Abstract
ADHD linkage findings have not all been consistently replicated, suggesting that other approaches to linkage analysis in ADHD might be necessary, such as the use of (quantitative) endophenotypes (heritable traits associated with an increased risk for ADHD). Genome-wide linkage analyses were performed in the Dutch subsample of the International Multi-Center ADHD Genetics (IMAGE) study comprising 238 DSM-IV combined-type ADHD probands and their 112 affected and 195 nonaffected siblings. Eight candidate neuropsychological ADHD endophenotypes with heritabilities > 0.2 were used as quantitative traits. In addition, an overall component score of neuropsychological functioning was used. A total of 5407 autosomal single-nucleotide polymorphisms (SNPs) were used to run multipoint regression-based linkage analyses. Two significant genome-wide linkage signals were found, one for Motor Timing on chromosome 2q21.1 (LOD score: 3.944) and one for Digit Span on 13q12.11 (LOD score: 3.959). Ten suggestive linkage signals were found (LOD scores ≥ 2) on chromosomes 2p, 2q, 3p, 4q, 8q, 12p, 12q, 14q, and 17q. The suggestive linkage signal for the component score that was found at 2q14.3 (LOD score: 2.878) overlapped with the region significantly linked to Motor Timing. Endophenotype approaches may increase power to detect susceptibility loci in ADHD and possibly in other complex disorders.
Main Text
Attention-Deficit/Hyperactivity Disorder (ADHD [MIM 143465]) is a neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity.1 Overall heritability estimates exceed 0.70.2 However, thus far the identified candidate genes only make a small contribution to the elevated risk for ADHD, and there is a large amount of genetic variance unaccounted for by these findings.3 Hence, it is to be expected that other, as yet unidentified genes may also increase susceptibility to ADHD. In addition, most of the regions of the genome identified by ADHD phenotype-based linkage scans have not been replicated. Therefore, other approaches to linkage analysis in ADHD are necessary.
It has been proposed that endophenotypes (or intermediate phenotypes), defined as heritable traits that are associated with an increased risk for developing a disorder, might be more suitable for detecting risk genes in ADHD than the disease phenotype, because they are genetically less complex than phenotypes by being etiologically closer to disease genes.3,4 Because of this, endophenotypes could prove helpful in identifying ADHD genes through linkage analysis; they might also provide more insight into the functional relevance of identified genes for ADHD by shedding light on the neuro(psycho/physio)logical mechanisms of disease.
In the current study, we used ten neuropsychological cognitive and motor measures that have shown to be candidate ADHD endophenotypes to select those endophenotypes most suitable for linkage analysis (Table 1).5–9 In addition, an overall measure of neuropsychological functioning, to which all ten individual measures were related (correlation coefficients between .53 and .79, all p values < .001, explaining 47% of the variance in the task measures), was used. This overall measure may be the most powerful neuropsychological instrument, because a composite score entails less error variance than do individual measures. The neuropsychological-task variables were normalized and standardized into z scores by the use of a Van der Waerden transformation (SPSS version 14). Endophenotypes were available for the Dutch subsample of the International Multi-Center ADHD Genetics (IMAGE) study comprising 238 DSM-IV combined-type ADHD probands and their 112 affected and 195 nonaffected siblings (for screening and diagnostic details, please see ref. 3). The study was approved by the local medical-ethics committee.
Table 1.
Description of the Neuropsychological Tasks
| Task | Aim of Measurement | Dependent Variable |
|---|---|---|
| Executive/cognitive tasks | ||
| Stop task8 | Inhibition | Stop signal reaction time |
| Shifting attentional set5 | Inhibition and cognitive flexibility | Percentage of errors |
| Time test9 | Time reproduction | Absolute discrepancy |
| Visuo-spatial sequencing8 | Visuo-spatial working memory | No. of correct targets in correct order |
| Digit span8 | Verbal working memory | Digit span backwards |
| Motor tasks | ||
| Pursuit7 | Motor control under continuous adaptation | Precision |
| Tracking7 | Motor control without continuous adaptation | Precision |
| Tapping6 | Self-generated motor output | Variability in tapping rate |
| Baseline speed6 | Motor output as response to external cue | Variability in reaction times |
| Motor timing6 | Timing of motor output | Variability in reaction times |
The full description of DNA extraction is provided by Brookes et al.10 As described in Asherson et al.,11 a total of 5545 autosomal single-nucleotide polymorphisms (SNPs) from the Illumina Linkage IVb SNP panel were successfully assayed, with a call rate of 99.6% and a reproduction rate of 99.994%. After data cleaning, 5407 autosomal SNPs with an average resolution of 1.66 SNPs/centimorgan (cM) were entered into the linkage analyses.
To select endophenotypes for linkage analysis, heritability of the traits was estimated (Table 2). The population parameters of mean, variance, and heritability of each measure were based on a control sample (n = 271) consisting of sibling pairs described in previous studies.5–9 Heritability estimates were estimated with the use of the linear mixed model implemented in SOLAR.12 Models were adjusted for age, sex, and scores on Conners' Inattention and Hyperactivity/Impulsivity subscales. QTL linkage was examined for eight of the ten individual task measures that showed a heritability estimate > 20%, as well as for the component score. Age was used as a covariate, because it had shown a large effect on neuropsychological performance.5–9 In addition, subscale (ADHD combined) totals of Conners' Parent Rating Scale and Conner's Teacher Rating Scale were used as covariates to prevent spurious association findings for SNPs and neuropsychological measures confounded by ADHD effects. Linkage analysis was performed with the use of Merlin-regress software, which implements a regression-based procedure using trait-squared sums and differences to predict identity by descent (IBD) sharing between relative pairs.13 Since the linkage disequilibrium (LD) between adjacent SNPs can lead to inflated LOD scores, we applied the criteria of r2 < .05 between SNPs to cluster SNPs into combined markers.14,15 Empirical p values were derived with the use of Merlin software by the running of 1000 simulations under the null hypothesis of no linkage while preserving the original phenotypes, family structures, allele frequencies, LD structures and missing-data patterns.13,15 In each simulated data set, the linkage score was defined as the peak LOD score equal or higher than the experimental LOD score rounded down to the nearest one decimal value (e.g., if the experimental LOD score was 3.99, the linkage score was 3.9). The threshold for significant linkage was defined as the LOD score occurring in 50 of the 1000 permutations, corresponding to a probability of 0.05 in a genome scan.16 The estimated thresholds for significant linkage for the individual neuropsychological traits plus the combined score ranged from 3.615 to 4.049. The cutoff for suggestive linkage was calculated as the score that was observed, on average, once per genome scan, thus representing the average maximum peak size expected once per genome scan by chance alone.16 The thresholds for suggestive linkage for the individual neuropsychological traits plus the combined score ranged from 1.785 to 2.011. We report all linkage signals above this value of 2.011.
Table 2.
Parameter Estimates for the Ten Individual Task Measures and the Combined Score
| Meana | Variancea | Heritabilityb(p Value) | Proportion of Variance Explainedc | |
|---|---|---|---|---|
| Executive/cognitive tasks | ||||
| Stop task | −0.264 | 0.784 | 0.235 (0.061) | 0.302 |
| Shifting attentional set | −0.255 | 0.881 | 0.236 (0.091) | 0.185 |
| Time test | −0.369 | 0.830 | 0.583 (3.1 × 10−4) | 0.207 |
| Visuo-spatial sequencing | 0.256 | 0.845 | 0.497 (0.001) | 0.410 |
| Digit span | 0.249 | 0.878 | 0.235 (0.076) | 0.338 |
| Motor tasks | ||||
| Pursuit | −0.075 | 0.905 | 0.567 (6.1 × 10−4) | 0.455 |
| Tracking | −0.294 | 1 | 0.440 (0.010) | 0.154 |
| Tapping | −0.041 | 0.926 | < 0.20 (> 0.1) | 0.206 |
| Baseline speed | −0.125 | 0.880 | < 0.20 (> 0.1) | 0.204 |
| Motor timing | −0.320 | 0.867 | 0.701 (3 × 10−6) | 0.256 |
| Combined score | 0.0 | 1 | 0.344 (0.027) | 0.605 |
Mean and variance based on control sample consisting of sibling pairs described elsewhere.5–9
Heritability models were adjusted by age, sex, and parent- and teacher-based Conners' questionnaires.
Proportion of variance due to all final covariates according to SOLAR models.
Table 3 and Figure 1 show the linkage-test results. Six of the eight measures with an estimated heritability of > 0.2 showed at least one linkage signal with a LOD score above 2.011. Motor Timing, the ability to produce a 1 s interval, showed genome-wide significant linkage to chromosome 2q21.1 (LOD score: 3.944; Figure 1A). Digit Span, a measure of verbal working memory, showed genome-wide significant linkage to chromosome 13q12.11 (LOD score 3.959; Figure 1B). In addition, eight suggestive linkage signals with LOD scores between 2.022 and 2.627 were found on chromosomes 2p (twice), 3p, 4q, 8q, 12q, 14q, and 17q (Table 3). Most of these findings did not overlap between measures, except for the suggestive findings for Motor Timing and Digit Span on chromosome 2p25 (see Figure S1, available online). The component score showed two suggestive linkage signals with LOD scores of 2.516 and 2.878 on 2q14.3 and 12p13.33, respectively, the former overlapping with the regions linked to Motor Timing on chromosome 2 (Figure 1A).
Table 3.
Linkage Results for the Neuropsychological Measures and the Combined Score
| Task | Chr.a | Position (cM) | Locationb | Marker | LOD Score | p Value | Empirical p Valuec |
|---|---|---|---|---|---|---|---|
| Executive/cognitive tasks | |||||||
| Stop task | 12 | 119.05 | 12q23.3 | rs1862032 | 2.627 | .0003 | ns |
| Shifting attentional set | - | - | - | - | - | - | - |
| Time test | 4 | 202.6 | 4q35.2 | rs996026 | 2.111 | .0009 | ns |
| Visuo-spatial sequencing | 3 | 44.01 | 3p24.3 | rs680930 | 2.112 | .0009 | ns |
| Digit span | 2 | 14.17 | 2p25.2 | rs1079417 | 2.022 | .0011 | ns |
| 13 | 0.0004 | 13q12.11 | rs1974047 | 3.959 | .00001 | .025 | |
| 14 | 102.71 | 14q32.13 | rs1007813 | 2.471 | .0004 | ns | |
| Motor tasks | |||||||
| Pursuit | 8 | 112.26 | 8q22.3 | rs1460239 | 2.570 | .0003 | ns |
| Tracking | - | - | - | - | - | - | - |
| Tapping | 9 | 51.1 | 9p21.2 | rs9103 | 2.337 | .0005 | ns |
| Baseline speed | - | - | - | - | - | - | - |
| Motor timing | 2 | 142.45 | 2q21.1 | rs985162 | 3.944 | .00001 | .042 |
| 2 | 27.34 | 2p25.1 | rs1309 | 2.195 | .0007 | ns | |
| 17 | 66.1 | 17q12 | rs1859212 | 2.338 | .0005 | ns | |
| Combined score | 2 | 135.13 | 2q14.3 | rs1028184 | 2.516 | .0003 | ns |
| 12 | 0.86 | 12p13.33 | rs2107614 | 2.878 | .00014 | ns | |
Chromosome.
Most likely cytogenetic location according to draft genome-sequence data.
Empirical p values were derived with the –simulate option featured in the Merlin software by the running of 1000 simulations under the null hypothesis of no linkage, while preserving the original phenotypes, family structures, allele frequencies, linkage disequilibrium (LD) structure, and missing-data pattern.
Figure 1.
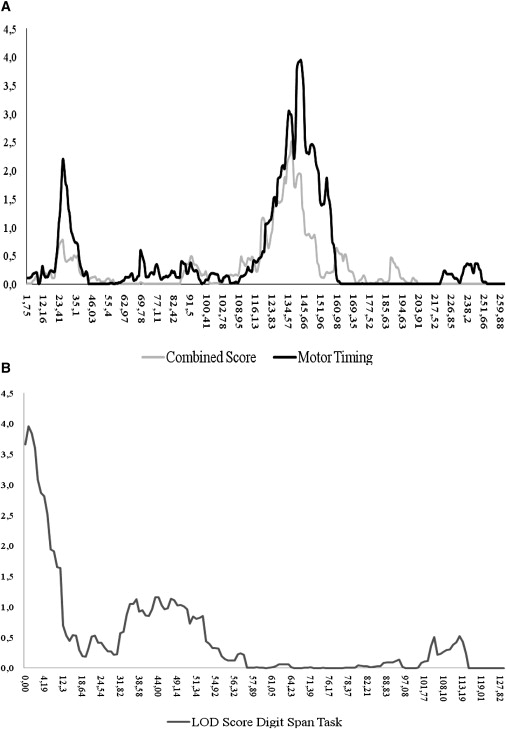
Graphs of Motor Timing and Digit Span LOD Scores
(A) Overlap of the LOD-score graphs of the motor timing scores and the combined scores on chromosome 2.
(B) LOD-score graph of digit span on chromosome 13.
The results of our study suggest the presence of candidate genes for ADHD neuropsychological endophenotypes in the two significantly linked chromosomal regions on chromosomes 2 and 13. These loci have not been previously reported in linkage studies using ADHD phenotypic measures. The 2q locus may be of special interest for neuropsychological functioning in ADHD, given that the linkage signal for the combined neuropsychological score overlapped with that of the Motor Timing score. The highest LOD score for Motor Timing was observed at SNP rs985162, which lies in a “gene desert” flanked by two hypothetical genes of unknown function, LOC151121 and LOC402102. Because the peak LOD score for the component score did not coincide with the peak score for Motor Timing, we selected a region of approximately 25 cM defined by the LOD-1 of the highest component-score peak, proximally, and that of the highest Motor Timing peak, distally, to search for candidate genes. According to the UCSC Genome Browser, 64 annotated (RefSeq) genes were present in this region. Several interesting candidate genes were found, which are listed in Table S1. Within the linkage peak on chromosome 13, 23 annotated genes were present, of which two may be likely candidate genes for neuropsychological dysfunctions in ADHD (Table S1). Of particular interest may be the genes of the TUBA3 family, because three TUBA3 genes are located within the two significant linkage peaks (TUBA3C [MIM 602528], on chromosome 13q12.11, and TUBA3D and TUBA3E, on chromosome 2q21.1). Genes of the TUBA1 family (previously known as the TUBA3 family, which is not the same family as that described here as TUBA3)17 have been associated with abnormalities in the laminar architecture of the hippocampus and the cortex accompanied by impaired neuronal migration in mouse mutants, and an association with lissencephaly [MIM 602529] has been shown in humans.18 It is not clear what role the genes of the (current) TUBA3 family play in this context, but they are expressed in the brain and their function is possibly related to that of the TUBA1 gene family.
In previous ADHD linkage studies using phenotypic measures of ADHD, one of the most reproducible linkage findings is the locus on chromosome 17p.11 This region has been hypothesized as being related to the serotonin-transporter gene (SLC6A4 [MIM 182138]) located on the other side of the centromere, on 17q11.1–q12.19 This is where we find suggestive linkage for Motor Timing (17q12), suggesting that the serotonin transporter may be related to the timing of motor output.
Loci in our study also show some overlap with other signals reported previously in ADHD linkage studies.11,20–22 For example, the linkage signal observed for Digit Span on chromosome 14q32 is located only 738 kb upstream of the signal found in the affected sib pair (ASP) study in the larger IMAGE sample.11 Furthermore, the linkage signal for Digit Span on chromosome 2p25.2 overlaps with the suggestive signal on 2p34.5 that was reported previously in the ASP study11 and also with the linkage signal on 2p25.1 reported for a latent-class measure of ADHD in combination with conduct disorder (CD).22 This could suggest that ADHD and CD might have a common basis in verbal-working-memory deficits related to chromosome 2p25. Previous studies have shown that ADHD in combination with CD (ADHD+CD) is not associated with more severe executive-functioning problems (like verbal working memory) than is ADHD alone,23 making this overlap in linkage signals more likely attributable to ADHD. Interestingly, overlap was also present between the region on chromosome 12p13 linked to overall neuropsychological functioning and the regions previously found for ADHD at 12p13.33,24 the latent class of ADHD+CD (12p13),22 the latent class of ADHD+nicotine dependence (12p12),22 and for a dichotomous measure of ADHD (12p13).25 This suggests that chromosome 12p12–p13 may harbor loci that relate to overall neuropsychological dysfunctioning and disorders characterized by impaired impulse control.
Two disorders, which share at least some susceptibility loci with ADHD,26 could be of particular interest for consideration in light of our linkage results: reading disability (RD [MIM 127700]) and autism [MIM 209850]. With respect to overlap with RD, we detected a LOD score of 2.02 for Digit Span (verbal working memory) and a score of 2.19 for Motor Timing on 2p25, a chromosomal location also reported for dyslexia (orthographic coding) [MIM 604254].25 Similarly, the locus for Visuo-Spatial Sequencing (visuo-spatial working memory) on chromosome 3p24 was close to a region linked to dyslexia (3p25, orthographic coding [MIM 606896]).25 Working-memory deficits and abnormalities in the timing of motor output have been reported in patients with RD, and the underlying brain-activation patterns during working-memory tasks and motor-timing tasks have also been reported to be abnormal in RD.27–30 These overlapping findings could suggest that loci on chromosomes 2p25 and 3p24–p25 might harbor pleiotropic loci that relate to deficits in working memory and motor timing seen in patients with ADHD and in those with RD.
Several overlapping regions were found between our study and those relating to autism as described in a recent review.31 The two most interesting loci are 13q12 and 14q32, which were related to Digit Span (verbal working memory) in our study and have been linked to autism.31 However, verbal working memory has generally been found to be intact in autism.32 It could be possible that loci on 13q12 and 14q32 have an effect on both ADHD and autism, but this effect is not comparable for both disorders (only in ADHD is this effect mediated by verbal-working-memory deficits). Such a differential effect could account for the fact that both disorders frequently co-occur (and share, in part, a common etiology) yet can be distinguished from each other in their neuropsychological and phenotypical presentations. Additional overlap with autism was found for the Time Test, on 4q35.31 We are not aware that a task similar to the one used here has ever been administered to children with autism for assessment of their time-reproduction abilities. However, given the fact that time reproduction is heavily related to executive function9 and that abnormalities in prefrontal circuits and executive functions have been found in autism,33 it may be expected that time reproduction is impaired in children with autism. Lastly, the loci for Visuo-Spatial Sequencing (visuo-spatial working memory), on 3p24, and for the Stop Task (inhibition), on 12q23, were in the vicinity of the linkage signals on 3p25 and 12q24 that have been reported repeatedly in relation to autism.31 Deficits in the underlying brain-activation patterns of visuo-spatial working memory and inhibition have indeed been reported in autism.34,35 In sum, several loci found in our study to be linked to neuropsychological traits have been reported previously in linkage studies in RD and autism, providing preliminary directions for future studies into the shared biological pathways of these disorders and ADHD.
Our findings should be viewed in the light of a few limitations. First, we chose not to adjust our findings for multiple testing. We refrained from doing so because the neuropsychological traits were correlated and because the current study is explorative in nature. However, the current findings need replication before firm conclusions can be drawn in terms of the full role of neuropsychological traits as relevant tools for finding genes related to ADHD risk. In addition, the neuropsychological measures used here are by no means representative of the full domain of neuropsychological functions and tasks relevant for ADHD and of ADHD's complete variability. Given this latter point, at this point we also cannot entirely exclude the possibility that not all linkage findings really explain variance of the trait that is relevant to ADHD. However, given that many linkage findings for neuropsychological traits reported here overlap with linkage signals in other studies in ADHD and related psychiatric disorders, this is not very likely for most findings.
In conclusion, candidate neuropsychological ADHD endophenotypes proved useful in ADHD linkage analyses. Two genome-wide significant linkage signals (2p21.1 and 13q12.11) and ten suggestive linkage signals have been found. These results suggest that candidate neuropsychological ADHD endophenotypes can aid in the discovery of ADHD genes through linkage analysis. Endophenotype approaches may increase power to detect susceptibility loci in other complex disorders as well. The identified loci in the current study may be targeted in future studies to gain a more detailed understanding into the genetic architecture of neuropsychological deficits in ADHD, including the mediating and moderating roles that environmental factors and gene-environment interactions might play.
Acknowledgments
The authors thank all of the parents, teachers, and children who participated. The authors are also grateful to Kaixin Zhou for providing the cleaned dataset and supporting the linkage analysis. N.R., M.A., E.F., C.B., P.A., S.V.F., J.O., and B.F. have no financial ties to disclose. J.K.B. has been a consultant to, member of the advisory board of, and/or speaker for Janssen Cilag BV, Eli Lilly, Bristol-Myer Squibb, UBC, Shire, and Medice. J.A.S. has been a member of the advisory board of Eli Lilly, Shire, and Janssen Cilag. This study was partly funded by a grant assigned to Stephen Faraone by the National Institute of Mental Health (NIH grant no. R01 MH62873-01A1).
Supplemental Data
Web Resources
The URLs for data presented herein are as follows:
IMAGE study, http://image.iop.kcl.ac.uk/
Merlin software, http://www.sph.umich.edu/csg/abecasis/Merlin/index.html
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
SOLAR, http://www.sfbr.org/solar/
UCSC Genome Browser, http://genome.ucsc.edu/cgi-bin/hgGateway
References
- 1.American Psychiatric Association . Fourth Edition. American Psychiatric Press; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Faraone S.V., Doyle A.E. Genetic influences on attention deficit hyperactivity disorder. Curr. Psychiatry Rep. 2000;2:143–146. doi: 10.1007/s11920-000-0059-6. [DOI] [PubMed] [Google Scholar]
- 3.Kuntsi J., Neale B.M., Chen W., Faraone S.V., Asherson P. The IMAGE project: Methodological issues for the molecular genetic analysis of ADHD. Behav. Brain Funct. 2006;2:27. doi: 10.1186/1744-9081-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 5.Rommelse N.N., Altink M.E., De Sonneville L.M., Buschgens C.J., Buitelaar J., Oosterlaan J., Sergeant J.A. Are motor inhibition and cognitive flexibility dead ends in ADHD? J. Abnorm. Child Psychol. 2007;35:957–967. doi: 10.1007/s10802-007-9146-z. [DOI] [PubMed] [Google Scholar]
- 6.Rommelse N.N., Altink M.E., Oosterlaan J., Beem L., Buschgens C.J., Buitelaar J., Sergeant J.A. Speed, variability, and timing of motor output in ADHD: Which measures are useful for endophenotypic research? Behav. Genet. 2007 doi: 10.1007/s10519-007-9186-8. Published online December 11, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rommelse N.N., Altink M.E., Oosterlaan J., Buschgens C.J., Buitelaar J., De Sonneville L.M., Sergeant J.A. Motor control in children with ADHD and non-affected siblings: deficits most pronounced using left hand. J. Child Psychol. Psychiatry. 2007;48:1071–1079. doi: 10.1111/j.1469-7610.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 8.Rommelse N.N.J., Altink M.E., Oosterlaan J., Buschgens C.J.M., Buitelaar J., Sergeant J.A. Support for an independent familial segregation of executive and intelligence endophenotypes in ADHD-families. Psychol. Med. 2008 doi: 10.1017/S0033291708002869. Published online February 8, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Rommelse N.N., Oosterlaan J., Buitelaar J., Faraone S.V., Sergeant J.A. Time reproduction in children with ADHD and their non-affected siblings. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:582–590. doi: 10.1097/CHI.0b013e3180335af7. [DOI] [PubMed] [Google Scholar]
- 10.Brookes K., Xu X., Chen W., Zhou K., Neale B., Lowe N., Anney R., Franke B., Gill M., Ebstein R. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: Association signals in DRD4, DAT1 and 16 other genes. Mol. Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 11.Asherson P., Zhou K., Anney R.J.L., Franke B., Buitelaar J., Ebstein R.P., Gill M., Altink M., Arnold R., Boer F. A high-density SNP linkage scan with 142 combined subtype ADHD sib pairs identifies replicated linkage regions on chromosomes 9 and 16. Mol. Psychiatry. 2008 doi: 10.1038/sj.mp.4002140. Published online January 8, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Almasy L., Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abecasis G.R., Cherny S.S., Cookson W.O., Cardon L.R. Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 14.Abecasis G.R., Wigginton J.E. Handling marker-marker linkage disequilibrium: Pedigree analysis with clustered markers. Am. J. Hum. Genet. 2005;77:754–767. doi: 10.1086/497345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou K., Asherson P., Sham P., Franke B., Anney R.J., Buitelaar J., Ebstein R., Gill M., Brookes K., Buschgens C. Linkage to chromosome 1p36 for attention deficit hyperactivity disorder traits in school and home settings. Biol. Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.024. Published online April 23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lander E., Kruglyak L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat. Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 17.Khodiyar V.K., Maltais L.J., Sneddon K.M.B., Smith J.R., Shimoyama M., Cabral F., Dumontet C., Dutcher S.K., Harvey R.J., Lafanechère L. A revised nomenclature for the human and rodent αtubulin gene family. Genomics. 2007;90:285–289. doi: 10.1016/j.ygeno.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Keays D.A., Tian G., Poirier K., Huang G.J., Siebold C., Cleak J., Oliver P.L., Fray M., Harvey R.J., Molnár Z. Mutations in α-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogdie M.N., Macphie I.L., Minassian S.L., Yang M., Fisher S.E., Francks C., Cantor R.M., McCracken J.T., McGough J.J., Nelson S.F. A genomewide scan for attention-deficit/hyperactivity disorder in an extended sample: Suggestive linkage on 17p11. Am. J. Hum. Genet. 2003;72:1268–1279. doi: 10.1086/375139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faraone S.V., Doyle A.E., Lasky-Su J., Sklar P.B., D'Angelo E., Gonzalez-Heydrich J., Kratochvil C., Mick E., Klein K., Rezac A.J., Biederman J. Linkage analysis of attention deficit hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007 doi: 10.1002/ajmg.b.30631. Published online December 14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebebrand J., Dempfle A., Saar K., Thiele H., Herpertz-Dahlmann B., Linder M., Kiefl H., Remschmidt H., Hemminger U., Warnke A. A genome-wide scan for attention-deficit/hyperactivity disorder in 155 German sib-pairs. Mol. Psychiatry. 2006;11:196–205. doi: 10.1038/sj.mp.4001761. [DOI] [PubMed] [Google Scholar]
- 22.Jain M., Palacio L.G., Castellanos F.X., Palacio J.D., Pineda D., Restrepo M.I., Muñoz J.F., Lopera F., Wallis D., Berg K. Attention-deficit/hyperactivity disorder and comorbid disruptive behavior disorders: Evidence of pleiotropy and new susceptibility loci. Biol. Psychiatry. 2007;61:1329–1339. doi: 10.1016/j.biopsych.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Oosterlaan J., Scheres A., Sergeant J.A. Which executive functioning deficits are associated with AD/HD, ODD/CD and comorbid AD/HD+ODD/CD? J. Abnorm. Child Psychol. 2005;33:69–85. doi: 10.1007/s10802-005-0935-y. [DOI] [PubMed] [Google Scholar]
- 24.Romanos M., Freitag C., Jacob C., Craig D.W., Dempfle A., Nguyen T.T., Halperin R., Walitza S., Renner T.J., Seitz C. Genome-wide linkage analysis of ADHD using high-density SNP arrays: Novel loci at 5q13.1 and 14q12. Mol. Psychiatry. 2008 doi: 10.1038/mp.2008.12. Published online February 26, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Fisher S.E., Francks C., Marlow A.J., MacPhie I.L., Newbury D.F., Cardon L.R., Ishikawa-Brush Y., Richardson A.J., Talcott J.B., Gayan J. Independent genome-wide scans identify a chromosome 18 quantitative-trait locus influencing dyslexia. Nat. Genet. 2002;30:86–91. doi: 10.1038/ng792. [DOI] [PubMed] [Google Scholar]
- 26.Banaschewski T., Hollis C., Oosterlaan J., Roeyers H., Rubia K., Willcutt E., Taylor E. Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Dev. Sci. 2005;8:132–140. doi: 10.1111/j.1467-7687.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- 27.Waber D.P., Weiler M.D., Bellinger D.C., Marcus D.J., Forbes P.W., Wypij D., Wolff P.H. Diminished motor timing control in children referred for diagnosis of learning problems. Dev. Neuropsychol. 2000;17:181–197. doi: 10.1207/S15326942DN1702_03. [DOI] [PubMed] [Google Scholar]
- 28.Smith-Spark J.H., Fisk J.E. Working memory functioning in developmental dyslexia. Memory. 2007;15:34–56. doi: 10.1080/09658210601043384. [DOI] [PubMed] [Google Scholar]
- 29.Vasic N., Lohr C., Steinbrink C., Martin C., Wolf R.C. Neural correlates of working memory performance in adolescents and young adults with dyslexia. Neuropsychologia. 2008;46:640–648. doi: 10.1016/j.neuropsychologia.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Nicolson R.I., Fawcett A.J., Berry E.L., Jenkins I.H., Dean P., Brooks D.J. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353:1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- 31.Yang M.S., Gill M. A review of gene linkage, association, and expression studies in autism and an assessment of convergent evidence. Int. J. Dev. Neurosci. 2007;25:69–85. doi: 10.1016/j.ijdevneu.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Williams D.L., Goldstein G., Minshew N.J. The profile of memory function in children with autism. Neuropsychol. 2006;20:21–29. doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loveland K.A., Bachevalier J., Pearson D.A., Lane D.M. Fronto-limbic functioning in children and adolescents with and without autism. Neuropsychologia. 2008;46:49–62. doi: 10.1016/j.neuropsychologia.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silk T.J., Rinehart N., Bradshaw J.L., Tonge B., Egan G., O'Boyle M.W., Cunnington R. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: A functional MRI study. Am. J. Psychiatry. 2006;163:1440–1443. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- 35.Sinzig J., Morsch D., Bruning N., Schmidt M.H., Lehmkuhl G. Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD-symptoms. Child Adolesc. Psychiatry Ment. Health. 2008;2:4. doi: 10.1186/1753-2000-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


