Abstract
The purpose of this study was to examine the relationships between lobar volumes and set shifting. We studied 101 subjects, including 36 normal controls, 16 patients with probable Alzheimer’s disease, 30 patients with frontotemporal dementia (FTD), and 19 patients with semantic dementia (SD), using a shifting paradigm that carefully controlled for component abilities. Subjects were administered two conditions of the Delis-Kaplan Executive Function System (D-KEFS) Design Fluency Test. In the control condition (DF:Control), examinees generated as many unique designs as possible in 60 s by drawing lines connecting only unfilled dots. In the switching condition (DF:Switch), examinees generated designs by drawing lines alternating between filled and unfilled dots. We used BRAINS2 software to generate volumes of the right and left frontal, temporal, and parietal lobes. Partial correlations and multiple regressions showed that, after controlling for Mini-Mental State Examination and DF:Control, only the right and left frontal lobe volumes significantly correlated with the DF:Switch, most clearly in the FTD and SD groups. Follow-up analyses indicated that frontal contributions to shifting were not related to working memory. Results highlight the importance of carefully controlling for component cognitive processes when studying executive functioning.
Keywords: FTD, AD, D-KEFS, Frontal lobes, BRAINS2, Design fluency
INTRODUCTION
Set shifting tasks are widely used in clinical and experimental neuropsychology. Subjects are typically required to shift their attention between different stimulus parameters or alternate between different response sets. Because these tasks rely heavily on mental flexibility and cognitive control, shifting tasks are considered excellent measures of executive functioning (Miyake et al., 2000; Monsell, 2003).
As an index of cognitive control, set shifting is presumed to be mediated by the prefrontal cortex (Brass et al., 2005; Nakahara et al., 2002; Ravizza & Ciranni, 2002). The literature on the relationship between shifting and frontal lobes is mixed, however. The Wisconsin Card Sorting Test (WCST; Heaton, 1993), for example, has been shown to be impaired in patients with focal frontal lesions, but the anatomic specificity of the WCST has been questioned by studies documenting impairments in patients with posterior lesions (Anderson et al., 1991; Barcelo & Santome-Calleja, 2000). Similarly, the relationship between the widely used Trail Making Test and the frontal lobes is controversial. Although some studies have used it as a proxy for frontal lobe functioning (Hanninen et al., 1997), several studies have failed to demonstrate a convincing association between test performance and the frontal lobes (Anderson et al., 1995; Reitan & Wolfson, 1995).
One reason for the discrepant literature may be methodological. Set shifting cannot be measured outside the context of more basic cognitive skills. For example, quickly drawing lines that serially alternate between number and letter sequences depends not only on executive ability but also visual scanning, hand-eye coordination, ability to count, facility with the alphabet, sustained attention, motor speed, and other skills (Arbuthnott & Frank, 2000). Successful performance on the WCST requires learning and problem solving. A deficit in any of these more basic skills will lower performance on the executive task, regardless of how preserved executive functioning might be. Thus, delineating and establishing scores for component skills on complex tasks is imperative to interpret the target behavior (Levine et al., 1995).
The purpose of this study was to determine the relationships between lobar volumes and set shifting using a behavioral paradigm that carefully controlled for component abilities. The task selected was a design fluency measure that required subjects to generate novel designs by connecting dots with four straight lines. By using two similar conditions that differed only in that one required shifting and the other did not, we were able to carefully control for the component skill of design fluency and more directly assess the response cost of having to shift. We also simultaneously considered the potential contribution of multiple brain regions because demonstrating an association with the frontal lobes is not sufficient to suggest anatomical specificity; it is also necessary to show that nonfrontal structures are not related to set shifting. Our primary hypothesis was that frontal volumes and not other lobar volumes would predict set shifting ability after carefully controlling for the component cognitive skills.
METHODS
Subjects
We studied 101 subjects recruited through the University of California at San Francisco (UCSF) Memory and Aging Center, including 36 normal controls, 16 patients with probable Alzheimer’s disease (AD), 30 patients with frontotemporal dementia (FTD), and 19 patients with semantic dementia (SD). Subjects were drawn from the Clinical Core of a Program Project Grant investigating frontotemporal lobar syndromes. All subjects underwent a standardized cognitive and neuroimaging evaluation. Inclusion criteria included a Mini-Mental State Examination (MMSE) score of 15 or higher and a magnetic resonance imaging (MRI) scan obtained within 90 days of the cognitive testing. The research diagnosis for each subject was made by a team consensus at the UCSF Memory and Aging Center based on medical, social, and psychiatric history; neurological evaluation; extensive family interview; visual inspection of a brain image [computed tomography (CT) or MRI], and mental status testing. The Neary criteria were used for the diagnosis of FTD and SD (Neary et al., 1998, 2000). Exclusionary features include early severe amnesia, early spatial disorientation, logoclonic speech, and myoclonus. Core diagnostic features of FTD include decline in social interpersonal conduct, impairment in regulation of personal conduct, emotional blunting, and loss of insight that occur very early in the course of the illness (e.g., first 1-2 years) in the absence of severe amnesia, aphasia, or visuospatial disorder. The presence or absence of these core features during the earliest stages of the patient’s neurodegenerative disease is determined through careful interview with informants and completion of the Neuropsychiatric Inventory (Cummings et al., 1994). A diagnosis of FTD is further supported by a presenile onset (late 50s or early 60s), the early presence of hyperorality and dietary changes, decline in personal hygiene, and predominant frontal and insular atrophy on neuroimaging. The primary clinical features of SD are a selective impairment of semantic memory; progressive, fluent, empty spontaneous speech; loss of word meaning; semantic paraphasias; anomia; and relative sparing of syntax and phonology. The National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984) were used for the diagnosis of probable AD. Table 1 summarizes the demographic, cognitive, and MRI data for each subject group. This study was approved by the Institutional Review Board at UCSF.
Table 1.
Demographic, design fluency, and MRI volumetric data (in cubic centimeters)
| Normals | AD | FTD | SD | |
|---|---|---|---|---|
| n | 36 | 16 | 30 | 19 |
| Age | 64.4 (10.5) | 60.8 (8.6) | 58.0 (6.9) | 61.9 (6.2) |
| Education | 17.2 (2.1) | 15.7 (3.0) | 16.2 (2.2) | 16.1 (3.2) |
| MMSE | 29.6 (0.6) | 22.8 (4.1) | 25.6 (3.7) | 24.1 (4.6) |
| Design fluency: Control | 11.1 (3.9) | 4.8 (2.3) | 6.0 (3.0) | 7.2 (2.8) |
| Design fluency: Shifting | 8.6 (2.8) | 2.6 (1.9) | 3.5 (3.2) | 5.8 (2.0) |
| Left frontal lobe | 184.3 (15.1) | 164.9 (16.7) | 157.7 (18.6) | 167.6 (21.3) |
| Right frontal lobe | 192.6 (13.2) | 179.5 (15.2) | 164.8 (21.5) | 183.5 (16.5) |
| Left temporal lobe | 108.1 (5.8) | 98.2 (7.8) | 99.8 (9.0) | 85.9 (11.0) |
| Right temporal lobe | 108.8 (3.7) | 99.7 (7.4) | 98.7 (8.0) | 94.0 (13.0) |
| Left parietal lobe | 111.6 (8.6) | 101.2 (7.8) | 106.7 (9.8) | 106.1 (9.5) |
| Right parietal lobe | 113.6 (8.8) | 104.4 (8.3) | 107.1 (9.7) | 112.2 (11.7) |
Note. MRI = magnetic resonance imaging; AD = Alzheimer’s disease; FTD = frontotemporal dementia; SD = semantic dementia; MMSE = Mini-Mental State Examination.
Set Shifting Procedure
The Delis-Kaplan Executive Function System (D-KEFS) Design Fluency Test (Delis et al., 2001) is a nonverbal analogue to verbal fluency and was administered according to standardized procedures. Two of the test’s three conditions consist of a row of stimulus boxes, each containing an array of 10 dots and requiring the examinee to construct unique designs by connecting dots with four straight lines. In each condition, the stimulus response box contains five filled dots and five empty dots (see Figure 1). In the control condition (DF:Control), examinees are asked to generate as many unique designs as possible in 60 s by drawing lines connecting only unfilled dots. In the switching condition (DF:Switch), examinees are asked to generate as many unique designs as possible in 60 s by drawing lines alternating between filled and unfilled dots. Each condition is preceded by detailed instructions and practice trials to ensure that the subject understands the task. Instructions emphasize the need to work quickly and to make each design different. The score for each condition is the total number of correct designs created in 60 s.
Fig. 1.
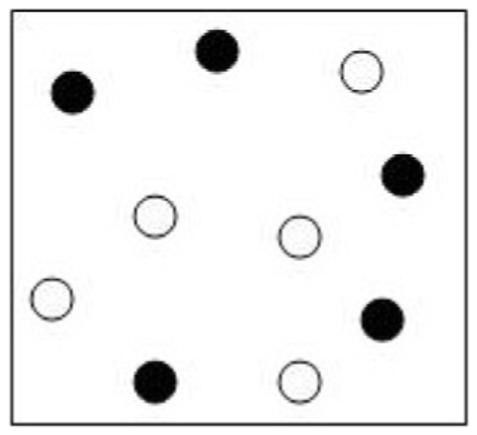
Example of design fluency stimulus.
Neuroimaging
MRI scans were obtained on a 1.5-T Magnetom VISION system (Siemens, Inc., Iselin, NJ) equipped with a standard quadrature head coil. Structural MRI sequences included (1) two-dimensional fast low-angle shot MRI along three orthogonal directions, 3-mm-thick slices, ∼15 slices in each direction to obtain scout views of the brain for positioning subsequent MRI slices. (2) A double spin echo sequence [repetition time/echo time 1/echo time 2 (TR/TE1/TE2) = 5000/20/80 ms] to obtain proton density and T2-weighted MRIs, 51 contiguous axial slices (3 mm) covering the entire brain and angulated -10° from the anterior commissure-posterior commissure line; 1.0 × 1.25 mm2 in-plane resolution. (3) Volumetric magnetization prepared rapid gradient echo MRI [MPRAGE, repetition time/echo time/inversion time (TR/TE/TI) = 10/4/300 ms] to obtain T1-weighted images of the entire brain, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution, and 1.5-mm slab thickness.
Magnetic resonance images were processed on Linux workstations using the BRAINS2 software package, which is developed and made freely available by the Mental Health-Clinical Research Center at the University of Iowa (Magnotta et al., 2002). The T1-weighted images were spatially normalized and resampled to 1.0-mm3 voxels so that the anterior-posterior axis of the brain was realigned parallel to the anterior commissure-posterior commissure line and the interhemispheric fissure was aligned on the other two axes. Next, the outermost boundaries of the cortex, as well as the anterior commissure and posterior commissure, are identified to warp the Talairach grid (Talairach & Tournoux, 1988) onto the current brain. The T2- and proton density-weighted images were then realigned to the spatially normalized T1-weighted image using an automated image registration program (Woods et al., 1992). The resampled images were then segmented into gray matter, white matter, and cerebrospinal fluid using the coregistered images and a discriminant analysis method based on automated training class selection (Harris et al., 1999). This tissue classification algorithm uses a Bayesian classifier based on discriminant analysis to reduce the variability in signal intensity across individual image sets and to correct for partial voluming. This step requires the manual tracing of venous blood, but is able to perform “plug” selection for gray matter, white matter, and cerebrospinal fluid automatically.
We generated a brain mask using a previously trained artificial neural network, one of the features of the BRAINS2 software package. Then, lobar volumes were calculated using an automated Talairach-based method of regional classification (Andreasen et al., 1996; Magnotta et al., 2002). Lobar volumes are normalized to correct for differences in overall head size by multiplying the absolute lobar volume by the average total intracranial volume (TIV) of our sample and dividing by the individual’s TIV.
Statistics
Our goal is to study the relationship between set shifting and lobar volumes after controlling for overall design fluency ability and other potential moderator variables. We first describe the bivariate correlations between the two fluency tasks, lobar volumes, demographic variables, and MMSE. We next carried out a second-order correlation between the set shifting condition and lobar volumes to partial out the effects of DF:Control and potential moderator variables. Finally, we used hierarchical multiple regression to determine whether frontal volumes predicted set shifting performance after controlling for the contribution of nonfrontal brain regions.
RESULTS
Bivariate correlations are summarized in Table 2. Both DF:Control and DF:Switch were significantly correlated with all six lobar volumes and MMSE score, but not age or education. Second-order correlations between DF:Switch and lobar volumes after partialling out the effects of DF:Control and MMSE are summarized in Table 2. Results indicate that, after controlling for MMSE and DF:Control, only the right and left frontal lobes were significantly correlated with the shifting condition.
Table 2.
Bivariate and partial correlations between lobar volumes and set shifting
| Bivariate correlations | Partial correlations | ||
|---|---|---|---|
| DF:Switch | DF:Control | DF:Switch | |
| DF:Control | .69** | ||
| Left frontal | .55** | .40** | .32** |
| Right frontal | .49** | .39** | .30* |
| Left temporal | .32* | .36** | -.07 |
| Right temporal | .38** | .37** | .10 |
| Left parietal | .42** | .41** | .09 |
| Right parietal | .40** | .35** | .19 |
| Age | .13 | .08 | |
| Education | .09 | .21 | |
| MMSE | .56** | .50** | |
Note. Partial correlations are the relationship between DF:Switch and lobar volumes after controlling for DF:Control, age, and MMSE. DF = design fluency; MMSE = Mini-Mental State Examination.
p <.01.
p <.001.
Although the partial correlations indicate a reasonably specific relationship between shifting and frontal volumes, we carried out an additional test of our hypothesis with hierarchical regression to control for whatever contribution temporal and parietal volumes might make. We explored two separate models, one for left frontal volumes and one for right frontal volumes, because of collinearity between the right and left frontal volumes (r = .78). In each model, performance on the shifting condition was the dependent variable. In the first step of each model, we entered DF:Control and MMSE, followed by right and left temporal and parietal volumes in the second step, and the right or left frontal volume in the final step.
In the first step of the model, DF:Control and MMSE explained 53.8% of the variance in shifting. The addition of the left and right temporal and parietal volumes did not contribute to a significant increase in explained variance (p > .30). In the model for the left frontal lobe, left frontal volume added an additional 5.9% of the variance in shifting (β = .320; Fchange = 14.4; p < .001). In the model for the right frontal lobe, right frontal volume added an additional 2.7% of the variance in shifting (β = .219; Fchange = 6.19; p < .05). A scatterplot illustrating the relationship between the set shifting condition (after partialling out the effect of DF:Control) and left frontal volumes is shown in Figure 2.
Fig. 2.
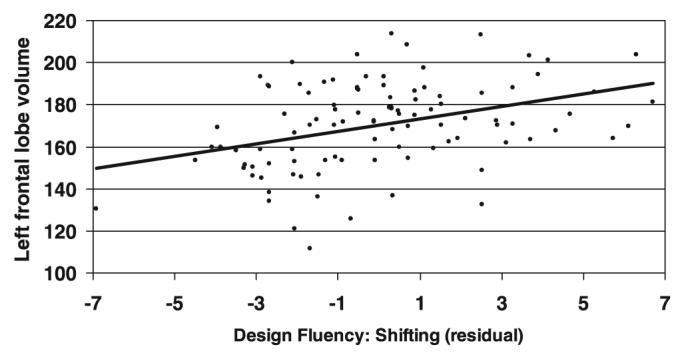
Scatterplot showing the relationship between left frontal lobar volumes and DF:Switch (after partialling out the contributions of DF:Control). DF, design fluency.
The ability to engage in cognitive set switching is functionally related to working memory (Ravizza & Ciranni, 2002). For example, the design fluency shifting condition requires the subject to simultaneously maintain instructions on-line and track progress while generating novel designs. Working memory has also been linked to dorsolateral prefrontal cortex (Cannon et al., 2005; Funahashi, 2005; Petrides, 2005), raising the possibility that our results might have been mediated by working memory. To address this possibility, we carried out an additional set of regression analyses entering backward digit span, a widely used auditory working memory task, into the model before entering the lobar volumes. Backward digit span data were available on 96 of the 101 subjects. Results were unchanged; both left (β = .325; p < .001) and right (β = .217; p < .05) frontal volumes were significant predictors of shifting, whereas backward digit span did not remain in the model (β’s < .04).
DISCUSSION
This study tested hypotheses about the neuroanatomical correlates of set shifting. There were two main findings. First, after carefully controlling for component cognitive tasks and global cognition, and simultaneously considering multiple brain regions, only left and right frontal volumes significantly correlated with the ability to shift during a design fluency task. Temporal and parietal volumes were not related to shifting after controlling for general design fluency ability. The second main finding is that the association between frontal volumes and shifting remained significant even after controlling for working memory.
The association between anterior brain structures and shifting ability is consistent with several studies that have found shifting impairment in patients with frontal damage (McDonald et al., 2005a,b; Pantelis et al., 1999; Stuss et al., 2001) and with functional imaging studies that report frontal activity during shifting tasks (Zakzanis et al., 2005). McDonald et al. (2005a) reported on two separate shifting paradigms, including the design fluency task used in the present study. They found that patients with frontal lobee pilepsy were impaired on the switching condition relative to patients with temporal lobe epilepsy and controls, whereas the two patient groups performed comparably to each other and controls on the baseline conditions of the test. Stuss et al. (2001) reported slower times on Trails B (relative to Trails A) in both right and left frontal lesion patients. Zakzanis et al. (2005) used functional MRI to study normal subjects while carrying out the Trail Making Test. They found left-sided dorsolateral and medial frontal activity when comparing Trails B to Trails A, although left middle and superior temporal gyrus activity was also noted. Our data with the design fluency paradigm build on these earlier studies by showing a direct relationship between shifting and frontal lobe volumes, and the absence of significant contributions from the temporal and parietal lobes.
Set shifting tasks are typically complex, requiring a host of perceptual, cognitive, and motor skills in addition to shifting. The broad spectrum of required skills can make identification of brain-behavior relationships more challenging. One important element common to several studies linking shifting with the frontal lobes is that the shifting task is compared with control tasks with either repeated-measures designs (McDonald et al., 2005b), proportional scores (Stuss et al., 2001), or, in the case of our study, partial correlations and regression models. This strategy better enables the parsing out of motor, perceptual, and other component skills that might otherwise obscure anatomic specificity.
The current study found bifrontal contributions to set shifting, although the relationship between set shifting and frontal volumes was slightly larger on the left. These findings are similar to other studies reporting bifrontal contributions (Aron et al., 2004; Stuss et al., 2001), although other researchers have found greater left-sided involvement (Moll et al., 2002; Zakzanis et al., 2005). Some investigators have proposed that both hemispheres contribute to cognitive set shifting but in different ways. Konishi et al. (2002), for example, found that right lateral frontal cortex was activated during exposure to negative feedback, and the corresponding region in the left hemisphere was activated during updating of behavior. Aron et al. (2004) also identified lateralized components of set shifting, with the right hemisphere, primarily inferior frontal gyrus, more involved in inhibiting task sets and/or responses, and the left hemisphere, particularly middle frontal gyrus, more involved in controlling task set.
The ability to engage in cognitive set shifting is anatomically and functionally related to working memory because of the need to attend simultaneously to multiple task parameters, track progress, and maintain task instructions on-line. When we included backward digit span, a widely used index of auditory working memory, in our regression models predicting shifting performance, we found that frontal volumes continued to be significant predictors of set shifting, whereas backward digit span did not remain in the model. These findings are consistent with those of Ravizza and Ciranni (2002), who reported that set shifting abilities remained impaired for patients with prefrontal damage regardless of working memory demands. These results suggest that, despite reported anatomical and conceptual overlap between set shifting and working memory, set shifting should be considered a distinct construct.
Some of the variable findings about set shifting reported in the literature may be attributable to the term “set shifting” being used in different ways. In the WCST, for example, shifting refers to the subjects’ ability to alter their sorting strategies when the reinforcement contingencies change. In contrast, tasks like Trail Making and D-KEFS Design Fluency require serial alternation between two different types of stimuli. Other researchers use the term to refer to performance on dual-task paradigms where subjects either simultaneously or alternately engage in two different tasks (e.g., unimanual tapping and verbal repetition; Crossley et al., 2004). It is likely that these different types of “set shifting” tasks have different underlying neuroanatomy. For example, extradimensional set shifting refers to when the correct response changes to a different stimulus dimension from previous trials, and intradimensional shifting refers to when the correct response changes to a different level within the same stimulus dimension. Extradimensional shifting has been linked to dorsolateral prefrontal cortex, whereas intradimensional shifting is thought to be mediated more by ventromedial frontal structures (Rogers et al., 2000).
Design fluency has been proposed as a behavioral index of right frontal lobe functioning. Jones-Gotman (1991) suggested that impairment on design fluency might be relatively specific to patients with excisions involving the right frontal lobes, and several studies of patients with presumed frontal pathology have been shown to perform poorly on design fluency tasks (Varney et al., 1996). Our lobar volume data suggest that general design fluency ability has the highest correlations with left and right frontal volumes; however, the correlation with left frontal volume was actually slightly higher than right frontal, and nonfrontal regions also significantly correlate with design fluency. Consequently, inferences about right frontal lobe functioning based on design fluency may not have a strong empirical basis.
The current study was based largely on patients with neurodegenerative disease, including AD and FTD. Differentiation between FTD and AD during life can be problematic, however, because both disorders have an insidious onset, produce a progressive dementia syndrome that can include executive dysfunction and language impairment, and can cause alterations in behavior (Mendez et al., 1993). Consequently, despite the careful application of the Neary criteria (Neary et al., 1998), some subjects might have been misdiagnosed. Implications for this study are negligible, however, because group differences were not the subject of analysis.
The neural networks underlying set shifting ability are complex. One limitation of the current study is that lobar volumes do not provide sufficient information about specific frontal regions. Although dorsolateral prefrontal cortex is often implicated in set shifting (Moll et al., 2002), other regions, including the bilateral inferior frontal junction (Derrfuss et al., 2005), anterior cingulate (Shafritz et al., 2005), medial prefrontal (Wager et al., 2004), ventrolateral prefrontal cortex (Shafritz et al., 2005), and even basal ganglia (Ravizza & Ciranni, 2002) have been implicated. Similarly, the act of shifting between two different response sets relies on a host of abilities, including mental flexibility, inhibition, conflict monitoring, attentional control, and working memory, and the structural substrates to these cognitive processes are only beginning to be understood.
ACKNOWLEDGMENTS
This work was supported by the National Institute on Aging (NIA) grants AG22983, P50-AG05142, the State of California Alzheimer’s Disease Research Center of California (ARCC) grant 01-154-20, and the Hillblom Network.
REFERENCES
- Anderson CV, Bigler ED, Blatter DD. Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatic brain-injured patients. Journal of Clinical and Experimental Neuropsychology. 1995;17:900–908. doi: 10.1080/01688639508402438. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. Journal of Clinical and Experimental Neuropsychology. 1991;13:909–922. doi: 10.1080/01688639108405107. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW, Jr., Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WT. Automatic atlas-based volume estimation of human brain regions from MR images. Journal of Computer Assisted Tomography. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Arbuthnott K, Frank J. Trail Making Test, part B as a measure of executive control: Validation using a set-switching paradigm. Journal of Clinical and Experimental Neuropsychology. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127(Pt 7):1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Santome-Calleja A. A critical review of the specificity of the Wisconsin Card Sorting Test for the assessment of prefrontal function. Revista de Neurologia. 2000;30:855–864. [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. Journal of Cognitive Neuroscience. 2005;17:1367–1375. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of General Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Crossley M, Hiscock M, Foreman JB. Dual-task performance in early stage dementia: Differential effects for automatized and effortful processing. Journal of Clinical and Experimental Neuropsychology. 2004;26:332–346. doi: 10.1080/13803390490510068. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan EB, Kramer J. The Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2005;139:251–261. doi: 10.1016/j.neuroscience.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Hanninen T, Hallikainen M, Koivisto K, Partanen K, Laakso MP, Riekkinen PJ, Sr., Soininen H. Decline of frontal lobe functions in subjects with age-associated memory impairment. Neurology. 1997;48:148–153. doi: 10.1212/wnl.48.1.148. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: A three-dimensional multispectral discriminant analysis method with automated training class selection. Journal of Computer Assisted Tomography. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test manual. Psychological Assessment Resources; Odessa, FL: 1993. [Google Scholar]
- Jones-Gotman M. Localization of lesions by neuropsychological testing. Epilepsia. 1991;32(Suppl 5):S41–S52. [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y. Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proceedings of the National Academy of Science of the United States of America. 2002;99:7803–7808. doi: 10.1073/pnas.122644899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Stuss DT, Milberg WP. Concept generation: Validation of a test of executive functioning in a normal aging population. Journal of Clinical and Experimental Neuropsychology. 1995;17:740–758. doi: 10.1080/01688639508405164. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui VJ. Discriminating patients with frontal-lobe epilepsy and temporal-lobe epilepsy: Utility of a multilevel design fluency test. Neuropsychology. 2005a;19:806–813. doi: 10.1037/0894-4105.19.6.806. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui-Madozi VI. Is impairment in set-shifting specific to frontal-lobe dysfunction? Evidence from patients with frontal-lobe or temporal-lobe epilepsy. Journal of the International Neuropsychological Society. 2005b;11:477–481. [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Selwood A, Mastri AR, Frey WH., Jr. Pick’s disease versus Alzheimer’s disease: A comparison of clinical characteristics. Neurology. 1993;43:289–292. doi: 10.1212/wnl.43.2.289. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Moll FT, Bramati IE, Andreiuolo PA. The cerebral correlates of set-shifting: an fMRI study of the Trail Making Test. Arquivos de Neuropsiquiatria. 2002;60:900–905. doi: 10.1590/s0004-282x2002000600002. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Science. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Mann DM. Classification and description of frontotemporal dementias. Annals of the New York Academy of Science. 2000;920:46–51. doi: 10.1111/j.1749-6632.2000.tb06904.x. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber FZ, Barnes TR, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophrenia Research. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: Architectonic and functional organization. Philosophical Transactions of the Royal Society of London Series B Biological Science. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Ciranni MA. Contributions of the prefrontal cortex and basal ganglia to set shifting. Journal of Cognitive Neuroscience. 2002;14:472–483. doi: 10.1162/089892902317361985. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. Category Test and Trail Making Test as measures of frontal lobe functions. The Clinical Neuropsychologist. 1995;9:50–56. [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Journal of Cognitive Neuroscience. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Kartheiser P, Belger A. Dissociation of neural systems mediating shifts in behavioral response and cognitive set. Neuroimage. 2005;25:600–606. doi: 10.1016/j.neuroimage.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The Trail Making Test: A study in focal lesion patients. Psychological Assessment. 2001;13:230–239. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system; An approach to cerebral imaging. George Thieme Verlag; Stuttgart, Germany: 1988. [Google Scholar]
- Varney NR, Roberts RJ, Struchen MA, Hanson TV, Franzen KM, Connell SK. Design fluency among normals and patients with closed head injury. Archives of Clinical Neuropsychology. 1996;11:345–353. [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43:1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]


