Abstract
Background
Structural magnetic resonance imaging (MRI) has been used to investigate the in vivo pathology of frontotemporal lobar degeneration. However, few neuroimaging studies have focused on white matter (WM) alterations in this disease.
Objectives
To use volumetric MRI techniques to identify the patterns of WM atrophy in vivo in 2 clinical variants of frontotemporal lobar degeneration—fronto-temporal dementia (FTD) and semantic dementia—and to compare the patterns of WM atrophy with those of gray matter (GM) atrophy in these diseases.
Design
Structural MRIs were obtained from patients with FTD (n=12) and semantic dementia (n=13) and in cognitively healthy age-matched controls (n=24). Regional GM and WM were classified automatically from high-resolution T1-, T2-, and proton density-weighted MRIs with Expectation-Maximization Segmentation and compared between the groups using a multivariate analysis of covariance model that included age and WM lesion volumes as covariates.
Results
Patients with FTD had frontal WM atrophy and frontal, parietal, and temporal GM atrophy compared with controls, who had none. Patients with semantic dementia had temporal WM and GM atrophy and patients with FTD had frontal GM atrophy. Adding temporal WM volume to temporal GM volume significantly improved the discrimination between semantic dementia and FTD.
Conclusions
These results show that patients with frontotemporal lobar degeneration who are in relatively early stages of the disease (Clinical Dementia Rating score, 1.0-1.2) have WM atrophy that largely parallels the pattern of GM atrophy typically associated with these disorders.
FRONTOTEMPORAL LOBAR DE-generation (FTLD) is a neurodegenerative disease characterized by atrophy in the frontal and anterior temporal lobes. It accounts for 10% to 15% of all cases of dementia. The clinical manifestations and the patterns of anatomic involvement in FTLD are heterogeneous. For example, some patients have atrophy predominantly in the frontal lobe while others have more severe atrophy in the temporal lobe.1,2 The patterns of anatomic involvement in FTLD can also be asymmetric.2,3 The neuropathology of FTLD may be as heterogeneous as the clinical spectrum of symptoms. Although the histopathologic subtypes of FTLD have generally been divided into those with tau-positive inclusions and those without tau abnormalities, clinicopathological and genetic studies have recently revealed that the majority of sporadic and familial frontotemporal dementia (FTD) cases are not obviously associated with tau pathology and/or tau gene mutations. Furthermore, some studies have linked several autosomal dominantly inherited familial FTD cases to a variety of gene loci on different chromosomes.4
Structural magnetic resonance imaging (MRI) techniques have long been used to investigate the pathology of FTLD in vivo. However, most of the neuroimaging work to date has focused on gray matter (GM) alterations in the brain.5-9 Thus, little is known about the regional patterns of white matter (WM) atrophy in these diseases in vivo. The primary goal of our study was to use volumetric MRI techniques to identify the patterns of WM atrophy in vivo in 2 clinical variants of FTLD: FTD and semantic dementia. A secondary aim was to compare the patterns of WM atrophy with the patterns of GM atrophy in these diseases.
Frontotemporal lobar degeneration has traditionally been regarded as a GM disease. However, there have been studies that reported WM pathology in FTLD. For example, 1 postmortem diffusion tensor imaging study reported WM abnormalities in the frontal lobes of a patient with FTD.10 Using tensor-based morphometry, our group has reported WM atrophy in vivo in the temporal lobes of patients with semantic dementia.11 Based on these findings, the first a priori hypothesis of our study is that patients with FTLD will exhibit significant WM atrophy relative to cognitively healthy control subjects.
Because myelin breakdown and the subsequent loss of or damage to WM would likely exhibit a regional pattern consistent with known FTLD pathology, our second a priori hypothesis is that the patterns of WM atrophy in FTLD will parallel the patterns of GM atrophy. Frontotemporal dementia has been described as the “frontal lobe variant of FTLD”12 because of the prominent frontal lobe damage associated with this disease.5,6,13,14 However, GM atrophy in the temporal lobes has also been noted in FTD.6,8,9,15 For this reason, we expect patients with FTD to exhibit WM atrophy in the frontal and temporal lobes relative to cognitively healthy subjects. Previous studies have also noted frontal lobe GM atrophy in FTD compared with semantic dementia cases.8 Therefore, we also expect patients with FTD to exhibit frontal WM atrophy relative to patients with semantic dementia. Semantic dementia has long been associated with anterior temporal lobe atrophy.16,17 However, recent voxel-based morphometry studies have shown frontal lobe involvement in semantic dementia as well.7,8,18 For this reason, we expect patients with semantic dementia to exhibit WM atrophy primarily in the temporal lobes, but also in the frontal lobes, relative to cognitively healthy patients.
METHODS
STUDY PARTICIPANTS
Twenty-five patients with FTLD (10 women; mean age, 63.6 years [SD, 7.0]) and 24 cognitively healthy control subjects (13 women; mean age, 66.3 years [SD, 10.8]) participated in the study. The patients with FTLD were recruited consecutively from the University of California San Francisco Memory and Aging Center. Of the 25 FLTD patients, 12 met Neary and colleagues’19 criteria for FTD: (1) insidious onset and gradual progression, (2) early decline in social, interpersonal conduct, (3) early impairment in regulation of personal conduct, (4) early emotional blunting, and (5) early loss of insight. Thirteen patients met Neary and colleagues’19 criteria for semantic dementia: (1) insidious onset and gradual progression, (2) a language disorder characterized by empty fluent speech, loss of word meaning, or semantic paraphasias, (3) a perceptual disorder characterized by impaired recognition of familiar faces or object identity, (4) preserved perceptual matching and drawing reproduction, (5) preserved single-word repetition, and (6) preserved ability to read aloud and write to dictation orthographically regular words. All patients were evaluated by a neurologist and a nurse and underwent neuropsychological evaluation to establish the pattern of cognitive and behavioral deficits. General intelligence was assessed using the Mini-Mental State Examination,20 and dementia severity was assessed using the Clinical Dementia Rating (CDR) scale.21
The 24 age-matched control subjects were chosen from participants enrolled in ongoing research on healthy aging at the University of California San Francisco Memory and Aging Center. The control subjects had no history of neurological or psychiatric disorders, no evidence of significant cognitive impairment (as confirmed by an informant), and no evidence of focal disease or subcortical WM ischemic changes on MRI. Control subjects underwent the same neuropsychological battery as the patient groups.
The committees for human research at University of California San Francisco and the San Francisco VAMC approved the protocol. Informed consent was obtained from each participant or a legal representative prior to the study.
MAGNETIC RESONANCE IMAGING
Coronal T1-weighted images (repetition time/inversion time/echo time=9/300/4 milliseconds; 1×1mm2 in-plane resolution; 1.5-mm slabs), axial proton density-weighted images, and T2-weighted images (repetition time/inversion time/echo time=5000/20/85 milliseconds; 1×1.25 mm2 in-plane resolution; 3-mm slabs) were obtained on a clinical 1.5-T magnetic resonance scanner (Vision; Siemens Medical Solutions, Iselin, New Jersey).
TISSUE SEGMENTATION
Cortical GM, WM, and cerebral spinal fluid were classified automatically using Expectation-Maximization Segmentation.22-24 Each subject’s T2- and proton density-weighted images were interpolated to the resolution of their T1-weighted image (1×1×1.5 mm) and coregistered to the T1-weighted image using Automated Image Registration, version 3.0.25 These 3 coregistered images were then normalized to a customized T1-weighted template (resolution, 2×2×2 mm), which was derived from the MRIs of 64 subjects (30 women and 34 men; mean age, 56.6 years [SD, 18.6]) with an affine coregistration algorithm and used as input for segmentation with Expectation-Maximization Segmentation. Each subject’s T2-weighted image, which has bright cerebral spinal fluid surrounding the brain, was used to capture the subarachnoid cerebral spinal fluid in its entirety. A customized digital brain atlas containing prior expectations about the spatial localization of different tissue classes was created from the same images used to create the T1-weighted template. These prior brain atlases were used to initialize the algorithm and to constrain the classification process during subsequent iterations. Figure 1A shows examples of a T1-weighted image and GM and WM segmentation in a control, FTD, and semantic dementia subject. The regional WM volumes analyzed in this study do not include WM signal hyperintensities or WM lesions, which Expectation-Maximization Segmentation identifies as outliers with respect to a statistical model of a healthy brain. Figure 1B shows an example of the spatial distribution of WM signal hyperintensities on a proton density-weighted image, as identified by Expectation-Maximization Segmentation.
Figure 1.
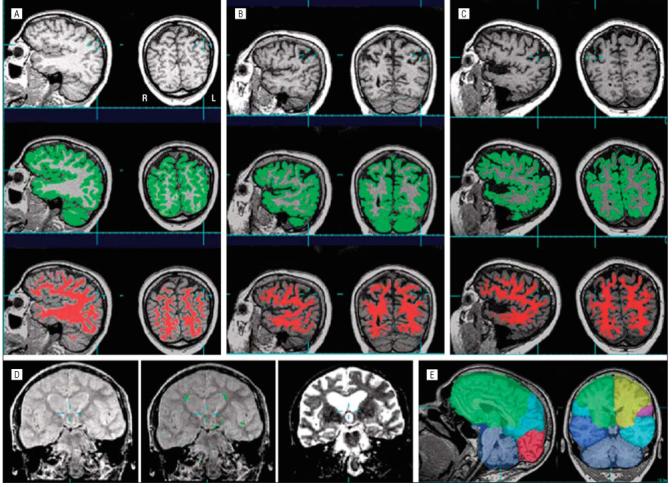
Examples of T-1 weighted images (top row) and T-1 weighted images showing gray matter (in green, second row) and white matter (in red, third row) segmentation in cognitively healthy (A), frontotemporal dementia (B), and semantic dementia (C) subjects. Note the coronal sections are displayed in radiological convention. D, Example of the spatial distribution of the white matter hyperintensities on a proton-density scan (left), the white matter hyperintensities identified by Expectation-Maximization Segmentation (in green in the middle), and a T2-weighted image (right), with bright cerebral spinal fluid surrounding the brain. E, Example of lobar markings. L indicates left; R, right.
To account for variations in head size between subjects, all regional brain volumes were scaled to each subjects’ total intracranial volume. The scaling for total intracranial volume was further normalized to the groups’ overall mean total intracranial volume value (patients and controls combined). Total intracranial volume was computed by summing the volume of all voxels classified as cerebral spinal fluid or brain tissue from the frontal, parietal, temporal, and occipital lobes (ie, cortical and subcortical GM, WM, and WM signal hyperintensities). Tissue and cerebral spinal fluid from the cerebellum and brain-stem were not included in the total intracranial volume calculation. Figure 1C shows an example of the lobar markings.
STATISTICAL ANALYSES
Data were analyzed with SPSS, version 12.0 (SPSS, Chicago, Illinois). Group differences in demographic and clinical variables were analyzed with a multivariate analysis of variance. In cases in which the omnibus multivariate analysis of variance yielded a significant main group effect, additional analyses of variance with the Tukey post hoc test were performed to further examine differences among FTD, semantic dementia, and control subjects.
Because previous MRI studies have reported age-related26 and WM lesion-related27 GM and WM loss, age and WM lesion (ie, WM signal hyperintensities) volumes were included as covariates in multivariate analyses of covariance of the GM and WM volumes. Furthermore, a Shapiro-Wilks test of normality revealed that the regional WM and GM volumes approached a normal distribution when age was included as an additive term in the model. Because of systematic errors partially introduced by the left/right asymmetry of the template used for warping, we combined volume data from both hemispheres in the analyses. To further examine group difference, analyses of covariance with the Tukey post hoc test were performed in cases in which the omnibus multivariate analysis of covariance yielded a significant main group effect.
We also estimated the powers of different regional WM volumetric measures to correctly differentiate each patient group from cognitively healthy controls and from each other (these were based on logistic regressions). Sensitivity and specificity of the classifications were expressed in terms of a receiver operator characteristics analysis as area under the curve. The logistic regressions were further adapted to a random leave-one-out procedure with 1000 runs for cross-validation of the classifications. The areas under the curve from the 1000 cross-validations were compared using Wilcoxon signed rank tests. The Wilcoxon signed rank test was also used to determine if the receiver operator characteristic distribution for GM volume was significantly different from the receiver operator characteristic for GM and WM volumes. These statistical computations were performed using Splus, version 6.3 (Insightful Corp, Seattle, Washington).
RESULTS
Demographic and clinical data are summarized in Table 1. There were significant group differences for Mini-Mental State Examination, CDR, and CDR sum of boxes scores. Post hoc tests revealed that control subjects had higher Mini-Mental State Examination and lower CDR and CDR sum of boxes scores than the other patient groups.
Table 1.
Demographic and Clinical Information in Subjects With Variants of Frontotemporal Lobar Degenerationa
| Characteristic | F Score | Controls (n=24) | Subjects With FTD (n=12) | Subjects With Semantic Dementia (n=13) |
|---|---|---|---|---|
| Age, y | F2,48=0.9 | 66.3 (10.8) | 61.9 (7.4) | 65.2 (6.4) |
| Age range, y | NA | 48-84 | 52-80 | 56-77 |
| Education, y | F2,48=0.7 | 16.9 (3.8) | 16.0 (2.4) | 15.6 (1.8) |
| MMSE score | F2,48=16.6b | 29.7 (0.6) | 23.4 (5.7)c | 19.1 (8.2)c |
| CDR score | F2,48=23.8b | 0.0 (0.0) | 1.2 (0.6)c | 1.0 (0.8)c |
| CDR sum of boxes | F2,48=22.3b | 0.1 (0.2) | 6.00 (3.2)c | 4.4 (3.7)c |
Abbreviations: CDR, Clinical Dementia Rating; FTD, frontotemporal dementia; MMSE, Mini-Mental Status Examination; NA, not applicable.
Values are mean (SD) unless otherwise indicated.
Multivariate analysis of variance statistic, P<.001.
Tukey post hoc test compared with control, P<.01.
The volumetric MRI data and results of the multivariate analysis of covariance and the Tukey post hoc test statistics are summarized in Table 2. There were significant group effects for frontal (P<.01) and temporal (P<.001) WM volumes. Post hoc tests revealed that patients with FTD had less left frontal WM volume (P=.02) than controls and that patients with semantic dementia had less temporal WM volume than control and FTD patients (P<.001).
Table 2.
Regional Volumes and Statistical Results in Variants of Frontotemporal Lobar Degeneration
| Mean Volume (SD), cm3 |
|||||
|---|---|---|---|---|---|
| Region | Tissue Type | F Score From MANCOVA | Controls (n=24) | Subjects With FTD (n=12) | Subjects With Semantic Dementia (n=13) |
| Frontal | GM | 22.532,48a | 208.5 (12.9) | 184.6 (11.0)a | 196.6 (8.5)b,c |
| WM | 5.442,48d | 202.4 (16.0) | 188.6 (9.2)b | 196.5 (13.1) | |
| Parietal | GM | 4.632,48e | 102.1 (6.2) | 95.3 (7.2)f | 98.9 (6.1) |
| WM | 1.862,48 | 78.6 (6.6) | 83.1 (9.3) | 78.1 (6.5) | |
| Temporal | GM | 44.502,48a | 134.7 (7.1) | 127.9 (9.0)g | 109.9 (8.6)a,h |
| WM | 39.372,48a | 61.5 (4.5) | 63.2 (5.9) | 48.2 (4.1)a,h | |
| Whole brain | WMSH | NA | 2.89i | 7.2 (1.9) | 9.3 (6.0) |
Abbreviations: FTD, frontotemporal dementia; GM, gray matter; MANCOVA, multivariate analysis of covariance; NA, not applicable; WM, white matter; WMSH, white matter T2-signal hyperintensities.
P≤.001.
P≤.01, Tukey post hoc test, compared with control.
P<.05, Tukey post hoc test, compared with FTD.
P≤.01, MANCOVA.
P<.05, MANCOVA.
P<.05, Tukey post hoc test, compared with control.
P=.05, MANCOVA.
P<.001, Tukey post hoc test, compared with control.
P=.07, MANCOVA.
There were main group effects for frontal, parietal, and temporal GM volumes. Post hoc tests revealed that patients with FTD had less frontal GM volume than controls (P<.001) and semantic dementia patients (P=.03); less parietal GM volume than controls (P=.02); and moderately (P=.051) less temporal GM volume than controls. Patients with semantic dementia had less frontal GM volume than controls (P=.01) and less temporal GM volume than controls and FTD patients (P<.001).
Finally, we performed exploratory analyses to examine the value of adding regional WM volumes to regional GM volumes for correctly differentiating each patient group from controls and from each other. Adding regional WM volume to regional GM volume did not improve the classification of any patient group from controls. However, adding temporal WM volume to temporal GM volume improved differentiating between FTD and semantic dementia; overall classification improved from 88% to 96%, with the area under the curve improving from 0.90 to 0.98. A Wilcoxon signed rank test revealed significant differences between the areas under the 2 receiver operator characteristic curves (P=.002) (Figure 2).
Figure 2.
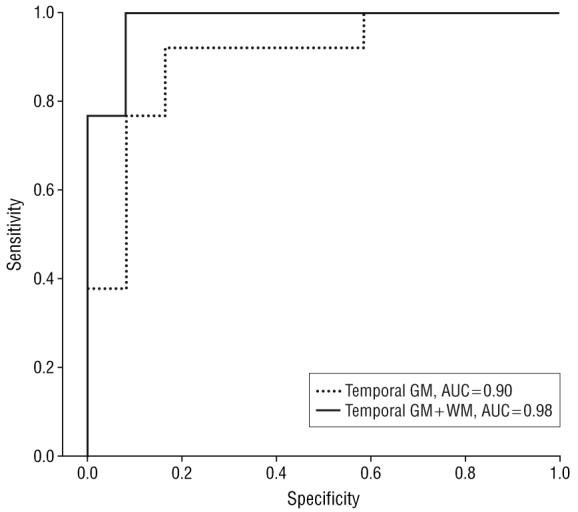
Receiver operating curve showing improved differentiation of frontotemporal dementia from semantic dementia when temporal white matter (WM) volumes are added to temporal gray matter (GM) volumes. AUC indicates area under the curve.
COMMENT
The major findings of this study are that (1) patients with FTD had frontal WM atrophy compared with cognitively healthy controls, who had none; (2) patients with semantic dementia had temporal WM atrophy relative to cognitively healthy and FTD patients; (3) adding temporal WM volume to temporal GM volume significantly improved the discrimination between semantic dementia and FTD patients; and (4) patients with FTD had parietal GM atrophy compared with cognitively healthy patients.
The primary goal of this study was to identify the regional patterns of WM atrophy in vivo in 2 subtypes of FTLD using volumetric MRI techniques. The first major finding is that FTD and semantic dementia patients exhibited significant WM atrophy. Compared with controls, FTD patients had WM atrophy in the frontal lobe, while semantic dementia patients had WM atrophy in the temporal lobes. This finding is consistent with previous reports of WM pathology in patients with FTLD10,14,28 and a postmortem diffusion tensor imaging study of an FTD patient that found WM abnormalities in the frontal lobes.10
Broe and colleagues29 have suggested that WM atrophy does not occur in FTD until later stages of the disease, roughly corresponding to CDR scores of 3 to 5, after the frontal and temporal lobes have been severely affected. However, the current results suggest that WM changes in FTD can be detected in vivo at earlier stages of the disease in patients with a mean CDR score of 1.2. At least 3 factors may contribute to these discrepant findings. First, we had smaller intervals between CDR rating and brain atrophy measurement. Second, the quantitative nature of the volumetric MRI analysis may be more sensitive than the visual atrophy measurements employed by Broe et al.29 Third, only 7 of the 24 cases that Broe and colleagues29 examined had CDR scores of 1 or 2 at the time of death. Moreover, the cause of death in each of these cases was a condition unrelated to FTD (eg, myocardial infarction and pulmonary embolism). Therefore, it may be possible that Broe et al29 did not have enough early FTD cases to detect significant WM changes.
Using deformation morphometry, we had previously reported significant WM atrophy in the superior temporal gyrus and the middle and inferior temporal gyri of semantic dementia patients relative to controls.11 In the current study, we extend this finding by showing that patients with semantic dementia also have significant temporal lobe WM atrophy compared with FTD patients. Moreover, logistic regression analysis revealed that adding temporal WM volume to temporal GM volume significantly improved the discrimination between semantic dementia and FTD. However, this may simply be a reflection of the overweighting of pathologic changes in the temporal lobe in patients with semantic dementia.
We hypothesized that WM atrophy would parallel GM atrophy in FTLD. However, the only region where patients with FTD showed significant GM and WM atrophy compared with controls was the frontal lobe. Patients with FTD also had significant GM but not WM atrophy in the parietal temporal lobes. Similarly, patients with semantic dementia had significant GM atrophy in temporal and frontal lobes, but only significant WM atrophy in the temporal lobe. One possible explanation for this pattern of results is that volumetric MRI cannot detect significant WM loss unless GM atrophy has reached a tipping point. For example, we only detected significant WM atrophy in regions of the brain where FTD and semantic dementia patients had the largest amount of GM atrophy (ie, >10% more than controls). Moreover, when regional GM volumes were included as covariates in the analyses, the WM volume differences between FTLD and controls were no longer significant.
One novel finding of this study is that patients with FTD had significant parietal GM atrophy compared with controls. Although parietal atrophy is not a feature typically associated with the disease, Figure 1A clearly shows parietal GM atrophy in the FTD patient compared with the control subject. Moreover, it is well known from research in monkeys that there are connections between some of the cytoarchitectonic areas of the parietal lobe and the anterior cingulate,30-33 frontal cortex,34,35 superior temporal sulcus, and the parahippocampal region.30,36,37 Given this intricate system of interconnections between frontal, parietal, and temporal neurons and that the frontal lobe and, to a lesser extent, the temporal lobe are affected in FTD, it should not be surprising that we also observed parietal GM atrophy in patients with FTD.
One limitation to consider when interpreting our results is the method we used to quantify WM volumes. T1-, T2-, and proton density-weighted MRI scans were used to compute WM volumes from large regions of the brain. Future studies with diffusion tensor imaging will afford a better opportunity to examine WM changes associated with these neurodegenerative diseases in more clearly defined brain regions. Another limitation is that, though the patterns of anatomic involvement in FTLD can be asymmetric,2,3 we were unable to examine hemispheric differences in WM and GM volumes owing to systematic asymmetries introduced by the warping process that we used. However, future studies with other kinds of software (eg, FreeSurfer38) may afford a better opportunity to examine the effects of these neurodegenerative diseases on cortical thickness and different cortical and subcortical regions. A third limitation of our study is the uncertainty of the true diagnosis or the underlying histopathology subtype of the patients studied. However, careful follow-up of patients without a change in their clinical diagnoses may improve the gold standard in clinical studies.39 A fourth limitation is that these findings were obtained from relatively small groups of patients; thus, the results may not generalize to more heterogeneous cohorts. For this reason, our findings need to be validated prospectively in larger patient populations. Finally, it is difficult to assess disease severity and duration reliably in FTD and semantic dementia. Mini-Mental State Examination scores are disproportionately sensitive to language deficits, while CDR scores are sensitive to memory impairment; behavioral changes are difficult to quantify. Therefore, volume differences between the different dementia groups could, at least in part, be explained by impairment severity rather than by the type of dementia. These limitations notwithstanding, our results clearly show that there is WM atrophy in vivo in FTLD patients who are in relatively early (CDR score, 1.0-1.2) stages of the disease. We found WM atrophy primarily in brain regions where there was also substantial GM loss (ie, >10% more than controls), which is suggestive of wallerian degeneration. However, we cannot rule out the possibility that the WM atrophy observed in our study is also partially caused by retrograde degeneration.
Acknowledgments
Funding/Support: This work was supported by grant P01 AG19724 from the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Neary D, Snowden JS, Mann DM. The clinical pathological correlates of lobar atrophy. Dementia. 1993;4(34):154–159. doi: 10.1159/000107315. [DOI] [PubMed] [Google Scholar]
- 2.Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain. 1997;120(pt 6):1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- 3.Boone KB, Miller BL, Lee A, Berman N, Sherman D, Stuss DT. Neuropsychological patterns in right versus left frontotemporal dementia. J Int Neuropsychol Soc. 1999;5(7):616–622. doi: 10.1017/s1355617799577047. [DOI] [PubMed] [Google Scholar]
- 4.Tolnay M, Probst A. Frontotemporal lobar degeneration: tau as a pied piper? Neurogenetics. 2002;4(2):63–75. doi: 10.1007/s10048-002-0140-x. [DOI] [PubMed] [Google Scholar]
- 5.Frisoni GB, Beltramello A, Geroldi C, Weiss C, Bianchetti A, Trabucchi M. Brain atrophy in frontemporal dementia. J Neurol Neurosurg Psychiatry. 1996;61(2):157–165. doi: 10.1136/jnnp.61.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukui T, Kertesz A. Volumetric study of lobar atrophy in Pick complex and Alzheimer’s disease. J Neurol Sci. 2000;174(2):111–121. doi: 10.1016/s0022-510x(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 7.Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47(1):36–45. [PubMed] [Google Scholar]
- 8.Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- 9.Boccardi M, Laakso MP, Bresciani L, et al. The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging. 2003;24(1):95–103. doi: 10.1016/s0197-4580(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 10.Larsson EM, Englund E, Sjobeck M, Lätt J, Brockstedt S. MRI with diffusion tensor imaging post-mortem at 3.0 T in a patient with frontotemporal dementia. Dement Geriatr Cogn Disord. 2004;17(4):316–319. doi: 10.1159/000077162. [DOI] [PubMed] [Google Scholar]
- 11.Studholme C, Cardenas V, Blumenfeld R, et al. Deformation tensor morphometry of semantic dementia with quantitative validation. Neuroimage. 2004;21(4):1387–1398. doi: 10.1016/j.neuroimage.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Miller BL, Gearhart R. Neuroimaging in the diagnosis of frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10(suppl 1):71–74. doi: 10.1159/000051217. [DOI] [PubMed] [Google Scholar]
- 13.Kitagaki H, Mori E, Yamaji S, et al. Frontotemporal dementia and Alzheimer disease: evaluation of cortical atrophy with automated hemispheric surface display generated with MR images. Radiology. 1998;208(2):431–439. doi: 10.1148/radiology.208.2.9680572. [DOI] [PubMed] [Google Scholar]
- 14.Larsson E, Passant U, Sundgren PC, et al. Magnetic resonance imaging and histopathology in dementia, clinically of frontotemporal type. Dement Geriatr Cogn Disord. 2000;11(3):123–134. doi: 10.1159/000017225. [DOI] [PubMed] [Google Scholar]
- 15.Galton CJ, Gomez-Anson B, Antoun N, et al. Temporal lobe rating scale: application to Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2001;70(2):165–173. doi: 10.1136/jnnp.70.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 17.Snowden JS, Neary D, Mann DMA, Goulding PJ, Testa HJ. Progressive language disorder due to lobar atrophy. Ann Neurol. 1992;31(2):174–183. doi: 10.1002/ana.410310208. [DOI] [PubMed] [Google Scholar]
- 18.Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57(2):216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- 19.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnosis criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(suppl 1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 22.Van Leemput K, Maes F, Vandermeulen D, Suetens P. A unifying framework for partial volume segmentation of brain MR images. IEEE Trans Med Imaging. 2003;22(1):105–109. doi: 10.1109/TMI.2002.806587. [DOI] [PubMed] [Google Scholar]
- 23.Van Leemput K, Maes F, Vandermeulen D, Colchester A, Suetens P. Automated segmentation of multiple sclerosis lesions by model outlier detection. IEEE Trans Med Imaging. 2001;20(8):677–688. doi: 10.1109/42.938237. [DOI] [PubMed] [Google Scholar]
- 24.Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans Med Imaging. 1999;18(10):897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- 25.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration, I: general methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22(1):139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain, part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002;23(8):1327–1333. [PMC free article] [PubMed] [Google Scholar]
- 27.Du AT, Schuff N, Chao LL, et al. White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging. 2005;26(4):553–559. doi: 10.1016/j.neurobiolaging.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Englund E, Brun A. Frontal lobe degeneration of non-Alzheimer type, IV: white matter changes. Arch Gerontol Geriatr. 1987;6(3):235–243. doi: 10.1016/0167-4943(87)90024-0. [DOI] [PubMed] [Google Scholar]
- 29.Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60(6):1005–1011. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- 30.Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Birkhauser; Boston, MA: 1993. pp. 249–284. [Google Scholar]
- 31.Morecraft RJ, Van Hoesen GW. Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res Bull. 1998;45(2):209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- 32.Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol. 1992;322(4):471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- 33.Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13(1):13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- 34.Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999;11(7):2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- 35.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363(4):615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex, II: cortical afferents. J Comp Neurol. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 37.Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey, II: cortical afferents. J Comp Neurol. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 39.Quizilbash N, Schneider LS, Chui H, et al. Evidence-based Dementia Practice. Blackwell Science; Oxford, England: 2002. [Google Scholar]


