Hayee A, Haque A, Anwarullah A, Rabbani M. Smoking enhances age related brain atrophy—a quantitative study with computed tomography. Bangladesh Med. Res. Counc. Bull. 2003;29:118–124. [PubMed] [Google Scholar]
- Heffernan TM, Ling J, Parrott AC, Buchanan T, Scholey AB, Rodgers J. Self-rated everyday and prospective memory abilities of cigarette smokers and non-smokers: a web-based study. Drug Alcohol Depend. 2005;78:235–241. doi: 10.1016/j.drugalcdep.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr., Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin. Exp. Res. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am. J. Epidemiol. 2002;156:936–944. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- Kubota K, Matsuzawa T, Fujiwara T, Yamaguchi T, Ito K, Watanabe H, Ono S. Age-related brain atrophy enhanced by smoking: a quantitative study with computed tomography. J. Exp. Med. 1987;153:303–311. doi: 10.1620/tjem.153.303. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Kondor N, Yousefi V, Green A, Wong F, Aquino-Parsons C. Reduction of carboxyhaemoglobin levels in the venous blood of cigarette smokers following the administration of carbogen. Radiother. Oncol. 2004;73:367–371. doi: 10.1016/j.radonc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rauch GM, Crawford K, Rauch RA, Konno S, Akiyama H, Terayama Y, Haque A. Risk factors accelerating cerebral degenerative changes, cognitive decline and dementia. Int. J. Geriatr. Psychiatry. 1999;14:1050–1061. doi: 10.1002/(sici)1099-1166(199912)14:12<1050::aid-gps56>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic. Biol. Med. 2003;35:582–588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict. Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Cohen RA, Williams LM, Niaura R, Pogun S, Clark RC, Gunstad J, Gordon E. J. Clin. Neurosci. 2006;13:457–465. doi: 10.1016/j.jocn.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Aubin HJ, Pomerleau OF. Self-reported alcohol use patterns in a sample of male and female heavy smokers. J. Addict. Dis. 1997;16:19–24. doi: 10.1300/J069v16n03_02. [DOI] [PubMed] [Google Scholar]
- Razani J, Boone K, Lesser I, Weiss D. Effects of cigarette smoking history on cognitive functioning in healthy older adults. Am. J. Geriatr. Psychiatry. 2004;12:404–411. doi: 10.1176/appi.ajgp.12.4.404. [DOI] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am. J. Public Health. 2003;93:994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed. Pharmacother. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am. J. Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Ansteny KJ, Parslow RA, Wen W, Maller J, Kumar R, Christensen H, Jorm AF. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement. Geriatr. Cogn. Disord. 2006;21:300–308. doi: 10.1159/000091438. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices the lifetime drinking history and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Humana Press Inc.; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J. Stud. Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. published erratum appears in J. Stud. Alcohol 1989, 50(1), 92. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Maudsley A, Weiner M. An intensity consistent filtering approach to the analysis of deformation tensor derived maps of brain shape. Neuroimage. 2003;19:1638–1649. doi: 10.1016/s1053-8119(03)00183-6. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Schuff N, Rosen H, Miller B, Weiner M. Detecting spatially consistent structural differences in Alzheimer's and fronto-temporal dementia using deformation morphometry.. Paper presented at the Proceedings of Medical Image Computing and Computer Assisted Interventions; Utrecht. 2001a. [Google Scholar]
- Studholme C, Cardenas V, Weiner M. Multi-scale image and multi-scale deformation of brain anatomy for building average brain atlases.. Paper presented at the SPIE Medical Imaging Conference..2001b. [Google Scholar]
- Sullivan EV. NIAAA research monograph no. 34: human brain vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J. Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol. Psychiatry. 2001;49:906–913. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]


