SUMMARY
Small bowel epithelium is at the frontline of intestinal barrier function. Restitution is considered to be the major determinant of epithelial repair as function recovers in parallel with restitution after acute injury. As such, studies of intact mucosa have largely been replaced by migration assays of cultured epithelia. These latter studies fail to account for the simultaneous roles played by villous contraction and paracellular permeability in recovery of barrier function. Non-steroidal anti-inflammatory drugs (NSAID) result in increased intestinal permeability and disease exacerbation in patients with IBD. Thus, we examined the reparative attributes of endogenous prostaglandins (PG) after injury of ileal mucosa by deoxycholate (6 mM) in Ussing chambers. Recovery of transepithelial resistance (TER) from 20–40 Ω.cm2 was abolished by indomethacin (INDO), whereas restitution of 40–100% of the villous surface was unaffected despite concurrent arrest of villous contraction. In the presence of PG, resident crypt and migrating epithelial cells were tightly apposed. In tissues treated with INDO, crypt epithelial cells had dilated intercellular spaces that were accentuated in the migrating epithelium. TER was fully rescued from the effects of INDO by osmotic-driven collapse of the paracellular space and PG-mediated recovery was significantly impaired by blockade of Cl− secretion. These studies are the first to clearly distinguish the relative contribution of paracellular resistance versus restitution to acute recovery of epithelial barrier function. Restitution was ineffective in the absence of PG-mediated paracellular space closure. Failure of PG-mediated repair mechanisms may underlie barrier failure resulting from NSAID use in patients with underlying enteropathy.
Keywords: resistance, permeability, tight junction, deoxycholate
INTRODUCTION
The single columnar epithelial lining of the small intestine is the primary barrier restricting transgression by luminal bacteria, endotoxin, bile, digestive enzymes and antigens while also serving as the principal site for selective transport of electrolytes and nutrients. Following mucosal injury to the small intestine, acute restoration of barrier function is thought to require the concerted action of three local repair mechanisms. These mechanisms include 1) villous contraction, which reduces the total and denuded surface area for repair (13,26), 2) epithelial cell migration (restitution) to seal exposed basement membrane (1,27), and 3) closure of leaky paracellular spaces and tight junctions between epithelial cells (6,14,21). The relative contribution of each mechanism to early recovery of barrier function is unknown.
Gut barrier failure can be a life-threatening consequence of non-steroidal anti-inflammatory drug (NSAID) use and results in more than 100,000 hospitalizations per year at an estimated cost of $2 billion (17). Substantial information has been gathered concerning the protective attributes of endogenous PG and the role of PG depletion in the genesis of peptic ulcer disease in patients receiving NSAIDs. Considerably less is known about the effects of endogenous PG on barrier function of the small intestine. It appears that simple inhibition of cyclooxygenase is insufficient to cause functional or morphologic injury to small intestinal mucosa. However, in the presence of pre-existing inflammatory bowel disease or luminal aggressive factors such as bile and bacteria, NSAID therapy can be associated with increases in intestinal permeability and disease exacerbation (4,5,12,19,20,33). Accordingly, PG may mediate reparative effects in the small intestine. Nonetheless, little attention has been given to clarifying the local mechanisms of PG action in the small intestine or to determination of whether NSAID result in inadequate repair mechanisms.
We hypothesized that increased synthesis of endogenous PG plays a key role in repair of pre-existing small intestinal injury. Accordingly, we examined the local effects of PG on epithelial repair after deoxycholate-induced injury to porcine ileal mucosa mounted in Ussing chambers. The ileum is a common site for intestinal inflammation, bacterial deconjugation of bile acids, and exposure to high concentrations of enterohepatically recirculated NSAID. After deconjugation, bile acids contribute to injury by triggering increases in tight junction permeability and epithelial cell loss (14). Our in vitro model retains the physiologic complexity of interactions between the lamina propria and overlying epithelium while eliminating effects mediated by PG in vivo such as leukocyte adhesion, mucosal blood flow, and cell proliferation (16). Consequently, local mechanisms that affect villus contraction, epithelial cell migration and tight junction permeability can be integrated and their contributions to recovery of barrier function quantified.
Results of the present study show that endogenous PG, released upon mucosal injury, mediate local repair of small intestinal epithelium after damage by the deconjugated bile salt, deoxycholate. While ongoing restitution and villous contraction were prominent features of repairing mucosa, acute recovery of barrier function was uniquely dependent on PG-mediated resealing of tight junctions and lateral intercellular space. Failure to repair increases in paracellular pathway permeability may underlie barrier failure resulting from NSAID use in patients with underlying enteropathy.
METHODS
Animals
Six to 8-wk old Yorkshire crossbred pigs were fed a commercial pelleted diet and had free access to water. Pigs were sedated (xylazine 1.5 mg/kg and ketamine 11 mg/kg IM; Fort Dodge, Iowa) and then euthanatized by intravenous pentobarbital injection (Vortech Pharm., Dearborn, MI). Approximately 30 cm of the distal ileum was then removed for in vitro studies. All protocols were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Ussing Chamber Studies
Ileal mucosa was separated from the seromuscular layers in an oxygenated (95% O2 − 5% CO2) Ringer solution and mounted in 3.14-cm2 aperture Ussing Chambers. Tissues were bathed on both serosal and mucosal sides by a Ringer solution containing (mmol/L): Na+, 142; K+, 5; Ca2+, 1.25; Mg2+, 1.1; Cl−, 124; HCO3−, 25; HPO42−, 1.65; and H2PO4−, 0.3. The serosal solution contained 10 mM glucose and the mucosal solution contained 10 mM mannitol. Solutions were oxygenated (95% O2 − 5% CO2) and circulated in water-jacketed reservoirs maintained at 37°C. Electrical recordings were performed every 15–30-min and included short circuit current (Isc) and spontaneous potential difference (PD). PD was measured using Ringer-agar bridges connected to calomel electrodes. If the PD was between −1.0 and 1.0 mV, tissues were current clamped at ±100 µA for PD measurement. PD was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrects for fluid resistance (World Precision Instruments; Sarasota, FA). Transepithelial electrical resistance (TER; Ω·cm2) was calculated from the spontaneous or clamped PD and Isc using Ohms’ law.
Isotopic flux studies of epithelial permeability were performed using 3H-labeled mannitol (2 µCi; 6.6mM; Dupont; Boston, MA) or 3H-labeled inulin (2 µCi; 0.2mM; Dupont; Boston, MA). Isotope was added to the mucosal reservoir immediately after removal of deoxycholate (time = 30-min). For 3H-labeled mannitol, three successive 60-min flux periods (from 30 to 210-min) were performed by taking paired samples from the serosal reservoir. For 3H-labeled inulin, five successive 30-min flux periods (from 60 to 210-min) were similarly performed. Samples were counted for 3H in a liquid scintillation counter (LKB Wallac; Turku, Finland). Flux of mannitol or inulin from mucosa to serosa was calculated using standard equations.
In Vitro Mucosal Injury
All tissues were allowed to equilibrate in the Ussing chamber for 15-min (time 0–15-min). Deoxycholate (6 mM; Sigma; St. Louis, MO) was then added to the mucosal reservoir for a duration of 15-min (time 15–30-min). Deoxycholate was removed and replaced by Ringer solution and tissue recovery was assessed over a 3-hr period (30–210-min). The 210-min length of study was chosen based on declining viability of uninjured ileum within the Ussing chamber after this time period.
To standardize the severity of injury, tissues having TER <16 or >28 Ω·cm2 immediately after removal of deoxycholate were discarded from analysis. This injury results in a uniform degree of epithelial damage which is partially repaired during the 3-hr recovery period. Details of our experimental model of acute mucosal injury and repair have been published previously (16). For each pig, uninjured and injured but untreated tissues were included in all experimental designs.
Morphometric Analyses
Tissues were removed from the Ussing chamber after specified time periods. A sample from each Ussing chamber was fixed in formalin, sectioned at 5 µm thickness, and stained with hematoxylin and eosin. Three sections from each tissue were examined without knowledge of prior treatment. Using an ocular micrometer and light microscope, the following measurements were recorded for 5 well-oriented villi and then averaged; 1) villus height measured from the crypt opening to villus tip, 2) crypt depth, 3) villus width at midpoint, 4) linear length of villus perimeter, and 5) linear length of denuded villus. To account for reductions in villus height and surface area that result from stripping and mounting of mucosa (24), measurements were compared to Ussing-chambered, but uninjured tissue, fixed at matching time points.
Villus surface area was calculated using a modified formula for calculating the surface area of a cylinder (3). The formula was modified by subtracting the area of each base of the cylinder, adding a factor accounting for the hemispherical shape of the apical villus, and multiplying by a factor accounting for the variable position at which each villus is frontally sectioned (22) as follows: villus surface area = (2π • 1/2[(4/π)d] h), where π = 3.14, d = villus diameter (width) at midpoint, and h = villus height.
Determination of the relative contributions of villi and crypts to total luminal surface area was performed as described by Collete et al for rat ileal mucosa (10). Briefly, paraffin-embedded tissues were sequentially sectioned in a transverse orientation every 20 µm beginning at the base of the crypts and extending to the villus tips. The crypt surface length was measured in each section from the base of the crypt to its opening at the surface. Surface length of villi were measured from the opening of the crypt to the tip of the villus. Measurements were made using a computer software package (Image-Pro® Plus, Media Cybernetics; Silver Spring, MD). Relative contributions of crypts and villi to total surface area were calculated as follows:
D = distance between sections (20µm)
lc = length of lumen of crypt
lv = length of periphery of villi
Transmission Electron Microscopy
To examine the ultrastructural effect of endogenous prostaglandins on intercellular space anatomy of deoxycholate-injured porcine ileum, tissues were removed after 210-min in the Ussing chamber and placed in Trump’s 4F:1G fixative at 4°C. Samples were processed for transmission electron microscopy using standard techniques (11).
Immunofluorescence Microscopy
Tissues were removed from the Ussing chamber after 210-min, embedded in optimal cutting temperature medium, and frozen-sectioned at 5 µm thickness. Epitope retrieval was performed by boiling the slide-mounted tissue sections in citrate buffer (pH=6) for 10-min. Sections were blocked with 2% BSA prior to incubation with rabbit anti-ZO-1 polyclonal antibody (1:250 in BLOTTO) or isotype control for rabbit primary antibody (Zymed Laboratories; San Francisco, CA) for 2-hrs at room temperature. After rinsing in BLOTTO, sections were incubated with goat anti-rabbit IgG Cy3 conjugate (1:300 in BLOTTO with 2% BSA; Zymed) for 45-minutes in the dark. Sections were rinsed in BLOTTO and PBS, counterstained with DAPI (50 µg/ml; Santa Cruz Biotechnology; Santa Cruz, CA) and coverslipped with aqueous media containing 2.5% DABCO (Sigma; St. Louis, MO). Sections were stored at −20°C. Well-oriented villi were examined using an immunofluorescence microscope.
Eicosanoid Analyses
For eicosanoid analyses, paired samples were taken from the serosal reservoir, gassed with N2, and frozen in liquid N2. Samples were stored at −20°C prior to assay. Samples were analyzed for concentration of PGE and 6-keto-PGF1α (the stable metabolite of PGI2) using commercial ELISA kits according to manufacturer instructions (Biomedical Technologies Inc.; Stoughton, MA).
Assessment of PG Effects on Epithelial Repair
Unless stated otherwise, treatments were added to tissues immediately after deoxycholate removal from the mucosal reservoir (time = 30-min). Treatments included 16,16-dimethyl-PGE2 (Sigma; St. Louis, MO) and the PGI2 analogue carbacyclin (Sigma; St. Louis, MO) (each 10−6 M serosal), and indomethacin (5×10−6 M mucosal and serosal). 16,16-dimethyl-PGE2 and carbacyclin were examined in combination based on prior studies demonstrating their synergistic action on recovery of barrier function after ischemic injury of porcine ileum (6) and increases in endogenous synthesis of both of these eicosanoids after deoxycholate injury in the present model.
Statistical Analysis
Data are reported as mean ± SE. For all analyses, P < 0.05 was considered significant. One-way or repeated-measures ANOVA and a post hoc Tukey’s test were used to compare differences between treatment and control tissues. Pearson correlation coefficient was used to examine relationships between TER and morphologic measurements under different treatment conditions. Number of pigs receiving treatment = n.
RESULTS
Local mechanisms of small intestinal repair
Addition of deoxycholate (6 mM for 15-min) to the luminal bathing solution of ileal mucosa mounted in Ussing chambers resulted in an acute decline in transepithelial electrical resistance (TER) and increased 3H-labeled mannitol permeability. After replacement of the deoxycholate with fresh Ringer’s solution, there was a gradual and significant recovery of barrier function over a 180-min time interval of repair (Figure 1).
Figure 1.
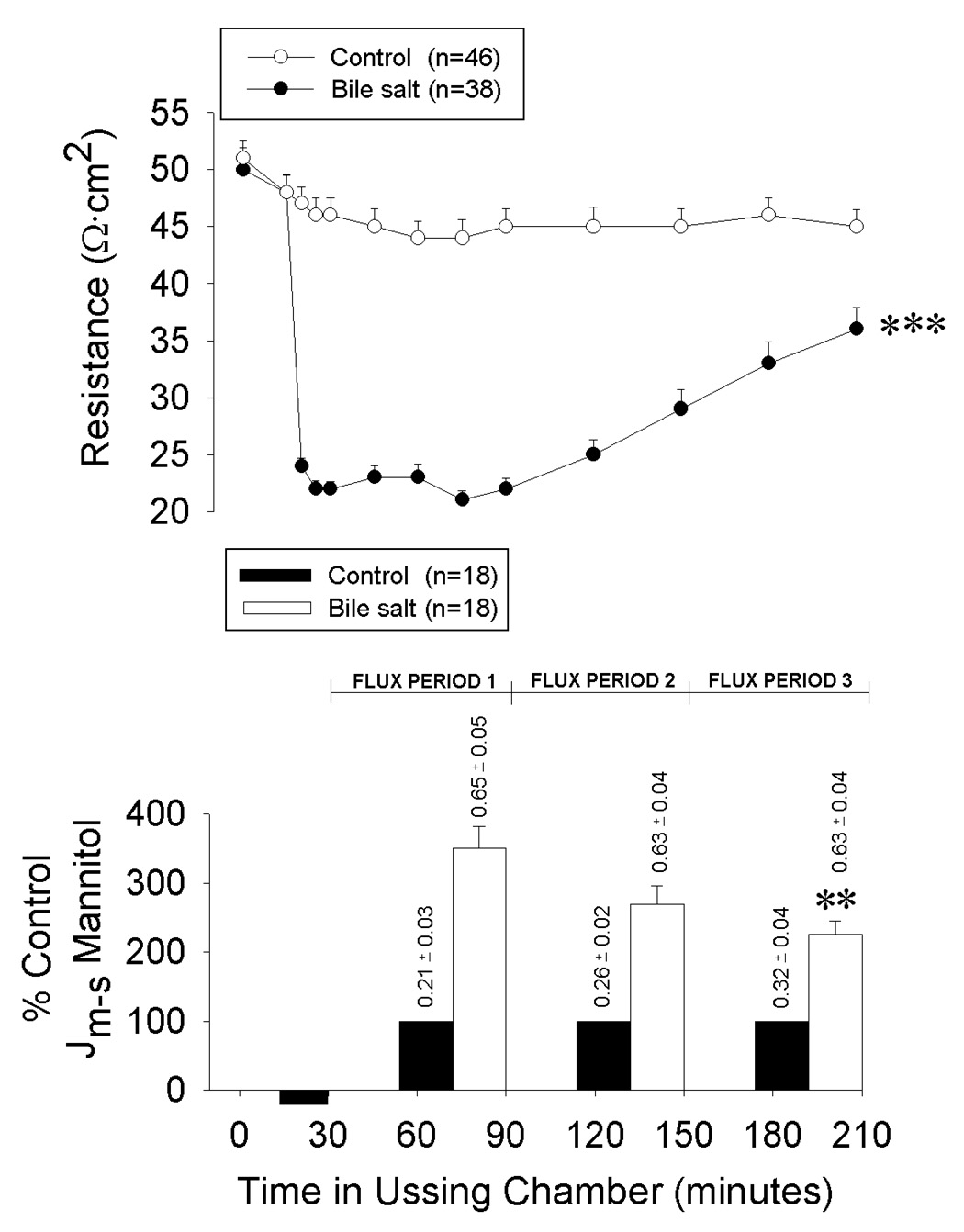
Transepithelial electrical resistance and mucosal-to-serosal flux (Jm→s) of 3H-labeled mannitol across porcine ileal mucosa mounted in Ussing chambers. Mucosae were acclimated for 15-min followed by a 15-min luminal exposure to 6 mM deoxycholate (black bar). Deoxycholate was then replaced by fresh Ringer’s solution (time = 30-min) and recovery of resistance and permeability were recorded over the following 180-min period (✱✱✱p<0.001; two-way repeated measures ANOVA)(✱✱ p<0.01 compared to injured mucosa at flux period 1 [values above bars indicate raw data]; one-way ANOVA). Values represent mean ± SE; n = number of pigs.
To determine the mechanisms responsible for recovery of barrier function, restitution was quantified by measuring the surface length of denuded and re-epithelialized villus perimeters using an ocular micrometer. Both injured and uninjured tissues were examined after 0, 30, 45, 75, 150 and 210-min in the Ussing chamber (Figure 2A). Immediately after replacement of deoxycholate with fresh Ringer’s solution (time = 30-min), there was detachment of enterocytes from the villus tips. Epithelial cell losses continued until time = 75-min. From 75 to 210-min there was ongoing restitution by migrating flattened to cuboidal absorptive cells and a significant decrease in denuded villus surface area (24,951 ± 5,480 µm2 at 75-min; 10,350 ± 2,702 µm2 at 210-min; n = 8 each; *p<0.05; one-way ANOVA) (Figure 2B). Uninjured tissue remained covered by confluent epithelium for over 210-min if left unperturbed within the Ussing chamber.
Figure 2.
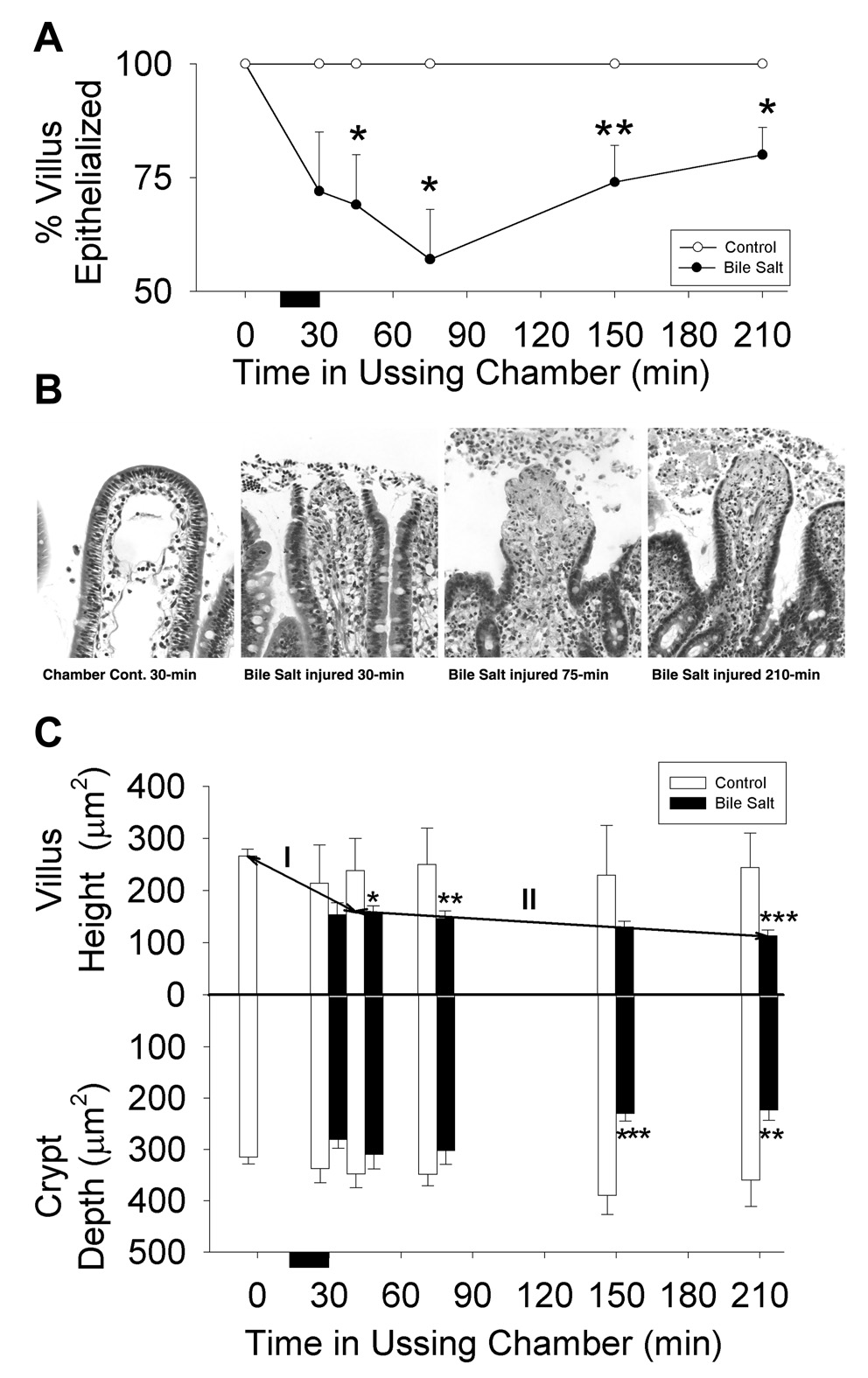
Restitution and villous contraction of deoxycholate injured porcine ileal mucosa mounted in Ussing chambers. (A) Percent villous epithelialization of mucosae removed from the Ussing chamber at specified time periods after deoxycholate injury. Bars represent mean ± SE; n = 4 pigs at 45 and 150-min; n = 8 pigs at 0, 75, and 210-min. (✱p<0.05,✱✱ p<0.01 –vs- time-matched, Ussing-chambered and uninjured control tissue (n = 4 each) (one-way ANOVA). (B) Histologic appearance of villi after removal from the Ussing chamber. Commensurate with deoxycholate injury (t = 30-min) there were epithelial losses from the tips of villi. Epithelial losses continued for the first 45-min of recovery culminating in peak injury at time = 75-min. Between 75 and 210-min there was partial restitution of the injured villi by flattened to cuboidal migrating enterocytes. Chamber-mounted, uninjured control tissue maintained epithelial continuity throughout the study period (chamber control). Magnification, 314X. (C) Villus and crypt measurements performed at specified time periods after deoxycholate injury. Bars represent mean ± SE. For injured tissue, n = 4 pigs at 45 and 150-min; n = 8 pigs at 30, 75, and 210-min. ✱ p<0.05, ✱✱ p<0.01, ✱✱✱ p<0.001–vs- time-matched, Ussing-chambered and uninjured control tissue (n = 18 at 0-min and n = 4 at each subsequent time period)(one-way ANOVA). There was a significant decrease in villous height of injured tissue between 45 and 210-min (II), ✱ p<0.05 (one-way ANOVA).
Villous contraction was quantified by measurements of villous height taken from injured and uninjured tissues after 0, 30, 45, 75, 150, and 210-min in the Ussing chamber. Deoxycholate injury and repair were associated with a rapid, followed by a gradual phase of villous contraction (Figure 2C). Initial contraction (I; 0–45-min) was acute and inclusive of deoxycholate injury. Subsequent contraction (II; 45–210-min) was ongoing throughout the repair period. Significant crypt contraction also accompanied mucosal repair. Morphometric analysis of injured tissue at 210-min revealed a villous to crypt surface area ratio of 1:1.8 indicating that the epithelialized crypts were responsible for the majority of the total mucosal surface area. Uninjured tissue retained 99.3 ± 13% of initial (post-stripping) villus height, and 101.7±9.5% of initial crypt depth, for over 210-min if left unperturbed within the chamber (n = 4).
Endogenous prostaglandins promote recovery of barrier function
Local synthesis of endogenous prostaglandins (PG) increased significantly after acute deoxycholate injury (PGE: control = 4,410 ± 1,667; injured = 10,218 ± 3,862 pg/ml and PGI2 [6-keto-PGF1α]: control = 16,769 ± 6,338; injured = 24,714 ± 9,341 pg/ml). Endogenous PG synthesis was inhibited by addition of a non-selective cyclooxygenase blocker immediately after injury (indomethacin [INDO]; 5×10−6M) (PGE = 496 ± 187; 6-keto-PGF1α = 1,726 ± 653 pg/ml) (n = 7 each at 210-min; ***p<0.001, Student’s paired t test).
To determine the role of increased PG synthesis in recovery of barrier function, INDO was added to the serosal and mucosal bathing solutions immediately after injury and prior to onset of repair (t = 30-min). Indomethacin abolished recovery of TER (Figure 3A) and increased permeability of the mucosa to 3H-labeled inulin (control = 1.47 ± 0.13×103 µmol/cm2.hr; injured = 3.04 ± 0.38×103 µmol/cm2.hr; injured + INDO = 4.2 ± 0.28 µmol/cm2.hr at 210-min, n = 7 each; *p<0.05, repeated measures ANOVA). Recognizing the dependence on endogenous PG for recovery of barrier function, we determined whether additional PG could augment repair. Addition of exogenous PG (PGE2 [10−6M] and carbacyclin [10−6M; an analogue of PGI2]) to the serosal bathing solution immediately after injury promoted an earlier increase in TER and decreased permeability to 3H-labeled inulin (control = 1.3 ± 0.11×103 µmol/cm2.hr; injured = 3.04 ± 0.38×103 µmol/cm2.hr; PG = 2.5 ± 0.23 µmol/cm2.hr @ 210-min, n = 9 each; *p<0.05, repeated measures ANOVA) (Figure 3A). Noteably, TER at the end of the 180-min repair period was not different between tissues having endogenous versus exogenous PG exposure. These findings suggested that endogenous PG synthesis was sufficient to mediate the maximal repair response observed at 210-min.
Figure 3.
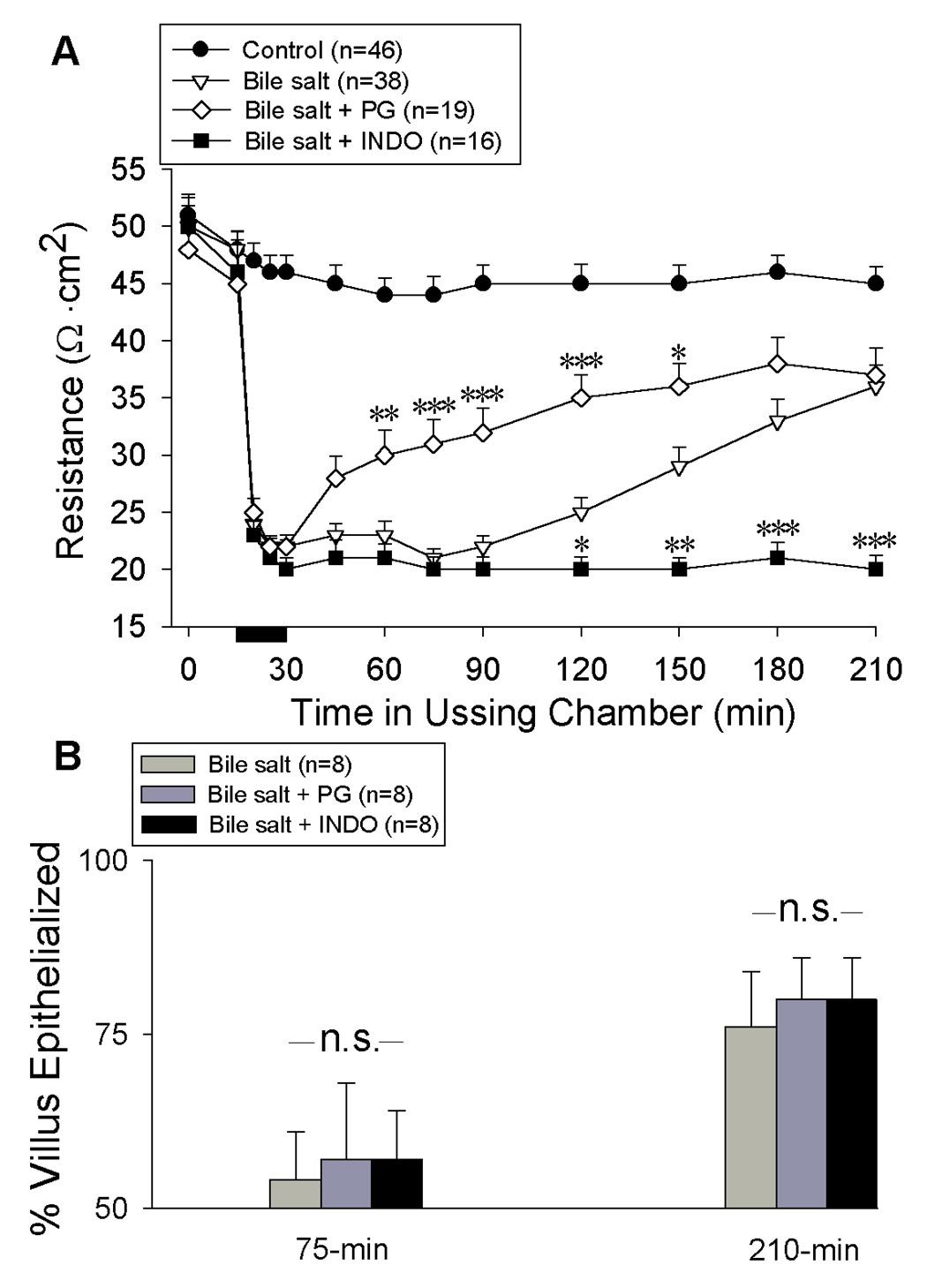
Recovery of transepithelial electrical resistance (A) and restitution (B) of Ussing chambered porcine ileal mucosa after deoxycholate injury and treatment with exogenous prostaglandins (PG) or indomethacin (INDO). PGE2 (10−6 M) and prostacyclin (10−6 M) or INDO (5×10−6M) were added immediately after injury and prior to onset of repair (time = 30-min). (A) INDO abolished recovery of TER while addition of exogenous PG resulted in an early elevation in TER that did not exceed baseline recovery at 210-min (✱ p<0.05 ✱✱ p<0.01 and ✱✱✱ p<0.001 –vs- injured control; one-way ANOVA). (B) Percent epithelialization of villi are shown at timepoints corresponding to maximal effects of exogenous PG (75-min) and INDO (210-min) on recovery of TER. Values represent mean ± SE; n= number of pigs; n.s. = not statistically significant.
Restitution rate is independent of prostaglandin synthesis
In migration assays of wounded epithelial monolayers, PG appear to either mediate the action of growth factors (36) or trigger the downstream expression of growth factors that promote cell migration (35). To determine if enhanced restitution was responsible for PG-mediated recovery of barrier function in the present model, surface re-epithelialization was quantified in untreated, INDO and PG-treated tissues at 75 and 210-min to examine the basis for differences in TER at these time points (Figure 3B). Neither INDO or PG had any effect on percent re-epithelialization of villi. However, in the presence of endogenous PG, TER appeared to relate positively with measures of epithelial restitution (including denuded villous surface length, calculated denuded villous surface area, and % villous re-epithelialization; p=0.10) while in the absence of PG, no relationship between TER and restitution could be demonstrated (Figure 4). Thus, we could not attribute the effect of PG on TER to an underlying difference in restitution rate.
Figure 4.
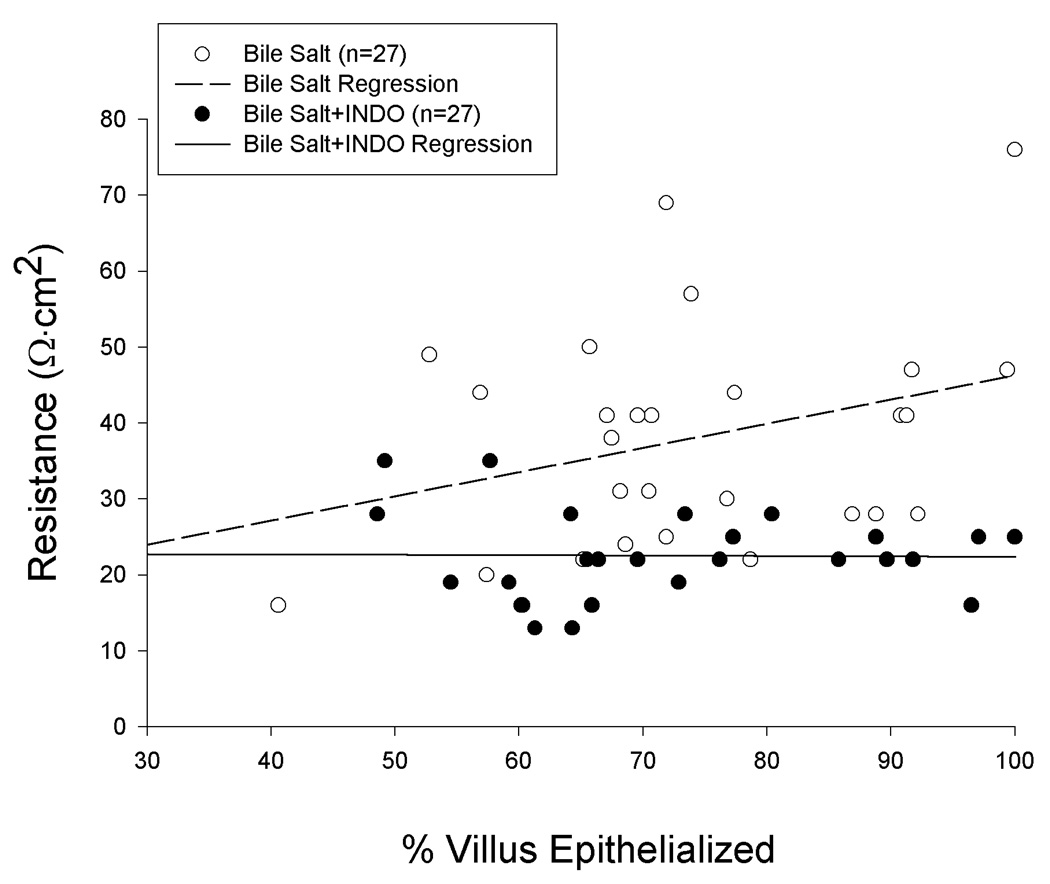
Transepithelial electrical resistance and degree of restitution attained by porcine ileal mucosa at peak repair (time = 210-min) after injury by deoxycholate in Ussing chambers. Although restitution was equivalent in the presence or absence of INDO (5 × 10−6M), there was no correlation between TER and restitution among mucosae treated with INDO (p>0.10; Pearson correlation coefficient).
Endogenous prostaglandins mediate villus and crypt contraction
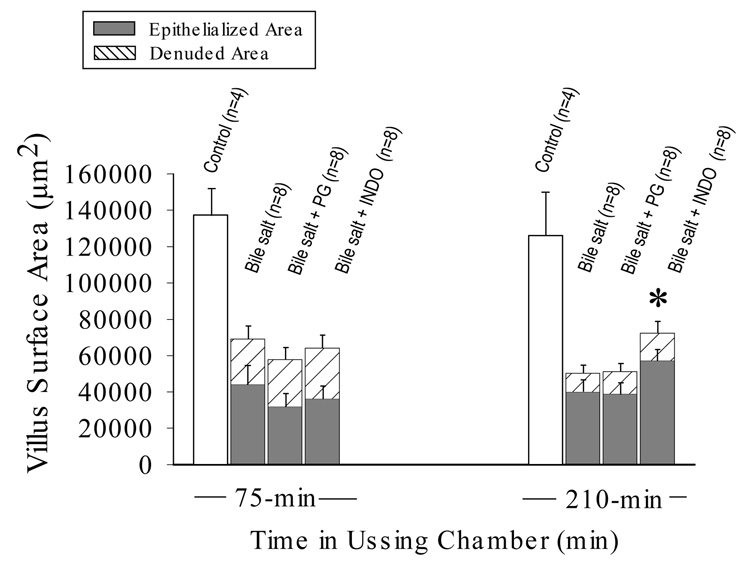
Prior in-vivo studies have demonstrated that PG mediate a modest villous contraction that is insufficient in magnitude to hasten re-epithelialization (13). However, because TER and isotopic permeability in-vitro are expressed per unit of serosal surface area (i.e. in reference to the luminal aperture of the Ussing chamber) we sought to examine whether villous contraction was responsible for PG-dependent increases in TER, perhaps by condensing the surface area of the paracellular pathway within the remaining epithelium. Addition of INDO immediately after deoxycholate injury significantly inhibited contraction of both villi and crypts seen during the repair phase. The resulting villi had a greater total surface area involving a proportionate increase in both the denuded and epithelialized surface (Figure 5). Villous contraction was not promoted by the addition of exogenous PG after injury. To determine whether PG additionally mediated the phase of villous contraction seen concurrent with deoxycholate injury, we treated tissues with INDO prior to injury and measured the height of villi immediately after removal of deoxycholate from the Ussing chamber. In the presence of INDO, the initial contractile phase of villi was not inhibited (villous height @ 30-min; injured = 153.3 ± 22.8 µm; n = 8; injured + INDO = 97.4 ± 9.2 µm; n = 7). The difference in TER observed between tissues treated with and without INDO was not related to any measure of mucosal surface amplification (including villus height, villus width, crypt depth, total villous surface length or calculated total villous surface area; p>0.10). Thus, PG-mediated effects on TER could not be attributed to stimulation of villous or crypt contraction.
Figure 5.
Relative contributions of epithelialized and denuded villus to total villous surface area of deoxycholate-injured mucosae at peak injury (time = 75-min) and maximal repair (time = 210-min). Indomethacin (INDO; 5×10−6M) arrested villous contraction during repair, resulting in a significant increase in total villous surface area compared to injured control or prostaglandin-treated (PG) tissue at 210-min (✱ p<0.05; one-way ANOVA). Restitution (% villous epithelialization) was unaffected by either treatment. Values represent mean ± SE; n = number of pigs.
Contribution of restitution to recovery of epithelial barrier function
We were surprised to observe that in the presence of INDO there was no recovery of TER despite an underlying 73% re-epithelialization of the villi by 210-min. To determine whether a greater degree of epithelialization could promote recovery of TER in the presence of INDO, we stimulated restitution after injury by treating Ussing chambered mucosa with L-arginine (ARG; 5 mM) and fetal bovine serum (FBS; 1%) as previously described (16). In the presence of INDO, ARG+FBS stimulated re-epithelialization of villi from 73 ± 3% to 90 ± 2% despite the greater surface area resulting from concurrent arrest of villous contraction (Figure 6 A,B). Even with restitution over a range of 49–100% of the villus surface, recovery of TER and decreases in 3H-labeled mannitol permeability were not observed in the absence of endogenous PG synthesis (Figure 6 C,D).
Figure 6.
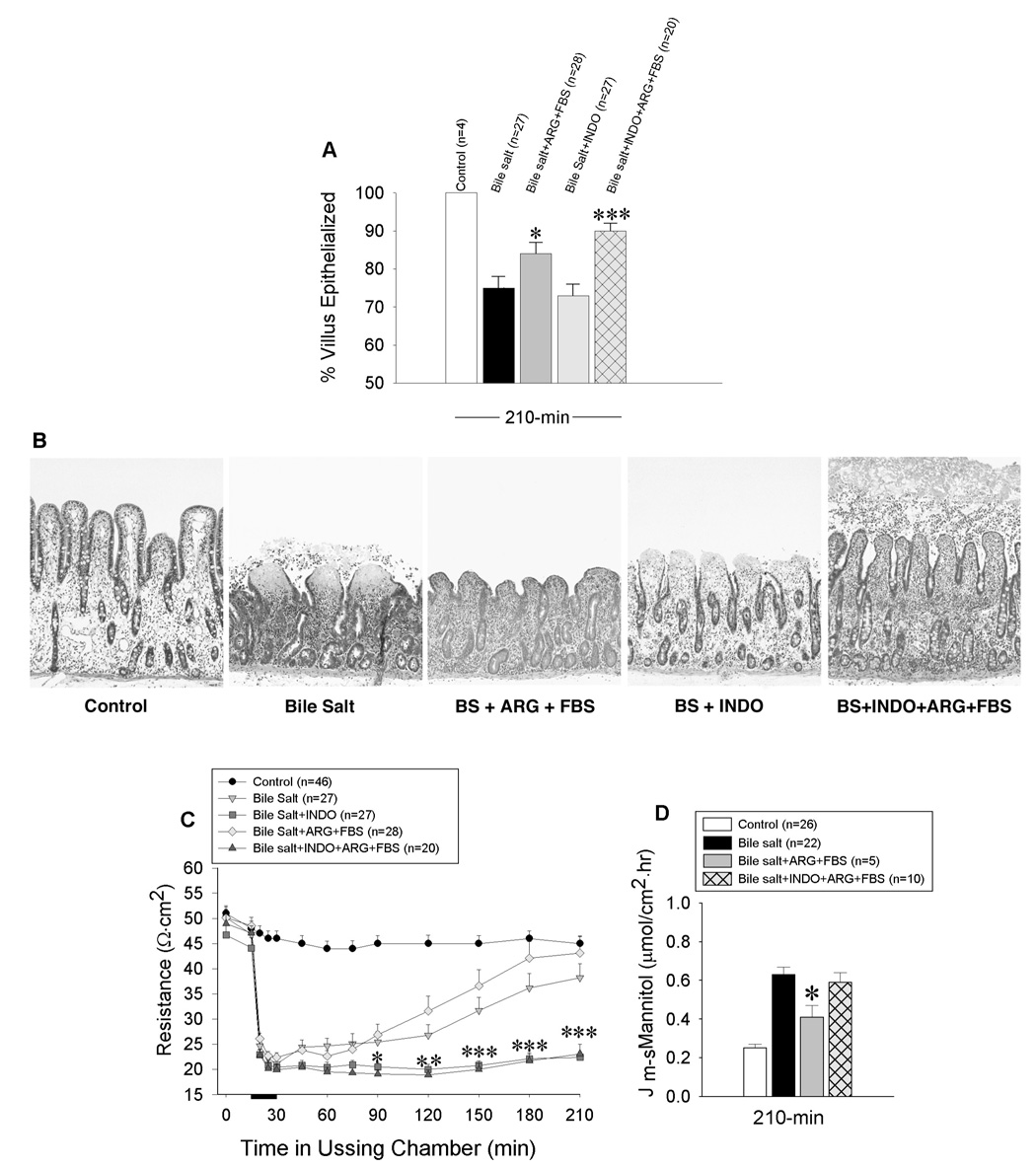
Effects of enhanced restitution on recovery of barrier function after deoxycholate injury to porcine ileal mucosa mounted in Ussing chambers. Restitution was stimulated by addition of L-arginine (ARG; 5 mM) and fetal bovine serum (FBS; 1%) to the serosal and mucosal reservoir immediately after injury and prior to onset of repair. Results are shown for mucosae at peak repair (A, B). Indomethacin (INDO; 5×10−6M) did not attenuate baseline or stimulated restitution despite arrest of villus contraction during recovery. Magnification, 250X. Barrier function, as determined by measures of transepithelial electrical resistance (TER)(C) and mucosal to serosal flux of 3H-mannitol (Jm→s) (D), was abolished in the absence of endogenous prostaglandin (PG) synthesis, despite nearly complete restitution of villi. (✱p<0.05, ✱✱p<0.01, ✱✱✱p<0.001; one-way ANOVA). Values represent mean ± SE; n = number of pigs. Bar = bile salt exposure.
Endogenous prostaglandins promote recovery of paracellular permeability
Because PG-dependent recovery of barrier function (210-min; Figure 3A) could not be accredited to effects on restitution rate or villous contraction, we surmised that PG exerted their effects on the paracellular pathway of the epithelium remaining after deoxycholate injury. To examine whether such a mechanism was reparative or could arise simply from effects of PG on normal epithelium, we examined the effect of INDO and exogenous PG on TER and 3H-labeled mannitol permeability of uninjured mucosa mounted in Ussing chambers. Neither INDO or exogenous PG had any effect on TER or permeability when left in contact with uninjured mucosa for over 210-min (control = 57 ± 3.6 Ω.cm2 and 0.14 ± 0.02 µmol/cm2.hr; PG = 54 ± 4.1Ω.cm2 and 0.15 ± 0.02 µmol/cm2.hr; INDO = 54 ± 4.3Ω.cm2 and 0.15 ± 0.01 µmol/cm2.hr at 210-min, n = 8 each). These results suggest that PG augment recovery of leaky epithelium remaining after acute mucosal injury.
To determine whether a change in paracellular permeability alone was capable of influencing TER by magnitudes similar to that mediated by endogenous PG, we evaluated the effect of osmotic gradient-driven paracellular collapse on recovery of TER after deoxycholate injury. When urea (150 and 300 mOsm) was added to the mucosal reservoir immediately after injury and in the presence of INDO, TER increased to values comparable to that mediated by endogenous PG. Conversely, addition of urea to the serosal reservoir in the presence of PG did not attenuate recovery of TER (Figure 7). The asymmetric responsiveness of TER to osmotic gradients is a reported characteristic of bile salt-induced increases in tight junction permeability and has been attributed to enhanced conductance of the lateral intercellular spaces (14). Because prior studies have shown that PG-mediated tight junction closure is dependent, in part, on Cl− secretion, we additionally examined the effect of the Cl− secretion blocker bumetanide (10−4 M) on recovery of TER after deoxycholate injury. Bumetanide significantly inhibited recovery of TER in the presence of endogenous PG and without having effects on concurrent restitution or villous contraction (control = 57 ± 3.3; injured = 40 ± 3; injured + BUM = 29 ± 3.8 Ω.cm2; *p<0.05, Student’s paired t test).
Figure 7.
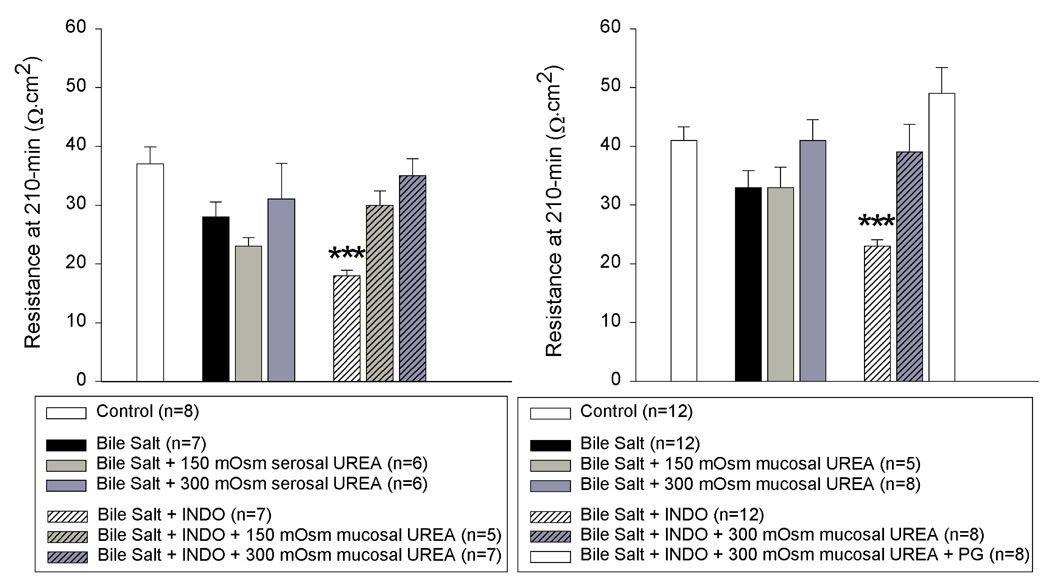
Transepithelial electrical resistance (TER) of porcine ileal mucosa at peak repair (time = 210-min) after injury by deoxycholate in Ussing chambers. A mucosal to serosal osmotic gradient of urea did not attenuate PG-mediated recovery of TER. Conversely, a serosal to mucosal osmotic gradient of urea could reproduce the effect of endogenous PG on recovery of TER after deoxycholate injury. Values represent mean ± SE; n = number of pigs. (✱✱✱p<0.001; one-way ANOVA).
We next sought to examine the effect of PG on the ultrastructural appearance of the tight junction and intercellular space of resident crypt and restituting epithelial cells at peak repair after deoxycholate injury (time = 210-min) (Figure 8). In the presence of PG, the lateral membranes of crypt epithelial cells were tightly apposed. Epithelial cells migrating along the villus remained contiguous, both apically and basally, and were closely adherent to the underlying basement membrane. In tissues treated with INDO however, crypt epithelium showed dilation of the lateral intercellular space below the tight junction. Epithelial cells migrating along the villus maintained only an apical attachment to neighboring cells with expansive separation of the lateral membranes and loss of contact with the basement membrane.
Figure 8.
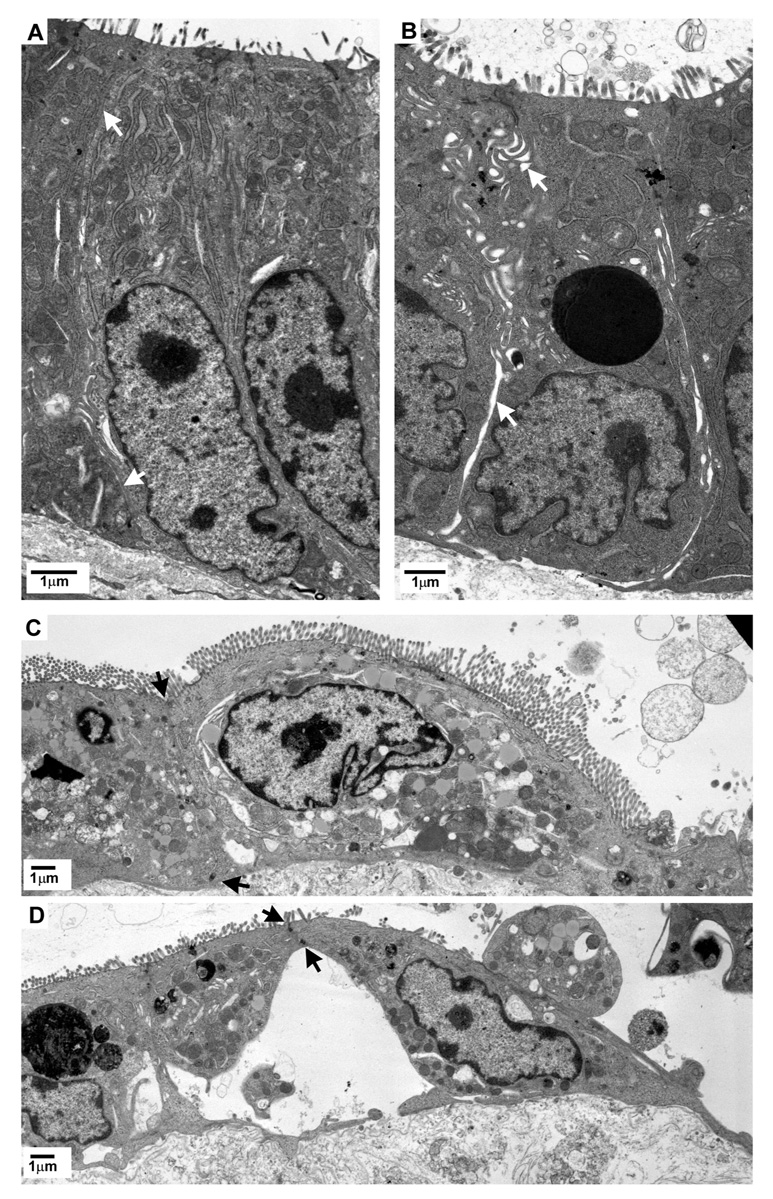
Transmission electron micrographs of crypt (A&B) and migrating villous enterocytes (C&D) from porcine ileal mucosa at peak repair after deoxycholate injury (time = 210-min). Mucosae shown in B & D were treated with indomethacin (INDO; 5×10−6M) immediately after injury and prior to onset of repair. White arrows indicate intercellular space. Black arrows indicate sites of lateral membrane attachment between adjacent, migrating enterocytes.
To determine if tight junction organization of the restituting villous epithelium was specifically altered in response to inhibition of PG synthesis, immunofluorescence microscopy for ZO-1 was performed using tissues obtained at peak repair (Figure 9). In uninjured mucosa, ZO-1 was circumferentially localized along the periapical tight junctions. At peak repair, restituting epithelium showed continuous periapical ZO-1 labeling along the sides of the villi that became localized to junctional foci as cells dispersed along the villus tip. In contrast, mucosa treated with INDO revealed ZO-1 labeling with relatively diminished intensity and continuity.
Figure 9.
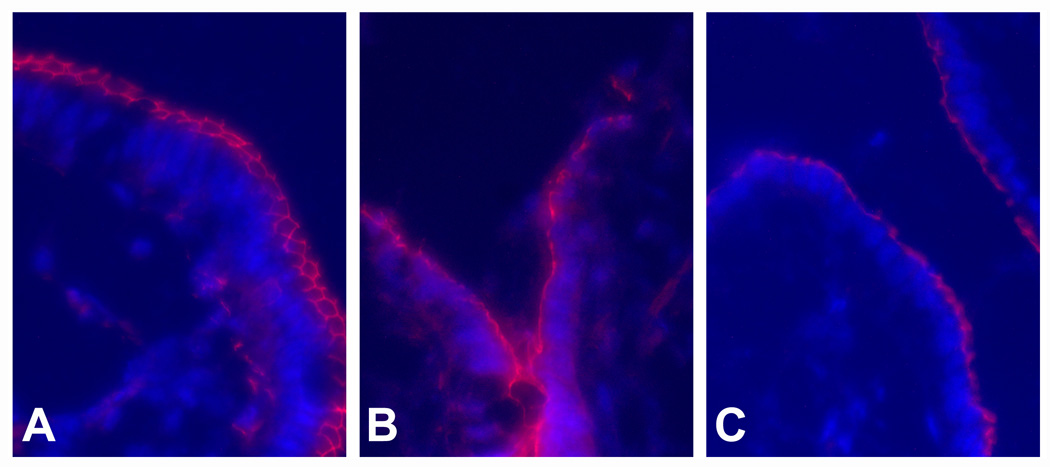
Immunofluorescence localization of the junctional complex protein ZO-1 in porcine ileal mucosa. (A) Uninjured villous mucosa. (B) Villous mucosa at peak repair after deoxycholate injury (time = 210-min). (C) Villous mucosa treated with indomethacin (INDO; 5×10−6M) immediately after deoxycholate injury and examined at peak repair (time = 210-min). Magnification, 314X.
DISCUSSION
Restitution is considered to be a major determinant of early recovery of gastrointestinal barrier function following an acute injury. This conclusion derives from numerous in vitro studies demonstrating that barrier function recovers in parallel with morphologic restitution after acute mucosal injury (27,31,34). Consequently, studies of gastrointestinal repair using intact mucosa have largely been replaced by migration assays of wounded epithelial monolayers (8,25,28,36). Regretably, these latter studies fail to account for the simultaneous roles played by villous contraction and tight junction permeability in recovery of small intestinal barrier function. For the present report we performed a detailed study of the independent and integrated contributions of restitution, villous contraction and paracellular permeability to early recovery of barrier function using an in vitro model of acute small intestinal mucosal repair. Results of these studies are the first to demonstrate that restoration of normal paracellular resistance and not restitution or villous contraction was the primary determinant in PG-mediated early recovery of barrier function.
This conclusion is based on the following observations. First, while recovery of TER over time was paralleled by ongoing restitution as seen in prior studies, villous re-epithelialization over a range of 49–100% was incapable of promoting recovery of barrier function in the absence of PG. Second, recovery of TER was not related to a decrease in total mucosal surface area. Third, in the absence of PG, independent manipulation of paracellular permeability with osmotic pressure gradients reproduced the TER measurements obtained in the presence of PG and without concurrent effects on restitution or villous contraction. Finally, paracellular space closure was mediated by an increase in endogenous PG synthesis after injury. Our finding that PG had no effect on restitution has been observed in other acute studies of intact mucosa (2,7,12). These results contrast those of PG effects on cell migration of wounded epithelial monolayers and may relate to the time required for induction of growth factor synthesis, which exceeds the duration of studies of early mucosal repair (< 4 hrs).
Nevertheless, it is surprising that unimpeded restitution contributed little to early recovery of barrier function or conversely how any recovery of TER was possible in the presence of frank epithelial denudation. Deliberation of these issues necessitates considering the known barrier attributes of simple intestinal epithelia. Transepithelial electrical resistance represents the competence of the epithelium to defend itself against the passive permeation of ions. As such, TER is considered to be the most sensitive measure of epithelial barrier function. There exists two parallel routes by which ions can passively traverse the epithelium. The transcellular pathway entails crossing both the apical and basal plasma membranes of the epithelial cell. The paracellular pathway involves passing through the tight junction and lateral intercellular space (9,23,24,30). Epithelial cell membranes have a high resistance to passive ion flow (1,000 to 10,000 Ω.cm2) while the overall resistance (TER) of mammalian small intestinal epithelium is only 21 to 100 Ω.cm2 (30). Accordingly, the major determinant of TER is the integrity of the paracellular pathway which has been calculated to account for 75–94% of the total passive ion flow across small intestinal epithelium (15,29,32). Thus, agents that affect transcellular resistance have little relative effect on TER of these so-called “leaky” epithelia.
An important limitation of TER measurements are their inability to distinguish between changes in tight junction permeability and the presence of epithelial cell loss, both of which contribute to the overall surface area of low-resistance paracellular pathway. Thus, recovery of TER after acute intestinal injury cannot be attributed to restitution alone without considering the possibility of simultaneous alterations in tight junction permeability; an assessment that requires a detailed dissection of the underlying mechanisms. Further, if early recovery of TER is to be attributed to resealing of tight junctions more so than to restitution, the injury must result in a greater increase in surface area of the tight junctional pathway than the area of denuded epithelium created at the apical villus. The latter is a feasible consideration because 63–73% of the total paracellular conductance of uninjured small intestinal epithelium is via a high linear density of tight junctions residing in the crypts (9,10,24) and following villous contraction, crypt epithelium accounts for the majority of surface area remaining after acute mucosal injury. In order to determine if changes in tight junction permeability could theoretically account for the magnitude of TER observed in our model, we approximated total tight junction surface area using Marcial’s measurements of 0.2180 µm paracellular pathway/µm2 surface area of villus and 0.7680 µm paracellular pathway/µm2 surface area of crypt (24)(Appendix 1). Given a total denuded and epithelialized villous surface area of 3.5 × 108 µm2 and 1.2 × 109 µm2 (i.e. 75% re-epithelialized) and total crypt surface area of 2.75 × 109 µm2 (64% of the total mucosal surface area) and the theoretical range in width of the tight junction (0.02 – 0.2 µm)(9), yields a range of tight junction surface area from 12 – 58% of the total surface area of low-resistance pathway (tight junction + denuded area). The 2.08-fold difference in total paracellular pathway resulting from changes in tight junction permeability well exceeds the 1.68-fold difference in TER measured in the presence versus absence of endogenous PG synthesis. Accordingly, a PG-induced change in TER due exclusively to closure of the tight junction pathway is a feasible consideration.
Failure to close the intercellular spaces created by migration of epithelium can additionally explain why restitution was incapable of promoting recovery of barrier function in the absence of PG. The intercellular space represents a series component in the pathway of paracellular resistance. Resistance of the intercellular space (Ri) is given by the equation Ri = ρL/ωlp, where ρ = resistivity of the bulk solution, L = length of the interspace, ω = width of the interspace, and lp = linear amount of paracellular pathway/cm2 epithelium (9). Under conditions of tight apposition of the lateral membranes (ω = 0.02 µm; (9)) approximations of intercellular space resistance may exceed the specific tight junction resistance depending on the length of the intercellular space. The latter measurement is difficult to accurately estimate due to extensive folding of the lateral membranes lining the intercellular space (Figure 8). However, once the intercellular space exceeds the physiologic range in width of the tight junction (0.02 – 0.2 µm)(9), Ri approaches zero. Thus, a PG-induced closure of intercellular space between resident and in particular, restituting epithelial cells is likely to have also contributed to recovery of TER after deoxycholate injury.
Although we did not conduct a detailed investigation of the mechanism underlying the paracellular effects of PG, prior studies of PG action after ischemic injury of porcine ileum have demonstrated that PGE and PGI2 act synergistically to promote an osmotic-driven collapse of the tight junction and lateral intercellular space (6). The initiating event appears to involve stimulation of Cl− secretion that promotes withdrawal of Na+ and water from the paracellular spaces of the crypt (7). Concurrently, PG inhibit neutral NaCl absorption resulting in decreased paracellular water absorption by the villus. Specific alterations by PG in tight junction structure are suggested by disruption of ZO-1 and occludin localization to the periapical tight junction in ischemia-injured tissues treated with indomethacin (21). Similar mechanisms are likely operative in the present model insofar as synthesis of both PGE and PGI2 were increased by deoxycholate injury, barrier function could be rescued in the absence of PG synthesis by application of a mucosal osmotic gradient, and PG-mediated recovery was partially inhibited by blockade of Cl− transport. Because localization of ZO-1 to periapical tight junctions was diminished after treatment of restituting mucosa with indomethacin, it is likely that a component of the action of PG on paracellular permeability in the present model involves restoration of normal tight junction structure.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (DK02868 and DK34987 to JLG) and the United States Department of Agriculture (9702239 to RAA). The authors thank Martha Armstrong, Marjory Gray, and Monica Mattmuller for excellent technical assistance; Dr. Michael Dykstra and Laura Reuss from the Laboratory for Advanced Electron and Light Optical Methods, North Carolina State University, College of Veterinary Medicine for assistance with transmission electron microscopy; and the NIH-Center for Gastrointestinal Biology and Disease Immunotechnology Core for performance of eicosanoid assays.
Appendix 1
Calculation of Villus and Crypt Surface Areas
Total villous surface area was calculated using the measured values of 1) average surface area of each villus, 2) number of villi per unit serosal surface and 3) size of the Ussing chamber aperture (314 mm2). The area of epithelialized and denuded villous surface were derived based on determination that villi were 75% re-epithelialized at 210-min.
| Measure @ 210-min | Deoxycholate injury |
|---|---|
| Average villus surface area (VSA) (n = 27) | 49863 ± 2334 µm2 |
| # villi per mm2 (n = 20) | 99 ± 7 |
| Total villous surface area = (VSA)•(# villi per mm2)•(314mm2) | 1.55 × 109 µm2 |
| -Epithelialized villous surface area = (0.75)(1.55 × 109 µm2) | 1.2 × 109 µm2 |
| -Denuded villous surface area = total VSA– epithelialized VSA | 3.5 × 108 µm2 |
Total crypt surface area was derived after determination that crypts contributed 64% and villi 36% to total mucosal surface area of deoxycholate-injured tissue after 210-min in the Ussing chamber.
| Total crypt surface area = (total VSA/0.36) – total VSA | 2.75 × 109 µm2 |
Calculation of Surface Area of Tight Junction Pathway
To estimate the surface area of tight junction pathway, we adopted the formula for linear density of tight junction pathway (lp; µm/µm2) as defined by Claude (9) and measurements taken by Marcial (24) of crypt and villus epithelium from Guinea pig ileum.
| Measure @ 210-min | Epithelialized Villus | Crypt |
|---|---|---|
| Density of tight junction pathway (lp; (9)) | 0.2180 µm/µm2 | 0.7680 µm/µm2 |
| Length of tight junction pathway = lp • (surface area) | 2.6 × 108 µm | 2.1 × 109 µm |
| Sum of tight junction length (villus + crypt) | 2.4 × 109 µm | |
| Surface area of tight junction pathway (villus + crypt) = tight junction length • tight junction width (0.02–0.2 µm) | 4.8 × 107 – 4.8 × 108 µm2 | |
Comparison of Contributions of Tight Junction Versus Denuded Surface Area to Total Low-Resistance Paracellular Pathway
| Measure at 210-min | Surface area (%) contribution to total low-resistance paracellular pathway | |
|---|---|---|
| Tight Junction Width (9) | 0.02 µm | 0.2 µm |
| Denuded villous surface area | 3.5 × 108 µm2 (88%) | 3.5 × 108 µm2 (42%) |
| Tight junction surface area | 4.8 × 107 µm2 (12%) | 4.8 × 108 µm2 (58%) |
| Total low-resistance paracellular pathway | 3.98 × 108 µm2 | 8.3 × 108 µm2 |
| Tight junction-mediated change in surface area of total paracellular pathway | 2.08X | |
| PG-mediated change in TER | 1.68X | |
REFERENCES
- 1.Argenzio RA, Henderson CK, Liacos JA. Restitution of barrier and transport function of porcine colon after acute mucosal injury. American Journal of Physiology. 1988;255:G62–G71. doi: 10.1152/ajpgi.1988.255.1.G62. [DOI] [PubMed] [Google Scholar]
- 2.Argenzio RA, Henderson CK, Liacos JA. Effect of prostaglandin inhibitors on bile salt-induced mucosal damage of porcine colon. Gastroenterology. 1989;96:95–109. doi: 10.1016/S0016-5085(89)80008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argenzio RA, Liacos JA, Levy ML, Meuten DJ, Lecce JG, Powell DW. Villous atrophy, crypt hyperplasia, cellular infiltration, and impaired glucose-Na absorption in enteric cryptosporidiosis of pigs. Gastroenterology. 1990;98:1129–1140. doi: 10.1016/0016-5085(90)90325-u. [DOI] [PubMed] [Google Scholar]
- 4.Bjarnason I, Williams P, Smethurst P, Peters TJ, Levi AJ. The effect of NSAIDs and prostaglandins on the permeability of the human small intestine. Gut. 1986;27:1292–1297. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- 6.Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Prostaglandins I2 and E2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. Journal of Clinical Investigation. 1997;100:1928–1933. doi: 10.1172/JCI119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. American Journal of Physiology. 1999;276:G28–G36. doi: 10.1152/ajpgi.1999.276.1.G28. [DOI] [PubMed] [Google Scholar]
- 8.Ciacci C, Lind SE, Podolsky DK. Transforming growth factor β regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology. 1993;105:93–101. doi: 10.1016/0016-5085(93)90014-4. [DOI] [PubMed] [Google Scholar]
- 9.Claude P. Morphological factors influencing transepithelial permeability:A model for the resistance of the Zonula Occludens. Journal of Membrane Biology. 1978;39:219–232. doi: 10.1007/BF01870332. [DOI] [PubMed] [Google Scholar]
- 10.Collette A, Walker D, Sims E, He YL, Speers P, Ayrton J, Rowland M, Warhurst G. Influence of morphometric factors on quantitation of paracellular permeability of intestinal epithelia in vitro. Pharmacology Research. 1997;14:767–773. doi: 10.1023/a:1012154506858. [DOI] [PubMed] [Google Scholar]
- 11.Dykstra MJ. A manual of applied techniques for biological electron microscopy. New York: Plenum Press; 1993. [Google Scholar]
- 12.Erickson RA. Effect of 16,16-dimethyl PGE2 and indomethacin on bile acid-induced intestinal injury and restitution in rats. Journal of Laboratory and Clinical Medicine. 1988;112:735–744. [PubMed] [Google Scholar]
- 13.Erickson RA, Tarnawski A, Dines G, Stachura JS. 16,16-dimethyl prostaglandin E2 induces villus contraction in rats without affecting intestinal restitution. Gastroenterology. 1990;99:708–716. doi: 10.1016/0016-5085(90)90959-5. [DOI] [PubMed] [Google Scholar]
- 14.Freel RW, Hatch M, Earnest DL, Goldner AM. Role of tight junctional pathways in bile salt-induced increases in colonic permeability. American Journal of Physiology. 1983;245:G816–G823. doi: 10.1152/ajpgi.1983.245.6.G816. [DOI] [PubMed] [Google Scholar]
- 15.Frizzell RA, Schultz SG. Ion conductances of extracellular shunt pathway in rabbit ileum. Journal of General Physiology. 1972;59:318–346. doi: 10.1085/jgp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gookin JG, Rhoads JM, Argenzio RA. Inducible nitric oxide synthase mediates early epithelial repair of porcine ileum. American Journal of Physiology. 2002;283:G157–G168. doi: 10.1152/ajpgi.00005.2001. [DOI] [PubMed] [Google Scholar]
- 17.Graumlich JF. Preventing gastrointestinal complications of NSAIDs: risk factors, recent advances, and latest strategies. Postgraduate Medicine. 2001;109:117–128. doi: 10.3810/pgm.2001.05.931. [DOI] [PubMed] [Google Scholar]
- 18.Joyce NC, Haire MF, Palade GE. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology. 1987;92:68–81. doi: 10.1016/0016-5085(87)90841-9. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann HJ, Taubin HL. Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease. Annals of Internal Medicine. 1987;107:513–516. doi: 10.7326/0003-4819-107-4-513. [DOI] [PubMed] [Google Scholar]
- 20.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduced arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 21.Little D, Dean RA, Young KM, McKane SA, Martin LA, Jones SL, Blikslager AT. Phosphatidylinositol-3-kinase (PI3’K) signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. American Journal of Physiology. 2003;284:G46–G56. doi: 10.1152/ajpgi.00121.2002. [DOI] [PubMed] [Google Scholar]
- 22.Loud AV, Anversa P. Biology of disease. Morphometric analysis of biologic processes. Laboratory Investigation. 1984;50:250–261. [PubMed] [Google Scholar]
- 23.Madara JL. Loosening tight junctions: lessens from the intestine. Journal of Clinical Investigation. 1989;83:1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: A hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. Journal of Membrane Biology. 1984;80:59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]
- 25.McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. American Journal of Physiology. 1992;263:G426–G435. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- 26.Moore R, Carlson S, Madara JL. Villus contraction aids repair of intestinal epithelium after injury. American Journal of Physiology. 1989;257:G274–G283. doi: 10.1152/ajpgi.1989.257.2.G274. [DOI] [PubMed] [Google Scholar]
- 27.Moore R, Carlson S, Madara JL. Rapid barrier restitution in an in vitro model of intestinal epithelial injury. Laboratory Investigation. 1989;60:237–244. [PubMed] [Google Scholar]
- 28.Noiri E, Peresleni T, Srivastava N, Weber P, Bahou WF, Peunova N, Goligorsky MS. Nitric oxide is necessary for a switch from stationary to locomoting phenotype in epithelial cells. American Journal of Physiology. 1996;270:C794–C802. doi: 10.1152/ajpcell.1996.270.3.C794. [DOI] [PubMed] [Google Scholar]
- 29.Okada Y, Irimajiri A, Inouye A. Electrical properties and active solute transport in the rat small intestine. II. Conductive properties of transepithelial routes. Journal of Membrane Biology. 1977;31:221–232. doi: 10.1007/BF01869406. [DOI] [PubMed] [Google Scholar]
- 30.Powell DW. Barrier function of epithelia. American Journal of Physiology. 1981;241:G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 31.Riegler M, Sedivy R, Sogukoglu T, Cosentini E, Bischof G, Teleky B, Feil W, Schiessel R, Hamilton G, Wenzl E. Epidermal growth factor promotes rapid response to epithelial injury in rabbit duodenum in vitro. Gastroenterology. 1996;111:28–36. doi: 10.1053/gast.1996.v111.pm8698221. [DOI] [PubMed] [Google Scholar]
- 32.Rose RC, Schultz SG. Studies on the electrical potential profile across rabbit ileum: effects of sugars and amino acids on transmural and transmucosal electrical potential differences. Journal of General Biology. 1971;57:639–663. doi: 10.1085/jgp.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigthorsson G, Tibble J, Hayllar J, Menzies I, Macpherson A, Moots R, Scott D, Gumperl MJ, Bjarnason I. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998;43:506–511. doi: 10.1136/gut.43.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svanes K, Ito S, Takeuchi K, Silen W. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982;82:1409–1426. [PubMed] [Google Scholar]
- 35.Takahashi M, Ota S, Hata Y, Mikami Y, Azuma N, Nakamura T, Terano A, Omata M. Hepatocyte growth factor as a key to modulate anti-ulcer action of prostaglandins in stomach. Journal of Clinical Investigation. 1996;98:2604–2611. doi: 10.1172/JCI119080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zushi S, Shinomura Y, Kiyohara T, Minami T, Sugimachi M, Higashimoto Y, Kanayama S, Matsuzawa Y. Role of prostaglandins in intestinal epithelial restitution stimulated by growth factors. American Journal of Physiology. 1996;270:G757–G762. doi: 10.1152/ajpgi.1996.270.5.G757. [DOI] [PubMed] [Google Scholar]