Abstract
Of 393 isolates of Streptococcus pneumoniae from U.S. children collected in 2005-2006, nonvaccine serotypes accounted for 89.1%, with serotype 19A the most prevalent, representing 30.5% of all isolates. The MIC90 of faropenem against serotype 19A isolates was 1 μg/ml, compared to ≥8 μg/ml against amoxicillin/clavulanate, cefdinir, cefuroxime axetil, and azithromycin.
Streptococcus pneumoniae continues to be a leading cause of respiratory tract infections in both children and adults (25). Although antimicrobial agents, such as the β-lactams and macrolides, have been widely used to manage such infections, their success continues to be compromised by increasing resistance, especially among isolates from young children (20). An alternative and attractive solution to the emergence of resistance has been the development and use of pneumococcal conjugate vaccines that would not only prevent a proportion of invasive pneumococcal disease (IPD), but also reduce the carriage of vaccine serotypes, including resistant phenotypes (31, 34). The heptavalent pneumococcal conjugate vaccine (PCV7) was approved in the United States in 2000 and recommended for use in all children less than 2 years old (2). At that time, the serotypes covered by PCV7 (4, 6B, 9V, 14, 18C, 19F, and 23F) accounted for more than 80% of isolates causing IPD among U.S. children (1, 6, 18). Widespread use of PCV7 was initially hailed as a success, resulting in a major decline in invasive pneumococcal infections, including those caused by resistant strains (4, 19, 32, 34). Unfortunately, there is now increasing concern regarding the development and spread of resistance among serotypes that are not covered by PCV7 (22, 24, 26). In particular, recent attention has focused on the nonvaccine serotype 19A strains of S. pneumoniae because of their resistance to currently approved antibiotics and their association with clinical failures in children with acute otitis media (AOM) (29).
Faropenem is an oral penem that is being developed for community-acquired respiratory tract infections in adults and children (30). The objective of this study was to assess the serotype distribution and antimicrobial susceptibilities of isolates of S. pneumoniae collected from 393 U.S. children in 2005-2006. The activities of faropenem and other agents against the most prevalent serotypes of S. pneumoniae, including 19A, were evaluated.
S. pneumoniae isolates were prospectively collected from 104 participating institutions geographically distributed across the United States. The isolates were limited to one per patient and were collected from a variety of specimen sources that were classified as noninvasive (sputum, bronchoalveolar lavage fluid, tracheal aspirate, ear, and sinus), invasive (blood), or carriage (nasopharynx). All isolates were shipped to a central laboratory (CMI, Inc., Wilsonville, OR), where each isolate was subcultured and reidentified by standard methods. All isolates were tested for susceptibility to faropenem, amoxicillin-clavulanate, cefdinir, cefuroxime, penicillin, azithromycin, telithromycin, levofloxacin, and trimethoprim-sulfamethoxazole (SXT). Antimicrobial susceptibility testing was conducted by determination of MICs by broth microdilution using frozen panels that were prepared at CMI, Inc., in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines (7). MICs were interpreted according to CLSI recommendations (8), with the exception of faropenem, for which no CLSI breakpoints are available. The breakpoints for penicillin were those described in CLSI document M100 S-17 (8).
All isolates were serotyped at a central laboratory (Case Western Reserve University, Cleveland, OH). Serotyping was conducted by the quellung reaction using type-specific antisera (Statens Serum Institute, Copenhagen, Denmark). Serotypes were classified into two groups: PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) and non-PCV7 serotypes (all other serotypes). Isolates identified as serotype 6A were recorded as serotype 6A/C, as the newly recognized serotype 6C cross-reacts with 6A (27).
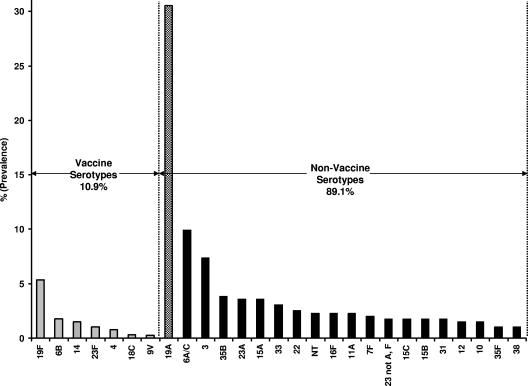
Eighty sites collected S. pneumoniae isolates from subjects ≤14 years old, with a mean number of five isolates per site. The breakdown of isolates by specimen sources was 17.8% (70 isolates) from invasive sources (blood); 63.1% (248 isolates) from noninvasive sources, including sputum, bronchoalveolar lavage fluid, tracheal aspirate, ear, and sinus; 18.8% (74 isolates) carriage isolates (nasopharynx); and 0.3% (1 isolate) from an unspecified specimen source. The results in Fig. 1 show the predominant serotype distributions for the 393 clinical isolates of S. pneumoniae collected in the study. Only 43 isolates (10.9%) were serotypes included in PCV7 (vaccine serotypes). The most prevalent vaccine serotype was 19F, accounting for 5.3% of all isolates. In contrast, 89.1% of the isolates were non-PCV7 serotypes. The most prevalent non-PCV7 serotype was 19A, accounting for 30.5% (120 isolates) of all isolates.
FIG. 1.
Serotype distribution of 393 pneumococcal isolates collected from U.S. children in 2005-2006.
In total, 70% (84 isolates) of the serotype 19A strains were isolated from children ≤2 years old. The prevalences of serotype 19A strains from children aged 3 to 5 years and 6 to 14 years were 22.5% and 7.5%, respectively. Analysis of isolate specimen sources showed that 23.3% of the isolates were from invasive sources, such as blood, while 60.8% were from noninvasive sources and 15% were from carriage sites. The prevalences of serotype 19A strains by U.S. geographic region were as follows: north central, 28.2%; northeast; 32.2%; northwest, 21.4%; south central, 37.1%; southeast, 26.5%; and southwest, 27.6% (P = 0.71).
The results in Table 1 show the comparative activities of faropenem and other agents against the most prevalent serotypes (those with ≥10 isolates) of S. pneumoniae (19A, 6A/C, 3, 19F, 35B, 23A, 15A, 33, and 22). Among the oral β-lactams, faropenem had the lowest MIC90 against the 120 serotype 19A isolates (1 μg/ml), eightfold lower than that of amoxicillin-clavulanate (8 μg/ml), with 35% of the isolates resistant to this agent. The MIC90s for cefdinir, cefuroxime axetil, azithromycin, and SXT against serotype 19A strains were >8, 16, >16, and > 8 μg/ml, respectively, with resistance rates that were >50% for all four agents. All serotype 19A isolates were susceptible to levofloxacin, and 99.2% of the isolates were susceptible to telithromycin. The other serotype with reduced susceptibility to many antimicrobial agents was serotype 19F, a PCV7 serotype. Faropenem also had the lowest MIC90 for oral β-lactams (1 μg/ml) against this serotype compared to 16 μg/ml for amoxicillin-clavulanate, with 47.6% of the isolates resistant to the latter agent. Cefdinir, cefuroxime axetil, azithromycin, and SXT had MIC90s of >8, 32, >16, and > 8 μg/ml against serotype 19F strains, with resistance rates of 67%, 67%, 71%, and 53%, respectively. The next serotype to demonstrate reduced susceptibility to commonly used agents was serotype 35B. The MIC90s for faropenem and amoxicillin-clavulanate were 0.5 and 2 μg/ml, respectively, and all isolates were susceptible to amoxicillin-clavulanate. Serotype 35B isolates had reduced susceptibility to cefdinir and azithromycin (MIC90s of 4 and 16 μg/ml, respectively) but were susceptible to SXT (MIC90, 1 μg/ml). Strains belonging to serotypes 3, 23A, 15A, 33, and 22 were all susceptible to penicillin and other β-lactams. Serotype 3 and 22 strains were the only serotypes in this group in which almost all isolates were susceptible to azithromycin (MIC90s of 0.5 and ≤0.12 μg/ml, respectively).
TABLE 1.
Antimicrobial susceptibilities of the nine most prevalent serotypes of S. pneumoniae collected from U.S. children in 2005-2006
| Serotype | No. of isolates | % Prevalence | MIC90a (% of isolates susceptible/intermediate/resistant)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Faropenemb | Amoxicillin/ clavulanate (≤2/4/≥8)d,e | Cefdinir (≤0.5/1/≥2) | Cefuroxime axetil (≤1/2/≥4) | Penicillin (≤0.06/0.12-1/≥2) | Azithromycin (≤0.5/1/≥2) | SXT (≤0.5/1-2/≥4)e | Telithromycin (≤1/2/≥4) | Levofloxacin (≤2/4/≥8) | |||
| 19A | 120 | 30.5 | 1 | 8 (62/3/35) | >8 (46/2/53) | 16 (46/2/52) | 4 (13/36/50) | >16 (36/0/64) | >8 (22/5/73) | 1 (99/1/0) | 1 (100/0/0) |
| 6A/C | 39 | 9.9 | 0.25 | 1 (97/0/3) | 2 (59/26/15) | 4 (64/23/15) | 1 (23/69/8) | 8 (31/0/69) | 8 (51/3/46) | 0.25 (100/0/0) | 1 (100/0/0) |
| 3 | 29 | 7.4 | 0.015 | ≤0.12 (100/0/0) | 0.12 (100/0/0) | ≤0.5 (100/0/0) | ≤0.03 (100/0/0) | 0.5 (93/3/4) | 0.25 (100/0/0) | ≤0.12 (100/0/0) | 2 (97/3/0) |
| 19Fc | 21 | 5.3 | 1 | 16 (47/5/48) | >8 (33/0/67) | 32 (33/0/67) | >4 (33/0/67) | >16 (29/0/71) | >8 (33/14/53) | 1 (100/0/0) | 1 (100/0/0) |
| 35B | 15 | 3.8 | 0.5 | 2 (100/0/0) | 4 (0/0/100) | 4 (0/0/100) | 2 (0/0/100) | 16 (73/0/27) | 1 (87/7/7) | 0.5 (100/0/0) | 1 (100/0/0) |
| 23A | 14 | 3.6 | 0.06 | 0.25 (100/0/0) | 0.5 (93/7/0) | 1 (93/7/0) | 0.5 (57/43/0) | >16 (79/21/0) | 0.5 (93/7/0) | ≤0.12 (100/0/0) | 1 (100/0/0) |
| 15A | 14 | 3.6 | 0.12 | 0.25 (100/0/0) | 1 (72/21/7) | 4 (72/7/21) | 0.5 (7/93/0) | >16 (7/0/93) | 4 (50/36/14) | ≤0.12 (100/0/0) | 1 (100/0/0) |
| 33 | 12 | 3.1 | 0.015 | ≤0.12 (100/0/0) | 0.12 (100/0/0) | ≤0.5 (100/0/0) | ≤0.03 (92/8/0) | 16 (42/58/0) | 2 (0/100/0) | 0.25 (100/0/0) | 1 (100/0/0) |
| 22 | 10 | 2.5 | 0.015 | ≤0.12 (100/0/0) | 0.12 (100/0/0) | ≤0.5 (100/0/0) | ≤0.03 (100/0/0) | ≤0.12 (100/0/0) | 0.25 (100/0/0) | ≤0.12 (100/0/0) | 1 (100/0/0) |
| All isolates | 393 | 100 | 1 | 8 (84/2/14) | >8 (68/4/28) | 16 (68/4/28) | 4 (49/26/25) | >16 (55/1/44) | 8 (56/9/35) | 1 (99/1/0) | 1 (99.7/0.3/0) |
MIC90s are expressed as micrograms per milliliter.
Breakpoints are not available for faropenem.
Serotype included in PCV7.
Susceptible/intermediate/resistant breakpoints (μg/ml).
Amoxicillin/clavulanate results are expressed as the amoxicillin component and SXT results as the trimethoprim component.
Analysis of resistance phenotypes among serotype 19A isolates showed that 76.7% were resistant to one or more antimicrobial agents, with 51.6% resistant to ≥3 classes of agents and 43.3% to 4 agents (penicillin, cefuroxime, azithromycin, and SXT).
The 30.5% prevalence of serotype 19A demonstrated in this study is 11.5% higher than the prevalence of this serotype reported in the PROTEKT US study conducted in 2004-2005 (14). There is increasing concern regarding the emergence of 19A serotypes because of their virulence and increased resistance to multiple classes of antimicrobial agents (13, 14, 23, 28, 29). Serotype 19A strains identified in one study were resistant to all antibiotics approved by the FDA for use in children with AOM (29). Furthermore, clinical failures were observed in this study with the use of high-dose amoxicillin, amoxicillin/clavulanate, and ceftriaxone in treating four children with AOM (29). An additional five children at the same practice with AOM caused by serotype 19A S. pneumoniae were successfully treated with levofloxacin, an agent unlikely to be widely used to treat pediatric infections because of concerns regarding safety and the potential for resistance development. Although serotype 19A and other non-PCV7 serotypes are pervasive among children, they are also increasingly prevalent in the adult and elderly populations (15, 24).
Several investigators have compared the antimicrobial susceptibilities of PCV7 and non-PCV7 serotypes; however, there is little information on how many of the common serotypes vary in their susceptibilities to antimicrobial agents. In this study, we found that serotypes 19F and 19A were the least susceptible to many commonly used antibiotics. Although the serotype 19F and 19A isolates in our study were not characterized molecularly, the majority of the isolates were resistant to azithromycin (52.4 and 73.3% resistant, respectively) and may contain both mef and erm resistance determinants, similar to the serotype 19 strains characterized in other studies (13, 33).
As more than 50% of the serotype 19A strains identified in this study were multiresistant to ≥3 antimicrobial classes, including all currently available oral β-lactams and macrolides, these strains are attracting considerable attention because they now represent the most prevalent serotype among U.S. pediatric isolates. All isolates were susceptible to levofloxacin and telithromycin, agents that are unlikely to be widely used in children because of safety concerns and because telithromycin is not available in pediatric formulation.
Faropenem appears to be a possible option for treating pediatric infections because it has low potential for resistance development (21), provided it achieves adequate in vivo activity against U.S. pediatric isolates, such as serotype 19A, that are notoriously resistant to other antimicrobial agents (10, 11). Phase 3 studies in adults have shown that faropenem has a good safety profile with a low incidence of diarrhea, nausea, and vomiting and no risk of cardiotoxicity or seizures (12, 16). Although the MIC90 for faropenem and serotype 19A strains is 1 μg/ml, faropenem has demonstrated in vivo efficacy against multidrug-resistant (MDR) S. pneumoniae strains, with MICs as high as 2 μg/ml in a murine neutropenic thigh infection model (9). In vivo pharmacodynamic (PD) studies of faropenem administered as the medoxomil ester have shown that the percent time above MIC (%T > MIC) for free faropenem required for stasis was similar for S. pneumoniae strains over a wide range of MICs, with a mean %T > MIC being required for 13.9% (95% confidence interval, 6.34; 21.5%) of the dosing interval (9). In another PD study, the %T > MIC for free faropenem was 16.4% for survival in a lethal murine Bacillus anthracis inhalation postexposure prophylaxis model (17).
In phase III clinical trials, the clinical and microbiological success rates for faropenem were 90% (19/21) for non-MDR S. pneumoniae and 80% (16/20) for MDR S. pneumoniae, while the MIC90s of faropenem against these groups were 0.25 and 2 μg/ml, respectively (35). Bacteriologic outcome data at days 4 to 6 from a dose-ranging study of children with AOM suggest that dosing regimens of ≥15 mg/kg of body weight/day in two divided doses will achieve the pharmacokinetic target required to eradicate MDR S. pneumoniae (3).
In conclusion, these results highlight the importance of continued surveillance, to monitor the emergence of not only resistance phenotypes, but also serotypes of S. pneumoniae. The non-PCV7 serotypes now account for the majority of serotypes, with multiresistant serotype 19A being the most prevalent, suggesting that vaccines may be only a partial solution to prevent IPD with MDR strains because of the continuing emergence of new vaccine escape recombinants (5). These data also highlight the need for new and safe agents that can be used in the pediatric setting with low propensity for resistance development. The investigational agent, faropenem, demonstrated lower MICs than other β-lactams and macrolides against serotype 19A strains. Further studies are warranted to evaluate the efficacy of faropenem against infections caused by resistant pneumococci, including the MDR serotype 19A strains.
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Alpern, E. R., E. A. Alessandrini, K. L. McGowan, L. M. Bell, and K. N. Shaw. 2001. Serotype prevalence of occult pneumococcal bacteremia. Pediatrics 108:e23. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases. 2000. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 106:362-366. [DOI] [PubMed] [Google Scholar]
- 3.Arguedas, A., R. Dagan, E. Wang, E. Leibovitz, I. A. Critchley, J. Song, R. Tosiello, and R. Echols. 2007. Dose-efficacy of faropenem medoxomil in the treatment of acute otitis media using double tympanocentesis to obtain middle ear fluid, abstr. G-987. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Black, S., H. Shinefield, R. Baxter, R. Austrian, L. Bracken, J. Hansen, E. Lewis, and B. Fireman. 2004. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr. Infect. Dis. J. 23:485-489. [DOI] [PubMed] [Google Scholar]
- 5.Brueggemann, A. B., R. Pai, D. W. Crook, and B. Beall. 2007. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS. Pathog. 3:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler, J. C., R. F. Breiman, H. B. Lipman, J. Hofmann, and R. R. Facklam. 1995. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: implications for development of a conjugate vaccine. J. Infect. Dis. 171:885-889. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Clinical Laboratory and Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Craig, W. A., and D. R. Andes. 2001. In vivo pharmacodynamic activity of faropenem against Streptococcus pneumoniae, abstr. A-2094. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 10.Critchley, I. A., S. D. Brown, M. M. Traczewski, G. S. Tillotson, and N. Janjic. 2007. National and regional assessment of antimicrobial resistance among community-acquired respiratory tract pathogens identified in a 2005-2006 U.S. Faropenem surveillance study. Antimicrob. Agents Chemother. 51:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Critchley, I. A., K. C. Stone, S. D. Brown, M. M. Traczewski, and N. Janjic. 2007. Antimicrobial resistance patterns among Streptococcus pneumoniae isolated from children in the U.S. 2005-2006 faropenem surveillance study, abstr. C2-200. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 12.Echols, R., and R. Tosiello. 2006. An integrated safety analysis of faropenem medoxomil: results of 5,023 subjects from phase II/III clinical data, abstr. P1717. Abstr. 16th Eur. Congr. Clin. Microbiol. Infect. Dis.
- 13.Farrell, D. J., and S. G. Jenkins. 2006. Emergence and spread of a multi-resistant Streptococcus pneumoniae serotype 19A clone in the United States: focus on the pediatric population, abstr. C2-0431. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 14.Farrell, D. J., K. P. Klugman, and M. Pichichero. 2007. Increased antimicrobial resistance among nonvaccine serotypes of Streptococcus pneumoniae in the pediatric population after the introduction of 7-valent pneumococcal vaccine in the United States. Pediatr. Infect. Dis. J. 26:123-128. [DOI] [PubMed] [Google Scholar]
- 15.Farrell, D. J., K. P. Klugman, M. Pichichero, and S. G. Jenkins. 2005. Impact of PCV7 in children on respiratory Streptococcus pneumoniae serotype distribution in the elderly, abstr. LB-21. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 16.Gill, S., and R. Tosiello. 2006. Pharmacokinetics of faropenem following oral administration of faropenem medoxomil in association with thorough QT/QTc evaluation, abstr. A-1942. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 17.Heine, H. S., C. M. Rubino, J. Bassett, L. Miller, P. G. Ambrose, S. M. Bhavnani, A. Forrest, G. L. Drusano, A. Beaudry, J. Li, N. Janjic, and S. Gill. 2007. Pharmacokinetic-pharmacodynamic (PK-PD) assessment of faropenem in a lethal murine-Bacillus anthracis inhalation post-exposure prophylaxis model, abstr A-1435. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 18.Joloba, M. L., A. Windau, S. Bajaksouzian, P. C. Appelbaum, W. P. Hausdorff, and M. R. Jacobs. 2001. Pneumococcal conjugate vaccine serotypes of Streptococcus pneumoniae isolates and the antimicrobial susceptibility of such isolates in children with otitis media. Clin. Infect. Dis. 33:1489-1494. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, S. L., E. O. Mason, Jr., E. R. Wald, G. E. Schutze, J. S. Bradley, T. Q. Tan, J. A. Hoffman, L. B. Givner, R. Yogev, and W. J. Barson. 2004. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 113:443-449. [DOI] [PubMed] [Google Scholar]
- 20.Karlowsky, J. A., C. Thornsberry, I. A. Critchley, M. E. Jones, A. T. Evangelista, G. J. Noel, and D. F. Sahm. 2003. Susceptibilities to levofloxacin in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children: results from 2000-2001 and 2001-2002 TRUST studies in the United States. Antimicrob. Agents Chemother. 47:1790-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosowska-Shick, K., C. Clark, K. Credito, B. Dewasse, L. Beachel, L. Ednie, and P. C. Appelbaum. 2008. In vitro capability of faropenem to select for resistant mutants of Streptococcus pneumoniae and Haemophilus influenzae. Antimicrob. Agents Chemother. 52:748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyaw, M. H., R. Lynfield, W. Schaffner, A. S. Craig, J. Hadler, A. Reingold, A. R. Thomas, L. H. Harrison, N. M. Bennett, M. M. Farley, R. R. Facklam, J. H. Jorgensen, J. Besser, E. R. Zell, A. Schuchat, and C. G. Whitney. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455-1463. [DOI] [PubMed] [Google Scholar]
- 23.McGee, L. 2007. The coming of age of niche vaccines? Effect of vaccines on resistance profiles in Streptococcus pneumoniae. Curr. Opin. Microbiol. 10:473-478. [DOI] [PubMed] [Google Scholar]
- 24.Moore, M. R., R. E. Gertz, R. L. Woodbury, G. A. Barkocy-Gallagher, W. Schaffner, C. Lexau, K. Gershman, A. Reingold, M. Farley, L. H. Harrison, J. H. Hadler, N. M. Bennett, A. R. Thomas, L. McGee, T. Pilishvili, A. B. Brueggemann, C. G. Whitney, J. H. Jorgensen, and B. Beall. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197:1016-1027. [DOI] [PubMed] [Google Scholar]
- 25.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-807. [DOI] [PubMed] [Google Scholar]
- 26.Pai, R., M. R. Moore, T. Pilishvili, R. E. Gertz, C. G. Whitney, and B. Beall. 2005. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J. Infect. Dis. 192:1988-1995. [DOI] [PubMed] [Google Scholar]
- 27.Park, I. H., D. G. Pritchard, R. Cartee, A. Brandao, M. C. Brandileone, and M. H. Nahm. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelton, S. I., H. Huot, J. A. Finkelstein, C. J. Bishop, K. K. Hsu, J. Kellenberg, S. S. Huang, R. Goldstein, and W. P. Hanage. 2007. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26:468-472. [DOI] [PubMed] [Google Scholar]
- 29.Pichichero, M. E., and J. R. Casey. 2007. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA 298:1772-1778. [DOI] [PubMed] [Google Scholar]
- 30.Schurek, K. N., R. Wiebe, J. A. Karlowsky, E. Rubinstein, D. J. Hoban, and G. G. Zhanel. 2007. Faropenem: review of a new oral penem. Exp. Rev. Anti. Infect. Ther. 5:185-198. [DOI] [PubMed] [Google Scholar]
- 31.Stephens, D. S., S. M. Zughaier, C. G. Whitney, W. S. Baughman, L. Barker, K. Gay, D. Jackson, W. A. Orenstein, K. Arnold, A. Schuchat, and M. M. Farley. 2005. Incidence of macrolide resistance in Streptococcus pneumoniae after introduction of the pneumococcal conjugate vaccine: population-based assessment. Lancet 365:855-863. [DOI] [PubMed] [Google Scholar]
- 32.Talbot, T. R., K. A. Poehling, T. V. Hartert, P. G. Arbogast, N. B. Halasa, E. Mitchel, W. Schaffner, A. S. Craig, K. M. Edwards, and M. R. Griffin. 2004. Reduction in high rates of antibiotic-nonsusceptible invasive pneumococcal disease in Tennessee after introduction of the pneumococcal conjugate vaccine. Clin. Infect. Dis. 39:641-648. [DOI] [PubMed] [Google Scholar]
- 33.Toltzis, P., M. Dul, M. A. O'Riordan, M. R. Jacobs, and J. Blumer. 2006. Serogroup 19 pneumococci containing both mef and erm macrolide resistance determinants in an American city. Pediatr. Infect. Dis. J. 25:19-24. [DOI] [PubMed] [Google Scholar]
- 34.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 35.Young, C. L., I. A. Critchley, and N. Janjic. 2006. Activity of faropenem against multi-drug-resistant Streptococcus pneumoniae identified in phase III respiratory clinical trials and correlation with clinical microbiological success, abstr. E-0224. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.