Abstract
Dopamine regulates movement, motivation, reward, and learning and is implicated in numerous neuropsychiatric and neurological disorders. The action of dopamine is mediated by a family of seven-transmembrane G protein-coupled receptors encoded by at least five dopamine receptor genes (D1, D2, D3, D4, and D5), some of which are major molecular targets for diverse neuropsychiatric medications. Dopamine receptors are present throughout the soma and dendrites of the neuron, but accumulating ultrastructural and biochemical evidence indicates that they are concentrated in dendritic spines, where most of the glutamatergic synapses are established. By modulating local channels, receptors, and signaling modules in spines, this unique population of postsynaptic receptors is strategically positioned to control the excitability and synaptic properties of spines and mediate both the tonic and phasic aspects of dopaminergic signaling with remarkable precision and versatility. The molecular mechanisms that underlie the trafficking, targeting, anchorage, and signaling of dopamine receptors in spines are, however, largely unknown. The present commentary focuses on this important subpopulation of postsynaptic dopamine receptors with emphases on recent molecular, biochemical, pharmacological, ultrastructural, and physiological studies that provide new insights about their regulatory mechanisms and unique roles in dopamine signaling.
1. The Central Dopaminergic Systems: An Overview
Dopamine (DA) is a prototypical slow neurotransmitter that plays pivotal roles in a variety of cognitive, motivational, neuroendocrine, and motor functions [1–2]. Two major groups of DA-containing neurons in the mammalian brain reside in the substantia nigra pars compacta (SN) and the ventral tegmental area (VTA) [3]. SN neurons form the nigrostriatal pathway, which projects mainly to the dorsal part of striatum where they control postural reflexes and initiation of movements. VTA neurons give rise to two pathways: the mesolimbic pathway, which projects to the subcortical and limbic nuclei, and the mesocortical pathway, which projects mainly to the cingulate, entorhinal and medial prefrontal cortices. Dysfunction of dopaminergic transmission in these major pathways has been implicated in an array of neurological and neuropsychiatric disorders, including Parkinson’s disease, Huntington’s diseases, schizophrenia, attention-deficit hyperactivity disorder (ADHD), and drug addiction [1–2]. Drugs targeting dopaminergic mechanisms are widely used to manage these and other conditions.
The action of DA is mediated by a family of seven-transmembrane G protein-coupled receptors (GPCRs) encoded by at least five DA receptor genes (D1, D2, D3, D4, and D5) in the mammalian brain [4]. These receptors are classified into two subfamilies, the D1-class and D2-class receptors based on deduced amino-acid sequence homologies and pharmacological and functional profiles. The D1-class receptors, including D1 and D5, couple to Gαs to stimulate adenylate cyclases, increase the production of cAMP, and activate protein kinase A (PKA) and subsequent downstream signaling systems, including extracellular signal-regulated kinase (ERK) (Figure 1A) [reviewed in 5]. The D2-class receptors, including D2, D3 and D4, are negatively coupled to this cAMP/PKA-dependent signaling. In addition, D2-class receptors modulate intracellular Ca2+ levels and a number of Ca2+-dependent intracellular signaling processes (Figure 1A). Through diverse cAMP- and Ca2+-dependent and -independent mechanisms, DA influences neuronal activity, synaptic plasticity, and behavior [6–9]. Like most GPCRs, the responsiveness of DA receptors is primarily controlled by the classical β arrestin- and GPCR kinase (GRK)-regulated desensitization process (Figure 1B) [10].
Figure 1. Key steps of dopaminergic signaling and regulation.
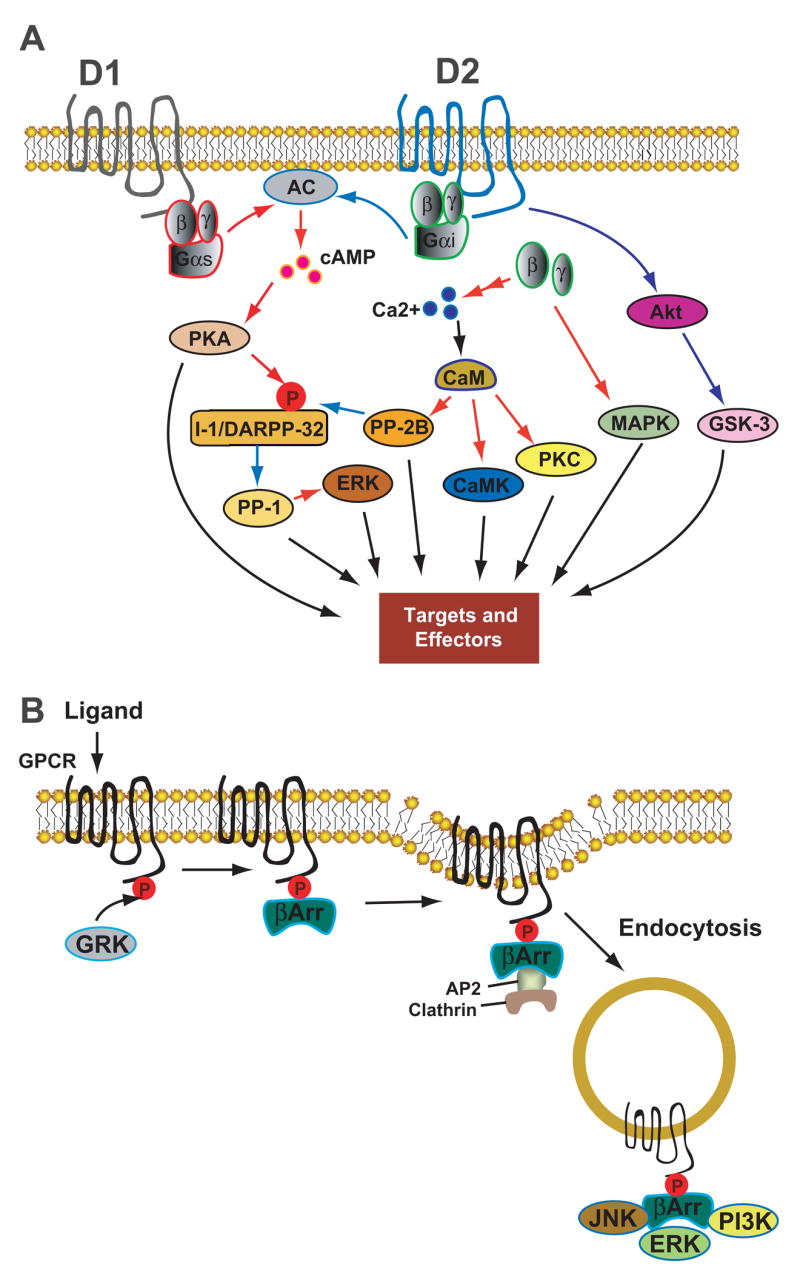
(A) Intracellular pathways mediated by D1- and D2-class DA receptors. Activation of D1-class receptors stimulates AC, increases the production of cAMP, and activates PKA. Activation of PKA phosphorylates I-1 and its homologue DARPP-32, potent inhibitors of PP-1 in their phosphorylated states. Activation of D2-class receptors inhibits AC and downregulates this cAMP-dependent pathway. D2-class receptors also couples to Ca2+ signaling through Gβγ subunits, which act on several types of ion channels and on intracellular Ca2+ stores, linking these receptors to downstream Ca2+-dependent effectors, e.g. calcineurin (PP-2B) and PKC. In addition, D2-class receptors can function through a novel protein kinase B (Akt)–GSK-3 signaling cascade. Downstream effectors of these multiple signaling pathways include, among others, voltage-dependent ion channels, ligand-gated glutamate receptors, and ion pumps. (B) Homologous desensitization process of GPCR. After ligand binding, GPCRs are rapidly phosphorylated by GRKs, followed by binding of the phosphorylated receptors to arrestins. The arrestin-GPCR interaction promotes recruitment of an endocytic complex, leading to receptor internalization via the dynamin-dependent, clathrin-coated vesicle-mediated endocytic pathway. Arrestins also serve as adaptors and scaffolds that interact with numerous signaling molecules and organiz some G protein-independent signaling pathways. Black arrows represent modulatory processes; red arrows indicate stimulating or positive actions; blue arrows indicate inhibitory actions. Abbrevations: AC, adenylyl cyclase; PKA, protein kinase A; PKC, protein kinase C; CaM, calmodulin; CaMK, Ca2+/CaM-dependent protein kinase; I-1, inhibitor-I; DARPP-32, dopamine and cyclic adenosine 3′,5′-monophosphate-regulated phosphoprotein, 32 kDa; PP-1, protein phosphatase 1; PP-2B, protein phosphatase 2B (calcineurin); MAPK, mitogen-activated protein kinase; Akt, protein kinase B (PKB); GSK-3, glycogen synthase kinase 3; GRK, GPCR kinase; βArr, beta arrestin; AP-2, adaptor protein 2; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; PI3K, Phosphoinositide 3-kinase.
DA receptors are distributed in the soma and across the dendritic tree of dopamineceptive neurons. However, a unique, disproportionately large population of DA receptors is localized in dendritic spines [11–14], where most of the excitatory glutamatergic synapses are formed. Moreover, a subset of DA receptor-containing spines is innervated simultaneously by both glutamatergic terminals and dopaminergic terminals [15–17]. DA receptors within dendritic spines differ from extraspinous receptors in that they are physically, and perhaps functionally, confined in the small compartment of spines. These receptors may shape the biophysical properties of individual spines that subserve various motivational and cognitive processes.
In this commentary, we summarize recent ultrastructural and biochemical studies concerning this unique population of DA receptors, assess current views about the targeting, trafficking and signaling of these receptors within dendritic spines, review recently identified DA receptor interacting proteins that may participate in DA receptor regulatory processes, and discuss how DA receptors within dendritic spines may mediate various aspects of DA action.
2. Dendritic Spines: Sites of Synaptic Transmission, Modulation and Plasticity
Dendritic spines are small protrusions from the dendritic surface of neurons that are the major sites of excitatory synaptic transmission in the mammalian CNS [18, 19]. More than 90% of asymmetric glutamatergic synapses are made on the heads of dendritic spines, which in the hippocampus exhibit a near one-to-one relationship to excitatory synapses. Almost all spines belong to pyramidal neurons and mediate virtually all glutamatergic inputs to these cells, at least in the cortex. Spines consist of a head (volume ~ 0.004–2 μm3) connected to the dendritic shaft by a thin neck (diameter ~ 0.04 –1 μm and length ~ 0.1–2 μm). Spines differ considerably in their morphological characteristics across different cell types. Based on their sizes and shapes spines can be classified as “mushroom”, “thin”, or “stubby”. Cortical spines, in general, display larger and more complex morphologies. This morphological heterogeneity may correspond to differences in strengths of synapses established on these spines. Spines show a remarkable dynamics in vivo and their formation, elimination, and morphological/structural plasticity are regulated during development, and by sensory experience [20].
A number of organelles are found in dendritic spines that support spines as structurally autonomous units for synaptic transmission, signaling, and plasticity [18–20]. All spines contain smooth endoplasmic reticulum (SER), which is involved in membrane synthesis and intracellular Ca2+ handling. Spine apparatus, a specialized SER derivative consisting of two or more disks of SER, is observed in larger and more mature spines. Polyribosomes are present in the head or base of most spines in the visual cortex and some spines of the hippocampus, indicating that local protein synthesis may take place within spines. The presence, size, and complexity of these organelles are generally proportional to the size and morphological complexity of spines, suggesting that different-sized spines may handle Ca2+ and structural plasticity differently.
The most prominent ultrastructural specialization of spines is the postsynaptic density (PSD), an electron-dense, disk-like organelle located just underneath the postsynaptic membrane in spine heads. PSD is a macromolecular assembly containing 400–500 proteins [21–23]. These proteins include glutamate receptors, ion channels (e.g. L- and R-type voltage-sensitive Ca2+ channels), protein kinases (e.g. PKA, protein kinase C (PKC), and Ca2+/calmodulin (Ca2+/CaM)-dependent kinase II (CaMKII)), phosphatases (e.g. protein phosphatase-1 (PP-1) and 2A (PP2A)), cytoskeletal components, and proteins involved in membrane trafficking [21, 23]. Within the PSD, receptors, channels, and kinases/phosphatases are likely organized into signaling complexes or modules by scaffolding and adaptor molecules, such as postsynaptic density protein 95 (PSD-95) [24–25]. Thus, the PSD can be thought of as a collection of signal processing devices associated with the maintenance and plasticity of synaptic function [23]. The organization and composition of the PSD is not static, but undergoes dynamic regulation [26], which may underlie the activity-dependent synaptic and structural plasticity in spines [20].
Experimental and theoretic work has suggested that dendritic spines subserve biochemical rather than electrical compartmentalization, and that the spine neck restricts diffusional exchange of signaling molecules between spine heads and parent dendrites [27]. It is estimated that the diffusional exchange between spine heads and dendrites is in the range of 20–200 ms for second messengers, such as intracellular free Ca2+ and inositol 1, 4, 5-trisphosphate (IP3), and small molecules, which is about 100 times slower than would be expected for free diffusion over the length of the spine neck [19]. This time scale should allow many biochemical reactions to take place. Larger cytoplasmic signaling molecules with slower diffusion coefficients (e.g. protein kinases and phosphatases) would be compartmentalized over longer periods of time. Membrane-bound receptors, such as N-methyl-D-aspartic acid receptors (NMDARs) and α-amino-3-hydroxy-5-methylisoxazole-4- propionic acid receptors (AMPARs), can diffuse in the plasma membrane between synaptic and extrasynaptic compartments within spines [28], exchange between different spines [29], and undergo dynamic endocytotic and excytotic membrane trafficking within spines [30]. Recent studies demonstrate that spontaneous activity at individual spines can confine the diffusional movement of AMPA-type glutamate receptor subunit 1 (GluR1) to restricted submicron domains at single synapses, providing a novel mechanism of receptor compartmentalization which may directly contribute to synaptic plasticity [29]. Collectively, the available evidence suggests that dendritic spines compartmentalize various components to limit activity-induced changes to individual spines – a process that could underlie input-specific synaptic activity and plasticity.
3. Dopaminergic Innervation and Receptor Localization
3.1. Dopaminergic Innervation Architecture
The midbrain DA systems project widely to a large number of postsynaptic target cells in multiple forebrain regions, including striatum, neocortex, hippocampus, and amygdala. For example, in the striatum, which is the most densely innervated DA target, virtually every medium spiny neuron (MSN) is innervated by DA due to the extensive ramification of dopaminergic axons. This level of ramification gives rise to extraordinarily dense DA-releasing varicosities; the average distance between release sites is estimated to be only 4 μm [31]. The MSN is the primary cell type in the striatum, accounting for 90–95% of total striatal cells, and is the major target of inputs from the cortex and thalamus. The glutamatergic inputs from these regions are mediated by the densely populated spines on the dendrites of MSNs. Most DA varicosities are in contact with spines, and to a lesser extent with dendritic shafts and cell bodies of MSNs, forming axosomatic, axodendritic, and axospinous synapses [15–16] (Figure 2A). The axodendritic synapses often form on the proximal dendritic shafts, whereas axospinous synapses are predominant on distal dendrites. A significant subpopulation of spines [15] is simultaneously contacted by both dopaminergic and glutamatergic varicosities [15–16], forming the “striatal microcircuit” or “synaptic triad” [17]. Similar DA innervation architecture is also observed in pyramidal neurons in the cortex and hippocampus, and magnocellualar neurons of basolateral amygdale [17,32]. This “triad” architecture can serve as a heterosynaptic co-incidence detector by which activation of the DA terminal selectively influences the simultaneously active converging glutamatergic input at the level of individual spines.
Figure 2. Dopaminergic innervation architecture in the striatum.
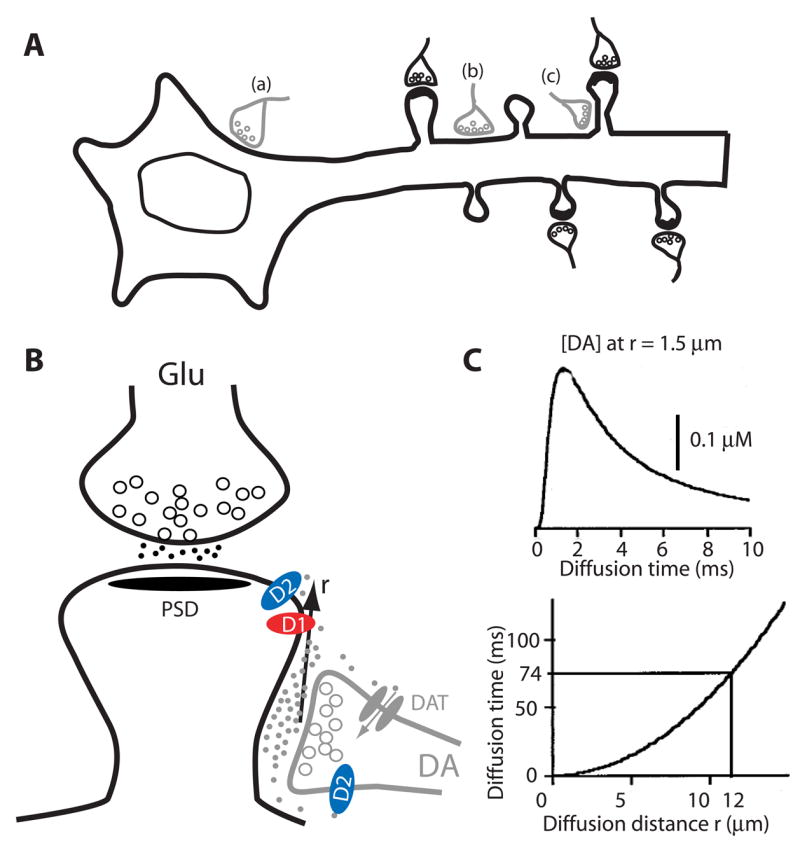
(A) Topographic illustration of dopaminergic synapses onto MSNs. MSNs receive dopaminergic input from the midbrain and glutamatergic input from cortex or thalamic regions. Depending on the subcellular regions on which DA terminals synapse, three distinct types, including axosomatic (a), axodendritic (b), or axospinous (c) are formed. This pattern is believed to exist for neurons in other DA target regions as well. Modified from [93]. (B) Synaptic triad. A MSN spine is contacted by a glutamatergic terminal at the head and a dopaminergic terminal at the neck. Postsynaptic D1- and D2-class receptors, presynaptc D2-class autoreceptors, and DAT localized in perisynaptic sites are depicted. DA released at the synaptic cleft can diffuse a distance (r) in the extrasynaptic space before being uptaken by DAT. (C) Kinetics of DA diffusion in the extracellular space. DA concentration at a receptor site resulting from the exocytosis of one DA vesicle in a release site located at a distance r = 1.5 μm (Upper). The DA concentration reaches its maximum at a time that depends on the square of the distance (Lower curve). In the striatum, DA has a measured half-life of 74 msec, which permits DA to diffuse for a maximal distance of 12 μm. Modified from [31]. Abbreviations: MSN, medium spiny neuron; DAT, DA transporter; PSD, postsynaptic density.
3.2. Subcellular localization of DA receptors
Molecular cloning of the five DA receptor genes and subsequent developments of receptor subtype-specific antibodies in the early 1990s permitted subcellular localization of these receptors by electron microscopy. The D1 receptor is expressed in multiple brain regions, including the cortex, hippocampus, amygdale, and most intensively, the striatum, olfactory bulb, and substantial nigra [11]. In the striatum, the D1 receptor is expressed in ~50% of MSNs, i.e. the striatonigral neurons that are associated with the direct pathway of the basal ganglion circuitry [38]. These receptors are localized primarily in the spine heads, shafts, and less commonly soma of MSNs [11–14]. In the cortex and hippocampus, D1 receptors are present in a subpopulation of interneurons [33], but are primarily expressed in pyramidal neurons, with a predominant subcellular localization in the spines of apical dendrites [14]. D5 receptors, while co-expressed with D1 receptors in cortical pyramidal neurons, are predominately localized in shafts [14], where inhibitory gamma-aminobutyric acid (GABA)-containing neurons form major postsynaptic contacts [34]. The segregation of D1 and D5 at the subcellular level suggests that these closely related receptors may mediate distinct excitatory and inhibitory functions, respectively.
The expression and localization of D2-class receptors have also been investigated at the cellular and subcellular levels [11–13, 35–37, 39–40]. D2 receptors, the predominant subtype of this class, are localized in both pre- and postsynaptic structures. Presynaptically, D2 receptors are associated with both forebrain projecting dopaminergic afferents and glutamatergic terminals in the striatum and prefrontal cortex (PFC). Postsynaptically, D2 receptors are concentrated in shafts and spines of both cortical pyramidal neurons and striatopallidal MSNs. While displaying similar postsynaptic localization profiles, D1 and D2 receptors appear to be distributed in distinct populations of spines, at least in the striatum [12]. This distribution may reflect the segregation of D1 and D2 into the direct and the indirect striatal pathways [38], respectively, rather than a differential transport of these receptors to specific spines within the same neurons. The D4 receptor, known to have an unusually high affinity for the atypical neuroleptical clozapine, is also localized in the shafts and spines of MSNs in the rat nucleus accumbens and pyramidal neurons in primate cerebral cortex [39–40]. The majority of D4 receptors, however, appears to be presynaptic and is expressed in GABAergic interneurons in the cortical, subcortical and basal ganglion circuits [40]. Hence, D2-class receptors likely serve both presynaptic and postsynaptic functions.
At least three lines of evidence show that DA receptors in dendritic spines are associated with PSD. First, western blot studies consistently detecte a substantial amount of D1 and D2 receptors in purified PSD fractions [41–43]. Second, DA receptors interact and co-localize with a number of PSD proteins in dendritic spines (see Section 6 below). Finally, immunoperoxidase electron microscopy reveals that PSD is intensively decorated with DA receptor immunoreactive complexes [11]. It is noteworthy, however, that pre-embedding immunogold electron microscopy shows that PSD is only modestly labeled by D1 and D2 receptor antibodies [12–13] and that in the primate PFC, D1 receptors appear to be displaced to the side of the asymmetric synapse [14]. These findings could be due to the low sensitivity of pre-embedding techniques to detecting PSD antigens. Post-embedding immunogold electron microscopy may help clarify this issue, and additional biochemical studies are needed to determine the strength of DA receptor association with PSD, as well as the mechanisms mediating the association.
3.3. Spine DA receptors mediate extrasynaptic transmission
Despite their presence in spines, the majority of postsynaptic DA receptors do not directly oppose, but are rather distant from, dopaminergic terminal varicosities in DA target areas [11, 17]. Dopaminergic transmission is hence characterized predominantly as non-synaptic or extrasynaptic volume transmission (Figure 2B). Dopaminergic neurons exhibit two activity patterns: tonic and phasic. At rest, these neurons fire tonically at low frequencies, evoking DA release at active varicosities. The released DA can diffuse a few micrometers away from its release sites before being taken up by DA transporters (DAT) localized presynaptically outside of synaptic contacts [44]. The relatively slow dynamics of reuptake allows DA to invade the extrasynaptic space for a maximum of 12 μm [31, 45] (Figure 2C), supplying a sustained, nearly homogenous, DA concentrations in the 10 nM range. This ambient concentration, which primarily acts on the D2 receptors in their high affinity state in the striatum [46], is believed to play an enabling role in movement, cognition, and motivation [47]. In addition to this tonic signaling, most dopaminergic neurons show phasic activation following primary rewards or environmental cues, with latencies of 50–110 ms and durations of < 200 ms. This phasic activation leads to spatially localized (4–12 μm within each activated axonal varicosity) and temporally restricted (500 – 600 ms) “DA waves” (peaking at 150–400 nM) in the striatum [47]. These waves can propagate out of the synaptic clefts before being extinguished by clearance mechanisms [31] (Figure 2B, C). Lower DA tone and more diffused extrasynaptic DA waves may occur in the frontal cortex, where both the DA release sites and transporters are sparser. This phasic DA signaling mediates DA’s limited spatial and temporal specificity and is presumably responsible for associating glutamatergic and dopaminergic information during reward or working memory tasks. The dynamics of the extrasynaptic volume transmission allows both tonic and phasic DA signaling to be detected and processed with remarkable efficiency.
4. Targeting and Trafficking of DA Receptors in Dendritic Spines
4.1. Dynamic trapping: a potential mechanism for DA receptor enrichment in spines?
Studies of a variety of neurotransmitter receptors have strengthened the view that receptor accumulation at synapses results from “dynamic trapping” of receptors by the submembranous PSD protein network [28]. The confinement of receptors, which are mobile even within synapses, may be achieved by interactions with scaffolding proteins and other obstacles as well as by weak molecular interactions that act as steric hinderers or by inter-molecule attractive potentials. Single molecule tracking techniques have demonstrated that receptors can enter or exit the synapse via “lateral diffusion” through the perisynaptic space, providing a potential mechanism underlying changes in receptor number at the synapse [30]. The number of receptors within synapses under basal condition (the equilibrium set point) is determined by the number of scaffolding molecules and by cellular mechanisms that regulate the influx and efflux of receptors. Neuronal activity disturbs this equilibrium, and during activity-dependent synaptic modification a new equilibrium set point may be established. In particular, extrasynaptic receptors may enter the synapse and integrate into the PSD, leading to long-term potentiation (LTP). Conversely, synaptic receptors may dissociate from the PSD and move to the extrasynaptic membrane, resulting in long-term depression (LTD) [28, 30].
This dynamic trapping scheme, which is based primarily on studies of fast neurotransmitters (e.g. glutamate, GABA, and glycine), may also apply to DA receptors. In cultured rat striatal slices, D1 receptors laterally diffuse in the dendritic plasma membrane and their mobility appears to be confined by ligand-occupied NMDARs, perhaps mediated by a direct D1-NMDAR interaction [48]. Interestingly, the action of NMDA in the synaptic recruitment of D1 receptors appears to be independent of Ca2+ flow through the NMDAR channel, but instead depends on an allostericl modification of NMDARs. Thus, NMDARs can act as scaffolds to recruit laterally diffusing D1 receptors to spines, providing a probable mechanism by which DA receptors can be enriched in the PSD [48].
4.2. Local DA receptor trafficking in spines
Endocytosis is a ubiquitous mechanism that allows cells to absorb external nutrients and regulate the level of specific cell-surface proteins. DA receptors, like most GPCRs, internalize via the dynamin-dependent, clathrin-mediated endocytic pathway [43] (Figure 1B). Endocytosis occurs frequently in dendritic shafts, and core endocytic proteins, including adaptor protein 2 (AP-2), clathrin, and dynamin-3, are present in the spine heads [49]. These proteins are systematically arrayed near, but not at, the PSD, to form stable endocytic zones near the PSD in the spine head [30]. The localization of these endocytic zones suggests that spine components are internalized locally within spines. This local endocytic machinery may mediate the constitutive and activity-dependent trafficking of AMPARs in spines and may play a role in synaptic plasticity [30].
Immunogold localization of D1 receptors in rat nucleus accumbens following administration of D1 agonists, however, reveals internalized D1 receptors associated with endocytic vesicles or endosomes in cell bodies and dendrites, but not spines [50]. In addition, snap shots of D2 receptor trafficking using high-resolution immunoelectron microscopy in the primate PFC have yet to capture any endocytosing or postendocytosing D2 receptors in spines; in contrast, clathrin-dependent endocytosis of D2 receptors in dendrites has been observed [51]. These observations raise the intriguing possibility that spine DA receptors may not internalize via the clathrin-dependent endocytic pathway in these cells. However, how do spine receptors adjust their responsiveness and signaling in response to constant or phasic DA stimulation? Do they desensitize at all? These questions are fundamentally important and await future investigations.
5. DA Signaling in Spines
Decades of investigation have elucidated a number of details concerning the molecular, cellular, biochemical, and pharmacological basis of DA signaling [1–2, 4]. Direct assessment of DA signaling in spines, however, is essentially lacking. It is not known, for example, which second messenger systems are linked to DA receptors in spines and what signaling systems they engage. There are several fundamental obstacles that hinder direct assessments of these questions. First, DA transmission is mediated not by the rapid opening of ligand-gated receptor channels, but through complex sequences of biochemical reactions involving second messengers, protein kinases, and protein phosphatases, thus preventing a direct application of electrophysiological approaches. Furthermore, spines are generally inaccessible to microelectrodes, making direct study of DA actions difficult. Finally, the lack of validated methods to isolate/purify dendritic spines prevents application of conventional biochemical and pharmacological approaches. These barriers notwithstanding, the available anatomical and functional evidence makes it highly likely that DA receptors in dendritic spines are functional and participate in DA signaling.
5.1. Anatomical evidence
Key components that mediate DA signaling (Figure 1) are well represented in dendritic spines. Multiple Ca2+/CaM-dependent and -independent adenylate cyclases, the first enzyme in the pathway that synthesizes cAMP, are spatially confined to the PSD and the spine head [52]. Other essential intermediates and effectors of G protein-coupled signaling systems, including heterotrimeric G proteins (Gαi in particular), cAMP-dependent phosphodiesterase, PKA and their anchoring proteins, Ca2+-responsive signaling molecules (e.g. CaM, CaM kinases, and PKC), major protein phosphatases (PP-1, PP-2A, and PP-2B), and components of the mitogen-activated protein kinase (MAPK) cascades, are also concentrated in the PSD and the spine head [21–23]. Many of these elements are strategically clustered at NMDARs and Ca2+ channels and could directly regulate synaptic transmission and plasticity. Ca2+ stores are also present in spines [19], making it possible for spine D2-class receptors to regulate local Ca2+ levels through postsynaptic phospholipase C-β (PLC-β) signaling. Recent characterization of DA receptor-interacting proteins (DRIPs, see below) has also identified a novel spine-localized D2 receptor-interacting protein, neuronal calcium sensor-1 (NCS-1) [53]. NCS-1 is mammalian ortholog of the Drosophila Ca2+-binding protein frequenin known to play a role in synaptic transmission, and NCS-1 inhibits D2 signaling in heterologous cells [53]. The apparent clustering of this rich repertoire of local signaling systems in dendritic spines suggests not only that the primary structural information has been encoded to render the cAMP/Ca2+-dependent systems responsive to spine DA receptor activation, but also that effectors are in place to permit these signals to be propagated.
5.2. Functional evidence
The following is a highly abbreviated summary of evidence supporting the existence of functional DA receptors in dendritic spines. We restrict our focus to electrophysiological studies in which synaptically evoked events were analyzed and dopaminergic actions could be carefully attributed to the postsynaptic site. For detailed reviews of DA modulation of receptor activity, neuronal excitability, and synaptic function, see [6–9].
DA modulation of postsynaptic responses
Brief bath application of low concentrations of D1-class agonists led to delayed onset and prolonged potentiation of NMDAR-mediated excitatory postsynaptic current (EPSC) in layer V PFC neurons. This potentiation was attributed to activation of postsynaptic D1-class receptors [54] and is consistent with the involvement of postsynaptic cAMP signaling [7]. Similarly, in upper cortical layers (II/III), DA, acting on postsynaptic D1-class receptors, enhanced both NMDAR- and AMPAR-mediated EPSCs, and these enhancements depended on postsynaptic Ca2+, PKA, and CaMKII signaling [55]. In contrast, activation of the postsynaptic D4 receptor in the PFC suppressed NMDA EPSC through inhibition of PKA, activation of PP-1, and reduction of CaMKII activity [56]. In hippocampal slices, selective activation of postsynaptic D1-class receptors potentiated both NMDAR- and AMPAR-mediated EPSCs in CA1 cells in a Ca2+-dependent manner [57]. In the nucleus accumbens, most studies report that DA attenuates corticoaccumbal glutamatergic input by acting on D1-class receptors [6]. This attenuation, according to one proposed mechanism, is mediated by an inhibitory feedback action of adenosine, liberated following facilitation of NMDARs by D1-mediated PKC activation in postsynaptic neurons [58]. However, this view remains controversial [59]. In the dorsal striatum, DA does not seem to have direct effects on postsynaptic NMDARs and AMPARs. Instead, postsynaptic DA receptors may regulate voltage-activated ion channels localized in spines (e.g. L-type Ca2+ channels) to indirectly regulate NMDAR- or AMPAR-mediated responses [reviewed in 6–7].
DA modulation of synaptic plasticity
Postsynaptic DA receptors play facilitating or permissive roles in synaptic plasticity in most DA target regions. In hippocampal slices, blockade of D1-class receptors attenuated, and activation of them enhanced the early (< 60 min) [60] and the late (> 2 hr), protein synthesis-dependent maintenance phases of LTP in CA1 synapses in a cAMP-dependent manner [61]. In addition, activation of D1-class receptors lowered the threshold for both LTP and LTD at CA1 synapses in vivo [62]. Similarly, in deep layer PFC synapses and hippocampal-PFC synapses, agonists of D1-class receptors facilitated, whereas antagonists impaired, NMDAR-dependent LTP via cAMP-dependent mechanisms [63–64]. Acting at both D1- and D2-class receptors, DA at high concentrations facilitates LTD, whereas at low concentrations promotes LTP [65]. These DA actions are likely mediated by the MAPK system downstream of cAMP signaling [65]. In the striatum, the role of DA in corticostriatal synaptic plasticity seems more crucial [reviewed in 9]. The predominant form of plasticity in this region is the high frequency stimulation-induced, endocannabinoid-mediated LTD. It is generally agreed that this LTD depends on activation of both D1- and D2-class receptors [9]. However, opinions differ with respect to the cell types that express this plasticity (striatopallidal vs. striatonigral), the sites of dopaminergic action (spines on postsynaptic MSN vs. presynaptic cholinergic interneurons), and the signaling mechanisms [66–67]. NMDAR-dependent striatal LTP can also be induced by high-frequency stimulation of corticostriatal glutamatergic inputs. This LTP requires D1-class receptors and is mediated by the postsynaptic cAMP/PKA/DA and cyclic adenosine 3′,5′-monophosphate-regulated phosphoprotein, 32 kDa (DARPP-32) cascade [9]. Taken as a whole, these findings suggest that DA receptors in dendritic spines are the most likely candidates for the postsynaptic DA modulations, although the exact signaling steps remain less well defined. However, because none of the studies to date has clearly defined the precise sites of DA actions, the contribution of extraspinous DA signaling can not be excluded.
6. DA Receptor-interacting Proteins (DRIPs)
As mentioned previously, the mechanisms regulating DA receptor targeting, trafficking, and signaling in dendritic spines are largely unexplored. Recent studies, however, have identified a family of proteins that associate with different subtypes of DA receptors, which could help elucidate these underlying mechanisms.
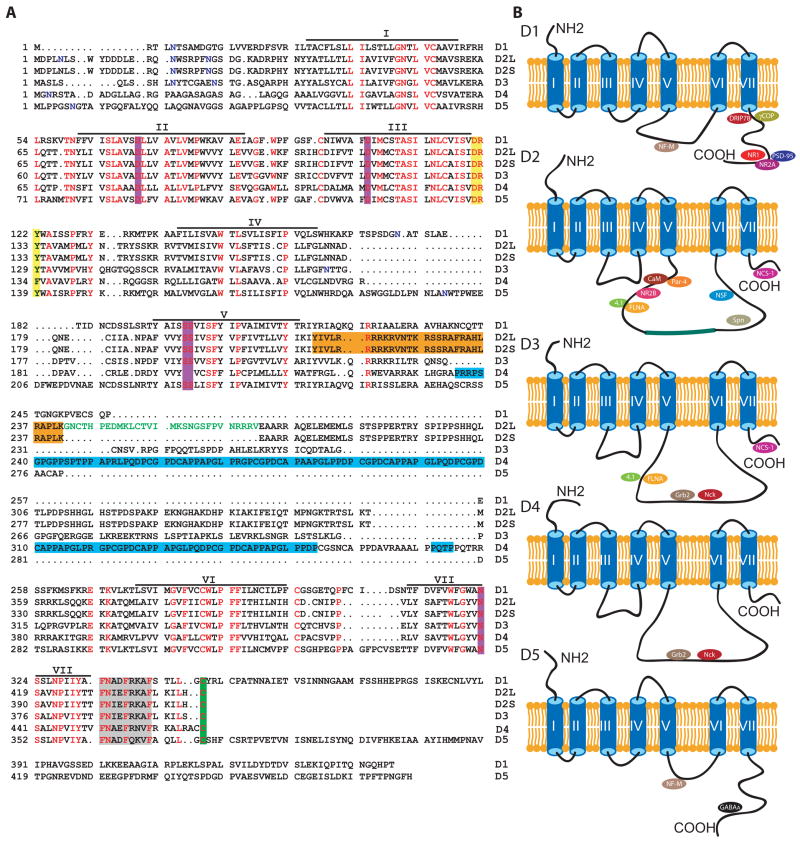
As members of the seven transmembrane (TM) GPCR family, DA receptors reside in the plasma membrane with three extracellular and three intracellular loops (ILs) linking the seven TM domains (Figure 3). D1- and D2-class receptors differ in the length of their intracellular segments. D1-class receptors have short IL3s and long carboxyl terminal cytoplasmic tails, whereas D2-class receptors have long IL3s and short carboxyl terminal tails (Figure 3). Besides short peptide sequences involved in G protein and arrestin binding, the remaining portions of these intracellular domains are presumably free to interact with other proteins. Using approaches such as co-immunoprecipitation and the yeast two-hybrid system, an increasing number of DRIPs are being identified [41–43, 53, 68–83]. The confirmed DRIPs and their suggested functions are summarized in Table 1. Consistent with the low homology among different DA receptor intracellular segments (Figure 3A), distinct sets of DRIPs associate with different receptor subtypes, suggesting that different receptors associate with distinct molecular complexes (Figure 3B). DRIPs interact primarily with the carboxyl terminal tails of D1-class receptors or the IL3s of D2-class receptors. These proteins can be further classified into several functional categories, including scaffolds and adaptors, signaling proteins, receptors and channels, and cytoskeletal components (Table 1). Functional studies suggest that DRIPs are involved in various aspects of DA receptor synthesis, trafficking, targeting, signaling, and crosstalk with other signaling systems. Significantly, a subset of DRIPs is concentrated in dendritic spines or PSD. These dendritic spine/PSD DRIPs could conceivably anchor DA receptors at the membrane-associated cytoskeleton, affect the equilibrium set point of DA receptors in the synapse, regulate local DA signaling, and mediate the interactions between DA receptors and other signaling modalities in the PSD and spines.
Figure 3. DA receptors and their associating proteins.
(A) Amino acid alignment of the human DA receptors. Identical residues are shown in red. Asparagine residues that form part of N-linked glycosylation consensus sequences are shown in blue. The DRY/F motif immediately following TM3 involved in interaction with G protein and β-arrestin is highlighted in yellow. Amino acids involved in agonist binding are shaded with purple. The conserved cysteine residue in the proximal region of carboxyl tails potentially involved in anchoring the receptors to the plasma membrane following palmitoylation is highlighted in green. The conserved carboxy-terminal hydrophobic ER-transport motif (FxxxFxxxF) is shaded with grey. CaM/Par-4 binding site on D2 is coded with orange. Putative SH3 binding domains (PxxP repeats) on D4 are coded with light blue. A 29-amino acid segment present in D2L, but not in D2S, is indicated in green. Black bars represent putative TM domains. (B) Schematic illustrations of DA receptor topology and identified proteins interacting with different intracellular fragments of these receptors. Detailed information about these DRIPs are summarized in Table 1. Abbreviations: TM, transmembrane; CaM, calmodulin; Par-4, prostate apoptosis response 4; D2L, D2 receptor long isoform; D2S, D2 receptor short isoform; ER, endoplasmic reticulum; SH3, Src homology 3.
Table 1.
Dopamine Receptor-Interacting Proteins (DRIPs)
| Proteins and Categories | Confirmed Interaction | Interaction motif | Effects on DA receptor | Locazed in PSD or spine?* | References |
|---|---|---|---|---|---|
| Scaffolds and adaptors | |||||
| PSD-95 | D1 | CT | Internalization | PSD | 43 |
| Spinophilin | D2 | IL3 | Organize D2 signaling in PSD | PSD | 68 |
| NCK | D4 | IL3 | Internalization | PSD | 69 |
| Grb2 | D3, D4 | IL3 | Internalization | PSD | 69 |
| DRIP78 | D1 | CT | ER export | unknown | 70 |
| NSF | D2 | IL3 | Uncouples D2 modulation of AMPAR trafficking | PSD | 71 |
| Signaling | |||||
| Calmodulin | D2 | IL3 | Ca2+ and cAMP signaling | PSD | 72 |
| NCS-1 | D2, D3, D5 | CT | Desensitization, Ca2+ signaling | spine | 53 |
| Receptors and channels | |||||
| NMDAR NR1-1a | D1 | CT | Trafficking and targeting | PSD | 73 |
| NMDAR NR2A | D1 | CT | Trafficking | PSD | 73 |
| NMDAR NR2B | D2 | IL3 | CaMKII signaling | PSD | 42 |
| GABAAR | D5 | CT | cAMP signaling | No | 74 |
| A1R | D1 | unknown | cAMP signaling | unknown | 75 |
| A2R | D2 | unknown | Desensitization and internalization | spine | 76 |
| SSTR5 | D2 | unknown | cAMP signaling | unknown | 77 |
| KIR3 | D2, D4 | unknown | Unknown | spine | 78 |
| Cytoskeleton | |||||
| NF-M | D1, D5 | IL3 | Internalization | PSD | 79 |
| 4.1N (also R, B, G) | D2, D3 | IL3 | Cell surface expression | PSD | 80 |
| Filamin A (ABP-280) | D2, D3 | IL3 | Cell surface expression; signaling | spine | 81 |
| gamma-COP | D1 | CT | ER transport | unknown | 82 |
| Apoptosis | |||||
| PAR-4 | D2 | IL3 | Desensitization and signaling | Unknown | 83 |
Only proteins that interact directly with DA receptors are listed. DA receptors are also known to form homo- or heterodimmers. DRIPs are classified based on their original functions. References are limited to those that reported the relevant protein interactions.
Whether a DRIP is localized in PSD or spine was determined by PubMeb searches.
Abbreviations not covered in text: Grb2, Growth factor receptor-bound protein 2; DRIP78, Dopamine receptor-interacting protein 78; NSF, N-ethylmaleimide-sensitive factor; GABAAR, GABAA receptor, A1R, Adenosine A1 receptor; A2R, Adenosine A2A receptor; SSTR5, Somatostatin sst5 receptor; KIR3, inwardly rectifying K channel; gamma COP, gamma subunit of the coatomer protein complex; PAR-4, Prostate apoptosis response 4.
6.1. D1 DRIPs in dendritic spines
A number of scaffolding, receptor, and cytoskeletal DRIPs present in the PSD have been shown to interact with D1-class receptors. Neurofilament M (NF-M), a major cytoskeletal element implicated in axonal and dendritic transport, has been shown to interact via IL3 with the D1 receptor [79]. In heterologous systems, NF-M reduces D1 receptor expression at the cell surface and promotes receptor accumulation in the cytosol. Surprisingly, it also inhibits agonist-induced D1 receptor desensitization, suggesting dual roles for NF-M in limiting receptor number at the cell surface and membrane trafficking.
Recent studies emphasize physical interactions between D1-class receptors and glutamate receptors independent of the classical second messenger systems. D1, but not D5 receptors, can interact with NMDAR subunit 1 (NR1) and subunit 2A (NR2A), but not subunit 2B (NR2B) through the C terminal tails of these receptors [73]. These direct protein-protein interactions allow D1 receptors to inhibit, rather than potentiate, NMDAR-mediated currents in cultured neurons. Conversely, association with NMDARs modifies intracellular D1 receptor trafficking in several ways. In heterologous cells, coexpression of D1 with NR1 and NR2B (to make functional NMDARs) abolished agonist-induced D1 receptor sequestration, suggesting that oligomerization with NMDARs could regulate D1 receptor desensitization. In addition, oligomerization with NMDARs facilitated D1 receptor targeting to the plasma membrane. When D1 and NR1 were coexpressed in individual cells in the absence of the NR2B subunit, both were retained in cytoplasmic compartments. However, in the presence of NR2B, the NR1-D1 receptor complex was translocated to the plasma membrane, suggesting that D1 and NMDARs were assembled within intracellular compartments as constitutive heteromeric complexes [41]. In both heterologous cells and cultured neurons, activation of NMDARs recruited D1 receptors to the plasma membrane via a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent mechanism, resulting in enhancement of D1-mediated cAMP accumulation [84]. These studies, while not entirely consistent mechanistically, all appear to support direct D1-NMDAR interaction.
In a phenotype-driven, microarray-based screen for mechanisms underlying psychostimulant-elicited plasticity, PSD-95 was identified as a regulator of DA signaling [85]. The level of PSD-95 is reduced in the striatum of several mouse models of DA supersensitivity, and mice lacking PSD-95 show enhanced behavioral responses to the psychostimulants cocaine and amphetamine [43, 85]. The role of PSD-95 in DA sensitivity is mediated by, at least in part, its interaction with the D1 receptor [43]. The D1-PSD-95 interaction does not require the well-characterized protein-protein interaction domains of PSD-95, but is instead mediated by the carboxyl terminal tail of the D1 receptor and the N-terminus of PSD-95. Co-expression of PSD-95 with D1 receptors in heterologous cells promotes D1 receptor internalization via a dynamin-dependent mechanism. Disruptions of the D1-PSD-95 interaction abolish the PSD-95-mediated enhancement of D1 endocytosis, demonstrating that the physical interaction is responsible for enhanced D1 internalization. It remains to be determined whether this mechanism holds true in vivo and whether PSD-95 acts as a scaffold to “stabilize” D1 receptors in the PSD.
Given the well-established association of PSD-95 with NMDARs and the concentration of this receptor-scaffold complex in the synapse, the results described above suggest that a tertiary protein complex containing the D1 receptor, NMDAR and PSD-95 may exist in the brain to regulate sorting and synaptic delivery of D1 receptors within spines. It is interesting to note that PSD-95 facilitation of D1 endocytosis opposes the NR1-dependent facilitation of D1 exocytosis. This antagonistic mechanism may play a role in D1-NMDAR interaction by de-coupling the positive feedback loop formed between NMDAR and the D1 receptor [86]. Specifically, D1 receptor activation potentiates NMDARs via the cAMP/PKA cascade [2]. On the other hand, NMDAR activation enhances D1 receptor recruitment to the plasma membrane. This positive feedback loop, if not controlled, might result in concomitant overactivation of both the D1 and the NMDAR systems, perhaps triggering NMDAR- and D1-dependent neurotoxicity and cell death [87–88]. Thus, D1-PSD-95 interaction may serve as a mechanism to counteract the NMDA-dependent surface delivery/anchorage of D1 receptors, thus preventing excessive D1 insertions to the synapse.
6.2. D2 DRIPs in dendritic spines
Several scaffolding, cytoskeletal, and receptor proteins in the PSD interact with D2-class receptors. The actin binding protein filamin A (ABP-280), a cytoskeleton-associated protein involved in cell morphology and motility, interacts with the IL3 of D2 and D3 receptors [81]. These same regions also mediate the interaction of D2/D3 receptors with protein 4.1N, the neuronal form of the 4.1 family of cytoskeletal proteins implicated in linking the spectrin-actin cytoskeleton to the plasma membrane [80]. Interactions with these structural components may anchor D2-class receptors to the actin cytoskeleton and stabilize them at the plasma membrane of dendritic spines. Recent studies revealed a direct interaction between D2 receptors and NR2B, which is mediated by the C-terminus of NR2B and the IL3 of the D2 receptor [42]. This interaction appears to modulate cocaine-elicited striatal NR2 phosphorylation and motor activities. Finally, the D2 receptor interacts with spinophilin/neurabin-II (neuronal actin binding protein II), an F-actin-associating postsynaptic scaffolding protein in the PSD [68]. Spinophilin itself interacts with PP-1, the major protein phosphatase in spines. The D2/spinophilin/PP-1 complex may provide a platform that links D2 receptors to the actin cytoskeleton and downstream PP-1 signaling in dendritic spines.
7. Dendritic Spine DA Receptors: Hypothetical Models and Behavioral Implications
Dopaminergic terminal fibers form symmetric synapses on the neck of a subpopulation of dendritic spines. Although not all spines receiving a glutamate afferent are contacted by a DA varicosity, spines that do receive a DA afferent invariably receive an asymmetric glutamate input [17]. Thus, spines can be classified as diads or triads, based on whether or not they receive direct dopaminergic innervation. Both diads and triads can have three possible configurations based on the types of DA receptor they contain: D1class-, D2 class-, and D1class/D2 class-containing spines (Figure 4). It should be noted that although spines containing either D1- or D2-class receptors exist [12], spines that contain both classes of receptors have not been reported. DA could statically or dynamically set the structurally distinct spines at different states, which could mediate the complex and state-dependent DA modulation of postsynaptic target cells.
Figure 4. DA receptor-containing spine configurations.
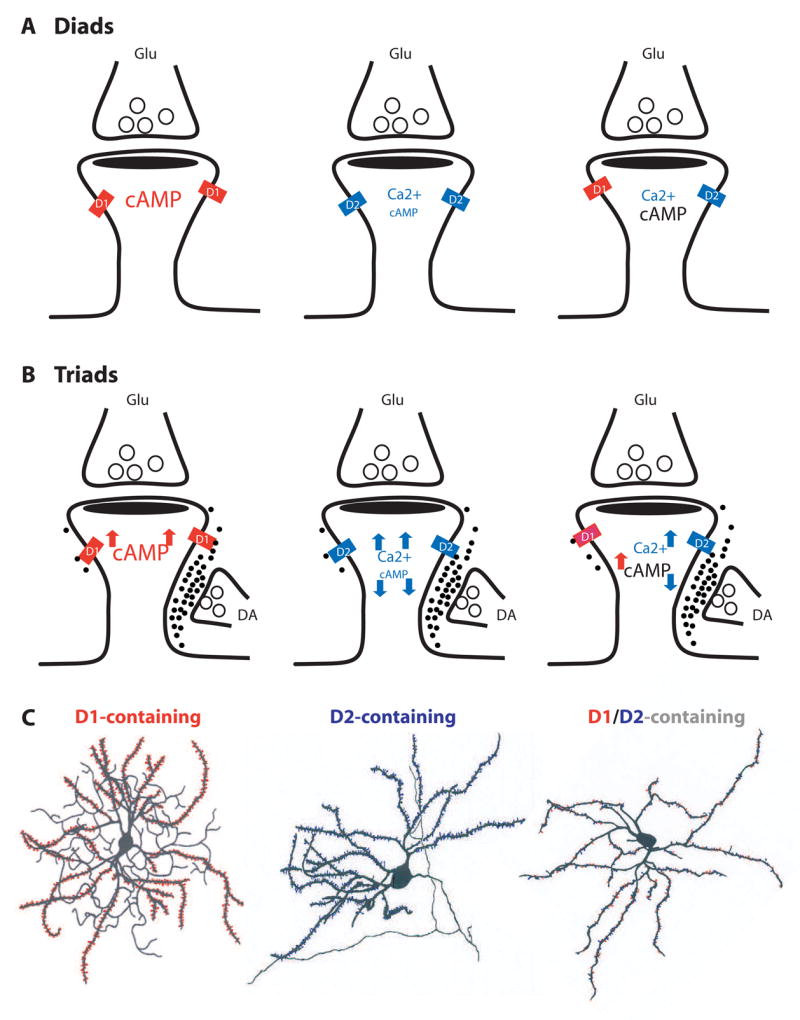
Six hypothetical configurations, based on the putative classes of DA receptors they contain, are grouped into diads (A) or triads (B). Diads are not directly contacted by dopaminergic terminals and mainly serve as synaptic detectors of tonic DA signaling. Triads receive dopaminergic innervation at the spine neck and may serve detectors for both tonic and phasic DA signals. D1-, D2, and D1/D2-containing spines respond differently to DA stimulation, thus may exhibit distinct synaptic physiology. D1 activation increases intraspine cAMP level, whereas D2 decreases it. D2 activation may contribute to spine Ca2+ signaling. Font sizes represent hypothetic levels of cAMP or Ca2+. (C) Hypothetical MSNs (modified from [94] and [95]) populated with D1 class- (red), D2 class- (blue), or D1/D2 (red and blue)-containing spines. D1 and D2 on illustrations denote D1- and D2-class receptors, respectively.
Diads
Synaptic diads (Figure 4A) are considered traditional excitatory synapses. Assuming that these synapses are beyond the DA diffusion range from the nearest releasing sites, they may be the principal synaptic detectors of background DA. As D1- and D2-coupled signaling differs considerably, the dopaminergic control of D1 and D2 diads should also differ. In addition, if D1-and D2-class receptors are proved to reside in the same spines, they would entitle these spines additional advantages: first, it permits the well-documented D1-D2 synergy/antagonism to occur at the level of individual spines; and second, given the different affinities to and activation profiles of different DA receptors by DA [46], these spines could code DA signaling at a range of concentrations. Regardless of the mechanisms, the same background DA tone may set D1-, D2-, and the putative D1/D2-containing spines at distinct biochemical, physiological, and activity states. Different basal states could determine how individual synapses undergo different experience-dependent changes [89].
Triads
Synaptic triads (Figure 4B) can be considered as heterosynaptic co-incidence detectors of concurrently active dopaminergic and glutamatergic inputs. The dimension of a typical spine is well within the influence of DA waves generated at the neck of the spine (Figure 2B, C). Schultz (2002) [47] has detailed how phasic DA signals can selectively modify a simultaneously active cortical input at striatal triads, which can be generalized to triads in other brain regions. Activation or inhibition of a DA neuron leads to a global, spatially unselective increase or decrease of DA release at most varicosities. Only those synapses that are activated by excitatory glutamatergic inputs at the same postsynaptic spines, however, would be influenced by the DA signal, whereas synapses not activated by coincident glutamatergic inputs would remain unchanged. There may be certain temporal specificity of this associative heterosynaptic modulation. Specifically, dopaminergic modifications of a glutamatergic input occur only when the DA input is active at about the same time as the glutamatergic input, or immediately following it. In the case of the DA input arriving after the glutamatergic input, the activated synapses will be “labeled”, or “tagged”, for later modifications. However, D1-, D2-, and D1/D2-containing triads could be differentially tuned or tagged following each DA signal. By modulating the postsynaptic activity, e.g. the dynamics of spike backpropagation through influencing the state of kinases and phosphatases, dopaminergic modulation/tagging could dynamically set different triads at different activity states, which may determine whether a synapse will be strengthened or weakened by activity [90].
Integrated dopaminergic modulation on neurons
The overall synaptic modulation of DA on a neuron is determined by the spatial and temporal summation of DA effect on all spines across the entire dendritic tree of the neuron. Thus, neurons populated with different patterns of D1-, D2-, and D1/D2-containing diads and triads (Figure 4C) could exhibit distinct cellular and synaptic properties, which could also contribute to the inherited complexity and some apparent contradictions regarding dopaminergic regulation of target neurons, as is often observed [reviewed in 6 and 7]. Based upon this analysis, one prediction is that D1-expressing MSNs in the striatonigral direct pathway and D2-expressing MSNs in the indirect striatopallidal pathway may show distinct physiological characteristic, e.g. intrinsic membrane excitability, basal bistability, responses to DA modulation, and plasticity. Recent studies using bacterial artificial chromosome (BAC) transgenic mice indeed support this idea [67, 91].
Behavioral and pathological implications of spine DA receptor function
The putative triadic DA-glutamate coincidence detector may provide a cellular substrate for cognitive processes gated or enabled by DA, such as reward and working memory [47, 92]. DA neurons are optimally activated by primary reward or reward-predicting stimuli [47]. Cortical inputs may code particular patterns of sensory information, e.g. smell, color, texture, or movement related to the same rewarding event. DA released in striatal triads following the phasic activation of DA neurons might selectively strengthen or weaken the corticostriatal glutamatergic synapses activated by the reward-associated sensory events, promoting reward.
In the PFC, neurons that display spatially tuned “memory fields” are considered to be the cellular basis for working memory [92]. During oculomotor delayed response task, these neurons increase firing when the behaving animals must retain the location of a target during a delay period between target presentation and time of response. D1 receptor antagonists can selectively potentiate the memory fields of these neurons but do not have effects on other cells [92]. These findings suggest that there is a direct gating of selective excitatory inputs to prefrontal neurons during cognition by the D1 receptors specifically localized in spines that receive inputs responsible for generating the delayed period activity [17].
DA receptors in dendritic spines may play a significant role in DA pathophysiology and diseases. DA dysfunction is associated with major neurological and neuropsychiatric disorders, such as Parkinson’s disease, Huntington’s disease, schizophrenia, ADHD, and addiction [1–2]. Despite distinct primary pathological characteristics for each disease, one common feature is that they all impair certain aspects of cognitive and motivational functions. Although DA-based therapies are effective in alleviating some of the core symptoms of these diseases, cognitive and motivational deficits are often not fully restored. In addition, DA-based pharmacological interventions often have severe unwanted effects, such as psychosis and dyskinesias for Parkinson’s disease and extrapyramidal side effects for schizophrenia. Perhaps, alterations of phasic dopaminergic transmission mediated exclusively at dendritic triads hold a key, because while exogenous DA or DA-based pharmacotherapies can in theory restore dopaminergic signaling at the diads and extraspinous loci, they can not in any simple manner restore phasic information transmitted by neuronal impulses at the synaptic triads. Considering that proper synaptic transmission and modulation at dendritic spines and synapses are essential for normal cognitive and motivational functions, abnormal spatial and temporal stimulation or inhibition of spine DA receptors by exogenous means is not likely to improve these functions.
8. Concluding remarks
DA receptors localized postsynaptically in dendritic spines may exert heterosynaptic influences on synaptic transmission and plasticity at the level of individual spines. This population of DA receptors may be particularly important in mediating the effect of DA in cognition, reward, and memory. The local rules governing DA receptor localization, trafficking, and anchorage in spines are poorly understood. The exact signaling cascades coupled to different DA receptors in dendritic spines also remain largely speculative. The precise dopaminergic regulation of dendritic function and physiology at single spines is essentially unexplored. Improved biochemical, electrophysiological, single molecule imaging techniques in individual spines should help address these issues. Identification of novel molecules that associate with various DA receptors, using candidate gene, yeast two-hybrid, and mass spectrometry approaches, and characterizations of their roles in DA receptor trafficking, targeting, and interaction with other neurotransmitter systems within spines will facilitate understanding of the biology of spine DA receptors. Progress along these lines is likely to uncover novel therapeutic targets for treatment of neurological and neuropsychiatric disorders.
Acknowledgments
We thank Ms. Donna Reed for her invaluable technical support. This work was supported by a National Center for Research Resources grant RR000168 (to the New England Primate Research Center), National Institute on Drug Abuse grants DA021420 (W.-D.Y.), DA011059, DA011928, DA017700, and DA024315 (R.D.S.), a National Alliance for Research on Schizophrenia and Depression Young Investigator Award, and Williams F. Milton Fund of Harvard University (to W.-D.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carlsson A. A paradigm shift in brain research. Science. 2001;294:1021–24. doi: 10.1126/science.1066969. [DOI] [PubMed] [Google Scholar]
- 2.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–47. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 3.Bj rklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Bj rklund A, Hkfelt T, editors. Handbook of Chemical Neuroanatomy: Classical Transmitter in the Rat. Vol. 2. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- 4.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 7.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–35. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–9. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 11.Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ, et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861–5. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–37. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–30. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- 14.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–36. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- 16.Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- 17.Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–71. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 19.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–53. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 21.Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–71. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Li KW, Hornshaw MP, Van Der Schors RC, Watson R, Tate S, Casetta B, Jimenez CR, Gouwenberg Y, Gundelfinger ED, Smalla KH, Smit AB. Proteomics analysis of rat brain postsynaptic density: Implications of the diverse protein functional groups for the integration of synaptic physiology. J Biol Chem. 2004;279:987–1002. doi: 10.1074/jbc.M303116200. [DOI] [PubMed] [Google Scholar]
- 23.Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003–11. doi: 10.1074/jbc.M400103200. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–4. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- 25.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 26.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–42. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 27.Koch C, Zador A. The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. J Neurosci. 1993;13:413–22. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–9. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–60. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–62. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J Neurosci. 1997;17:5972–8. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Muly EC, 3rd, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci. 1998;18:10553–65. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa E. From GABAA receptor diversity emerges a unified vision of GABAergic inhibition. Annu Rev Pharmacol Toxicol. 1998;38:321–50. doi: 10.1146/annurev.pharmtox.38.1.321. [DOI] [PubMed] [Google Scholar]
- 35.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442:392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- 37.Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488:464–75. doi: 10.1002/cne.20601. [DOI] [PubMed] [Google Scholar]
- 38.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 39.Svingos AL, Periasamy S, Pickel VM. Presynaptic dopamine D (4) receptor localization in the rat nucleus accumbens shell. Synapse. 2000;36:222–32. doi: 10.1002/(SICI)1098-2396(20000601)36:3<222::AID-SYN6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–8. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 41.Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem. 2003;278:20196–202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- 42.Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SG, Miller GM, Isacson O, Caron MG, Yao WD. Inhibition of the dopamine D1 receptor signaling by PSD-95. J Biol Chem. 2007;282:15778–89. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14:6084–93. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience. 1989;30:767–77. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- 47.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 48.Scott L, Zelenin S, Malmersjo S, Kowalewski JM, Markus EZ, Nairn AC, Greengard P, Brismar H, Aperia A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc Natl Acad Sci USA. 2006;103:762–7. doi: 10.1073/pnas.0505557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–8. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- 50.Dumartin B, Caille I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J Neurosci. 1998;18:1650–61. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paspalas CD, Rakic P, Goldman-Rakic PS. Internalization of D2 dopamine receptors is clathrin-dependent and select to dendro-axonic appositions in primate prefrontal cortex. Eur J Neurosci. 2006;24:1395–403. doi: 10.1111/j.1460-9568.2006.05023.x. [DOI] [PubMed] [Google Scholar]
- 52.Mons N, Harry A, Dubourg P, Premont RT, Iyengar R, Cooper DM. Immunohistochemical localization of adenylyl cyclase in rat brain indicates a highly selective concentration at synapses. Proc Natl Acad Sci USA. 1995;92:8473–7. doi: 10.1073/pnas.92.18.8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, Levenson R. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J Neurosci. 2002;22:8476–86. doi: 10.1523/JNEUROSCI.22-19-08476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA. 2001;98:301–6. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci. 2003;23:867–75. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci. 2003;23:9852–61. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang SN. Sustained enhancement of AMPA receptor- and NMDA receptor-mediated currents induced by dopamine D1/D5 receptor activation in the hippocampus: an essential role of postsynaptic Ca2+ Hippocampus. 2000;10:57–63. doi: 10.1002/(SICI)1098-1063(2000)10:1<57::AID-HIPO6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci. 1997;17:5271–80. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci. 1999;19:1437–45. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92:2446–50. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–9. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci USA. 2004;101:3236–41. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otani S, Daniel H, Roisin MP, Crepel F. Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex. 2003;13:1251–6. doi: 10.1093/cercor/bhg092. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–52. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 68.Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem. 1999;274:19894–900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- 69.Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, Pawson T, von Zastrow M, Van Tol HH. SH3 binding domains in the dopamine D4 receptor. Biochemistry. 1998;37:15726–36. doi: 10.1021/bi981634+. [DOI] [PubMed] [Google Scholar]
- 70.Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;3:492–8. doi: 10.1038/35074561. [DOI] [PubMed] [Google Scholar]
- 71.Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, Wan Q, Liu F. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J Neurosci. 2005;25:4385–95. doi: 10.1523/JNEUROSCI.5099-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672–80. doi: 10.1074/jbc.M002780200. [DOI] [PubMed] [Google Scholar]
- 73.Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–30. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- 74.Liu F, Wan Q, Pristupa ZB, Yu XM, Wang YT, Niznik HB. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature. 2000;403:274–80. doi: 10.1038/35002014. [DOI] [PubMed] [Google Scholar]
- 75.Gines S, Hillion J, Torvinen M, Le Crom S, Casado V, Canela EI, Rondin S, Lew JY, Watson S, Zoli M, Agnati LF, Verniera P, Lluis C, Ferre S, Fuxe K, Franco R. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA. 2000;97:8606–11. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–7. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 77.Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–7. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 78.Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem. 2002;277:46010–9. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 79.Kim OJ, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002;22:5920–30. doi: 10.1523/JNEUROSCI.22-14-05920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol. 2002;62:507–13. doi: 10.1124/mol.62.3.507. [DOI] [PubMed] [Google Scholar]
- 81.Li M, Bermak JC, Wang ZW, Zhou QY. Modulation of dopamine D(2) receptor signaling by actin-binding protein (ABP-280) Mol Pharmacol. 2000;57:446–52. doi: 10.1124/mol.57.3.446. [DOI] [PubMed] [Google Scholar]
- 82.Bermak JC, Li M, Bullock C, Weingarten P, Zhou QY. Interaction of gamma-COP with a transport motif in the D1 receptor C-terminus. Eur J Cell Biol. 2002;81:77–85. doi: 10.1078/0171-9335-00222. [DOI] [PubMed] [Google Scholar]
- 83.Park SK, Nguyen MD, Fischer A, Luke MP, Affar el B, Dieffenbach PB, Tseng HC, Shi Y, Tsai LH. Par-4 links dopamine signaling and depression. Cell. 2005;122:275–87. doi: 10.1016/j.cell.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 84.Pei L, Lee FJ, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci. 2004;24:1149–58. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–38. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 86.Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;2006:pe20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- 87.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–34. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 88.Bozzi Y, Borrelli E. Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci. 2006;29:167–74. doi: 10.1016/j.tins.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Ward B, McGuinness L, Akerman CJ, Fine A, Bliss TV, Emptage NJ. State-dependent mechanisms of LTP expression revealed by optical quantal analysis. Neuron. 2006;52:649–61. doi: 10.1016/j.neuron.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 90.Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–41. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- 91.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–9. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 92.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–5. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 93.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–65. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 94.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 95.Chang HT, Wilson CJ, Kitai ST. A Golgi study of rat neostriatal neurons: light microscopic analysis. J Comp Neurol. 1982;208:107–26. doi: 10.1002/cne.902080202. [DOI] [PubMed] [Google Scholar]