Abstract
In the present study, we used fMRI to examine the influence of age on two other known risk factors for Alzheimer’s Disease (AD), APOE genotype and parental history of AD (FH status), during episodic encoding (ENC) and metacognitive self-appraisal (SA) paradigms. These paradigms have previously been shown to evoke activity from brain regions that are implicated in AD. First we examined the effect of age across the adult lifespan (age 18 to 84) on cerebral activity in a large sample (n=231) of cognitively healthy individuals. Next we examined a subset (n=155) on whom APOE status and FH status were known. For ENC, we found that increasing age was associated with reduced activity in the ventral temporal lobes and hippocampus. Our analysis of risk factors suggested that FH and age exerted independent effects, but APOE interacted with age such that APOE e4 carriers exhibit age-related increases in activity in the hippocampus. For the metacognitive SA task, increasing age was found to be associated with reduced activity in the medial prefrontal cortex, and increased activity in the mesial temporal lobe, posterior orbital cortex and striatum. Neither AD risk factor significantly modified age-related changes in brain activity during SA. These results suggest that FH and aging are exerting independent effects in both tasks while APOE affected the relationship with age in the hippocampus in one of the two tasks given.
Keywords: aging, Alzheimer Disease, hippocampus, functional imaging, self, APOE genotype
1. Introduction
It is well known that increasing age is associated with a host of changes in cognitive functions including reduced information processing speed and reduced episodic memory ability. Several studies have documented age-related declines in memory ability using word-list learning tasks and other memory paradigms. Brain imaging studies have also revealed age-related reductions in cerebral blood flow and cerebral metabolic rate of oxygen consumption (Pantano et al., 1984), glucose metabolism in the frontal and temporal lobes (De Santi et al., 1995), white matter integrity measured using diffusion tensor imaging (DTI) (Ardekani, Kumar, Bartzokis, & Sinha, 2007; see Malloy, Correia, Stebbins, & Laidlaw, 2007 for review), and volume reductions in brain regions important for episodic memory such as the medial temporal lobes (MTL) and prefrontal cortex (see Raz, Rodrigue, & Haacke, 2007 for review). Recent evidence from functional MRI studies of aging suggest that increasing age is also associated with changes in brain activity across the lifespan on several tasks including both episodic and working memory paradigms (see Persson & Nyberg, 2006 for reveiw).
Age is also the greatest risk factor for the development of Alzheimer’s disease (AD). Recent studies have suggested that the neuropathologic changes in AD may begin decades prior to symptomatic disease (Reiman et al., 1996; Reiman et al., 2004; Sager, Hermann, & La Rue, 2005). If this is true, then it is likely that people destined to develop AD will have a different trajectory of brain aging than those who are not predisposed. In our laboratory, we have begun to address this by enrolling people with risk factors for AD and analyzing cross-sectional differences in fMRI activation patterns associated with risk.
We have used 1) an episodic encoding paradigm (ENC) that activates the MTL, and 2) a meta-cognitive, self-appraisal paradigm (SA) that results in robust activity in medial frontal and temporal cortex as well as posterior cingulate cortex. These tasks were selected because the regions they activate are known to be particularly vulnerable to early AD pathology (Buckner et al., 2005). In our encoding studies, we have used a novel/familiar picture discrimination paradigm to evaluate changes in brain activity in individuals with risk factors for AD. We have reported reduced activation in the MTL during ENC in individuals with mild cognitive impairment-amnesic type (AMCI) (Johnson et al., 2006a), a prodromal stage of AD (see also Johnson et al., 2004; Machulda et al., 2003), and in cognitively healthy and asymptomatic middle-aged (mean age 54) individuals with a positive parental history of AD (Johnson et al., 2006b). The latter effect was found to be more pronounced for people with a parental history of AD that were also carriers of the APOE e4 allele (Trivedi et al., 2006).
In our studies of metacognitive SA, we have used a paradigm in which participants are asked to make self-referential and semantic decisions on trait adjectives (Schmitz, Rowley, Kawahara, & Johnson, 2006). In these studies we have also observed that parental history of AD reduces activity. In this case the effect was in the hippocampus and medial parietal lobe in middle-aged asymptomatic adults (Johnson et al., in press). Because the accuracy of self-appraisals (or insight) is a meta-cognitive function that may be impaired in AD and AMCI (Vogel et al., 2004), we next conducted a study in which we behaviorally assessed AMCI patients regarding insight into their cognitive deficits, and correlated this insight score against fMRI activity in the self-appraisal task described above. FMRI activation in patients with AMCI correlated with the degree of insight that these individuals had regarding their cognitive deficits. Those AMCI patients who were less aware of their cognitive deficits also had less activity in the posterior cingulate cortex and medial frontal lobe (Ries et al., 2007).
The current study presents data regarding the interaction between age, parental history of AD, and APOE genotype and the independent effects of age on fMRI activation during memory encoding and self appraisal in a large sample of cognitively healthy individuals between 18 and 83 years of age. For this report, the data from healthy, cognitively normal adults (Johnson et al., in press; Johnson et al., 2006a; Johnson et al., 2006b; Ries et al., 2007; Trivedi et al., 2006) were utilized to examine the effect of age of fMRI activation. This included cognitively normal healthy elderly with and without risk factors for AD, a large group of healthy middle-aged subjects with and without risk factors for AD, and young adults. The combined dataset included 231 healthy subjects 18-84 years of age. Based on previous studies from our lab and others we hypothesized that increasing age would be associated with reduced activation in MTL and ventral temporal regions during memory encoding and in prefrontal cortex and posterior cingulate regions important for self appraisal, and that this age-related reduction would be significantly influenced by AD risk factors.
2. Materials and Methods
2.1. Subjects
Two hundred thirty-one subjects were studied with ENC and SA fMRI tasks (see Table 1). All participants were cognitively healthy individuals between 18 and 84 years of age. Recruitment was through advertisement in the community, and through the Wisconsin Registry for Alzheimer’s Prevention (WRAP) (Sager et al., 2005), a longitudinal cohort study of adults with or without risk factors for AD. The inclusion criteria for all subjects consisted of the following: normal cognitive function determined by neuropsychological evaluation (described below), no current diagnosis of major psychiatric disease or other major medical conditions (e.g., diabetes, myocardial infarction, or recent history of cancer), no history of head trauma, and MRI scanner compatibility. All subjects included in the overall statistical analysis were required to have useable imaging data (movement in the x, y, and z plane < 3 mm). For the AD risk factor analyses APOE genotype and known parental FH status were also required.
Table 1.
Demographic and cognitive characteristics
| Mean (SD) | Range/% | |
|---|---|---|
| Total Sample | ||
|
| ||
| N=230 | ||
| Age | 49.1 (17.0) | 18-84 |
| Sex | 139 f / 91 m | 60% f |
| Education | 15.9 (2.5) | 10-22 |
| Subjects receiving ENC | N=208 | 90% |
| Subjects receiving SA | N=203 | 88% |
| Risk Factors Analysis | Mean | Range |
|
| ||
| N=155 | ||
| Age | 57.2 (8.9) | 41-84 |
| Sex | 96 f / 59 m | 62% f |
| Education | 16.2 (3.9) | 10-22 |
| APOE e4+ | N=52 | 34% |
| Parental FH+ | N=68 | 44% |
| WRAT-III Reading SS | 108.7 (7.9) | |
| Trails A (seconds) | 29.0 (10.3) | |
| Trails B (seconds) | 64.0 (22.4) | |
| RAVL 1-5 (total) | 48.9 (7.9) | |
| RAVL T7 (words recalled) | 9.6 (2.7) | |
| Subjects receiving ENC | N=148 | 95% |
| Subjects receiving SA | N=150 | 97% |
The brief neuropsychological test battery used to ensure normal cognitive function included the Rey Auditory Verbal Learning Test (RAVLT) or California Verbal Learning Test for the younger adults; the Wide Range Achievement Test-3 (WRAT-3) reading subtest, and Trail Making Test A and B. We have previously reported that there were no meaningful significant baseline differences between cognitively healthy individuals with and without AD risk factors (Johnson et al., 2006a; Sager et al., 2005; Trivedi et al., 2006).
2.2. Design
We conducted two analyses with each of the fMRI tasks. The main analysis examined the effect of age alone on the cerebral response. Of the 231 unique subjects, 208 had the ENC task and 203 subjects had the SA task. The majority of subjects (181 subjects) received both tasks; SA followed by ENC during the same fMRI scanning session.
The second analysis examined a subset of the total sample. This analysis utilized the AD risk factors of APOE epsilon4 status (present or absent) and parental family history of AD (FH; present or absent) to determine whether these influenced the slope of aging. There were 155 subjects (age range = 41-84 years) on whom APOE genotype and parental history of AD was known (148 and 150 subjects for ENC and SA tasks respectively). 44% (n=68) had a positive parental history of AD which was verified by consensus panel review of medical records. The remaining group of participants self-reported a negative parental history of AD in a detailed medical history questionnaire and interview. There were 52 subjects who were APOE4 positive (34%). This is higher than the population base-rate of 15% because we recruited from a cohort of subjects at risk for AD (Sager et al., 2005).
2.3. fMRI Tasks
2.3.1. Episodic Memory Encoding ENC
This task has been described previously (Johnson et al., 2006b; Trivedi et al., 2006). Briefly, the task consisted of serial presentations of line drawings (Snodgrass & Vanderwart, 1980) during which participants distinguished between novel (NV) and previously learned (PL) items. PL items were presented in two separate training sessions (45 and 15 minutes prior to the task). The training items were presented iteratively in pseudorandom fashion for a total of 30 exposures to each item. The participants were instructed to view the pictures and try to remember them. During the fMRI test session, items were presented at 3000 ms intervals, and each picture was presented for 2800 ms with a 200 ms interstimulus interval. NV pictures were intermixed with the presentation of PL pictures using a variable-length block (boxcar) paradigm. The task, regardless of condition was always to decide if the current picture was old or new. Therefore, the subjects were always engaged in the same cognitive set for the duration of the scan. Epoch length ranged from 1 to 5 events that were appropriately balanced across blocks and conditions. Two alternate forms of the task were presented (order counterbalanced) using the same PL items, but different NV items. Responses to each item were made with a two-button MRI compatible response device held in the right hand. Task duration was 9 minutes and 24 seconds, over the two runs.
2.3.2. Meta-cognitive Self-appraisal
The SA task (described in Johnson et al., in press; Ries et al., 2007; Schmitz & Johnson, 2006) employed a block (boxcar) design that consisted of two conditions: a metacognitive self-appraisal (self) condition and a baseline (semantic decision) condition. For the self condition, trait adjectives were presented on the screen one at a time, and participants made a yes/no decision about whether the word described them. In the semantic decision condition, participants were presented with the same trait adjectives seen during the self condition, and asked to decide whether the presented word was positive or not. In both conditions, the adjectives were presented every 4000 ms (displayed for 3000 ms followed by a 1000 ms second interstimulus interval) in blocks of six. First presentation of the items was counterbalanced across condition to avoid confounding with novelty. An index finger button press indicated “no”, and the middle finger indicated “yes”. Two alternate forms of the task with identical timing were presented sequentially (order was counterbalanced) using a discrete 30-adjective set. The text color for each condition was slightly different and a prompt was displayed at the top of the screen to inform participants about the condition. The combined task duration was 8 min 16 s.
2.4. Imaging Procedures
A GE 3.0 Tesla long bore MRI scanner outfitted with an MR-compatible button-box and high-resolution goggles set at 800 × 600 (Resonance Technology; Northridge, CA) were used for fMRI imaging and stimulus presentation. The software Presentation (www.neurobs.com) was used to deliver visual stimuli and record responses in synchrony with slice acquisition and stimulus delivery. A T2*-weighted gradient-echo, echo-planar image (EPI) pulse sequence was obtained after higher order shimming and acquisition of a gradient echo phase map (see Johnson et al., 2006). EPI parameters included: flip angle = 90°; acquisition matrix = 64 × 64 voxels; field of view (FOV) = 240 mm; echo time (TE) = 30 ms; repetition time (TR) = 2000 ms. Thirty sagittal slices of the brain were acquired within the TR at each time point, with a voxel resolution of 3.75 × 3.75 × 4 mm and a 1-mm skip between slices.
The fMRI data were processed using Statistical Parametric Mapping (SPM2) statistical software. The time series images were motion-corrected to reduce the effects of head movement during the scan session. 3D field maps across the brain taken co-planar with the fMRI slices were used to correct inhomogeneity induced distortions in the image files. The images were then normalized into standard atlas space (using the T2* weighted template from SPM2) and written out at a 2 × 2 × 2 voxel resolution, and then smoothed with an 8 mm full-width, half-maximum Gaussian kernel.
Statistical analyses of the time-series data were performed using the General Linear Model in SPM2. For each participant, the time-series statistical model included convolution with the canonical hemodynamic response function and high frequency signal filtering (high pass filter = 128 seconds). Temporal autocorrelation was estimated using a first-order autoregression estimation on supra-threshold voxels.
2.5. Second-level Analyses
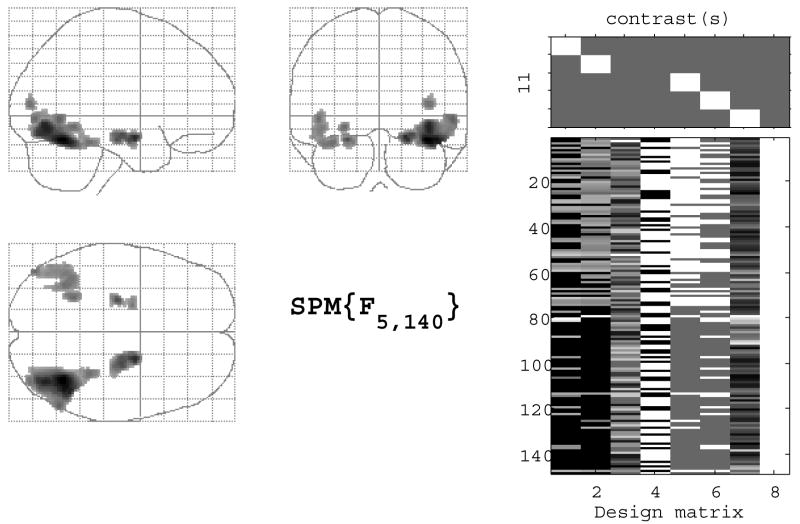
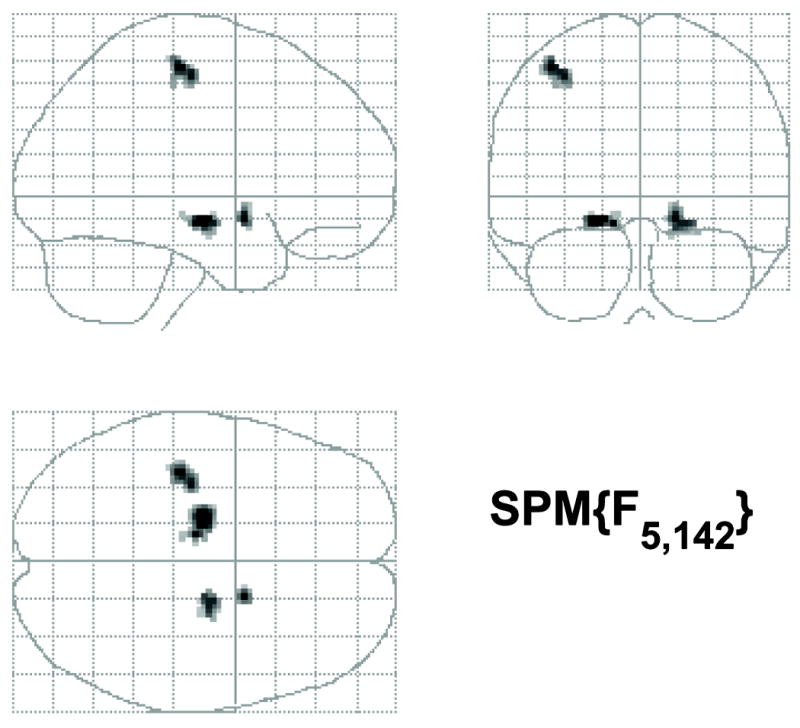
The contrasts examined in this study included NV versus PL, for ENC, and the self condition relative to the baseline semantic decision condition for SA. For the second-level group analyses, we employed a non-parametric rank-based multiple regression method customized for use in SPM2 (Gleason et al., 2006). The main analysis of aging effects on fMRI activation included education and gender as covariates. A second non-parametric multiple regression analysis that was restricted to only subjects with known AD risk factor status of FH and APOE genotype was next conducted for ENC and SA tasks. These analyses included the risk factors Age, FH, APOE as well as the interactions between these risk factors. Gender, education and the constant (see Fig. 3) were also included. Two F contrasts were specified: the ‘full’ model consisted of any risk factor (see Fig. 3). The second F contrast was reduced to only the age × APOE and age × FH interaction columns and only included voxels identified in the full model.
Fig. 3.
The left panel is the maximum intensity projection F-map for global risk (n = 148) for ENC; the right panel displays the design matrix of the statistical model for this analysis.
In order to avoid spurious results, and to ensure that findings were interpretable with regard to the cognitive task that was performed, the regression analyses were restricted to regions in which there was a significant main effect of task across all subjects using a false-discovery rate (FDR) corrected p-value of 0.05, which corresponded to a critical t-value of 2.25 for ENC and 4.62 for SA. This procedure reduces the risk of false positive errors from the thousands of voxel-wise tests that are performed by reducing the search region to those voxels in which the entire group of subjects displayed significant activation for a given contrast at a liberal threshold. It also facilitates interpretation of the findings by ensuring that the available voxels for analysis are only those that are responsive to the task. To examine aging and risk factor effects, a statistical threshold of p < 0.001 (uncorrected) was used for the regression analyses examining age-related changes in brain activity and the interactions between APOE genotype, FH status, and age.
3. Results
3.1. Behavioral Performance
The reaction times for the main aging analysis (n = 208) of the ENC task was 0.90 seconds (0.2) for NV items and 0.80 seconds (0.1) for the PL items. Accuracy was 98% (4.0) for NV items, and 98% (4.2) for PL items. The reaction times for the main aging analysis (n = 203) of the self-appraisal task were 1.61 seconds (0.2) for self condition and 1.71 seconds (0.3) for the semantic condition.
3.2. fMRI Non-parametric Multiple Regression Analyses
3.2.1. Episodic Encoding Task
The main effect of task for ENC across all 208 subjects is displayed in Fig. 1 and Table 2. This is the average effect of task across all subjects and included most prominently the ventral and medial temporal lobes bilaterally.
Fig. 1.
T maps of the main effect of task for ENC task (n = 208). The statistical threshold was set at p = 0.05, FDR corrected for multiple comparisons.
Table 2.
Locations and statistics for the fMRI tasks across all subjects.
| Region (k) | MNI coordinates
|
Peak T value | P-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
|
ENC Encoding task (n = 208)
|
|||||
| R ventral temporal cortex (38661) | 32 | -50 | -18 | 20.63 | < 0.0001 |
| - L hippocampus | -24 | -8 | -20 | 10.24 | < 0.0001 |
| - R hippocampus | 28 | -6 | -22 | 9.94 | < 0.0001 |
| L superior frontal gyrus (2690) | -8 | 20 | 48 | 5.51 | < 0.0001 |
| L lateral parietal cortex (290) | -60 | -28 | 36 | 4.03 | < 0.0001 |
|
|
|||||
|
SA Self-appraisal task (n = 203)
|
|||||
| L retrosplenial cortex (14724) | -6 | -58 | 22 | 22.30 | < 0.0001 |
| - L hippocampus | -24 | -6 | -16 | 9.63 | < 0.0001 |
| - R hippocampus | 22 | -4 | -14 | 5.24 | < 0.0001 |
| L medial prefrontal cortex (12205) | -8 | 56 | 8 | 16.76 | < 0.0001 |
| L occipital gyrus (810) | -52 | -72 | 28 | 12.47 | < 0.0001 |
| Posterior cingulate gyrus (461) | 0 | -16 | 36 | 8.87 | < 0.0001 |
| L lateral parietal cortex | -48 | -24 | 58 | 3.44 | 0.003 |
The statistical threshold was set at p (FDR) < 0.05.
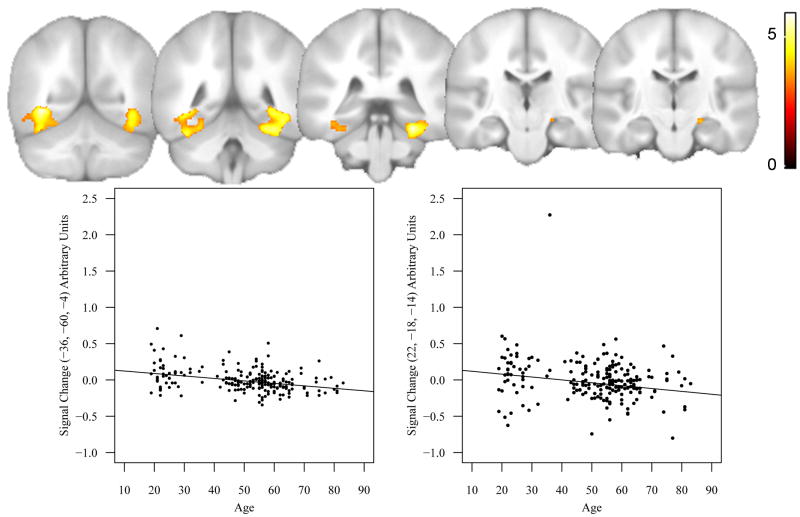
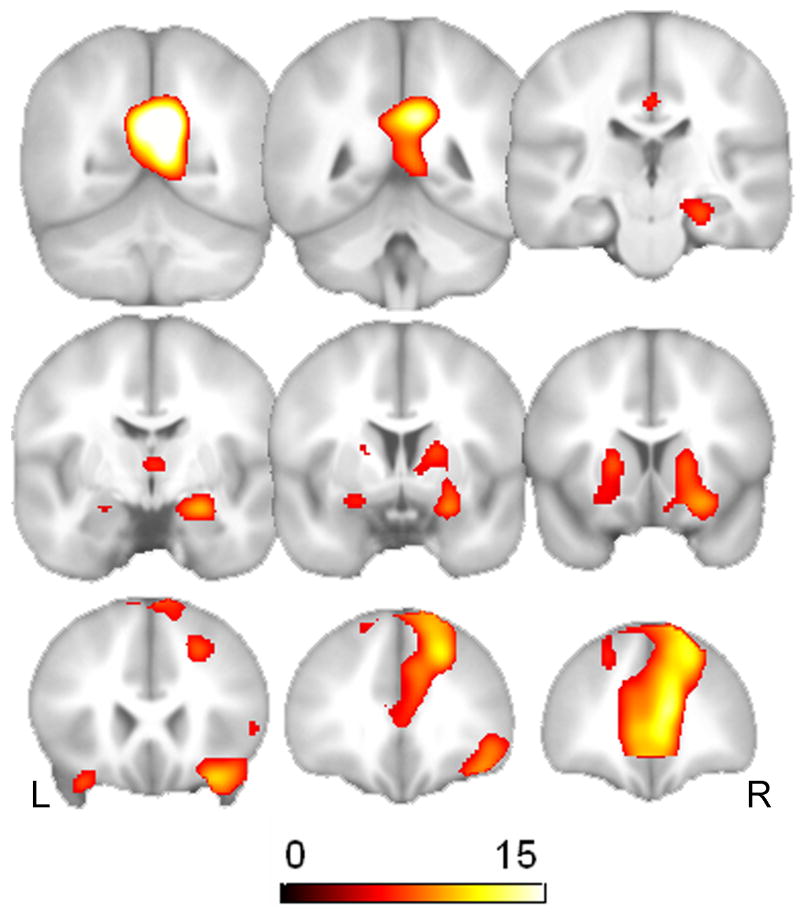
The multiple regression analysis modeled the effect of age, controlling for education and gender. Fig. 2 displays the significant main effect of age in the right (x, y, z = 28, -38, -20, t = 5.85, p < 0.0001, uncorrected) and left ventral temporal lobes (x, y, z = -36, -60, -4, t = 5.85, p < 0.0001, uncorrected). In addition, we also found a main effect of age in the right hippocampus (x, y, z = 22, -18, -14, t = 3.97, p < 0.0001, uncorrected). Table 3 provides statistics, locations, and cluster size for all significantly activated regions. There were no regions found to display significant age-related increases in activation for ENC.
Fig. 2.
The top panel shows the T maps for the results of the multiple regression analysis of aging effects (n = 208) for the ENC task. The statistical threshold was set at p = 0.001, uncorrected for multiple comparisons. The analyses were restricted to voxels that displayed a main effect of task, which are displayed in Fig. 1. Also shown are scatter plots of age-related decline in signal change in the left ventral temporal cortex (left panel) and the right hippocampus (right panel). The adjusted rank-estimation fitted lines are also shown.
Table 3.
Montreal Neurological Institute (MNI) coordinates for the regression analyses (k = cluster size).
| Region (k) | MNI coordinates
|
Peak T value | P-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
|
ENC Encoding task (n = 208)
|
|||||
| Main effect of age (decrease) | |||||
| R ventral temporal lobe (1821) | 28 | -38 | -20 | 5.85 | < 0.0001 |
| L ventral temporal lobe (1890) | -36 | -60 | -4 | 5.85 | < 0.0001 |
| R Hippocampus (28) | 22 | -18 | -14 | 3.97 | < 0.0001 |
|
SA Self-appraisal Task (n = 203)
|
|||||
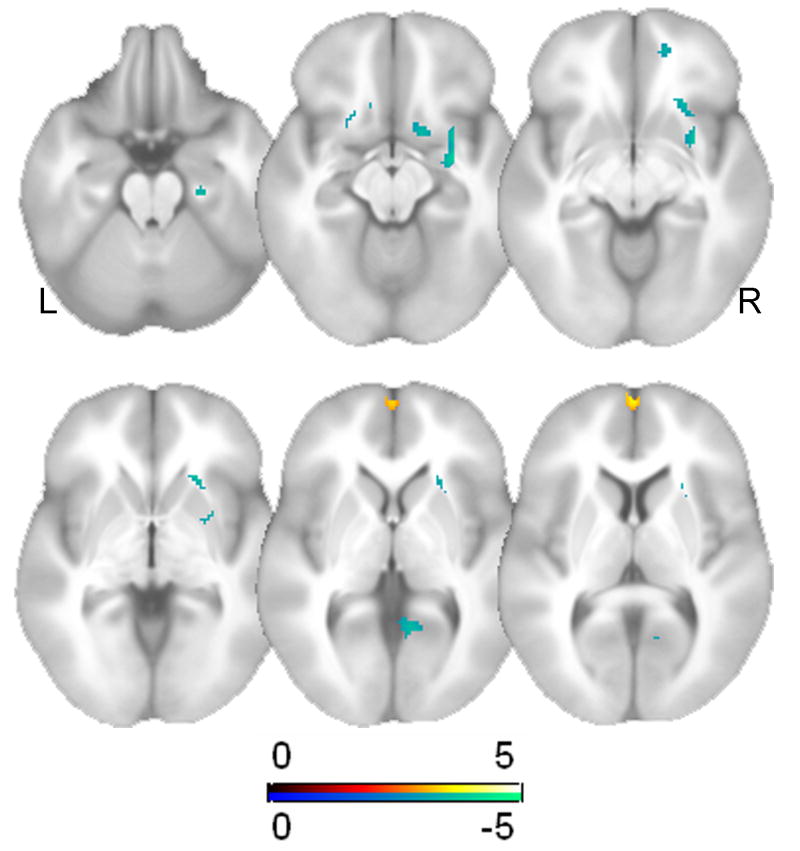
| Main effect of age (increase) | |||||
| L lateral parietal cortex (208) | -50 | -20 | 56 | 4.84 | < 0.0001 |
| R amygdala/posterior orbitofrontal gyrus (107) | 30 | -4 | -12 | 4.06 | < 0.0001 |
| L orbitofrontal gyrus (44) | -12 | 20 | -16 | 3.98 | < 0.0001 |
| R basal forebrain (27) | 14 | 8 | -14 | 3.80 | < 0.0001 |
| R occipital gyrus (29) | 10 | -52 | 2 | 3.54 | <0.0001 |
| Main effect of age (decrease) | |||||
| Medial prefrontal cortex | 0 | 62 | 6 | 3.82 | < 0.0001 |
The statistical threshold was set at p < 0.001, uncorrected
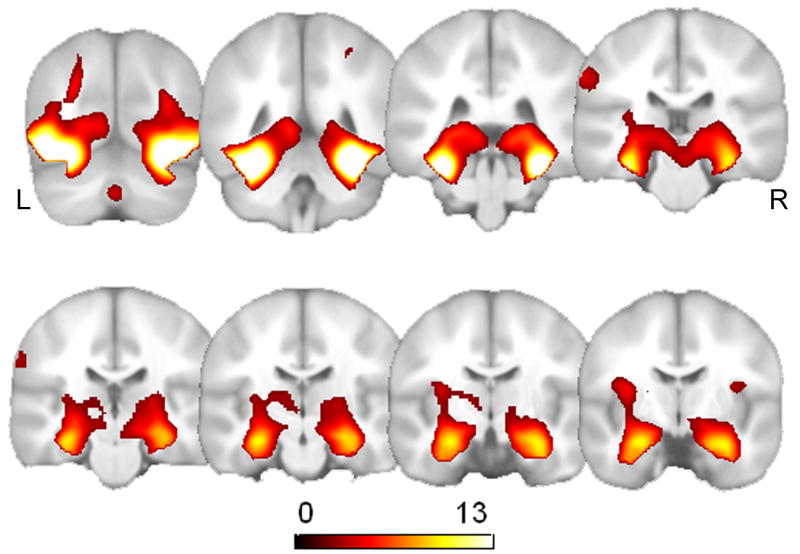
To more closely evaluate the effects of the risk factors and the interactions between them, an F-test was conducted on the columns contain risk factors (age, FH, and APOE) and their interactions age × FH status and age × APOE genotype interactions. This F-contrast would tell us where any of the risk factors or the interactions between them displayed an effect in the brain. The global risk factor analysis revealed five clusters that exhibited a global effect of risk: bilaterally in the ventral temporal cortex (x, y, z = 38, -58, -18, F = 10.90; x, y, z = -46, -80, -2, F = 6.31), bilaterally in the hippocampus (x, y, z = 20, -4, -16, F = 8.71; x, y, z = -24, -18, -18, F = 7.11), and the left fusiform gyrus (x, y, z = -26, -50, -8, F = 6.37).
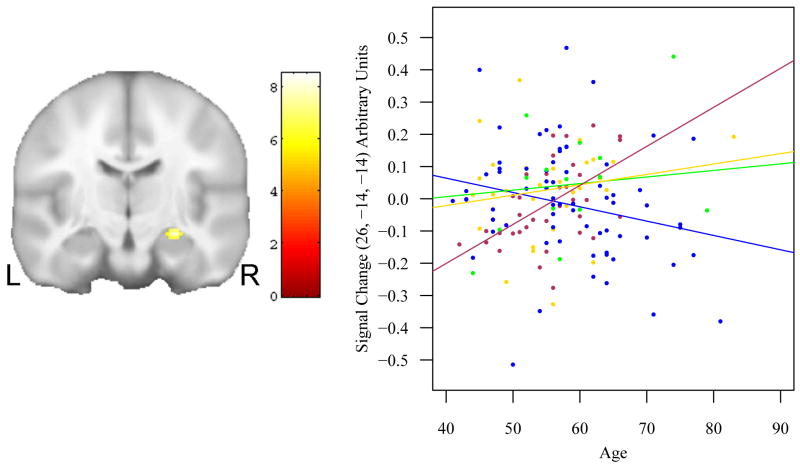
We then tested the age × APOE genotype and age × FH status interactions in those voxels showing any affect of risk. This analysis allowed us to determine whether any of the voxels active in Fig 3 were attributable to two or three way risk factor interactions with age. This analysis failed to reveal any brain regions displaying interaction effects. We lowered the statistical threshold to p < 0.005, uncorrected, and found a two-way interaction in the head of the right hippocampus (x, y, z = 26, -14, -14, F = 8.14, p < 0.0001, uncorrected) (see Fig. 4). The scatter plots in Fig. 4 indicate that APOE e4 carriers who were also FH+ displayed age-related increases in activation at this location. It is notable that this cluster is small in comparison to the F test for any risk factor shown in Fig. 3, suggesting that the age × risk interactions are fairly small and focal in this sample, and only present at a more liberal threshold; as such, this result will be interpreted with circumspection.
Fig. 4.
A coronal view indicates the single region (right hippocampus), that exhibited a significant risk × age interaction for ENC. The scatter-plot and rank-estimation fitted lines to the right further describes the result. Maroon: FH+, APOE4+; green: FH-, APOE4+; gold: FH+, APOE4-; blue: FH-, APOE4-. Both APOE4 positive groups show an increase with age; the FH-, APOE4- group exhibited a decrease with age. The result is focal in comparison to Fig. 3, suggesting the main effects of APOE and FH only weakly interact with age.
3.2.2. Metacognitive Self Appraisal task
Fig 5. displays the average effect of task for the self versus semantic contrast for all 203 subjects. As described previously (e.g. Johnson et al in press; Schmitz and Johnson 2006), the task activates the medial prefrontal cortex and posterior cingulate as well as anterior medial temporal and subcortical regions (see Table 2).
Fig. 5.
T map of the main effect of task for SA (n = 203). The statistical threshold was set at p = 0.05, FDR corrected for multiple comparisons.
The nonparametric multiple regression analysis in these subjects revealed a significant main effect of age after adjusting for education and gender for SA. Fig. 6 displays a cluster in the medial prefrontal cortex (x, y, z = 0, 62, 6, t = 3.82, p < 0.0001, uncorrected) that exhibited a significant age-related decline in brain activation. By contrast, several regions were found to display age-related increases in brain activation including the lateral parietal cortex, amygdala, orbitofrontal cortex, and basal forebrain (also shown in Fig 6). Table 3 provides the statistics, locations, and cluster size for all regions that displayed age-related changes in brain activation. Fig. 7 provides plots for some of these regions.
Fig. 6.
Displays T maps for the results of the multiple regression analysis of aging effects (n = 203) for SA. The analyses were restricted to voxels that displayed a main effect of task, which are displayed in Fig. 5. Hot colors represent age-related declines in brain activity, whereas cold colors represent age-related increases in brain activity.
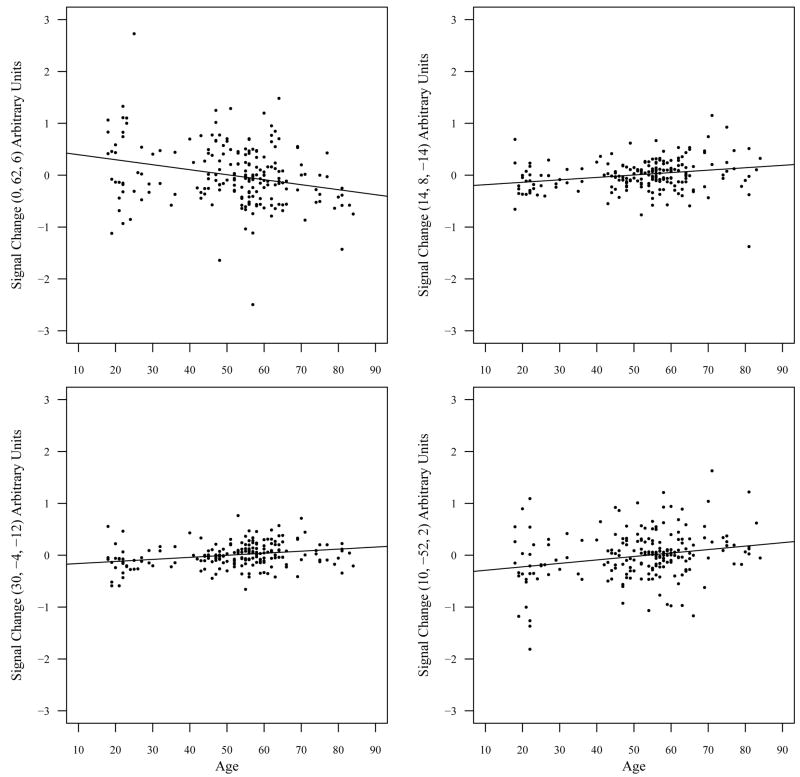
Fig. 7.
Scatter plots with adjusted rank estimation fit of age-related changes in brain activity for the self versus semantic contrast. The upper left panel is a scatter plot of age-related declines in signal change in the medial prefrontal cortex and the other three panels are scatter plots of age-related increases in signal change in right basal forebrain (upper right panel), right amygdala/orbitofrontal gyrus (lower left panel), and the right cuneus (lower right panel).
The AD risk factor analysis followed the same approach that was used to evaluate the influence of the AD risk factors for ENC (described above). We found a global effect of risk bilaterally (see Fig. 8) in the hippocampus (x, y, z = -20, -14, -10, F = 8.56, p < 0.0001, uncorrected; 20, -12, -12, F = 6.67, p < 0.0001), the left lateral parietal cortex (x, y, z = -40, -26, 58, F = 7.30, p < 0.0001, uncorrected), and the right globus pallidus (x, y, z = 16, 4, -10, F = 6.67, p < 0.0001, uncorrected). There were no age × APOE genotype or age × FH status interactions in any of these locations, nor did we find a three way interaction. This indicates that the main risk effects (age, FH or APOE) are driving the significance of the omnibus effects that we detected, rather than interactions of risk and age.
Fig. 8.
Maximum intensity projections of the global risk F test (n = 150) for the SA task. Bilateral results are observed in the medial temporal lobe.
4. Discussion
Increasing age is associated with reduced episodic memory functions including encoding, and a host of cerebral changes including reduced WM integrity, reduced CBF, reduced glucose metabolism in frontal and temporal lobes, reduced volume in MTL and prefrontal regions critical for episodic memory, and alterations in the cerebrovascular system (D’Esposito, Deouell, & Gazzaley, 2003). Age is also the largest single risk factor for AD and is associated with a number of other neurodegenerative diseases and cerebrovascular disease. Functional MRI studies have revealed age-related changes in brain activation during a variety of cognitive tasks including episodic memory tasks (see Persson & Nyberg, 2006 for review). In the present study we examined age-related changes in brain activation across the adult lifespan in a large sample of cognitively healthy individuals between the ages of 18 and 83 years using an episodic encoding paradigm found to yield robust activity in the MTL, and a metacognitive self-appraisal paradigm that results in robust activity in posterior cingulate and medial frontal and subcortical regions important for metacognitive executive functions. The sample was enriched with subjects at risk for AD, enabling us to determine whether risk factors influenced the effect of aging.
D’Esposito et al. (2003) have suggested that age-related changes in the blood-oxygenation level dependent (BOLD) fMRI signal are more difficult to interpret because of changes in the cerebrovascular system (e.g., altered neurovascular coupling) that might influence accurate measurement of the BOLD signal. For example, normal aging is associated with increased incidence of several conditions that might influence the BOLD signal such as chronic cerebral ischemia, atherosclerosis, small vessel disease, reduced resting blood flow, and changes in specific neurotransmitter systems. In order to study these decoupling changes prospectively, it is necessary to identify asymptomatic people at risk for AD and follow them longitudinally with imaging exams including perfusion imaging and BOLD, which are sensitive to change. Such work is underway in our laboratory.
4.1 Episodic Encoding
In a series of previous studies using the same task, we have demonstrated reduced brain activity in the MTL in individuals with certain risk factors for AD including individuals with AMCI (Johnson et al., 2006a), middle-aged individuals with a positive parental history of AD (Johnson et al., 2006b), and carriers of the APOE e4 allele (Trivedi et al., 2006). In the present study, we observed significant age-related declines in fMRI activation bilaterally in ventral temporal lobe regions important for object identification and the hippocampus during episodic memory encoding in a large sample of cognitively healthy adults.
The results of the analyses restricted to the 148 participants in the AD risk factor analysis indicated there were several locations in the medial and ventral temporal lobe that were related to task activation, but there was no significant interaction between age and FH or APOE risk factors. At a more liberal threshold we found that APOE e4 carriers who were also FH+ displayed age-related increases in fMRI activation in the right hippocampus. In conjunction with our prior results, these results suggest that FH status continues to exert an effect but appears to be separate from the effects of age (in the age range that we studied), while APOE may interact with age.
The effect of APOE on the brain response is still unclear in both the direction of the effect and its potential meaning. Few if any fMRI studies have examined the interaction between age and APOE in a large sample. Studies of the effect of APOE alone have provided mixed results. Lind et al. (2006) found that APOE e4 carriers (age range: 49 – 74 years) displayed reduced activation in the left inferior parietal cortex, bilateral anterior cingulate cortex, and the right hippocampus during a novel versus familiar word encoding paradigm (see also Trivedi et al., 2006). In contrast, other studies have reported increased activation in elderly APOE e4 carriers (mean age > 75 years) using a verbal paired-associate paradigm (Han et al., 2006) and a novel versus familiar picture encoding paradigm (Bondi, Houston, Eyler, & Brown, 2005), though the APOE4 positive subjects also performed significantly better on neuropsychological tests of encoding. The meaning of increased signal with risk has been hypothesized to reflect a compensatory mechanism or mechanisms. Others have suggested an alternative hypothesis (Mondadori et al., 2006) involving an interaction with age, where a beneficial effect may be present early in life and deleterious effect in late life. These hypotheses now require more clarification with operational criteria for further direct testing and validation, and characterization of the temporal profile over the lifespan.
Prior fMRI studies of age-related changes in brain activity during episodic memory have also yielded conflicting data. Several studies have reported reduced activation in elderly relative to young individuals in certain brain areas important for episodic memory function, such as the prefrontal cortex and MTL (e.g., Grady et al., 1995; Logan, Sanders, Snyder, Morris, & Buckner, 2002; Stebbins et al., 2002). In contrast, other studies have reported greater activity in these same regions in older individuals during episodic memory tasks (Cabeza, Anderson, Locantore, & McIntosh, 2002; Grady, Bernstein, Beig, & Siegenthaler, 2002; Gutchess et al., 2005; Morcom, Good, Frackowiak, & Rugg, 2003). One recent study examined age-related changes in brain activity in three groups of individuals aged 20-30 years, 40-60 years, and 65-87 years. These authors reported linear increases in fMRI activation in the medial frontal and parietal regions at both encoding and recognition, whereas activation in the dorsolateral prefrontal cortex was found to linearly decrease with age (Grady, Springer, Hongwanishkul, McIntosh, & Winocur, 2006). The results of the present study indicate that FH status and APOE genotype do not strongly affect age-related changes in brain activation using the episodic encoding paradigm employed in this study. More studies are needed to clarify the conflicting data regarding age-related changes in brain activity during episodic memory function.
It is important to note that laboratories using fMRI to investigate aging and risk-factors for AD have used episodic memory tasks which vary along several experimental (e.g., task difficulty, task timing, use of a ‘resting state’ baseline, or different statistical analysis techniques) and clinical (e.g., alterations in cerebral hemodynamics and energy metabolism, age, degree of cognitive impairment, and other health characteristics) dimensions that may influence the accurate measurement of task-related changes in the BOLD hemodynamic response. In addition, a recent fMRI study of paired-associate encoding and recall by Celone et al. (2006) emphasizes the possibility that the effect of disease severity may be nonlinear.
Methodological and clinical variability limits the extent to which one study can be generalized to another. Large studies are needed in which these factors can be modeled or manipulated experimentally, possibly using a non-parametric approach similar to the one employed in this study. FMRI studies of cognitive operations are prone to the same problems and issues as any other neuropsychological test when applied to patient groups or individuals with risk factors for disease. Therefore, a psychometric approach would also be useful if fMRI is to be successfully used to examine functional changes in the brain during episodic memory or on other cognitive tasks in the elderly or individuals with risk factors for AD.
4.2. Meta-cognitive self-appraisal
The most prominent cognitive deficit in AMCI is a robust impairment in episodic encoding and memory that typically progresses to other aspects of cognitive function. However, awareness of cognitive dysfunction in individuals with AD and AMCI is often quite variable, ranging from clear insight and marked concern about cognitive difficulties to frank anosognosia (i.e., a patient’s unawareness of deficits resulting from brain disease or injury) (Vogel, Hasselbalch, Gade, Ziebell, & Waldemar, 2005; Vogel et al., 2004). Previous findings from our laboratory indicate that individuals with AMCI display reduced brain activation in medial prefrontal and posterior cingulate regions (Ries et al., 2007) using the same meta-cognitive self-appraisal task used in the present study. Ries et al. (2007) also found that activation of both medial prefrontal and posterior cingulate regions in individuals with AMCI was strongly related with level of insight into cognitive difficulties (indexed by a discrepancy score between patient and informant ratings of cognitive decline in each AMCI participant, i.e., the informant questionnaire on cognitive decline in the elderly (Jorm, Christensen, Korten, Jacomb, & Henderson, 2000) such that individuals with poor insight also displayed the least amount of brain activation in these cortical midline structures. Importantly, this relationship existed even after controlling for extent of memory impairment.
In the present study, we sought to determine if fMRI activation in metacognitive regions such as the prefrontal cortex and posterior cingulate gyrus were modulated by risk factors for AD in cognitively healthy individuals. We found that increasing age was associated with reduced activity in the medial prefrontal cortex. By contrast, we also found that increasing age was associated with increased activity in the lateral parietal cortex, amygdala, orbitofrontal cortex, and basal forebrain across 203 individuals. In the follow-up analysis, restricted to the 150 participants with known AD risk factor status, we failed to demonstrate any evidence that these age-related changes in brain activation during self-appraisal were modulated by APOE genotype or FH status. Although, activity in certain brain regions such as the medial prefrontal cortex appears to decline over the lifespan, other brain regions such as the lateral parietal cortex appear to exhibit an increase in brain activity over the lifespan. These results only generalize to cognitively healthy people, a different pattern will likely be observed in symptomatic participants along the AD continuum.
4.3. Limitations
There are several limitations to the present study. First, the FH status and APOE genotype were not available for all subjects. Therefore, we were not able to determine the influence of these variables across the entire lifespan. Second, the risk factor of parental FH is quite vague and likely comprises unidentified genes and environmental factors. Only 10% of the FH+ individuals had parents with autopsy confirmed AD, whereas a consensus diagnostic conference using published DSM-IV and NINDS-ADRDA criteria was used for the remaining FH+ individuals. Although not likely to have a significant effect on the results, our FH+ cohort may have been contaminated with subjects whose demented parents did not actually have AD pathology. Third, we cannot exclude the possibility that the FH- group may have contained persons whose parents may eventually develop AD. Finally, the design of this study was cross-sectional. It will be more informative to examine rates of change within individuals and these studies are underway in our laboratory.
4.4. Summary
Although risk factors such as FH and APOE have previously been shown to affect neural activation, the results of this study indicate that age-related changes in brain activation during episodic encoding and metacognitive appraisals are not strongly modulated by FH status or APOE genotype. We found age-related declines in brain activation in the ventral temporal lobes and the hippocampus during novel picture encoding and we showed that these regions were affected by AD risk factors (Fig 3), and we provided limited evidence for an interaction between APOE genotype and age. We also found evidence for age-related declines in the medial prefrontal cortex during metacognitive self-appraisal and age-related increases in brain activation in the parietal cortex, orbital cortex and amygdala. There was no evidence that these age-related changes in brain activation during SA were modulated by FH status or APOE genotype. Given the current state of the literature, we suggest that future functional imaging studies in this area adopt psychometric methods familiar to neuropsychologists with large sample sizes and normative data. We also suggest that future studies involve a longitudinal component so that endpoints of AD status can be included in statistical models of early AD brain changes, thereby improving internal validity. Other imaging modalities such as perfusion and amyloid imaging should also be utilized in examining the early brain changes that occur prior to the onset of clinical AD.
Acknowledgments
This study was supported in part by the National Institutes of Health R01 AG021155 and R01 MH65723 to SCJ. This study was also supported with resources and use of facilities at the William S. Middleton Memorial Veterans Hospital, Madison, WI. The assistance of Gemma Gliori, MS, Brent Thiel, Erik Kastman, Andrew Alexander, PhD, Michael Anderle, Ron Fisher, and the Waisman Center for Brain Imaging was greatly appreciated. We especially thank all the participants that made this study possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging. 2007;25(2):154–167. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH, Sinaiko E, Wang GJ, Bartlet E, Volkow N. Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiatr Q. 1995;66(4):357–370. doi: 10.1007/BF02238755. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Schmitz TW, Hess T, Koscik RL, Trivedi MA, Ries ML, Carlsson CM, Sager MA, Asthana S, Johnson SC. Hormone effects on fMRI and cognitive measures of encoding: importance of hormone preparation. Neurology. 2006;67(11):2039–2041. doi: 10.1212/01.wnl.0000247277.81400.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychol Aging. 2002;17(1):7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269(5221):218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey-Bloom J, Salmon DP, Thal LJ, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42(7):980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Ries ML, Hess TM, Carlsson CM, Gleason CE, Alexander AL, Rowley HA, Asthana S, Sager MA. Alzheimer Disease Risk in Healthy Middle-Aged Adults Affects Brain Function During Self Appraisal. Archives of General Psychiatry. doi: 10.1001/archpsyc.64.10.1163. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML, Asthana S, Chen K, Reiman EM, Alexander GE. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006a;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006b;26(22):6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Informant ratings of cognitive decline in old age: validation against change on cognitive tests over 7 to 8 years. Psychol Med. 2000;30(4):981–985. doi: 10.1017/s0033291799002299. [DOI] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Backman L, Nilsson LG, Petersson KM, Nyberg L. Reduced functional brain activity response in cognitively intact apolipoprotein E {varepsilon}4 carriers. Brain. 2006 doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR., Jr Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy P, Correia S, Stebbins G, Laidlaw DH. Neuroimaging of white matter in aging and dementia. Clin Neuropsychol. 2007;21(1):73–109. doi: 10.1080/13854040500263583. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropoulos A, Henke K. Better Memory and Neural Efficiency in Young Apolipoprotein E {varepsilon}4 Carriers. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126(Pt 1):213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15(4):635–641. doi: 10.1161/01.str.15.4.635. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L. Altered brain activity in healthy seniors: what does it mean? Prog Brain Res. 2006;157:45–56. doi: 10.1016/s0079-6123(06)57004-9. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann N Y Acad Sci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries ML, Jabbar BM, Schmitz TW, Trivedi MA, Gleason CE, Carlsson CM, Rowley HA, Asthana S, Johnson SC. Anosognosia in mild cognitive impairment: Relationship to activation of cortical midline structures involved in self-appraisal. J Int Neuropsychol Soc. 2007;13(3):450–461. doi: 10.1017/S1355617707070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18(4):245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. NeuroImage. 2006;30:1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Rowley HA, Kawahara TN, Johnson SC. Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia. 2006;44(5):762–773. doi: 10.1016/j.neuropsychologia.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Snodgrass J, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, Bennett DA, Wilson RS, Glover G, Gabrieli JD. Aging effects on memory encoding in the frontal lobes. Psychol Aging. 2002;17(1):44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer’s disease: a cross-sectional study. BMC Med. 2006;4:1. doi: 10.1186/1741-7015-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, Hasselbalch SG, Gade A, Ziebell M, Waldemar G. Cognitive and functional neuroimaging correlate for anosognosia in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2005;20(3):238–246. doi: 10.1002/gps.1272. [DOI] [PubMed] [Google Scholar]
- Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer’s disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17(3):181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]