Abstract
The yellow fever mosquito, Aedes aegypti, the global vector of dengue and yellow fever, is inexorably linked to water-filled human-made containers for egg laying and production of progeny. Oviposition is stimulated by cues from water containers, but the nature and origin of these cues have not been elucidated. We showed that mosquito females directed most of their eggs to bamboo and white-oak leaf infusions, and only a small fraction of the eggs were laid in plain water containers. In binary choice assays, we demonstrated that microorganisms in leaf infusions produced oviposition-stimulating kairomones, and using a combination of bacterial culturing approaches, bioassay-guided fractionation of bacterial extracts, and chemical analyses, we now demonstrate that specific bacteria-associated carboxylic acids and methyl esters serve as potent oviposition stimulants for gravid Ae. aegypti. Elucidation of these compounds will improve understanding of the chemical basis of egg laying behavior of Ae. aegypti, and the kairomones will likely enhance the efficacy of surveillance and control programs for this disease vector of substantial global public health importance.
Keywords: kairomone, semiochemical, mosquito, egg-laying, dengue vector control
The mosquito Aedes aegypti is the principal vector of important global diseases, including dengue and yellow fever viruses. Dengue fever is a major public health problem in tropical countries worldwide, and the World Health Organization estimates that 51 million infections occur annually and 2.5–3 billion people are at risk in the 100 countries where dengue fever occurs (1). The dramatic rise in the number of cases of dengue hemorrhagic fever in Asia and its recent introduction into Central and South America (1) have stimulated interest in biorational approaches, including identification and implementation in mosquito-control programs of behavior-modifying compounds, such as host attractants, repellents, and oviposition-site attractants and stimulants. Oviposition-site attractants and stimulants, especially, have great potential not only in detection and surveillance of mosquito populations and associated pathogenic viruses, but also in sustainable vector and disease suppression by targeting gravid mosquito females, which are epidemiologically the most important component of the mosquito population (2).
Ae. aegypti females lay eggs in human-made containers placed in residential landscapes. Each female normally does not lay her entire batch of eggs in one location but, rather, distributes them in multiple water-filled containers, a behavior called “skip-oviposition” (3). However, augmentation of some containers with organic material can counteract skip oviposition and significantly increase the numbers of eggs in target containers (4, 5) because more gravid females are attracted and induced to lay eggs (5, 6). The oviposition behavior of mosquitoes is mediated by various cues associated with the aquatic habitat where larvae mature (7). Visual cues associated with the oviposition site attract gravid mosquitoes from a distance, and olfactory cues guide the female to water-filled containers; upon landing, contact with the water surface stimulates the female to oviposit. Presumably, contact-mediated chemoreception and mechanoreception mediate the oviposition response (7).
Gravid Ae. aegypti females orient to and prefer to oviposit in organic infusions of fermenting leaves in water (8, 9). Microbial metabolites have been implicated as mosquito attractants, but these odorants do not induce egg laying (6). Using a combination of culturing approaches, bioassay-guided fractionation of extracts of cultured bacteria, and chemical analyses, we demonstrate that specific carboxylic acids and methyl esters of bacterial origin are potent oviposition kairomones for gravid Ae. aegypti.
Results and Discussion
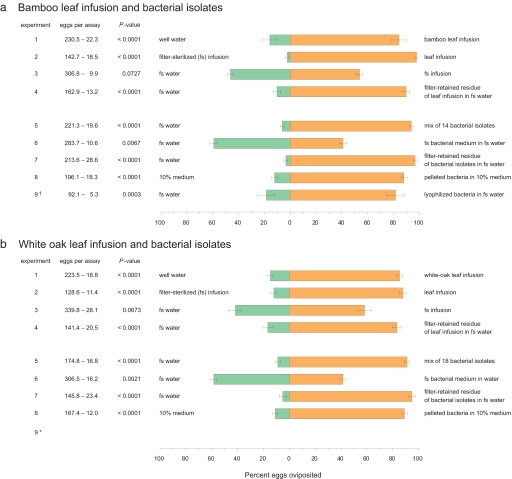
Oviposition Responses to Leaf Infusions.
Using binary choice behavioral assays (5), we screened bamboo (Arundinaria gigantea) leaf infusions and found that Ae. aegypti females directed 84.3 ± 5.6% (SEM) of their eggs to 1-week-old infusions, and only a small fraction of the eggs were laid in control containers holding water (t = 5.697, df = 11, P < 0.0001) (Fig. 1a, experiment 1). To evaluate the role of microorganisms in egg laying, gravid Ae. aegypti females were offered a choice of a bamboo infusion and an identical infusion that had been filtered through a 0.22-μm filter to remove microorganisms. Significantly more eggs were laid in bioassay cups containing the unfiltered infusion than in cups holding filter-sterilized infusion (Fig. 1a, experiment 2). Because some organic infusions contain odorants that can attract gravid females (8), we compared egg laying in filter-sterilized infusions, which remained attractive to mosquitoes and, filter-sterilized water, which was comparatively less attractive. No significant difference was found in the number of eggs deposited in these containers (Fig. 1a, experiment 3), indicating that filtration removed the oviposition cues and, therefore, that these cues likely were not solubilized in the infusion, but rather associated with the filtered microbes. We also compared the attractiveness of filter-sterilized infusion to sterile water (same procedure as in experiment 3), using the olfactory bioassay. Importantly, the filter-sterilized bamboo leaf infusions did not lose their attractiveness to mosquitoes: The sticky screen positioned just above the infusion surface trapped 5.6 ± 0.5 (77.4%) of 10 females in the infusion cup and only 1.8 ± 0. 5 females in the control water cup (t = 4.171, df = 11, P < 0.0001). By comparing the attractiveness of an unfiltered bamboo-leaf infusion and a filter-sterilized infusion, we confirmed that oviposition attractants, unlike oviposition stimulants, were dissolved in the organic infusion and could not be removed solely by filtering the microorganisms (52.0 ± 4.5% vs. 48.0%, respectively, t = 0.411, df = 11, P = 0.3443).
Fig. 1.
Egg laying responses of Ae. aegypti to bamboo leaf infusions (a), white oak leaf infusions (b), and mixes of bacteria cultured from the infusions, in 24-h oviposition bioassays. Experiment 1: leaf infusion versus well water; experiment 2: leaf infusion versus filter-sterilized infusion; experiment 3: filter-sterilized infusion versus sterile water; experiment 4: filter-retained residue (filtrand) of leaf infusion resuspended in sterile water versus filter-retained residue from a second filtration of the same leaf infusion (rendered sterile after the first filtration) resuspended in sterile water; experiment 5: in each test cup, 5 ml of 2-day-old medium containing a mix of bacterial species (109 cells per ml) was added to 25 ml of sterile water (final concentration, 108 cells per ml) versus 5 ml of fresh sterile R2A medium added to 25 ml of sterile water; experiment 6: in each test cup, 5 ml of filter-sterilized bacterial culture medium was added to 25 ml of sterile water versus 5 ml of fresh sterile medium added to 25 ml of sterile water; experiment 7: filter-retained residue of 5 ml of medium containing a mix of bacterial species resuspended in 30 ml of sterile water versus filter-retained residue obtained from a second filtration of filter-sterilized medium resuspended in sterile water; experiment 8: bacteria harvested from 2-day cultures by centrifugation (1,254 × g, 10 min, 4°C) were resuspended in sterile 10% R2A medium (108 cells per ml) versus 30 ml of bacteria-free sterile 10% medium; experiment 9: response of single gravid females (denoted with †) to lyophilized bacteria added to sterile water versus lyophilized bacteria-free medium added to sterile water. Experiment 9 was not conducted with white-oak leaf infusion *, P value represents results of a one-tailed paired t test of arcsin√x transformed data.
Next, we conducted a replacement experiment to confirm that microorganisms mediated oviposition decisions. We consecutively filter-sterilized bamboo-leaf infusions using two fresh-filter membranes, and the filtered microorganisms were resuspended in sterile water that had been filtered once. Ae. aegypti laid significantly more eggs in filter-sterilized water augmented with the first filter membrane (with microorganisms) than in sterilized infusion with the contents of the second filter membrane (Fig. 1a, experiment 4). Taken together, the results of these behavioral assays indicate that cues associated with microorganisms in plant infusions direct gravid Ae. aegypti females to deposit >90% of their eggs in microbe-enriched containers. Although microorganisms also produce volatile metabolites that attract gravid females to infusions, these odorants do not induce egg-laying. We obtained similar results in an identical set of experiments conducted with white-oak (Quercus alba) leaf infusions (Fig. 1b, experiments 1–3), indicating that the cues that mediate oviposition decisions in female Ae. aegypti are not unique to bamboo-leaf infusions.
Oviposition Responses to Cultured Bacteria.
Several investigations have linked bacteria to various phases of mosquito orientation and acceptance of oviposition substrates (reviewed in ref. 10). We therefore cultured, purified, and identified 14 bacterial isolates from bamboo infusions and 18 isolates from white-oak leaf infusions using enrichment, agar-plate methods, and 16S rRNA gene sequencing. All isolates were assigned to recognized phyla of domain bacteria [see supporting information (SI) Table S1]. Nine bamboo isolates and 8 white oak isolates had ≥98% sequence identity to described bacterial species, and 5 bamboo isolates and 10 white-oak isolates were considered previously unrecognized based on ≤97% match with described bacterial species. Particularly notable is the large number of members of the phylum Proteobacteria (affiliated with subdivisions Alpha and Gamma). Members of this phylum have been shown to be widely distributed on leaf surfaces in different plants (11). In experiment 5 (Fig. 1 a and b), gravid females were offered a choice between containers holding fresh sterile medium and culture medium containing a mix of the bacterial species. Ae. aegypti laid 93.9 ± 1.5% and 91.1 ± 2.0% of their eggs in cups containing the bacteria from bamboo and white-oak leaf infusions, respectively, and only 6.5% and 9.8% of the total number of eggs in control containers, showing that both sets of bacterial isolates produced highly stimulatory oviposition kairomones. Although other microbes (fungi, uncultivatable bacteria) might further stimulate egg laying, the cultured bacterial mixes that we used contained potent oviposition cues that directed Ae. aegypti females to concentrate their eggs in favorable habitats. Nevertheless, some individual isolates failed to stimulate egg laying at several cell densities, indicating that only certain bacterial isolates produce the egg-laying cues.
We followed the same experimental design with the bacterial isolates as with the leaf infusions to confirm a central role for bacteria in egg-laying behavior of Ae. aegypti. First, we showed that filter-sterilized bacterial cultures lost their oviposition-stimulating activity; removing microbes from either bamboo or oak-leaf cultures rendered them deterrent (Fig. 1 a and b, experiment 6). However, supplementing sterile water with the filtered bacteria completely recovered the egg-laying activity to the same level as the mix of bacterial isolates (compare experiments 7 and 6). Likewise, more eggs were laid by Ae. aegypti in cups containing a centrifuged and resuspended bacterial pellet than in cups containing bacteria-free sterile medium (experiment 8), showing again that cues associated with bacterial cells were responsible for stimulating egg laying in Ae. aegypti, that these cues were not released into the medium, and therefore, they could be removed and then restored with the bacteria.
Two major types of cues might be involved: chemical and tactile. In preparation for bioassay-guided fractionation of bacteria extracts, we developed procedures for retaining their kairomonal activity during the harvesting and storage process. Many more eggs were laid in medium augmented with pelleted and reconstituted lyophilized bacteria from bamboo-leaf infusion than in control medium that was augmented with lyophilized medium (Fig. 1a, experiment 9). Starting with this experiment, we also modified the bioassay to a single gravid female per cage to minimize the effects of social interactions on oviposition (e.g., social facilitation or antagonism).
Isolation and Identification of Bacteria-Associated Oviposition Stimulants.
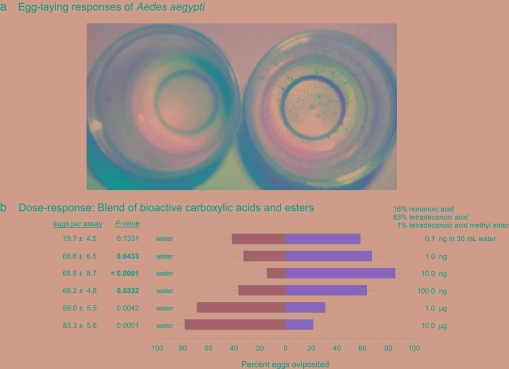
Preliminary bioassays indicated that methanol was more effective than hexane or dichloromethane in extracting the egg-laying kairomones from lyophilized bacteria. Lyophilized bacterial cells were extracted with methanol, and the crude extract was used in bioassays and chemical analyses. In dose–response studies, we found that a methanol extract of 0.05 ml equivalents of the bacterial cells (106 cells per ml) was highly stimulatory in binary choice oviposition assays, with 81.6 ± 6.7% of the eggs laid in the extract-treated cup (n = 17 assays with single gravid females, P < 0.0001). The methanol extract was fractionated on a C18 Sep-Pak, a bioactive 95% acetonitrile fraction (78.6 ± 8.7% of the eggs were oviposited in cups containing this fraction; n = 17, P < 0.0001) was subjected to reverse-phase HPLC, and a 5-min bioactive fraction [89.6 vs. 19.4% (SEM = 6.3), t = 4.756, df = 22, P < 0.0001] was further fractionated on RP-HPLC into 1-min fractions. GC-MS analysis of a 1-ml RP-HPLC fraction that contained bioactive compounds [76.8 vs. 23.2% (SE = 7.5), t = 3.110, df = 17, P = 0.0032] revealed a mix of carboxylic acids ranging from nonanoic acid to octadecanoic acid and several carboxylic acid methyl esters, which are listed in Table S2. Acid–base partitioning of this fraction showed that the acid fraction contained oviposition kairomones, whereas the basic fraction, which also contained neutral compounds, was deterrent (Table S3).
Synthetic compounds and blends were bioassayed at 1, 10, and 100 ng in cups filled with 30 ml of water (0.14, 1.46, and 14.6 nM for tetradecanoic acid). Most fatty acids and esters were ineffective at any concentration (Table S4). However, others, namely nonanoic acid, tetradecanoic acid, and methyl tetradecanoate, were highly effective at inducing egg laying but at extremely narrow dosage ranges. Tetradecanoic acid, at 10 ng, diverted the greatest amount of oviposition to the treated water. These assays, using single females, were conducted for 72 h, and females laid 22.6 ± 0.6 eggs per 24 h, on average, whereas in previous 24-h assays with five females, each female deposited 43.3 ± 3.4 eggs. To confirm that these compounds were effective in assays of shorter duration, we repeated these assays using five females in 24-h assays. Under these conditions, 1 ng of tetradecanoic acid was highly stimulatory, with a clear decline in the oviposition responses at both lower and higher doses (Table S5), and each of the five females averaged 43.4 ± 3.9 eggs in 24 h.
We also bioassayed a blend of synthetic carboxylic acids at their natural ratio in bacteria, as determined from GC-MS analysis of methanol extracts of a lyophilized bacterial mix that was highly stimulatory in oviposition assays (Fig. 1a, experiment 9). A blend of bioactive compounds, consisting of 16% nonanoic acid, 83% tetradecanoic acid, and 1% methyl tetradecanoate (Table S3) was highly stimulatory to Ae. aegypti females. A curvilinear response was observed, with the highest percentage of eggs (85.5 ± 11%) laid in cups containing 10 ng of the blend (Fig. 2b). Notably, blending the three bioactive compounds in their natural ratio did not synergize the activity of the blend beyond what was observed for tetradecanoic acid alone. However, the dose–response curve to the blend of three components spanned over two log scales (1–100 ng, with tetradecanoic acid at 0.12–12.12 nM), whereas the oviposition response to individual components was limited to narrow dosage ranges.
Fig. 2.
Egg-laying responses of Ae. aegypti. (a) A bioassay from experiment 8 (Fig. 1a), showing that most of the eggs were laid in a mix of 14 bacterial isolates from bamboo-leaf infusion (right cup), and few eggs were placed in the control cup (left cup). (b) Egg laying bioassays of Ae. aegypti, showing dose–response patterns to blends of two bioactive carboxylic acids and a methyl ester. Detailed bioassay results with carboxylic acids and methyl esters are described in Tables S4 and S5.
Our results show that gravid Ae. aegypti perceive specific carboxylic acids and esters associated with microorganisms in their oviposition habitat, and these kairomones radically alter their oviposition decisions. Although some bacteria-associated cues induce oviposition at specific concentrations, the same cues at higher concentrations (e.g., tetradecanoic acid), or other cues produced by either the same or different bacteria (e.g., hexadecanoic acid methyl ester), deter oviposition. Indeed, when tested as individual isolates in preliminary bioassays, some of the 14 bacterial species that we cultured from a bamboo leaf infusion stimulated oviposition, whereas others deterred egg laying; nonetheless, even stimulatory bacteria became deterrent at high concentrations. Based on these observations, we postulate that the final decision by a gravid female Ae. aegypti to accept or reject an oviposition site might involve a two-step process: First, upon alighting on water within a container, she determines the presence and measures the relative concentration of various bacteria and associated semiochemicals (which are likely structural components of the bacterial cell wall) using contact chemoreceptors, most likely on her antennae, mouthparts, tarsi, or ovipositor (12, 13). She thus obtains information about the composition and density of the microbial community in the water container. It will be important to delineate whether the female also obtains mechanosensory information from microbes and whether she engages in behaviors that facilitate the flux of bacteria across mechano- and chemosensilla. In a second step, the female integrates sensory information from chemostimulatory and chemodeterrent cues, together with other chemical (e.g., pH, alkalinity) and mechanical cues (7), to resolve whether the water container represents a suitable oviposition site. There is strong selection pressure on gravid females for accurate egg-laying decisions because the microbial community within the oviposition container must support growth and development of her offspring, which graze on microbes (14). Thus, the concentration and relative amounts of keystone fatty acids and esters might represent to the female a suitable microbial community at an acceptable cell density. Conversely, the presence of deterrent compounds or even stimulatory fatty acids at high concentrations, likely represents to the Ae. aegypti female a more eutrophic container community that is less suitable for her larvae.
The semiochemicals that we identified from extracts of bioactive bacteria have great potential in mosquito-abatement programs. A pivotal life-history trait of Ae. aegypti is skip oviposition (3), where gravid females disperse single eggs or small groups of eggs into multiple water containers (15). That is, each oviposition bout is punctuated by appetitive flight seeking another oviposition site. The oviposition-stimulating compounds might (i) bias females' decisions in favor of oviposition over flight and (ii) cause a quantitative shift from egg retention to oviposition. Both effects would result in longer contact with the water surface and possibly the inner container walls. Of several practical implications, two stand out as having potentially far-reaching effects on mosquito populations and hence on the epidemiology of vectored diseases. First, increasing the numbers of eggs laid in target containers would likely enhance the sensitivity of oviposition traps that are used to detect and monitor the activity of Ae. aegypti in disease-endemic regions (16). Second, increasing the residence time within containers would assure that females receive ample exposure to traps impregnated with lethal toxicants (17, 18) or with biologically active materials, such as insect growth regulators, that can be horizontally transferred to adjacent water containers through skip-oviposition (19, 20). In addition to their potential in mosquito management programs, the oviposition-inducing semiochemicals will facilitate investigations of contact-mediated chemoreception in a mosquito vector of substantial public health importance globally.
Materials and Methods
Origin and Maintenance of Mosquito Colonies.
Ae. aegypti colonies were established from field-collected eggs from New Orleans, LA, in 2003. Larvae were reared as described by Trexler et al. (21), at ≈28°C, ≈75% relative humidity, and a photoperiod of 14-h light/10-h dark, including two twilight periods (60 min each). Mosquitoes were bloodfed on a human arm 4–5 d before each experiment.
Preparation of Plant Infusions.
Plant infusions were prepared by fermenting 16.8 g of white oak or 33.6 g of bamboo senescent leaves in 4 liters of well water in separate Teflon bags. Bamboo and white-oak leaves were obtained from separate residential areas in Raleigh, NC. We found 1-week-old bamboo and 2-week-old white-oak leaf infusion to be attractive to Aedes mosquitoes in behavioral bioassays. Before use in bioassays, whether they were filtered or not, infusions were diluted 1:1 with well water.
Bioassay Methods.
Modifications of the olfactory- and contact-mediated bioassays previously described (5) were used to measure the response of gravid mosquitoes to plant infusions or bacterial compounds. Briefly, two 125-ml polypropylene cups (test and control) were placed randomly in diagonal corners of a Plexiglas cage (30 × 30 × 30 cm) that was fitted with a stockinet cloth sleeve (AlbaHealth). Each cup was filled with 30 ml of either test or control solution. White paper sleeves were placed around each bioassay cup to mask visual cues presented by darkly colored leaf infusions. Adults had ad libitum access to water contained in a polypropylene cup that was fitted with a lid with a cotton wick protruding through a hole in the lid.
For egg-laying bioassays, either one or five gravid females were released in each replicate cage (see Results for details). Cages were placed randomly on racks in a room where environmental conditions were the same as in the insectary. After a 24-h exposure period, eggs laid on the surface of the water in the test and control cups (see Fig. 2a) were counted.
In olfactory bioassays, 10 gravid females were released in each bioassay cage. A circular metal wire-mesh screen covered with insect glue (Tanglefoot; Tanglefoot Co.) was inserted in each cup on top of an insert cut from a 120-ml polypropylene cup. Sticky screens were placed above test and control media, 2.5 cm below the lip of each cup. The mesh size of the metal screen prevented females from entering without landing on the screen. Therefore, positive or negative responses to the test infusions were measured by the numbers of females trapped during a 24-h exposure period on the sticky screen in the test and control cups.
Statistical Analysis.
For each replicate bioassay cage, the numbers of eggs laid in the test and control cups were converted to the proportion of the total number of eggs laid. The proportions were subjected to an arcsin√x transformation to achieve approximate normality. A one-tailed paired t test was used to determine whether differences in the mean transformed proportion of eggs deposited in test and control cups were statistically significant.
Purification of Bacterial Isolates.
Bacterial cells from plant infusions were cultured in R2A medium (22). Enrichment cultures were established by inoculating 1 ml each of undiluted 1- to 2-week old bamboo-leaf and white-oak leaf infusions into separate 250-ml flasks, each containing 100 ml of culture medium. Bacterial cultures were grown for 2 days at 28°C with constant shaking (120 rpm). Enriched cultures were serially diluted to 10−7 with sterile peptone water 0.1% (wt/vol), and 100 μl of each of the last three dilutions was separately spread on two replicate R2A agar plates to isolate bacterial species, after which the plates were incubated at 28°C. Similarly, 1 ml of each plant infusion was also serially diluted up to 10−5 and spread-plated on R2A agar (22). Colonies with visually distinct morphologies were restreaked several times on R2A agar plates. In total, 14 and 18 different bacterial isolates from bamboo-leaf and white-oak leaf infusions, respectively, were purified. Bacterial isolates from each plant infusion were mixed (14 isolates from bamboo-leaf infusions and 18 isolates from white-oak leaf infusions), and grown for 2-days in R2A medium, and the bacterial cells in these cultures were used in contact chemoreception bioassays.
PCR Amplification.
Pure colonies of each bacterial isolate were boiled in 50 μl of sterile distilled water for 10 min and immediately cooled on ice for 5 min. After centrifugation (1,960 × g, 30 sec), 2 μl of supernatant was used as DNA template in PCRs. The primer sets 63f (5′-CAGGCCTAACACATGCAAGTC-3′) (23) and 1492r (5′-GGTTACCTTGTTACGACTT-3′) (24) were used to amplify the 16S rRNA gene. The PCR mixture contained 2 μl of template DNA, 0.2 μmol concentrations of each primer, 5 μl of 10× Pfx amplification buffer, 1.5 μl of 10 mM dNTP mixture, 1 μl of 50 mM MgSO4, and 1 unit of Platinum Pfx DNA Polymerase (Invitrogen), and sterile deionized water was added to achieve a final volume of 50 μl. After an initial step consisting of 3 min at 94°C, 30 cycles of amplification were performed; each amplification cycle consisted of 45 s at 94°C, 1 min at 55°C, and 1 min at 72°C. A final elongation step was carried out for 10 min at 72°C. PCR products were electrophoresed in a 1.2% agarose gel, followed by ethidium bromide staining. Amplicon size and yield were determined by comparison to molecular mass standards (Low DNA Mass Ladder; GIBCO–BRL).
Sequencing and Identification of Bacterial Isolates.
Amplified PCR products were purified by using the QIAquik PCR purification kit (Qiagen). Sequencing of the 16S rRNA gene fragments was performed on an ABI Prism 377 automated sequencer (Genomics Research Laboratory at North Carolina State University) with the BigDye Terminator v3.1 Cycle Sequencing Reaction kit (Applied Biosystems). The dye terminator cycle sequencing reactions were performed according to the manufacturer's guidelines with the primers 63f, 518r (5′-ATTACCGCGGCTGCTGG-3′) and 520f (5′-CAGCAGCCGCGGTAATAC-3′) (23, 24). Sequences were first checked for chimeras by using the CHECK-CHIMERA program of the Ribosomal Database Project (http://rdp8.cme.msu.edu/cgis/chimera.cgi?su = SSU). The 16S rRNA gene sequences were compared with sequences for cultured bacteria on the EzTaxon Server (www.eztaxon.org) (25). The Basic Local Alignment Search Tool (BLAST) was also used to search for sequence homologies (26).
Isolation, Identification, and Bioassay of Oviposition Stimulants.
Aliquots of 60-ml cultures (109 cells per ml) were centrifuged at 1,254 × g for 10 min, the supernatant was decanted, and cells were suspended in 0.6 ml of fresh R2A medium and transferred to 8-ml glass vials. The vials were quick-frozen at −80°C and lyophilized (Bench Top 6; Virtis; cold trap = −50° to −60°C, ≈200 mTorr, ambient temperature, 23°C) for 18 h. Vials with dehydrated bacteria were then sealed and stored at −5°C until needed. Freeze-dried preparations were reconstituted by adding 60 ml of sterile water, and 5 ml of the reconstituted culture suspension was bioassayed immediately in sterile water (Fig. 1, experiment 9).
Preliminary bioassays indicated that methanol was more effective than hexane or dichloromethane in extracting the egg-laying kairomones from lyophilized bacteria. The lyophilized bacterial cells in 0.6 ml of R2A medium (equivalent to 60 ml of 109 cells per ml) were extracted with 30 ml of methanol, and the crude extract was centrifuged (1,960 × g, 4°C). The supernatant was rotary evaporated at 40°C and used in bioassays and chemical analyses. In dose–response studies, 5.0, 0.5, 0.05, and 0.005 ml equivalents of lyophilized bacterial cells were reduced under a gentle stream of N2, and the extract was resuspended in 1.0 ml of ether and aliquoted into eight bioassay cups; control cups received the identical treatment with evaporated methanol resuspended in ether. All cups were aerated in a fume hood for at least 1 h to evaporate ether before bioassays.
The methanol extracts were fractionated on a reverse-phase C18 Sep-Pak (Waters) with stepwise elutions of 50%, 75%, 95%, and 100% acetonitrile in water. Each fraction was evaporated in a rotary evaporator to remove acetonitrile and water and redissolved in ether. The 95% acetonitrile fraction was subjected to two rounds of reverse-phase HPLC (Hewlett Packard 1050) on a Phenomenex Zorbax ODS column (250 mm × 4.6 mm, acetonitrile/water gradient elution: 50–100% acetonitrile in 20 min at 1.0 ml per min, monitored at 265 nm).
GC-MS analysis was conducted on an Agilent 5975 mass selective detector, operated in electron-impact ionization mode and coupled to an Agilent 6890 GC. The GC was operated in splitless injection mode and fitted with a 30 m × 0.25 mm × 0.25 μm DB-5MS column programmed from 40°C to 250°C at 10°C per min after an initial delay of 2 min, and held at 250°C for 20 min. Injector, MS quad, MS source, and transfer line temperatures were 280°C, 150°C, 230°C, and 250°C, respectively. Mass spectra were compared to Wiley7/NIST05 mass spectra libraries.
Synthetic compounds were bioassayed by placing hexane solutions on the surface of sterile distilled water; water in control cups was treated with hexane alone. The hexane was evaporated in a fume hood before bioassays commenced. Each bioassay was with a single female and lasted 72 h.
For quantitative chemical analysis, 0.6 ml of lyophilized bacterial cells (equivalent to 60 ml of 109 cells per ml) was extracted in 30 ml of methanol. After centrifugation, the supernatant was transferred to a pear-shaped flask, and methanol was rotary evaporated. The residue was suspended in 20 ml of 0.5 M HCl, and the aqueous solution was extracted twice with 10 ml of ether to obtain neutral and acidic compounds. Acidic compounds were extracted from the combined ether layer with 20 ml of 0.5 M NaOH. Then, the alkaline aqueous layer was acidified with 5 M HCl and extracted twice with 10 ml of ether to recover the acidic compounds. The ether layer was washed with brine until the pH became neutral and dried over anhydrous Na2SO4. An aliquot of the obtained acidic fraction was reacted with trimethylsilyl diazomethane to derivatize fatty acids to the corresponding methyl esters. The ether layer, from which acidic compounds had been extracted, and which contained neutral compounds, including esters, was washed with brine until the pH became neutral, and dried over anhydrous Na2SO4. An aliquot of the neutral fraction was subjected to GC-MS analysis in SIM mode (m/z 74 and molecular masses of target compounds).
Methyl esters were derivatized as follows: A sample in 100 μl of ether, which was equivalent to 3 ml of bacterial culture, was placed in a conical vial, the ether was evaporated with a N2 stream, and 1 μg of hendecanoic acid (internal standard) was added in 20 μl of hexane, followed by 5 μl of methanol and 5 μl of 2.3% diazomethane in hexane. The reaction mixture was stirred vigorously and incubated at room temperature for 30 min. The reaction mixture was diluted 10-fold and 1 μl of the diluted sample was injected into the GC-MS in SIM mode. The undiluted reaction mixture was also subjected to GC-MS analysis in SCAN mode for qualitative analysis.
Supplementary Material
Acknowledgments.
We thank Luma Abu Ayyash, who expertly reared mosquitoes. This work was supported in part by a National Institutes of Health–National Institute of Allergy and Infectious Diseases Grant U01-AI-58303-01, the Blanton J. Whitmire Endowment, and the W. M. Keck Center for Behavioral Biology at North Carolina State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802505105/DCSupplemental.
References
- 1.Gubler DJ. The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comp Immunol Microbiol Infect Dis. 2004;27:319–330. doi: 10.1016/j.cimid.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Reiter P. Oviposition, dispersal, and survival in Aedes aegypti: Implications for the efficacy of control strategies. Vector-Borne Zoon Dis. 2007;7:261–273. doi: 10.1089/vbz.2006.0630. [DOI] [PubMed] [Google Scholar]
- 3.Colton YM, Chadee DD, Severson DW. Natural “skip oviposition” of the mosquito Aedes aegypti as evidenced by codominant genetic markers. Med Vet Entomol. 2003;17:195–201. doi: 10.1046/j.1365-2915.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 4.Reiter P, Amador MA, Colon N. Enhancement of CDC ovitrap with bay infusion for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc. 1991;7:52–55. [PubMed] [Google Scholar]
- 5.Trexler JD, Apperson CS, Schal C. Laboratory and field evaluations of oviposition responses of Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) to oak leaf infusions. J Med Entomol. 1998;35:967–976. doi: 10.1093/jmedent/35.6.967. [DOI] [PubMed] [Google Scholar]
- 6.Benzon GL, Apperson CS. Reexamination of chemically mediated oviposition behavior in Aedes aegypti (L.) (Diptera: Culicidae) J Med Entomol. 1988;25:158–164. doi: 10.1093/jmedent/25.3.158. [DOI] [PubMed] [Google Scholar]
- 7.Bentley MD, Day JF. Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol. 1989;34:401–421. doi: 10.1146/annurev.en.34.010189.002153. [DOI] [PubMed] [Google Scholar]
- 8.Allan SA, Kline DL. Evaluation of organic infusions and synthetic compounds mediating oviposition in Aedes albopicuts and Aedes aegypti (Diptera: Culicidae) J Chem Ecol. 1995;21:1847–1860. doi: 10.1007/BF02033681. [DOI] [PubMed] [Google Scholar]
- 9.Sant'Ana AL, Roque RA, Eiras AE. Characteristics of grass infusions as oviposition attractants to Aedes (Stegomyia) (Diptera: Culicidae) J Med Entomol. 2006;43:214–220. doi: 10.1603/0022-2585(2006)043[0214:cogiao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Clements AN. The Biology of Mosquitoes, Sensory Reception and Behaviour. Vol 2. Oxford: CABI, Oxford Univ Press; 1999. pp. 564–566. [Google Scholar]
- 11.Yang CH, Crowley DE, Borneman J, Keen NT. Microbial phyllosphere populations are more complex than previously realized. Proc Natl Acad Sci USA. 2001;98:3889–3894. doi: 10.1073/pnas.051633898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossignol PA, McIver SB. Fine structure and role in behavior of sensilla on the terminalia of Aedes aegypti (L.) (Diptera: Culicidae) J Morphol. 1977;151:419–438. doi: 10.1002/jmor.1051510307. [DOI] [PubMed] [Google Scholar]
- 13.McIver SB, Siemicki R. Fine structure of tarsal sensilla of Aedes aegypti (L.) (Diptera: Culicidae) J Morphol. 1978;155:137–154. doi: 10.1002/jmor.1051550202. [DOI] [PubMed] [Google Scholar]
- 14.Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- 15.Apostol BL, Black WC, IV, Reiter P, Miller BR. Use of randomly amplified polymorphic DNA amplified by polymerase chain reaction markers to estimate the number of Aedes aegypti families at oviposition sites in San Juan, Puerto Rico. Am J Trop Med Hyg. 1994;51:89–97. doi: 10.4269/ajtmh.1994.51.89. [DOI] [PubMed] [Google Scholar]
- 16.Chen CD, et al. Dengue vectors surveillance in endemic areas in Kuala Lumpur city centre and Selangor State, Malaysia. Dengue Bull. 2006;30:197–203. [Google Scholar]
- 17.Zeichner BC, Perich MJ. Laboratory testing of a lethal ovitrap for Aedes aegypti. Med Vet Entomol. 1999;13:234–238. doi: 10.1046/j.1365-2915.1999.00192.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams CR, Ritchie SA, Long SA, Dennison N, Russell RC. Impact of a bifenthrin-treated lethal ovitrap on Aedes aegypti oviposition and mortality in north Queensland, Australia. J Med Entomol. 2007;44:256–262. doi: 10.1603/0022-2585(2007)44[256:ioablo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Chism BD, Apperson CS. Laboratory evaluation of the horizontal transfer of the insect growth regulator pyriproxyfen to larval microcosms by gravid Aedes albopictus and Ochlerotatus triseriatus. Med Vet Entomol. 2003;17:211–220. doi: 10.1046/j.1365-2915.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 20.Sihuincha M, et al. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J Med Entomol. 2005;42:620–630. doi: 10.1093/jmedent/42.4.620. [DOI] [PubMed] [Google Scholar]
- 21.Trexler JD, et al. Role of bacteria in mediating the oviposition responses of Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2003;40:841–848. doi: 10.1603/0022-2585-40.6.841. [DOI] [PubMed] [Google Scholar]
- 22.Reasoner DJ, Geldreich EE. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchesi JR, et al. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane DJ. In: Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt E, Goodfellow M, editors. Chichester, UK: Wiley; 1991. pp. 115–175. [Google Scholar]
- 25.Chun J, et al. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol. 2007;57:2259–2261. doi: 10.1099/ijs.0.64915-0. [DOI] [PubMed] [Google Scholar]
- 26.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Molec Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.