Abstract
The association between trimethoprim-sulfamethoxazole use and resistance among the major respiratory tract pathogens was investigated by comparing regional consumption of the drug to regional resistance in the following year in 21 central hospital districts in Finland. A total of 23,530 Streptococcus pneumoniae isolates, 28,320 Haemophilus influenzae isolates, and 14,138 Moraxella catarrhalis isolates were tested for trimethoprim-sulfamethoxazole susceptibility during the study period (1998-2004). Among the S. pneumoniae isolates, a statistically significant connection was found between regional consumption and resistance. No statistically significant connection was found between regional trimethoprim-sulfamethoxazole use and resistance among H. influenzae and M. catarrhalis isolates. According to our results, it seems that only in pneumococci can the development of trimethoprim-sulfamethoxazole resistance be influenced by restricting its use. However, trimethoprim-sulfamethoxazole remains an important antimicrobial agent because of its reasonable price. Hence, resistance to trimethoprim-sulfamethoxazole among these pathogens needs continuous monitoring.
Sulfonamides were the first class of antimicrobial agents introduced into clinical use in 1935. Trimethoprim (TMP) was introduced in 1962, and the combination trimethoprim-sulfamethoxazole (SXT) was brought into clinical use in 1968. The antibacterial spectrum of the combination is directed at Escherichia coli, other species in the Enterobacteriaceae family, Staphylococcus aureus, Staphylococcus saprophyticus, and the principal respiratory tract pathogens Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.
Hence, the most important fields of use of SXT combinations are in the treatment of urinary tract infections and upper respiratory tract infections. According to a point prevalence study conducted in the Finnish primary health care system, by indication, 81% of all SXT prescriptions were for respiratory tract infections and 15% were for urinary tract infections (21). Since it is a relatively inexpensive drug, this combination has been used widely in developing countries. There are also a few special indications for SXT use, such as the prophylaxis of Pneumocystis carinii infections among AIDS patients and the treatment of infections caused by Stenotrophomonas maltophilia (13).
Emerging resistance among the major respiratory tract pathogens S. pneumoniae, H. influenzae, and M. catarrhalis has undoubtedly decreased the use of SXT. This resistance, however, varies worldwide. In the late 1990s, 31.9 to 88.6% of European and 24.2 to 89.4% of Asian S. pneumoniae isolates were susceptible to SXT. Among H. influenzae strains, a similar variation was noticed (22). According to a recent report from the United States, resistance among pneumococci is decreasing after peaking in 1999-2000 (9). A very small proportion of M. catarrhalis isolates, 0.1 to 2.6%, in the SENTRY surveillance program in 1997-1999 (12) showed resistance to SXT.
The relationship between antimicrobial use and resistance has been evaluated in several studies, using different approaches and analytical methods. Many studies have shown the connection between increased consumption and increased resistance of certain antimicrobial agents (4, 6, 11). As Baquero et al. point out in their review (2), an increase in use has been linked to an increase in resistance at all ecological levels, including patients, small communities, different areas of the same country, the national level, and also the international level. The effect of reduced antimicrobial consumption on the prevalence of resistance has not always been as obviously demonstrated. Seppälä and coworkers (23) showed a significant decline in erythromycin resistance among Streptococcus pyogenes isolates after a reduction in macrolide use in Finland. In Iceland, the prevalence of non-penicillin-susceptible pneumococci even increased in certain areas after significant reductions in total antimicrobial use owing to the spread of a resistant clone (1). On the other hand, resistance can be extremely persistent; in England, sulfonamide resistance persisted in urinary Escherichia coli isolates as long as 9 years after prescription of SXT was formally restricted (3). This had, however, already been predicted earlier (14).
The present study was undertaken to investigate whether the outpatient consumption of SXT is associated with resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. We analyzed this relationship by comparing regional resistances of the three pathogens separately to local SXT consumption in 21 central hospital districts in Finland.
(Preliminary results of this work were presented as a poster at the 15th European Congress of Clinical Microbiology and Infectious Diseases, Copenhagen, Denmark [P1456].)
MATERIALS AND METHODS
Antimicrobial consumption.
Consumption data for SXT at the regional and national levels were provided by the Finnish National Agency of Medicines and are expressed as defined daily doses (DDD) per 1,000 inhabitants per day. The consumption figures used in this study in comparing regional use to regional resistance are based on sales from wholesalers to pharmacies (use in hospitals is not included) on the assumption that this portion represents the use of these antibiotics in community health care. These regional consumption data were collected over a 7-year period (1997-2003) from 21 central hospital districts. The total use of SXT and TMP in Finland is also presented for the years 1990 to 2003. In this case, we collected sales figures for pharmacies and for hospitals.
Antimicrobial resistance.
The regional SXT resistance data between 1998 and 2004 for S. pneumoniae, H. influenzae, and M. catarrhalis were obtained from 26 clinical microbiology laboratories belonging to the FiRe network (Finnish Study Group for Antimicrobial Resistance). The reporting activity varied annually. For two central hospital districts (Kainuu and Ahvenanmaa), no resistance data for this period were available. When several laboratories reported results from a particular central hospital district, the data were combined. Resistance rates based on at least 30 tested strains per laboratory per year were included in the study. The resistance data consist of data for all consecutive (isolated from both inpatients and outpatients) clinical isolates in each laboratory over the whole year.
The national resistance data presented for the years 1988 to 2004 were obtained from the FINRES report (http://www.ktl.fi/portal/english/projects/fire/finres).
The resistance data are based on routine susceptibility results from the participating laboratories. The methods were based on the CLSI (formerly NCCLS) guidelines (8), with some exceptions: in 1998 and 1999, 20/26 laboratories performed these tests according to CLSI methods. Five laboratories used modified disc diffusion methods for H. influenzae (Isosensitest agar or PDM agar with supplements or chocolate agar), and one laboratory used Etest and chocolate agar for testing. Since 2000, all laboratories have basically followed the CLSI standard. The quality control program includes susceptibility testing of S. pneumoniae ATCC 49619 and H. influenzae ATCC49247 on a weekly basis as internal quality controls and proficiency testing programs by Labquality (www.labquality.fi) and UKNEQAS (www.ukneqasmicro.org).
Statistical analysis.
The resistance rates of S. pneumoniae, H. influenzae, and M. catarrhalis in the 21 central hospital districts were compared to local consumption figures for SXT in the previous year.
A linear mixed model for repeated measures was used to model the association between regional resistance and regional antimicrobial consumption of the previous year. The percentage of resistant strains was taken as the dependent variable, while antimicrobial consumption and time were the explanatory variables. A random coefficient model with random intercept and slope was fitted. Mixed models were fitted using the Proc Mixed in SAS system for Windows, version 9.1. The level of statistical significance was set to 0.05.
RESULTS
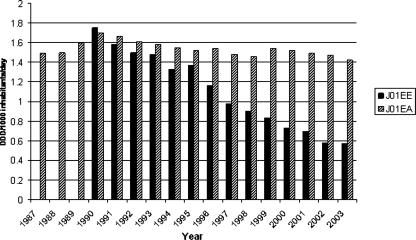
The use of SXT in community health care decreased in all 21 health care districts during the study period (1997-2003) (Table 1). At the national level, total use, including sales to hospitals in addition to pharmacies, fell from 1.95 DDD in 1990 to 0.57 in 2003 (Fig. 1). The total use of TMP alone remained very constant in Finland from 1990 to 2003. Sulfonamides alone were used in very minor amounts in the 1990s, and since 1996 no use has been registered because the compounds are no longer on the market.
TABLE 1.
SXT consumption for outpatient care in 21 central hospital districts in Finland (1997-2003)
| Central hospital district | SXT use (DDD/1,000 inhabitants/day)
|
||||||
|---|---|---|---|---|---|---|---|
| 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | |
| Varsinais-Suomi | 0.85 | 0.73 | 0.71 | 0.63 | 0.58 | 0.52 | 0.49 |
| Satakunta | 0.85 | 0.93 | 0.79 | 0.68 | 0.68 | 0.57 | 0.56 |
| Kanta-Häme | 0.92 | 0.86 | 0.68 | 0.58 | 0.56 | 0.50 | 0.46 |
| Pirkanmaa | 1.10 | 0.90 | 0.84 | 0.76 | 0.77 | 0.66 | 0.62 |
| Päijät-Häme | 0.85 | 0.70 | 0.69 | 0.62 | 0.58 | 0.43 | 0.44 |
| Kymenlaakso | 0.95 | 0.80 | 0.84 | 0.76 | 0.73 | 0.62 | 0.68 |
| Etelä-Karjala | 0.66 | 0.64 | 0.57 | 0.55 | 0.58 | 0.44 | 0.42 |
| Etelä-Savo | 0.72 | 0.59 | 0.62 | 0.58 | 0.56 | 0.47 | 0.51 |
| Itä-Savo | 0.77 | 0.75 | 0.81 | 0.72 | 0.58 | 0.48 | 0.49 |
| Pohjois-Karjala | 0.75 | 0.75 | 0.80 | 0.61 | 0.62 | 0.49 | 0.48 |
| Pohjois-Savo | 1.04 | 0.93 | 0.86 | 0.72 | 0.67 | 0.57 | 0.57 |
| Keski-Suomi | 0.65 | 0.55 | 0.52 | 0.48 | 0.48 | 0.43 | 0.39 |
| Etelä-Pohjanmaa | 1.11 | 1.01 | 0.91 | 0.82 | 0.80 | 0.65 | 0.65 |
| Vaasa | 0.59 | 0.51 | 0.45 | 0.42 | 0.43 | 0.32 | 0.34 |
| Keski-Pohjanmaa | 0.73 | 0.70 | 0.63 | 0.56 | 0.57 | 0.52 | 0.59 |
| Pohjois-Pohjanmaa | 1.03 | 0.90 | 0.86 | 0.76 | 0.70 | 0.58 | 0.56 |
| Kainuu | 1.19 | 1.21 | 0.97 | 0.93 | 0.97 | 0.75 | 0.78 |
| Länsi-Pohja | 0.73 | 0.66 | 0.60 | 0.56 | 0.53 | 0.43 | 0.45 |
| Lappi | 0.98 | 0.91 | 0.80 | 0.73 | 0.58 | 0.46 | 0.45 |
| Ahvenanmaa | 0.61 | 0.67 | 0.54 | 0.50 | 0.48 | 0.44 | 0.28 |
| Helsinki and Uusimaa | 0.82 | 0.73 | 0.66 | 0.59 | 0.55 | 0.44 | 0.43 |
FIG. 1.
SXT and TMP consumption in Finland from 1987 to 2003 (DDD/1,000 inhabitants/day), including sales figures for pharmacies and hospitals. J01EE, all SXT combinations; J01EA, TMP. Figures for SXT for the period 1987-1989 were not available.
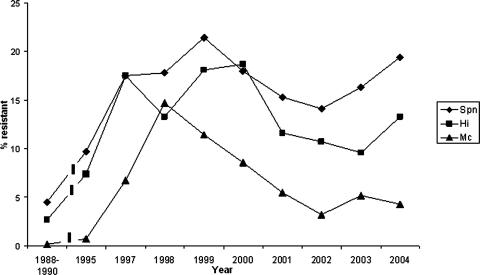
The susceptibility results for 23,530 S. pneumoniae isolates (annual variation, 1,928 to 4,238 isolates), 28,320 H. influenzae isolates (annual variation, 3,043 to 5,116 isolates), and 14,138 M. catarrhalis isolates (annual variation, 1,298 to 2,731 isolates) were included in the analysis. There was considerable variation between laboratories in the number of annually tested strains owing to the variation in population size in the areas they serve. Resistance rates varied from 14.1 to 21.4% for S. pneumoniae, 9.7 to 18.7% for H. influenzae, and 3.2 to 14.5% for M. catarrhalis in the whole country during the study period (Fig. 2). The annual regional resistance rates for the three pathogens are presented in Tables 2, 3, and 4.
FIG. 2.
SXT resistance (%) in S. pneumoniae (Spn), H. influenzae (Hi), and M. catarrhalis (Mc) in Finland from 1988 to 2004.
TABLE 2.
Regional SXT resistance in Streptococcus pneumoniae (1998-2004)
| Central hospital district | No. of isolates (% resistant)
|
||||||
|---|---|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | |
| Varsinais-Suomi | 194 (25.8) | 250 (30.0) | 138 (11.6) | 269 (18.6) | 239 (18.9) | 241 (19.1) | |
| Satakunta | 221 (15.0) | 139 (15.8) | 111 (15.3) | 117 (21.4) | 126 (19.0) | 137 (16.8) | 120 (25.8) |
| Kanta-Häme | 116 (15.5) | 72 (29.2) | 93 (23.7) | 79 (19.0) | 73 (16.4) | 98 (9.2) | 103 (12.6) |
| Pirkanmaa | 356 (17.4) | 349 (18.1) | 380 (21.1) | 320 (20.9) | 289 (18.0) | 275 (17.1) | 336 (18.8) |
| Päijät-Häme | 52 (5.8) | 82 (14.6) | 159 (13.2) | 183 (9.8) | 112 (9.8) | 116 (12.9) | 64 (23.4) |
| Kymenlaakso | 121 (45.0) | 134 (16.4) | 96 (11.5) | 110 (7.3) | 74 (13.5) | 70 (12.9) | 59 (18.6) |
| Etelä-Karjala | 88 (15.9) | 101 (17.8) | 96 (12.5) | 95 (17.9) | |||
| Etelä-Savo | 140 (11.0) | 207 (14.0) | 118 (16.1) | 115 (5.2) | 92 (22.8) | 99 (11.1) | 83 (16.9) |
| Itä-Savo | 70 (35.7) | 55 (12.7) | 40 (10.0) | 48 (4.2) | 42 (9.5) | ||
| Pohjois-Karjala | 210 (10.0) | 199 (13.6) | 179 (19.6) | 167 (15.6) | 103 (6.8) | 127 (9.4) | 102 (11.8) |
| Pohjois-Savo | 40 (25.0) | 108 (16.0) | 336 (13.1) | 337 (12.8) | 281 (10.3) | 363 (14.6) | 258 (17.4) |
| Keski-Suomi | 150 (14.7) | 151 (13.9) | 119 (11.8) | ||||
| Etelä-Pohjanmaa | |||||||
| Vaasa | 89 (15.7) | 132 (10.6) | 141 (4.3) | 113 (9.7) | 83 (14.5) | ||
| Keski-Pohjanmaa | 9 (0) | ||||||
| Pohjois-Pohjanmaa | 789 (17.0) | 1,000 (16.0) | 754 (13.9) | 529 (16.3) | 479 (23.4) | ||
| Kainuu | |||||||
| Länsi-Pohja | 48 (12.5) | ||||||
| Lappi | 29 (24.1) | 24 (25) | |||||
| Ahvenanmaa | |||||||
| Helsinki and Uusimaa | 672 (16.2) | 1,067 (26.1) | 645 (15.5) | 1,397 (16.3) | 1,075 (13.7) | 1,501 (19.3) | 1,562 (20.5) |
TABLE 3.
Regional SXT resistance in Haemophilus influenzae (1998-2004)
| Central Hospital district | No. of isolates (% resistant)
|
||||||
|---|---|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | |
| Varsinais-Suomi | 422 (19.2) | 334 (21.3) | 425 (31.5) | 260 (21.5) | 129 (15.5) | 234 (10.3) | 211 (19) |
| Satakunta | 117 (21.4) | 100 (11.0) | 99 (7.1) | 86 (4.7) | 93 (11.8) | 77 (10.4) | |
| Kanta-Häme | 164 (18.0) | 80 (10.0) | 130 (21.5) | 152 (6.6) | 101 (6.9) | 107 (9.3) | 83 (10.8) |
| Pirkanmaa | 351 (12.0) | 352 (17.0) | 305 (15.4) | 302 (7.9) | 238 (8.0) | 217 (8.3) | 158 (15.8) |
| Päijät-Häme | 125 (18.0) | 110 (13.6) | 102 (21.6) | 130 (11.5) | 74 (9.5) | 75 (13.3) | 54 (22.2) |
| Kymenlaakso | 158 (19.0) | 134 (20.9) | 133 (10.5) | 77 (6.5) | 89 (9) | 112 (11.6) | |
| Etelä-Karjala | 190 (15.0) | 156 (16.7) | 147 (17.7) | 192 (10.9) | 97 (13.4) | 86 (7) | 67 (11.9) |
| Etelä-Savo | 170 (14.0) | 181 (15.5) | 143 (15.4) | 166 (15.7) | 87 (6.9) | 96 (8.3) | 83 (4.8) |
| Itä-Savo | 53 (18.9) | 42 (2.4) | 34 (8.8) | 40 (5) | 33 (24.2) | ||
| Pohjois-Karjala | 174 (14.0) | 173 (15.0) | 136 (15.4) | 120 (11.7) | 85 (14.1) | 103 (6.8) | 85 (17.6) |
| Pohjois-Savo | 505 (11.0) | 482 (13.9) | 343 (14.9) | 346 (10.1) | 229 (12.2) | 338 (10.7) | 278 (17.3) |
| Keski-Suomi | 242 (11.0) | 242 (19.4) | 175 (12.0) | 175 (20.6) | 118 (11.9) | 133 (9) | 98 (13.3) |
| Etelä-Pohjanmaa | 209 (13.4) | 197 (17.8) | 183 (9.8) | 117 (6.0) | 132 (12.1) | 113 (9.7) | |
| Vaasa | 18 (6.0) | 168 (10.1) | 123 (12.2) | 130 (8.5) | 137 (14.6) | 158 (9.5) | 90 (10) |
| Keski-Pohjanmaa | 54 (9.3) | ||||||
| Pohjois-Pohjanmaa | 469 (10.0) | 441 (11.1) | 826 (16.7) | 676 (8.9) | 538 (8.0) | 516 (5.6) | 398 (7.5) |
| Kainuu | |||||||
| Länsi-Pohja | 98 (6.0) | 82 (7.3) | 44 (9.1) | 55 (1.8) | 48 (8.3) | 50 (8) | 28 (7.1) |
| Lappi | 77 (10.0) | 62 (8.1) | 59 (11.9) | 52 (17.3) | 36 (11.1) | 26 (7.7) | 17 (11.8) |
| Ahvenanmaa | |||||||
| Helsinki and Uusimaa | 1,341 (13.6) | 1,132 (26.8) | 1,674 (20.0) | 1,220 (12.7) | 812 (13.5) | 1,251 (9.3) | 1,120 (13.8) |
TABLE 4.
Regional SXT resistance in Moraxella catarrhalis (1998-2004)
| Central Hospital district | No. of isolates (% resistant)
|
||||||
|---|---|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | |
| Varsinais-Suomi | 257 (21.2) | 248 (20.6) | 294 (27.9) | 115 (3.5) | 99 (4.0) | 133 (10.5) | 121 (15.7) |
| Satakunta | 66 (6.1) | 35 (2.9) | 50 (2.0) | 45 (0.0) | 37 (0.0) | 39 (2.6) | |
| Kanta-Häme | 67 (25.0) | 66 (15.2) | 46 (13.0) | 63 (9.5) | 24 (4.2) | 70 (15.7) | 42 (7.1) |
| Pirkanmaa | 98 (8.2) | 101 (3.0) | 105 (5.7) | 75 (8.0) | 83 (4.8) | ||
| Päijät-Häme | 145 (5.0) | 90 (3.3) | 143 (3.5) | 121 (7.4) | 78 (5.1) | 72 (8.3) | 33 (0.0) |
| Kymenlaakso | 72 (1.4) | 66 (0.0) | 42 (2.4) | 33 (6.1) | 36 (5.6) | 29 (3.4) | |
| Etelä-Karjala | 82 (1.0) | 84 (7.1) | 106 (8.5) | 96 (4.2) | 48 (2.1) | 31 (12.9) | 28 (7.1) |
| Etelä-Savo | 39 (8.0) | 70 (12.9) | 67 (6.0) | 64 (12.5) | 27 (0.0) | 24 (8.3) | 30 (3.3) |
| Itä-Savo | 44 (4.5) | 27 (3.7) | 20 (5.0) | 11 (0.0) | 16 (6.3) | ||
| Pohjois-Karjala | 72 (14.0) | 77 (0.0) | 74 (5.4) | 60 (3.3) | 42 (0.0) | 44 (4.5) | 45 (11.1) |
| Pohjois-Savo | 285 (4.0) | 212 (7.1) | 181 (3.3) | 165 (6.1) | 119 (4.2) | 157 (6.4) | 141 (4.3) |
| Keski-Suomi | 117 (3.0) | 92 (2.2) | 85 (3.5) | 106 (2.8) | 67 (1.5) | 61 (0.0) | 58 (0.0) |
| Etelä-Pohjanmaa | 123 (9.8) | 97 (14.4) | 91 (4.4) | 80 (3.8) | 60 (6.7) | 50 (2.0) | |
| Vaasa | 84 (2.4) | 68 (1.5) | 89 (5.6) | 68 (4.4) | 81 (3.7) | 52 (3.8) | |
| Keski-Pohjanmaa | |||||||
| Pohjois-Pohjanmaa | 184 (4.0) | 200 (5.0) | 396 (2.3) | 327 (4.3) | 57 (3.5) | 109 (8.3) | 158 (7.6) |
| Kainuu | |||||||
| Länsi-Pohja | 46 (0.0) | 38 (0.0) | 53 (3.8) | 41 (9.8) | 29 (0.0) | 28 (0.0) | 24 (4.2) |
| Lappi | 45 (7.0) | 35 (14.3) | 25 (16.0) | 25 (0.0) | 21 (0.0) | 15 (0.0) | |
| Ahvenanmaa | |||||||
| Helsinki and Uusimaa | 723 (25.2) | 603 (19.1) | 853 (8.8) | 665 (6.6) | 336 (2.7) | 792 (2.3) | 854 (2.2) |
The relationships between regional SXT consumption and resistance are summarized in Table 5. The regional consumption of SXT seems to have an effect on regional resistance among S. pneumoniae isolates (P = 0.007). The association is positive, indicating that the greater the consumption, the higher the resistance in the following year. The change of resistance over time was not significant (P = 0.452). The significance in change of resistance over time was borderline for H. influenzae (P = 0.051), but consumption of SXT failed to explain the level of resistance (P = 0.808). No significant change in SXT resistance was seen among M. catarrhalis isolates (P = 0.349), and again, consumption failed to explain the level of resistance (P = 0.744).
TABLE 5.
Connection between SXT use and resistance among S. pneumoniae, H. influenzae, and M. catarrhalis isolates
| Organism and effect | Parameter estimate | SE | df | P value |
|---|---|---|---|---|
| S. pneumoniae | ||||
| Intercept | 3.21 | 6.05 | 15 | 0.604 |
| Time | 0.45 | 0.59 | 14 | 0.452 |
| Consumption | 17.08 | 6.07 | 60 | 0.007 |
| H. influenzae | ||||
| Intercept | 16.62 | 4.70 | 18 | 0.002 |
| Time | −0.79 | 0.38 | 17 | 0.051 |
| Consumption | −1.22 | 5.02 | 80 | 0.808 |
| M. catarrhalis | ||||
| Intercept | 6.89 | 5.75 | 17 | 0.248 |
| Time | −0.47 | 0.49 | 16 | 0.349 |
| Consumption | 1.97 | 6.00 | 65 | 0.744 |
DISCUSSION
Our study over a 7-year period shows a positive association between the regional consumption of SXT and resistance in the following year for S. pneumoniae but not for H. influenzae or M. catarrhalis.
SXT resistance in Finland increased among pneumococci from the end of the 1980s until 1999 and after that decreased slightly, only to rise again. The same pattern was seen for H. influenzae. For M. catarrhalis, resistance increased in the 1990s but has since decreased to a very low level (4.3% in 2004). At the regional level, time on its own did not explain changes in resistance among S. pneumoniae and M. catarrhalis isolates, and among H. influenzae isolates, the change in resistance was borderline. When the resistance levels are estimated at the national level, it seems that a decreasing trend exists for M. catarrhalis. For H. influenzae or S. pneumoniae, such conclusions cannot be drawn.
There are several studies showing significant correlations between antimicrobial consumption and resistance in S. pneumoniae. Most often, the use of macrolides has been linked to erythromycin resistance. The coselection of penicillin-resistant pneumococci has been linked to the use of several drugs or drug classes (7). In Finland (20), the regional resistance rates of consecutive pneumococcal isolates were compared to rates of use of several antimicrobials for the two previous years, and a statistically significant correlation was found between SXT consumption and resistance (P = 0.043). The connection between the use of SXT and resistance in pneumococci has also been shown at the individual level (16). In contrast, no link between macrolide use and azithromycin resistance was observed among invasive or noninvasive H. influenzae isolates in Slovenia (6). In Finland (19), the increase in use of cephalosporins was linked to increased prevalence of β-lactamases in M. catarrhalis but not in H. influenzae. In Sweden, the production of β-lactamase was compared to the total use of antibiotics in areas of high consumption and low consumption (18). Among M. catarrhalis isolates, the number of β-lactamase producers was significantly higher in the area of highest mean consumption of all antibiotics. Among H. influenzae isolates, no significant difference was shown, although larger numbers of β-lactamase-positive isolates were detected in the areas of high antimicrobial consumption.
In the 1970s and 1980s, TMP resistance was linked to increased consumption of TMP alone and of SXT for E. coli (15), but not later, for E. coli isolates from urinary tract infections (17). Thus, it seems that restricting the use of SXT may result in resistance declining, but only in pneumococci. On the other hand, M. catarrhalis may be subject to selection pressure, but not with SXT. In the case of H. influenzae, the present study supports the results of the few previous studies, showing that resistance in H. influenzae is not highly susceptible to selective pressure.
Despite the decrease in consumption at the national level, increases in resistance among pneumococci and also among H. influenzae isolates from 2002 to 2004 were evident (Fig. 2). In Iceland, the spread of an international multiresistant clone explained the increased carriage of non-penicillin-susceptible pneumococci in an area of low and declining use of antimicrobials (1). Resistance against erythromycin, tetracycline, chloramphenicol, and SXT was very common among these isolates. Similarly, in Spain, dissemination of several multiresistant pneumococcal clones was associated with a high prevalence of macrolide resistance in pneumococci (5). We are not able to analyze multiresistance among single S. pneumoniae isolates from our resistance data, and the isolates have not been serotyped. Consequently, we cannot confirm the occurrence of multiresistant clones in Finland, which in our opinion may explain this increase in resistance. Our view is supported indirectly by the increase in penicillin nonsusceptibility (from 8.5% to 10.8%) and erythromycin resistance (from 15.4% to 20.2%) between 2002 and 2004 in all S. pneumoniae isolates (http://www.ktl.fi/portal/english/projects/fire/finres). The clonality among multidrug-resistant H. influenzae strains is not as obvious as that among pneumococci (10). Therefore, the reason for the increase in SXT resistance remains open at this point.
Our method differs from that of many studies in that we compared consumption and resistance over a relatively long period (7 years), and also geographically. The consumption data used in our analysis (sales from wholesalers) are estimated to represent at least 99% of the actual sales. Resistance data were not available from two (2/21 districts) central hospital districts. The remaining 19 districts cover ∼98% of the population in Finland. Thus, our data have an extensive coverage. In Finland, resistance data have been collected annually from all health care districts since 1997. As shown in Tables 2, 3, and 4, the reporting activity for SXT resistance has varied, especially in the case of pneumococci. The large amount of missing information may have influenced the analysis of pneumococci. Also, there are other factors possibly biasing the results. The resistance data are not based on a one-per-patient principle; a common practice in Finnish laboratories, however, is to perform susceptibility testing only once if the same pathogen is cultured from several samples during an infection episode. On the other hand, the regional consumption figures may include prescriptions purchased in one health care district but used elsewhere. Parts of prescriptions may even remain unused. S. pneumoniae, H. influenzae, and M. catarrhalis cause infections in and are carried mainly by young children; therefore, antimicrobial consumption by this age group can be considered most important for the development and spread of resistance. Our consumption data and resistance data were, however, collected during the study period as total figures, and we are not able to analyze the connection by age groups. From the FINRES data of 2005 (http://www.ktl.fi/portal/english/projects/fire/finres), we know that SXT resistance was higher (23.1%) among young children (<5 years) than in the rest of the population (16.2%). It is also known that 60% of SXT has been prescribed for otitis media, an infection presenting mostly in children of <5 years old (21). However, we do not know how these figures have altered during the study period. Therefore, the effect of age on our results cannot reliably be evaluated.
In conclusion, the use of SXT explains changes in resistance in pneumococci but not in H. influenzae and M. catarrhalis. The selection of coresistance and persistence of resistance mechanisms may increase and maintain resistance against SXT in respiratory tract pathogens despite declining consumption of the drug.
Acknowledgments
This study was supported by a special government grant (EVO grant) from the Päijät-Häme Health Care District (PK), by the Turku University Foundation, by The Finnish Medical Foundation, by the Research Foundation of Orion Corporation (MB), and by the Academy of Finland (PH).
Members of the FiRe network in 2004 were Petteri Carlson and Merja Rautio (Jorvi Hospital), Risto Renkonen and Anna Muotiala (Medix-Diacor Laboratories), Martti Vaara and Eveliina Tarkka (HUSLAB), Riitta Karttunen and Tarja Ojanen (Kanta-Häme Central Hospital), Jari Karhukorpi (Pohjois-Karjala Central Hospital), Antti Nissinen (Keski-Suomi Central Hospital), Pekka Ruuska (Kainuu Central Hospital), Henrik Jägerroos (Lappi Central Hospital), Martti Larikka (Länsi-Pohja Central Hospital), Simo Räisänen (Keski-Pohjanmaa Central Hospital), Ulla Larinkari and Benita Forsblom (Kymenlaakso Central Hospital), Ulla Kärkkäinen (Kuopio University Hospital), Hannu Sarkkinen and Pauliina Kärpänoja (Päijät-Häme Central Hospital), Maritta Kauppinen and Seppo Paltemaa (Etelä-Karjala Central Hospital), Päivi Kärkkäinen (Mikkeli Central Hospital and Savonlinna Central Hospital), Markku Koskela (Oulu University Hospital), Sini Pajarre and Raija Manninen (Satakunta Central Hospital), Sinikka Oinonen and Virpi Ratia (Seinäjoki Central Hospital), Paul Grönroos (Health Center Koskiklinikka), Risto Vuento and Oili Liimatainen (Tampere University Hospital), Maj-Rita Siro (Health Center Pulssi); Erkki Eerola (Turku University), Olli Meurman and Kaisu Rantakokko-Jalava (Turku University Hospital), Suvi-Sirkku Kaukoranta (Vaasa Central Hospital), and Pentti Huovinen and Katrina Lager (National Public Health Institute).
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Arason, V. A., A. Gunnlaugsson, J. A. Sigurdson, H. Erlendsdottir, S. Gudmundsson, and K. G. Kristinsson. 2002. Clonal spread of resistant pneumococci despite diminished antimicrobial use. Microb. Drug Resist. 8:187-192. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, F., G. Baquero-Artiago, R. Canton, and C. Garcia-Rey. 2002. Antibiotic consumption and resistance selection in Streptococcus pneumoniae. J. Antimicrob. Chemother. 50(Suppl. S2):27-37. [DOI] [PubMed] [Google Scholar]
- 3.Bean, D. C., D. M. Livermore, I. Papa, and L. M. C. Hall. 2005. Resistance among Escherichia coli to sulphonamides and other antimicrobials now little used in man. J. Antimicrob. Chemother. 56:962-964. [DOI] [PubMed] [Google Scholar]
- 4.Bergman, M., S. Huikko, M. Pihlajamäki, P. Laippala, E. Palva, P. Huovinen, H. Seppälä, and the Finnish Study Group for Antimicrobial Resistance (FiRe Network). 2004. Effect of macrolide consumption on erythromycin resistance in Streptococcus pyogenes in Finland in 1997-2001. Clin. Infect. Dis. 38:1251-1256. [DOI] [PubMed] [Google Scholar]
- 5.Calatayud, L., C. Ardanuy, E. Cercenado, A. Fenoll, E. Bouza, R. Pallares, R. Martin, and J. Linares. 2007. Serotypes, clones and mechanisms of resistance of erythromycin-resistant Streptococcus pneumoniae isolates collected in Spain. Antimicrob. Agents Chemother. 51:3240-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cizman, M., M. Pokorn, K. Seme, A. Orazem, and M. Paragi. 2001. The relationship between trends in macrolide use and resistance to macrolides of common respiratory pathogens. J. Antimicrob. Chemother. 47:475-477. [DOI] [PubMed] [Google Scholar]
- 7.Cizman, M. 2003. The use and resistance to antibiotics in the community. Int. J. Antimicrob. Agents 21:297-307. [DOI] [PubMed] [Google Scholar]
- 8.Clinical Laboratory Standards Institute. 2006. Methods for dilution antimicrobial tests for bacteria that grow aerobically; approved standard—7th ed. Clinical Laboratory Standards Institute document M7-A7. CLSI, Wayne, PA.
- 9.Doern, G. V., S. S. Richter, A. Miller, N. Miller, C. Rice, K. Heilman, and S. Beekman. 2005. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin. Infect. Dis. 41:139-148. [DOI] [PubMed] [Google Scholar]
- 10.Fuste, M. C., M. A. Pineda, J. Palomar, M. Vinas, and J. G. Loren. 1996. Clonality of multidrug-resistant nontypeable strains of Haemophilus influenzae. J. Clin. Microbiol. 34:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Rey, C., L. Aguilar, F. Baquero, J. Casal, and J. E. Martin. 2002. Pharmacoepidemiological analysis of provincial differences between consumption of macrolides and rates of erythromycin resistance among Streptococcus pyogenes isolates in Spain. J. Clin. Microbiol. 40:2959-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoban, D. J., G. V. Doern, A. C. Fluit, M. Roussel-Delvallez, and R. N. Jones. 2001. Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in the SENTRY antimicrobial surveillance program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S81-S93. [DOI] [PubMed] [Google Scholar]
- 13.Huovinen, P. 2001. Resistance to SXT. Clin. Infect. Dis. 32:1608-1614. [DOI] [PubMed] [Google Scholar]
- 14.Huovinen, P., L. Sundström, G. Swedberg, and O. Sköld. 1995. Trimethoprim and sulfonamide resistance. Antimicrob. Agents Chemother. 39:279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huovinen, P., L. Pulkkinen, H.-L. Helin, M. Mäkilä, and P. Toivanen. 1986. Emergence of trimethoprim resistance in relation to drug consumption in a Finnish hospital from 1971 through 1984. Antimicrob. Agents Chemother. 29:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordano, Q., V. Falco, B. Almirante, A. M. Planes, O. del Valle, E. Ribera, O. Len, C. Pigrau, and A. Pahissa. 2004. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 38:1623-1628. [DOI] [PubMed] [Google Scholar]
- 17.Kahlmeter, G., P. Menday, and O. Cars. 2003. Non-hospital antimicrobial usage and resistance in community-acquired Escherichia coli urinary tract infection. J. Antimicrob. Chemother. 52:1005-1010. [DOI] [PubMed] [Google Scholar]
- 18.Mölstad, S., E. Arvidsson, I. Eliasson, B. Hovelius, C. Kamme, and C. Schalen. 1992. Production of betalactamase by respiratory tract bacteria in children: relationship to antibiotic use. Scand. J. Prim. Health Care 10:16-20. [DOI] [PubMed] [Google Scholar]
- 19.Nissinen, A., P. Grönroos, P. Huovinen, E. Herva, M.-L. Katila, T. Klaukka, S. Kontiainen, O. Liimatainen, S. Oinonen, and P. H. Mäkelä. 1995. Development of (-lactamase-mediated resistance to penicillin in middle-ear isolates of Moraxella catarrhalis in Finnish children, 1978-1993. Clin. Infect. Dis. 21:1193-1196. [DOI] [PubMed] [Google Scholar]
- 20.Pihlajamäki, M., P. Kotilainen, T. Kaurila, T. Klaukka, E. Palva, P. Huovinen, and the Finnish Study Group for Antimicrobial Resistance (FiRe Network). 2001. Macrolide-resistant Streptococcus pneumoniae and use of antimicrobial agents. Clin. Infect. Dis. 33:483-488. [DOI] [PubMed] [Google Scholar]
- 21.Rautakorpi, U.-M., T. Klaukka, P. Honkanen, M. Mäkelä, T. Nikkarinen, E. Palva, R. Roine, H. Sarkkinen, and P. Huovinen on behalf of the MIKSTRA Collaborative Study Group. 2001. Antibiotic use by indication: a basis for active antibiotic policy in the community. Scand. J. Infect. Dis. 33:920-926. [DOI] [PubMed] [Google Scholar]
- 22.Sahm, D. F., M. E. Jones, M. L. Hickey, D. R. Diakun, S. V. Mani, and C. Thornsberry. 2000. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in Asia and Europe, 1997-1998. J. Antimicrob. Chemother. 45:457-466. [DOI] [PubMed] [Google Scholar]
- 23.Seppälä, H., T. Klaukka, J. Vuopio-Varkila, A. Muotiala, H. Helenius, K. Lager, P. Huovinen, and the Finnish Study Group for Antimicrobial Resistance. 1997. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N. Engl. J. Med. 337:441-446. [DOI] [PubMed] [Google Scholar]