Abstract
The sense organs of the vertebrate head arise predominantly from sensory placodes. The sensory placodes have traditionally been grouped as structures that share common developmental and evolutionary characteristics. In attempts to build a coherent model for development of all placodes, the fascinating differences that make placodes unique are often overlooked. Here I review olfactory placode development with special attention to the origin and cell movements that generate the olfactory placode, the derivatives of this sensory placode, and the degree to which it shows plasticity during development. Next, through comparison with adenohypophyseal, and lens placodes I suggest we revise our thinking and terminology for these anterior placodes, specifically by: 1) referring to the peripheral olfactory sensory system as neural ectoderm because it expresses the same series of genes involved in neural differentiation and differentiates in tandem with the olfactory bulb, 2) grouping the anterior placodes with their corresponding central nervous system structures and emphasizing patterning mechanisms shared between placodes and these targets. Sensory systems did not arise independent of the central nervous system; they are part of a functional unit composed of peripheral sensory structures and their targets. By expanding our analyses of sensory system development to also include cell movements, gene expression and morphological changes observed in this functional unit, we will better understand the evolution of sensory structures.
Keywords: GnRH, sensory neuron, Kallman, hypothalamus
Introduction
The peripheral nervous system of vertebrate animals arises from the interaction of cranial neural crest and placodes. Placodes were originally described as ectodermal thickenings that will contribute the majority of the cell types found in head sensory structures. The embryonic origins of the sensory placodes, elucidated through fate map analysis in amphibians, birds and fish, lie at the edge of the anterior neural plate. The cranial placodes generate very different derivatives: the adenohypophysis is endocrine, the olfactory epithelium contains sensory neurons with a regenerative properties, and the lens of the eye is non-neural, generating crystalline containing cells. Thus, despite their commonality of origin at the border of neurectoderm, they generate very different cell populations. Given the contiguity of placodal precursors with neural plate precursors, I suggest that we re-examine the term "placode" and work to develop a terminology more reflective of the unique characteristics of cells they generate.
The olfactory sensory epithelium has been reported to give rise to the regenerating sensory neurons, support cells, glia cells, and neuroendocrine cells containing gonadotropin-releasing hormone (GnRH). In our analysis of olfactory sensory system development, we have shown that the GnRH neuroendocrine cells originate in the flanking adenohypophyseal and cranial neural crest domains. The intimacy of the developing functional fields (olfactory, endocrine, and neural crest) can be observed early in development prior to the formation of neural tube and the appearance of sensory placodes, thus we need more careful analysis of the fate of these precursors before the formation of definitive structures.
Finally, as the olfactory sensory system differentiates the environment can mold the nervous system by effecting changes in gene expression during early development. Our analysis of olfactory sensory neuron differentiation argues that patterns of gene expression can be biased by interactions with the environment. Whether these changes are embedded in the genome by processes such a genetic imprinting and thus are potentially heritable is unknown at this time. The combination of fundamental mechanisms that generate sensory structures with mechanisms that allow for plasticity to react to changes in the environment, results in the evolution of organisms as unique individuals.
Development of the Olfactory Placode
Development of the placodes can be divided roughly into four phases: 1) initiation of the placode field in the forming neural ectoderm, 2) movement of cells within placode domains, 3) appearance (often transient) of a localized thickening of cells, 4) differentiation of the sensory structure as evidenced by cell types specific to the placodal structure (Fig. 1). Because of the well-understood mechanisms leading to the induction of the lens placode of the eye it was originally thought (and is still commonly misunderstood) that the olfactory placodes arose via the induction of overlying ectoderm by the underlying neural tube, much like the lens of the eye. The majority of the fate maps elucidating the embryonic origins of placodal precursors have been executed by the transplantation and or labeling of groups of cells in the developing chick, Xenopus 4 and zebrafish embryos [11,26,27]. In these fate maps as well as others [3,21,27,38,59] there is general agreement that the adenohypophyseal, olfactory lens and otic placodes arise from the region at the border of the anterior neural plate and, in addition share common features of receiving inductive signals from the underlying mesoderm and endoderm [19].
Figure 1.
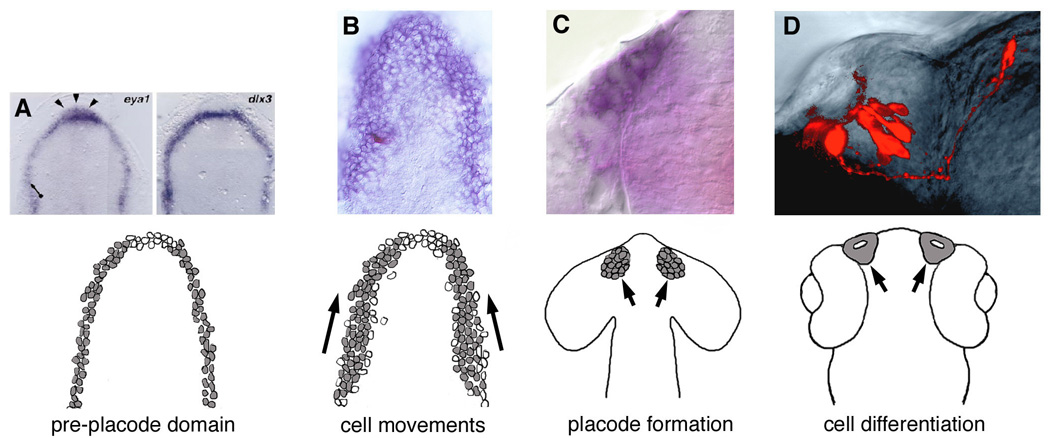
A model for development of olfactory sensory epithelia. (A) Initiation of the olfactory epithelia precursors at border of neural/non-neural ectoderm; Olfactory epithelia competence as reflected by expression of eya1 and dlx3 in the anterior neural plate. (B) Movement of cells within placode domains; Olfactory epithelia precursors as reflected by dlx3 expression (blue, with single cell labeled in field, red). (C) Appearance (often transient) of a localized thickening of cells; Olfactory epithelia commitment and formation with dlx3 expressed in the olfactory placode (blue). (D) Differentiation of the sensory structure as evidenced by cells types specific to the placodal structure; Olfactory sensory neurons in the olfactory organ of a 2 day old zebrafish (red). (A1–D1), Diagram depicting the sequential appearance of the olfactory epithelial precursors in grey (A, from [39]; D, from [58]).
Initiation of the placode field in the forming neural/non-neural ectoderm: the preplacode domain?
Analysis of the region flanking the anterior neural plate that gives rise to sensory placodes has led to the proposal of a placode domain: a region specified early to give rise to the sensory placodes (for reviews [3,43,46]. Originally this proposal was based on experimental analysis using transplants to demonstrate that the ability to generate sensory structures is specific to regions in the anterior neural plate [46]. More recently, analyses of gene expression domains in the anterior region of the forming neural plate-neural tube have confirmed that patterns of gene expression delineate the anterior border of the neural plate [43]. At the neural plate stage, specific genes are expressed in a strip, like an upside-down horseshoe, wrapping around the anterior end of the forming neural plate (Fig. 1A). The genes expressed in this region are in general transcription factors such as distal-less-3, (dlx3) [1], distal-less 7, (dlx7) [13], eyes absent, (eya1) [39] and six4.1 [24]. These genes along with others [3], appear to be co-expressed in a common region at the anterior end of the neural plate [43]. This region of gene expression correlates with the previously described placode domain (for thorough reviews see [42,43]. Many of the genes expressed in the placode domain are not 5 specific to this region of developing embryos but, like many important developmental transcription factors, are also expressed in other tissues (Six1 and Eya1 in organogenesis and myogenesis for example). These genes, reported as reflecting "molecular anatomy", work to restrict developmental potential, thus defining the region that will generate placodes. In our single cell fate map of the region giving rise to the olfactory organ we correlated the fate map with the expression of dlx3 a placode domain gene [59] (Fig. 1B), showing that a large region of this expression domain will generate the olfactory organ as well as olfactory bulbs.
Movement of cells within placode domains: The olfactory placode
The olfactory organs differentiate from olfactory placodes, transient, paired structures evident lying laterally at the anterior end of the forming neural tube. In zebrafish, early fate maps from 60–90% epiboly show that the olfactory placode/telencephalic tissues arise from a region clustered at the animal pole with the olfactory placodes arising from the lateral regions [26]. Based on previous research carried out primarily in amphibians and birds, the olfactory placodes were proposed to originate from discrete regions of the anterior neural plate where a small piece of neural plate which becomes isolated by differential cell movements and is then surrounded by non-neuronal tissue as development proceeds (see for review: [14]. The use of more refined techniques for labeling and following cells in the early embryo, suggests the olfactory placodes arise from the neural ridge or neural plate as opposed to the non-neural ectoderm. We completed a single cell fate map of the olfactory placode domains at 4–5 somite-stage zebrafish embryo (~11–12 hours post-fertilization at 28.5C) and demonstrated that the olfactory placode develops from a field of cells within the edge of the anterior neural plate which is initially continuous with the pre-migratory cranial neural crest and the adenohypophyseal placode [59], for reviews see [51–53]. Our model has also been supported by work in the chick studying the formation of a similarly patterned sensory placode, the otic placode. By labeling small groups of cells with DiI, (carbocyanine lipophilic fluorescent dye), at the 4-somite stage it was demonstrated that the cells converge to form the otic placodes in their final position adjacent to rhombomeres 5–6 through extensive cell movements [45]. Thus, it appears that both the olfactory and otic placodes form through the movement of fields of cells during neurulation.
Having defined the border separating the olfactory placode and cranial neural crest domains, we examined the interactions of these to cellular domains during neural tube formation. The migratory routes of cranial neural crest cells passing ventral-posterior to the developing eye are well characterized [27]. In contrast, migratory route of cranial neural crest cells passing dorsal to the eye and populating the frontal mass is not well understood. Using a transgenic sox10-GFP line of zebrafish [48] (GFP expressed in the neural crest) we characterized the development of cranial neural crest cells to determine whether there was any cell mixing of olfactory placodes and cranial neural crest during formation of the olfactory sensory system [17,57]. At approximately 13 hours post-fertilization (6–8 somites), the neural crest cells migrate anteriorly as a group and separate at the anterior end of the neural tube. By co-localizing the sox10-GFP expression with olfactory placode markers dlx3b [1] and six4.1 [24] we observed little cell mixing occurs during this process. Furthermore, we find that the olfactory placode markers dlx3b and six4.1 are localized to different domains of the developing olfactory placode suggesting that they may give positional information to the differentiating sensory neurons [17], unpublished data).
Appearance (often transient) of a localized thickening of cells
The movement of the olfactory sensory precursors results in a neurectodermal thickening, i.e. the olfactory placode (Fig. 1C). Originally the formation of this thickened piece of neurectoderm was thought to result from cell division but there is little cell division until after the formation of the olfactory placode [59] and the cells maintain contact through gap junction [49]. Once this cohesive unit of cells is formed there is an increase in cell division and expression of neurogenic genes [33,34].
Differentiation of cell types specific to the olfactory system: olfactory sensory neurons and neuroendocrine cells
Olfactory Sensory Neurons
Once the olfactory placode is evident in the developing zebrafish (about 17–18 somites) it is possible to localize pioneer neurons, a class of neurons lacking dendrites, that maintain the connection between the peripherally differentiating olfactory epithelium and the developing olfactory bulb [32,58]. The pioneer neurons undergo programmed cell death as the olfactory sensory neurons differentiate (Fig. 1D, 2A, [16,58,59]), express olfactory receptors [4] and send axons centrally. To better understand the potential role of the olfactory epithelium in olfactory memory we developed a paradigm for testing olfactory imprinting in zebrafish. We demonstrated that zebrafish make and retain memories and that this process is correlated with changes in gene expression in the developing olfactory epithelium [17,55]. Furthermore, analysis of neurogenic genes and Olfactory Marker Protein support the hypothesis that the induced changes in gene expression are in sensory neuron precursors [17]. This coupled with our subsequent analysis of immediate early gene expression in the olfactory epithelium 24–72 hours post fertilization [30], McKenzie and Whitlock unpublished data) suggests that the developing olfactory sensory epithelium is plastic and can respond to signals from the environment during early development through changes in gene expression. These data taken in light of recent reports showing that mammals display inherited epigenetic variation [37,50] suggests that the environment may play a role in forming the inter-individual variability of olfactory receptor expression such as has been reported in humans [31].
Figure 2.
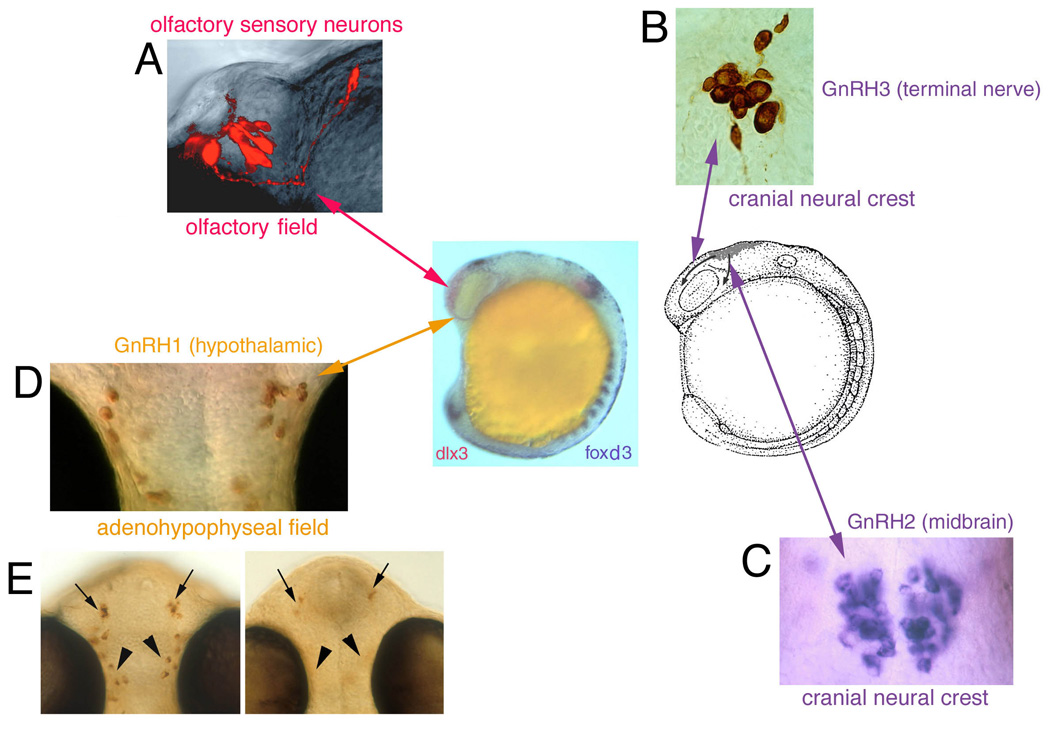
Summary diagram indicating origin of cells associated with the olfactory sensory epithelia. (A) Olfactory sensory neurons differentiate within this tissue. (B) Terminal nerve GnRH3 cells (purple arrow) arise from cranial neural crest and migrate rostrally dorsal to the eye (arrow) entering the region of the forming olfactory system (center diagram; dlx3 expression, red). (C) Midbrain GnRH2 expressing cells (purple arrow) lying on either side of the midline also arise from cranial neural crest and migrate behind the developing eye (arrow). (D) Hypothalamic GnRH cells arise from the adenohypophyseal region (orange arrow; see [60]. (E) Hypothalamic GnRH cells are lost when interfering with gli signaling (you-too mutant, [60]), or knocking down kal1a gene function [57]. Center diagram: Lateral view of an embryo showing olfactory field (Left, red, dlx3 expression) and migration routes (Right: arrows) of cranial neural crest.
GnRH Cells
When comparing sensory placodes, the olfactory placode is unusual in that, it has been reported to give rise to an unusual array of cell types not recorded for other sensory structures including cells including those containing peptides, such as gonadotropin releasing hormone (GnRH), [44,62,63]. These investigations were done using tissue fixed after the formation of the olfactory placode, thus after the completion of cell movements necessary for the development of the olfactory sensory system, pituitary and hypothalamus. In contrast, experiments in chick and zebrafish [12,59], done well before the appearance of the olfactory placodes, suggest the olfactory sensory system does not generate GnRH cells. Both physical (axolotl [35]) and genetic (mouse [8]) ablation have been used to remove the olfactory placodes during development. In the case of axolotl, the ablation resulted in loss of the olfactory epithelium, nerve, bulb, and the GnRH cells. Because the ablation occurred after placode formation it may have disrupted the migration route rather than the origin of the GnRH cells. Genetic ablation of the olfactory placode using mutant mice homozygous for Pax6 gene (small-eye (Sey) results in loss of both the olfactory placode and GnRH cells. However, Pax6 is expressed in a variety of tissues in the developing mouse including the developing adenohypophysis, which is disrupted in the Sey mutant [23]. Thus, the loss of GnRH cells in the Pax6 mutant does not conclusively demonstrate that these endocrine cells arise from the olfactory placode. Rather, the Pax6 phenotype suggests a developmental link between the GnRH cell development and adenohypophyseal development.
Analysis of GnRH gene expression has provided insight into potential embryonic origins of these endocrine cells. In the chick GnRH1 is first expressed along the neural fold, becomes localized to the anterior neural folds, and subsequently is expressed in bilateral clusters of cells within the most anterior neural folds [61]. In medaka, a species-specific form of GnRH1 has been cloned [36] and it is expressed in the hypothalamus. The differences in timing between the onset of GnRH1 and GnRH3 gene expression led to the proposal that the terminal nerve (GnRH3) and hypothalamic (GnRH1) cells do not share a common origin in the olfactory placode but have separate origins [9,36]. Consistent with this hypothesis, we have found that in zebrafish GnRH3 cells of the terminal nerve and GnRH2 cells of the midbrain (Fig. 2B,C) have their origin in the cranial neural crest [53,57,60] whereas the GnRH cells of the hypothalamus arise from the region of the adenohypophyseal placode [54,56,57,60] (Fig. 2D). To date a gene coding for a third form of GnRH (equivalent to mammalian GnRH1) has not been isolated in zebrafish. Given the incomplete nature of the zebrafish genome this is not particularly surprising. The differential expression of GnRH in situ probes (GnRH2, GnRH3) and immunocytochemical labeling with antibodies recognizing GnRH1 in mammals or multiple forms of GnRH suggests that zebrafish like other many fishes may have a third form of GnRH [47,57]. Thus, analysis in chick and fish indicates that the GnRH cells arise from the border of the neural plate suggesting that the neural ridge has the potential to generate GnRH cells across vertebrates [54].
Further analysis of GnRH cell origin
Two of the genes known to result in loss of GnRH cells in humans when mutated are KAL1 (kallman/anosmin1) and KAL2 (fibroblast growth factor receptor 1, fgfr1). We have analyzed development of the hypothalamic GnRH cells through disruption of kallman and fgfr1 gene function in the developing zebrafish. We used morpholinos (modified oligonucleotides; MO) to block protein translation of kal1a, kal1b, and fgfr1. The greatest effect observed to date in the “knockdown” of gene function is the loss or reduction of endocrine GnRH (hypothalamic) cells in animals deficit in kal1a protein. Strikingly there was had no effect on neuromodulatory midbrain or nervus terminalis GnRH cells [57]. The olfactory nerves of these animals were disrupted but not absent. Our data indicate that knockdown of kal1a, kal1b, and fgfr1 results in a different GnRH cell and olfactory sensory system phenotype for each gene ([22], Kim and Whitlock, unpublished). We also examined the developmental origins of endocrine GnRH cells in relation to adenohypophyseal and hypothalamic development. Adenohypophyseal development was not greatly disrupted in kal1a MO injected fish, yet a hypothalamic marker was completely absent in many injected fish [22]. Our data suggest kal1a gene is involved in both endocrine GnRH neuron and hypothalamic development, suggesting the precursors of GnRH cells may lie in the anterior hypothalamus [22] rather than the adenohypophysis as we previously reported [60].
Adenohypophysis and olfactory organ: neighbors in the early embryo
The adenohypophysis arises from a field of cells located on the midline at the anterior end of the neural plate [18,20,41]. In our work [60], we demonstrated using the you-too and detour mutants [20] that the loss of the adenohypophysis results in the loss of the GnRH cells of the hypothalamus but not the GnRH cells of the terminal nerve (Fig. 2DE). Because the olfactory organs develop normally in these mutants, the loss of GnRH cells cannot be due to loss of the olfactory placode. We have used a line of transgenic fish with the proopiomelanocortin (POMC) gene promoter linked to green fluorescent protein (GFP) [28] to visualize the development of adenohypophyseal lineages and found transient expression of the POMC-GFP expressing cells in the olfactory placode [56]. Our observations do not suggest that the olfactory placodes generate POMC-GFP containing neurons but rather the perdurance of the GFP signal reflects the prior history of the cell: the precursor started out destined to be an adenohypophyseal cell type but as the olfactory and adenohypophyseal precursors separated the (initially) POMC-GFP cells ending up in the olfactory placodes down-regulated the POMC expression. The idea that there is an association of endocrine tissue (adenohypophyseal and possibly anterior hypothalamus) with the olfactory precursors is consistent with the hypothesis that neurohypophyseal duct/stomodeum of urochordates/cephalochordates is homologous to the olfactory/adenohypophyseal placodes and hypothalamus of craniates [29,40]. Because GnRH is a molecule found in living representatives of ancestral vertebrates such as amphioxus, the assumption is that GnRH predates the appearance of the jawed vertebrates. Therefore it is most parsimonious to propose that each group of animals (all having pituitaries and hypothalami) may share common mechanisms for co-opting GnRH into the hypothalamic system. To date we do not have the equivalent data at the same developmental stages across fish, birds and mammals necessary to draw firm conclusions about the embryonic origins of GnRH across animals.
Why separate the target from the source?
In analyzing the concept of placode domains we are overlooking not only the dramatic differences amongst the placodes but also the characteristics shared between placodal structures and their post-synaptic targets in the central nervous system. For example, the gene dlx3 is expressed not only in the olfactory placode domain but also in the developing telencephalon in both zebrafish and Xenopus [15,59]. In zebrafish embryos processed for dlx3 expression, the expression is stronger at the border of the forming neural tube (placode domain) but it is also expressed in the telencephalic domain with a distinct posterior border [59]. Furthermore, in our fate mapping studies no clear division could be observed in the living embryos between the cellular fields giving rise to the telencephalon and those giving rise to the olfactory organ (Fig. 3). At this time we do not have enough clones from the telencephalic domain to know whether there are precursors giving rise to progeny spanning both the olfactory organ and olfactory bulb. More recently it was demonstrated that when the migration of olfactory placode precursors is blocked by interfering with chemokine signaling the olfactory sensory neurons wrap around the anterior end of the neural tube, thus remaining associated with the forming olfactory bulbs [32].
Figure 3.
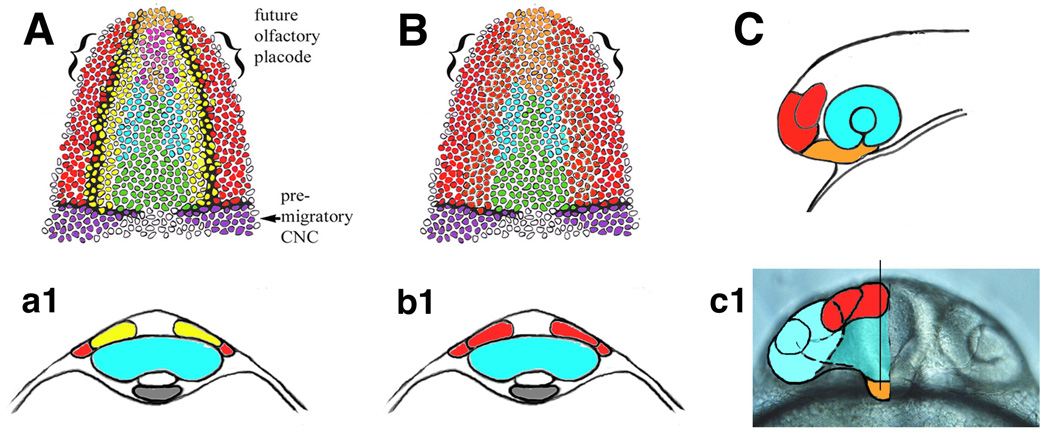
Placodes are part of a functional unit. (A) Fate map of the cellular fields giving rise to the olfactory epithelium (red) olfactory bulb (yellow) and adenohypophysis (orange) (a1) cross section of embryos at level of forming optic primordia (blue). Traditional view separates peripheral derivatives (sensory epithelia, red) from central targets (olfactory bulb, yellow). (B) Proposed new conceptual model for model for analyzing development of placodal structures. Olfactory sensory epithelia and olfactory bulb would be considered function unit (red, b1, red). Likewise adenohypophysis and endocrine hypothalamus would be considered functional endocrine unit (orange). (C) Olfactory placode (red) and adenohypophysis (orange) are functional units of the forebrain. (c1) Proposed functional units in overlay of frontal view of 22 hour post fertilization zebrafish head.
Several studies in both zebrafish and chick have suggested that the lens and adenohypophysis represent equivalent pre-placode domains. What is often overlooked is that the transformation of adenohypophysis into lens affects primarily the anterior region of the adenohypophysis as reflected by expresses beta-crystallin expression [25]. This could be a result of either the anterior adenohypophysis lying at the ectodermal/neurectodermal border, or, reflect a duo origin of the adenohypophyseal placode. Further analyses in chick suggest that the lens is a “ground state” of placodes [2], although these data are difficult to interpret due to use of common neuronal markers and a controversial marker for olfactory placode precursors. A recent model proposing a non-neural ectoderm “outer pre-placode” domain, as reflected by Pitx3 expression [10,64] is intriguing in that it suggests changes in fates within the placode domain are in fact decisions made between generating neuronal fates (retina, olfactory epithelium) or ectodermal fates (lens, parts of adenohypophysis), Thus it appears that an important decision in forming “placodal” structure is whether the cells are in an ectodermal or neurectodermal differentiation pathway as defined by their final differentiated state.
The genes expressed at the border of the neural plate, delineating the placode domain [43] may be imparting position information in the manner of timing of differentiation relative to the central nervous system. In regard to neural differentiation within the peripheral sensory system the same genes, such as Notch Delta, and Hu that are used in the central nervous system are also used to control neural differentiation. Thus one could organize ones thoughts around an "olfactory domain" (Fig. 3B,b1, C, c1, red) of the nervous system which is subdivided into first order neurons (olfactory epithelium), second neurons (olfactory bulb) and so on. In the case of the adenohypophysis, it becomes part of a nervous system neuroendocrine domain (Fig. 3B, C, c1. orange) with the adenohypophysis abutting the hypothalamus. This idea is supported by fate mapping experiments using chick–quail chimeras demonstrating that the region of the hypothalamus is continuous with the region giving rise to the adenohypophyseal placode as is the ventral telencephalon (precursor of olfactory bulbs) with olfactory placodes [7]. These tissues are co-organized developmentally by inductive signals from ectoderm and mesoderm as well as midline signaling genes.
Is the nose an eye?
A common theme in the analysis of placode development is to streamline characteristics of the placodes into a unifying theme. This unifying theme, namely that all placodes are fundamentally equivalent, also calls to question the basic differences between the peripheral and central nervous system [5]. For example the fact that Six1, Six4 and Eya are all expressed in the placode domain is highlighted as promoting generic placode properties such as cell proliferation, cell shape changes and specification of neurons [42]. Yet these generic properties of placodes are in fact generic properties of the nervous system as a whole. Our affection for placodes stems in part from the fact that we can see them in the developing embryo, a historical fact, and that they give us a tangible border separating the central from peripheral nervous systems during development. In examining eye development [6] there is no question that a non-neural, crystalline containing structure is induced from the overlying ectoderm by the optic vesicle. Unlike the lens, the olfactory placode is not induced in the ectoderm by the underlying olfactory bulb. Rather, it appears to arise through the sequential specification of neural identity [46] similar to the interactions that induce and pattern the anterior neural plate. The olfactory sensory precursors move with the olfactory bulb precursors (see fate maps Fig. 3) during neural tube formation keeping the differentiating olfactory sensory neurons in register with their future targets as the olfactory system forms. Thus, the movements of the developing olfactory sensory epithelia and bulb precursors are not directly analogous to the formation of the optic cup: a folding and extension of the neural tube with the optic vesicle triggering the final differentiation of the lens from a ”lens-biased” ectoderm.
One gets the impression from the literature that placodes arose as little structures pasted on to the nervous system and held there by the "glue" of neural crest. Perhaps “placodes” are an outdated term and it would be more useful to think in terms of the nervous system as a whole. In this manner we would look at the evolution of olfactory placodes not just in terms of their relationship to their neighboring placodes such as the adenohypophysis, but equally weight their underlying post-synaptic target, the central nervous system.
Acknowledgements
I would like to thank the organizers of the 5th European Conference on Comparative Neuroscience, Sylvie Retaux, Philippe Vernier and Joana Osorio for the invitation to participate and for creating a welcoming and intellectually stimulating environment. I thank the members my previous laboratory in the United States for their contributions to the work I have discussed. Support: NIH DC0421801, NIH/HD050820, FONDICYT 1071071, Millennium Nucleus in Cellular Genomics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akimenko MA, Ekker M, Wegner J, Lin W, Westerfield M. Combinatorial expression of three zebrafish genes related to distal-less: Part of a homeobox gene code for the head. The Journal of Neuroscience. 1994;14:3475–3486. doi: 10.1523/JNEUROSCI.14-06-03475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Developmental Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Developmental Biology. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- 4.Barth AL, Justice NJ, Ngai J. Asynchronous onset of odorant receptor expression in the developing zebrafish olfactory system. Neuron. 1996;16:23–34. doi: 10.1016/s0896-6273(00)80020-3. [DOI] [PubMed] [Google Scholar]
- 5.Begbie AL, Graham A. The ectodermal placodes: a dysfunctional family. Philosophical Transactions Royal Society of London B. 2001;356:1655–1660. doi: 10.1098/rstb.2001.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuang JC, Raymond PA. Embryonic origin of the eyes in teleost fish. Bioessays. 2002;24:519–529. doi: 10.1002/bies.10097. [DOI] [PubMed] [Google Scholar]
- 7.Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Developmental Biology. 1985;110:422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- 8.Dellovade TL, Pfaff DW, Schwanzel-Fukuda M. The gonadotropin-releasing hormone system does not develop in Small-Eye (Sey) mouse phenotype. Brain Research Developmental Brain Research. 1998;107:233–240. doi: 10.1016/s0165-3806(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 9.Dubois DA, Zandbergen MA, Peute J, Goos HJ. Evolutionary development of three gonadotropin-releasing hormone (GnRH) systems in vertebrates. Brain Research Bulletin. 2002;57:413–418. doi: 10.1016/s0361-9230(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 10.Dutta S, Dietrich JE, Aspöck G, Burdine RD, Schier AW, Westerfield M, Varga ZM. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132:1579–1590. doi: 10.1242/dev.01723. [DOI] [PubMed] [Google Scholar]
- 11.Eagleson G, Ferreiro B, Harris WA. Fate of the anterior neural ridge and the morphogenesis of the Xenopus forebrain. Journal of Neurobiology. 1995:146–158. doi: 10.1002/neu.480280203. [DOI] [PubMed] [Google Scholar]
- 12.el Amraoui A, Dubois PM. Experimental evidence for an early commitment of gonadotropin-releasing hormone neurons, with special regard to their origin from the ectoderm of nasal cavity presumptive territory. Neuroendocrinology. 1993;57:991–1002. doi: 10.1159/000126490. [DOI] [PubMed] [Google Scholar]
- 13.Ellies DL, Stock DW, Hatch G, Giroux G, Weiss KM, Ekker M. Relationship between the genomic organization and the overlapping embryonic expression patterns of the zebrafish dlx genes. Genomics. 1997;45:580–590. doi: 10.1006/geno.1997.4978. [DOI] [PubMed] [Google Scholar]
- 14.Farbman AI. Cell Biology of Olfaction. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 15.Franco MD, Pape MP, Swiergiel JJ, Burd GD. Differential and overlapping expression patterns of X-dll3 and Pax-6 genes suggest distinct roles in olfactory system development of the African clawed frog Xenopus laevis. Journal of Experimental Biology. 2001;204:2049–2061. doi: 10.1242/jeb.204.12.2049. [DOI] [PubMed] [Google Scholar]
- 16.Hansen A, Zeiske E. Development of the olfactory organ in the zebrafish, Brachydanio rerio. Journal of Comparative Neurology. 1993;333:289–300. doi: 10.1002/cne.903330213. [DOI] [PubMed] [Google Scholar]
- 17.Harden MV, Yang Z, Lin S, Whitlock KE. Association for Chemoreception Sciences. Vol. 31. Sarasota, Fl: Chemical Senses; 2006. Formation of the Olfactory Placode in the Zebrafish, Danio rerio; pp. A136–A137. [Google Scholar]
- 18.Herzog W, Zeng X, Lele Z, Sonntag C, Ting JW, Chang CY, Hammerschmidt M. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Developmental Biology. 2003;254:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson AG. The Determination and Positioning of the Nose, Lens and Ear. Journal of Experimental Zoology. 1963;154:273–283. doi: 10.1002/jez.1401540303. [DOI] [PubMed] [Google Scholar]
- 20.Karlstrom RO, Talbot WS, Schier AF. Comparative synteny cloning of zebrafish you-too: mutations in the Hedgehog target gli2 affect ventral forebrain patterning. Genes & Development. 1999;13:388–393. doi: 10.1101/gad.13.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura K, Kouki T, Kawahara G, Kikuyama S. Hypophyseal development in vertebrates from amphibians to mammals. General and Comparative Endocrinology. 2002;126:130–135. doi: 10.1006/gcen.2002.7784. [DOI] [PubMed] [Google Scholar]
- 22.Kim HK, Smith KM, Whitlock KE. Association for Chemoreception Sciences. Sarasota, Fl: 2006. Disruption of Kallmann and fgfr1 gene function in zebrafish diferentially afects GnRH an olfactory cell development; p. A136. [Google Scholar]
- 23.Kioussi C, O'Connell S, St-Onge L, Treier M, Gleiberman AS, Gruss P, Rosenfeld MG. Pax6 is essential for establishing ventral-dorsal cell boundaries in pituitary gland development. Proceedings of the National Academy of Sciences U S A. 1999;96:14378–14382. doi: 10.1073/pnas.96.25.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Osanai H, Kawakami K, Yamamoto M. Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mechanisms of Development. 2000;98:151–155. doi: 10.1016/s0925-4773(00)00451-2. [DOI] [PubMed] [Google Scholar]
- 25.Kondoh H, Uchikawa M, Yoda H, Takeda H, Furutani-Seiki M, Karlstrom RO. Zebrafish mutations in Gli-mediated hedgehog signaling lead to lens transdifferentiation from the adenohypophysis anlage. Mechanisms of Development. 2000;96:165–174. doi: 10.1016/s0925-4773(00)00387-7. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochemical Cell Biology. 1997;75:551–562. [PubMed] [Google Scholar]
- 27.Le Douarin NM, Kalcheim C. The Neural Crest. Second edn. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 28.Liu NA, Huang H, Yang Z, Herzog W, Hammerschmidt M, Lin S, Melmed S. Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Molecular Endocrinology. 2003;17:959–966. doi: 10.1210/me.2002-0392. [DOI] [PubMed] [Google Scholar]
- 29.Manni L, Agnoletto A, Zaniolo G, Burighel P. Stomodeal and neurohypophysial placodes in Ciona intestinalis: insights into the origin of the pituitary gland. Journal of Experimental Zoology B Mol Dev Evol. 2005;304:324–339. doi: 10.1002/jez.b.21039. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie MG, Harden MV, Whitlock KE. Association for Chemoreception Sciences. Vol. 31. Sarasota, Fl: Chemical Senses; 2006. Odorant modulation of Immediate Early Gene expression in the zebrafish olfactory epithelia. [Google Scholar]
- 31.Menashe I, Man O, Lancet D, Gilad Y. Different noses for different people. Nature Genetics. 2003;34:143–144. doi: 10.1038/ng1160. [DOI] [PubMed] [Google Scholar]
- 32.Miyasaka N, Knaut H, Yoshihara Y. Cxcl12/Cxcr4 chemokine signaling is required for placode assembly and sensory axon pathfinding in the zebrafish olfactory system. Development. 2007;134:2459–2468. doi: 10.1242/dev.001958. [DOI] [PubMed] [Google Scholar]
- 33.Mueller T, Wullimann MF. Anatomy of neurogenesis in the early zebrafish brain. Brain Research Developmental Brain Research. 2003;140:137–155. doi: 10.1016/s0165-3806(02)00583-7. [DOI] [PubMed] [Google Scholar]
- 34.Mueller T, Wullimann MF. Atlas of Early Zebrafish Brain Development. Amsterdam: Elsevier B. V.; 2005. p. 183. [Google Scholar]
- 35.Northcutt RG, Muske LE. Multiple embryonic origins of gonadotropin-releasing hormone (GnRH) immunoreactive neurons. Brain Research Developmental Brain Research. 1994;78:279–290. doi: 10.1016/0165-3806(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 36.Parhar IS, Soga T, Ishikawa Y, Nagahama Y, Sakuma Y. Neurons synthesizing gonadotropin-releasing hormone mRNA subtypes have multiple developmental origins in the medaka. Journal of Comparative Neurology. 1998;401:217–226. [PubMed] [Google Scholar]
- 37.Richards EJ. Inherited epigenetic variation--revisiting soft inheritance. Nature Reviews Genetics. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 38.Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annual Review of Neuroscience. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- 39.Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Development, Genes and Evolution. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- 40.Satoh G. A trajectory of increasing activity and the elaboration of chemosensory modality: a new perspective on vertebrate origins. Zoological Science. 2005;22:613–626. doi: 10.2108/zsj.22.613. [DOI] [PubMed] [Google Scholar]
- 41.Sbrogna JL, Barresi MJ, Karlstrom RO. Multiple roles for Hedgehog signaling in zebrafish pituitary development. Developmental Biology. 2003;254:19–35. doi: 10.1016/s0012-1606(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 42.Schlosser G. Evolutionary origins of vertebrate placodes: insights from developmental studies and from comparisons with other deuterostomes. Journal of Experimental Zoology B (Mol Dev Evol) 2005;304:347–399. doi: 10.1002/jez.b.21055. [DOI] [PubMed] [Google Scholar]
- 43.Schlosser G. Induction and specification of cranial placodes. Developmental Biology. 2006 doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Research Molecular Brain Research. 1989;6:311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- 45.Streit A. Extensive cell movements accompany formation of the otic placode. Developmental Biology. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- 46.Torres M, Giraldez F. The development of the vertebrate inner ear. Mechanisms of Development. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 47.Twomey S, Illing N, Brideau NJ, Smith KM, Whitlock KE. Association for Chemoreception Sciences. Vol. 31. Sarasota: 2006. Expression of gonadotropin-releasing hormone (GnRH) and gonadotropin releasing hormone receptors (GnRH-R) in the zebrafish. [Google Scholar]
- 48.Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- 49.Weber SA, Ross LS. Gap junctional coupling in the olfactory organ of zebrafish embryos. Brain Research Developmental Brain Research. 2003;143:25–31. doi: 10.1016/s0165-3806(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 50.Whitelaw NC, Whitelaw E. How lifetimes shape epigenotype within and across generations. Human Molecular Genetics. 2006;15:R131–R137. doi: 10.1093/hmg/ddl200. [DOI] [PubMed] [Google Scholar]
- 51.Whitlock KE. Making Scents: Development and Function of the Olfactory Sensory System. In: Gong Z, Korzh V, editors. Fish Developmental Biology and Genetics. Vol. 2. Singapore: World Scientific; 2004a. pp. 216–260. [Google Scholar]
- 52.Whitlock KE. A new model for olfactory placode development. Brain, Behavior and Evolution. 2004b;64:1–11. doi: 10.1159/000079742. [DOI] [PubMed] [Google Scholar]
- 53.Whitlock KE. Development of the nervus terminalis: Origin and migration. Microscopy Research and Techniques. 2004c;65(1–2):2–12. doi: 10.1002/jemt.20094. [DOI] [PubMed] [Google Scholar]
- 54.Whitlock KE. Origin and Development of GnRH Neurons. Trends in Endocrinology and Metabolism. 2005;16:145–151. doi: 10.1016/j.tem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Whitlock KE. The Sense of Scents. 2006;3:203–213. doi: 10.1089/zeb.2006.3.203. [DOI] [PubMed] [Google Scholar]
- 56.Whitlock KE, Illing N, Brideau N, Smith KM, Twomey S. Development of GnRH cells: Setting the stage for puberty. Molecular and Cellular Endocrinology. 2006 doi: 10.1016/j.mce.2006.04.038. in press. [DOI] [PubMed] [Google Scholar]
- 57.Whitlock KE, Smith KM, Kim H, Harden MR. A role for foxd3 and sox10 in the differentiation of gonadotropin-releasing hormone (GnRH) cells in the zebrafish. Danio rerio, Development. 2005;132:5491–5502. doi: 10.1242/dev.02158. [DOI] [PubMed] [Google Scholar]
- 58.Whitlock KE, Westerfield M. A transient population of neurons pioneers the olfactory pathway in the zebrafish. Journal of Neuroscience. 1998;18:8919–8927. doi: 10.1523/JNEUROSCI.18-21-08919.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitlock KE, Westerfield M. The olfactory placode of the zebrafish form by convergence of cellular fields at the edge of the neural plate. Development. 2000;127:3645–3653. doi: 10.1242/dev.127.17.3645. [DOI] [PubMed] [Google Scholar]
- 60.Whitlock KE, Wolf CD, Boyce ML. Gonadotropin-releasing hormone (GnRH) cells arise from cranial neural crest and adenohypophyseal regions of the neural plate in the zebrafish, danio rerio. Developmental Biology. 2003;257:140–152. doi: 10.1016/s0012-1606(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 61.Witkin JW, Dao D, Livne I, Dunn IC, Zhou XL, Pula K, Silverman AJ. Early expression of chicken gonadotropin-releasing hormone-1 in the developing chick. Journal of Neuroendocrinlogy. 2003;15:865–870. doi: 10.1046/j.1365-2826.2003.01073.x. [DOI] [PubMed] [Google Scholar]
- 62.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proceedings of the National Academy of Sciences USA. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wray S, Nieburgs A, Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Research Developmental Brain research. 1989;46:309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- 64.Zilinski CA, Shah R, Lane ME, Jamrich M. Modulation of zebrafish pitx3 expression in the primordia of the pituitary, lens, olfactory epithelium and cranial ganglia by hedgehog and nodal signaling. Genesis. 2005;41:33–40. doi: 10.1002/gene.20094. [DOI] [PubMed] [Google Scholar]


